Design, Synthesis, and Anti-Tyrosinase, Anti-Melanogenic, and Antioxidant Activities of Novel (Z)-3-Benzyl-5-Benzylidene-2-Thioxothiazolidin-4-One Analogs
Abstract
1. Introduction
2. Results and Discussion
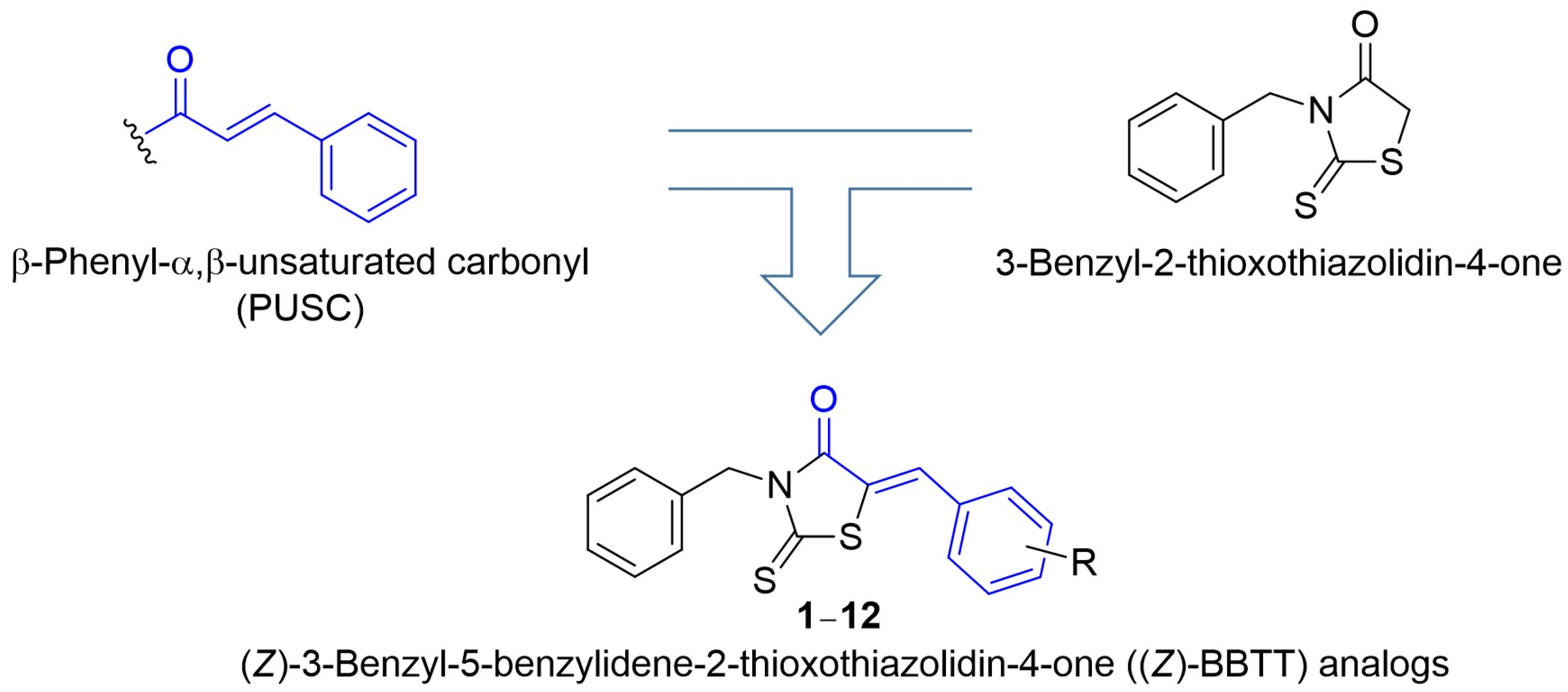
2.1. Synthesis of Target Compounds (Z)-BBTT Analogs 1–12
2.2. Inhibitory Activity of (Z)-BBTT Analogs 1–12 Against Mushroom Tyrosinase
2.3. Kinetic Analysis of Mushroom Tyrosinase Using Lineweaver–Burk Plots
2.4. In Silico Docking Simulation of (Z)-BBTT Analogs and Mushroom Tyrosinase
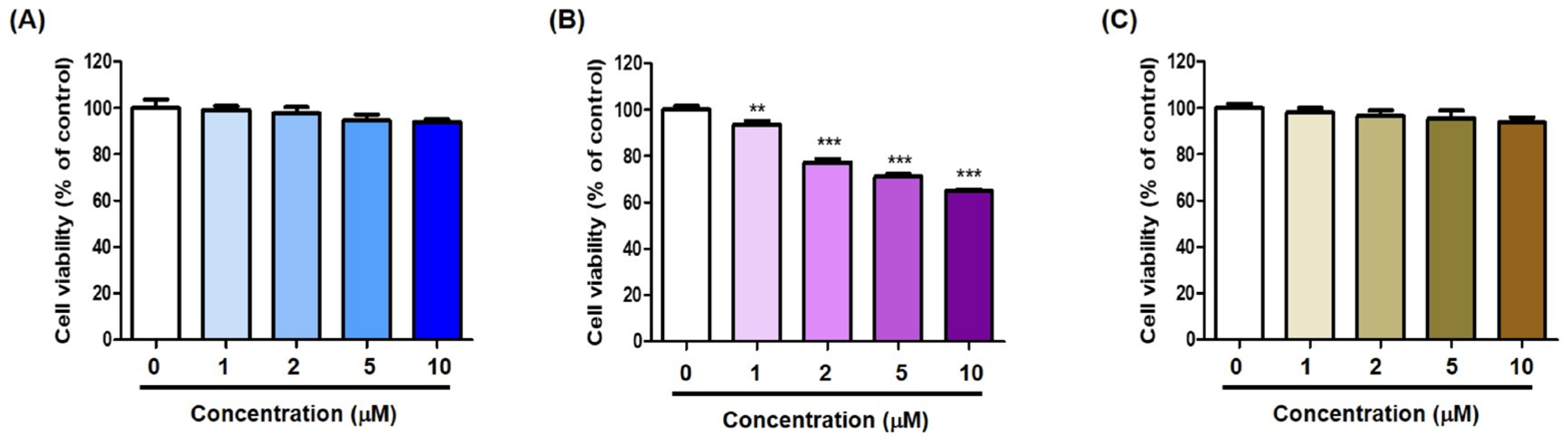
2.5. Cell Viability of (Z)-BBTT Analogs on B16F10 Cells
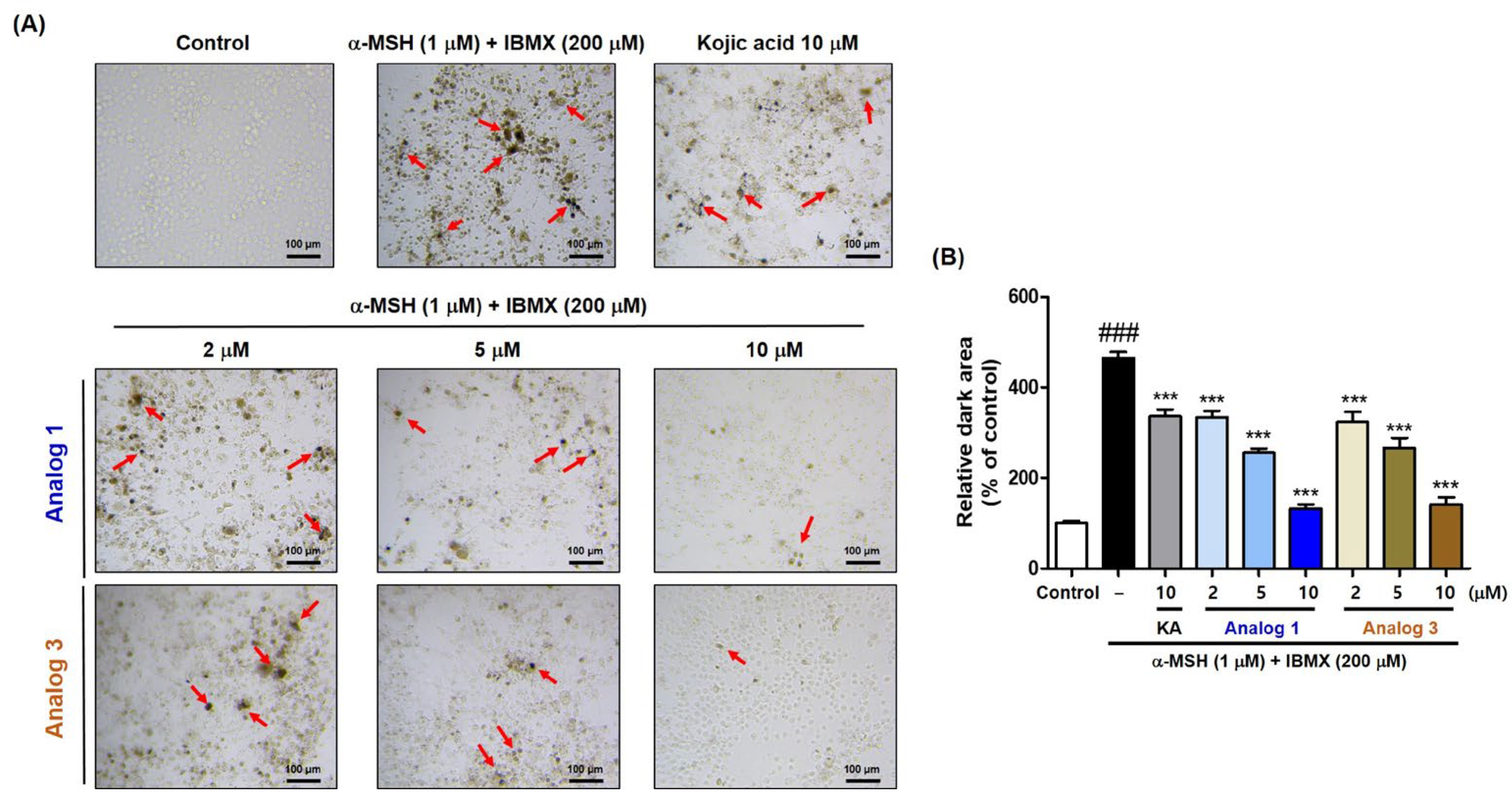
2.6. Effect of (Z)-BBTT Analogs 1 and 3 on Melanin Production in B16F10 Cells
2.7. Effect of (Z)-BBTT Analogs 1 and 3 on Tyrosinase Activity in B16F10 Cells
2.8. In Situ B16F10 Cell Tyrosinase Inhibitory Activity of (Z)-BBTT Analogs 1 and 3
2.9. Antioxidant Capacity of (Z)-BBTT Analogs 1–12
2.9.1. ABTS+ Radical-Scavenging Capacity
2.9.2. DPPH Radical-Scavenging Capacity
2.9.3. ROS-Scavenging Capacity
2.10. Effects of Analogs 1–3 on HaCaT Cell Viability
3. Materials and Methods
3.1. Synthesis
3.1.1. General Methods
3.1.2. Synthesis of 3-Benzyl-2-Thioxothiazolidin-4-One (13) [34,35]
3.1.3. General Preparation of (Z)-BBTT Analogs 1–12
- (Z)-3-Benzyl-5-(4-hydroxybenzylidene)-2-thioxothiazolidin-4-one (1).1H NMR (dimethyl sulfoxide [DMSO]-d6, 500 MHz) δ 10.51 (s, 1H, OH), 7.76 (s, 1H, vinyl H), 7.52 (d, 2H, J = 8.5 Hz, 2′-H, 6′-H), 7.35–7.26 (m, 5H, Ph), 6.94 (d, 2H, J = 8.5 Hz, 3′-H, 5′-H), 5.23 (s, 2H, benzyl H2); 13C NMR (DMSO-d6, 125 MHz) δ 193.7, 167.5, 161.2, 135.5, 134.7, 133.9, 129.0, 128.1 (2 × C), 124.4, 117.9, 117.1, 47.5; LR-MS (ESI−) m/z 326 (M − H)−; yield, 76%; molecular formula, C17H13NO2S2; HR-MS (EDA) m/z C17H14NO2S2 (M + H)+ calcd. 328.0466, obsd. 328.0460; melting point, 199–201 °C; brown solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.38.
- (Z)-3-Benzyl-5-(3,4-dihydroxybenzylidene)-2-thioxothiazolidin-4-one (2).1H NMR (DMSO-d6, 500 MHz) δ 10.06 (s, 1H, OH), 9.59 (s, 1H, OH), 7.68 (s, 1H, vinyl H), 7.35–7.25 (m, 5H, Ph), 7.08–7.04 (m, 2H, 2′-H, 6′-H), 6.90 (d, 1H, J = 8.5 Hz, 5′-H), 5.23 (s, 2H, benzyl H2); 13C NMR (DMSO-d6, 125 MHz) δ 193.7, 167.5, 150.1, 146.6, 135.5, 135.1 (2 × C), 129.0, 128.1, 125.9, 124.8, 117.7, 117.2, 117.0, 47.5; LR-MS (ESI−) m/z 342 (M − H)−; yield, 84%; molecular formula, C17H13NO3S2; HR-MS (EDA) m/z C17H14NO3S2 (M + H)+ calcd. 344.0415, obsd. 344.0410; melting point, 140–142 °C; brown solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.10.
- (Z)-3-Benzyl-5-(2,4-dihydroxybenzylidene)-2-thioxothiazolidin-4-one (3).1H NMR (DMSO-d6, 500 MHz) δ 10.76 (s, 1H, OH), 10.42 (s, 1H, OH), 7.99 (s, 1H, vinyl H), 7.35–7.25 (m, 5H, Ph), 7.22 (d, 1H, J = 8.5 Hz, 6′-H), 6.45–6.43 (m, 2H, 3′-H, 5′-H), 5.22 (s, 2H, benzyl H2); 13C NMR (DMSO-d6, 125 MHz) δ 193.9, 167.7, 163.3, 160.5, 135.6, 132.1, 130.4, 128.9, 128.0 (2 × C), 115.6, 112.4, 109.5, 102.9, 47.3; LR-MS (ESI−) m/z 342 (M − H)−; yield, 74%; molecular formula, C17H13NO3S2; HR-MS (EDA) m/z C17H14NO3S2 (M + H)+ calcd. 344.0415, obsd. 344.0412; melting point, 191–193 °C; copper-colored solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.10.
- (Z)-3-Benzyl-5-(4-hydroxy-3-methoxybenzylidene)-2-thioxothiazolidin-4-one (4).1H NMR (CDCl3, 500 MHz) δ 7.67 (s, 1H, vinyl H), 7.47 (d, 2H, J = 7.0 Hz, 2-H, 6-H), 7.33–7.28 (m, 3H, 3-H, 4-H, 5-H), 7.08 (brd, 1H, J = 8.0 Hz, 6′-H), 7.00 (d, 1H, J = 8.0 Hz, 5′-H), 6.95 (s, 1H, 2′-H), 6.04 (s, 1H, OH), 5.32 (s, 2H, benzyl H2), 3.95 (s, 3H, OCH3); 13C NMR (CDCl3, 125 MHz) δ 193.0, 167.9, 148.6, 147.1, 134.9, 133.9, 129.0, 128.6, 128.1, 126.4, 126.0, 119.8, 115.4, 112.0, 56.1, 47.5; LR-MS (ESI−) m/z 356 (M − H)−; yield, 68%; molecular formula, C18H15NO3S2; melting point, 157–159 °C (lit. [19] 151–154 °C); brown solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.29.
- (Z)-3-Benzyl-5-(3-hydroxy-4-methoxybenzylidene)-2-thioxothiazolidin-4-one (5).1H NMR (CDCl3, 500 MHz) δ 7.64 (s, 1H, vinyl H), 7.47 (d, 2H, J = 7.0 Hz, 2-H, 6-H), 7.33–7.27 (m, 3H, 3-H, 4-H, 5-H), 7.07–7.04 (m, 2H, 2′-H, 6′-H), 6.92 (d, 1H, J = 8.0 Hz, 5′-H), 5.72 (s, 1H, OH), 5.32 (s, 2H, benzyl H2), 3.95 (s, 3H, OCH3); 13C NMR (CDCl3, 125 MHz) δ 193.3, 168.0, 148.9, 146.2, 134.9, 133.6, 129.0, 128.6, 128.1, 126.9, 124.6, 120.7, 116.1, 111.0, 56.2, 47.5; LR-MS (ESI−) m/z 356 (M − H)−; yield, 54%; molecular formula, C18H15NO3S2; melting point, 173–175 °C; yellow solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.28.
- (Z)-3-Benzyl-5-(4-methoxybenzylidene)-2-thioxothiazolidin-4-one (6).1H NMR (CDCl3, 500 MHz) δ 7.70 (s, 1H, vinyl H), 7.47 (d, 2H, J = 7.0 Hz, 2-H, 6-H), 7.45 (d, 2H, J = 9.0 Hz, 2′-H, 6′-H), 7.33–7.27 (m, 3H, 3-H, 4-H, 5-H), 6.99 (d, 2H, J = 9.0 Hz, 3′-H, 5′-H), 5.32 (s, 2H, benzyl H2), 3.87 (s, 3H, OCH3); 13C NMR (CDCl3, 125 MHz) δ 193.2, 168.0, 161.8, 135.0, 133.5, 132.8, 129.0, 128.6, 128.1, 126.0, 119.9, 115.0, 55.6, 47.5; yield, 69%; molecular formula, C18H15NO2S2; HR-MS (EDA) m/z C18H16NO2S2 (M + H)+ calcd. 342.0622, obsd. 342.0619; melting point, 146–148 °C (lit. [19] 142–145 °C); copper-colored solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.63.
- (Z)-3-Benzyl-5-(3,4-dimethoxybenzylidene)-2-thioxothiazolidin-4-one (7).1H NMR (CDCl3, 500 MHz) δ 7.69 (s, 1H, vinyl H), 7.47 (d, 2H, J = 7.0 Hz, 2-H, 6-H), 7.33–7.27 (m, 3H, 3-H, 4-H, 5-H), 7.13 (d, 1H, J = 8.5 Hz, 6′-H), 6.97 (s, 1H, 2′-H), 6.95 (d, 1H, J = 8.5 Hz, 5′-H), 5.32 (s, 2H, benzyl H2), 3.94 (s, 6H, 2 × OCH3); 13C NMR (CDCl3, 125 MHz) δ 193.0, 167.9, 151.6, 149.5, 134.9, 133.7, 129.0, 128.6, 128.1, 126.3, 125.7, 120.1, 112.4, 111.5, 56.1, 56.0, 47.5; LR-MS (ESI+) m/z 372 (M + H)+, 394 (M + Na)+; yield, 52%; molecular formula, C19H17NO3S2; melting point, 166–168 °C; yellow solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.45.
- (Z)-3-Benzyl-5-(2,4-dimethoxybenzylidene)-2-thioxothiazolidin-4-one (8).1H NMR (CDCl3, 500 MHz) δ 8.07 (s, 1H, vinyl H), 7.48 (d, 2H, J = 7.5 Hz, 2-H, 6-H), 7.33–7.25 (m, 4H, 3-H, 4-H, 5-H, 6′-H), 6.57 (dd, 1H, J = 9.0, 2.0 Hz, 5′-H), 6.45 (d, 1H, J = 2.0 Hz, 3′-H), 5.32 (s, 2H, benzyl H2), 3.88 (s, 3H, OCH3), 3.86 (s, 3H, OCH3); 13C NMR (CDCl3, 125 MHz) δ 194.0, 168.1, 163.9, 160.4, 135.1, 132.1, 129.6, 129.0, 128.5, 128.0, 119.4, 115.7, 105.9, 98.5, 55.7, 55.6, 47.5; LR-MS (ESI+) m/z 372 (M + H)+, 394 (M + Na)+; yield, 81%; molecular formula, C19H17NO3S2; melting point, 156–158 °C; brown solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.76.
- (Z)-3-Benzyl-2-thioxo-5-(3,4,5-trimethoxybenzylidene)thiazolidin-4-one (9).1H NMR (CDCl3, 500 MHz) δ 7.65 (s, 1H, vinyl H), 7.46 (d, 2H, J = 7.0 Hz, 2-H, 6-H), 7.33–7.25 (m, 3H, 3-H, 4-H, 5-H), 6.70 (s, 2H, 2′-H, 6′-H), 5.32 (s, 2H, benzyl H2), 3.92 (s, 3H, OCH3), 3.91 (s, 6H, 2 × OCH3); 13C NMR (CDCl3, 125 MHz) δ 192.9, 167.7, 153.7, 140.6, 134.8, 133.6, 129.0, 128.7, 128.6, 128.2, 121.8, 107.9, 61.1, 56.3, 47.6; LR-MS (ESI+) m/z 402 (M + H)+, 424 (M + Na)+; yield, 78% molecular formula, C20H19NO4S2; melting point, 108–110 °C; copper-colored solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.78.
- (Z)-3-Benzyl-5-(4-hydroxy-3,5-dimethoxybenzylidene)-2-thioxothiazolidin-4-one (10).1H NMR (CDCl3, 500 MHz) δ 7.63 (s, 1H, vinyl H), 7.46 (d, 2H, J = 7.0 Hz, 2-H, 6-H), 7.33–7.25 (m, 3H, 3-H, 4-H, 5-H), 6.71 (s, 2H, 2′-H, 6′-H), 5.96 (s, 1H, OH), 5.31 (s, 2H, benzyl H2), 3.94 (s, 6H, 2 × OCH3); 13C NMR (CDCl3, 125 MHz) δ 192.9, 167.8, 147.5, 137.9, 134.9, 134.0, 128.9, 128.6, 128.1, 124.9, 120.1, 107.8, 56.5, 47.5; LR-MS (ESI+) m/z 388 (M + H)+, 410 (M + Na)+; LR-MS (ESI−) m/z 386 (M − H)−; yield, 92%; molecular formula, C19H17NO4S2; melting point, 151–153 °C; copper-colored solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.68.
- (Z)-3-Benzyl-5-(3-bromo-4-hydroxybenzylidene)-2-thioxothiazolidin-4-one (11).1H NMR (CDCl3, 500 MHz) δ 7.62 (s, 1H, 2′-H), 7.60 (s, 1H, vinyl H), 7.46 (d, 2H, J = 7.0 Hz, 2-H, 6-H), 7.37 (d, 1H, J = 8.5 Hz, 6′-H), 7.33–7.27 (m, 3H, 3-H, 4-H, 5-H), 7.11 (d, 1H, J = 8.5 Hz, 5′-H), 5.93 (brs, 1H, OH), 5.32 (s, 2H, benzyl H2); 13C NMR (CDCl3, 125 MHz) δ 192.6, 167.8, 154.4, 134.8, 134.5, 131.9, 131.4, 129.0, 128.6, 128.2, 127.6, 121.8, 117.0, 111.4, 47.6; LR-MS (ESI−) m/z 404 (M − H)−, 406 (M − H + 2)−; yield, 47%; molecular formula, C17H12BrNO2S2; melting point, 154–156 °C; yellow solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.34.
- (Z)-3-Benzyl-5-(3,5-dibromo-4-hydroxybenzylidene)-2-thioxothiazolidin-4-one (12).1H NMR (DMSO-d6, 500 MHz) δ 10.95 (brs, 1H, OH), 7.81 (s, 2H, 2′-H, 6′-H), 7.74 (s, 1H, vinyl H), 7.36–7.26 (m, 5H, Ph), 5.23 (s, 2H, benzyl H2); 13C NMR (DMSO-d6, 125 MHz) δ 193.1, 167.2, 153.7, 135.3, 134.8, 131.3 (2 × C), 129.0, 128.1, 127.8, 121.8, 112.9, 47.6; LR-MS (ESI−) m/z 482 (M − H)−, 484 (M − H + 2)−, 486 (M − H + 4)−; yield, 80%; molecular formula, C17H11Br2NO2S2; melting point, 179–181 °C; copper-colored solid; Rf (hexane:ethyl acetate = 3:1 on silica gel TLC) = 0.26.
3.2. Mushroom Tyrosinase Inhibition Assay [36,37]
3.3. Kinetic Experiment in the Presence of (Z)-BBTT Analogs 1–3 and 6 Using Mushroom Tyrosinase [38,39,40]
3.4. Docking Simulation of Mushroom Tyrosinase and (Z)-BBTT Analogs 1–3 and 6 [41,42]
3.5. Cell Culture [42,43]
3.6. Cytotoxicity Assay on B16F10 Murine Melanoma Cells [44]
3.7. Cellular Melanin Level Measurement in the Presence of (Z)-BBTT Analogs 1 and 3 in B16F10 Cells [44]
3.8. Cellular Tyrosinase Activity Measurement in the Presence of (Z)-BBTT Analogs 1 and 3 in B16F10 Cells [44]
3.9. Measurement of In Situ Cellular Tyrosinase Activity in B16F10 Cells [44,45]
3.10. ABTS•+ Scavenging Activity [46,47]
3.11. DPPH Radical-Scavenging Activity [48,49]
3.12. ROS-Scavenging Activity [50,51]
3.13. Cytotoxicity Assay on HaCaT Cells [42]
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, R.; Shanmuga, S.C.; Srinivas, C. Update on photoprotection. Indian J. Dermatol. 2012, 57, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678s–1688s. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, ultraviolet radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2014, 810, 1–16. [Google Scholar] [PubMed]
- Kaidbey, K.H.; Agin, P.P.; Sayre, R.M.; Kligman, A.M. Photoprotection by melanin—A comparison of black and Caucasian skin. J. Am. Acad. Dermatol. 1979, 1, 249–260. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment. Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M.C. Melanosomes at a glance. J. Cell Sci. 2008, 121, 3995–3999. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Human hair melanins: What we have learned and have not learned from mouse coat color pigmentation. Pigment. Cell Melanoma Res. 2011, 24, 63–74. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Tsukamoto, K. Enzymatic control of pigmentation in mammals. FASEB J. 1991, 5, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. A Chemist’s View of Melanogenesis. Pigment. Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef]
- Decker, H.; Schweikardt, T.; Tuczek, F. The first crystal structure of tyrosinase: All questions answered? Angew. Chem. Int. Ed. Engl. 2006, 45, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem.–A Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Rodríguez-López, J.; Serna-Rodríguez, P.; Tudela, J.; Varón, R.; Garcia-Cánovas, F. A continuous spectrophotometric method for the determination of diphenolase activity of tyrosinase using 3, 4-dihydroxymandelic acid. Anal. Biochem. 1991, 195, 369–374. [Google Scholar] [CrossRef]
- Gentili, V.; Turrin, G.; Marchetti, P.; Rizzo, S.; Schiuma, G.; Beltrami, S.; Cristofori, V.; Illuminati, D.; Compagnin, G.; Trapella, C.; et al. Synthesis and biological evaluation of novel rhodanine-based structures with antiviral activity towards HHV-6 virus. Bioorganic Chem. 2022, 119, 105518. [Google Scholar] [CrossRef]
- Mandal, S.P.; Garg, A.; Sahetya, S.S.; Nagendra, S.R.; Sripad, H.S.; Manjunath, M.M.; Soni, M.; Baig, R.N.; Kumar, S.V.; Kumar, B.P. Novel rhodanines with anticancer activity: Design, synthesis and CoMSIA study. RSC Adv. 2016, 6, 58641–58653. [Google Scholar] [CrossRef]
- Han, L.; Zhao, L.; Wang, H.; Dou, T.; Guo, F.; Qi, J.; Xu, W.; Piao, L.; Jin, X.; Fen’er, C.; et al. Synthesis, Antibacterial and Antifungal Evaluation of Rhodanine Derivatives Bearing Quinoxalinyl Imidazole Moiety as ALK5 Inhibitors. Chin. J. Org. Chem. 2021, 41, 4428–4436. [Google Scholar] [CrossRef]
- Martinez, A.; Alonso, M.; Castro, A.; Dorronsoro, I.; Gelpí, J.L.; Luque, F.J.; Pérez, C.; Moreno, F.J. SAR and 3D-QSAR studies on thiadiazolidinone derivatives: Exploration of structural requirements for glycogen synthase kinase 3 inhibitors. J. Med. Chem. 2005, 48, 7103–7112. [Google Scholar] [CrossRef] [PubMed]
- Dastyafteh, N.; Noori, M.; Montazer, M.N.; Zomorodian, K.; Yazdanpanah, S.; Iraji, A.; Ghomi, M.K.; Javanshir, S.; Asadi, M.; Dianatpour, M.; et al. New thioxothiazolidinyl-acetamides derivatives as potent urease inhibitors: Design, synthesis, in vitro inhibition, and molecular dynamic simulation. Sci. Rep. 2023, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Griffett, K. Targeting Nuclear Receptors for Chronic Inflammatory Pain: A Potential Alternative. ACS Pharmacol. Transl. Sci. 2022, 5, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zou, Y.; Chen, X.; Chen, J.; Wang, B.; Tian, J.; Ye, F.; Lu, Y.; Huang, H.; Lu, Y.; et al. Design, synthesis and biological evaluation of 3-substituted-2-thioxothiazolidin-4-one (rhodanine) derivatives as antitubercular agents against Mycobacterium tuberculosis protein tyrosine phosphatase B. Eur. J. Med. Chem. 2023, 58, 115571. [Google Scholar] [CrossRef]
- Ullah, S.; Kang, D.; Lee, S.; Ikram, M.; Park, C.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in α-MSH-stimulated B16F10 melanoma cells. Eur. J. Med. Chem. 2019, 161, 78–92. [Google Scholar] [CrossRef]
- Choi, H.; Ryu, I.Y.; Choi, I.; Ullah, S.; Jung, H.J.; Park, Y.; Hwang, Y.; Jeong, Y.; Hong, S.; Chun, P.; et al. Identification of (Z)-2-benzylidene-dihydroimidazothiazolone derivatives as tyrosinase inhibitors: Anti-melanogenic effects and in silico studies. Comput. Struct. Biotechnol. J. 2022, 20, 899–912. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, Y.; Jung, H.J.; Ullah, S.; Ko, J.; Kim, G.Y.; Yoon, D.; Hong, S.; Kang, D.; Park, Y.; et al. Anti-tyrosinase flavone derivatives and their anti-melanogenic activities: Importance of the β-phenyl-α,β-unsaturated carbonyl scaffold. Bioorganic Chem. 2023, 135, 106504. [Google Scholar] [CrossRef]
- Yoon, D.; Kang, M.K.; Jung, H.J.; Ullah, S.; Lee, J.; Jeong, Y.; Noh, S.G.; Kang, D.; Park, Y.; Chun, P.; et al. Design, Synthesis, In Vitro, and In Silico Insights of 5-(Substituted benzylidene)-2-phenylthiazol-4(5H)-one Derivatives: A Novel Class of Anti-Melanogenic Compounds. Molecules 2023, 28, 3293. [Google Scholar] [CrossRef]
- Park, Y.J.; Jung, H.J.; Kim, H.J.; Park, H.S.; Lee, J.; Yoon, D.; Kang, M.K.; Kim, G.Y.; Ullah, S.; Kang, D.; et al. Thiazol-4(5H)-one analogs as potent tyrosinase inhibitors: Synthesis, tyrosinase inhibition, antimelanogenic effect, antioxidant activity, and in silico docking simulation. Bioorganic Med. Chem. 2024, 98, 117578. [Google Scholar] [CrossRef]
- Vögeli, U.; von Philipsborn, W.; Nagarajan, K.; Nair, M.D. Structures of Addition Products of Acetylenedicarboxylic Acid Esters with Various Dinucleophiles. An application of C, H-spin-coupling constants. Helv. Chim. Acta 1978, 61, 607–617. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wu, Z.; Zheng, R.; Yin, N.; Han, F.; Zhao, Z.; Dai, M.; Han, D.; Wang, W.; Niu, L. Inhibition mechanism of melanin formation based on antioxidant scavenging of reactive oxygen species. Analyst 2022, 147, 2703–2711. [Google Scholar] [CrossRef]
- Roosta, A.; Alizadeh, A.; Rezaiyehraad, R.; Khanpour, M. Efficient and Chemoselective Procedure for Conversion of Rhodanine Derivatives into 1,3-Thiazolidine-2,4-diones via 1,3-Dipolar Cycloaddition Reaction and Rearrangement Sequences. ChemistrySelect 2020, 5, 12531–12534. [Google Scholar] [CrossRef]
- Alizadeh, A.; Chelebari, E.A.; Rezaiyehraad, R. Regio-and Chemoselective Synthesis of 4,6-Dithia-1,2,9-triazaspiro [4.4]non-2-en-8-ones through an Ultrasound-Promoted One-Pot Sequential Pseudo-Five-Component Reaction. Synthesis 2024, 56, 3199–3205. [Google Scholar] [CrossRef]
- Hyun, S.K.; Lee, W.-H.; Jeong, D.M.; Kim, Y.; Choi, J.S. Inhibitory Effects of Kurarinol, Kuraridinol, and Trifolirhizin from Sophora flavescens on Tyrosinase and Melanin Synthesis. Biol. Pharm. Bull. 2008, 31, 154–158. [Google Scholar] [CrossRef]
- Bang, E.; Noh, S.G.; Ha, S.; Jung, H.J.; Kim, D.H.; Lee, A.K.; Hyun, M.K.; Kang, D.; Lee, S.; Park, C.; et al. Evaluation of the Novel Synthetic Tyrosinase Inhibitor (Z)-3-(3-bromo-4-hydroxybenzylidene)thiochroman-4-one (MHY1498) In Vitro and In Silico. Molecules 2018, 23, 3307. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, H.J.; Park, H.S.; Kim, G.Y.; Park, Y.J.; Lee, J.; Kang, M.K.; Yoon, D.; Kang, D.; Park, Y.; et al. Highly potent anti-melanogenic effect of 2-thiobenzothiazole derivatives through nanomolar tyrosinase activity inhibition. Bioorganic Chem. 2024, 150, 107586. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, H.J.; Kim, Y.E.; Jeong, D.; Park, H.S.; Park, H.S.; Kang, D.; Park, Y.; Chun, P.; Chung, H.Y.; et al. Investigation of the Efficacy of Benzylidene-3-methyl-2-thioxothiazolidin-4-one Analogs with Antioxidant Activities on the Inhibition of Mushroom and Mammal Tyrosinases. Molecules 2024, 29, 2887. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Park, H.S.; Park, H.S.; Kim, H.J.; Yoon, D.; Park, Y.; Chun, P.; Chung, H.Y.; Moon, H.R. Exploration of Compounds with 2-Phenylbenzo[d]oxazole Scaffold as Potential Skin-Lightening Agents through Inhibition of Melanin Biosynthesis and Tyrosinase Activity. Molecules 2024, 29, 4162. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin Suppresses α-MSH-Induced Melanogenesis in B16F10 Mouse Melanoma Cells by Inhibiting Tyrosinase Activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef]
- Moon, K.M.; Yang, J.H.; Lee, M.K.; Kwon, E.B.; Baek, J.; Hwang, T.; Kim, J.I.; Lee, B. Maclurin Exhibits Antioxidant and Anti-Tyrosinase Activities, Suppressing Melanogenesis. Antioxidants 2022, 11, 1164. [Google Scholar] [CrossRef]
- Moon, S.Y.; Akter, K.M.; Ahn, M.J.; Kim, K.D.; Yoo, J.; Lee, J.H.; Lee, J.H.; Hwangbo, C. Fraxinol Stimulates Melanogenesis in B16F10 Mouse Melanoma Cells through CREB/MITF Signaling. Molecules 2022, 27, 1549. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of Scavenging Activity of Phenolic Compounds Using the ABTS + Assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1, 1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- LeBel, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef]
- Ali, S.F.; LeBel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar] [PubMed]








 | |||
|---|---|---|---|
| Compound | R | IC50 (μM) | |
| l-Tyrosine | l-Dopa | ||
| a Kojic acid | 19.22 ± 1.29 | 26.54 ± 0.17 | |
| 1 | 4-Hydroxy | 4.69 ± 0.58 | 38.10 ± 2.26 |
| 2 | 3,4-Dihydroxy | 5.75 ± 0.81 | 27.47 ± 1.84 |
| 3 | 2,4-Dihydroxy | 0.09 ± 0.01 | 0.27 ± 0.04 |
| 4 | 4-Hydroxy-3-methoxy | 45.52 ± 2.49 | 121.24 ± 4.90 |
| 5 | 3-Hydroxy-4-methoxy | 39.01 ± 2.15 | 91.01 ± 8.55 |
| 6 | 4-Methoxy | 19.11 ± 2.03 | 33.54 ± 0.30 |
| 7 | 3,4-Dimethoxy | 56.19 ± 1.98 | 70.64 ± 1.25 |
| 8 | 2,4-Dimethoxy | 18.57 ± 1.23 | 51.14 ± 2.05 |
| 9 | 3,4,5-Trimethoxy | >200 | 97.23 ± 7.73 |
| 10 | 4-Hydroxy-3,5-dimethoxy | >200 | >200 |
| 11 | 3-Bromo-4-hydroxy | 66.26 ± 2.11 | 114.88 ± 2.28 |
| 12 | 3,5-Dibromo-4-hydroxy | >200 | >200 |
 | |
|---|---|
| Substitution | a Tyrosinase Inhibition |
| R2 = OH | ↑↑↑↑ when R4 = OH |
| R3 = OH | ↓↓ when R4 = OMe |
| ↑ when R4 = OH | |
| R4 | OH > MeO in the presence of l-tyrosine |
| OH ≈ MeO in the presence of l-dopa | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.S.; Jung, H.J.; Park, H.S.; Kim, H.J.; Park, Y.; Chun, P.; Chung, H.Y.; Moon, H.R. Design, Synthesis, and Anti-Tyrosinase, Anti-Melanogenic, and Antioxidant Activities of Novel (Z)-3-Benzyl-5-Benzylidene-2-Thioxothiazolidin-4-One Analogs. Molecules 2025, 30, 517. https://doi.org/10.3390/molecules30030517
Park HS, Jung HJ, Park HS, Kim HJ, Park Y, Chun P, Chung HY, Moon HR. Design, Synthesis, and Anti-Tyrosinase, Anti-Melanogenic, and Antioxidant Activities of Novel (Z)-3-Benzyl-5-Benzylidene-2-Thioxothiazolidin-4-One Analogs. Molecules. 2025; 30(3):517. https://doi.org/10.3390/molecules30030517
Chicago/Turabian StylePark, Hyeon Seo, Hee Jin Jung, Hye Soo Park, Hye Jin Kim, Yujin Park, Pusoon Chun, Hae Young Chung, and Hyung Ryong Moon. 2025. "Design, Synthesis, and Anti-Tyrosinase, Anti-Melanogenic, and Antioxidant Activities of Novel (Z)-3-Benzyl-5-Benzylidene-2-Thioxothiazolidin-4-One Analogs" Molecules 30, no. 3: 517. https://doi.org/10.3390/molecules30030517
APA StylePark, H. S., Jung, H. J., Park, H. S., Kim, H. J., Park, Y., Chun, P., Chung, H. Y., & Moon, H. R. (2025). Design, Synthesis, and Anti-Tyrosinase, Anti-Melanogenic, and Antioxidant Activities of Novel (Z)-3-Benzyl-5-Benzylidene-2-Thioxothiazolidin-4-One Analogs. Molecules, 30(3), 517. https://doi.org/10.3390/molecules30030517







