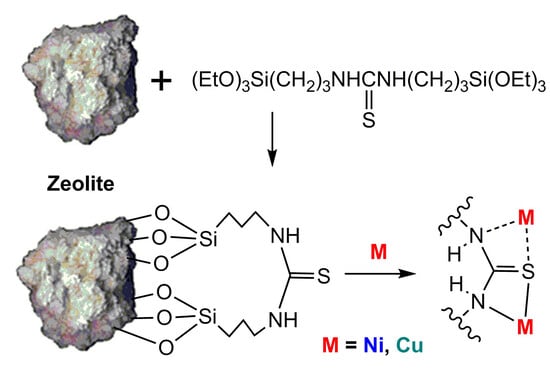
Zeolite Heulandite Modified with N,N′-bis(3-Triethoxysilylpropyl)thiourea—Adsorption of Ni(II) and Cu(II) Ions: A Quantum Chemical Insight into the Mechanism
Abstract
1. Introduction
2. Results and Discussion
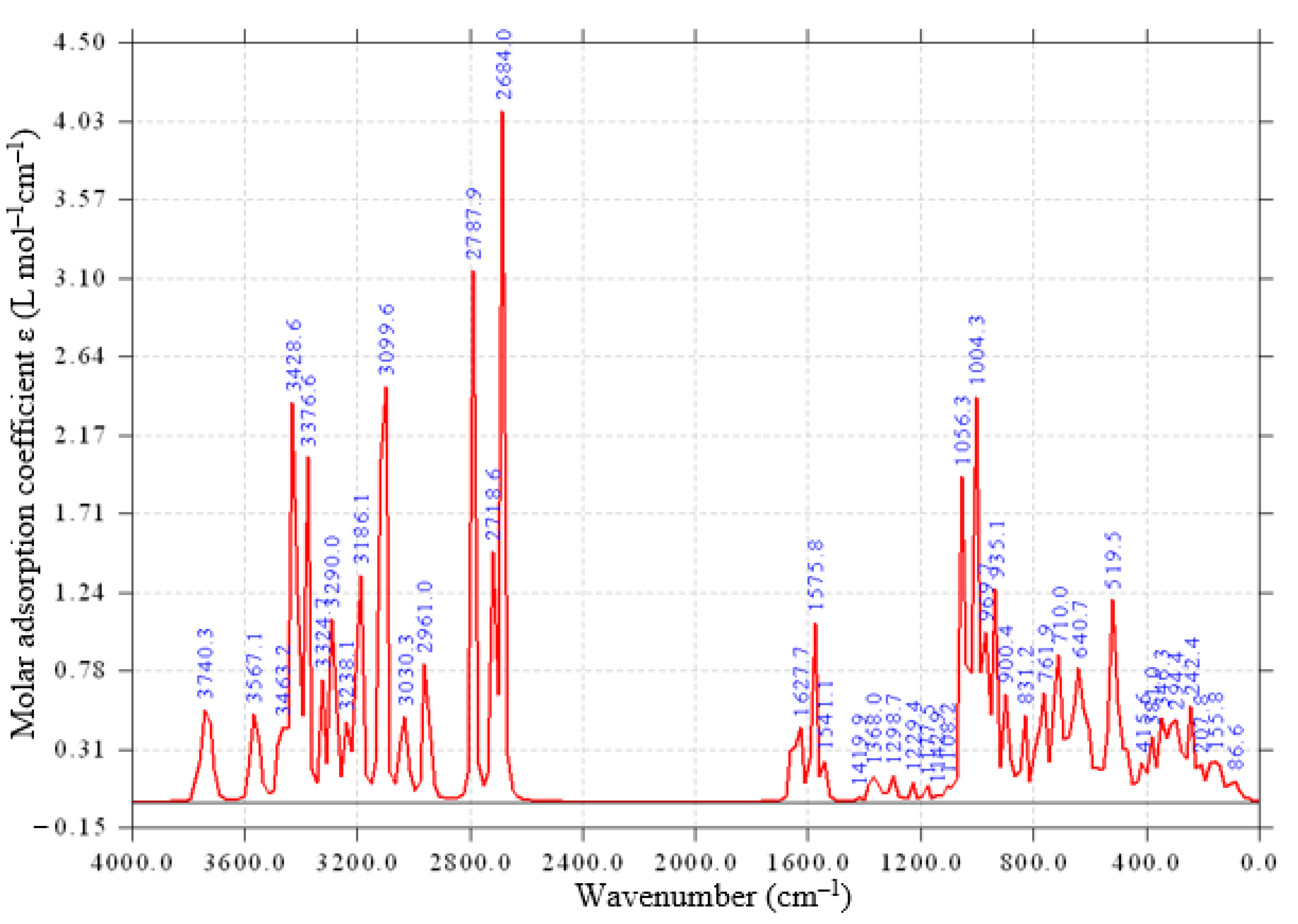
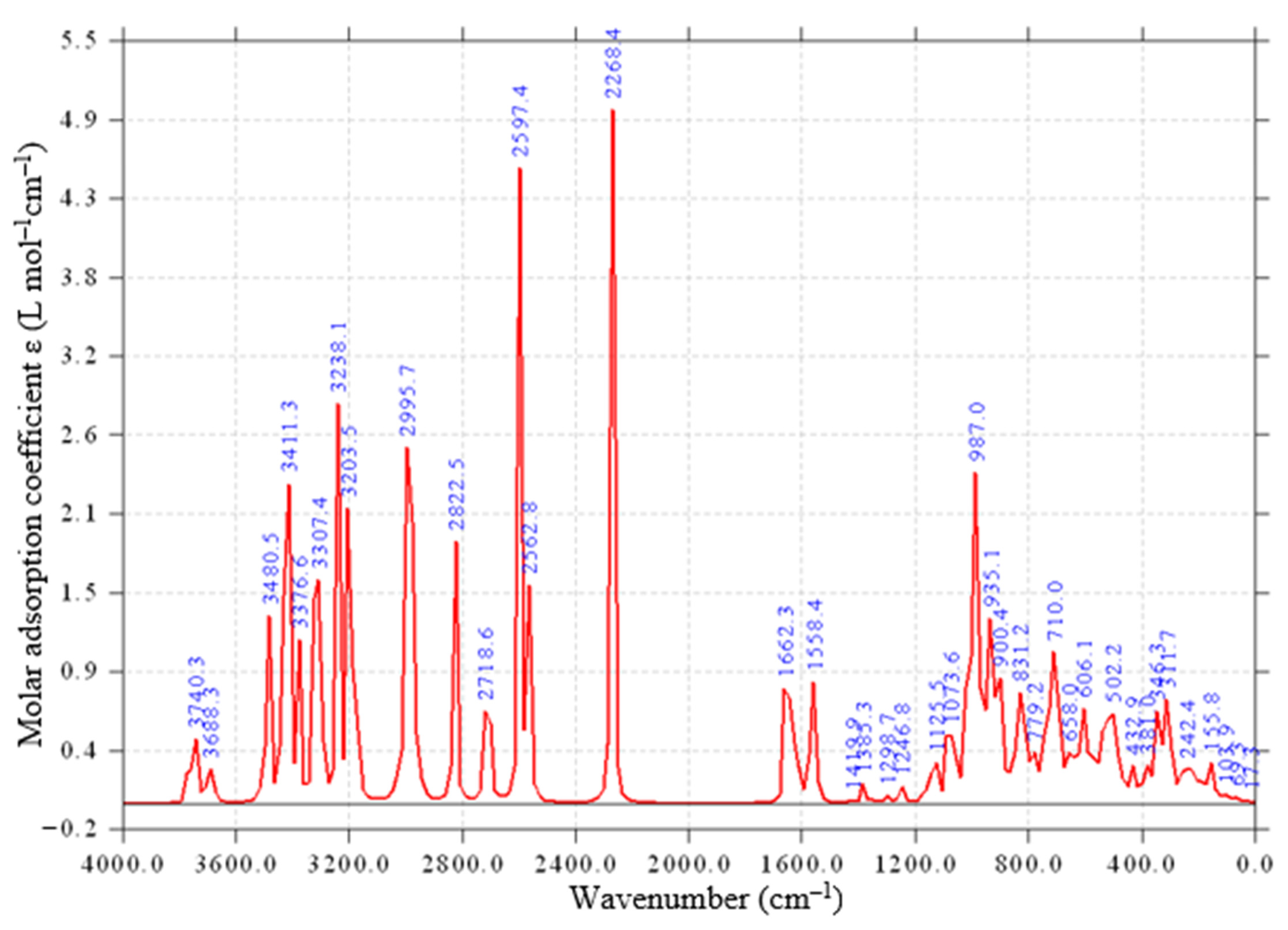
2.1. Composition and Structure of GS
2.2. Adsorption Properties of GS
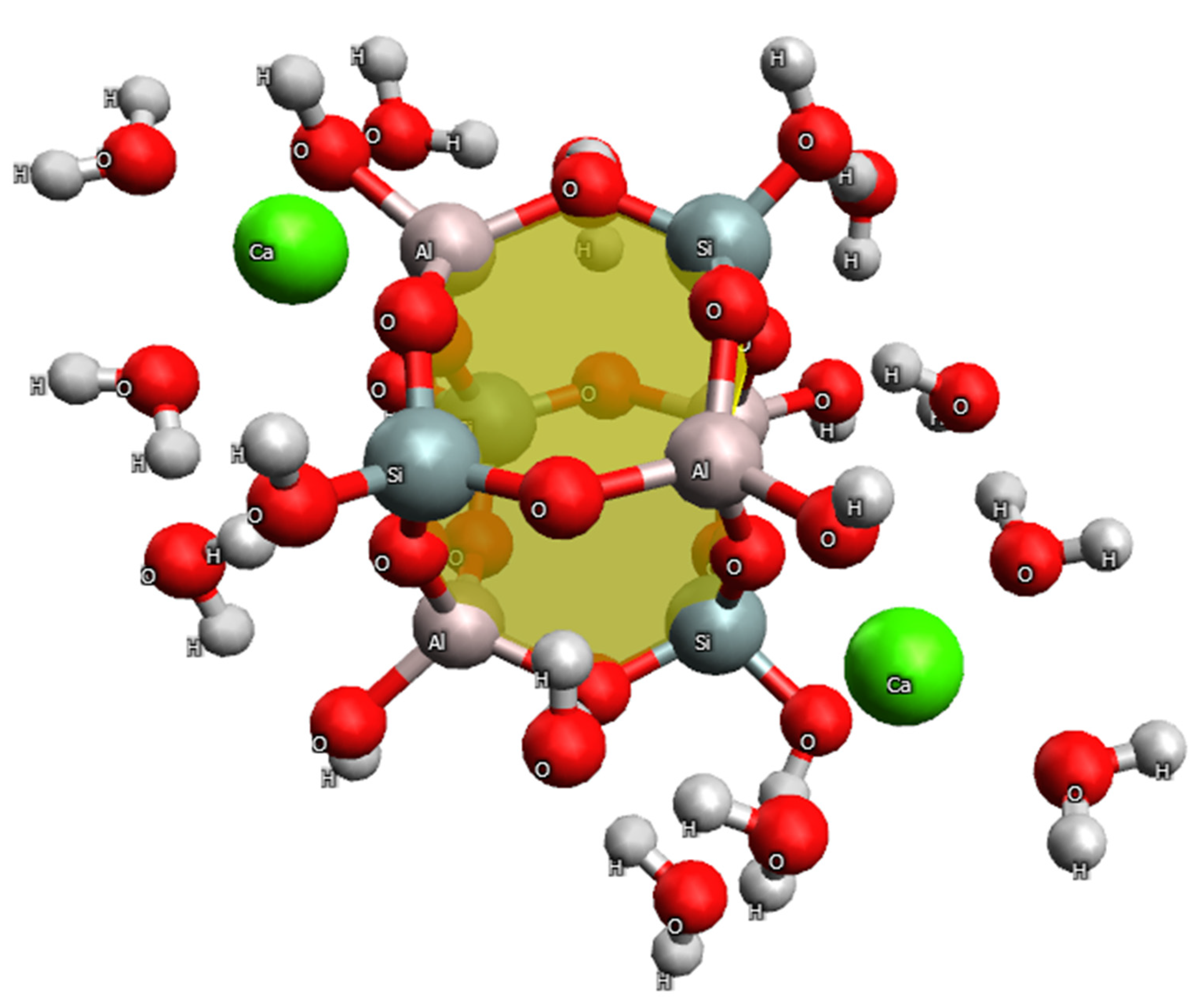
2.3. Models of Adsorption
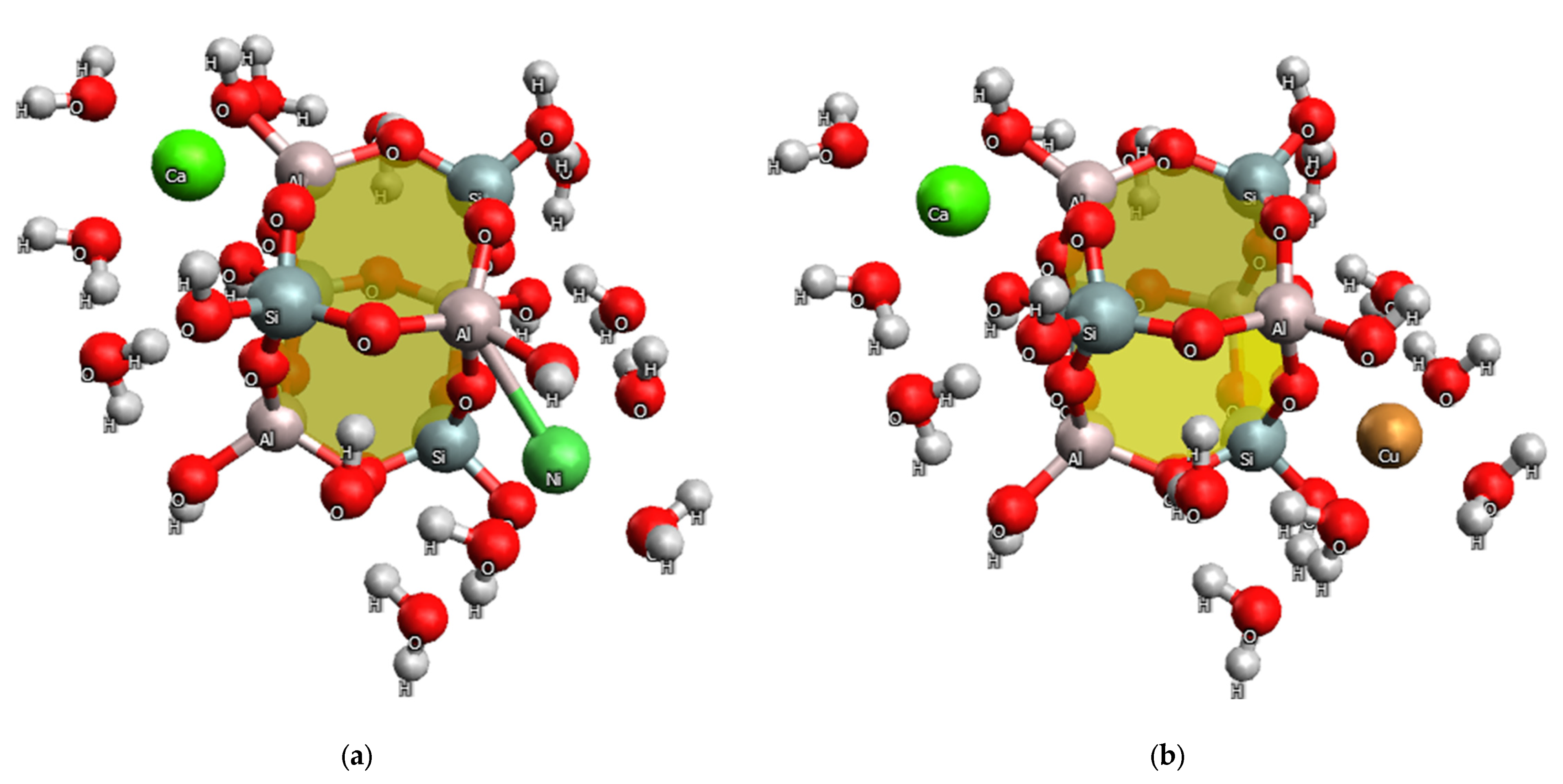
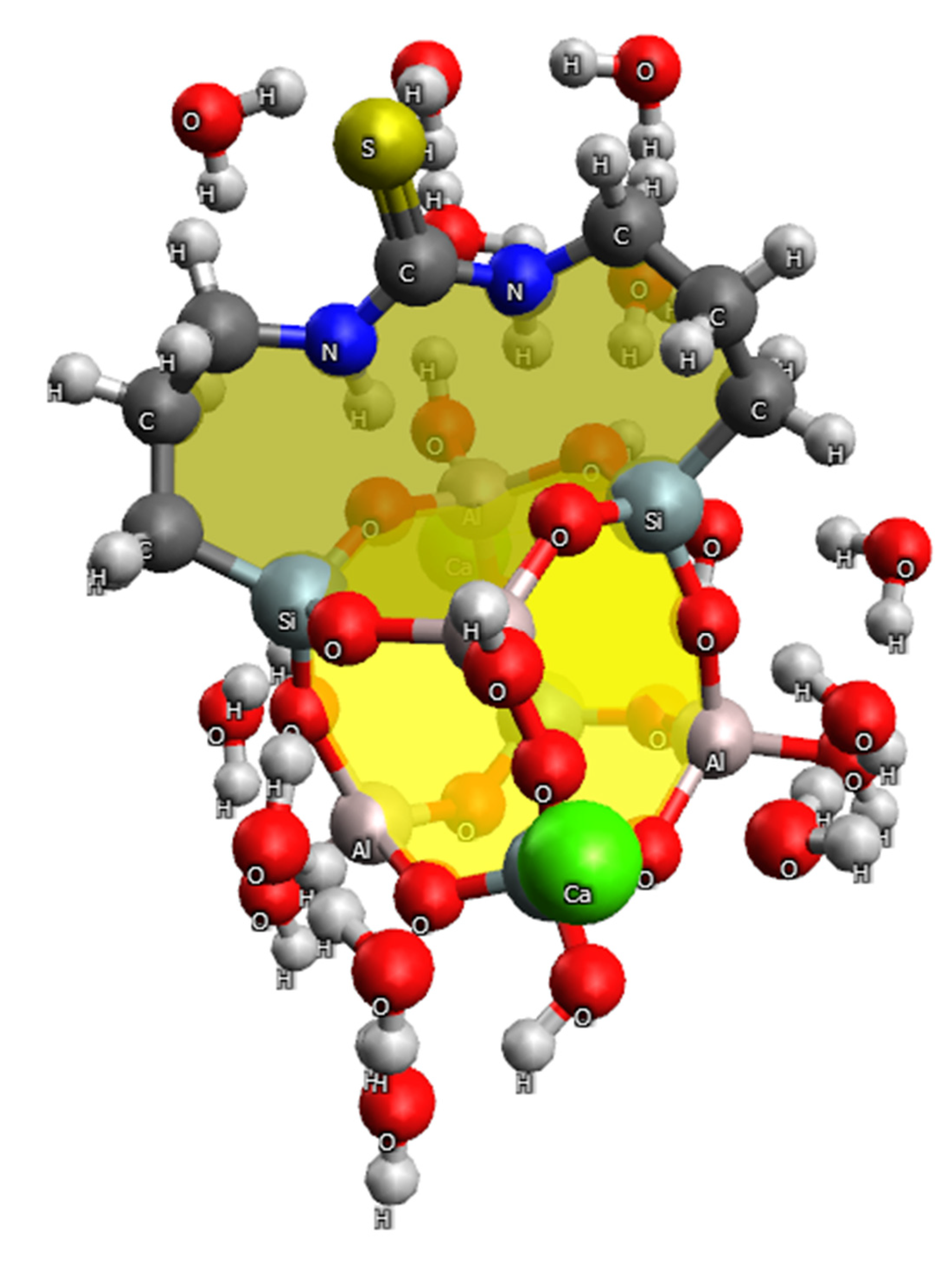
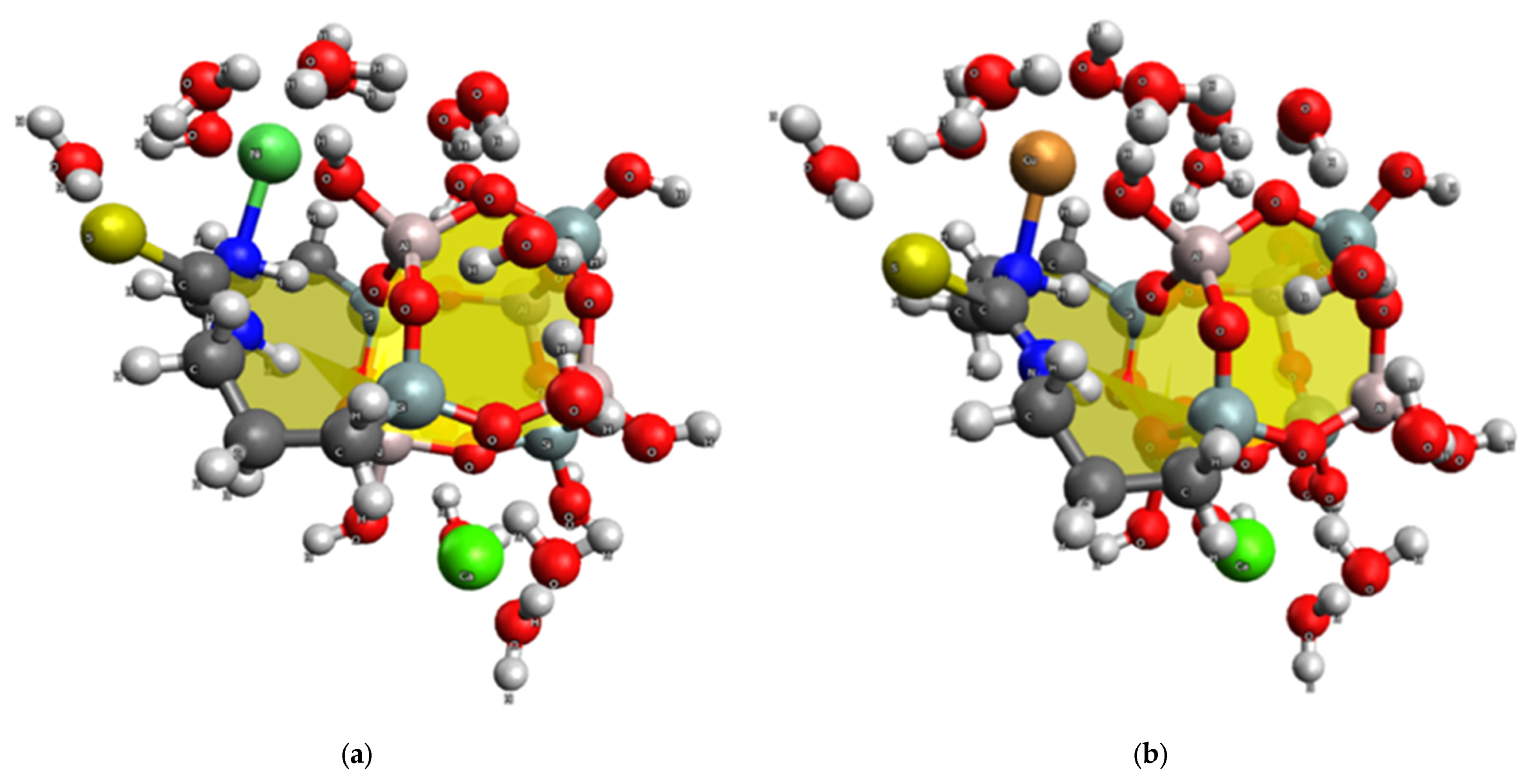
2.4. Quantum Chemical Study of the Adsorption Mechanism of GS by Heavy Metal Ions
- 1.
- Thermodynamics of HMIs adsorption on the GS cluster without the desorption of calcium ions Ca(II).
- 2.
- Thermodynamics of HMIs adsorption on the GS cluster with the desorption of calcium ions Ca(II).
- 3.
- Thermodynamics of HMIs adsorption on the GS cluster with parallel desorption of calcium ions Ca(II) (ion exchange process).
3. Materials and Methods
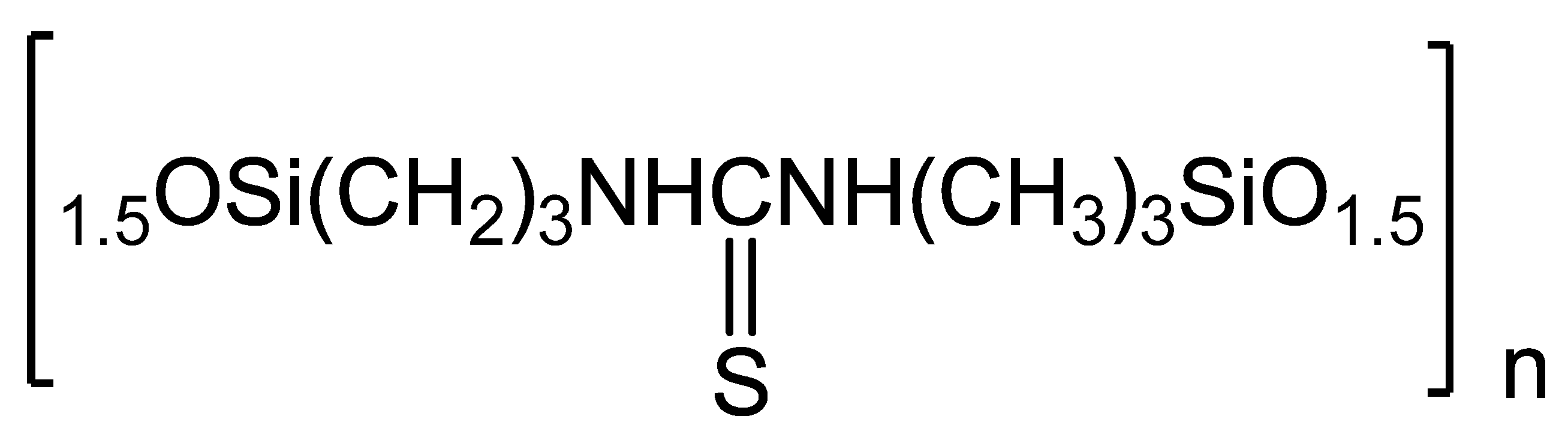
3.1. Synthesis of N,N-bis(3-Triethoxysilylpropyl)thiocarbamide (S) [34]
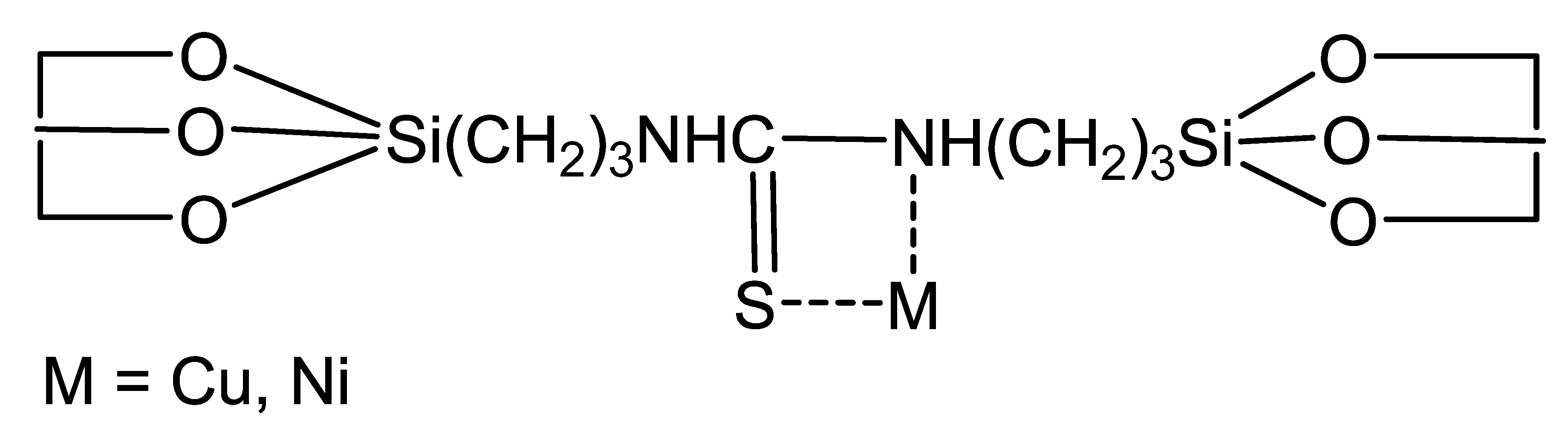
3.2. Synthesis of Sorbent (GS)
3.3. Determination of Morphology
3.4. Evaluation of the Textural Features of Heulandite and GS
3.5. PXRD
3.6. Adsorption Study
3.7. Mathematical Modeling of Experimental Adsorption Data
3.8. Computer Modeling (See Supplementary Materials)
4. Conclusions
- A new adsorbent (GS) was obtained by modifying the zeolite “heulandite” (G) with N,N′-bis(3-triethoxysilylpropyl)thiocarbamide (S). The composition, structure, and surface morphology of the adsorbent GS were confirmed using elemental analysis, IR-, NMR-spectroscopy, XRD, SEM, EDX spectra, elemental mapping, and nitrogen adsorption/desorption by the BET method. The potential of the obtained materials for the application as sorbents for the removal of Cu(II) and Ni(II) ions from concentrated solutions has been revealed. The nature of HMIs adsorption was investigated using the Langmuir, Freundlich, and Dubinin–Radushkevich models.
- The adsorption capacity of Cu(II) and Ni(II) ions by GS sorbents was found to be 1.7 and 2.1 times higher than that of heulandite G, amounting to 0.128 mmol/g (8.1 mg/g) and 0.214 mmol/g (12.6 mg/g), respectively. It was established that the free energy of adsorption E during the adsorption of Cu(II) and Ni(II) ions was 12.5 and 16.2 kJ/mol, respectively. Calculations of changes in Gibbs energy based on quantum chemical modeling results ( = −38.5 kJ/mol for Ni and = −56.5 kJ/mol for Cu) confirmed that adsorption of heavy metal ions onto the GS sample occurs through the formation of metal ion coordination complexes with the sorbent’s functional groups (chemosorption).
- It has been found that in heulandite G, Ca(II) ions are strongly bound to oxygen atoms in the aluminosilicate framework through electrostatic, closed-shell interactions (ionic bonds). Modification of the heulandite structure with N,N′-bis(3-triethoxysilylpropyl)thiocarbamide S weakens the bond between the oxygen and calcium atoms. This increases the desorption capacity of calcium ions, promoting the subsequent adsorption of nickel and copper ions. Changes in Gibbs energy calculated based on the results of quantum chemical modeling ( = −18.55 kJ/mol for nickel and = −2.08 kJ/mol for copper) confirm that nickel adsorption is an ion-exchange process, while the adsorption Cu(II) ion is a physical process involving the micropores of the heulandite G sorbent.
- The adsorption of Ni(II) and Cu(II) ions on GS occurs primarily through the donor-acceptor interactions between these ions and the nitrogen atom, as well as the OH group at the heulandite aluminum atom. There are no bonds with sulfur; however, electrostatic interactions occur between the metal ion and the electron-rich group of aluminosilicate [AlO3(OH)]−.
- The presence of strong polarization effects and donor-acceptor interactions has been proven to cause good contact between Ni(II) and Cu(II) ions, as well as nitrogen and sulfur atoms in structures modified with N′-bis(3-triethoxysilylpropyl)thiocarbamide. Interaction with oxygen atoms of heulandite is not always favorable. Based on the topological analysis of electron density values, it can be concluded that calcium ions prefer to interact with oxygen atoms, whereas nickel and copper ions prefer to interact with nitrogen atoms.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goswami, N.; Barman, P. Schiff base metal complexes as alternative heterocatalysts in water remediation: Photocatalytic degradation of toxic organic pollutants. J. Mol. Struct. 2026, 1349, 143925. [Google Scholar] [CrossRef]
- Adnan, M.A.M.; Majnis, M.F.; Adnan, W.N.W.M.; Abdullah, N.H.; Baharom, A.S.; Julkapli, N.M. Deep insights into the integration of Artificial Neural Networks (ANNs) for predicting the photocatalytic activities of metal-based catalysts in water pollutant reduction. J. Environ. Chem. Eng. 2025, 13, 116350. [Google Scholar] [CrossRef]
- Mandal, W.; Fajal, S.; Desai, A.V.; Ghosh, S.K. Metal-organic frameworks (MOFs) and related other advanced porous materials for sequestration of heaetvy metal-based toxic oxo-pollutants from water. Coord. Chem. Rev. 2025, 524, 216326. [Google Scholar] [CrossRef]
- Hejji, L.; Ali, Y.A.E.H.; Azzouz, A.; Raza, N.; Villarejo, L.P.; Kailasa, S.K. Recent insights into molecularly imprinted membrane technology for removal of pollutants from environmental water: From organic molecules to metal ions. J. Water Process Eng. 2024, 58, 104852. [Google Scholar] [CrossRef]
- Teotia, S.; Verma, D.; Ting, K.P.; Kumari, V.; Ahmed, M.U.; Mukherjee, M.D. Engineered 2D smart nanomaterials and nanocomposites: Advanced frontiers in heavy metal ion detection for water purification. J. Environ. Chem. Eng. 2025, 13, 119367. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Shah, M.U.H. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 343–379. [Google Scholar] [CrossRef]
- Sanchez-Olegario, E.; Pelicano, C.M. Characterization of philippine natural zeolite and its application for heavy metal removal from acid mine drainage (AMD). Key Eng. Mater. 2017, 737, 407–411. [Google Scholar] [CrossRef]
- Sanchez-Olegario, E.; Pelicano, C.M.; Felizco, J.C.; Mendoza, H. Thermal stability and heavy metal (As5+, Cu2+, Ni2+, Pb2+ and Zn2+) ions uptake of the natural zeolites from the Philippines. Mater. Res. Express 2019, 6, 085204. [Google Scholar] [CrossRef]
- Filatova, E.G.; Pozhidaev, Y.N.; Pomazkina, O.I. Investigation of adsorption of heavy metal ions by natural aluminosili-cate. Prot. Met. Phys. Chem. Surf. 2016, 52, 438–442. [Google Scholar] [CrossRef]
- Merrikhpour, H.; Jalali, M. Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite. Clean Technol. Environ. Policy 2013, 15, 303–316. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Faustino, B.; Mae Cobo, D.; Vequizo, R.; Candidato, R. Enhanced heavy metal adsorption capacity of surface-functionalized Philippine natural zeolite in simulated wastewater. J. Open Ceramics 2024, 18, 100612. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Z.; Dai, H.; Lu, X.; Peng, L.; Tan, X.; Shi, L.; Fahim, R. Preparation and application of modified zeolites as adsorbents in wastewater treatment. Water Sci. Technol. 2018, 3, 621–635. [Google Scholar] [CrossRef]
- Dignos, E.C.G.; Gabejan, K.E.A.; Olegario-Sanchez, E.M.; Mendoza, H.D. The comparison of the alkali-treated and acid-treated naturally mined Philippine zeolite for adsorption of heavy metals in highly polluted waters. IOP Conf. Ser. Mater. Sci. Eng 2019, 478, 012030. [Google Scholar] [CrossRef]
- Bakatula, E.M.; Mosai, A.K.; Tutu, H. Removal of uranium from aqueous solutions using ammonium- modified zeolite: Research article. S. Afr. J. Chem. 2015, 68, 165–171. [Google Scholar] [CrossRef]
- Kragović, M.; Pašalić, S.; Marković, M.; Petrović, M.; Nedeljković, B.; Momčilović, M.; Stojmenović, M. Natural and Modified Zeolite—Alginate Composites. Application for Removal of Heavy Metal Cations from Contaminated Water Solutions. Minerals 2018, 8, 11. [Google Scholar] [CrossRef]
- Camacho, L.M.; Parra, R.R.; Deng, S. Arsenic removal from groundwater by MnO2-modified natural clinoptilolite zeolite: Effects of pH and initial feed concentration. J. Hazard. Mater. 2011, 189, 286–293. [Google Scholar] [CrossRef]
- Pei, Y.; Mo, S.; Xie, Q.; Chen, N.; Yang, Z.; Huang, L.; Ma, L. Stellerite-seeded facile synthesis of zeolite X with excellent aqueous Cd2+ and Ni2+ adsorption performance. Chin. J. Chem. Eng. 2022, 51, 61–74. [Google Scholar] [CrossRef]
- Tertykh, V.A.; Polishchuk, L.M.; Yanovska, E.S.; Dadashev, A.D. Concentration of anions by silica adsorbents with immobilized nitrogen-containing polymers. Adsorpt. Sci. Technol. 2008, 26, 59–68. [Google Scholar] [CrossRef]
- Wingenfelder, U.; Nowack, B.; Furrer, G.; Schulin, R. Adsorption of Pb and Cd by amine-modified zeolite. Water Res. 2005, 39, 3287–3297. [Google Scholar] [CrossRef]
- Lin, J.; Zhan, Y.; Zhu, Z. Adsorption characteristics of copper (II) ions from aqueous solution onto humic acid-immobilized surfactant-modified zeolite. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 9–16. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.; Li, J.; Zhan, X.; Chen, J.; Yang, J. Tuning the hydrophobicity of ZSM-5 zeolites by surface silanization using alkyltrichlorosilane. Appl. Surf. Sci. 2011, 257, 9525–9531. [Google Scholar] [CrossRef]
- Li, Y.; Guan, H.-M.; Chung, T.-S.; Kulprathipanja, S. Effects of novel silane modification of zeolite surface on polymer chain rigidification and partial pore blockage in polyethersulfone (PES)–zeolite. A mixed matrix membranes. J. Membr. Sci. 2006, 275, 17–28. [Google Scholar] [CrossRef]
- Wei, P.; Qu, X.; Dong, H.; Zhang, L.; Chen, H.; Gao, C. Silane-modified NaA zeolite/PAAS hybrid pervaporation membranes for the dehydration of ethanol. Appl. Polym. Sci. 2013, 128, 3390–3397. [Google Scholar] [CrossRef]
- Kim, S.H.; Semenya, D.; Castagnolo, D. Antimicrobial drugs bearing guanidine moieties: A review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef]
- Walczak-Nowicka, L.; Biernasiuk, A.; Ziemichód, W.; Karczmarzyk, Z.; Kwaśnik, M.; Kozyra, P.; Wysocki, W.; Stenzel-Bembenek, A.; Kowalczuk, D.; Herbet, M.; et al. N-Substituted 2-(Benzenosulfonyl)-1-Carbotioamide Derivatives Exert Antimicrobial and Cytotoxic Effects via Aldehyde Dehydrogenase Pathway: Synthesis, In Silico and In Vitro Studies. Pharmaceuticals 2023, 16, 1706. [Google Scholar] [CrossRef]
- Bahojb Noruzi, E.; Kheirkhahi, M.; Shaabani, B.; Geremia, S.; Hickey, N.; Asaro, F.; Nitti, P.; Kafil, H.S. Design of a Thiosemicarbazide-Functionalized Calix[4]arene Ligand and Related Transition Metal Complexes: Synthesis, Characterization, and Biological Studies. Front. Chem. 2019, 7, 663. [Google Scholar] [CrossRef]
- Acharya, P.T.; Bhavsar, Z.A.; Jethava, D.J.; Patel, D.B.; Patel, H.D. A review on development of bio-active thiosemicarbazide derivatives: Recent advances. J. Mol. Struct. 2021, 1226, 129268. [Google Scholar] [CrossRef]
- Adamovich, S.N.; Filatova, E.G.; Pozhidaev, Y.N.; Ushakov, I.A.; Chugunov, A.D.; Oborina, E.N.; Rozentsveig, I.B.; Verpoort, F. Natural zeolite modified with 4-(3-triethoxysilylpropyl) thiosemicarbazide as an effective adsorbent for Cu(II), Co(II) and Ni(II). J. Taiwan Inst. Chem. Eng. 2021, 129, 396–409. [Google Scholar] [CrossRef]
- Filatova, E.G.; Chugunov, A.D.; Pozhidaev, Y.N.; Adamovich, S.N. Natural aluminosilicates modified with organosilicon thiosemicarbazides for the extraction of nickel(II) ions. Prot. Met. Phys. Chem. Surf. 2022, 58, 469–477. [Google Scholar] [CrossRef]
- Namiecińska, E.; Sobiesiak, M.; Małecka, M.; Guga, P.; Rozalska, B.; Budzisz, E. Antimicrobial and structural properties of metal ions complexes with thiosemicarbazide motif and related heterocyclic compounds. Curr. Med. Chem. 2019, 26, 664–693. [Google Scholar] [CrossRef] [PubMed]
- Pomazkina, O.I.; Filatova, E.G.; Pozhidaev, Y.N. Adsorption of Ni(II), Cu(II), and Zn(II) ions by natural alumosilicate modified with N,N’-bis(3-triethoxysilylpropyl)thiocarbamide. Prot. Met. Phys. Chem. Surf. 2017, 53, 416–421. [Google Scholar] [CrossRef]
- Filatova, E.G.; Pomazkina, O.I.; Pozhidaev, Y.N. Adsorption of nickel(II) and copper(II) ions by modified aluminosilicates. Prot. Met. Phys. Chem. Surf. 2017, 53, 999–1004. [Google Scholar] [CrossRef]
- Pomazkina, O.I.; Filatova, E.G.; Pozhidaev, Y.N. Antibacterial properties of modified alumosilicates. J. Water Chem. Technol. 2018, 40, 196–200. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O. Modification of natural zeolites and their applications for heavy metal removal from polluted environments: Challenges, recent advances, and perspectives. Heliyon 2024, 10, 25303. [Google Scholar] [CrossRef]
- Garcia-Ratés, M.; Neese, F. Effect of the Solute Cavity on the Solvation Energy and its Derivatives within the Framework of the Gaussian Charge Scheme. J. Comput. Chem. 2020, 41, 922–939. [Google Scholar] [CrossRef]
- Yang, C.S.; Mora-Fonz, J.M.; Catlow, C.R.A. Modeling the polymerization of aluminosilicate clusters. J. Phys. Chem. 2012, 116, 22121–22128. [Google Scholar] [CrossRef]
- Mehrizi, E.A.; Sadani, M.; Karimaei, M.; Ghahramani, E.; Ghadiri, K.; Taghizadeh, M.S. Isotherms and kinetics of lead and cadmium uptake from the waste leachate by natural absorbent. World Appl. Sci. J. 2011, 15, 1678–1686. [Google Scholar]
- Nezamzadeh-Ejhieh, A.; Kabiri-Samani, M. Effective removal of Ni(II) from aqueous solutions by modification of nano particles of clinoptilolite with dimethylglyoxime. J. Hazard. Mater. 2013, 260, 339–349. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E.A. A Review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Douven, S.C.; Paez, A.; Gommes, C.J. The range of validity of sorption kinetic models. J. Colloid Interface Sci. 2015, 448, 437–450. [Google Scholar] [CrossRef]
- Sparks, D.L. Kinetics of Soil Chemical Processes; Academic Press: New York, NY, USA, 1989; p. 210. [Google Scholar] [CrossRef]
- Senila, M.; Kovacs, E.; Senila, L. Silver-Exchanged Zeolites: Preparation and Applications—A Review. Materials 2025, 18, 4779. [Google Scholar] [CrossRef] [PubMed]
- Pomazkina, O.I.; Filatova, E.G.; Pozhidaev, Y.N. Adsorption of copper(II) ions by calcium heulandite. Prot. Met. Phys. Chem. Surf. 2015, 51, 518–522. [Google Scholar] [CrossRef]
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Frolov, Y.G. Course of Colloid Chemistry. In Surface Phenomena and Dispersed Systems; Chemistry: Moscow, Russia, 1989; p. 462. [Google Scholar]
- Shchukin, E.D.; Pertsov, A.V.; Amelina, E.A. Colloid Chemistry; Higher School: Moscow, Russia, 1990; p. 463. [Google Scholar]
- Anari-Anaraki, M.; Nezamzadeh-Ejhieh, A. Modification of an Iranian clinoptilolite nano-particles by hexadecyltrimethyl ammonium cationic surfactant and dithizone for removal of Pb(II) from aqueous solution. J. Colloid Interface Sci. 2015, 440, 272–281. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves; John Wiley & Sons: Hoboken, NJ, USA, 1973; p. 781. [Google Scholar]
- Morozov, I.V.; Boltalin, A.I.; Karpova, E.V. Oxidation-Reduction Processes; Moscow University Press: Moscow, Russia, 2003; p. 79. [Google Scholar]
- Nah, I.W.; Hwang, K.-Y.; Shul, Y.-G. A simple synthesis of magnetically modified zeolite. Powder Technol. 2007, 2, 99–101. [Google Scholar] [CrossRef]
- Aslamova, V.S.; Shalunc, L.V.; Aslamov, A.F.; Grabel’nykh, V.A. Computer simulation of the sorption of heavy metal ions by a sulphur-containing modified zeolite. Proceedings Univ. Appl. Chem. Biotechnol. 2020, 10, 564–572. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, 1606. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Madsen, G.K. Functional form of the generalized gradient approximation for exchange: The PBE α functional. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 195108. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Bannwarth, C.; Grimme, S. Extension of the D3 dispersion coefficient model. J. Chem. Phys. 2017, 147, 034112. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A generally applicable atomic-charge dependent London dispersion correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Mewes, J.M.; Ehlert, S.; Grimme, S. Extension and evaluation of the D4 London-dispersion model for periodic systems. Phys. Chem. Chem. Phys. 2020, 22, 8499–8512. [Google Scholar] [CrossRef]
- Wittmann, L.; Gordiy, I.; Friede, M.; Helmich-Paris, B.; Grimme, S.; Hansen, A.; Bursch, M. Extension of the D3 and D4 London dispersion corrections to the full actinides series. Phys. Chem. Chem. Phys. 2024, 26, 21379–21394. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The SHARK integral generation and digestion system. J. Comput. Chem. 2023, 44, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Garcia-Ratés, M.; Neese, F. Efficient implementation of the analytical second derivatives of hartree–fock and hybrid DFT energies within the framework of the conductor-like polarizable continuum model. J. Comput. Chem. 2019, 40, 1816–1828. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]








| Zeolite | Idealized Molecular Formula | Typical Si/Al Ratio | Fraction Diameter, mm | Specific Surface Area, cm2/g | Average Pore Size, nm |
|---|---|---|---|---|---|
| Clinoptilolite | Na6[(AlO2)6(SiO2)30]·24H2O | 6.5 | 0.5–1 | 34 | 1.7 |
| Heulandite | Ca4[(A1O2)8(SiO2)28]·24H2O | 3.5 | 0.5–1 | 32 | 1.8 |
| A∞, mmol/g | A∞, mg/g | K·10−3 | Linear Form of the Langmuir Equation | RL | R2 | |
|---|---|---|---|---|---|---|
| Cu(II) ions | ||||||
| G | 0.087 | 5.5 | 33.80 | 1/A = 0.342·1/Ceq + 11.552 | 0.04 | 0.999 |
| GS | 0.468 | 29.7 | 14.16 | 1/A = 0.151·1/Ceq + 2.138 | 0.09 | 0.996 |
| Ni(II) ions | ||||||
| G | 0.150 | 8.8 | 14.07 | 1/A = 0.474·1/Ceq + 6.670 | 0.06 | 0.992 |
| GS | 0.781 | 46.1 | 8.06 | 1/A = 0.159·1/Ceq + 1.281 | 0.10 | 0.947 |
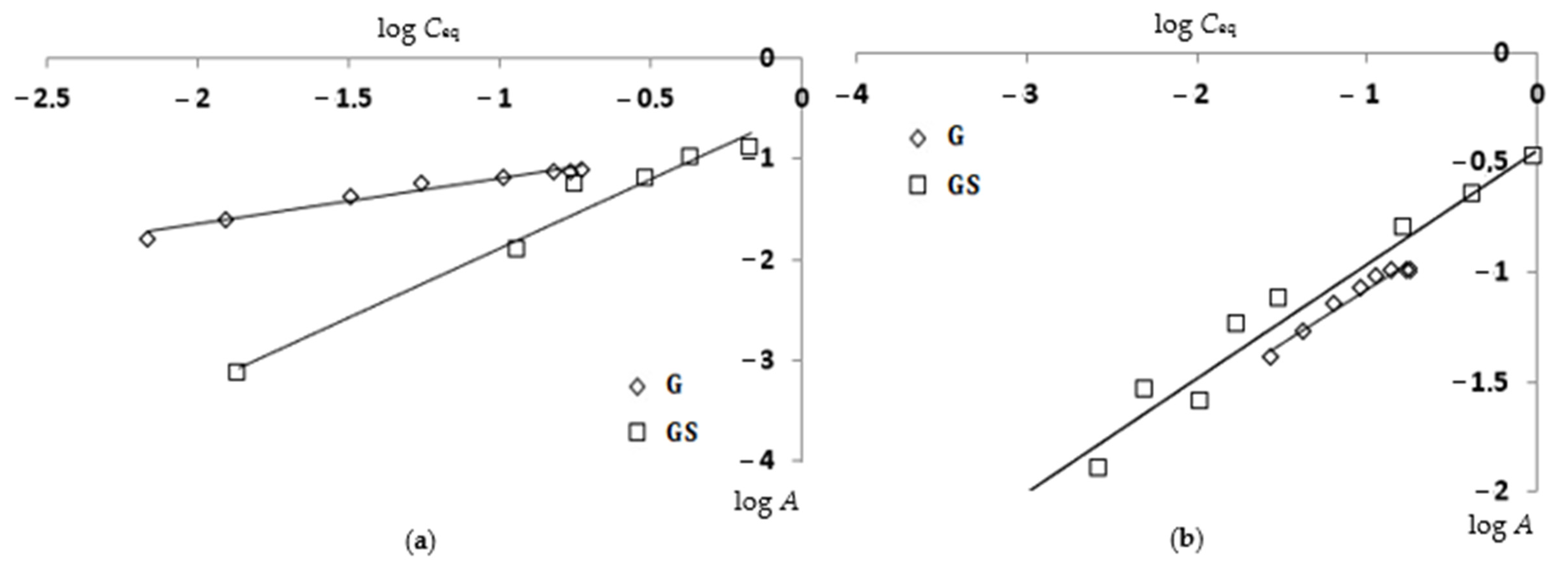
| Sorbents | Linear Form of the Freundlich Equation | Kf | n | R2 |
|---|---|---|---|---|
| Cu(II) ions | ||||
| G | log A = −0.741 + 0.455·log Ceq | 0.182 | 2.20 | 0.969 |
| GS | log A = −0.513 + 1.379·log Ceq | 0.307 | 0.73 | 0.966 |
| Ni(II) ions | ||||
| G | log A = −0.581 + 0.493·log Ceq | 0.262 | 2.03 | 0.962 |
| GS | log A = −0.446 + 0.520·log Ceq | 0.358 | 1.92 | 0.954 |
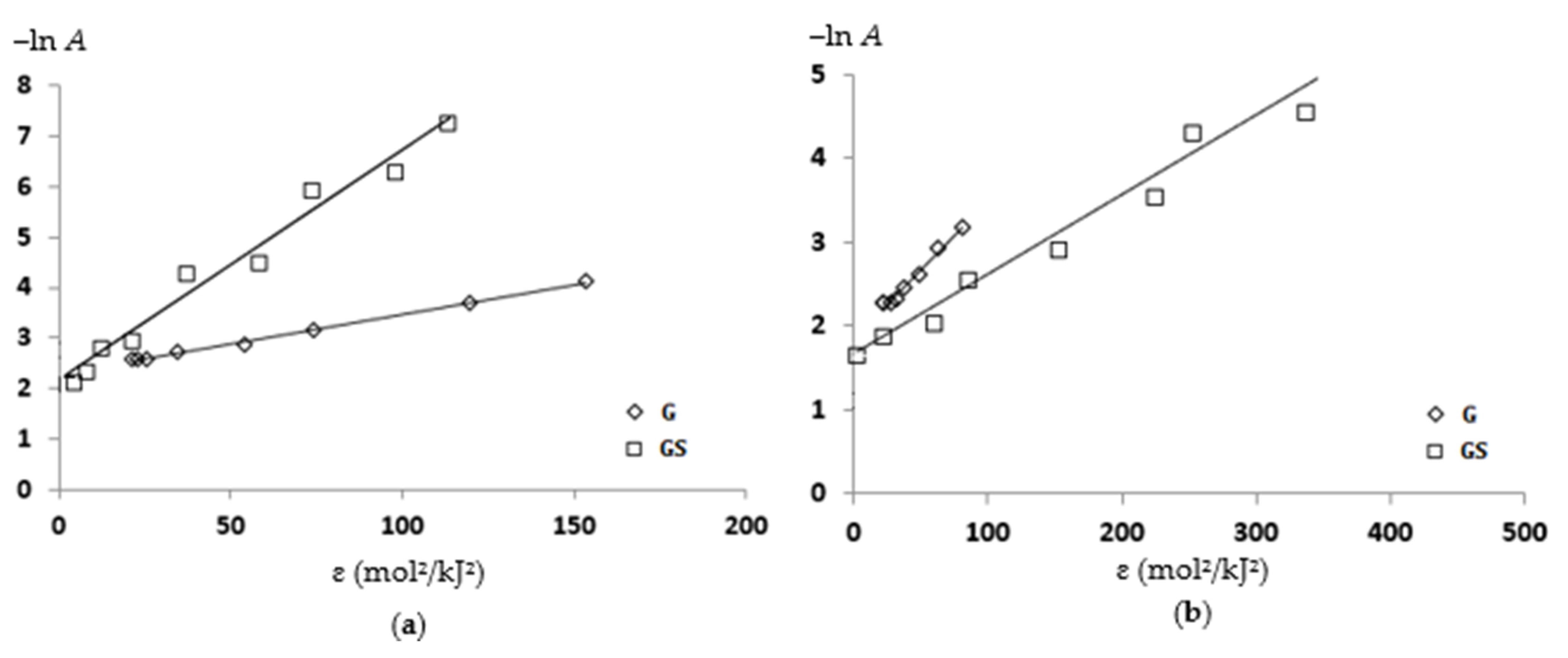
| Sorbents | Linear Form of the Dubinin–Radushkevich Equation | Am, mmol/g | k, mol2/kJ2 | E, kJ/mol | R2 |
|---|---|---|---|---|---|
| Cu(II) ions | |||||
| G | −ln A = −2.295 + 0.008 ɛ2 | 0.101 | 0.008 | 7.9 | 0.998 |
| GS | −ln A = −2.639 + 0.003 ɛ2 | 0.071 | 0.003 | 12.5 | 0.934 |
| Ni(II) ions | |||||
| G | −ln A = −1.874 + 0.007 ɛ2 | 0.154 | 0.007 | 8.5 | 0.987 |
| GS | −ln A = −1.608 + 0.0023 ɛ2 | 0.200 | 0.002 | 14.7 | 0.928 |
| Thermodynamic Functions | HMI | |
|---|---|---|
| Ni(II) Ions | Cu(II) Ions | |
| , kJ/mol | −19.42 | +0.12 |
| , J/mol·K | −2.94 | +7.38 |
| , kJ/mol | −18.55 | −2.08 |
| Thermodynamic Functions | HMI | |
|---|---|---|
| Ni(II) Ions | Cu(II) Ions | |
| , kJ/mol | +63.17 | +80.73 |
| , J/mol·K | +2.00 | +32.74 |
| , kJ/mol | +60.01 | +70.97 |
| Thermodynamic Functions | Values of the First Ion of Ca(II) |
|---|---|
| , kJ/mol | −16.58 |
| , J/mol·K | +49.59 |
| , kJ/mol | −31,36 |
| Thermodynamic Functions | HMI | |
|---|---|---|
| Ni(II) Ions | Cu(II) Ions | |
| , kJ/mol | +54.66 | +68.34 |
| , J/mol·K | +48.36 | +66.06 |
| , kJ/mol | +40.24 | +48.94 |
| Thermodynamic Functions | HMI | |
|---|---|---|
| Ni(II) Ions | Cu(II) Ions | |
| , kJ/mol | −58.58 | −73.26 |
| , J/mol·K | −67.38 | −56.06 |
| , kJ/mol | −38.5 | −56.54 |
| Structure | GS + Ni(II)–Ca(II) | GS + Cu(II)–Ca(II) | |
|---|---|---|---|
| Ρ | Me—H2O | 0.074 | 0.081 |
| Me—OAl | 0.079 | 0.088 | |
| Me—N | 0.062 | 0.068 | |
| Me—CH2 | 0.007 | 0.007 | |
| Δρ | Me—H2O | 0.397 | 0.407 |
| Me—OAl | 0.430 | 0.444 | |
| Me—N | 0.237 | 0.254 | |
| Me—CH2 | 0.022 | 0.020 | |
| J | Me—H2O | −0.106 | −0.156 |
| Me—OAl | −0.124 | −0.176 | |
| Me—N | −0.133 | −0.180 | |
| Me—CH2 | +0.043 | +0.042 | |
| Atomic charge | Me | +1.131 | +1.076 |
| S | −0.011 | +0.011 | |
| N | −1.132 | −1.118 | |
| OAl | −1.421 | −1.400 | |
| Hydroxide | pH of the Beginning of Hydrate Formation | pH Max of Hydroxide Release | pH of the Beginning of Hydroxide Dissolution |
|---|---|---|---|
| Cu(OH)2 | 5.3 | 8.0–9.5 | >9 |
| Ni(OH)2 | 6.0 | 9.25–10 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filatova, E.G.; Nalibayeva, A.M.; Lebedeva, O.V.; Beznosyuk, S.A.; Ryabykh, A.V.; Oborina, E.N.; Abdikalykov, Y.N.; Turmukhanova, M.Z.; Rozentsveig, I.B.; Adamovich, S.N. Zeolite Heulandite Modified with N,N′-bis(3-Triethoxysilylpropyl)thiourea—Adsorption of Ni(II) and Cu(II) Ions: A Quantum Chemical Insight into the Mechanism. Molecules 2025, 30, 4811. https://doi.org/10.3390/molecules30244811
Filatova EG, Nalibayeva AM, Lebedeva OV, Beznosyuk SA, Ryabykh AV, Oborina EN, Abdikalykov YN, Turmukhanova MZ, Rozentsveig IB, Adamovich SN. Zeolite Heulandite Modified with N,N′-bis(3-Triethoxysilylpropyl)thiourea—Adsorption of Ni(II) and Cu(II) Ions: A Quantum Chemical Insight into the Mechanism. Molecules. 2025; 30(24):4811. https://doi.org/10.3390/molecules30244811
Chicago/Turabian StyleFilatova, Elena G., Arailym M. Nalibayeva, Oksana V. Lebedeva, Sergey A. Beznosyuk, Andrey V. Ryabykh, Elizaveta N. Oborina, Yerlan N. Abdikalykov, Mirgul Zh. Turmukhanova, Igor B. Rozentsveig, and Sergey N. Adamovich. 2025. "Zeolite Heulandite Modified with N,N′-bis(3-Triethoxysilylpropyl)thiourea—Adsorption of Ni(II) and Cu(II) Ions: A Quantum Chemical Insight into the Mechanism" Molecules 30, no. 24: 4811. https://doi.org/10.3390/molecules30244811
APA StyleFilatova, E. G., Nalibayeva, A. M., Lebedeva, O. V., Beznosyuk, S. A., Ryabykh, A. V., Oborina, E. N., Abdikalykov, Y. N., Turmukhanova, M. Z., Rozentsveig, I. B., & Adamovich, S. N. (2025). Zeolite Heulandite Modified with N,N′-bis(3-Triethoxysilylpropyl)thiourea—Adsorption of Ni(II) and Cu(II) Ions: A Quantum Chemical Insight into the Mechanism. Molecules, 30(24), 4811. https://doi.org/10.3390/molecules30244811