Superior Oxidase-Mimetic Activity of Co-MOF Nanozyme for Smartphone-Based Visually Colorimetric Assay of Mancozeb
Abstract
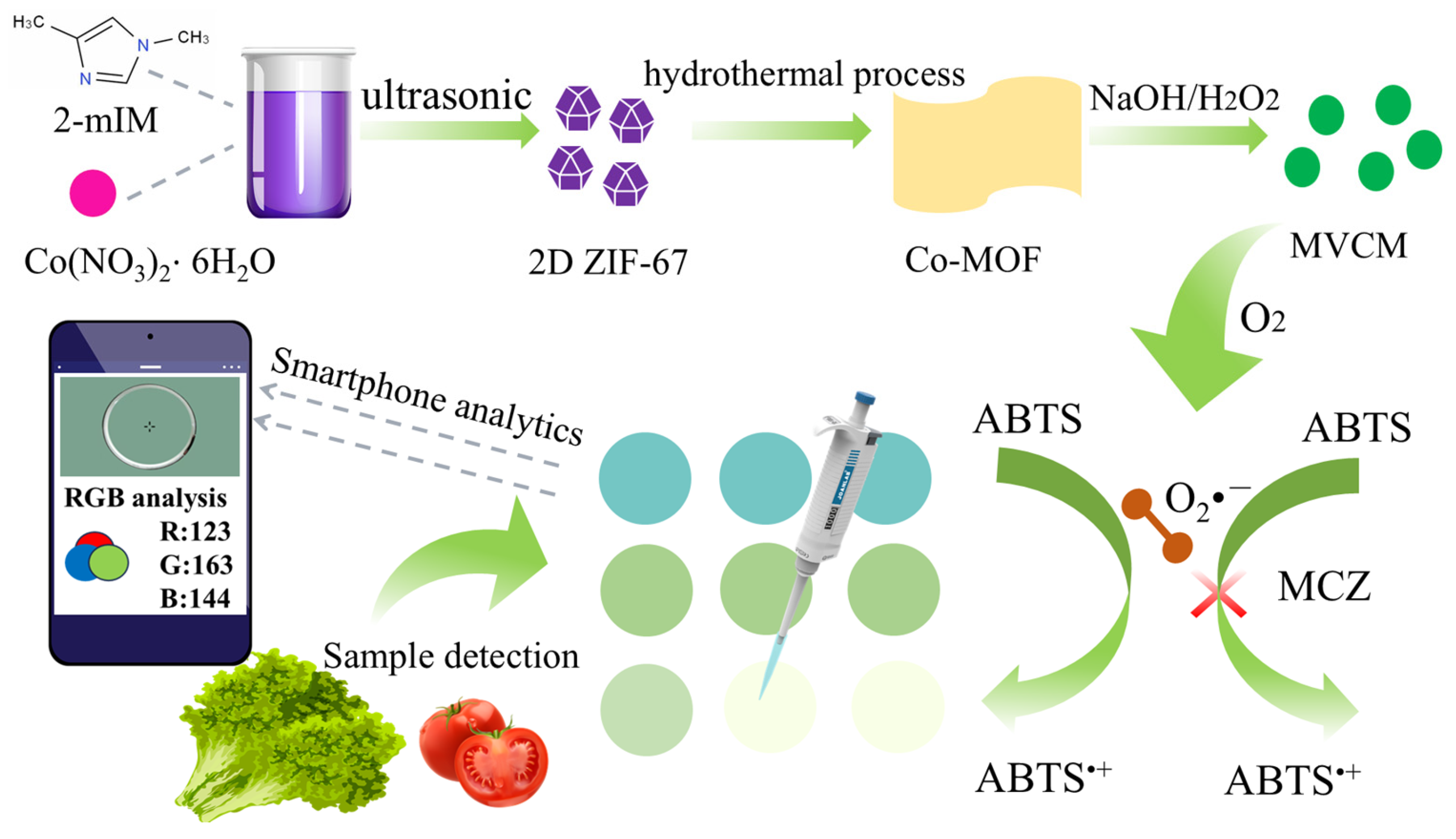
1. Introduction
2. Results and Analysis
2.1. Characterization of MVCM
2.2. The Oxidase-like Activity of MVCM
2.3. Oxidase Simulation Mechanism of MVCM
2.4. Steady-State Dynamics Analysis
2.5. Optimization of Reaction Conditions
2.6. Colorimetric Detection of MCZ
2.7. Mancozeb Detection by Smartphone-Test Paper Platform
2.8. Real Sample Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instruments and Equipment
3.3. Preparation of MVCM
3.4. Study on the Oxidase-like Activity of MVCM
3.5. Steady-State Kinetics Study on MVCM
3.6. Colorimetric Assay for MCZ
3.7. Selectivity and Anti-Interference Ability Analysis
3.8. Visual Detection of MCZ Based on Smart Phone
3.9. Analysis of Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Katna, S.; Dubey, J.K.; Sharma, S.; Istatu, P.S.; Devi, N.; Brar, G.B.; Kumar, A.; Singh, S.; Prashad, H. Residue behaviour and health risk assessment of chlorpyrifos and mancozeb in apple fruits and soil. Environ. Monit. Assess. 2023, 196, 58. [Google Scholar] [CrossRef]
- Bano, F.; Mohanty, B. Thyroid disrupting pesticides mancozeb and fipronil in mixture caused oxidative damage and genotoxicity in lymphoid organs of mice. Environ. Toxicol. Pharmacol. 2020, 79, 103408. [Google Scholar] [CrossRef] [PubMed]
- Gündel, S.S.; Reis, T.R.D.; Copetti, P.M.; Favarin, F.R.; Sagrillo, M.R.; Silva, A.S.; Segat, J.C.; Baretta, D.; Ourique, A.F. Evaluation of cytotoxicity, genotoxicity and ecotoxicity of nanoemulsions containing mancozeb and eugenol. Ecotoxicol. Environ. Saf. 2019, 169, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Castellani, F.; Filippo, P.D.; Riccardi, C.; Pomata, D.; Risoluti, R.; Buiarelli, F.; Sonego, E. Determination of mancozeb, a pesticide used worldwide in agriculture: Comparison among GC, LC, and CE. Curr. Anal. Chem. 2020, 16, 1041–1053. [Google Scholar] [CrossRef]
- da Silva, R.C.; Wickert, C.; Pizzutti, I.R.; de Kok, A. Clean-up strategy for dithiocarbamate fungicide determination in soybean by GC-ITD-MS and GC-PFPD: Method development and validation. J. Agric. Food Chem. 2021, 69, 11485–11493. [Google Scholar] [CrossRef] [PubMed]
- Tsen, C.; Yu, C.; Chen, S.; Lin, C.; Chuang, C. Application of surface-enhanced raman scattering in rapid detection of dithiocarbamate pesticide residuesin foods. Appl. Surf. Sci. 2021, 558, 149740. [Google Scholar] [CrossRef]
- Mozzaquatro, J.O.; César, I.A.; Pinheiro, A.E.B.; Caldas, E.D. Pesticide residues analysis in passion fruit and its processed products by LC-MS/MS and GC-MS/MS: Method validation, processing factors and dietary risk assessment. Food Chem. 2022, 375, 131643. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Liu, H.; Yang, J.; Feng, D.; Hou, K.; Wang, X.; Wu, W. A dual-optical sensor for mancozeb by UCNP@PVP@MnO2 nanozyme. Food Chem. 2023, 409, 135255. [Google Scholar] [CrossRef]
- Guo, T.; Qu, Y.; Ning, B.; Song, Q.; Wan, J.; Lin, X.; Zhuang, S.; Lin, P.; Yang, J.; Huang, G. A novel sulfur quantum dots-based electrochemical sensor for highly sensitive and specific detection of mancozeb. Microchem. J. 2025, 209, 112642. [Google Scholar] [CrossRef]
- Jin, R.; Wang, F.; Li, Q.; Yan, X.; Liu, M.; Chen, Y.; Zhou, W.; Gao, H.; Sun, P.; Lu, G. Construction of multienzyme-hydrogel sensor with smartphone detector for on-site monitoring of organophosphorus pesticide. Sens. Actuators B 2021, 327, 128922. [Google Scholar] [CrossRef]
- He, D.; Yan, M.; Sun, P.; Sun, Y.; Qu, L.; Li, Z. Recent progress in carbon-dots-based nanozymes for chemosensing and biomedical applications. Chin. Chem. Lett. 2021, 32, 2994–3006. [Google Scholar] [CrossRef]
- Ling, P.; Qian, C.; Yu, J.; Gao, F. Artificial nanozyme based on platinum nanoparticles anchored metal-organic frameworks with enhanced electrocatalytic activity for detection of telomeres activity. Biosens. Bioelectron. 2020, 149, 111838. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cheng, D.; Li, P.; Xu, Z.; Zhu, X.; Zhang, Y.; Li, H.; Liu, X.; Liu, M.; Yao, S. Au/Metal–Organic Framework Nanocapsules for Electrochemical Determination of Glutathione. ACS Appl. Nano Mater. 2021, 4, 4853–4862. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chai, L.; Liu, J.; Huang, S.; Liu, F.; Zhu, X.; Zhang, Y.; Liu, M.; Yao, S. Total antioxidant capacity assessment and accurate quantification of ascorbic acid and glutathione utilizing the enhanced multi-mimetic of Cu-CeO2 NPs decorated PCN-224. Sens. Actuators B 2025, 437, 137709. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.; Yang, Q.; Liu, J.; Liu, F.; Zhu, X.; Huang, S.; Yin, P.; Wang, X.; Li, H.; et al. Multifunctional porphyrinic metal-organic framework-based nanoplatform regulating reactive oxygen species achieves efficient imaging-guided cascaded nanocatalytic therapy. J. Colloid Interface Sci. 2025, 684, 423–438. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Pan, Y.; Zhang, Y.; Le, T. Interactions of metal-based nanozymes with aptamers, from the design of nanozyme to its application in aptasensor: Advances and perspectives. Talanta 2025, 286, 127450. [Google Scholar] [CrossRef]
- Song, C.; Ding, W.; Liu, H.; Zhao, W.; Yao, Y.; Yao, C. Label-free colorimetric detection of deoxyribonuclease I activity based on the DNA-enhanced peroxidase-like activity of MIL-53(Fe). New J. Chem. 2019, 43, 12776–12784. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Liu, P.; Qin, X.; Liu, G. Colorimetric quantification of sodium benzoate in food by using d-amino acid oxidase and 2D metal organic framework nanosheets mediated cascade enzyme reactions. Talanta 2022, 237, 122906. [Google Scholar] [CrossRef]
- Yuan, Z.; Chai, H.; Huang, Y.; Zhang, Z.; Tan, W.; Sun, Y.; Ma, J.; Zhang, G. Porphyrin-engineered metal−organic frameworks for photo/electrochemical sensing: Preparation and mechanisms. Coord. Chem. Rev. 2025, 527, 216385. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Cheng, P.; Zaworotko, M.J.; Chen, Y.; Zhang, Z. Post-synthetic modifications of metal–organic cages. Nat. Rev. Chem. 2022, 6, 339–356. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, S.; Ye, F.; Su, L.; Zhang, C.; Shen, S.; Zhao, S. Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem. Commun. 2015, 51, 4635–4638. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, Y.; Yu, L.; Qu, L.; Li, Z.; Zhang, L. A Co-based MOF as nanozyme with enhanced oxidase-like activity for highly sensitive and selective colorimetric differentiation of aminophenol isomers. Talanta 2023, 255, 124219. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, J.; Dong, X.; Chen, J.; Wang, G.; Wang, R. Stabilizing Pt3Co intermetallic nanocrystals on Co3ZnC/Co in N-doped carbon for efficient oxygen reduction reaction and high-performance zinc-air batteries. Carbon 2023, 214, 118321. [Google Scholar] [CrossRef]
- Yu, Z.; Bai, Y.; Zhang, S.; Liu, Y.; Zhang, N.; Wang, G.; Wei, J.; Wu, Q.; Sun, K. Metal–Organic Framework-Derived Co3ZnC/Co Embedded in Nitrogen-Doped Carbon Nanotube-Grafted Carbon Polyhedra as a High-Performance Electrocatalyst for Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Max, J.-J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Comparison between Different Acids and Their Salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Nagalakshmi, P.; Rajaputra, S.S.; Brahman, P.K. Development of ternary Pd-Co-Ir metal nanoparticles decorated on graphene-CNTs hybrid support: An efficient electrocatalyst for hydrogen production from methanol reformation. Electrochim. Acta 2022, 432, 141229. [Google Scholar] [CrossRef]
- Švegl, F.; Orel, B.; Bukovec, P.; Kalcher, K.; Hutchins, M.G. Spectroelectrochemical and structural properties of electrochromic Co (Al) -oxide and Co (Al,Si) -oxide films prepared by the sol-gel route. J. Electroanal. Chem. 1996, 418, 53–66. [Google Scholar] [CrossRef]
- Han, M.; Ren, M.; Li, Z.; Qu, L.; Yu, L. A two-dimensional thin Co-MOF nanosheet as a nanozyme with high oxidase-like activity for GSH detection. New J. Chem. 2022, 46, 10682–10689. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Li, Y.; Zhang, X.; Huang, Y. 2D bimetallic Ni/Fe MOF nanosheet composites as a peroxidase-like nanozyme for colorimetric assay of multiple targets. Anal. Methods 2021, 13, 2066–2074. [Google Scholar] [CrossRef]
- Fu, M.; Xu, F.; Yan, J.; Wang, C.; Fan, G.; Song, G.; Chai, B. Mixed valence state cerium metal organic framework with prominent oxidase-mimicking activity for ascorbic acid detection: Mechanism and performance. Colloids Surf. A 2022, 641, 128610. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Shao, Z.; Gu, R. Single gold nanoparticle-driven heme cofactor nanozyme as an unprecedented oxidase mimetic. Chem. Commun. 2021, 57, 3399–3402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Z.; Sun, Y.; Xu, Z.; Liu, J. The most active oxidase-mimicking Mn2O3 nanozyme for biosensor signal generation. Chem.-Eur. J. 2021, 27, 9597–9604. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Su, L.; Yang, C.; Ma, Y.; Zhang, H.; Chen, X. Colorimetric Detection of Sulfite in Foods by a TMB–O2–Co3O4 Nanoparticles Detection System. J. Agric. Food Chem. 2014, 62, 5827–5834. [Google Scholar] [CrossRef] [PubMed]
- Vallabani, N.V.S.; Karakoti, A.; Singh, S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological Ph. Colloids Surf. B 2017, 153, 52–60. [Google Scholar] [CrossRef]
- Gu, Y.; Cao, Z.; Zhao, M.; Xu, Y.; Lu, N. Single-Atom Fe Nanozyme with Enhanced Oxidase-like Activity for the Colorimetric Detection of Ascorbic Acid and Glutathione. Biosensors 2023, 13, 487. [Google Scholar] [CrossRef]
- Yaqub, A.; Gilani, S.R.; Bilal, S.; Hayat, A.; Asif, A.; Siddique, S.A. Efficient Preparation of a Nonenzymatic Nanoassembly Based on Cobalt-Substituted Polyoxometalate and Polyethylene Imine-Capped Silver Nanoparticles for the Electrochemical Sensing of Carbofuran. ACS Omega 2022, 7, 149–159. [Google Scholar] [CrossRef]
- Jiao, Z.; Hou, S.; Kang, X.; Yang, X.; Zhao, B. Recyclable Luminescence Sensor for Dinotefuran in Water by Stable Cadmium–Organic Framework. Anal. Chem. 2021, 93, 6599–6603. [Google Scholar] [CrossRef]
- Li, C.; Zeng, J.; Guo, D.; Liu, L.; Xiong, L.; Luo, X.; Hu, Z.; Wu, F. Cobalt-Doped Carbon Quantum Dots with Peroxidase-Mimetic Activity for Ascorbic Acid Detection through Both Fluorometric and Colorimetric Methods. ACS Appl. Mater. Interfaces 2021, 13, 49453–49461. [Google Scholar] [CrossRef]
- Chang, A.S.; Tahira, A.; Chang, F.; Solangi, A.G.; Bhatti, M.A.; Vigolo, B.; Nafady, A.; Ibupoto, Z.H. Highly Heterogeneous Morphology of Cobalt Oxide Nanostructures for the Development of Sensitive and Selective Ascorbic Acid Non-Enzymatic Sensor. Biosensors 2023, 13, 147. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Q.; Zou, T.; Kong, Y.; Su, L.; Ma, D.; Wang, Y. Dual-emission ratiometric fluorescent detection of dinotefuran based on sulfur-doped carbon quantum dots and copper nanocluster hybrid. Sens. Actuators B 2020, 321, 128534. [Google Scholar] [CrossRef]
- López-Fernández, O.; Rial-Otero, R.; González-Barreiro, C.; Simal-Gándara, J. Surveillance of fungicidal dithiocarbamate residues in fruits and vegetables. Food Chem. 2012, 134, 366–374. [Google Scholar] [CrossRef]
- López-Fernández, O.; Barroso, M.F.; Fernandes, D.; Rial-Otero, R.; Simal-Gándara, J.; Morais, S.; Nouws, H.; Freire, C.; Delerue-Matos, C. Voltammetric analysis of mancozeb and its degradation product ethylenethiourea. J. Electroanal. Chem. 2015, 758, 54–58. [Google Scholar] [CrossRef]
- Zamora, R.; Masís-Meléndez, F.; Phillips, H.; Alvarado-Marchena, L.A.; Starbird, R. Development of poly (3,4-ethylenedioxythiophene (PEDOT)/carbon nanotube electrodes for electrochemical detection of Mancozeb in water. Int. J. Electrochem. Sci. 2018, 13, 1931–1944. [Google Scholar] [CrossRef]
- Rohit, J.V.; Solanki, J.N.; Kailasa, S.K. Surface modification of silver nanoparticles with dopamine dithiocarbamate for selective colorimetric sensing of mancozeb in environmental samples. Sens. Actuators B 2014, 200, 219–226. [Google Scholar] [CrossRef]
- Asghar, M.; Yaqoob, M.; Munawar, N.; Nabi, A. Determination of Mancozeb and Maneb using flow injection chemiluminescence detection. Int. J. Environ. Anal. Chem. 2020, 102, 2586–2606. [Google Scholar] [CrossRef]
- NY/T 788-2018; Guideline for the Testing of Pesticide Residues IN Crops. China Agriculture Press: Beijing, China, 2018.
- GB 2763-2021; National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. China Agriculture Press: Beijing, China, 2021.
- Lu, W.; Zhang, J.; Li, N.; You, Z.; Feng, Z.; Natarajan, V.; Chen, J.; Zhan, J. Co3O4@β-cyclodextrin with synergistic peroxidase-mimicking performance as a signal magnification approach for colorimetric determination of ascorbic acid. Sens. Actuators B 2020, 303, 127106. [Google Scholar] [CrossRef]
- Qin, C.; Wang, B.; Wu, N.; Han, C.; Wang, Y. General strategy to fabricate porous Co- based bimetallic metal oxide nanosheets for high-performance CO sensing. ACS Appl. Mater. Interfaces 2021, 13, 26318–26329. [Google Scholar] [CrossRef]
- Wan, J.; Zou, J.; Zhou, S.; Pan, F.; Hua, F.; Zhang, Y.; Nie, J.; Zhang, Y. A bimetallic (Ni/Co) metal–organic framework with excellent oxidase-like activity for colorimetric sensing of ascorbic acid. Anal. Methods 2023, 15, 1819–1825. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Xia, Q.; Yang, D.; Yang, Y. Zirconium-porphyrin-MOF-based oxidase-like nanozyme with oxygen vacancy for aflatoxin B1 colorimetric sensing. J. Food Sci. 2024, 89, 3618–3628. [Google Scholar]
- Yuan, B.; Chou, H.; Peng, Y. Disclosing the origin of transition metal oxides as peroxidase (and catalase) mimetics. ACS Appl. Mater. Interfaces 2021, 14, 22728–22736. [Google Scholar] [CrossRef]
- Liao, J.; Gao, Z.; Liu, Z.; Wu, H.; Liu, L.; Zeng, Z.; Yang, X.; Wang, H.; Du, J.; Zheng, B.; et al. A multienzyme cascade nanoplatform based on cerium metal organic framework for dual-channel visual detection of organophosphorus pesticides in food samples. Sens. Actuators B 2025, 426, 137052. [Google Scholar] [CrossRef]





| Nanozymes | Km/(mM) | Vmax/(10−8 M S−1) | Reference |
|---|---|---|---|
| Heme-AuNPS | 0.168 | 1.68 | [31] |
| Mn2O3 | 0.130 | 15 | [32] |
| Co3O4 NPS | 0.051 | 3.3 | [33] |
| Fe3O4 | 0.370 | 2.60 | [34] |
| Fe-N-C SAzyme | 0.253 | 4.11 | [35] |
| Nanomaterials | Methods | Detection Limit (μM) | Reference |
|---|---|---|---|
| CoPOM/AgNP | electrochemical | 100 | [36] |
| Cd-MOF | luminous | 2.09 | [37] |
| Co-CQDs/Fe3+ | luminous | 18 | [38] |
| Co3O4 | electrochemical | 1 | [39] |
| S-CQDs/CuNCs | luminous | 7.04 | [40] |
| Methods | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| HPLC-DAD | 0.5–9.3 mg/kg | 0.04 mg/kg | [41] |
| voltammetry | 10–90 μM | 7 μM | [42] |
| electrochemical method | 25–150 μM | 10 μM | [43] |
| colorimetric method | 50–300 μM | 21.1 μM | [44] |
| flow injection chemiluminescence | 0.0092–0.9218 μM | 0.003 μM | [45] |
| Sample | Added (μM) | UV–vis Spectroscopy | Smartphone-Based Colorimetric Method | ||||
|---|---|---|---|---|---|---|---|
| Found (μM) | RSD (%) | Recovery (%) | Found (μM) | RSD (%) | Recovery (%) | ||
| Lettuce | 15 | 14.71 | 0.23 | 98.11 | 15.18 | 1.18 | 101.21 |
| 30 | 29.79 | 0.85 | 99.30 | 29.68 | 2.50 | 98.95 | |
| 60 | 60.84 | 1.16 | 101.41 | 49.30 | 5.82 | 82.17 | |
| 90 | 85.27 | 1.06 | 94.75 | 88.46 | 1.03 | 98.29 | |
| Tomato | 15 | 14.92 | 1.15 | 99.50 | 15.23 | 3.21 | 101.54 |
| 30 | 30.72 | 0.51 | 102.41 | 27.77 | 2.57 | 92.56 | |
| 60 | 64.26 | 3.76 | 107.11 | 65.83 | 3.70 | 109.71 | |
| 90 | 100.07 | 0.92 | 111.18 | 98.66 | 5.46 | 109.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, S.; Chen, L.; Liu, Y.; Lu, X.; Liu, H.; Shu, Y.; Bai, H.; Wang, J.; Shi, D. Superior Oxidase-Mimetic Activity of Co-MOF Nanozyme for Smartphone-Based Visually Colorimetric Assay of Mancozeb. Molecules 2025, 30, 4758. https://doi.org/10.3390/molecules30244758
Pang S, Chen L, Liu Y, Lu X, Liu H, Shu Y, Bai H, Wang J, Shi D. Superior Oxidase-Mimetic Activity of Co-MOF Nanozyme for Smartphone-Based Visually Colorimetric Assay of Mancozeb. Molecules. 2025; 30(24):4758. https://doi.org/10.3390/molecules30244758
Chicago/Turabian StylePang, Shuyue, Lina Chen, Yangyuxin Liu, Xiuting Lu, Hongfei Liu, Yuting Shu, Helong Bai, Jing Wang, and Dongfang Shi. 2025. "Superior Oxidase-Mimetic Activity of Co-MOF Nanozyme for Smartphone-Based Visually Colorimetric Assay of Mancozeb" Molecules 30, no. 24: 4758. https://doi.org/10.3390/molecules30244758
APA StylePang, S., Chen, L., Liu, Y., Lu, X., Liu, H., Shu, Y., Bai, H., Wang, J., & Shi, D. (2025). Superior Oxidase-Mimetic Activity of Co-MOF Nanozyme for Smartphone-Based Visually Colorimetric Assay of Mancozeb. Molecules, 30(24), 4758. https://doi.org/10.3390/molecules30244758





