Application of Gas Chromatographic Retention Indices to GC and GC–MS Identification with Variable Limits for Deviations Between Their Experimental and Reference Values
Abstract
1. Introduction
1 − 0.05|ΔRI|, if 5 < |ΔRI| ≤ 20 = ΔRIlim
0, if |ΔRI| > 20,
- The choice of fixed value ΔRIlim = 20 i.u. seems to be illogical because the differences between experimental and reference data can take both larger and smaller values depending on the chemical nature of analytes and types of stationary phases under comparison;
- Condition (2) implies the symmetrical distribution of ΔRI, in other words, the equally probable deviations of experimental and reference data both at ΔRI < 0 and ΔRI > 0, but in the general case, it is not obvious.
- It should not include any fixed limiting values of possible deviations between experimental and reference RI values;
- It should be applicable to asymmetrically distributed reference RI values;
- It should be applicable to both statistically processed reference data and the results of single measurements;
- It should be applicable to compare the RI values determined using columns with semi-standard stationary phases (terminology used in database [2]) with reference data for standard nonpolar stationary phases and vice versa;
- It should be applicable to RI values measured for both capillary and packed chromatographic columns at various temperatures without any artificial restrictions.
2. Results and Discussion
2.1. Optimization of Comparing the Experimental and Reference Values of GC Retention Indices
2.2. Application of the Algorithm of Comparing the Experimental and Reference GC Retention Indices to Multicomponent Mixtures
3. Materials and Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovats’ Retention Index System. In Encyclopedia of Chromatography, 3rd ed.; Cazes, J., Ed.; Taylor & Francis: New York, NY, USA, 2010; Volume 2, pp. 1304–1310. [Google Scholar]
- The NIST Mass Spectral Library (NIST/EPA/NIH EI MS Library, 2017 Release). Software/Data Version; NIST Standard Reference Database, Number 69, August 2017. National Institute of Standards and Technology: Gaithersburg, MD, USA. Available online: http://webbook.nist.gov (accessed on 30 September 2025).
- Sokolow, S.; Karnofsky, J.; Gustafson, P. The Finnigan Library Search Program. Finnigan Applied Report No. 2; Finnigan Corporation: San Jose, CA, USA, 1978; 45p. [Google Scholar]
- Zenkevich, I.G.; Ukolova, E.S. Dependence of chromatographic retention indices on a ratio of amounts of target and reference compounds. J. Chromatogr. A 2012, 1265, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Rospondek, M.J.; Narynowski, L.; Chachaj, A.; Gora, M. Novel aryl polycyclic aromatic hydrocarbons: Phenylphenanthrene and phenylanthracene identification, occurrence and distribution in sedimentary rocks. Org. Geochem. 2009, 40, 986–1004. [Google Scholar] [CrossRef]
- Costa, R.; Pizzimenti, F.; Marotta, F.; Dugo, P.; Santi, L.; Mondelo, L. Volatiles from steam-distilled leaves of some plant species from Madagascar and New Zealand and evaluation of their biological activity. Nat. Prod. Commun. 2010, 5, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Yu, W.-J.; Zhou, Q.; Han, R.-F.; Huang, D.F. Metabolic response of pakchoi leaves to amino acid nitrogen. J. Integr. Agric. 2014, 13, 778–788. [Google Scholar] [CrossRef]
- Bodner, M.; Vagalinski, B.; Makarov, S.E.; Antic, D.Z.; Vujisic, L.V.; Leis, H.-J.; Raspotnig, G. “Quinone millipedes” reconsidered: Evidence for a mosaic-like taxonomic distribution of phenol-based secretion across the julidae. J. Chem. Ecol. 2016, 42, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.P.; Alves, R.C.; de Carvalho Rocha, W.F.; da Silva Monteiro, S.; de Oliveira Ribeiro, L.; Moreira, R.F.A. Chemical profile of the volatile fraction of Bauhinia forficata leaves: An evaluation of commercial and in natura samples. Food Sci. Technol. 2022, 42, e34122. [Google Scholar] [CrossRef]
- Trovato, E.; Micalizzi, G.; Dugo, P.; Utczas, M.; Mondello, L. Use of linear retention indices in GC-MS. In Handbook of Essential Oils. Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, CA, USA, 2015. [Google Scholar]
- Weissburg, J.R.; Johanningsmeier, S.D.; Dean, L.L. Volatile compounds profiles of raw and roasted peanut seeds of the runner and Virginia market-types. J. Food Res. 2023, 12, 47–68. [Google Scholar] [CrossRef]
- Wesolowska, A.; Jadczak, D.; Zyburtowich, K. Influence of distillation time and distillation apparatus on the chemical composition and quality of Lavandula angustifolia Mill, essential oil. Polish J. Chem. Technol. 2023, 25, 36–43. [Google Scholar] [CrossRef]
- Albarico, G.; Unbanova, K.; Houdkova, M.; Bande, M.; Tulin, E.; Kokoskova, T.; Kokoska, L. Evaluation of chemical composition and anti-staphylococcal activity of essential oils from leaves of two indigenous plant species, Litsea leytensis and Piper philippinum. Plants 2024, 13, 3555. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.N.; Calva, J.; Ramirez, J.; Vidari, G.; Arnijos, C. Chemical analysis of the essential oils from three populations of Lippia dulcis Trevir. grown at different locations in southern Ecuador. Plants 2024, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Achenbach, M.; Yeager, W.I.; Andersson, P.J.; Fitch, W.H.; Rindfleisch, T.C. Quantitative comparison of combined gas chromatographic/mass spectrometric profiles of complex mixtures. Anal. Chem. 1977, 49, 1623–1632. [Google Scholar] [CrossRef]
- Zenkevich, I.G.; Babushok, V.I.; Linstrom, P.J.; White, V.E.; Stein, S.E. Application of histograms in evaluation of large collections of gas chromatographic indices. J. Chromatogr. A 2009, 1216, 6651–6661. [Google Scholar] [CrossRef] [PubMed]
- Bizzo, H.R.; Brilhante, N.S.; Nolvachai, Y.; Marriott, P.J. Use and abuse of retention indices in gas chromatography. J. Chromatogr. A 2023, 1708, 464376. [Google Scholar] [CrossRef] [PubMed]
- Matheson, A. Identifying and rectifying the misuse of retention indices in GC. LCGC Int. 2024, 20, 2–8. [Google Scholar]
- Adams, R. Identification of Essential Oils Components by GC/MS, Version 4; Allured Publ. Corp.: Carol Stream, IL, USA, 2007; 804p. [Google Scholar]
- Brown, I. The composition of a Lurgi brown coal tar. III. The neutral oil fraction boiling from 30 to 130 °C and 130–172 °C. Austr. J. Appl. Chem. 1960, 11, 403–433. [Google Scholar]
- Linnik, Y.V. Least Squares Method and Fundamentals of Processing the Observations; Physical-Mathematical Literature Press: Moscow, Russia, 1958; 334p. (In Russian) [Google Scholar]
- Engewald, W.; Knobloch, T.; Haufe, G.; Muller, M.; Pohris, V. A novel method for terpene pattern determination of essential oils by selectivity tuning in GC. Fresenius’ J. Anal. Chem. 1991, 341, 641–643. [Google Scholar] [CrossRef]
- Benadi, T.; Lemhadri, A.; Harboul, K.; Chtibi, H.; Khabbach, A.; Jadouali, S.M.; Quesada-Romero, L.; Louahliq, S.; Hammani, K.; Ghaleb, A.; et al. Chemical profiling and biological properties of essential oils of Lavandula stolchas L., collected from three Moroccan sites: In vitro and in silico investigations. Plants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Obistioiu, D.; Cristina, R.T.; Schmerold, I.; Chizzola, R.; Stolze, K.; Nichita, I.; Chiurciu, V. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Zenkevich, I.G. Non-traditional criteria for the identification of organic compounds by chromatography and chromatography—Mass spectrometry. J. Anal. Chem. 1998, 53, 725–731. (In Russian) [Google Scholar]
- Wilson, M.B.; Barnes, B.B.; Boswell, P.S. What experimental factors influence the accuracy of retention projections in gas chromatography—Mass spectrometry. J. Chromatogr. A 2014, 1373, 179–189. [Google Scholar] [CrossRef] [PubMed]
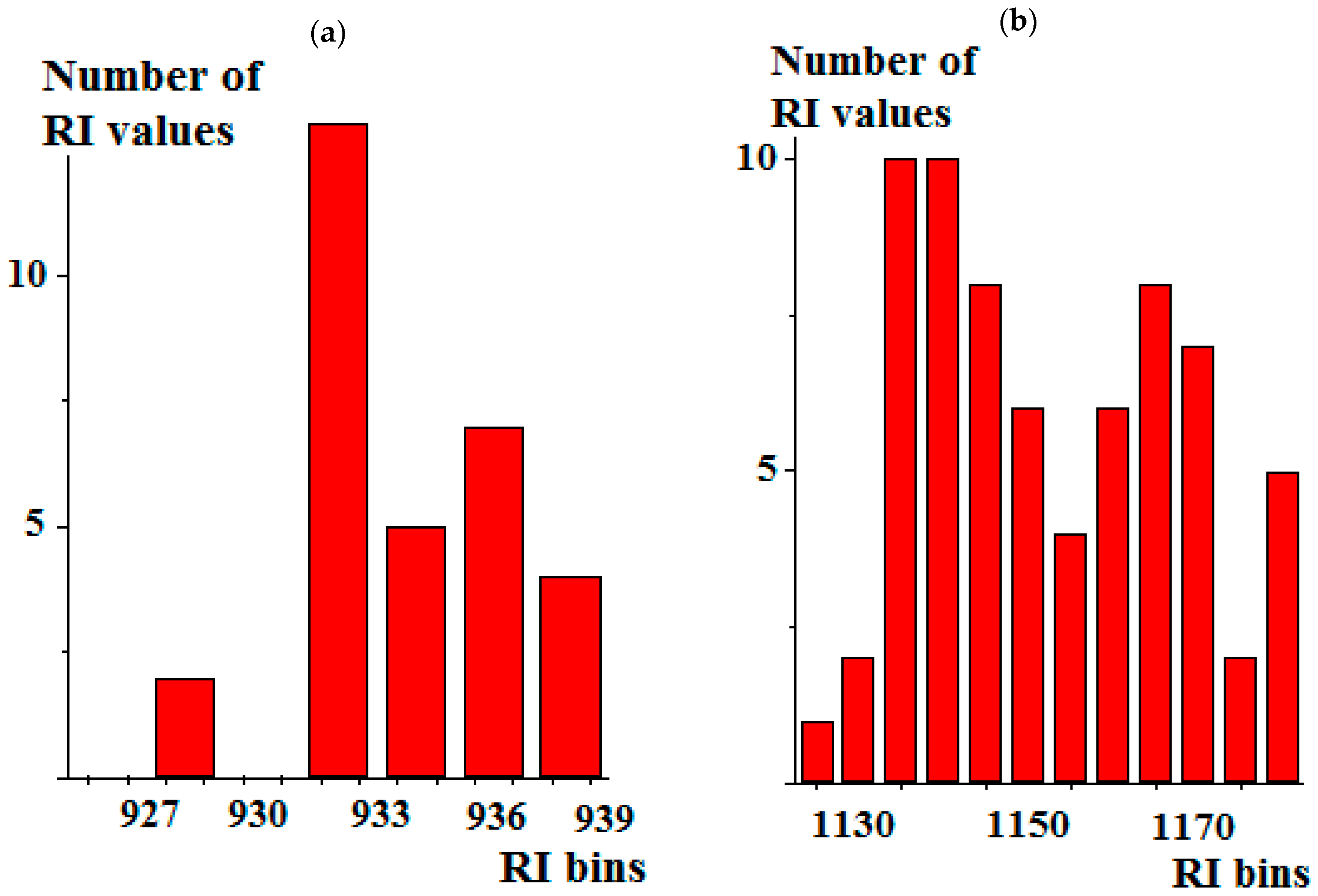
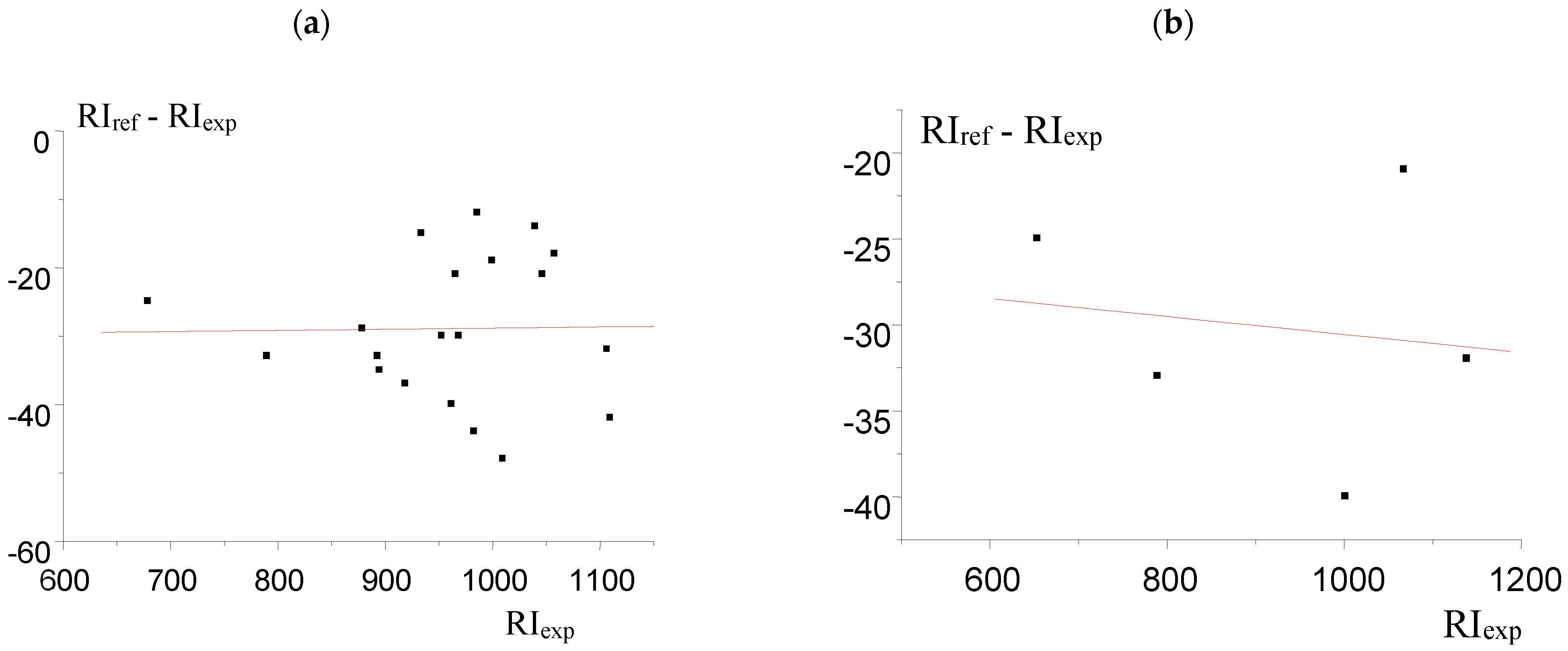
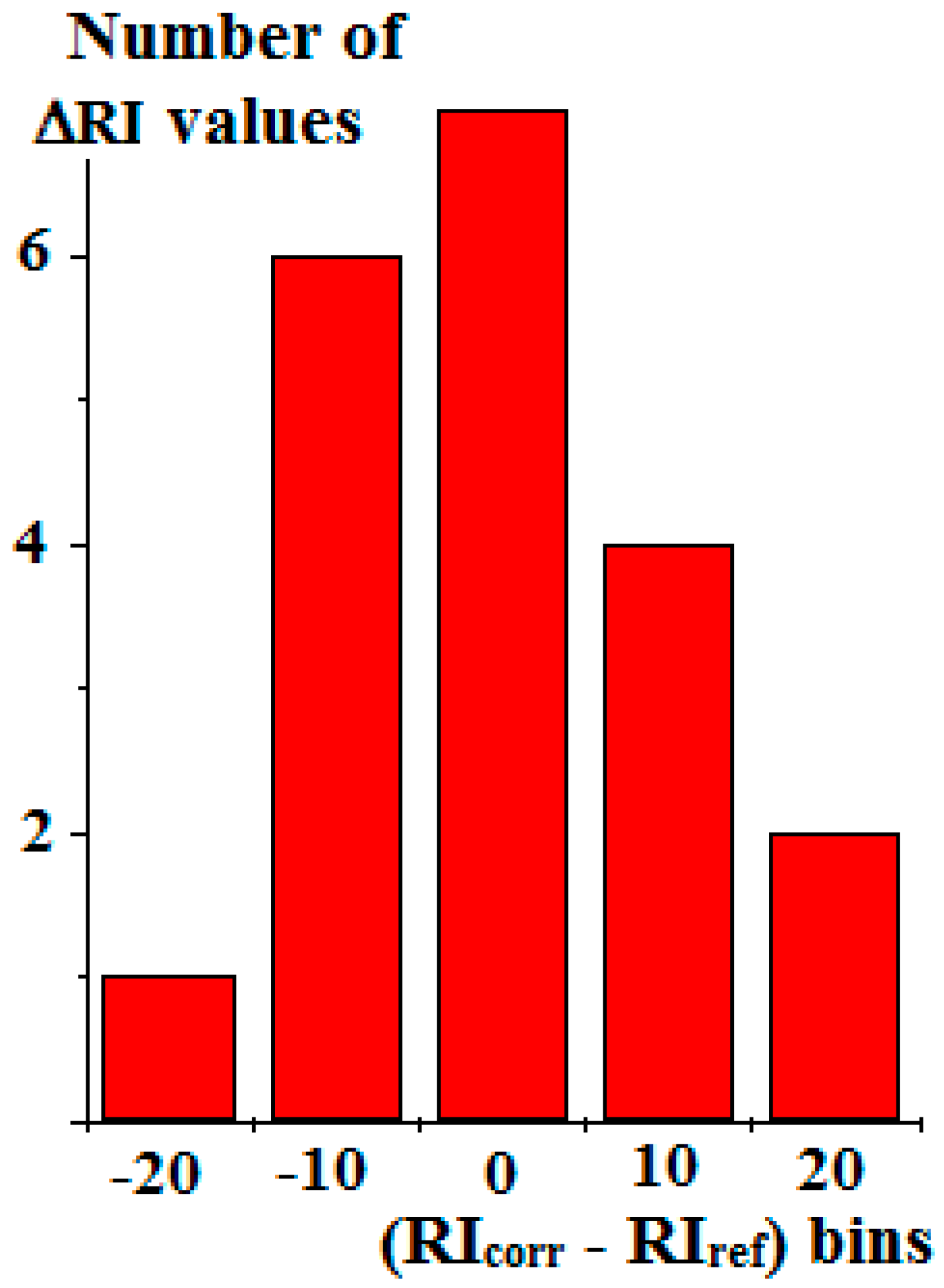
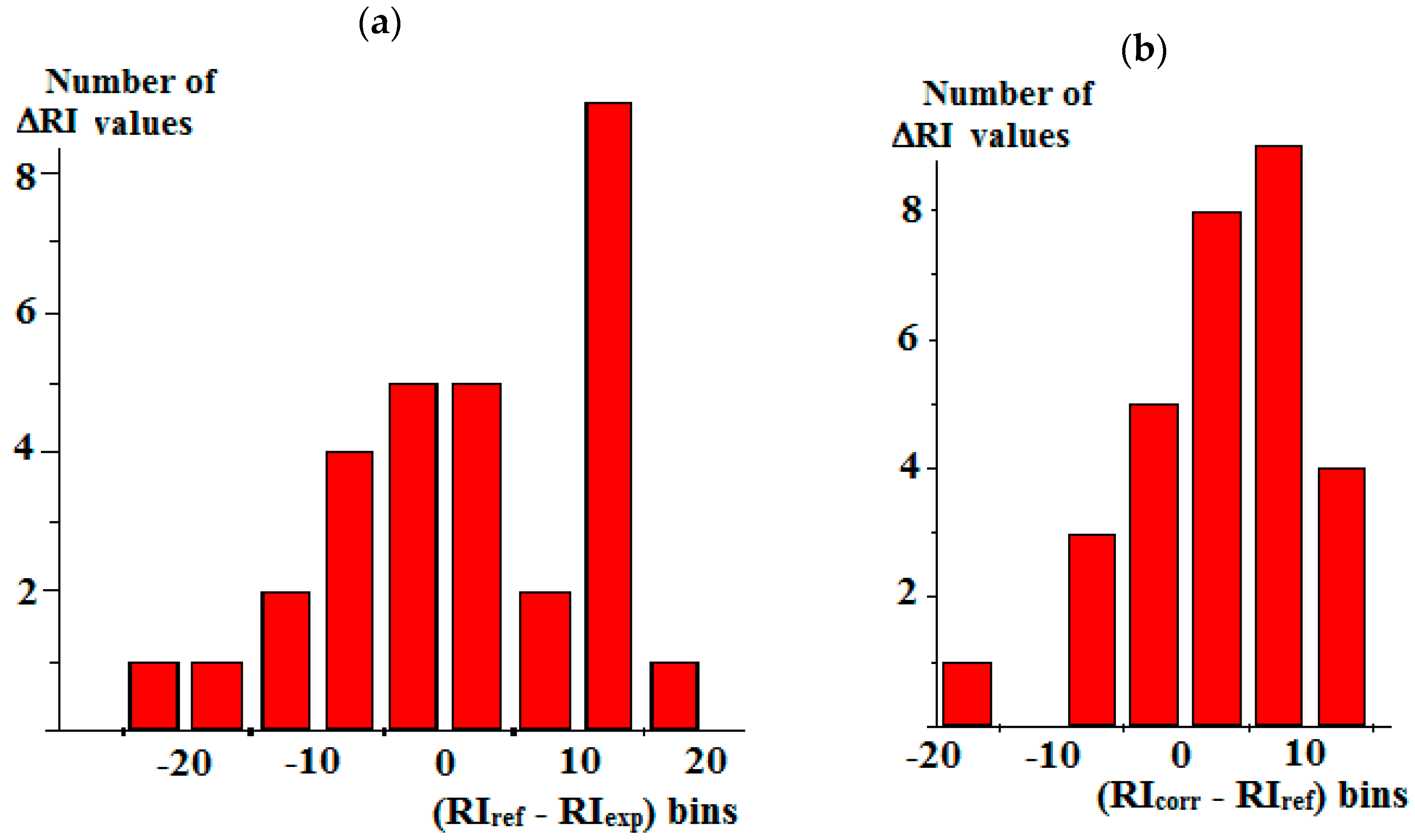
| Compound | RIexp [20] | RIref [2] | Δref-exp | Complete Set of Reference Data | Reduced Set of Reference Data | ||
|---|---|---|---|---|---|---|---|
| RIcorr | Δcorr-ref | RIcorr * | Δcorr-ref | ||||
| Benzene | 679 | 654 ± 7 | −25 | 650 | −4 | 650 | −4 |
| Toluene | 790 | 757 ± 6 | −33 | 761 | +4 | 760 | +3 |
| Ethylbenzene | 879 | 850 ± 6 | −29 | 850 | 0 | 849 | −1 |
| m-Xylene | 893 | 860 ± 6 | −35 | 866 | +6 | 865 | +5 |
| p-Xylene | 893 | 860 ± 6 | −33 | 864 | +4 | 863 | +3 |
| o-Xylene | 919 | 881 ± 6 | −37 | 890 | +9 | 889 | +8 |
| Isopropylbenzene | 934 | 919 ± 7 | −15 | 905 | −14 | 904 | −15 |
| Propylbenzene | 966 | 945 ± 5 | −21 | 937 | −8 | 936 | −9 |
| 1-Methyl-4-ethylbenzene | 983 | 953 ± 5 | −30 | 954 | +1 | 953 | 0 |
| tert-Butylbenzene | 998 | 986 ± 7 | −12 | 969 | −17 | 968 | −18 |
| 1-Methyl-2-ethylbenzene | 999 | 969 ± 5 | −30 | 970 | +1 | 969 | 0 |
| 1,3,5-Trimethylbenzene | 1002 | 962 ± 6 | −40 | 974 | +12 | 972 | +10 |
| sec-Butylbenzene | 1019 | 1000 ± 5 | −19 | 991 | −9 | 989 | −11 |
| 1,2,4-Trimethylbenzene | 1027 | 983 ± 5 | −44 | 999 | +16 | 997 | +14 |
| 1,3-Diethylbenzene | 1054 | 1040 ± 5 | −14 | 1026 | −14 | 1023 | −17 |
| 1,2,3-Trimethylbenzene | 1058 | 1010 ± 6 | −48 | 1030 | +20 | 1027 | +17 |
| Butylbenzene | 1068 | 1047 ± 6 | −21 | 1040 | −7 | 1037 | −10 |
| 1-Methyl-2-propylbenzene | 1076 | 1058 ± 5 | −18 | 1048 | −10 | 1045 | −13 |
| 1,2,4,5-Tetramethylbenzene | 1139 | 1107 ± 5 | −32 | 1111 | +4 | 1108 | +1 |
| 1,2,3,5-Tetramethylbenzene | 1152 | 1110 ± 6 | −42 | 1124 | +14 | 1121 | +11 |
| Average standard deviation of reference RI values, sRI | 5.8 | ||||||
| Average difference Δref-exp: | −29 ± 10 | ||||||
| Average difference Δcorr-ref: | 9 ± 6 (0 ± 11) ** | 8 ± 6 * (−1 ± 10) ** | |||||
| Compound | RIexp | RIref [2] | Δref-exp | Complete Set of Reference Data | Reduced Set of Reference Data | ||
|---|---|---|---|---|---|---|---|
| RIcorr | Δcorr-ref | RIcorr * | Δcorr-ref * | ||||
| α-Pinene ** | 950 | 933 ± 4 | −17 | 936 | +3 | 930 | −3 |
| Camphene | 969 | 946 ± 5 | −23 | 956 | +10 | 950 | +4 |
| Myrcene | 985 | 983 ± 3 | −2 | 970 | −15 | 967 | −18 |
| 3-Carene | 1022 | 1006 ± 5 | −16 | 1010 | +4 | 1005 | −1 |
| Limonene | 1038 | 1017 ± 3 | −21 | 1026 | +9 | 1022 | +5 |
| Linalool | 1089 | 1086 ± 3 | −3 | 1077 | −9 | 1076 | −10 |
| Isofenchol | 1114 | - | - | - | - | - | - |
| Fenchone | 1120 | 1105 ± 6 | −15 | 1109 | +4 | 1108 | +3 |
| Citronellal | 1138 | 1134 ± 4 | −4 | 1127 | −7 | 1127 | −7 |
| (Z)-Verbenol | 1144 | 1133 ± 5 | −11 | 1133 | 0 | 1148 | +15 |
| Camphor | 1146 | 1123 ± 6 | −23 | 1135 | +12 | 1136 | +13 * |
| (Z)-Pinocarveol *** | 1146 | 1135 ± 7 | −11 | 1135 | 0 | 1136 | +1 |
| 1126 ± 6 | −20 | 1135 | +9 | 1136 | +10 | ||
| (E)-Verbenol | 1147 | 1133 ± 5 | −14 | 1136 | +3 | 1137 | +4 |
| Isopulegol | 1148 | 1144 ± 5 | −4 | 1137 | −7 | 1138 | −6 |
| (Z)-Pinocamphone | 1160 | 1140 ± 5 | −20 | 1149 | +9 | 1150 | +10 |
| Borneol | 1171 | 1151 ± 18 | −20 | 1161 | +10 | 1162 | +11 |
| Menthol *** | 1174 | 1157 ± 2 | −17 | 1164 | +7 | 1165 | +8 |
| 1165 ± 6 | −9 | 1164 | −1 | 1165 | 0 | ||
| Terpinen-4-ol | 1178 | 1164 ± 5 | −14 | 1168 | +4 | 1169 | +5 |
| Carenol | 1188 | - | - | - | - | - | - |
| α-Terpineol | 1189 | 1175 ± 5 | −14 | 1179 | +4 | 1181 | +6 |
| Neocarveol | 1189 | - | - | - | - | - | - |
| Myrtenol | 1195 | 1181 ± 5 | −14 | 1185 | +4 | 1187 | +6 |
| Verbenone | 1202 | 1184 ± 7 | −18 | 1092 | +8 | 1194 | +10 |
| Neral | 1223 | 1218 ± 5 | −5 | 1213 | −5 | 1216 | −2 |
| Carvone | 1230 | 1218 ± 6 | −12 | 1220 | +2 | 1227 | +9 |
| Geraniol | 1238 | 1237 ± 4 | −1 | 1229 | −8 | 1232 | −5 |
| Linalyl acetate | 1242 | 1241 ± 2 | −1 | 1233 | −8 | 1236 | −5 |
| Geranial | 1250 | 1249 ± 8 | −1 | 1241 | −8 | 1245 | −4 |
| Safrol | 1275 | 1269 ± 7 | −6 | 1266 | −3 | 1271 | +2 |
| Bornyl acetate | 1280 | 1270 ± 5 | −10 | 1271 | +1 | 1276 | +6 |
| (Z)-Pinocarvyl acetate | 1301 | - | - | - | - | - | - |
| Isosafrole | 1357 | 1327 ± 31 | −30 | 1349 | +22 4* | 1357 | +30 4* |
| 1358 ± 6 5* | +1 | 1349 | −9 | 1357 | −1 | ||
| Average standard deviation of reference RI values, sRI | 6.3 | ||||||
| Average difference Δref-exp: | 13.2 | ||||||
| Average difference Δcorr-ref: | 7 ± 5 | 6 ± 4 | |||||
| Compound | RIexp | RIref [19] | Δref-exp | Complete set of Reference Data | Reduced set of Reference Data | ||
|---|---|---|---|---|---|---|---|
| RIcorr | Δcorr-ref | RIcorr * | Δcorr-ref * | ||||
| α-Pinene ** | 950 | 932 | −18 | 933 | +1 | 933 | +1 |
| Camphene | 969 | 946 | −23 | 953 | +7 | 954 | +8 |
| Myrcene | 985 | 988 | +3 | 970 | −18 *** | 972 | −16 *** |
| 3-Carene | 1022 | 1008 | −14 | 1010 | +2 | 1012 | +4 |
| Limonene | 1038 | 1024 | −14 | 1028 | +4 | 1029 | +5 |
| Linalool | 1089 | 1095 | +6 | 1083 | −12 | 1085 | −10 |
| Isofenchol | 1114 | 1114 | 0 | 1110 | −4 | 1112 | −2 |
| Fenchone | 1120 | 1118 | −2 | 1116 | −2 | 1119 | +1 |
| Citronellal | 1138 | 1148 | +10 | 1136 | −12 | 1139 | −9 |
| (Z)-Verbenol | 1144 | 1137 | −7 | 1142 | +5 | 1145 | +8 |
| Camphor | 1146 | 1141 | −5 | 1144 | +3 | 1147 | +6 |
| (Z)-Pinocarveol | 1146 | 1135 | −11 | 1144 | +9 | 1147 | +12 |
| (E)-Verbenol | 1147 | 1140 | −7 | 1145 | +5 | 1148 | +8 |
| Isopulegol | 1148 | 1145 | −3 | 1146 | +1 | 1149 | +4 |
| (Z)-Pinocamphone | 1160 | 1172 | +12 | 1159 | −13 | 1163 | −9 |
| Borneol | 1171 | 1165 | −6 | 1171 | +6 | 1175 | +10 |
| Menthol | 1174 | 1167 | −7 | 1174 | +7 | 1178 | +11 |
| Terpinen-4-ol | 1178 | 1174 | −4 | 1179 | +5 | 1182 | +8 |
| Carenol | 1188 | - | - | - | - | - | - |
| α-Terpineol | 1189 | 1189 | 0 | 1191 | +2 | 1194 | +5 |
| Neocarveol | 1189 | - | - | - | - | - | - |
| Myrtenol | 1195 | 1194 | −1 | 1197 | +3 | 1201 | +6 |
| Verbenone | 1202 | 1204 | +2 | 1205 | +1 | 1208 | +4 |
| Neral | 1223 | 1235 | +12 | 1227 | −8 | 1231 | −4 |
| Carvone | 1230 | 1239 | +9 | 1235 | −4 | 1239 | 0 |
| Geraniol | 1238 | 1249 | +11 | 1244 | −5 | 1248 | −1 |
| Linalyl acetate | 1242 | 1254 | +12 | 1248 | −6 | 1252 | −2 |
| Geranial | 1250 | 1264 | +14 | 1257 | −7 | 1261 | −3 |
| Safrol | 1275 | 1285 | +10 | 1284 | −1 | 1288 | +3 |
| Bornyl acetate | 1280 | 1284 | +4 | 1289 | +5 | 1293 | +11 |
| (Z)-Pinocarvyl acetate | 1301 | 1311 | +10 | 1312 | +1 | 1316 | +5 |
| Isosafrole | 1357 | 1373 | +16 | 1372 | −1 | 1377 | +4 |
| Average difference Δref-exp: | 8.4 | ||||||
| Average difference Δcorr-ref: | 5 ± 4 | 6 ± 4 | |||||
| RIexp | RIref [19] | ΔRIref-exp | RIcorr | ΔRIcorr | |
|---|---|---|---|---|---|
| Camphene | 949 | 946 | −3 | 948 | +2 |
| Myrcene | 993 | 988 | −5 | 992 | −1 |
| (E)-Ocimene | 1038 | 1044 | +6 | 1037 | −1 |
| γ-Terpinene | 1049 | 1054 | +5 | 1048 | −1 |
| Linalool | 1100 | 1095 | −5 | 1098 | +3 |
| Alloocimene | 1136 | 1128 | −8 | 1134 | −2 |
| Linalyl acetate | 1256 | 1254 | −2 | 1253 | −3 |
| C15H24 * | 1365 | - | - | 1361 | - |
| C15H24 * | 1384 | - | - | 1380 | - |
| Aromadendrene | 1443 | 1439 | −4 | 1439 | −4 |
| Average difference Δref-exp: | 4.8 | ||||
| Average difference Δcorr-ref: | 2 ± 1 | ||||
| RI | Compound | Number of RI Values for Standard/Semi-Standard Phases in [2] |
|---|---|---|
| 1376 [2] | α-Copaene | 377/698 |
| 1377 [19] | Silfiperfol-6-ene | None |
| 1379 [19] | β-Patchoulene | 10/23 |
| 1380 [19] | Daucene | 16/14 |
| 1381 [19] | β-Panansinene | 1/5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenkevich, I.G. Application of Gas Chromatographic Retention Indices to GC and GC–MS Identification with Variable Limits for Deviations Between Their Experimental and Reference Values. Molecules 2025, 30, 4706. https://doi.org/10.3390/molecules30244706
Zenkevich IG. Application of Gas Chromatographic Retention Indices to GC and GC–MS Identification with Variable Limits for Deviations Between Their Experimental and Reference Values. Molecules. 2025; 30(24):4706. https://doi.org/10.3390/molecules30244706
Chicago/Turabian StyleZenkevich, Igor G. 2025. "Application of Gas Chromatographic Retention Indices to GC and GC–MS Identification with Variable Limits for Deviations Between Their Experimental and Reference Values" Molecules 30, no. 24: 4706. https://doi.org/10.3390/molecules30244706
APA StyleZenkevich, I. G. (2025). Application of Gas Chromatographic Retention Indices to GC and GC–MS Identification with Variable Limits for Deviations Between Their Experimental and Reference Values. Molecules, 30(24), 4706. https://doi.org/10.3390/molecules30244706






