Acetylbinankadsurin A Decreases Macrophage Glycolysis and Pro-Inflammatory Phenotype Polarization via Inhibiting HIF-1α to Alleviate Hepatic Fibrosis in Mice
Abstract
1. Introduction
2. Results
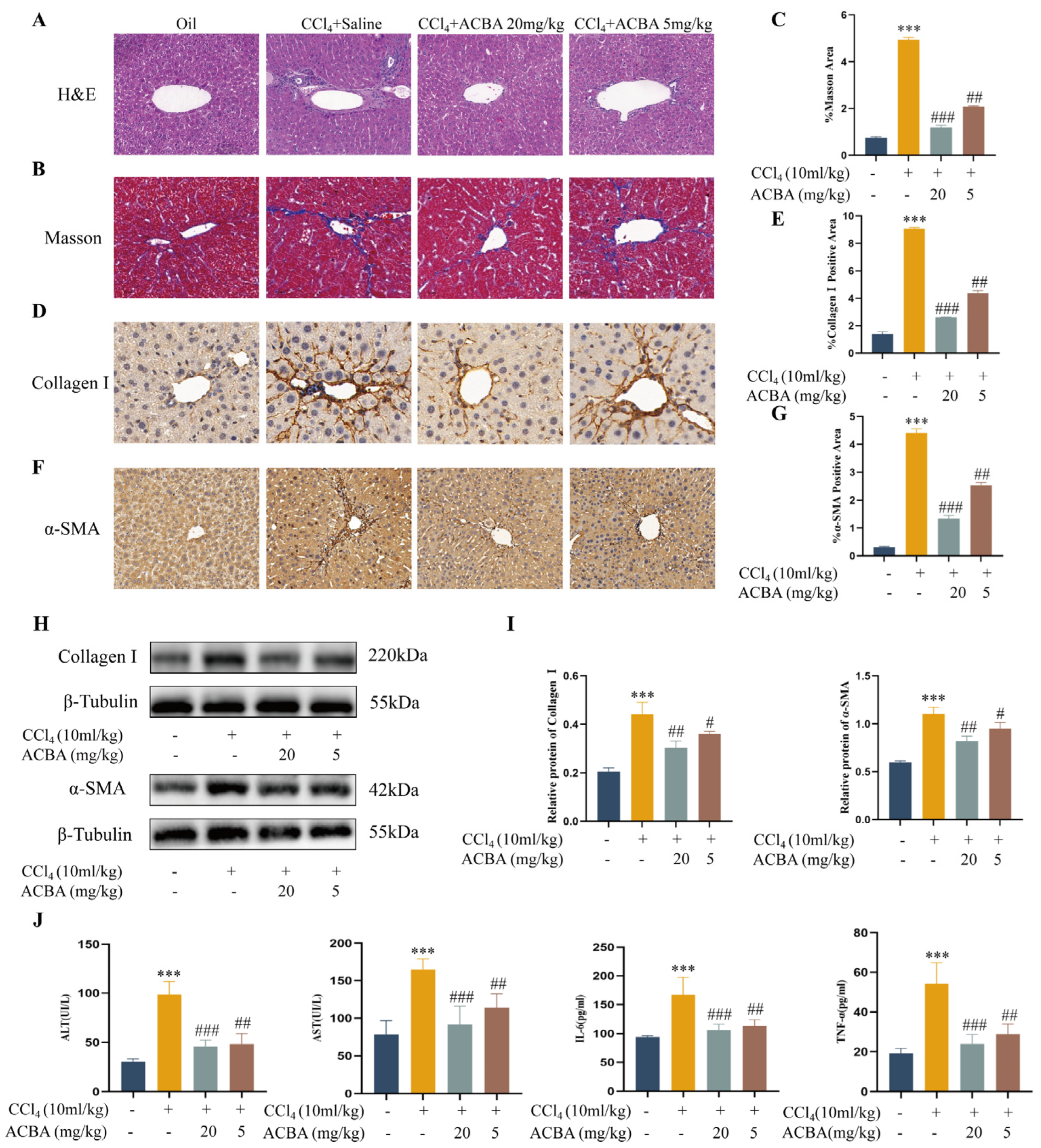
2.1. ACBA Effectively Alleviates CCl4-Induced Inflammation and Hepatic Fibrosis in Mice
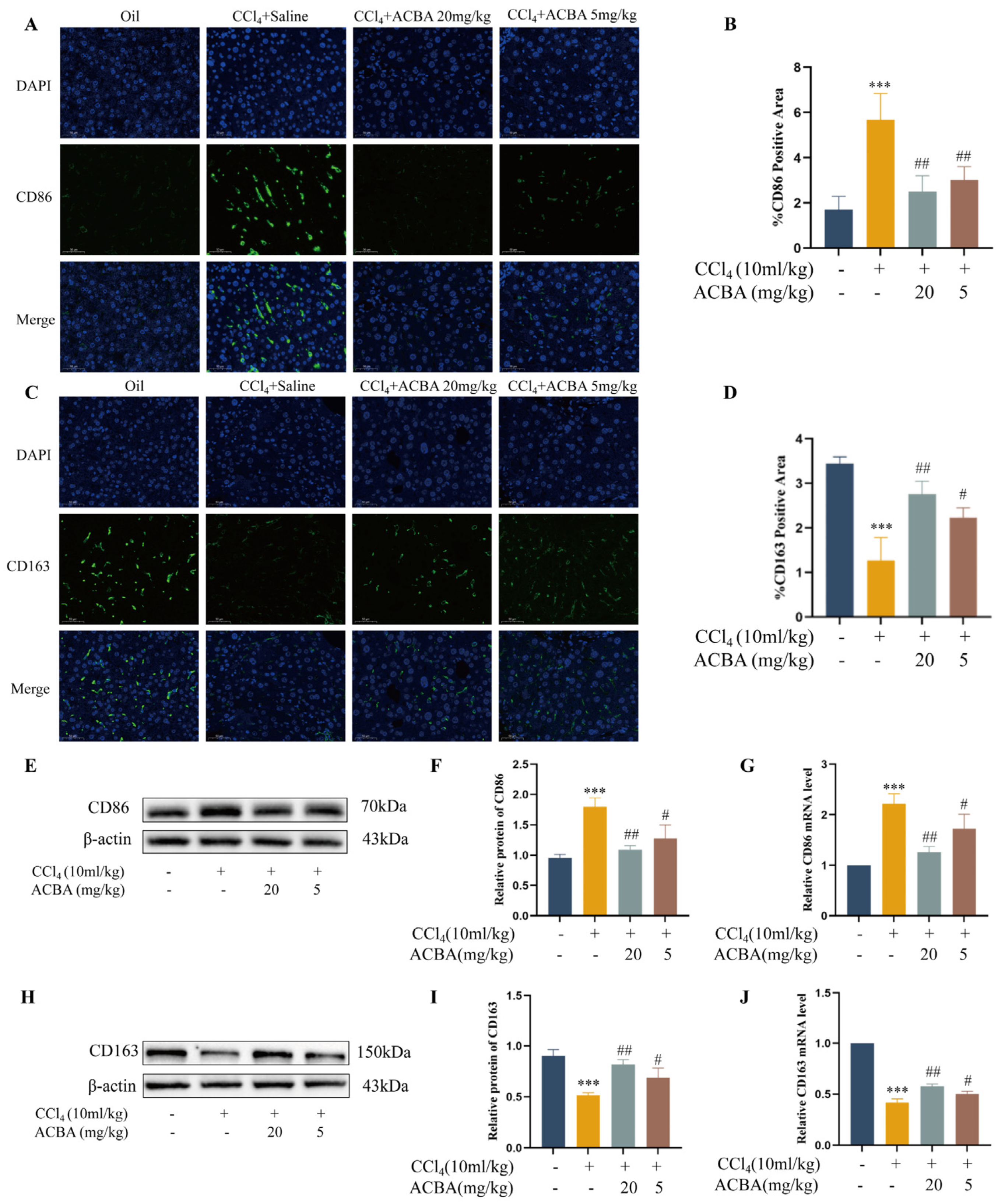
2.2. ACBA Inhibits M1 Macrophage Polarization in CCl4-Induced Mice Hepatic Fibrosis Tissue
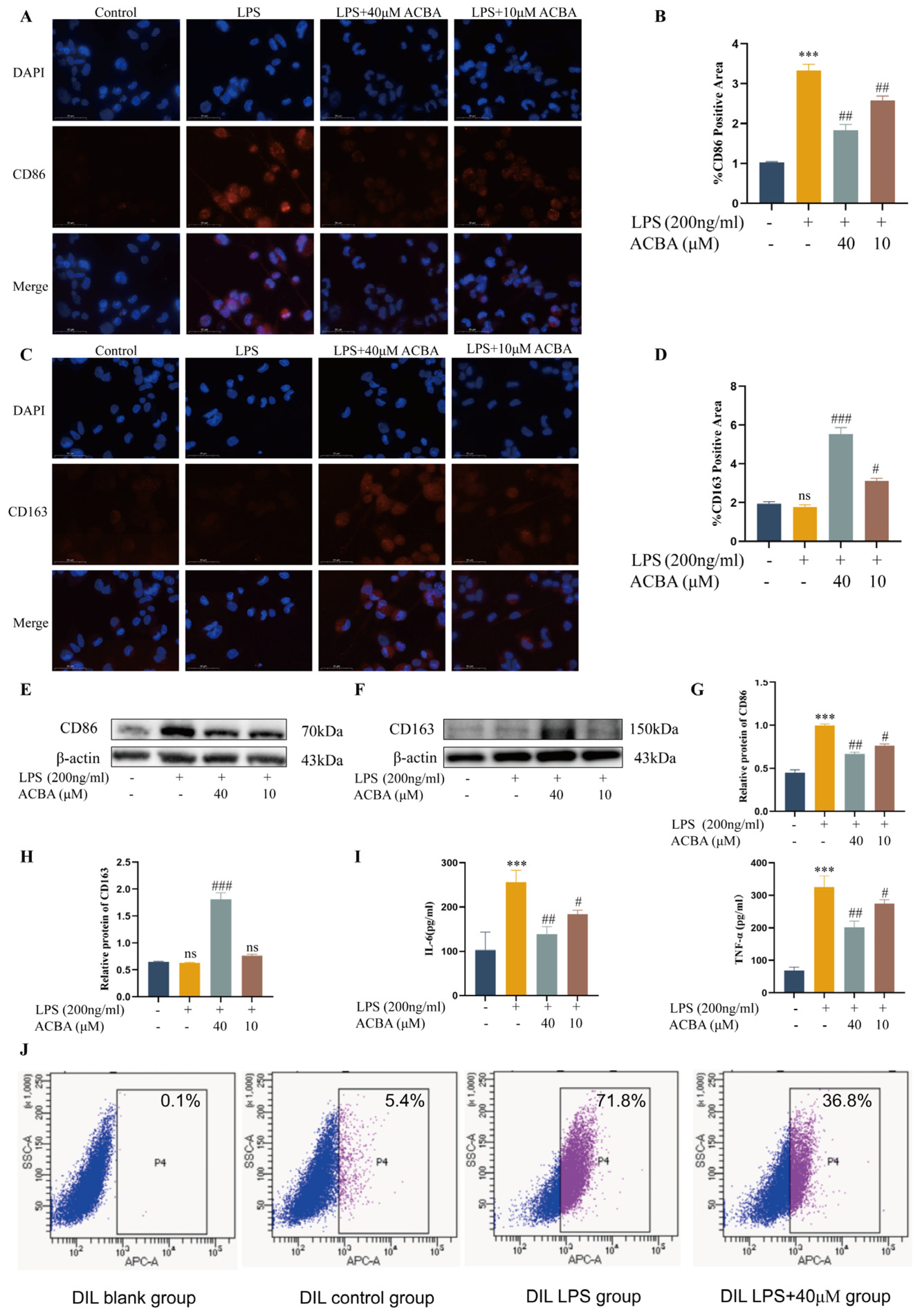
2.3. ACBA Inhibits Pro-Inflammatory Phenotype and Function in LPS-Induced THP-1 Cells
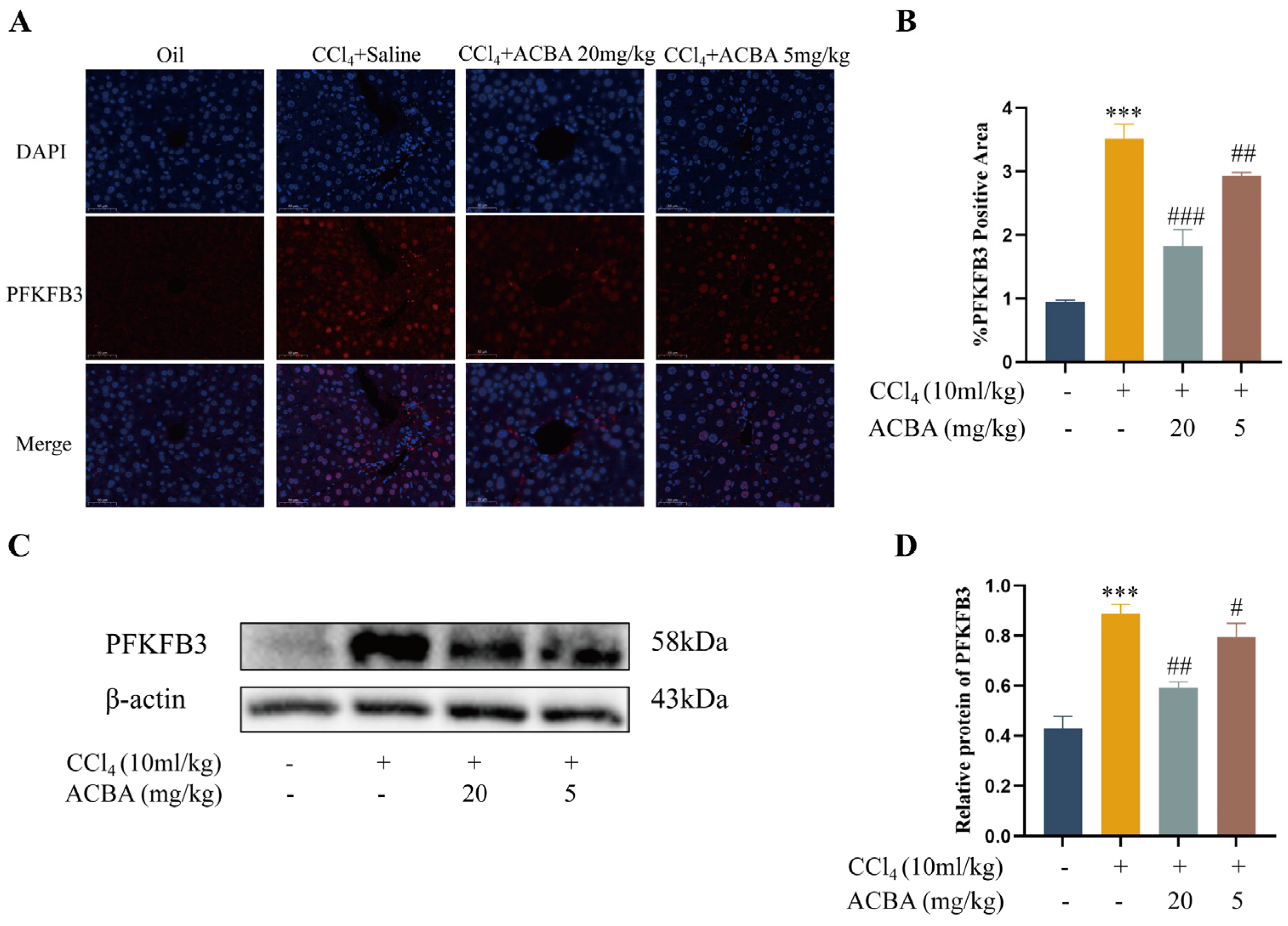
2.4. ACBA Decreases the Expression of PFKFB3 in CCl4-Induced Mouse Hepatic Fibrosis Tissue
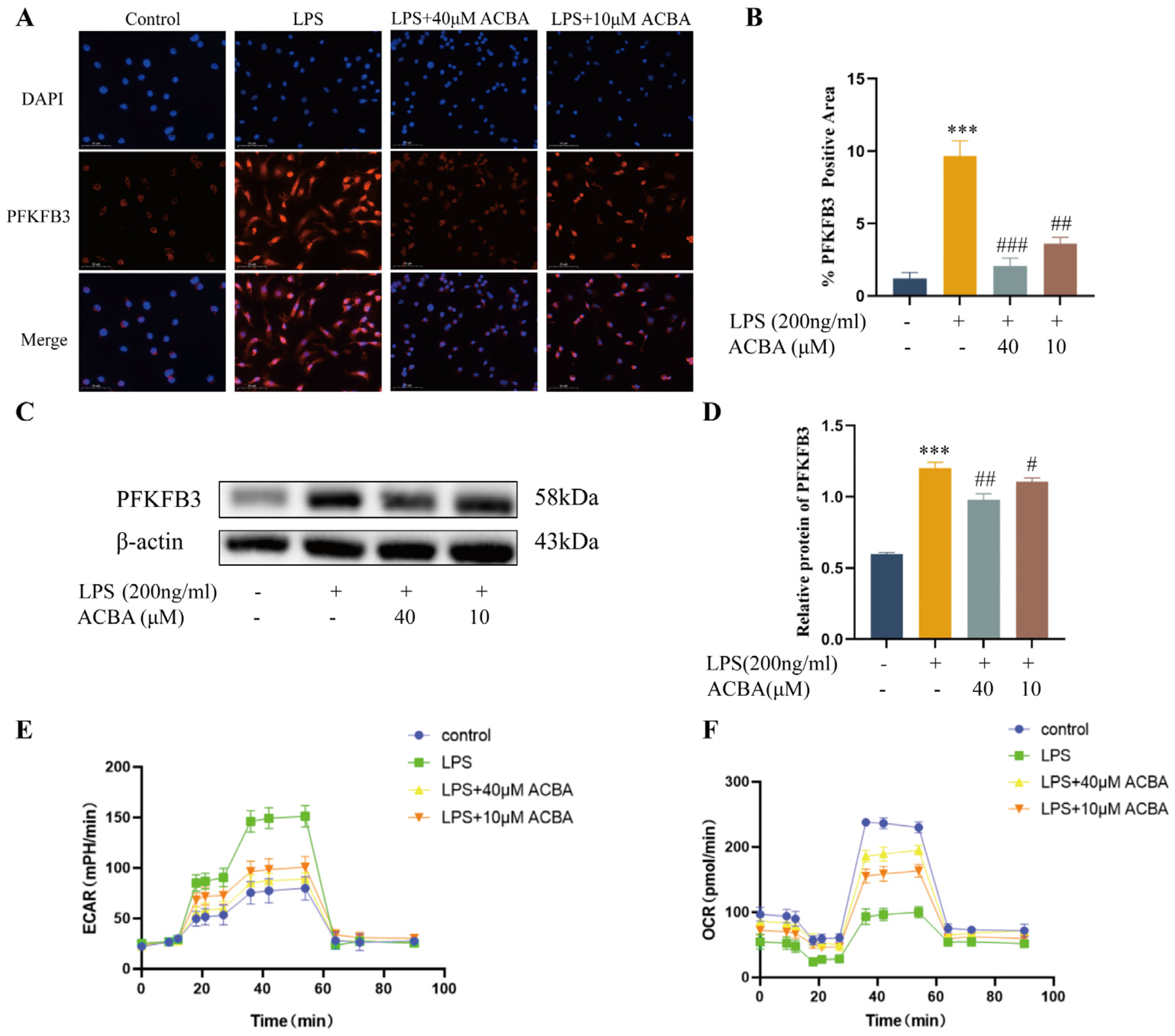
2.5. ACBA Inhibits the Expression of PFKFB3 and Glycolysis Levels in LPS-Induced THP-1 Cells
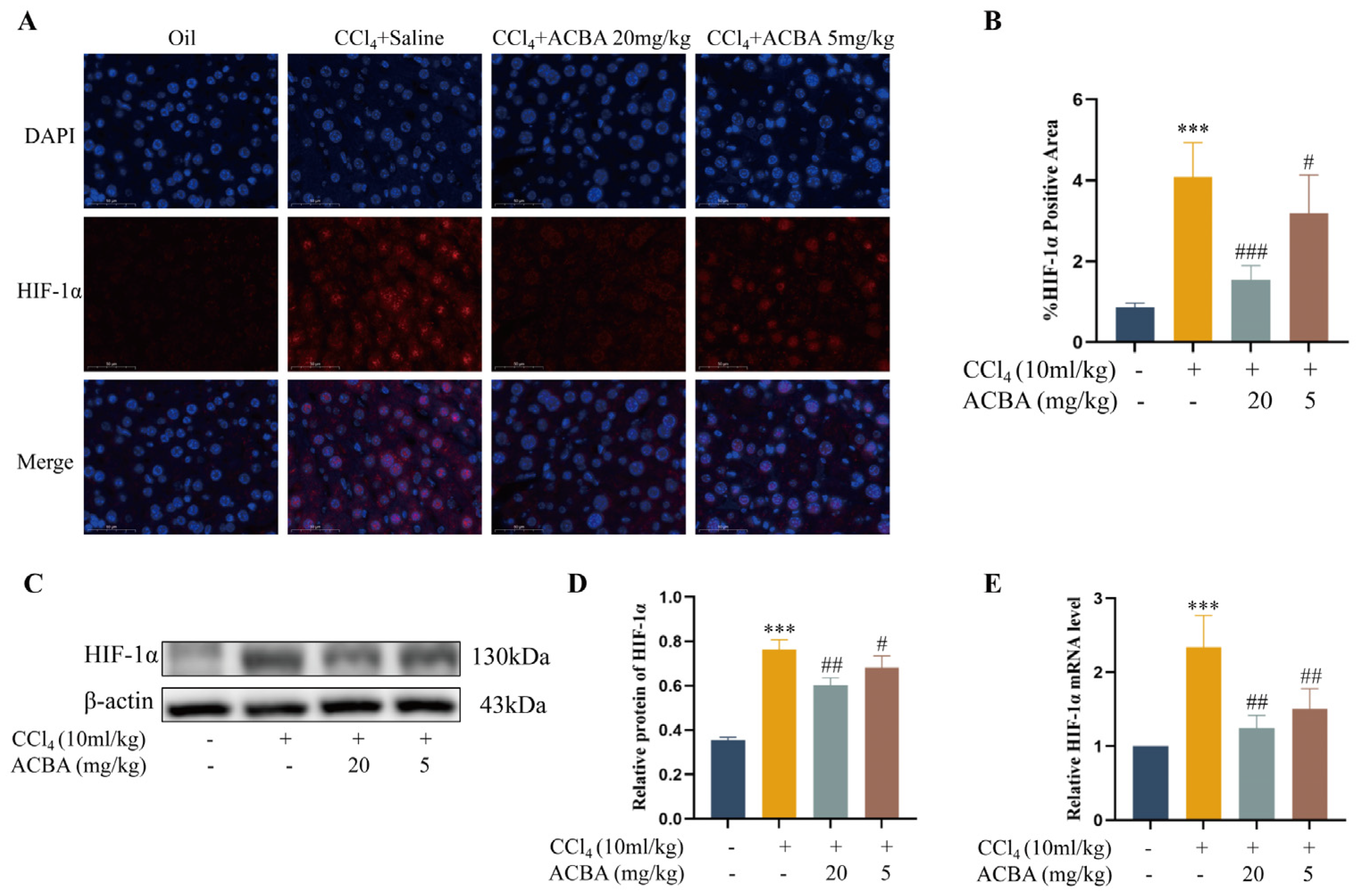
2.6. ACBA Inhibits the Expression of HIF-1α in CCl4-Induced Hepatic Fibrosis Tissue in Mice
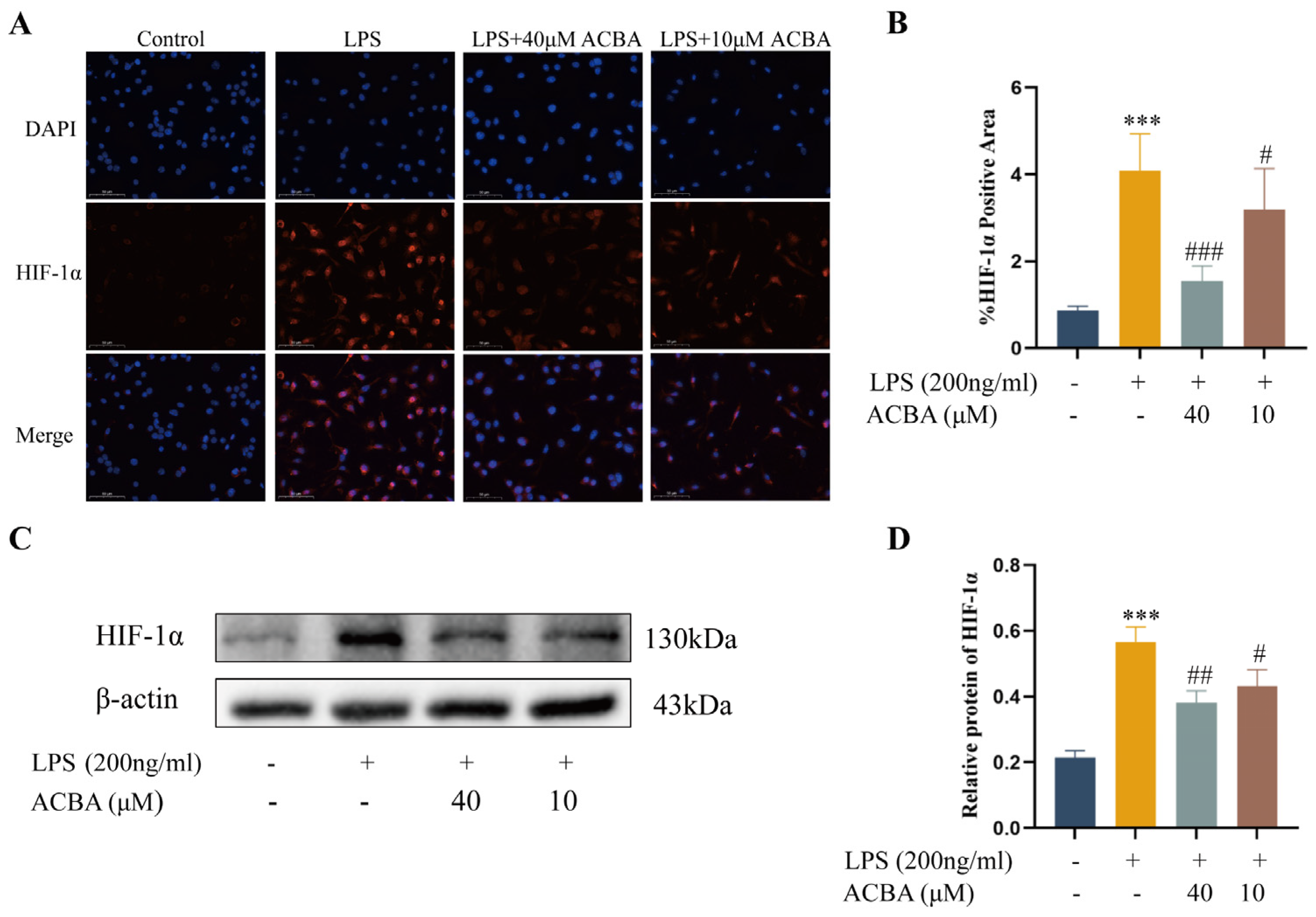
2.7. ACBA Inhibits the Expression of HIF-1α in LPS-Induced THP-1 Cells
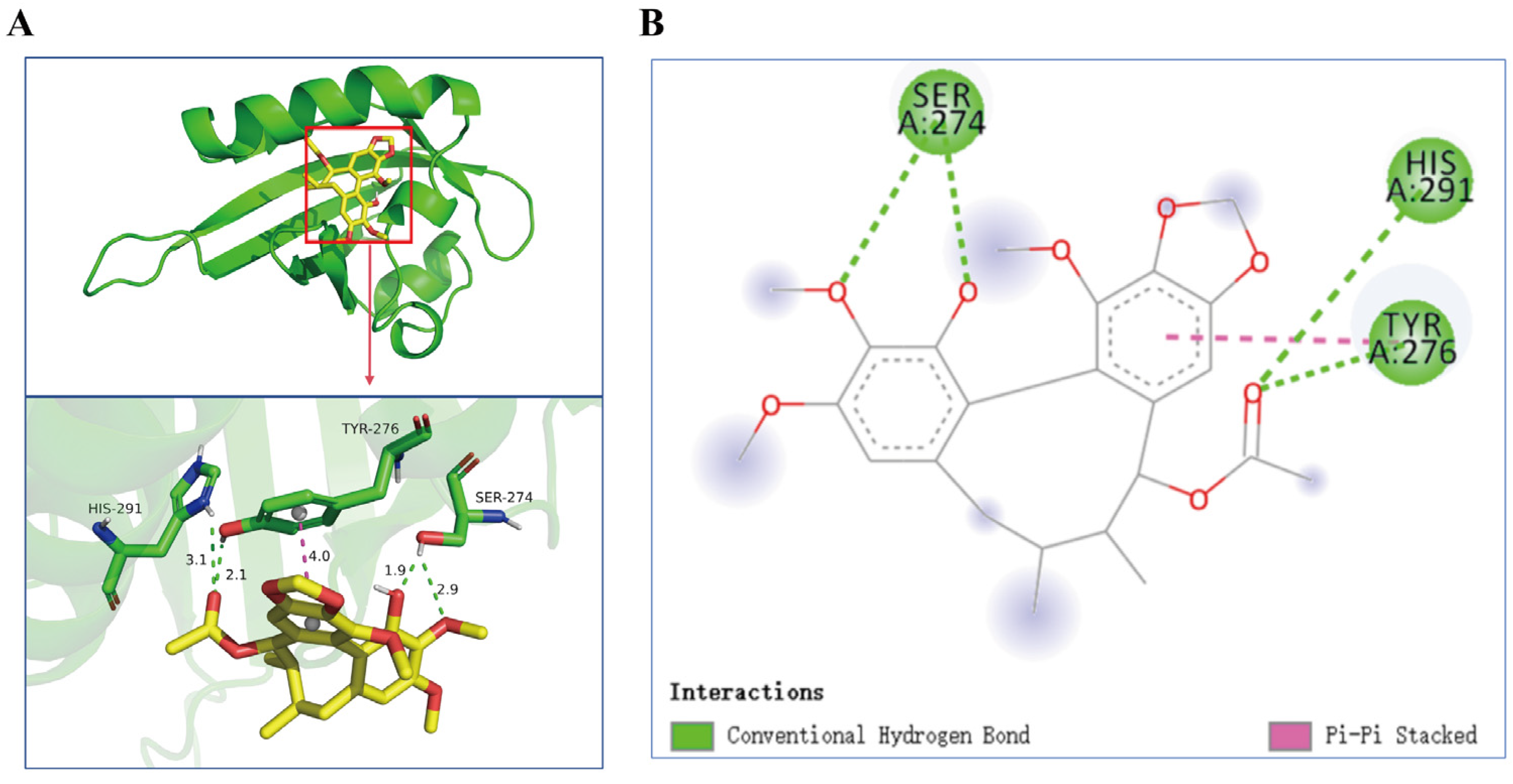
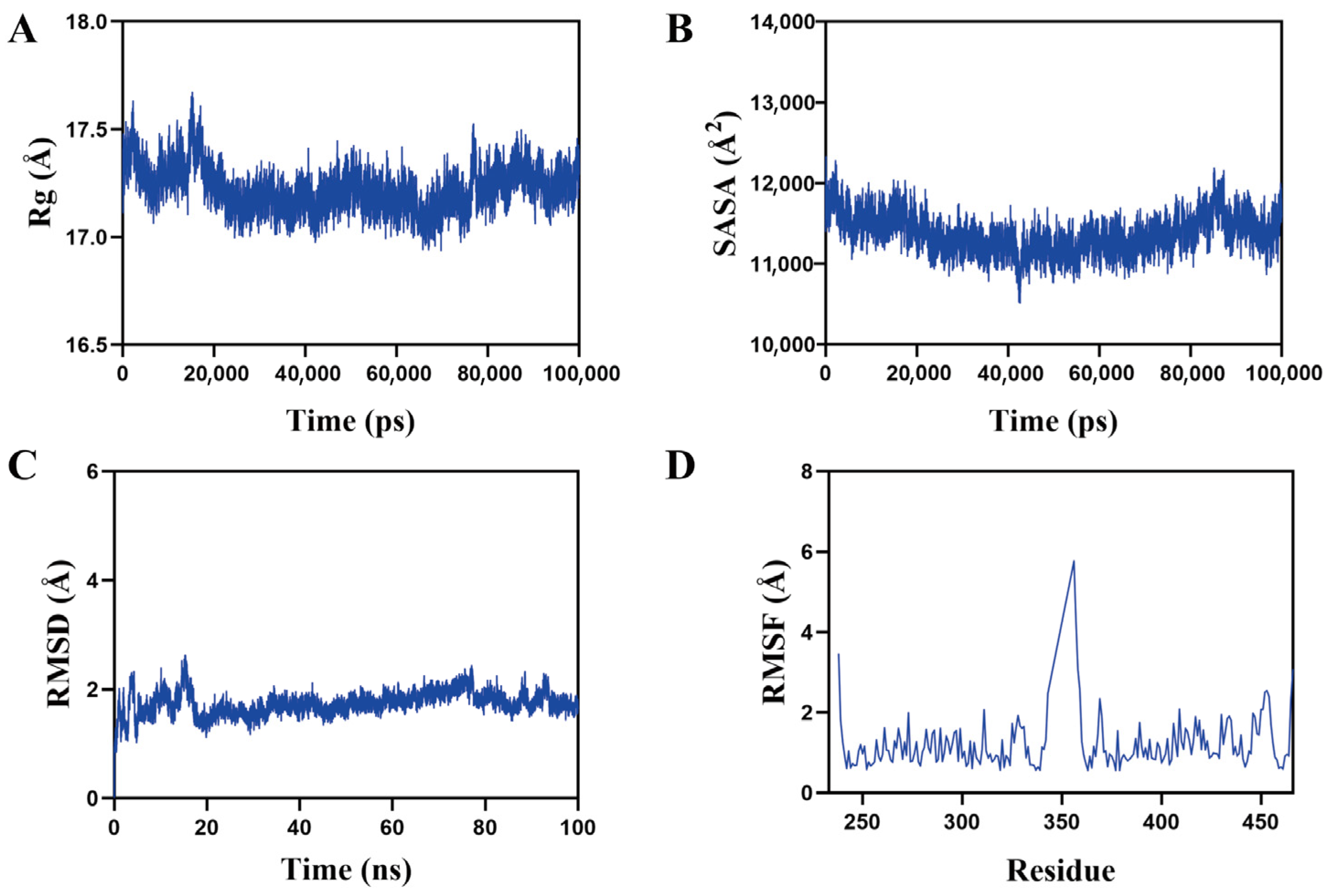
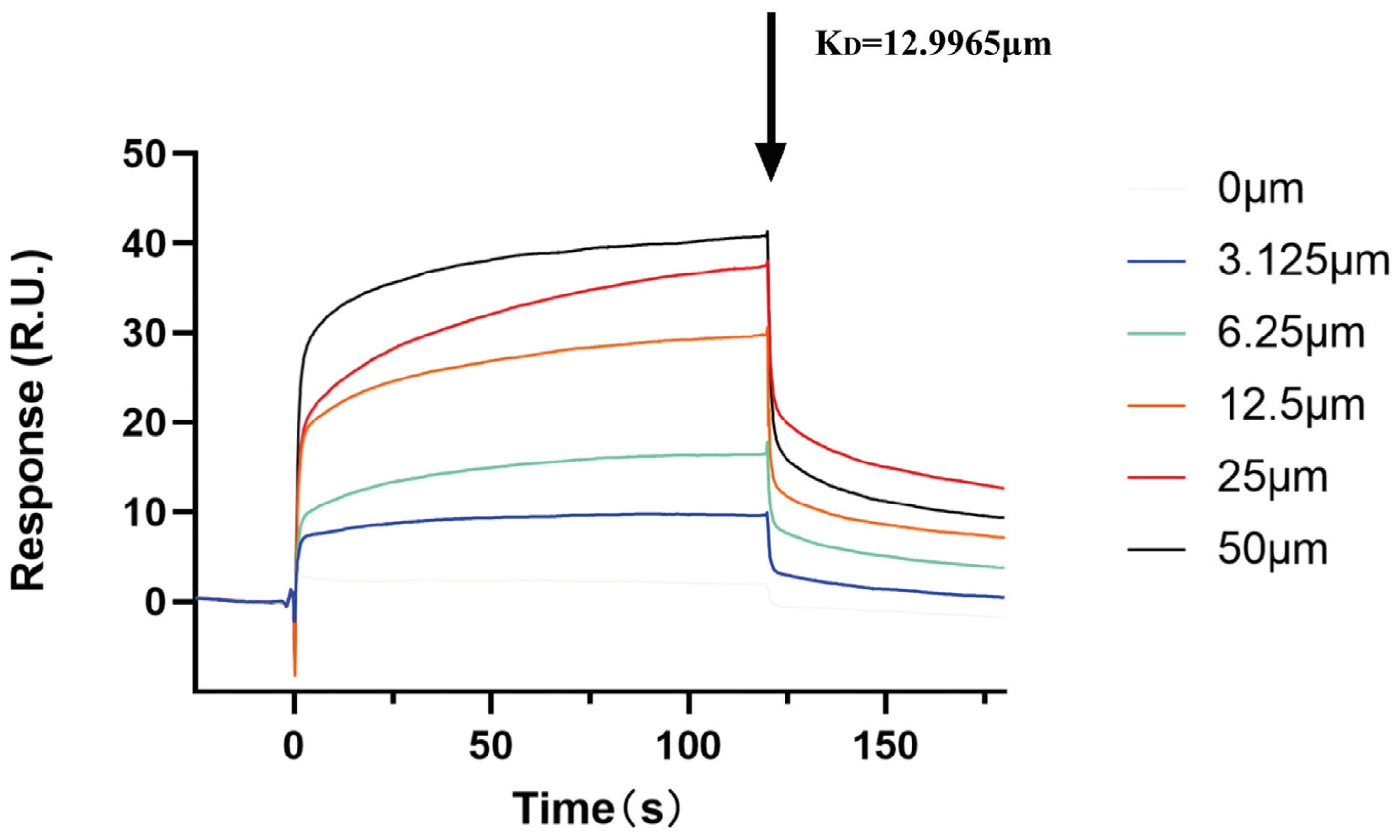
2.8. Analysis of ACBA and HIF-1α Interaction
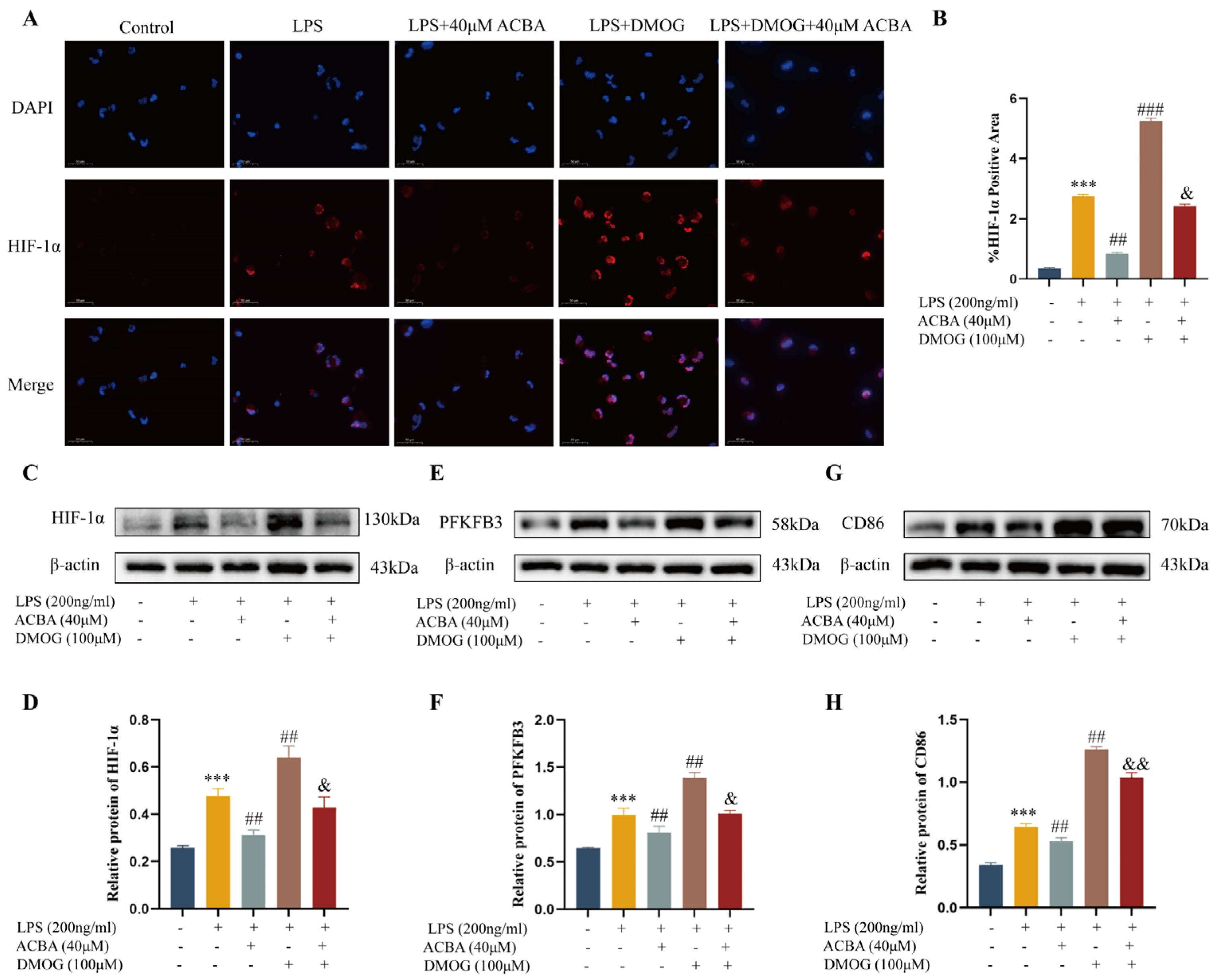
2.9. DMOG Antagonized the Inhibitory Effect of ACBA on M1 Polarization in LPS-Induced THP-1 Cells
2.10. ADMET Properties of ACBA
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Experiments
4.3. Cell Culture
4.4. H&E and Masson’s Trichrome Staining
4.5. Immunohistochemistry
4.6. Western Blot
4.7. ELISA
4.8. Immunofluorescence
4.9. RNA Extraction and RT-qPCR
4.10. Flow Cytometry
4.11. Real-Time Cell Metabolism Assay
4.12. Molecular Docking
4.13. Molecular Dynamics Simulations
4.14. SPR Assay
4.15. ADMET Properties Prediction
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Hepatic fibrosis |
| HIF-1α | Hypoxia inducible factor-1α |
| ACBA | Acetylbinankadsurin A |
| CCl4 | Carbon tetrachloride |
| SPR | Surface plasmon resonance |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| TNF-α | Tumor necrosis factor-alpha |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 |
| ECARs | Extracellular acidification rates |
| OCRs | Oxygen consumption rates |
| DMOG | Dimethyloxallyl glycine |
| HSCs | Hepatic stellate cells |
| ECM | Extracellular matrix |
| KCs | Kuffer cells |
| Rg | Radius of gyration |
| SASA | Solvent-accessible surface area |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| KD | Equilibrium dissociation constant |
| MoMFs | Monocyte-derived macrophages |
| PAS | Periodicity-ARNT-Single-minded |
| MW | Molecular weight |
| RBs | Rotatable bonds |
| HBAs | Hydrogen bond acceptors |
| HBDs | Hydrogen bond donors |
| ESOL Log S | Estimated solubility |
| ESOL | Estimated aqueous solubility |
| SAscore | Synthetic accessibility score |
| GI | Gastrointestinal absorption |
| BBB | Blood–brain barrier |
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Taru, V.; Szabo, G.; Mehal, W.; Reiberger, T. Inflammasomes in Chronic Liver Disease: Hepatic Injury, Fibrosis Progression and Systemic Inflammation. J. Hepatol. 2024, 81, 895–910. [Google Scholar] [CrossRef]
- Chen, L.; Guo, W.; Mao, C.; Shen, J.; Wan, M. Liver Fibrosis: Pathological Features, Clinical Treatment and Application of Therapeutic Nanoagents. J. Mater. Chem. B 2024, 12, 1446–1466. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Cheng, D.; Chai, J.; Wang, H.; Fu, L.; Peng, S.; Ni, X. Hepatic Macrophages: Key Players in the Development and Progression of Liver Fibrosis. Liver Int. 2021, 41, 2279–2294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, K.; Jin, N.; Tang, B.; Zhang, W. Macrophage in Liver Fibrosis: Identities and Mechanisms. Int. Immunopharmacol. 2023, 120, 110357. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, D.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Diseases. Front. Immunol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Liu, Y.; Ding, X.; Yang, Y.; Chen, S.; Cao, J.; Tacke, F.; Dong, W.; Lan, T. Macrophage Heterogeneity in Liver Fibrosis. Front. Immunol. 2025, 16, 1639455. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (HIF): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Tawakol, A.; Singh, P.; Mojena, M.; Pimentel-Santillana, M.; Emami, H.; MacNabb, M.; Rudd, J.H.F.; Narula, J.; Enriquez, J.A.; Través, P.G.; et al. HIF-1α and PFKFB3 Mediate a Tight Relationship Between Proinflammatory Activation and Anerobic Metabolism in Atherosclerotic Macrophages. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1463–1471. [Google Scholar] [CrossRef]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory Mechanisms of Hypoxia-Inducible Factor 1 Activity: Two Decades of Knowledge. Cancer Sci. 2018, 109, 560–571. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The Effect of HIF on Metabolism and Immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef]
- Wu, D.; Potluri, N.; Lu, J.; Kim, Y.; Rastinejad, F. Structural Integration in Hypoxia-Inducible Factors. Nature 2015, 524, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, M.; Xia, S.; Zeng, F.; Liu, Q. Systematic and Comprehensive Insights into HIF-1 Stabilization under Normoxic Conditions: Implications for Cellular Adaptation and Therapeutic Strategies in Cancer. Cell. Mol. Biol. Lett. 2025, 30, 2. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Zhang, J.; Lin, Y.; Xie, J.; Yin, D.; Zhu, Y. Research and Application of Medicines for Treating Liver Fibrosis: Current Status and Prospects. Front. Pharmacol. 2025, 16, 1582258. [Google Scholar] [CrossRef]
- Kuete, J.B.T.; Kuete, J.R.N.; Mbaveng, A.T.; Kuete, V.; Efferth, T.; Weiskirchen, R. A Three-Year Review (2023–2025) on the Effectiveness of Natural Products from Plants in Treating Major Types of Fibrosis. Phytomedicine 2025, 148, 157242. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xia, X.; Liao, S.; Wu, T.; Wang, L.; Chen, Q.; Wei, S.; Gu, X.; Zhu, Z. Physicochemical Characterization and Antioxidant and Hypolipidaemic Activities of a Polysaccharide from the Fruit of Kadsura coccinea (Lem.) A. C. Smith. Front. Nutr. 2022, 9, 903218. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-Z.; Yang, Y.-P.; Cheng, S.-W.; Cao, L.; Xie, Q.-L.; Wang, M.-Y.; Li, B.; Jian, Y.-Q.; Liu, B.; Peng, C.-Y.; et al. Heilaohuguosus A-S from the Fruits of Kadsura coccinea and Their Hepatoprotective Activity. Phytochemistry 2021, 184, 112678. [Google Scholar] [CrossRef]
- Yang, Y.; Jian, Y.; Cheng, S.; Jia, Y.; Liu, Y.; Yu, H.; Cao, L.; Li, B.; Peng, C.; Iqbal Choudhary, M.; et al. Dibenzocyclooctadiene Lignans from Kadsura coccinea Alleviate APAP-Induced Hepatotoxicity via Oxidative Stress Inhibition and Activating the Nrf2 Pathway in Vitro. Bioorg. Chem. 2021, 115, 105277. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Liu, S.-Q.; Yao, Y.-X.; Zhang, S.; Li, D.-H.; Li, H.-Y.; Yu, H.-H.; Yuan, H.-W.; Wang, W.; Li, B.; et al. Integrated Network Pharmacology and Metabolomics to Explore the Mechanism of Kadsura coccinea Root in Alleviating Acetaminophen-Induced Liver Injury. J. Ethnopharmacol. 2025, 352, 120278. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Tasneem, S.; Hussain, N.; Daniyal, M.; Yuan, H.; Xie, Q.; Liu, B.; Sun, J.; Jian, Y.; et al. Lignans from Tujia Ethnomedicine Heilaohu: Chemical Characterization and Evaluation of Their Cytotoxicity and Antioxidant Activities. Molecules 2018, 23, 2147. [Google Scholar] [CrossRef]
- Peng, W.; Yang, Y.; Lu, H.; Shi, H.; Jiang, L.; Liao, X.; Zhao, H.; Wang, W.; Liu, J. Network Pharmacology Combines Machine Learning, Molecular Simulation Dynamics and Experimental Validation to Explore the Mechanism of Acetylbinankadsurin A in the Treatment of Liver Fibrosis. J. Ethnopharmacol. 2024, 323, 117682. [Google Scholar] [CrossRef]
- Sakai, M.; Troutman, T.D.; Seidman, J.S.; Ouyang, Z.; Spann, N.J.; Abe, Y.; Ego, K.M.; Bruni, C.M.; Deng, Z.; Schlachetzki, J.C.M.; et al. Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Immunity 2019, 51, 655–670.e8. [Google Scholar] [CrossRef]
- Chi, G.; Pei, J.; Li, X. The Imbalance of Liver Resident Macrophages Polarization Promotes Chronic Autoimmune Hepatitis Development in Mice. PeerJ 2023, 11, e14871. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.-F.; Gao, C.-C.; Yi, J.; Zhao, J.-L.; Liang, S.-Q.; Zhao, Y.; Ye, Y.-C.; Bai, J.; Zheng, Q.-J.; Dou, K.-F.; et al. Cytotherapy with M1-Polarized Macrophages Ameliorates Liver Fibrosis by Modulating Immune Microenvironment in Mice. J. Hepatol. 2017, 67, 770–779. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Li, X.; Zhang, L.; Yao, S.; Wang, Y.; Tuo, B.; Jin, H. Advances in the Understanding of the Role and Mechanism of Action of PFKFB3-mediated Glycolysis in Liver Fibrosis (Review). Int. J. Mol. Med. 2024, 54, 105. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.A.; Fisher-Wellman, K.H.; Neufer, P.D. From OCR and ECAR to Energy: Perspectives on the Design and Interpretation of Bioenergetics Studies. J. Biol. Chem. 2021, 297, 101140. [Google Scholar] [CrossRef]
- Minchenko, A.; Leshchinsky, I.; Opentanova, I.; Sang, N.; Srinivas, V.; Armstead, V.; Caro, J. Hypoxia-Inducible Factor-1-Mediated Expression of the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase-3 (PFKFB3) Gene. Its Possible Role in the Warburg Effect. J. Biol. Chem. 2002, 277, 6183–6187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Wu, W.; Liao, L.; Zhou, M.; Wang, X.; Tan, Z.; Zhang, G.; Bai, Y.; Li, X.; et al. Hypoxia Activates Macrophage-NLRP3 Inflammasome Promoting Atherosclerosis via PFKFB3-Driven Glycolysis. FASEB J. 2024, 38, e23854. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.-Y.; Yang, X.-M.; Chen, W.-C.; Zhang, M.-J.; Zhong, X.-D.; Chen, W.-G.; Zhong, B.-L.; He, S.-Q.; Sun, H.-T. Paeoniflorin Coordinates Macrophage Polarization and Mitigates Liver Inflammation and Fibrogenesis by Targeting the NF-[Formula: See Text]B/HIF-1α Pathway in CCl4-Induced Liver Fibrosis. Am. J. Chin. Med. 2023, 51, 1249–1267. [Google Scholar] [CrossRef]
- Nowak-Stępniowska, A.; Osuchowska, P.N.; Fiedorowicz, H.; Trafny, E.A. Insight in Hypoxia-Mimetic Agents as Potential Tools for Mesenchymal Stem Cell Priming in Regenerative Medicine. Stem Cells Int. 2022, 2022, 8775591. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, O.; Issaoui, N.; Al-Dossary, O. DFT and Molecular Docking Study of Chloroquine Derivatives as Antiviral to Coronavirus COVID-19. J. King Saud. Univ. Sci. 2021, 33, 101248. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Temraz, A. Anti-Fibrotic Activity of Natural Products, Herbal Extracts and Nutritional Components for Prevention of Liver Fibrosis: Review. Arch. Physiol. Biochem. 2022, 128, 382–393. [Google Scholar] [CrossRef]
- Li, J.-Z.; Chen, N.; Ma, N.; Li, M.-R. Mechanism and Progress of Natural Products in the Treatment of NAFLD-Related Fibrosis. Molecules 2023, 28, 7936. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-P.; Hussain, N.; Zhang, L.; Jia, Y.-Z.; Jian, Y.-Q.; Li, B.; Iqbal Choudhary, M.; Rahman, A.-U.; Wang, W. Kadsura coccinea: A Rich Source of Structurally Diverse and Biologically Important Compounds. Chin. Herb. Med. 2020, 12, 214–223. [Google Scholar] [CrossRef]
- Sun, Q.-Z.; Chen, D.-F.; Ding, P.-L.; Ma, C.-M.; Kakuda, H.; Nakamura, N.; Hattori, M. Three New Lignans, Longipedunins A-C, from Kadsura longipedunculata and Their Inhibitory Activity against HIV-1 Protease. Chem. Pharm. Bull. 2006, 54, 129–132. [Google Scholar] [CrossRef]
- Conte, E. Targeting Monocytes/Macrophages in Fibrosis and Cancer Diseases: Therapeutic Approaches. Pharmacol. Ther. 2022, 234, 108031. [Google Scholar] [CrossRef]
- Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Resolution of Liver Fibrosis: Basic Mechanisms and Clinical Relevance. Semin. Liver Dis. 2015, 35, 119–131. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhu, X.; Xu, C.; Lin, J.; Zhao, M.; Cheng, Y. New Insights into Therapeutic Strategies for Targeting Hepatic Macrophages to Alleviate Liver Fibrosis. Int. Immunopharmacol. 2025, 158, 114864. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic Reprogramming of Macrophages: Glucose Transporter 1 (GLUT1)-Mediated Glucose Metabolism Drives a Proinflammatory Phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef]
- Pavlou, S.; Wang, L.; Xu, H.; Chen, M. Higher Phagocytic Activity of Thioglycollate-Elicited Peritoneal Macrophages Is Related to Metabolic Status of the Cells. J. Inflamm. 2017, 14, 4. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, H.; Sun, M.; Wang, Y.; Meng, Q.; Guo, P.; Cao, Y.; Chen, J.; Gao, X.; Li, E.; et al. PFKFB3-Driven Macrophage Glycolytic Metabolism Is a Crucial Component of Innate Antiviral Defense. J. Immunol. 2016, 197, 2880–2890. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, Y.; Wang, D.; Zhang, L.; Qiu, Y.; Cui, J.; Xie, F.; Zhu, N.; Qin, M.; Wang, Y. PFKFB3-Inhibitor 3PO-Mediated Glycolytic Reprogramming Promotes Inflammatory Dental Pulp Repair: An In Vitro and In Vivo Study. Int. Endod. J. 2025, 58, 1872–1889. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, J.; Jiao, W.; Liu, L.; Yu, J.; Zhang, M.; Chen, G. Suppression of Macrophage Activation by Sodium Danshensu via HIF-1α/STAT3/NLRP3 Pathway Ameliorated Collagen-Induced Arthritis in Mice. Molecules 2023, 28, 1551. [Google Scholar] [CrossRef]
- Wu, K.K.-L.; Xu, X.; Wu, M.; Li, X.; Hoque, M.; Li, G.H.Y.; Lian, Q.; Long, K.; Zhou, T.; Piao, H.; et al. MDM2 Induces Pro-Inflammatory and Glycolytic Responses in M1 Macrophages by Integrating iNOS-Nitric Oxide and HIF-1α Pathways in Mice. Nat. Commun. 2024, 15, 8624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, Z.; Li, X.; Wang, F.; Wei, X.; Kang, Y.; Mo, C.; Jiang, J.; Liang, H.; Ye, L. Activation of the JNK/COX-2/HIF-1α Axis Promotes M1 Macrophage via Glycolytic Shift in HIV-1 Infection. Life Sci. Alliance 2023, 6, e202302148. [Google Scholar] [CrossRef]
- Zeng, X.; Li, T.; Yang, K.; Jiang, Y.; Chen, S.; Yang, S.; Zou, S.; Liu, J.; Duan, P. Natural Compound Phloretin Restores Periodontal Immune Homeostasis via HIF-1α-Regulated PI3K/Akt and Glycolysis in Macrophages. Int. Immunopharmacol. 2024, 141, 112933. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Dong, X.; He, H.; Jiang, J.-L.; Guan, Z.-J.; Li, X.; Lu, L.; Li, H.; Huang, Y.-S.; Xian, S.-X.; et al. A Simplified Herbal Decoction Attenuates Myocardial Infarction by Regulating Macrophage Metabolic Reprogramming and Phenotypic Differentiation via Modulation of the HIF-1α/PDK1 Axis. Chin. Med. 2024, 19, 75. [Google Scholar] [CrossRef]
- Moon, J.-O.; Welch, T.P.; Gonzalez, F.J.; Copple, B.L. Reduced Liver Fibrosis in Hypoxia-Inducible Factor-1alpha-Deficient Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G582–G592. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Bai, S.; Moon, J.-O. Hypoxia-Inducible Factor-Dependent Production of Profibrotic Mediators by Hypoxic Kupffer Cells. Hepatol. Res. 2010, 40, 530–539. [Google Scholar] [CrossRef]
- Xu, M.; Warner, C.; Duan, X.; Cheng, Z.; Jeyarajan, A.J.; Li, W.; Wang, Y.; Shao, T.; Salloum, S.; Chen, P.-J.; et al. HIV Coinfection Exacerbates HBV-Induced Liver Fibrogenesis through a HIF-1α- and TGF-Β1-Dependent Pathway. J. Hepatol. 2024, 80, 868–881. [Google Scholar] [CrossRef]
- Yang, Q.; Huo, E.; Cai, Y.; Zhang, Z.; Dong, C.; Asara, J.M.; Shi, H.; Wei, Q. Myeloid PFKFB3-Mediated Glycolysis Promotes Kidney Fibrosis. Front. Immunol. 2023, 14, 1259434. [Google Scholar] [CrossRef] [PubMed]
- Reda, D.; Elfiky, A.A.; Elnagdy, M.; Khalil, M.M. Molecular Docking and Molecular Dynamics of Hypoxia-Inducible Factor (HIF-1alpha): Towards Potential Inhibitors. J. Biomol. Struct. Dyn. 2024, 42, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Sanjay, K.S.; Kumar, P.; Singh, S.; Thareja, S. Molecular Dynamics and 3D-QSAR Studies on Indazole Derivatives as HIF-1α Inhibitors. J. Biomol. Struct. Dyn. 2023, 41, 3524–3541. [Google Scholar] [CrossRef] [PubMed]
- Salamat, A.; Kosar, N.; Mohyuddin, A.; Imran, M.; Zahid, M.N.; Mahmood, T. SAR, Molecular Docking and Molecular Dynamic Simulation of Natural Inhibitors against SARS-CoV-2 Mpro Spike Protein. Molecules 2024, 29, 1144. [Google Scholar] [CrossRef]
| Mode | Affinity (kcal/mol) | Rmsd l.b. | Rmsd u.b. |
|---|---|---|---|
| 1 | −6.0 | 0.000 | 0.000 |
| 2 | −5.874 | 1.745 | 6.466 |
| 3 | −5.867 | 25.61 | 27.99 |
| 4 | −5.797 | 15.84 | 18.73 |
| 5 | −5.538 | 6.878 | 10.39 |
| 6 | −5.508 | 24.58 | 26.76 |
| 7 | −5.425 | 5.503 | 9.721 |
| 8 | −5.42 | 20.29 | 23.51 |
| 9 | −5.389 | 25.48 | 27.47 |
| 10 | −5.277 | 16.24 | 19.54 |
| 11 | −5.27 | 19.63 | 22.73 |
| 12 | −5.242 | 9.833 | 13.6 |
| 13 | −5.139 | 19.31 | 22.29 |
| 14 | −5.127 | 25.21 | 27.62 |
| 15 | −5.104 | 4.821 | 8.209 |
| 16 | −5.092 | 25.64 | 27.09 |
| 17 | −5.06 | 21.89 | 23.67 |
| 18 | −4.997 | 19.92 | 22.06 |
| 19 | −4.997 | 19.52 | 22.93 |
| 20 | −4.988 | 11.52 | 15.57 |
| Molecules | MW | RB | HBA | HBD | Log p | ESOL Log S | ESOL (mol/L) | Bioavailability Score | P-gp Substrate | SA Score | GI | BBB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACBA | 444.47 | 5 | 8 | 1 | 3.57 | −5.23 | 5.93 × 10−6 | 0.55 | No | 4.88 | High | No |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| CD86 | TGGGCTTGGCAATCCTTATCTT | CCAGCTCACTCAGGCTTATGTTT |
| CD163 | AGGAAACCAATCCCAGACACTA | CGACCACCTCCACCTACCAA |
| HIF-1α | AACCCATTCCTCATCCGTCAA | TTCAACCCAGACATATCCACCTC |
| β-actin | GTGACGTTGACATCCGTAAAGA | GTAACAGTCCGCCTAGAAGCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Peng, W.; Lu, H.; Yang, Y.; Rao, Z.; Xie, X.; Huang, D.; Wang, W.; Yan, J.; Liu, J. Acetylbinankadsurin A Decreases Macrophage Glycolysis and Pro-Inflammatory Phenotype Polarization via Inhibiting HIF-1α to Alleviate Hepatic Fibrosis in Mice. Molecules 2025, 30, 4571. https://doi.org/10.3390/molecules30234571
Yao Q, Peng W, Lu H, Yang Y, Rao Z, Xie X, Huang D, Wang W, Yan J, Liu J. Acetylbinankadsurin A Decreases Macrophage Glycolysis and Pro-Inflammatory Phenotype Polarization via Inhibiting HIF-1α to Alleviate Hepatic Fibrosis in Mice. Molecules. 2025; 30(23):4571. https://doi.org/10.3390/molecules30234571
Chicago/Turabian StyleYao, Qiang, Wangxia Peng, Huaguan Lu, Yupei Yang, Ziti Rao, Xin Xie, Dan Huang, Wei Wang, Jianye Yan, and Jianjun Liu. 2025. "Acetylbinankadsurin A Decreases Macrophage Glycolysis and Pro-Inflammatory Phenotype Polarization via Inhibiting HIF-1α to Alleviate Hepatic Fibrosis in Mice" Molecules 30, no. 23: 4571. https://doi.org/10.3390/molecules30234571
APA StyleYao, Q., Peng, W., Lu, H., Yang, Y., Rao, Z., Xie, X., Huang, D., Wang, W., Yan, J., & Liu, J. (2025). Acetylbinankadsurin A Decreases Macrophage Glycolysis and Pro-Inflammatory Phenotype Polarization via Inhibiting HIF-1α to Alleviate Hepatic Fibrosis in Mice. Molecules, 30(23), 4571. https://doi.org/10.3390/molecules30234571







