Composition of Volatiles, Phytochemical Analysis, Antioxidant and Anticancer Activity of Euryops floribundus Ne.Br. Leaves (Asteraceae)
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
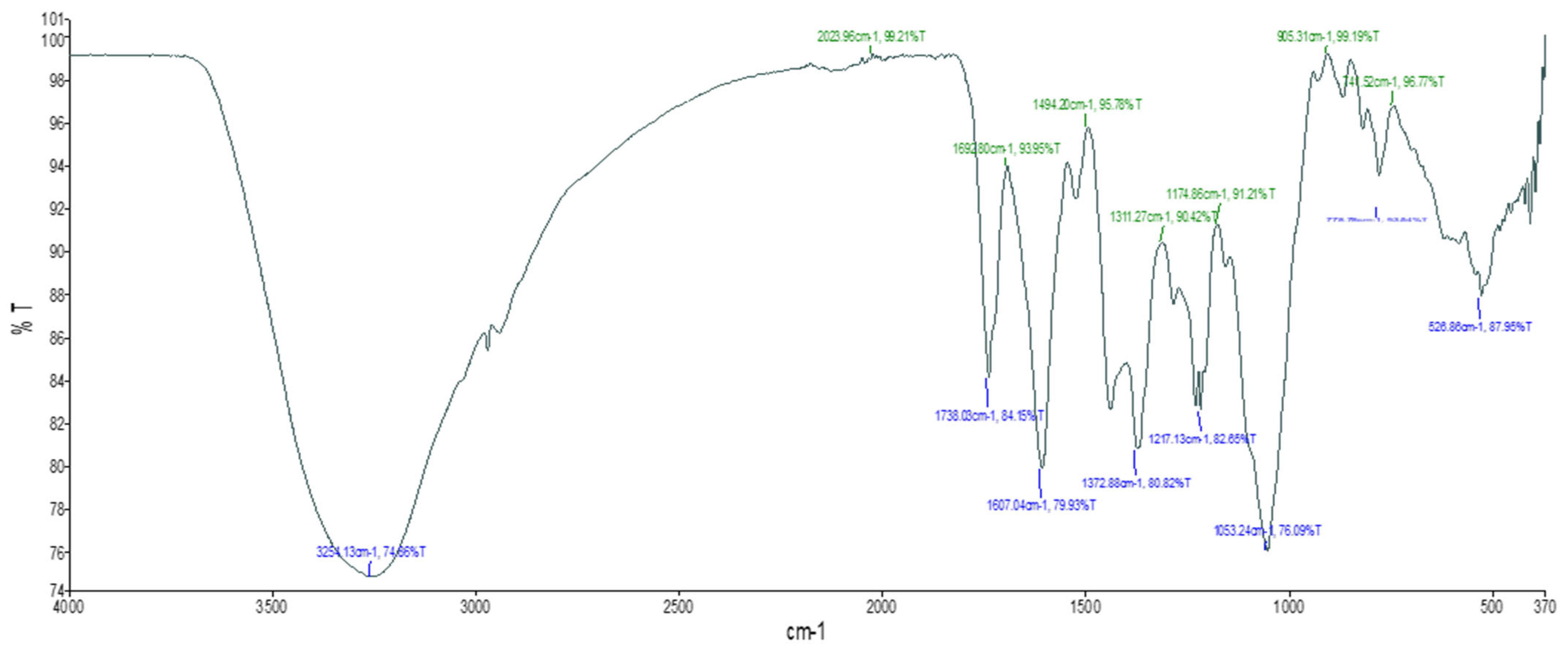
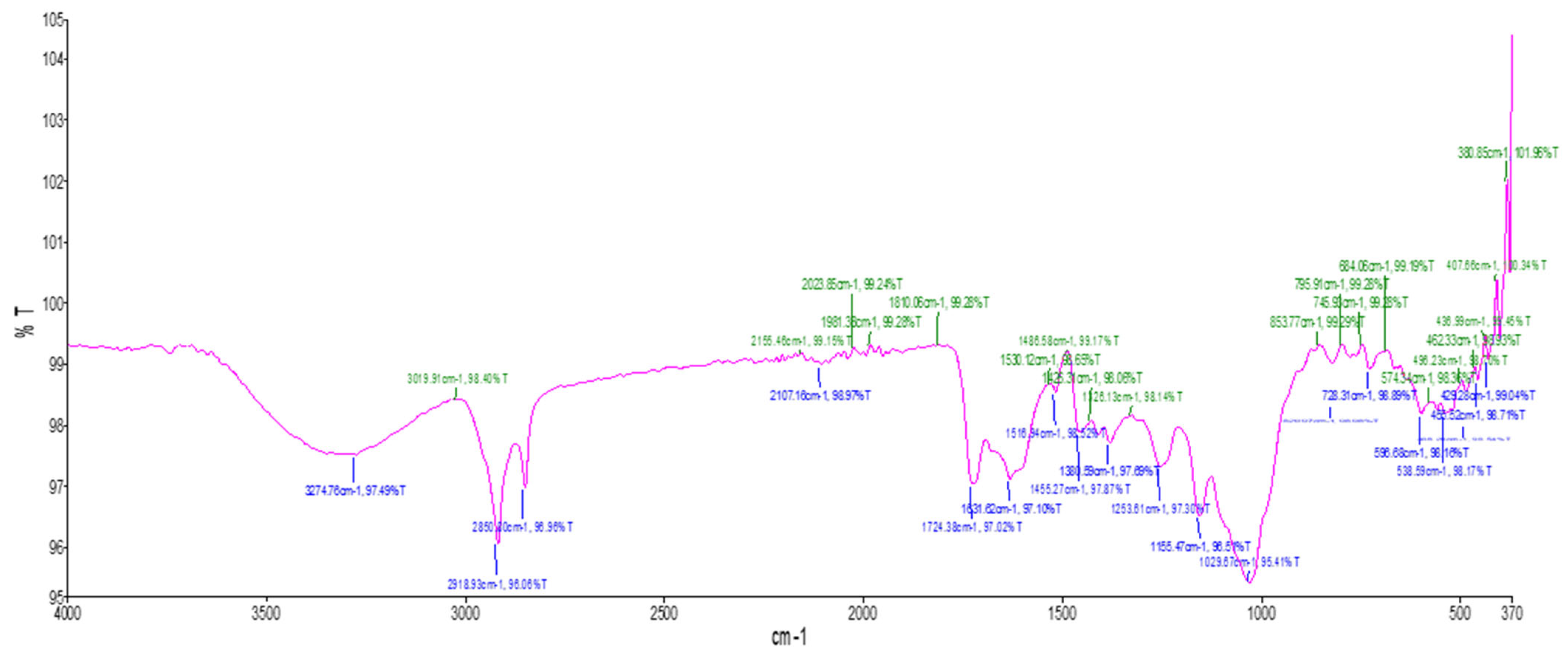
2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.1. Aqueous Extract
2.2.2. Dry Powder
2.3. Essential Oil Composition
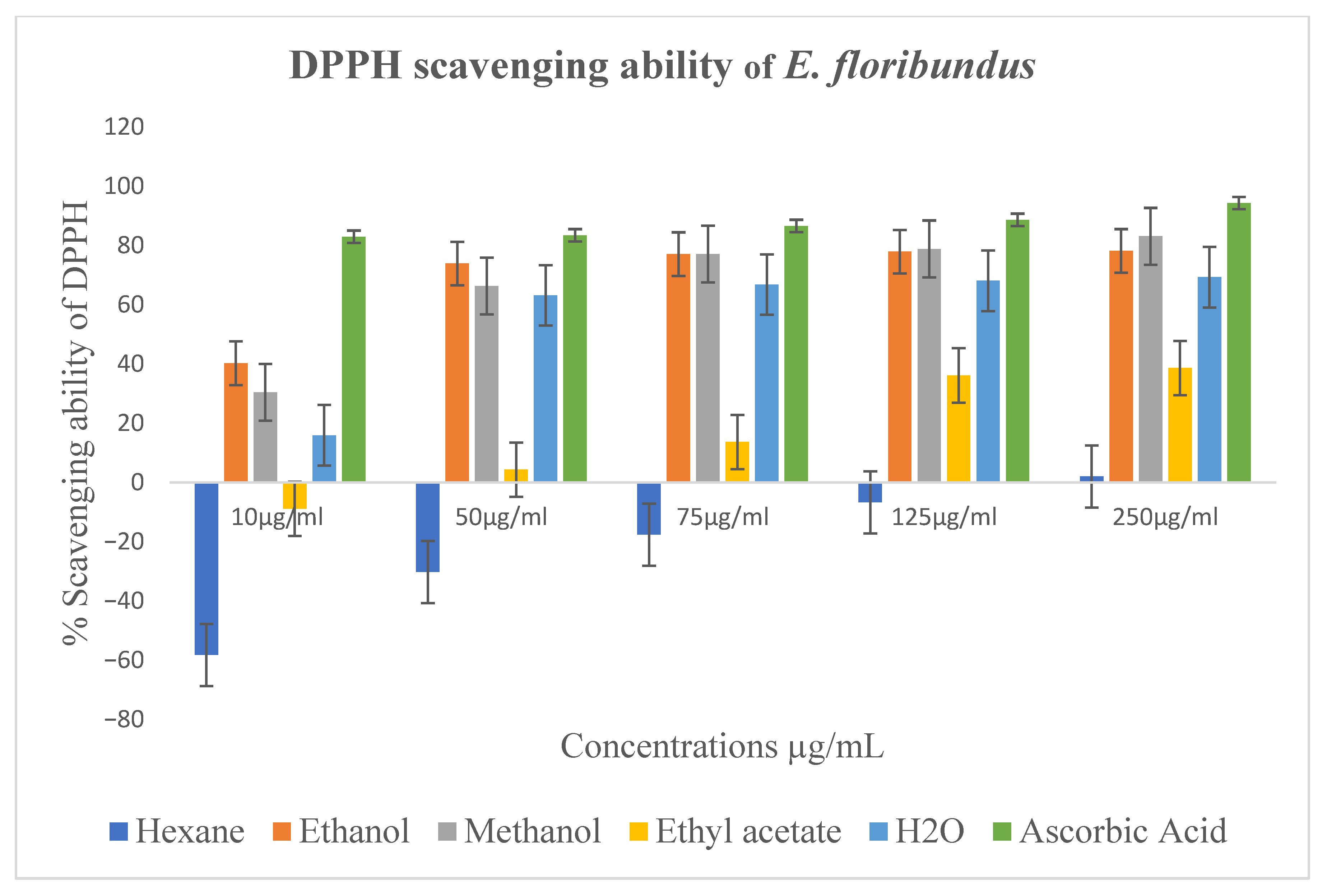
2.4. Antioxidant Activity
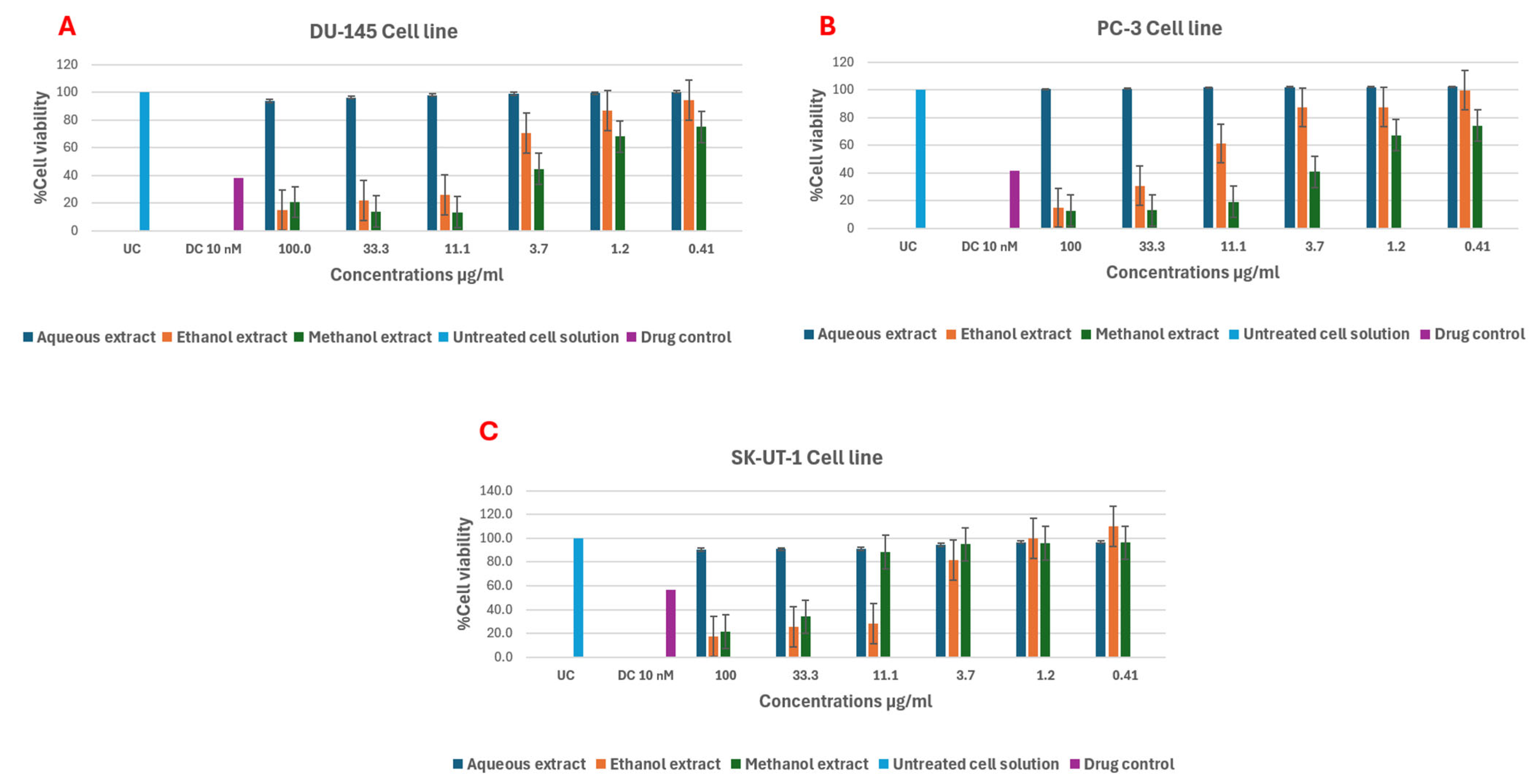
2.5. In Vitro Anticancer Activity
3. Discussion
3.1. Phytochemical Analysis
3.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.3. Gas Chromatograph Mass Spectroscopy of Volatiles
3.4. In Vitro Antioxidant Activity
3.5. In Vitro Anticancer Activity
3.6. Limitations and Future Directions
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Preparation of the Extracts
4.3. Extraction of Essential Oil
4.4. Phytochemical Screening
4.5. Fourier Transform Infrared Spectroscopy Analysis
4.6. Gas Chromatograph Mass Spectroscopy of Volatiles
4.7. Antioxidant Assay
4.7.1. DPPH Radical Scavenging Assay
4.7.2. Nitric Oxide (NO) Scavenging Activity
4.8. Anticancer Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical Constituents of Some Nigerian Medicinal Plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Rajeh, M.A.B.; Zuraini, Z.; Sasidharan, S.; Latha, L.Y.; Amutha, S. Assessment of Euphorbia hirta L. Leaf, Flower, Stem and Root Extracts for Their Antibacterial and Antifungal Activity and Brine Shrimp Lethality. Molecules 2010, 15, 6008–6018. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Boukhobza, Z.; Boulenouar, N.; Abdelkrim, C.; Kadri, Z. Essential Oil of Rosmarinus officinalis L. from West Highlands of Algeria: Chemical Characterization and in Vitro Antifungal Activity against Fusarium oxysporum f. sp. Albedinis. Nat. Volatiles Essent. Oils 2021, 8, 44–55. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Gxasheka, M.; Mbita, Z.; Laka, K.; Mndela, M.; Dlamini, P. Phytochemical Analysis and Allelopathic Potential of an Aggressive Encroacher Shrub, Euryops floribundus (Asteraceae). Plants 2025, 14, 601. [Google Scholar] [CrossRef]
- Nikolić, M.; Stevović, S. Family Asteraceae as a Sustainable Planning Tool in Phytoremediation and Its Relevance in Urban Areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Dlamini, P.; Mbanjwa, V.; Gxasheka, M.; Tyasi, L.; Sekhohola-Dlamini, L. Chemical Stabilisation of Carbon Stocks by Polyvalent Cations in Plinthic Soil of a Shrub-Encroached Savanna Grassland, South Africa. Catena 2019, 181, 104088. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Shackleton, S.; Campbell, B.; Lotz-Sisitka, H.; Shackleton, C. Links between the Local Trade in Natural Products, Livelihoods and Poverty Alleviation in a Semi-Arid Region of South Africa. World Dev. 2008, 36, 505–526. [Google Scholar] [CrossRef]
- Rossi, P.G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; Serra, D.D.R.; Gonny, M.; Bolla, J.M. Antibacterial Action of Essential Oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Senatore, F.; Rosselli, S. Chemical Composition of Essential Oils of Anthemis secundiramea Biv. subsp. Secundiramea (Asteraceae) Collected Wild in Sicily and Their Activity on Micro-Organisms Affecting Historical Art Craft. Nat. Prod. Res. 2019, 33, 970–979. [Google Scholar] [CrossRef]
- Mziouid, A.; Chebli, B.; Berrabah, M.; Chebli, H.; Heimeur, N.; Bounimi, S.; Hassan, E. Phytochemical screening and antioxidant activity of four Moroccan aromatic plant methanolic extracts and essential oils. Arab. J. Med. Aromat. Plants 2022, 8, 117–132. [Google Scholar]
- Paulraj, J.; Jeyashree, T.; Yuvashree, C.S.; Shanmugam, R.; Maiti, S. Investigating the Potential of Acacia Nilotica-Enriched Glass Ionomer Cement: An Analysis of Antimicrobial Activity and Compressive Strength. Cureus 2024, 16, e54821. [Google Scholar] [CrossRef]
- Santana, C.M.; Ferrera, Z.S.; Padrón, M.E.T.; Rodríguez, J.J.S. Methodologies for the Extraction of Phenolic Compounds from Environmental Samples: New Approaches. Molecules 2009, 14, 298–320. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J.; Nikabadi, S.; Varma, A. Importance of Medicinal and Aromatic Plants in Human Life. In Medicinal Plants and Environmental Challenges; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–23. ISBN 9783319687179. [Google Scholar]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-Fabrication of Silver Nanoparticles Using the Leaf Extract of an Ancient Herbal Medicine, Dandelion (Taraxacum officinale), Evaluation of Their Antioxidant, Anticancer Potential, and Antimicrobial Activity against Phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Wintola, O.A.; Olajuyigbe, A.A.; Afolayan, A.J.; Coopoosamy, R.M.; Olajuyigbe, O.O. Chemical Composition, Antioxidant Activities and Antibacterial Activities of Essential Oil from Erythrina caffra Thunb. Growing in South Africa. Heliyon 2021, 7, e07244. [Google Scholar] [CrossRef] [PubMed]
- Lamula, S.Q.N.; Taliwe, A.; Buwa-Komoreng, L.V. Pharmacological Properties of Platycarpha Glomerata Extracts—A Plant Used to Treat and Manage Elephantiasis. Int. J. Mol. Sci. 2025, 26, 646. [Google Scholar] [CrossRef]
- Mouhid, L.; Gómez De Cedrón, M.; Vargas, T.; García-Carrascosa, E.; Herranz, N.; García-Risco, M.; Reglero, G.; Fornari, T.; De Molina, A.R. Identification of Antitumoral Agents against Human Pancreatic Cancer Cells from Asteraceae and Lamiaceae Plant Extracts. BMC Complement. Altern. Med. 2018, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.N.; do Nascimento, R.M.C.; Rodrigues, D.C.d.N.; Ferreira, P.M.P.; Pessoa, C.; Lima, D.J.B.; de Moraes Filho, M.O.; de Almeida, R.M.; Ferreira, S.R.; Fujiwara, R.T.; et al. In Vitro Activity Evaluation of Seven Brazilian Asteraceae against Cancer Cells and Leishmania amazonensis. S. Afr. J. Bot. 2019, 121, 267–273. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 1973. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 11th ed.; Baillière Tindall: London, UK, 1989. [Google Scholar]
- Sofowora, A. Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa; Spectrum Books Ltd.: Oyo, Nigeria, 1993; pp. 150–156. [Google Scholar]
- Madikizela, B.; McGaw, L.J. In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities of Pittosporum viridiflorum Sims and Hypoxis colchicifolia Baker Used Traditionally against Cancer in Eastern Cape, South Africa. S. Afr. J. Bot. 2019, 126, 250–255. [Google Scholar] [CrossRef]
- Berry, J.M.; Huebner, E.; Butler, M. The Crystal Violet Nuclei Staining Technique Leads to Anomalous Results in Monitoring Mammalian Cell Cultures. Cytotechnology 1996, 21, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
| Phytochemical | Aqueous | Methanol | Ethanol | Chloroform |
|---|---|---|---|---|
| Terpenoids | + | + | + | + |
| Flavonoids | + | + | + | + |
| Glycosides | + | + | + | + |
| Tannins | + | + | + | + |
| Saponins | + | + | + | + |
| Steroids | + | + | + | + |
| Alkaloids | − | − | − | − |
| Anthraquinones | − | − | − | − |
| SI. No. | Absorption Peak (cm−1) (Test Sample) | Functional Groups |
|---|---|---|
| 1 | 526.86 | C-I, C-Cl |
| 2 | 1053.24 | C-O stretching |
| 3 | 1217.13 | C-O stretching |
| 4 | 1372.88 | O-H bend, Alcoholic group |
| 5 | 1607.04 | C=O stretch |
| 6 | 1738.03 | C=O stretching |
| 7 | 3254.13 | O-H stretch, Carboxylic group |
| SI. No. | Absorption Peak (cm−1) | Functional Groups |
|---|---|---|
| 1 | 429.28 | C-Halogen |
| 2 | 455.52 | C-Halogen |
| 3 | 538.59 | C-I, C-Cl |
| 4 | 596.68 | C-I, C-Cl |
| 5 | 728.31 | C-Cl |
| 6 | 1029.67 | PO3 stretching |
| 7 | 1155.47 | C-N stretch |
| 8 | 1253.61 | C-N stretch |
| 9 | 1380.59 | O-H bend, alcoholic group |
| 10 | 1455.27 | C=C aromatic ring stretching |
| 11 | 1516.94 | C=C stretch |
| 12 | 1631.62 | C=O stretching |
| 13 | 1724.38 | C=O stretch |
| 14 | 2107.16 | Carbon-Carbon triple bond |
| 15 | 2850.80 | Symmetrical stretching of -CH2(CH2) vibration |
| 16 | 2918.93 | Asymmetric stretching of CH(CH2) vibration |
| 17 | 3274.76 | O-H stretch, Carboxylic group |
| Retention Time | Constituent | % Total |
|---|---|---|
| 10.6464 | α-Thujene | 1.01 |
| 10.8745 | α-Pinene | 9.53 |
| 12.3700 | sabinene | 27.86 |
| 12.4613 | β-Pinene | 1.61 |
| 13.0473 | Myrcene | 1.39 |
| 13.9426 | α-Terpinene | 3.97 |
| 14.7088 | trans-β-Ocimene | 8.06 |
| 15.0724 | cis-Ocimene | 1.17 |
| 15.4215 | γ-Terpinene | 5.02 |
| 16.4253 | Terpinolene | 1.24 |
| 19.2891 | Terpinene-4-ol | 13.63 |
| 19.7266 | α-Terpinene | 0.76 |
| 27.7060 | Germacrene D | 0.64 |
| 28.6867 | σ-Cadinene | 0.67 |
| 31.8052 | T-Muurolo | 0.95 |
| 31.9416 | α-Gurjunene | 1.88 |
| 37.8521 | Cyclooctane,1,4-dimethyl-trans- | 1.73 |
| 38.0106 | Palmitic acid | 8.64 |
| 38.3583 | 3-Ethyl-5-methyl-1-propyl-Cyclohexane | 1.98 |
| 38.6926 | 2,3,4-trimethyl-4,5-methylenetetradecane | 1.98 |
| 39.0258 | cis, trans-1,2,3-trimethyl-cyclohexane | 1.36 |
| 39.2887 | 1,1′-bicyclohexanyl, 2-propyl-, cis- | 1.59 |
| 41.1914 | 3,7,11-trimethyldodeca-7(Z),10-diene | 0.94 |
| Extracts | DPPH Assay (IC50 µg/mL) | NO Assay (IC50 in µg/mL) |
|---|---|---|
| Aqueous | 2.41 ± 3.4 | 316.1 ± 10.9 |
| Ethanol | 0.56 ± 3.2 | 386.9 ± 12.0 |
| Methanol | 1.54 ± 1.9 | 514.5 ± 13.8 |
| Ethyl acetate | 2.26 ± 4.1 | - |
| Ascorbic acid | - | 431.4 ± 12.6 |
| Cell Lines | ||||||
|---|---|---|---|---|---|---|
| DU-145 | PC-3 | SK-UT-1 | DU-145 | PC-3 | SK-UT-1 | |
| Plant Extracts | IC50 μg/ml | % Inhibition at 100 µg/ml | ||||
| Aqueous | - | - | - | 6.1 | - | 9.5 |
| Ethanol | 2.1 ± 0.02 | 2.7 ± 0.03 | 1.7 ± 0.02 | 84.1 | 85.3 | 82.6 |
| methanol | 3.2 ± 0.02 | 2.7 ± 0.51 | 2.2 ± 0.02 | 79.3 | 87.4 | 78.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhinana, Z.; Mayekiso, B.; Lamula, S.; Bhanisa, T.; Wium, M.; Paccez, J.; Zerbini, L.; Bvenura, C.; Buwa-Komoreng, L. Composition of Volatiles, Phytochemical Analysis, Antioxidant and Anticancer Activity of Euryops floribundus Ne.Br. Leaves (Asteraceae). Molecules 2025, 30, 4555. https://doi.org/10.3390/molecules30234555
Mhinana Z, Mayekiso B, Lamula S, Bhanisa T, Wium M, Paccez J, Zerbini L, Bvenura C, Buwa-Komoreng L. Composition of Volatiles, Phytochemical Analysis, Antioxidant and Anticancer Activity of Euryops floribundus Ne.Br. Leaves (Asteraceae). Molecules. 2025; 30(23):4555. https://doi.org/10.3390/molecules30234555
Chicago/Turabian StyleMhinana, Zoleka, Buyisile Mayekiso, Siphamandla Lamula, Thando Bhanisa, Martha Wium, Juliano Paccez, Luiz Zerbini, Callistus Bvenura, and Lisa Buwa-Komoreng. 2025. "Composition of Volatiles, Phytochemical Analysis, Antioxidant and Anticancer Activity of Euryops floribundus Ne.Br. Leaves (Asteraceae)" Molecules 30, no. 23: 4555. https://doi.org/10.3390/molecules30234555
APA StyleMhinana, Z., Mayekiso, B., Lamula, S., Bhanisa, T., Wium, M., Paccez, J., Zerbini, L., Bvenura, C., & Buwa-Komoreng, L. (2025). Composition of Volatiles, Phytochemical Analysis, Antioxidant and Anticancer Activity of Euryops floribundus Ne.Br. Leaves (Asteraceae). Molecules, 30(23), 4555. https://doi.org/10.3390/molecules30234555







