Post–Synthetic Modification of MOF–808 for Mixed Matrix Membranes with High and Stable Ion Separation Capacity
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Dosage Determination of Spiropyran (SP) Molecules
2.1.1. Synthesis of SP Molecules
2.1.2. Determination of the Optimal Dosage of SP Molecules in the Modification Process
2.2. Characterization of MOF–808–SP MMMs
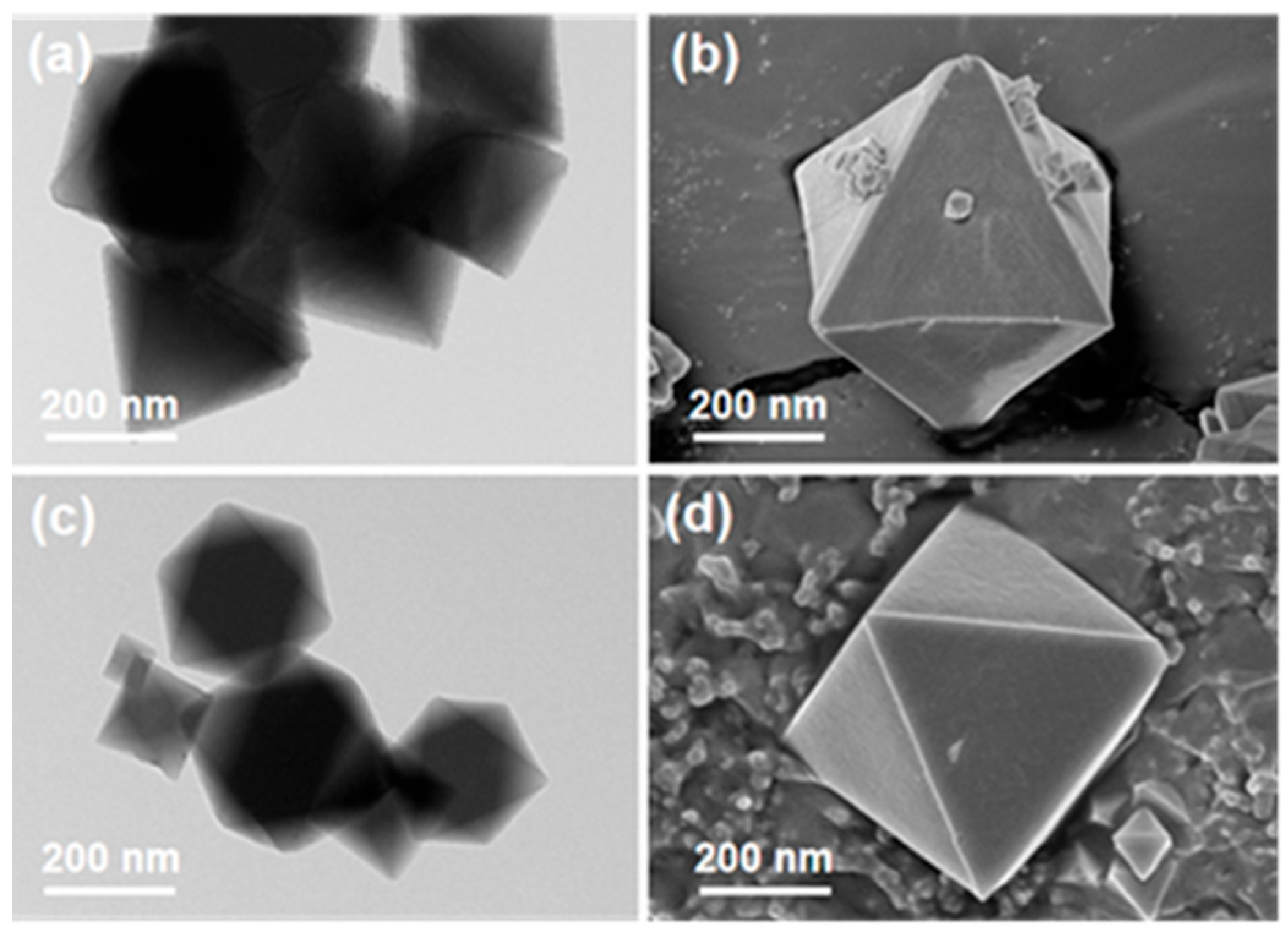
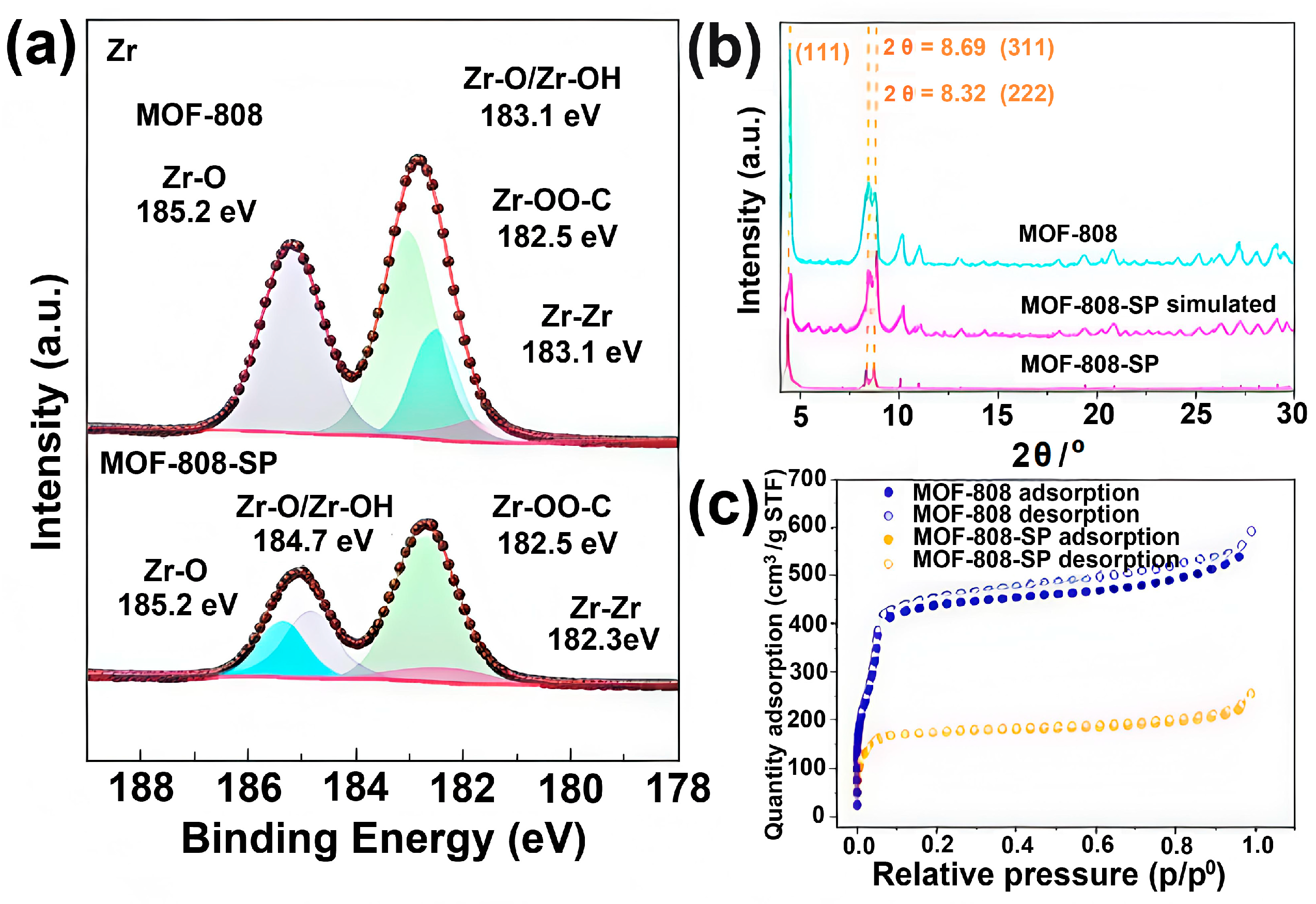
2.2.1. Characterization of MOF–808 and MOF–808–SP
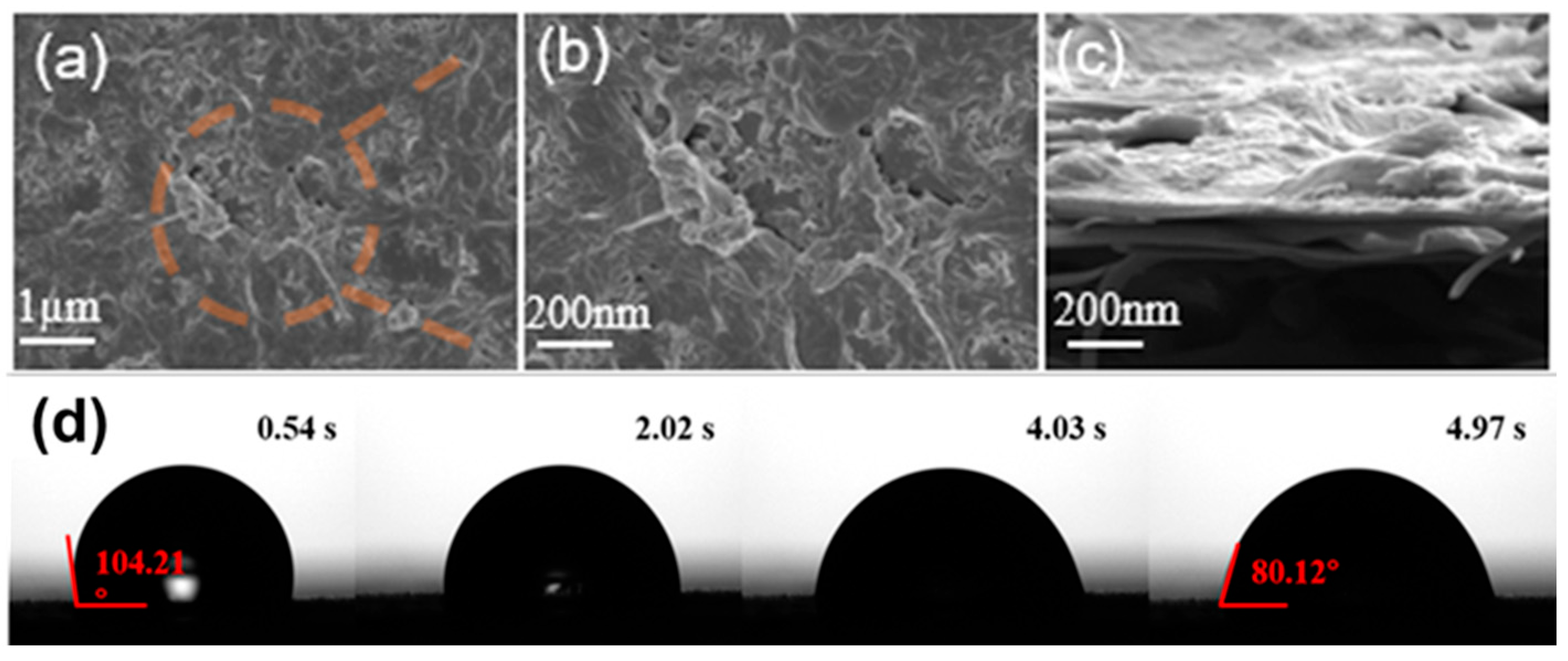
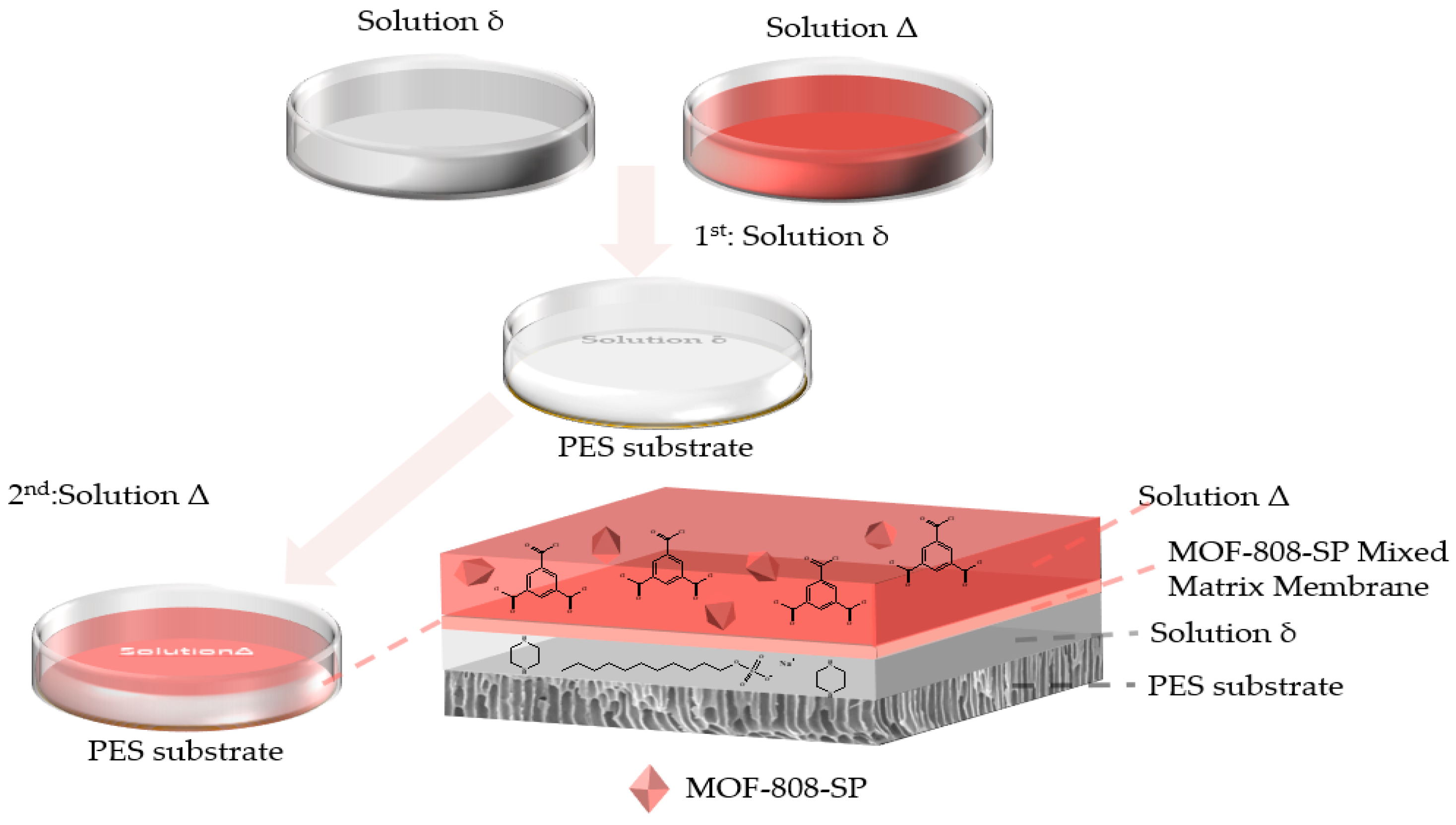
2.2.2. Characterization of Mixed Matrix Membrane
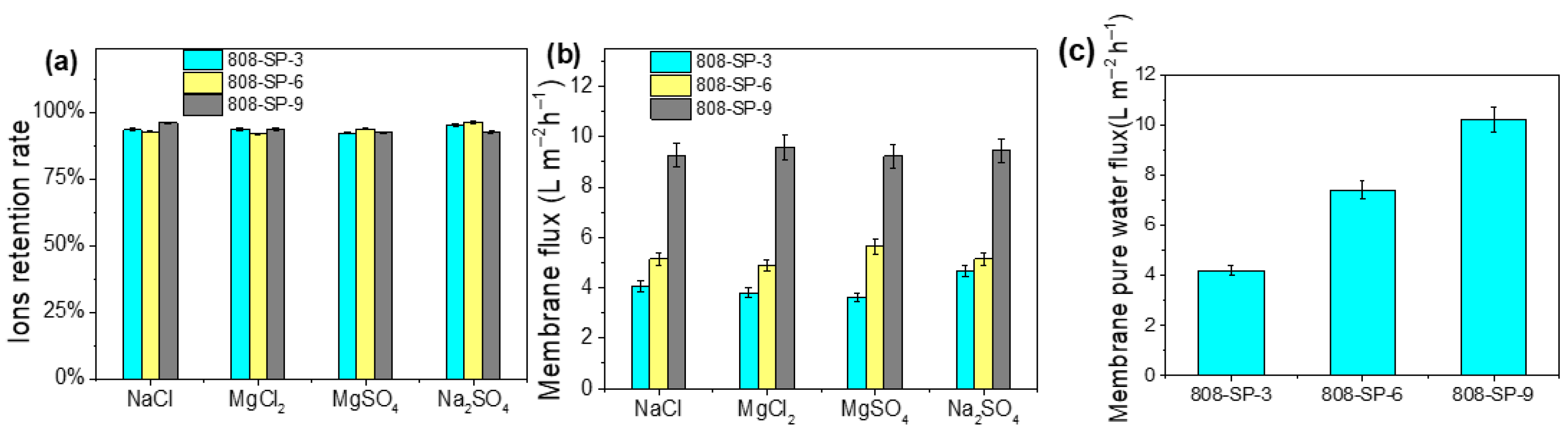
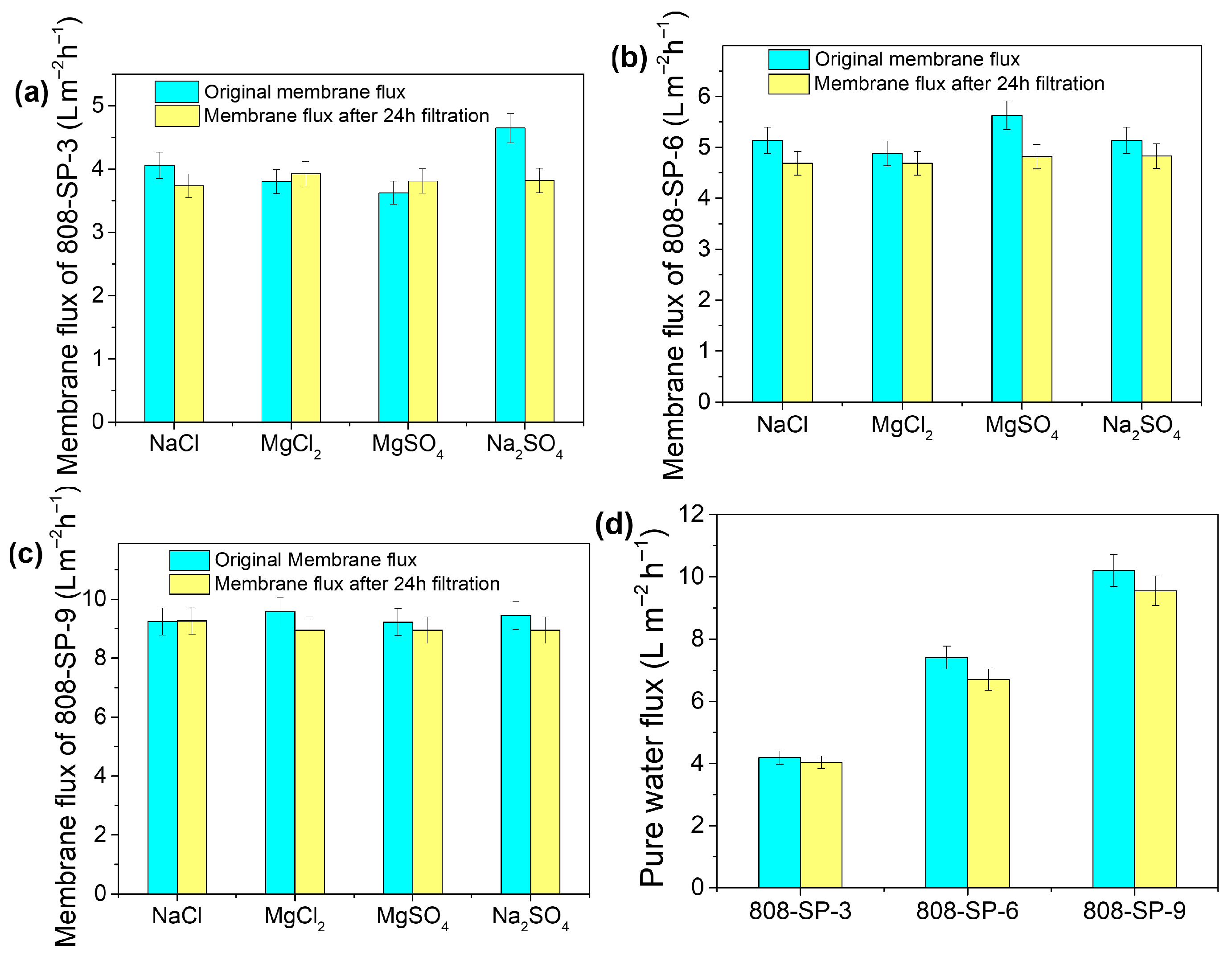
2.3. Membrane Filtration Performance Test
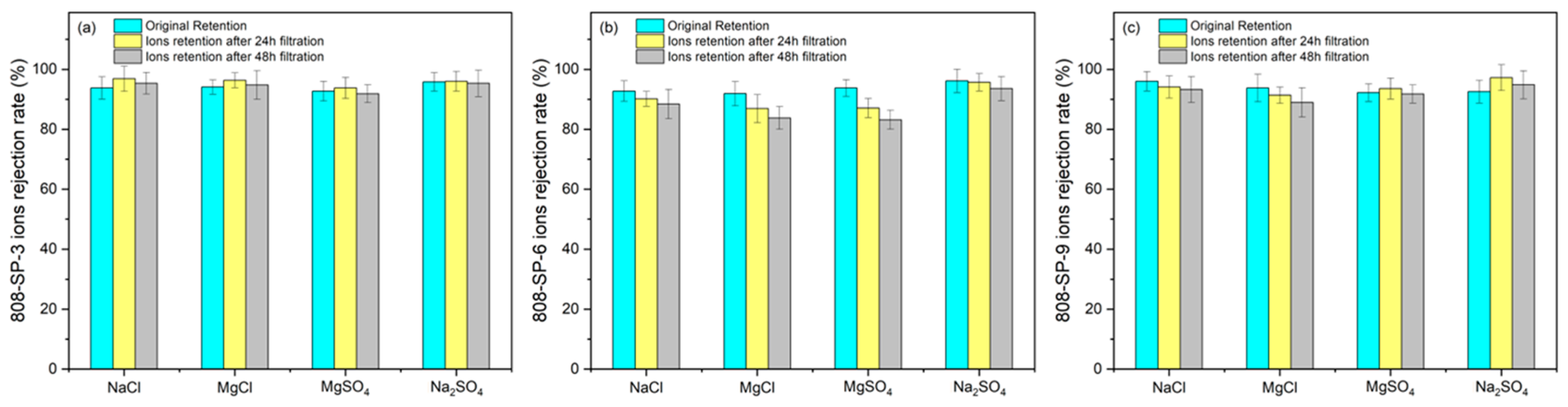
2.4. Membrane Stability Test
2.5. Seawater Treatment Performance
3. Experimental
3.1. Materials
3.2. Syntheses
3.2.1. Synthesis of Spiropyran (SP)
3.2.2. Synthesis of MOF–808
3.2.3. Synthesis of MOF–808–SP
3.3. Fabrication of MOF–808–SP Mixed Matrix Membranes (MMMs)
3.4. Characterizations
3.5. Membrane Filtration Performance Test
- (1)
- Membrane flux (Q) was calculated according to the following equation:
- (2)
- Rejection rate (R) was calculated using the following equation:
3.6. Membrane Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xevgenos, D.; Moustakas, K.; Malamis, D.; Loizidou, M. An overview on desalination & sustainability: Renewable energy-driven desalination and brine management. Desalination Water Treat. 2014, 57, 2304–2314. [Google Scholar] [CrossRef]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Hilal, N. Green Approaches for Sustainable Development of Liquid Separation Membrane. Membranes 2021, 11, 235. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Wang, X.; Ma, H.; Chu, B.; Hsiao, B.S. Thin-film nanofibrous composite reverse osmosis membranes for desalination. Desalination 2017, 420, 91–98. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Han, X.; Liu, Y.; Wang, C.; Yan, F.; Wang, J. Regulating the interfacial polymerization process toward high performance polyamide thin film composite reverse osmosis and nanofiltration membranes: A review. J. Membr. Sci. 2021, 640, 119765. [Google Scholar] [CrossRef]
- Yu, C.; Li, H.; Zhang, X.; Lü, Z.; Yu, S.; Liu, M.; Gao, C. Polyamide thin film composite membrane fabricated through interfacial polymerization coupled with surface amidation for improved reverse osmosis performance. J. Membr. Sci. 2018, 566, 87–95. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2016, 46, 62–80. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Japip, S.; Jiang, S.D.; Chung, T.S.; Zhang, S.; Zhao, D. Advanced Porous Materials in Mixed Matrix Membranes. Adv. Mater. 2018, 30, 1802401. [Google Scholar] [CrossRef]
- Cheng, Y.; Datta, S.J.; Zhou, S.; Jia, J.; Shekhah, O.; Eddaoudi, M. Advances in metal-organic framework-based membranes. Chem. Soc. Rev. 2022, 51, 8300–8350. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yue, D.; Zhang, J.; Wang, J.; Li, B.; Yang, Y.; Cui, Y.; Qian, G. Flexible Metal-Organic Framework-Based Mixed Matrix Membranes: A New Platform for H2S Sensors. Small 2018, 14, 1801563. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhao, L.; Zhang, L.; Chen, H.; Gao, C.; Winston Ho, W.S. High flux reverse osmosis membranes incorporated with NaY zeolite nanoparticles for brackish water desalination. J. Membr. Sci. 2015, 476, 373–383. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, G.; Liu, Z.; Gao, C. Effect of MCM-48 nanoparticles on the performance of thin film nanocomposite membranes for reverse osmosis application. Desalination 2016, 394, 72–82. [Google Scholar] [CrossRef]
- Dorosti, F.; Ge, L.; Wang, H.; Zhu, Z. A path forward: Understanding and mitigating defects in polycrystalline membranes. Prog. Mater. Sci. 2023, 137, 101123. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quemener, D. MOF-mixed matrix membranes: Precise dispersion of MOF particles with better compatibility via a particle fusion approach for enhanced gas separation properties. J. Membr. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, T.; Li, Z.; Wang, S.; Li, Y.; Li, Z.; Ouyang, J.; Jiang, Z.; Wu, H. Mixed matrix membranes composed of sulfonated poly(ether ether ketone) and a sulfonated metal-organic framework for gas separation. J. Membr. Sci. 2015, 488, 67–78. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, X.; Wang, X.; Wang, Q.; Ji, Z.; Wang, X.; Wu, T.; Gao, C. Highly and Stably Water Permeable Thin Film Nanocomposite Membranes Doped with MIL-101 (Cr) Nanoparticles for Reverse Osmosis Application. Materials 2016, 9, 870. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High performance polyamide thin film nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Membr. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Zhu, C.; Pan, Y.; Cheng, S.; Ren, X.; Guo, M.; Zhong, J.; Liu, Q.; Liu, G. UiO-66-NH2 MOF/organosilica mixed matrix membrane for water desalination. J. Membr. Sci. 2023, 685, 121913. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal-organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Bentz, K.C.; Cohen, S.M. Defect-Free MOF-Based Mixed-Matrix Membranes Obtained by Corona Cross Linking. ACS Appl. Mater. Interfaces 2019, 11, 13029–13037. [Google Scholar] [CrossRef]
- Begum, M.; Wang, F.; Saboor, A.; Khan, A.; Lv, G.; Oussama, L.; Shen, J.; Bai, J. A critical review on the modification and application of MOF–808 frameworks. Sep. Purif. Technol. 2025, 368, 133046. [Google Scholar] [CrossRef]
- Jun, H.J.; Yoo, D.K.; Jhung, S.H. Metal-organic framework (MOF–808) functionalized with ethyleneamines: Selective adsorbent to capture CO2 under low pressure. J. CO2 Util. 2022, 58, 101932. [Google Scholar] [CrossRef]
- Najafi, M.; Kulak, H.; Landa, H.O.R.; Vankelecom, I.F.J.; Denayer, J.F.M. Appraising separation performance of MOF–808-based adsorbents for light olefins and paraffins. Micropor. Mesopor. Mat. 2024, 367, 112961. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, Y.; Chung, T.S. Poly-/metal-benzimidazole nano-composite membranes for hydrogen purification. Energy Environ. Sci. 2011, 4, 4171–4180. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Refaat, D.; Yahia, M.; Martínez-Hernández, H.D.; Jimenez-Ruiz, M.; Galván, V.; Petrenko, V.; de Luis, R.F.; Coronas, J. Mixed matrix membranes of PIM-1 incorporating MOF–808 functionalized with amino acids for enhanced CO2/CH4 separation. J. Mater. Chem. A 2025. submitted. [Google Scholar] [CrossRef]
- del Castillo-Velilla, I.; Romero-Muñiz, I.; Marini, C.; Montoro, C.; Platero-Prats, A.E. Copper single-site engineering in MOF–808 membranes for improved water treatment. Nanoscale 2024, 16, 6627–6635. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Wang, X.; Wang, Z. Synthesis, structure and properties of Pd@MOF–808. J. Mater. Sci. 2019, 54, 12911–12924. [Google Scholar] [CrossRef]
- Imato, K.; Momota, K.; Kaneda, N.; Imae, I.; Ooyama, Y. Photoswitchable Adhesives of Spiropyran Polymers. Chem. Mater. 2022, 34, 8289–8296. [Google Scholar] [CrossRef]
- Zhang, W.; Bu, A.; Ji, Q.; Min, L.; Zhao, S.; Wang, Y.; Chen, J. pK(a)-Directed Incorporation of Phosphonates into MOF–808 via Ligand Exchange: Stability and Adsorption Properties for Uranium. ACS Appl. Mater. Interfaces 2019, 11, 33931–33940. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, M.; Xie, Z.; Li, Y.; Zhang, A. Performance of defective Zr-MOFs for the adsorption of anionic dyes. J. Mater. Sci. 2022, 57, 5438–5455. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Wang, L.; Gao, G.; Xu, H.; Huang, W.; Yan, N.; Wang, H.; Qu, Z. FeS(x)@MOF–808 composite for efficient As(III) removal from wastewater: Behavior and mechanism. J. Hazard. Mater. 2023, 446, 130681. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Karadeniz, B.; Biliškov, N.; Lončarić, I.; Muratović, S.; Žilić, D.; Avdoshenko, S.M.; Roslova, M.; Popov, A.A.; Užarević, K. Tunable Fulleretic Sodalite MOFs: Highly Efficient and Controllable Entrapment of C60 Fullerene via Mechanochemistry. Chem. Mater. 2020, 32, 10628–10640. [Google Scholar] [CrossRef]
- Sun, Q.; Du, J.; Yao, A.; Zhang, Y.; Yu, B.; Lim, W.; Hassan, S.U.; Guan, J.; Dou, P.; Liu, J. Tunable Hydrated Channels in Covalent Organic Framework Membrane for Seawater Desalination. ACS Nano 2025, 19, 18409–18420. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Wang, Y.; Li, R.; Gu, X.; Yuan, Y.D.; Qian, Y.; Hu, Z.; Zhao, D. Improving Water-Treatment Performance of Zirconium Metal-Organic Framework Membranes by Post-synthetic Defect Healing. ACS Appl. Mater. Interfaces 2017, 9, 37848–37855. [Google Scholar] [CrossRef]
- Thur, R.; Van Velthoven, N.; Lemmens, V.; Bastin, M.; Smolders, S.; De Vos, D.; Vankelecom, I.F.J. Modulator Mediated Functionalization of MOF–808 as a Platform Tool to Create High Performance Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2019, 11, 44792–44801. [Google Scholar] [CrossRef]
- Landaburu Aguirre, J.; García Pacheco, R.; Molina, S.; Rodríguez Sáez, L.; Rabadán, J.; García Calvo, E. Fouling prevention, preparing for reuse and membrane recycling. Towards circular economy in RO desalination. Desalination 2016, 393, 16–30. [Google Scholar] [CrossRef]
- Matin, A.; Laoui, T.; Falath, W.; Farooque, M. Fouling control in reverse osmosis for water desalination & reuse: Current practices & emerging environment friendly technologies. Sci. Total Environ. 2021, 765, 142721. [Google Scholar] [CrossRef]
- Harun Kulak, R.T.; Vankelecom, I.F.J. MOF/Polymer Mixed Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes 2022, 12, 425. [Google Scholar] [CrossRef]
- Ly, H.G.T.; Fu, G.; Kondinski, A.; Bueken, B.; De Vos, D.; ParacVogt, T.N. Superactivity of MOF–808 toward Peptide Bond Hydrolysis. J. Am. Chem. Soc. 2018, 140, 6325–6335. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadeniz, B.; Fang, H.-L.; He, Y.-Y.; Ye, Q.-L.; Chen, J.-Y.; Lü, J. Post–Synthetic Modification of MOF–808 for Mixed Matrix Membranes with High and Stable Ion Separation Capacity. Molecules 2025, 30, 4554. https://doi.org/10.3390/molecules30234554
Karadeniz B, Fang H-L, He Y-Y, Ye Q-L, Chen J-Y, Lü J. Post–Synthetic Modification of MOF–808 for Mixed Matrix Membranes with High and Stable Ion Separation Capacity. Molecules. 2025; 30(23):4554. https://doi.org/10.3390/molecules30234554
Chicago/Turabian StyleKaradeniz, Bahar, Han-Liang Fang, Yi-Ying He, Qi-Lin Ye, Jun-Yu Chen, and Jian Lü. 2025. "Post–Synthetic Modification of MOF–808 for Mixed Matrix Membranes with High and Stable Ion Separation Capacity" Molecules 30, no. 23: 4554. https://doi.org/10.3390/molecules30234554
APA StyleKaradeniz, B., Fang, H.-L., He, Y.-Y., Ye, Q.-L., Chen, J.-Y., & Lü, J. (2025). Post–Synthetic Modification of MOF–808 for Mixed Matrix Membranes with High and Stable Ion Separation Capacity. Molecules, 30(23), 4554. https://doi.org/10.3390/molecules30234554




