Influence of Copigmentation on the Stability and Oxidative Stress of Anthocyanins from Purple Corn and Camu-Camu
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC-MS Anthocyanin Characterization
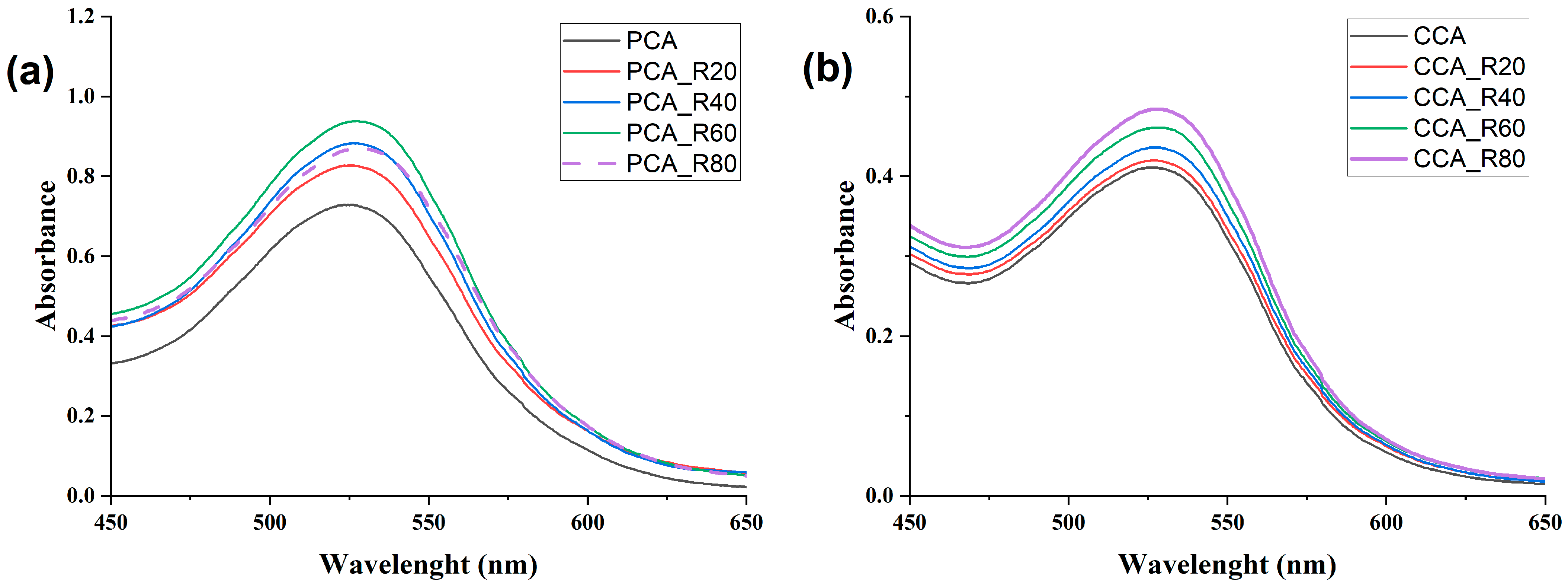
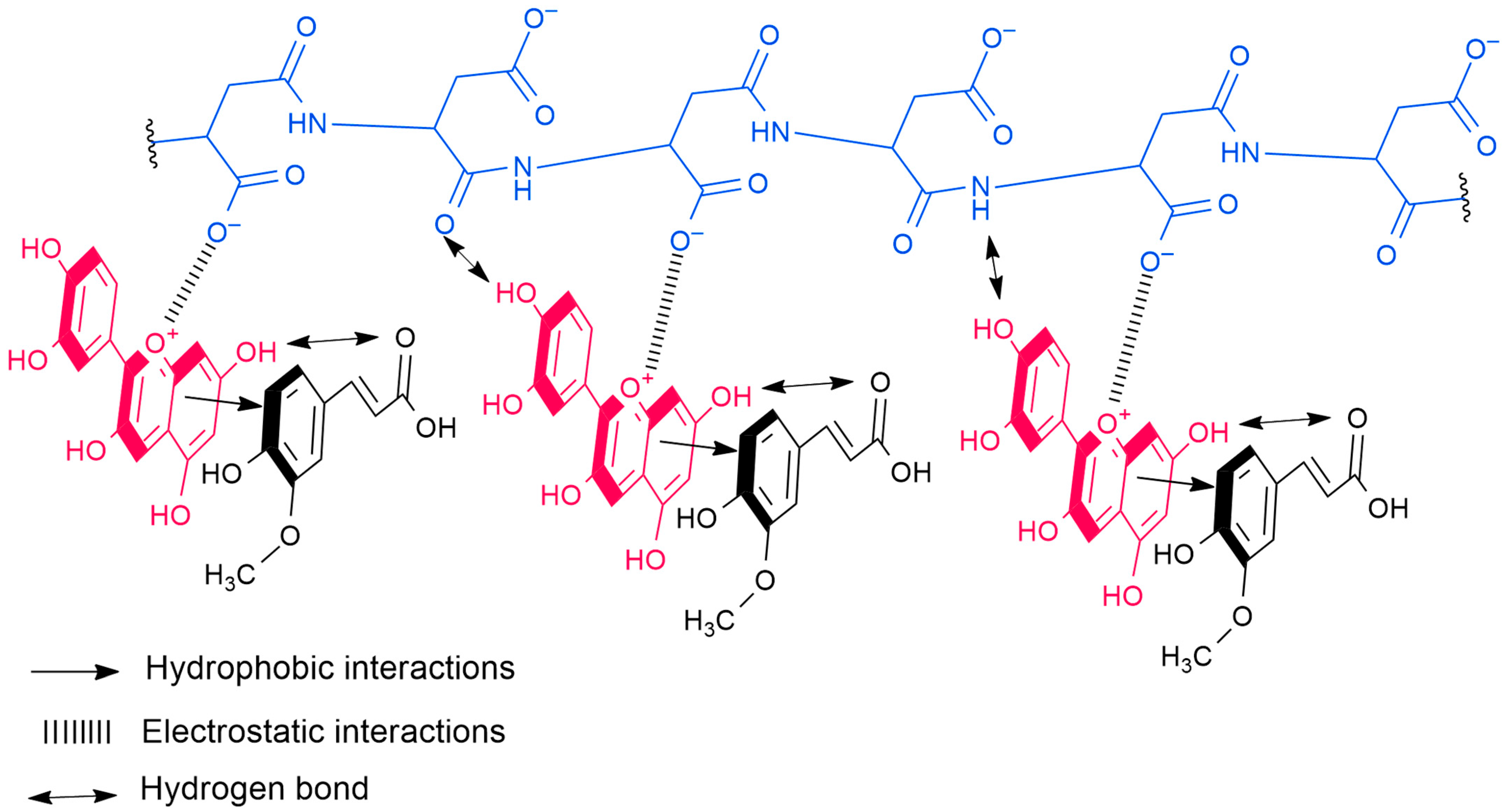
2.2. Anthocyanin Copigmentation
2.2.1. Copigmentation with Phenolic Copigments and Polyaspartic Acid
2.2.2. Double Copigmentation
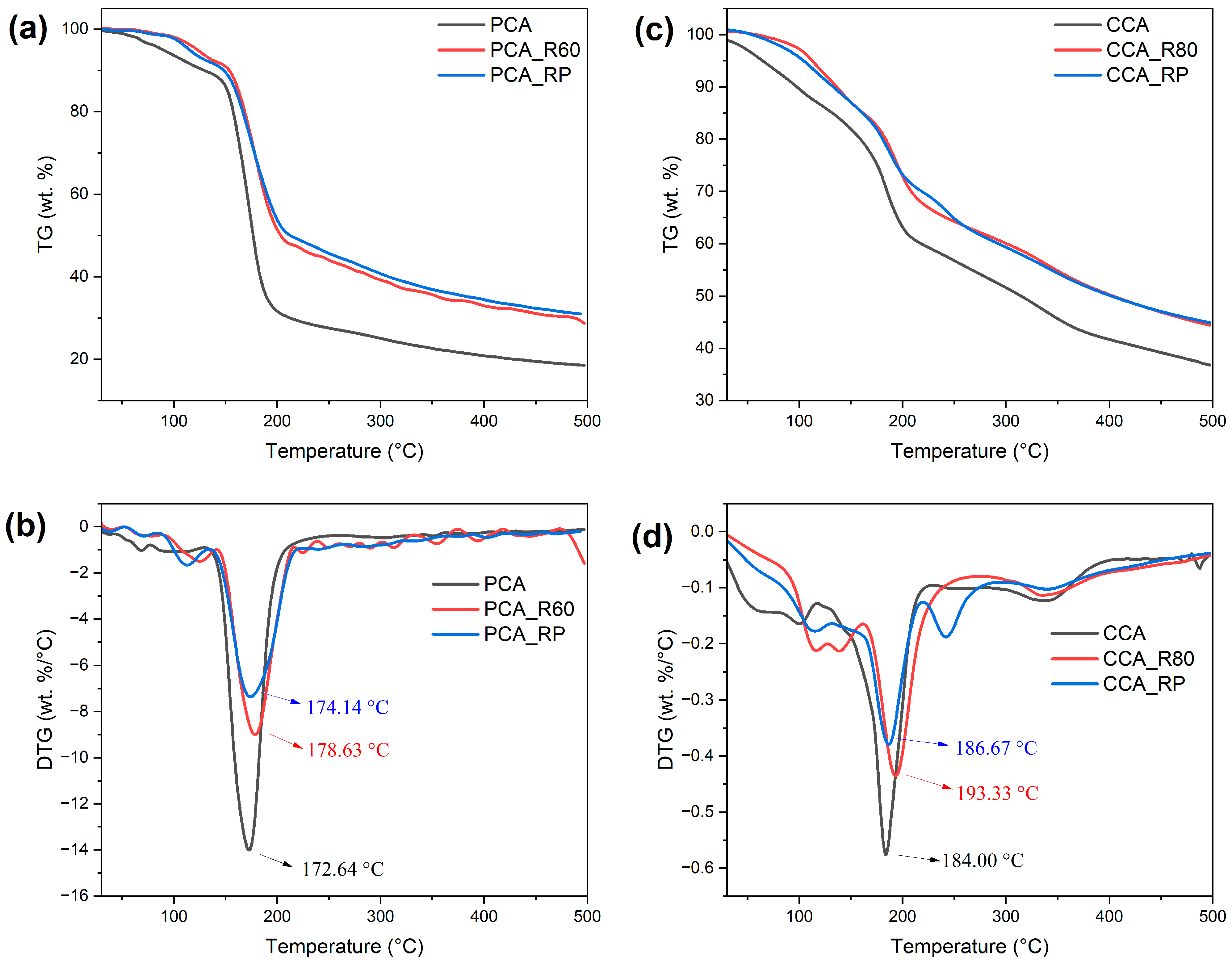
2.3. Thermogravimetric Analysis
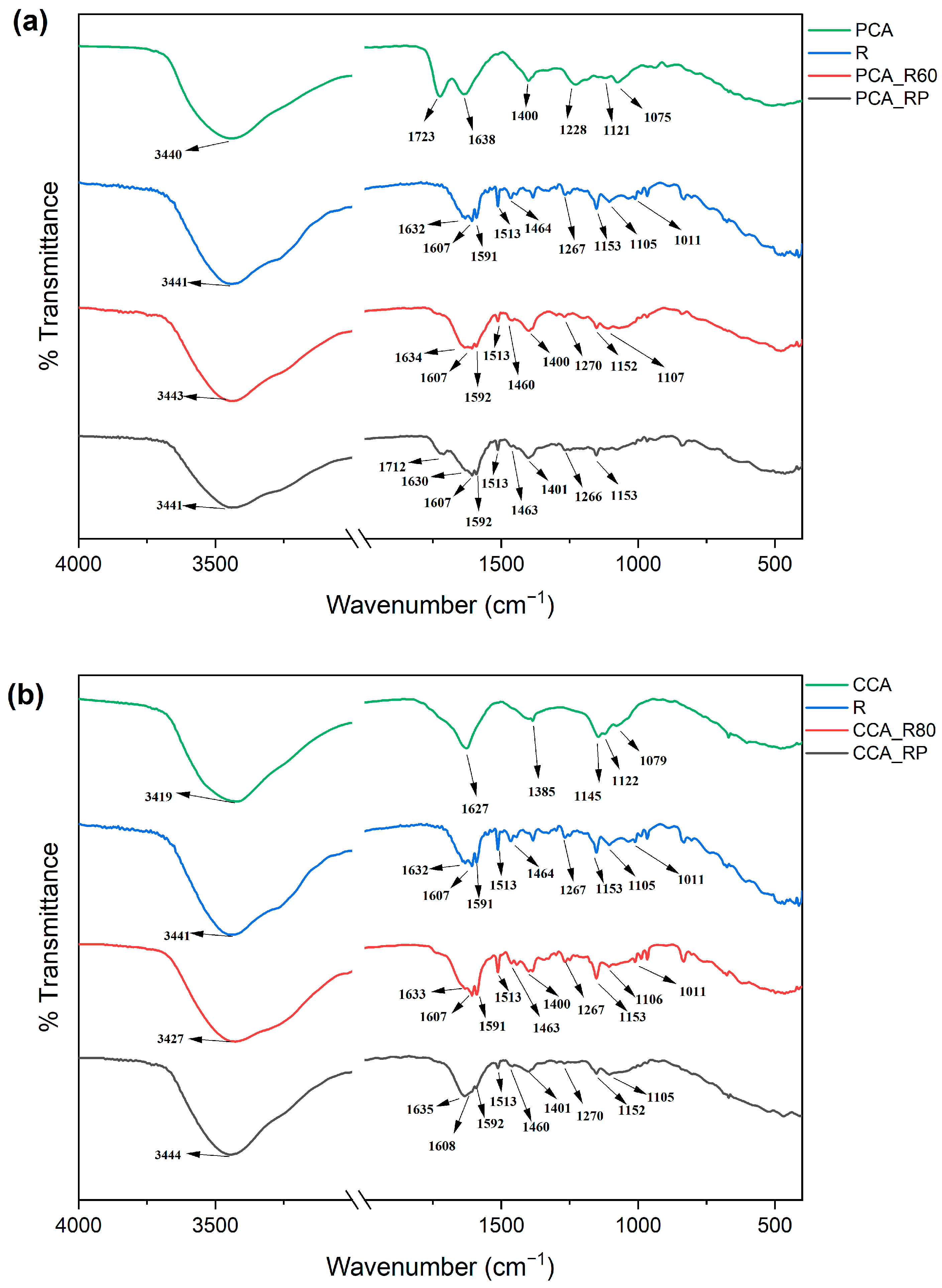
2.4. FTIR Analysis
2.5. Copigmented Anthocyanin Stability at pH 7.4
2.6. In Vitro Oxidative Stress Assessment by TBARS Method
2.7. Potential Neurobiological Implications in Alzheimer’s Disease
3. Materials and Methods
3.1. Materials
3.2. Extraction and Purification of Anthocyanins from Purple Corn Cob and Camu-Camu Peel
3.3. HPLC-MS Characterization
3.4. Anthocyanin Copigmentation
3.5. Thermogravimetric Analysis (TG) and Its Derivative (DTG)
3.6. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.7. Experimental Determination of Copigmented Anthocyanin Stability at pH 7.4
3.8. TBARS Assay for In Vitro Oxidative Stress Determination
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans-Lacko, S.; Aguzzoli, E.; Read, S.; Comas-Herrera, A.; Farina, N. World Alzheimer Report 2024: Global Changes in Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2024. [Google Scholar]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative Stress in Alzheimer’s Disease: Why Did Antioxidant Therapy Fail? Oxid. Med. Cell. Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhai, W. Identification and Antioxidant Activity of Anthocyanins Extracted from the Seed and Cob of Purple Corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Langley, P.C.; Pergolizzi, J.V.; Taylor, R.; Ridgway, C. Antioxidant and Associated Capacities of Camu Camu (Myrciaria dubia): A Systematic Review. J. Altern. Complement. Med. 2015, 21, 8–14. [Google Scholar] [CrossRef]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in Metabolites of Purple Corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- Conceição, N.; Albuquerque, B.R.; Pereira, C.; Corr, C.G.; Lopes, C.B.; Calhelha, R.C.; Jos, M.; Barros, L.; Ferreira, I.C.F.R. By-Products of Camu-Camu [Myrciaria dubia (Kunth) McVaugh ] as Promising Sources of Bioactive High Added-Value Food Ingredients: Functionalization of Yogurts. Molecules 2020, 25, 70. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Sun, B.; Yang, Y.; Wang, S.; Feng, Z.; Li, J. The Structure of Anthocyanins and the Copigmentation by Common Micromolecular Copigments: A Review. Food Res. Int. 2024, 176, 113837. [Google Scholar] [CrossRef]
- Constantinescu, T.; Mihis, A.G. Resveratrol as a Privileged Molecule with Antioxidant Activity. Food Chem. Adv. 2023, 3, 100539. [Google Scholar] [CrossRef]
- Chávez, B.Y.; Paz, J.L.; Gonzalez-Paz, L.A.; Alvarado, Y.J.; Contreras, J.S.; Loroño-González, M.A. Theoretical Study of Cyanidin-Resveratrol Copigmentation by the Functional Density Theory. Molecules 2024, 29, 2064. [Google Scholar] [CrossRef] [PubMed]
- Heras-Roger, J.; Alonso-Alonso, O.; Gallo-Montesdeoca, A.; Díaz-Romero, C.; Darias-Martín, J. Influence of Copigmentation and Phenolic Composition on Wine Color. J. Food Sci. Technol. 2016, 53, 2540–2547. [Google Scholar] [CrossRef]
- Qian, B.J.; Liu, J.H.; Zhao, S.J.; Cai, J.X.; Jing, P. The Effects of Gallic/Ferulic/Caffeic Acids on Colour Intensification and Anthocyanin Stability. Food Chem. 2017, 228, 526–532. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Lou, L.; Chen, Y.; Ye, X.; Chen, J. Copigmentation Effect of Three Phenolic Acids on Color and Thermal Stability of Chinese Bayberry Anthocyanins. Food Sci. Nutr. 2020, 8, 3234–3242. [Google Scholar] [CrossRef]
- He, Y.; Wen, L.; Yu, H.; Zheng, F.; Wang, Z.; Xu, X.; Zhang, H.; Cao, Y.; Wang, B.; Chu, B.; et al. Effects of High Hydrostatic Pressure-Assisted Organic Acids on the Copigmentation of Vitis Amurensis Rupr Anthocyanins. Food Chem. 2018, 268, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Y.; Xie, P.; Zhang, L.; Li, Y.; Zhou, J. Copigmentation Effects of Phenolics on Color Enhancement and Stability of Blackberry Wine Residue Anthocyanins: Chromaticity, Kinetics and Structural Simulation. Food Chem. 2019, 275, 299–308. [Google Scholar] [CrossRef]
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Regenstein, J.M.; Laokuldilok, T. Copigmentation of Cyanidin 3-O-Glucoside with Phenolics: Thermodynamic Data and Thermal Stability. Food Biosci. 2019, 30, 100419. [Google Scholar] [CrossRef]
- Azman, E.M.; Yusof, N.; Chatzifragkou, A.; Charalampopoulos, D. Stability Enhancement of Anthocyanins from Blackcurrant (Ribes nigrum L.) Pomace through Intermolecular Copigmentation. Molecules 2022, 27, 5489. [Google Scholar] [CrossRef]
- Azargoonjahromi, A.; Abutalebian, F. Unraveling the Therapeutic Efficacy of Resveratrol in Alzheimer’s Disease: An Umbrella Review of Systematic Evidence. Nutr. Metab. 2024, 21, 15. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Chen, Q.; Lu, J.; Rapposelli, S.; Pi, R. A Review on the Hybrids of Hydroxycinnamic Acid as Multi-Target-Directed Ligands against Alzheimer’s Disease. Bioorganic Med. Chem. 2018, 26, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Muronetz, V.I.; Barinova, K.; Kudryavtseva, S.; Medvedeva, M.; Melnikova, A.; Sevostyanova, I.; Semenyuk, P.; Stroylova, Y.; Sova, M. Natural and Synthetic Derivatives of Hydroxycinnamic Acid Modulating the Pathological Transformation of Amyloidogenic Proteins. Molecules 2020, 25, 4647. [Google Scholar] [CrossRef]
- Tan, C.; Dadmohammadi, Y.; Lee, M.C.; Abbaspourrad, A. Combination of Copigmentation and Encapsulation Strategies for the Synergistic Stabilization of Anthocyanins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3164–3191. [Google Scholar] [CrossRef]
- Sharma, S.; Dua, A.; Malik, A. Polyaspartic Acid Based Superabsorbent Polymers. Eur. Polym. J. 2014, 59, 363–376. [Google Scholar] [CrossRef]
- Tudorachi, N.; Chiriac, A.P. TGA/FTIR/MS Study on Thermal Decomposition of Poly(Succinimide) and Sodium Poly(Aspartate). Polym. Test. 2011, 30, 397–407. [Google Scholar] [CrossRef]
- Zhao, L.; Pan, F.; Mehmood, A.; Zhang, H.; Ur Rehman, A.; Li, J.; Hao, S.; Wang, C. Improved Color Stability of Anthocyanins in the Presence of Ascorbic Acid with the Combination of Rosmarinic Acid and Xanthan Gum. Food Chem. 2021, 351, 129317. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Brás, N.F.; Oliveira, J.; Mateus, N.; De Freitas, V. Impact of a Pectic Polysaccharide on Oenin Copigmentation Mechanism. Food Chem. 2016, 209, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and Characteristics of Alginate and Anthocyanin Complexes. Int. J. Biol. Macromol. 2020, 164, 726–734. [Google Scholar] [CrossRef]
- Jeong, D.; Na, K. Chondroitin Sulfate Based Nanocomplex for Enhancing the Stability and Activity of Anthocyanin. Carbohydr. Polym. 2012, 90, 507–515. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, X.; Yang, T.; Wang, Z.; Chen, Q.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Enhancing the Storage Stability of Mulberry Anthocyanin Extract through Ternary Complex with Whey Protein Isolate and Ferulic Acid at Neutral PH: Investigation of Binding Mechanisms. Food Hydrocoll. 2024, 149, 109560. [Google Scholar] [CrossRef]
- Tan, C.; Selig, M.J.; Abbaspourrad, A. Anthocyanin Stabilization by Chitosan-Chondroitin Sulfate Polyelectrolyte Complexation Integrating Catechin Co-Pigmentation. Carbohydr. Polym. 2018, 181, 124–131. [Google Scholar] [CrossRef]
- Terefe, N.S.; Netzel, G.A.; Netzel, M.E. Copigmentation with Sinapic Acid Improves the Stability of Anthocyanins in High-Pressure-Processed Strawberry Purees. J. Chem. 2019, 2019, 3138608. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase Activity of Selected Phenolic Acids and Flavonoids—Interaction Testing in Model Solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and Bound Phenolic Compounds in Different Bolivian Purple Corn (Zea mays L.) Cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Carrera, E.J.; Cejudo-bastante, M.J.; Hurtado, N.; Heredia, F.J.; Zea, L. Revalorization of Colombian Purple Corn Zea mays L. by-Products Using Two-Step Column Chromatography. Food Res. Int. 2023, 169, 112931. [Google Scholar] [CrossRef]
- Sánchez-Ilárduya, M.B.; Sánchez-Fernández, C.; Viloria-Bernal, M.; López-Márquez, D.M.; Berrueta, L.A.; Gallo, B.; Vicente, F. Mass Spectrometry Fragmentation Pattern of Coloured Flavanol-Anthocyanin and Anthocyanin-Flavanol Derivatives in Aged Red Wines of Rioja. Aust. J. Grape Wine Res. 2012, 18, 203–214. [Google Scholar] [CrossRef]
- González-Manzano, S.; Pérez-Alonso, J.J.; Salinas-moreno, Y.; Mateus, N.; Gonza, S.; Silva, A.M.S.; De Freitas, V.; Santos-Buelga, C. Flavanol—Anthocyanin Pigments in Corn: NMR Characterisation and Presence in Different Purple Corn Varieties. J. Food Compos. Anal. 2008, 21, 521–526. [Google Scholar] [CrossRef]
- Zhao, X.; Corrales, M.; Zhang, C.; Hu, X.; Ma, Y.; Tauscher, B. Composition and Thermal Stability of Anthocyanins from Chinese Purple Corn (Zea mays L.). J. Agric. Food Chem. 2008, 56, 10761–10766. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and Antioxidant Activity in Coloured Waxy Corn at Different Maturation Stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, Y.; He, Z.; Zhai, W.; Gong, H.; Yang, Z. Effect of Ferulic Acid on the Formation of Pyranoanthocyanins from Purple Corn (Zea mays L.) Cob in a Model System and Their Effects on Color. Int. J. Food Prop. 2016, 19, 847–858. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akillioǧlu, G.; Gökmen, V.; Vančetović, J. Phenolic Compounds, Carotenoids, Anthocyanins, and Antioxidant Capacity of Colored Maize (Zea mays L.) Kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Optimization of the Extraction of Phenolic Compounds from Purple Corn Cob (Zea mays L.) by Sequential Extraction Using Supercritical Carbon Dioxide, Ethanol and Water as Solvents. J. Supercrit. Fluids 2016, 116, 10–19. [Google Scholar] [CrossRef]
- Pedreschi, R.; Cisneros-Zevallos, L. Phenolic Profiles of Andean Purple Corn (Zea mays L.). Food Chem. 2007, 100, 956–963. [Google Scholar] [CrossRef]
- Chen, L.; Yang, M.; Mou, H.; Kong, Q. Ultrasound-Assisted Extraction and Characterization of Anthocyanins from Purple Corn Bran. J. Food Process. Preserv. 2018, 42, e13377. [Google Scholar] [CrossRef]
- Fernandez-Aulis, F.; Hernandez-Vazquez, L.; Aguilar-Osorio, G.; Arrieta-Baez, D.; Navarro-Ocana, A. Extraction and Identification of Anthocyanins in Corn Cob and Corn Husk from Cacahuacintle Maize. J. Food Sci. 2019, 84, 954–962. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, W. Optimization of Microwave-Assisted Extraction of Anthocyanins from Purple Corn (Zea mays L.) Cob and Identification with HPLC-MS. Innov. Food Sci. Emerg. Technol. 2010, 11, 470–476. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Yang, W.; Mu, B.; Jiao, Y.; Zhou, X.; Zhang, C.; Fan, Y.; Chen, R. Synthesis of Seed-Specific Bidirectional Promoters for Metabolic Engineering of Anthocyanin-Rich Maize. Plant Cell Physiol. 2018, 59, 1942–1955. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.F.; March, R.E.; Longerich, H.P.; Stadey, C.J. A Mass Spectrometric Study of Glucose, Sucrose, and Fructose Using an Inductively Coupled Plasma and Electrospray Ionization. Int. J. Mass Spectrom. 2005, 243, 71–84. [Google Scholar] [CrossRef]
- Sun, J.; Lin, L.Z.; Chen, P. Study of the Mass Spectrometric Behaviors of Anthocyanins in Negative Ionization Mode and Its Applications for Characterization of Anthocyanins and Non-Anthocyanin Polyphenols. Rapid Commun. Mass Spectrom. 2012, 26, 1123–1133. [Google Scholar] [CrossRef]
- Zanatta, C.F.; Cuevas, E.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of Anthocyanins from Camu-Camu (Myrciaria dubia) by HPLC-PDA, HPLC-MS, and NMR. J. Agric. Food Chem. 2005, 53, 9531–9535. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic Acid Derivatives, Ellagitannins, Proanthocyanidins and Other Phenolics, Vitamin C and Antioxidant Capacity of Two Powder Products from Camu-Camu Fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef]
- Bingöl, A.; Türkyılmaz, M.; Özkan, M. Increase in Thermal Stability of Strawberry Anthocyanins with Amino Acid Copigmentation. Food Chem. 2022, 384, 132518. [Google Scholar] [CrossRef]
- Yaranga Chavez, B. Estudio DFT-TD-SCF, Docking y Dinámica Molecular Del Proceso de Copigmentación de Antocianinas, Ácidos Orgánicos y El Resveratrol. El Uso Del Poliaspartato Como Agente Encapsulante; Universidad Nacional Mayor de San Marcos: Lima, Peru, 2024. [Google Scholar]
- Nave, F.; Brás, N.F.; Cruz, L.; Teixeira, N.; Mateus, N.; Ramos, M.J.; Di Meo, F.; Trouillas, P.; Dangles, O.; De Freitas, V. Influence of a Flavan-3-Ol Substituent on the Affinity of Anthocyanins (Pigments) toward Vinylcatechin Dimers and Proanthocyanidins (Copigments). J. Phys. Chem. B 2012, 116, 14089–14099. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Da Silva, P.; Lima, J.C.; Freitas, A.A.; Shimizu, K.; Maçanita, A.L.; Quina, F.H. Charge-Transfer Complexation as a General Phenomenon in the Copigmentation of Anthocyanins. J. Phys. Chem. A 2005, 109, 7329–7338. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Brás, N.F.; Mateus, N.; De Freitas, V. Understanding the Molecular Mechanism of Anthocyanin Binding to Pectin. Langmuir 2014, 30, 8516–8527. [Google Scholar] [CrossRef] [PubMed]
- Buchweitz, M.; Speth, M.; Kammerer, D.R.; Carle, R. Impact of Pectin Type on the Storage Stability of Black Currant (Ribes nigrum L.) Anthocyanins in Pectic Model Solutions. Food Chem. 2013, 139, 1168–1178. [Google Scholar] [CrossRef]
- Cai, X.; Du, X.; Cui, D.; Wang, X.; Yang, Z.; Zhu, G. Improvement of Stability of Blueberry Anthocyanins by Carboxymethyl Starch/Xanthan Gum Combinations Microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Dong, J.; Li, S.; Zhang, J.; Liu, A.; Ren, J. Thermal Degradation of Cyanidin-3-O-Glucoside: Mechanism and Toxicity of Products. Food Chem. 2022, 370, 131018. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Dara, P.K.; Perumcherry Raman, S.; Vijayan, D.K.; Sadasivam, J.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Nanoencapsulation in Low-Molecular-Weight Chitosan Improves in Vivo Antioxidant Potential of Black Carrot Anthocyanin. J. Sci. Food Agric. 2021, 101, 5264–5271. [Google Scholar] [CrossRef]
- Bhushan, B.; Bibwe, B.; Pal, A.; Mahawar, M.K.; Dagla, M.C.; KR, Y.; Jat, B.S.; Kumar, P.; Aggarwal, S.K.; Singh, A.; et al. FTIR Spectra, Antioxidant Capacity and Degradation Kinetics of Maize Anthocyanin Extract under Variable Process Conditions: Anthocyanin Degradation under Storage. Appl. Food Res. 2023, 3, 100282. [Google Scholar] [CrossRef]
- Kim, H.J.; Bin, Y.T.; Karthick, S.N.; Hemalatha, K.V.; Raj, C.J.; Venkatesan, S.; Park, S.; Vijayakumar, G. Natural Dye Extracted from Rhododendron Species Flowers as a Photosensitizer in Dye Sensitized Solar Cell. Int. J. Electrochem. Sci. 2013, 8, 6734–6743. [Google Scholar] [CrossRef]
- Zegarra-Urquia, C.L.; Santiago, J.; Bumgardner, J.D.; Vega-Baudrit, J.; Hernández-Escobar, C.A.; Zaragoza-Contreras, E.A. Synthesis of Nanoparticles of the Chitosan-Poly((α,β)-DL-Aspartic Acid) Polyelectrolite Complex as Hydrophilic Drug Carrier. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 497–506. [Google Scholar] [CrossRef]
- Güder, A.; Korkmaz, H.; Gökce, H.; Alpaslan, Y.B.; Alpaslan, G. Isolation, Characterization, Spectroscopic Properties and Quantum Chemical Computations of an Important Phytoalexin Resveratrol as Antioxidant Component from Vitis labrusca L. and Their Chemical Compositions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 378–395. [Google Scholar] [CrossRef]
- Kalinowska, M.; Świsłocka, R.; Lewandowski, W. The Spectroscopic (FT-IR, FT-Raman and 1H, 13C NMR) and Theoretical Studies of Cinnamic Acid and Alkali Metal Cinnamates. J. Mol. Struct. 2007, 834–836, 572–580. [Google Scholar] [CrossRef]
- Hanai, K.; Kuwae, A.; Takai, T.; Senda, H.; Kunimoto, K.K. Comparative Vibrational and NMR Study of Cis-Cinnamic Acid Polymorphs and Trans-Cinnamic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 513–519. [Google Scholar] [CrossRef]
- Sebastian, S.; Sundaraganesan, N.; Manoharan, S. Molecular Structure, Spectroscopic Studies and First-Order Molecular Hyperpolarizabilities of Ferulic Acid by Density Functional Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 312–323. [Google Scholar] [CrossRef]
- Ijod, G.; Nawawi, N.I.M.; Qoms, M.S.; Rashedi Ismail Fitry, M.; Rahim, M.H.A.; Charalampopoulos, D.; Sulaiman, R.; Adzahan, N.M.; Azman, E.M. Synergistic Effects of Intermolecular Copigmentation and High-Pressure Processing on Stabilizing Mangosteen Pericarp Anthocyanins. Food Chem. 2025, 480, 143888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Q.; Zhou, P.P.; Li, N.N.; Han, S.Y. Copigmentation Evidence of Oenin with Phenolic Compounds: A Comparative Study of Spectrographic, Thermodynamic and Theoretical Data. Food Chem. 2020, 313, 126163. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S.; Dong, L.; Wu, L.; Shen, X. Nanoparticles Based on the Complex of Chitosan and Polyaspartic Acid Sodium Salt: Preparation, Characterization and the Use for 5-Fluorouracil Delivery. Eur. J. Pharm. Biopharm. 2007, 67, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and Biotransformation of Various Dietary Anthocyanins in Vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Aggregation State and PKa Values of (E)-Resveratrol as Determined by Fluorescence Spectroscopy and UV-Visible Absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef]
- Cahyana, Y.; Gordon, M.H. Interaction of Anthocyanins with Human Serum Albumin: Influence of PH and Chemical Structure on Binding. Food Chem. 2013, 141, 2278–2285. [Google Scholar] [CrossRef]
- Menegas, S.; Ferreira, C.L.; Cararo, J.H.; Gava, F.F.; Dal-Pont, G.C.; Gomes, M.L.; Agostini, J.F.; Schuck, P.F.; Scaini, G.; Andersen, M.L.; et al. Resveratrol Protects the Brain against Oxidative Damage in a Dopaminergic Animal Model of Mania. Metab. Brain Dis. 2019, 34, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Bayram, I.; Decker, E.A. Underlying Mechanisms of Synergistic Antioxidant Interactions during Lipid Oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Agarwal, M.; Alam, M.R.; Haider, M.K.; Malik, M.Z.; Kim, D.K. Alzheimer’s Disease: An Overview of Major Hypotheses and Therapeutic Options in Nanotechnology. Nanomaterials 2021, 11, 59. [Google Scholar] [CrossRef]
- Suresh, S.; Begum, R.F.; Singh, A.; Chitra, V. Anthocyanin as a Therapeutic in Alzheimer’s Disease: A Systematic Review of Preclinical Evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef]
- Liu, X.; Baxley, S.; Hebron, M.; Turner, R.S.; Moussa, C. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5044. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hussain, M.H. Multi-Target Drug Design in Alzheimer’s Disease Treatment: Emerging Technologies, Advantages, Challenges, and Limitations. Pharmacol. Res. Perspect. 2025, 13, e70131. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Giusti, M.M. Effects of Extraction Conditions on Improving the Yield and Quality of an Anthocyanin-Rich Purple Corn (Zea mays L.) Color Extract. J. Food Sci. 2007, 72, 363–368. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, A.D.; Reig, M.; Mayor, L.; Ortiz-Climent, M.; Garcia-Castello, E.M. Characterization of Ionic Exchange and Macroporous Resins for Their Application on the Separation and Recovery of Chlorogenic Acid from the Wastewater of Artichoke Blanching. Sustainability 2021, 13, 8928. [Google Scholar] [CrossRef]
- González-Arostegui, L.G.; Cerón, J.J.; Gök, G.; Neselioglu, S.; Erel, O.; Rubio, C.P. Validation of Assays for Measurement of Oxidant Compounds in Saliva of Pigs: Thiobarbituric Acid Reactive Substances (TBARS), Carbonyl, and Reactive Oxygen Species (ROS). Res. Vet. Sci. 2023, 165, 105069. [Google Scholar] [CrossRef] [PubMed]
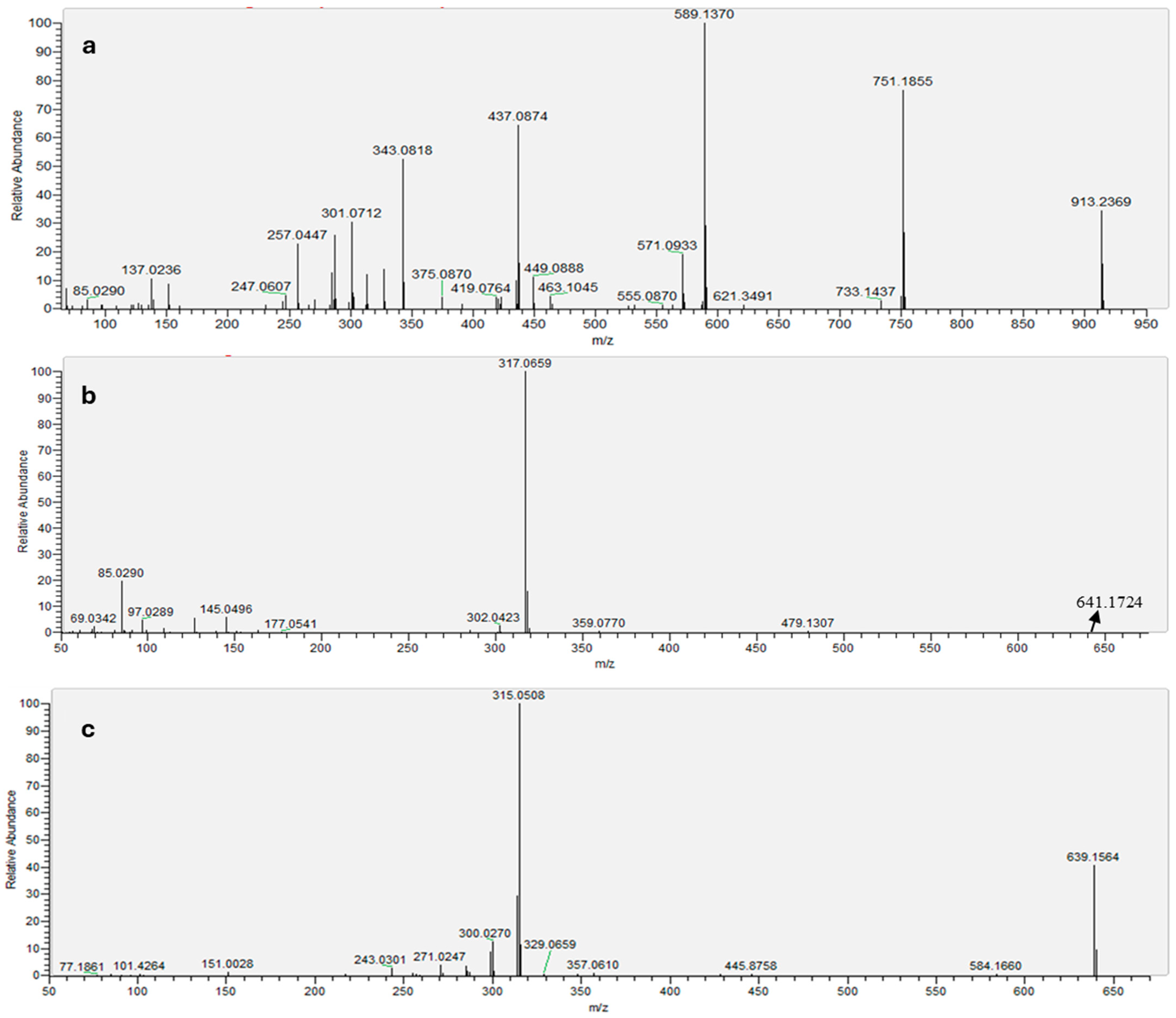
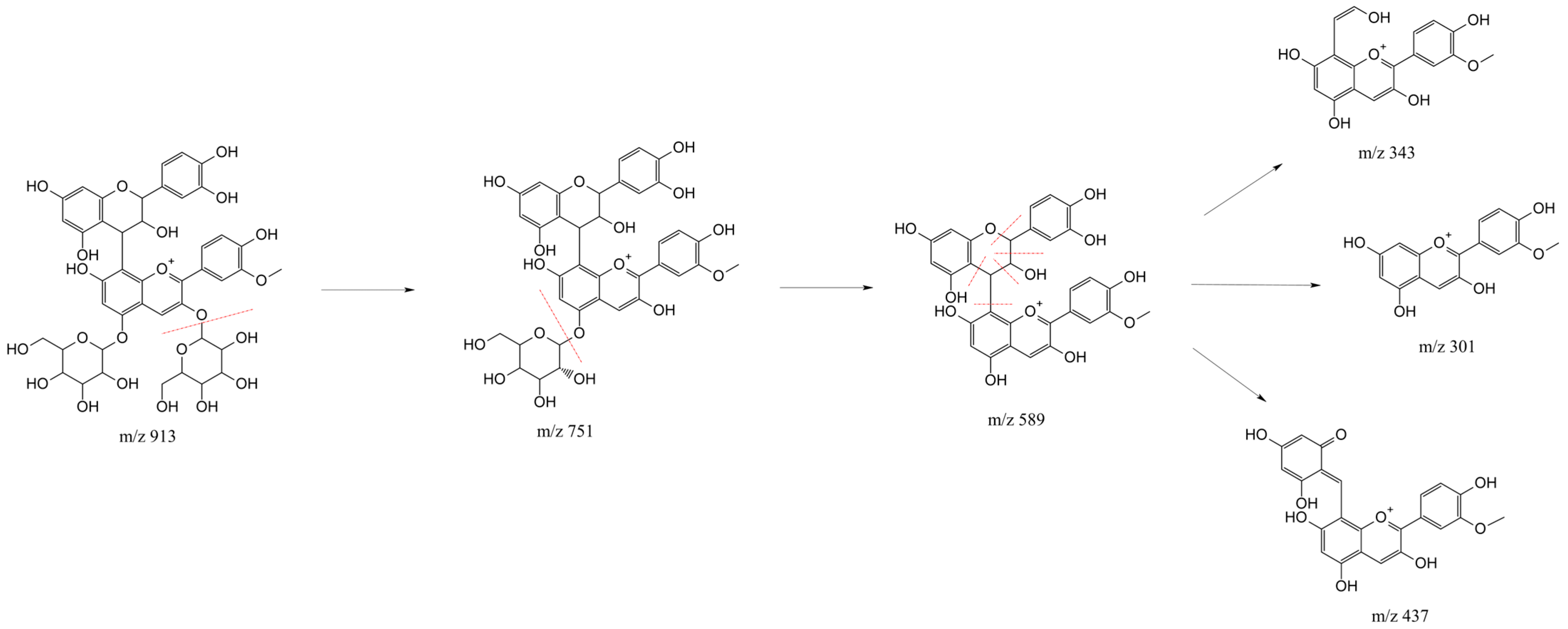
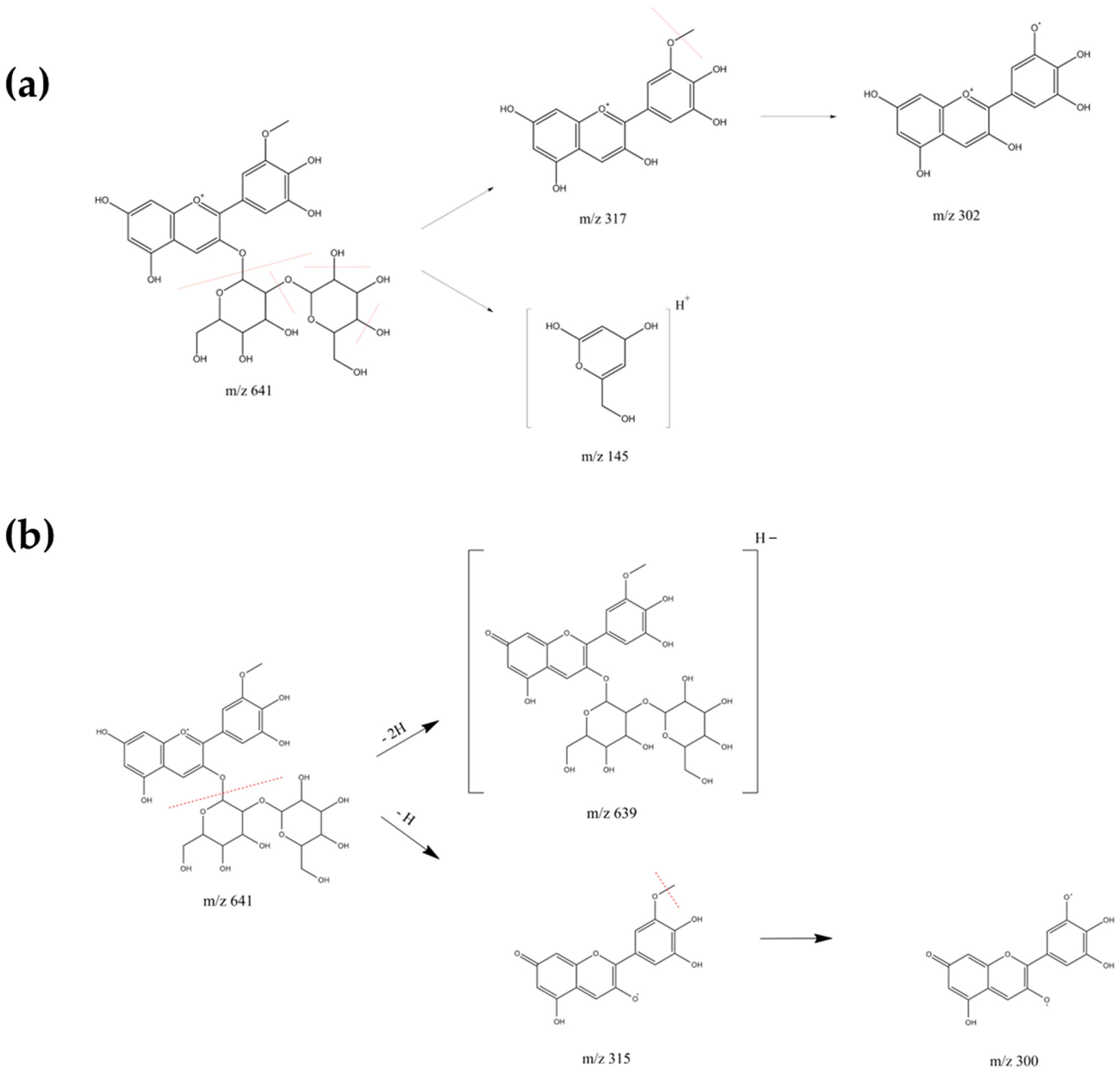
| Anthocyanin | Retention Time (min) | Molecular Ion [M]+ (m/z) | MS Ions (m/z) |
|---|---|---|---|
| Cyanidin-3-malonylglucoside | 3.53 | 535 | 177, 287 |
| Peonidin-3-glucoside | 3.57 | 463 | 112, 286, 301, 383 |
| Cyanidin-3-glucoside | 3.64 | 449 | 167, 244, 287 |
| Catechin-(4,8)-pelargonidin-3,5-diglucoside | 3.67 | 883 | 271, 313, 407, 559, 721 |
| Pelargonidin-3-glucoside | 4.16 | 433 | 68, 130, 235, 271, 307 |
| Catechin-(4,8)-peonidin-3,5-diglucoside | 4.47 | 913 | 301, 343, 437, 589, 751 |
| Cyanidin-3-malonylglucosyl-5-glucoside | 4.52 | 697 | 177, 287, 449, 535 |
| Pelargonidin-3-malonylglucoside | 15.34 | 519 | 187, 271, 433, 475 |
| Peonidin-3-malonylglucoside | 15.76 | 549 | 185, 286, 301, 505 |
| Pelargonidin-3-dimalonylglucoside | 16.62 | 605 | 137, 177, 271, 425 |
| Peonidin-3-dimalonylglucoside | 16.81 | 635 | 137, 177, 301, 436 |
| Petunidin sophoroside | 17.57 | 641 639 * | 145, 302, 317, 479 300 *, 315 * |
| Anthocyanin | Retention Time (min) | Molecular Ion [M]+ (m/z) | MS Ions (m/z) |
|---|---|---|---|
| Cyanidin-3-glucoside | 3.50 | 449 | 68, 153, 229, 287, 375 |
| Delphinidin-3-glucoside | 16.82 | 465 | 85, 153, 267, 303 |
| PCA Samples | %A | %λ | CCA Samples | %A | %λ |
|---|---|---|---|---|---|
| PCA_P8 | 7.67 ± 0.26 d | 0.16 ± 0.05 d | CCA_P16 | 25.09 ± 4.37 a | 0.19 ± 0.00 a |
| PCA_C80 | 18.37 ± 1.79 c | 0.45 ± 0.06 bc | CCA_C80 | 9.73 ± 0.98 bcd | 0.32 ± 0.05 a |
| PCA_CP | 49.24 ± 1.49 a | 0.38 ± 0.05 c | CCA_CP | 7.38 ± 0.51 cd | −1.08 ± 0.15 c |
| PCA_F80 | 20.62 ± 0.10 c | 0.51 ± 0.00 abc | CCA_F80 | 15.70 ± 3.05 b | 0.29 ± 0.00 a |
| PCA_FP | 51.63 ± 0.64 a | 0.60 ± 0.00 a | CCA_FP | 5.43 ± 0.61 d | −0.45 ± 0.06 b |
| PCA_R60 | 31.38 ± 2.66 b | 0.57 ± 0.06 ab | CCA_R80 | 15.25 ± 3.00 b | 0.32 ± 0.05 a |
| PCA_RP | 29.54 ± 1.03 b | 0.57 ± 0.10 ab | CCA_RP | 12.25 ± 0.57 bc | −0.51 ± 0.22 b |
| PCA Samples | Remaining Anthocyanins (%) | CCA Samples | Remaining Anthocyanins (%) |
|---|---|---|---|
| PCA | 24.26 ± 1.08 e | CCA | 45.61 ± 1.56 b |
| PCA_P8 | 30.17 ± 0.57 d | CCA_P16 | 51.55 ± 2.63 a |
| PCA_C80 | 38.82 ± 0.88 bc | CCA_C80 | 54.72 ± 1.08 a |
| PCA_CP | 46.02 ± 0.68 a | CCA_CP | 55.61 ± 1.49 a |
| PCA_F80 | 40.86 ± 1.39 b | CCA_F80 | 56.57 ± 2.78 a |
| PCA_FP | 48.31 ± 1.28 a | CCA_FP | 56.10 ± 2.19 a |
| PCA_R60 | 38.85 ± 1.49 bc | CCA_R80 | 53.92 ± 1.36 a |
| PCA_RP | 37.16 ± 1.31 c | CCA_RP | 55.84 ± 1.02 a |
| Sample | TBARS (nmol/g) | Sample | TBARS (nmol/g) |
|---|---|---|---|
| Control | 106.50 ± 7.70 bc | Control | 106.50 ± 7.70 b |
| SI | 172.64 ± 14.16 a | SI | 172.64 ± 14.16 a |
| PCA Samples | CCA Samples | ||
| P | 76.12 ± 6.13 d | P | 54.27 ± 11.45 de |
| C | 83.77 ± 9.84 cd | C | 98.85 ± 3.94 b |
| F | 88.51 ± 2.91 cd | F | 77.94 ± 7.76 bcd |
| R | 51.14 ± 6.82 ef | R | 44.80 ± 5.14 e |
| PCA | 120.56 ± 7.55 b | CCA | 99.50 ± 13.12 b |
| PCA_P8 | 100.90 ± 5.44 bc | CCA_P16 | 97.68 ± 14.14 b |
| PCA_C80 | 90.25 ± 7.28 cd | CCA_C80 | 77.80 ± 7.31 bcd |
| PCA_CP | 105.48 ± 5.91 bc | CCA_CP | 67.82 ± 11.24 cde |
| PCA_F80 | 115.46 ± 8.73 b | CCA_F80 | 60.61 ± 10.45 cde |
| PCA_FP | 105.41 ± 9.25 bc | CCA_FP | 86.47 ± 8.01 bc |
| PCA_R60 | 48.00 ± 3.72 f | CCA_R80 | 44.43 ± 3.03 e |
| PCA_RP | 73.28 ± 6.78 de | CCA_RP | 40.36 ± 1.46 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera Bless, G.; Muñoz Aguilar, V.; Suarez-Cunza, S.; Pumacahua-Ramos, A.; Santiago Contreras, J. Influence of Copigmentation on the Stability and Oxidative Stress of Anthocyanins from Purple Corn and Camu-Camu. Molecules 2025, 30, 4553. https://doi.org/10.3390/molecules30234553
Nájera Bless G, Muñoz Aguilar V, Suarez-Cunza S, Pumacahua-Ramos A, Santiago Contreras J. Influence of Copigmentation on the Stability and Oxidative Stress of Anthocyanins from Purple Corn and Camu-Camu. Molecules. 2025; 30(23):4553. https://doi.org/10.3390/molecules30234553
Chicago/Turabian StyleNájera Bless, Giulliano, Victoria Muñoz Aguilar, Silvia Suarez-Cunza, Augusto Pumacahua-Ramos, and Julio Santiago Contreras. 2025. "Influence of Copigmentation on the Stability and Oxidative Stress of Anthocyanins from Purple Corn and Camu-Camu" Molecules 30, no. 23: 4553. https://doi.org/10.3390/molecules30234553
APA StyleNájera Bless, G., Muñoz Aguilar, V., Suarez-Cunza, S., Pumacahua-Ramos, A., & Santiago Contreras, J. (2025). Influence of Copigmentation on the Stability and Oxidative Stress of Anthocyanins from Purple Corn and Camu-Camu. Molecules, 30(23), 4553. https://doi.org/10.3390/molecules30234553






