From Extraction to Valorization: Unlocking the Potential of Bark-Derived Extraction Residues for Sustainable Material Development
Abstract
1. Introduction
2. Chemical Composition of Tree Bark: Extraction Potential
3. Utilization Strategies for Tree Bark as a Byproduct Value
3.1. Materials Industry
3.2. Farming and Gardening
3.3. Energy Sector
3.4. Dietary Supplements, Cosmetics, and Pharmaceuticals
3.5. Lignin as a High-Value Component of Bark Residues
3.6. Solvent Recovery and Recycling Considerations
4. Tree Bark Extraction Methods
4.1. Traditional Methods
4.1.1. Hot Water Extraction (HWE)
4.1.2. Solvent Extraction
4.1.3. Alkaline Extraction
4.2. Modern Methods
4.2.1. Organosolv Extraction
4.2.2. Supercritical Carbon Dioxide (ScCO2) Extraction
4.2.3. Enzymatic Hydrolysis
4.2.4. Energy-Assisted Methods—Steam Explosion and Microwave- and Ultrasound-Assisted Extraction
4.2.5. Energy and Scalability Constraints in Green Extraction Methods
4.3. Life Cycle and Techno-Economic Considerations
5. Waste Extraction
5.1. Lignocellulosic Waste
5.2. Resin and Wax Waste
5.3. Wastes with Residual Active Compounds
5.4. Future Perspectives and Research Gaps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pieratti, E.; Paletto, A.; De Meo, I.; Fagarazzi, C.; Giovannini, M.R.M. Assessing the Forest-Wood Chain at Local Level: A Multi-Criteria Decision Analysis (MCDA) Based on the Circular Bioeconomy Principles. Ann. For. Res. 2019, 62, 123–138. [Google Scholar] [CrossRef]
- Le, T.M.; Tran, U.P.N.; Duong, Y.H.P.; Nguyen, K.T.; Tran, V.T.; Le, P.K. Development of a Paddy-Based Biorefinery Approach toward Improvement of Biomass Utilization for More Bioproducts. Chemosphere 2022, 289, 133249. [Google Scholar] [CrossRef]
- Sakib, S.M.N. The Role of Innovation in Driving the Bioeconomy: The Challenges and Opportunities; IGI Global Scientific Publishing: Palmdale, Pennsylvania, 2023. [Google Scholar]
- Yalçın, Ö.Ü.; Sahin, H.T.; Türker, Y.Ş. A Waste Lignocellulosic Material: Tree Bark. In Proceedings of the International Symposium Ecology 2018, Kastamonu, Turkey, 19–23 June 2018. [Google Scholar]
- Wenig, C.; Reppe, F.; Horbelt, N.; Spener, J.; Berendt, F.; Cremer, T.; Frey, M.; Burgert, I.; Eder, M. Adhesives Free Bark Panels: An Alternative Application for a Waste Material. PLoS ONE 2023, 18, e0280721. [Google Scholar] [CrossRef] [PubMed]
- Kwan, I.; Huang, T.; Ek, M.; Seppänen, R.; Skagerlind, P. Bark from Nordic Tree Species—A sustainable Source for Amphiphilic Polymers and Surfactants. Nord. Pulp Pap. Res. J. 2022, 37, 566–575. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Yuan, Z.; Leitch, M.; Xu, C.C. Valorization of Bark for Chemicals and Materials: A Review. Renew. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
- Edward, C. Agriculture, Forestry and Fishery Statistics: 2020 Edition; Publications Office of the European Union: Luxembourg, 2020; p. 234. [Google Scholar]
- Eurostat. Forestry in the EU and the World; Eurostat: Luxembourg, 2011; ISBN 9789279199882. [Google Scholar]
- Thorenz, A.; Lars Wietschel, D.S.; Resource, A.T. Assessment of Agroforestry Residue Potentials for the Bioeconomy in the European Union. J. Clean. Prod. 2018, 176, 348–359. [Google Scholar] [CrossRef]
- REHAP Horizon 2020 programme under grant agreement No. 723670 D1.2 Forecasting of Future Waste Arisings in Agriculture and Forestry. Available online: https://rehap.eu.com/wp-content/uploads/2017/11/rehap-a1-poster_flat.pdf (accessed on 21 October 2025).
- Liobikienė, G.; Miceikienė, A. Contribution of the European Bioeconomy Strategy to the Green Deal Policy: Challenges and Opportunities in Implementing These Policies. Sustainability 2023, 15, 7139. [Google Scholar] [CrossRef]
- Jafari, Y.; Shang, L.; Kuhn, A.; Heckelei, T. The National and Regional Impact of the EU Bioeconomy Strategies on the Agri-Food Sector: Insights from Germany. Ger. J. Agric. Econ. 2023, 72, 73–90. [Google Scholar] [CrossRef]
- Philippidis, G.; M’barek, R.; van Zeist, W.-J. Bioeconomy Transition Pathways—Potential Impacts for the EU Bio-Based Chemicals Sector; [Voies de Transition Bioéconomique—Impacts Potentiels Pour Le Secteur Européen Des Produits Chimiques d’origine Biologique]; [Wege in Die Bioökonomie—Mögliche Auswirkungen auf den EU-Sektor für biobasierte Chemikalien]. EuroChoices 2023, 22, 28–36. [Google Scholar] [CrossRef]
- Ronzon, T.; Sanjuán, A.I. Friends or Foes? A Compatibility Assessment of Bioeconomy-Related Sustainable Development Goals for European Policy Coherence. J. Clean. Prod. 2020, 254, 119832. [Google Scholar] [CrossRef]
- Giuntoli, J.; Barredo, J.I.; Avitabile, V.; Camia, A.; Cazzaniga, N.E.; Grassi, G.; Jasinevičius, G.; Jonsson, R.; Marelli, L.; Robert, N.; et al. The Quest for Sustainable Forest Bioenergy: Win-Win Solutions for Climate and Biodiversity. Renew. Sustain. Energy Rev. 2022, 159, 112180. [Google Scholar] [CrossRef]
- Paleari, S. The EU Policy on Climate Change, Biodiversity and Circular Economy: Moving towards a Nexus Approach. Environ. Sci. Policy 2024, 151, 103603. [Google Scholar] [CrossRef]
- Öhlinger, E.-M.; Lehner, O.M. Aligning Policy and Science: A Teleological Analysis of Biodiversity Accounting and Accountability under the European Green Deal. Sustain. Account. Manag. Policy J. 2025, 16, 62–97. [Google Scholar] [CrossRef]
- Hlásny, T.; Perunová, M.; Modlinger, R.; Blake, M.; Brazaitis, G.; Csóka, G.; de Groot, M.; Duduman, M.-L.; Faccoli, M.; Georgieva, M.; et al. Perspectives: State of National Forest Damage Survey Programmes in Europe and Ways toward Improved Harmonization and Data Sharing. For. Ecol. Manag. 2025, 597, 123111. [Google Scholar] [CrossRef]
- Tamantini, S.; Del Lungo, A.; Romagnoli, M.; Paletto, A.; Keller, M.; Bersier, J.; Zikeli, F. Basic Steps to Promote Biorefinery Value Chains in Forestry in Italy. Sustainability 2021, 13, 11731. [Google Scholar] [CrossRef]
- Ratajczak, E.; Szostak, A.; Bidzińska, G.; Herbeć, M. Potential Resources of Post-Consumer Wood Waste in Poland. J. Mater. Cycles Waste Manag. 2018, 20, 402–413. [Google Scholar] [CrossRef]
- Bauer, R.; Billard, A.; Mothe, F.; Longuetaud, F.; Houballah, M.; Bouvet, A.; Cuny, H.; Colin, A.; Colin, F. Modelling Bark Volume for Six Commercially Important Tree Species in France: Assessment of Models and Application at Regional Scale. Ann. For. Sci. 2021, 78, 104. [Google Scholar] [CrossRef]
- Pásztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The Utilization of Tree Bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Wolski, P. Assessment of Waste Management in Poland. Econ. Environ. 2025, 93, 1030. [Google Scholar] [CrossRef]
- Ptak, M.; Suchorab, N.; Kaczan, W. Do We Have to Waste the Waste? (Poland). In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2020; Volume 2209. [Google Scholar]
- Tsimnadis, K.; Kyriakopoulos, G.L. Investigating the Role of Municipal Waste Treatment within the European Union through a Novel Created Common Sustainability Point System. Recycling 2024, 9, 42. [Google Scholar] [CrossRef]
- Correani, L.; Morganti, P.; Benedetti, I.; Crescenzi, F. Insights on the Social and Economic Factors of the Circular Economy: A Study of the Italian Industrial and Urban Waste Recycling Sector. Waste Manag. 2025, 193, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Cehlár, M.; Taušová, M.; Ivanková, V.; Khouri, S. Municipal Waste Recycling in the EU: A Multi-Method Analysis of Determinants and Country Profiles (2005–2023). Front. Environ. Sci. 2025, 13, 1670365. [Google Scholar] [CrossRef]
- Wunderlich, J.; Armstrong, K.; Buchner, G.A.; Styring, P.; Schomäcker, R. Integration of Techno-Economic and Life Cycle Assessment: Defining and Applying Integration Types for Chemical Technology Development. J. Clean. Prod. 2021, 287, 125021. [Google Scholar] [CrossRef]
- Longo, S.; Cellura, M.; Luu, L.Q.; Nguyen, T.Q.; Rincione, R.; Guarino, F. Circular Economy and Life Cycle Thinking Applied to the Biomass Supply Chain: A Review. Renew. Energy 2024, 220, 119598. [Google Scholar] [CrossRef]
- Samson-Bręk, I.; Owczuk, M.; Matuszewska, A.; Biernat, K. Environmental Assessment of the Life Cycle of Electricity Generation from Biogas in Polish Conditions. Energies 2022, 15, 5601. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Musademba, D. Lifecycle Assessment and Techno-Economic Analysis of Biofuel Production from Lignocellulosic Biomass. In Biofuels Production from Lignocellulosic Materials; Woodhead Publishing: Sawston, CA, USA, 2024; pp. 283–315. [Google Scholar]
- Van den Auwelant, E.; Nimmegeers, P.; Van Passel, S. Life Cycle Assessment and Circular Practices in the Woodworking Sector: A Systematic Review. Clean Technol. Environ. Policy 2024, 27, 1673–1692. [Google Scholar] [CrossRef]
- Świsłowski, P.; Kříž, J.; Rajfur, M. The Use of Bark in Biomonitoring Heavy Metal Pollution of Forest Areas on the Example of Selected Areas in Poland. Ecol. Chem. Eng. 2020, 27, 195–210. [Google Scholar] [CrossRef]
- Martynov, V.V.; Shchemelinina, T.N.; Anchugova, E. Potential of Utilizing Aged Bark-and-Wood Waste through Mycological Degradation as a Biotechnological Process. Biol. Bull. 2024, 52, 221. [Google Scholar] [CrossRef]
- Maksymiuk, G.; Jeżo, A.; Rižikovs, J. Selected Physical and Mechanical Properties of Particleboards Manufactured with Addition of Betula Bark Post-Extraction Residues. Eur. J. Wood Wood Prod. 2024, 82, 1981–1992. [Google Scholar] [CrossRef]
- Jeżo, A.; Wronka, A. Post-Extraction Birch Bark Residues as a Potential Binder in Particleboards. Ann. Warsaw Univ. Life Sci.—SGGW For. Wood Technol. 2022, 118, 35–47. [Google Scholar] [CrossRef]
- Makars, R.; Rizikovs, J.; Godina, D.; Paze, A.; Merijs-Meri, R. Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards. Polymers 2022, 14, 2304. [Google Scholar] [CrossRef]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark Residues Valorization Potential Regarding Antioxidant and Antimicrobial Extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Ryazanova, T.V.; Shchipko, M.L.; Kuznetsova, S.A.; Veprikova, E.V.; Chuprova, N.A. Optimisation of Thermal and Biochemical Methods for Recovery of Wastes from Extraction Processing of Birch Bark. Chem. Sustain. Dev. 2005, 13, 439–447. [Google Scholar]
- Sen, U.; Esteves, B.; Pereira, H. Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review. Energies 2023, 16, 4848. [Google Scholar] [CrossRef]
- Kulikowska, D.; Bernat, K. Waste Willow-Bark from Salicylate Extraction Successfully Reused as an Amendment for Sewage Sludge Composting. Sustainability 2021, 13, 6771. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce Bark as an Industrial Source of Condensed Tannins and Non-Cellulosic Sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Raymond, L.G.; Hill, S.J.; Grigsby, W.J.; Bogun, B.R. A Chemometric Approach for the Segregation of Bark Biomass Based on Tree Height and Geographic Location. J. Wood Chem. Technol. 2020, 40, 361–369. [Google Scholar] [CrossRef]
- Laitinen, J.; Julkunen-Tiitto, R.; Rousi, M.; Heinonen, J.; Tahvanainen, J. Ontogeny and Environment as Determinants of the Secondary Chemistry of Three Species of White Birch. J. Chem. Ecol. 2005, 31, 2243–2262. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.W. Highly Variable Bark-Wood Density Relationships across Tree Species Reflect Tradeoffs in Evolved Tolerances to Environmental Stressors. Trees—Struct. Funct. 2024, 38, 1223–1239. [Google Scholar] [CrossRef]
- Brennan, M.; Fritsch, C.; Cosgun, S.; Dumarcay, S.; Colin, F.; Gérardin, P. Yield and Compositions of Bark Phenolic Extractives from Three Commercially Significant Softwoods Show Intra- and Inter-Specific Variation. Plant Physiol. Biochem. 2020, 155, 346–356. [Google Scholar] [CrossRef]
- Santana, A.N.; Tanajura Mendes, J.O.; de Godoi Pereira, M.; Alvarenga, Y.A.; Boffo, E.F.; da Silva Ramos, F.; El-Bachá, R.S.; Araújo, F.M.; de Jesus Correia Torquato, S.; Lima Cruz Santos, M.H.; et al. Influence of Seasonality and Habitat on Chemical Composition, Cytotoxicity and Antimicrobial Properties of the Libidibia Ferrea. Heliyon 2024, 10, e30632. [Google Scholar] [CrossRef]
- Fromm, J.; Lautner, S. Abiotic Stresses on Secondary Xylem Formation. In Secondary Xylem Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 59–71. [Google Scholar] [CrossRef]
- Monteverde-Calderón, E.G.; Palacios-Ramos, S.C.; Chavesta-Custodio, M. Anatomical Characterization of the Bark of Five Tree Species in a Neotropical Savanna in the Peruvian Amazon. Floresta 2025, 55, 96883. [Google Scholar] [CrossRef]
- Spier, L.; Van Dobben, H.; Van Dort, K. Is Bark PH More Important than Tree Species in Determining the Composition of Nitrophytic or Acidophytic Lichen Floras? Environ. Pollut. 2010, 158, 3607–3611. [Google Scholar] [CrossRef]
- Grootemaat, S.; Wright, I.J.; Van Bodegom, P.M.; Cornelissen, J.H.C.; Shaw, V. Bark Traits, Decomposition and Flammability of Australian Forest Trees. Aust. J. Bot. 2017, 65, 327–338. [Google Scholar] [CrossRef]
- Nie, W.; Liu, Y.; Tan, C.; Wang, Y.; Liu, J.; Zhao, X.; Jiang, Z.; Jia, Z. Characteristics and Factors Driving the Variations in Bark Thickness of Major Woody Plants in China. Ecol. Indic. 2022, 144, 109447. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Lu, F.; Tao, W.; Li, J.; Huang, F.; Guo, Y.; Xiang, W.; Li, X. Variations in Bark Thickness of Common Tree Species in the Karst Seasonal Rainforest of Nonggang and Their Environmental Explanations. Chinese J. Ecol. 2025, 44, 1436–1447. [Google Scholar] [CrossRef]
- Rosell, J.A. Bark Thickness across the Angiosperms: More than Just Fire. New Phytol. 2016, 211, 90–102. [Google Scholar] [CrossRef]
- Schubert, A.T.; Nano, C.E.M.; Clarke, P.J.; Lawes, M.J. Evidence for Bark Thickness as a Fire-Resistance Trait from Desert to Savanna in Fire-Prone Inland Australia. Plant Ecol. 2016, 217, 683–696. [Google Scholar] [CrossRef]
- Anderson, A.B. Silvichemicals from the Forest. Econ. Bot. 1967, 21, 15–30. [Google Scholar] [CrossRef]
- Williams, A.H. Chemical Evidence from the Flavonoids Relevant to the Classification of Malus Species. Bot. J. Linn. Soc. 1982, 84, 31–39. [Google Scholar] [CrossRef]
- Rosell, J.A.; Olson, M.E.; Anfodillo, T.; Martínez-Méndez, N. Exploring the Bark Thickness–Stem Diameter Relationship: Clues from Lianas, Successive Cambia, Monocots and Gymnosperms. New Phytol. 2017, 215, 569–581. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Steiger, P.; Börner, A. Bark Anatomy of Trees and Shrubs in the Temperate Northern Hemisphere; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Quilhó, T.; Pereira, H.; Georg Richter, H. Within-Tree Variation in Phloem Cell Dimensions and Proportions in Eucalyptus Globulus. IAWA J. 2000, 21, 31–40. [Google Scholar] [CrossRef]
- Vázquez-Segovia, K.; Olson, M.E.; Campo, J.; Ángeles, G.; Martínez-Garza, C.; Vetter, S.; Rosell, J.A. Tip-to-Base Bark Cross-Sectional Areas Contribute to Understanding the Drivers of Carbon Allocation to Bark and the Functional Roles of Bark Tissues. New Phytol. 2025, 245, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.; Valenzuela, P.; Gacitúa, W. Eucalyptus Nitens: Nanomechanical Properties of Bark and Wood Fibers. Appl. Phys. A Mater. Sci. Process. 2012, 108, 1007–1014. [Google Scholar] [CrossRef]
- Tanabe, J.; Ishiguri, F.; Tamura, A.; Takashima, Y.; Ohshima, J.; Iizuka, K.; Yokota, S. Within-Tree Radial and among-Family Variations in Wood Density, Microfibril Angle, and Mechanical Properties in Picea Glehnii. Silva Fenn. 2018, 52, 9914. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Pereira, H. Chemical Composition of Lipophilic Extractives from Six Eucalyptus Barks. Wood Sci. Technol. 2018, 52, 1685–1699. [Google Scholar] [CrossRef]
- Masendra; Ashitani, T.; Takahashi, K.; Lukmandaru, G. Lipophilic Extractives of the Inner and Outer Barks from Six Different Pinus Species Grown in Indonesia. J. For. Res. 2018, 29, 1329–1336. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Cavaleiro, J.A.S. Lipophilic Extractives of the Inner and Outer Barks of Eucalyptus Globulus. Holzforschung 2002, 56, 372–379. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Kustas, S. Chemical Analysis of Tree Barks Using ATR-FTIR Spectroscopy and Conventional Techniques. BioResources 2017, 12, 9143–9151. [Google Scholar] [CrossRef]
- Szmechtyk, T.; Małecka, M. Phytochemicals from Bark Extracts and Their Applicability in the Synthesis of Thermosetting Polymers: An Overview. Materials 2024, 17, 2123. [Google Scholar] [CrossRef]
- Del Vecchio, G.; Zhang, L.; Sinan, K.I.; Terzic, M.; Zengin, G.; Bene, K. Different Extraction Methods Shape the Phenolic Signature and Biological Activity of Morinda Lucida Extracts: A Novel Source of Bioactive Compounds Preparing Functional Applications. Food Chem. 2025, 462, 140956. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; Díaz-Reinoso, B.; Domínguez, H. Pressurized Solvent Extraction of Paulownia Bark Phenolics. Molecules 2022, 27, 254. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Domingues, R.M.A.; Oliveira, C.S.D.; Villaverde, J.J.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Secondary Metabolites from Eucalyptus Grandis Wood Cultivated in Portugal, Brazil and South Africa. Ind. Crops Prod. 2017, 95, 357–364. [Google Scholar] [CrossRef]
- Cardoso, S.; Ferreira, J.; Miranda, I.; Pereira, H. Age Variation of Douglas-Fir Bark Chemical Composition. J. Wood Chem. Technol. 2018, 38, 385–396. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Ravber, M.; Volf, I.; Knez, Ž.; Popa, V.I. Isolation of Bioactive Compounds from Spruce Bark Waste Using Sub- and Supercritical Fluids. J. Supercrit. Fluids 2016, 117, 243–251. [Google Scholar] [CrossRef]
- Zekkori, B.; Bentayeb, A.; Ed-Dra, A.; Filali, F.R.; Sbai, A.; Chlif, N.; El Omari, M.; Khallouki, F. Effect of Hydro-Alcohol Solvent Polarity on the Antioxidant, Antibacterial and Anti-Inflammatory Activities of Four Moroccan Lettuce Varieties (Lactuca Sativa l.): A Comparative Study. Carpathian J. Food Sci. Technol. 2021, 13, 133–149. [Google Scholar] [CrossRef]
- Kaiser, C.S.; Römpp, H.; Schmidt, P.C. Pharmaceutical Applications of Supercritical Carbon Dioxide. Pharmazie 2001, 56, 907–926. [Google Scholar]
- Fernández, K.; Kappes, T.; González, N.; Gutiérrez, C. Influence of Tree Height on the Hydrophilic and Lipophilic Composition of Bark Extracts from Eucalyptus Globulus and Eucalyptus Nitens. Holzforschung 2019, 73, 705–713. [Google Scholar] [CrossRef]
- Sapouna, I.; van Erven, G.; Heidling, E.; Lawoko, M.; McKee, L.S. Impact of Extraction Method on the Structure of Lignin from Ball-Milled Hardwood. ACS Sustain. Chem. Eng. 2023, 11, 15533–15543. [Google Scholar] [CrossRef]
- Godina, D.; Makars, R.; Paze, A.; Rizhikovs, J. Analytical Method Cluster Development for Comprehensive Characterisation of Suberinic Acids Derived from Birch Outer Bark. Molecules 2023, 28, 2227. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Jiménez-Moreno, N.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. A Systematic Review of the Potential Uses of Pine Bark in Food Industry and Health Care. Trends Food Sci. Technol. 2019, 88, 558–566. [Google Scholar] [CrossRef]
- Janceva, S.; Andersone, A.; Spulle, U.; Tupciauskas, R.; Papadopoulou, E.; Bikovens, O.; Andzs, M.; Zaharova, N.; Rieksts, G.; Telysheva, G. Eco-Friendly Adhesives Based on the Oligomeric Condensed Tannins-Rich Extract from Alder Bark for Particleboard and Plywood Production. Material 2022, 15, 3894. [Google Scholar] [CrossRef]
- Fedorov, V.S.; Ryazanova, T.V. Bark of Siberian Conifers: Composition, Use, and Processing to Extract Tannin. Forests 2021, 12, 1043. [Google Scholar] [CrossRef]
- Wijeyekoon, S.; Suckling, I.; Fahmy, M.; Hall, P.; Bennett, P. Techno-Economic Analysis of Tannin and Briquette Co-Production from Bark Waste: A Case Study Quantifying Symbiosis Benefits in Biorefinery. Biofuels Bioprod. Biorefining 2021, 15, 1332–1344. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Patankar, S.C.; Chandra, R.P.; Sathitsuksanoh, N.; Saddler, J.N.; Renneckar, S. Valorization of Bark Using Ethanol−Water Organosolv Treatment: Isolation and Characterization of Crude Lignin. ACS Sustain. Chem. Eng. 2020, 8, 4745–4754. [Google Scholar] [CrossRef]
- Grzybek, J.; Sepperer, T.; Petutschnigg, A.; Schnabel, T. Organosolv Lignin from European Tree Bark: Influence of Bark Pretreatment. Materials 2021, 14, 7774. [Google Scholar] [CrossRef]
- Rietzler, B.; Karlsson, M.; Kwan, I.; Lawoko, M.; Ek, M. Fundamental Insights on the Physical and Chemical Properties of Organosolv Lignin from Norway Spruce Bark. Biomacromolecules 2022, 23, 3349–3358. [Google Scholar] [CrossRef]
- Fradinho, D.M.; Neto, C.P.; Evtuguin, D.; Jorge, F.C.; Irle, M.A.; Gil, M.H.; Pedrosa de Jesus, J. Chemical Characterisation of Bark and of Alkaline Bark Extracts from Maritime Pine Grown in Portugal. Ind. Crops Prod. 2002, 16, 23–32. [Google Scholar] [CrossRef]
- Li, D.; Moriana, R.; Ek, M. From Forest Residues to Hydrophobic Nanocomposites with High Oxygen-Barrier Properties. Nord. Pulp Pap. Res. J. 2016, 31, 261–269. [Google Scholar] [CrossRef]
- Pals, M.; Lauberts, M.; Zijlstra, D.S.; Ponomarenko, J.; Arshanitsa, A.; Deuss, P.J. Mild Organosolv Delignification of Residual Aspen Bark after Extractives Isolation as a Step in Biorefinery Processing Schemes. Molecules 2022, 27, 3185. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Garcia, H.; Sousa, A.F.; Freire, C.S.R.; Silvestre, A.J.D.; Rebelo, L.P.N.; Silva Pereira, C. Isolation of Suberin from Birch Outer Bark and Cork Using Ionic Liquids: A New Source of Macromonomers. Ind. Crops Prod. 2013, 44, 520–527. [Google Scholar] [CrossRef]
- Dönmez, İ.E.; Önem, E. Chemical Composition and in Vitro Antibacterial Activity of Bark Extractives and Suberin Monomers from Pinus Brutia and Pinus Nigra. Eur. J. Wood Wood Prod. 2024, 82, 231–240. [Google Scholar] [CrossRef]
- Cho, S.H.; Yoon, B.; Lee, S.K.; Do Nam, J.; Suhr, J. Natural Cork Suberin-Originated Ecofriendly Biopolyester Syntactic Foam. ACS Sustain. Chem. Eng. 2022, 10, 7508–7514. [Google Scholar] [CrossRef]
- Makars, R.; Rizikovs, J.; Paze, A.; Godina, D.; Berzins, R. Birch Outer Bark Characterisation After Extraction and its Potential for Obtaining Suberin Fatty Acids. In Proceedings of the 31st European Biomass Conference and Exhibition, Bologna, Italy, 5–8 June 2023; pp. 1113–1116. [Google Scholar] [CrossRef]
- Tahoun, M.; Gee, C.T.; McCoy, V.E.; Stoneman, M.; Raicu, V.; Engeser, M.; Müller, C.E. Suberin, the Hallmark Constituent of Bark, Identified in a 45-Million-Year-Old Monkeyhair Tree from Geiseltal, Germany. Sci. Rep. 2024, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Ankita, N.; Rashmi, K. Suberin—A Potential Source of Renewable Chemicals for Industrial Applications. Res. J. Biotechnol. 2022, 17, 182–191. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The Biopolyester at the Frontier of Plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef]
- Križková, L.; Lopes, M.H.; Polónyi, J.; Belicová, A.; Dobias, J.; Ebringer, L. Antimutagenicity of a Suberin Extract from Quercus Suber Cork. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 1999, 446, 225–230. [Google Scholar] [CrossRef]
- Lopes, M.H.; Gil, A.M.; Silvestre, A.J.D.; Neto, C.P. Composition of Suberin Extracted upon Gradual Alkaline Methanolysis of Quercus Suber L. Cork. J. Agric. Food Chem. 2000, 48, 383–391. [Google Scholar] [CrossRef]
- Sut, S.; Maccari, E.; Zengin, G.; Ferrarese, I.; Loschi, F.; Faggian, M.; Paolo, B.; De Zordi, N.; Dall’Acqua, S. “Smart Extraction Chain” with Green Solvents: Extraction of Bioactive Compounds from Picea Abies Bark Waste for Pharmaceutical, Nutraceutical and Cosmetic Uses. Molecules 2022, 27, 6719. [Google Scholar] [CrossRef]
- Pals, M.; Lauberte, L.; Arshanitsa, A.; Vevere, L.; Jurkjane, V.; Telysheva, G. Organosolv Delignification of Residual Plantation Willow Bark after Extractive Removal. In Proceedings of the Annual 26th International Scientific Conference Proceedings 2020, Online, 7–12 December 2020. [Google Scholar] [CrossRef]
- Shen, J. Extraction Method for Chinese Parasol Tree Bark Tannin. CN Patent CN103768108A, 7 May 2014. Available online: https://patents.google.com/patent/CN103768108A/en (accessed on 21 October 2025).
- Demets, O.V.; Kassenov, A.T.T.R.Z.; Aliyeva, M.R. Methods of Betulin Extraction from Birch Bark. Molecules 2022, 27, 3621. [Google Scholar] [CrossRef]
- Borah, A.; Selvaraj, S.; Holla, S.R.; De, S. Extraction and Characterization of Total Phenolic and Flavonoid Contents from Bark of Swietenia Macrophylla and Their Antimicrobial and Antioxidant Properties. Arab. J. Chem. 2022, 15, 104370. [Google Scholar] [CrossRef]
- Bautista, G.F.M.; Musl, O.; Easson, M.L.A.E.; Kruse, L.H.; Gordon, H.; Bacher, M.; Sumerskii, I.; Watrelot, A.A.; Bohlmann, J.; Potthast, A.; et al. Strong Association between Proanthocyanidins and Polysaccharides in the Cell Walls of Western Redcedar Bark. Biomacromolecules 2025, 26, 5601–5613. [Google Scholar] [CrossRef]
- Niu, X.; He, Y.; Musl, O.; Bautista, G.F.M.; Xie, Q.; Wu, Y.; Guo, J.; Rojas, O.J. Bark Extractives as Sources of Carbon-Efficient Functional Precursors and Materials. Innov. Mater. 2024, 2, 100074. [Google Scholar] [CrossRef]
- Neiva, D.M.; Ek, M.; Sels, B.F.; Samec, J.S.M. Toward Sustainable Upgrading of Bark. Chem Catal. 2024, 4, 101022. [Google Scholar] [CrossRef]
- Bento, A.; Escórcio, R.; Tomé, A.S.; Robertson, M.; Gaugler, E.C.; Malthus, S.J.; Raymond, L.G.; Hill, S.J.; Silva Pereira, C. Pinus Radiata Bark Sequentially Processed Using scCO2 and An Ionic Liquid Catalyst Yields Plentiful Resin Acids and Alkanoic Acids Enriched Suberin. Ind. Crops Prod. 2022, 185, 115172. [Google Scholar] [CrossRef]
- Normand, M.L.; Krogell, J.; Willför, S.; Holmbom, B.; Ek, M. Hot-Water Extraction and Characterization of Hemicelluloses and Pectins from Bark of Norway Spruce (Picea Abies). In Proceedings of the 11th European Workshop on Lignocellulosics and Pulp, Hamburg, Germany, 16–19 August 2010; pp. 243–246. [Google Scholar]
- Normand, M.L.; Edlund, U.; Holmbom, B.; Ek, M. Hot-Water Extraction and Characterization of Spruce Bark Non-Cellulosic Polysaccharides. Nord. Pulp Pap. Res. J. 2012, 27, 18. [Google Scholar] [CrossRef]
- Gong, C.; Bujanovic, B.M. Impact of Hot-Water Extraction on Acetone-Water Oxygen Delignification of Paulownia Spp. and Lignin Recovery. Energies 2014, 7, 857–873. [Google Scholar] [CrossRef]
- Therasme, O.; Volk, T.A.; Cabrera, A.M.; Eisenbies, M.H.; Amidon, T.E. Hot Water Extraction Improves the Characteristics of Willow and Sugar Maple Biomass with Different Amount of Bark. Front. Energy Res. 2018, 6, 93. [Google Scholar] [CrossRef]
- Geoffroy, T.R.; Fortin, Y.; Stevanovic, T. Hot-Water Extraction Optimization of Sugar Maple (Acer Saccharum Marsh.) and Red Maple (Acer rubrum L.) Bark Applying Principal Component Analysis. J. Wood Chem. Technol. 2017, 37, 261–272. [Google Scholar] [CrossRef]
- Amândio, M.S.T.; Rocha, J.M.S.; Xavier, A.M.R.B. Enzymatic Hydrolysis Strategies for Cellulosic Sugars Production to Obtain Bioethanol from Eucalyptus globulus Bark. Fermentation 2023, 9, 241. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Mazlina, M.K.S.; Radiah, A.B.D.; Syafiie, S. Microwave-Assisted Dilute Acid Pretreatment and Enzymatic Hydrolysis of Sago Palm Bark. BioResources 2016, 11, 5687. [Google Scholar] [CrossRef]
- Borrero-Lopez, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications of Lignocellulose-Based Polyurethanes: A Review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Arshadi, M.; Eriksson, D.; Isacsson, P.; Bergsten, U. Bark Assortments of Scots Pine and Norway Spruce as Industrial Feedstock for Tall Oil Production. Forests 2018, 9, 332. [Google Scholar] [CrossRef]
- Moodley, R.S.; Andrew, J.E.; Sithole, B.B. Beneficiation Opportunities for Bark from South African Grown Eucalyptus grandis and Pinus patula. J. Sci. Ind. Res. 2018, 77, 176–180. [Google Scholar]
- Strizincova, P.; Haz, A.; Sládková, A.; Šurina, I. Total Phenolic Content in Spruce Bark. J. Hyg. Eng. Des. 2018, 25, 69–74. [Google Scholar]
- Ajao, O.; Benali, M.; Faye, A.; Li, H.; Maillard, D.; Ton-That, M.T. Multi-Product Biorefinery System for Wood-Barks Valorization into Tannins Extracts, Lignin-Based Polyurethane Foam and Cellulose-Based Composites: Techno-Economic Evaluation. Ind. Crops Prod. 2021, 167, 113435. [Google Scholar] [CrossRef]
- Sinan, K.I.; Dall’acqua, S.; Ferrarese, I.; Mollica, A.; Stefanucci, A.; Glamočlija, J.; Sokovic, M.; Nenadić, M.; Aktumsek, A.; Zengin, G. Lc-Ms Based Analysis and Biological Properties of Pseudocedrela kotschyi (Schweinf.) Harms Extracts: A Valuable Source of Antioxidant, Antifungal, and Antibacterial Compounds. Antioxidants 2021, 10, 1570. [Google Scholar] [CrossRef]
- Shtein, I.; Gričar, J.; Lev-Yadun, S.; Oskolski, A.; Pace, M.R.; Rosell, J.A.; Crivellaro, A. Priorities for Bark Anatomical Research: Study Venues and Open Questions. Plants 2023, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Rencoret, J.; Chemetova, C.; Gominho, J.; Gutiérrez, A.; Del Río, J.C.; Pereira, H. Lignin Composition and Structure Differs between Xylem, Phloem and Phellem in Quercus Suber L. Front. Plant Sci. 2016, 7, 1612. [Google Scholar] [CrossRef]
- Jové, P.; Olivella, M.À.; Cano, L. Study of the Variability in Chemical Composition of Bark Layers of Quercus Suber L. from Different Production Areas. BioResources 2011, 6, 1806–1815. [Google Scholar] [CrossRef]
- Ogunwusi, A. Potentials of Industrial Utilization of Bark. J. Nat. Sci. Res. 2013, 3, e2921. [Google Scholar]
- Wang, X.-M.; Feng, H.F.M.; Zhang, Y.; Yan, N. Manufacturing Medium-Density Particleboards from Wood–Bark Mixture and Different Adhesive Systems. For. Prod. J. 2015, 65, 20–25. [Google Scholar] [CrossRef]
- Brito, E.O.; Batista, D.C.; Vidaurre, G.B.; Sampaio, L. de C. Chapas de Madeira Aglomerada de Uma Camada de Pinus Elliottii Engelm. Com a Adição Das Cascas de Eucalyptus Pellita F. Muell. Cerne 2005, 11, 369–375. [Google Scholar]
- Trianoski, R.; Iwakiri, S.; de Matos, J.L.M.; Prata, J.G. Propriedades Físicas e Mecânicas de Painéis de Madeira Aglomerada de Acrocarpus Fraxinifolius, Compostos Com Diferentes Percentuais de Casca. Ciência Florest. 2013, 23, 761–769. [Google Scholar] [CrossRef]
- Kain, G.; Barbu, M.-C.; Teischinger, A.; Musso, M.; Petutschnigg, A. Substantial Bark Use as Insulation Material. For. Prod. J. 2012, 62, 480–487. [Google Scholar] [CrossRef]
- Tudor, E.M.; Dettendorfer, A.; Kain, G.; Barbu, M.C.; Réh, R.; Krišt’ák, L. Sound-Absorption Coefficient of Bark-Based Insulation Panels. Polymers 2020, 12, 1012. [Google Scholar] [CrossRef]
- Busquets Ferrer, M.; Solt-Rindler, A.; Vay, O.; Hansmann, C.; Gindl-Altmutter, W. Bark Based Porous Materials Obtained with a Simple Mechanical Foaming Procedure. Eur. J. Wood Wood Prod. 2023, 81, 61–71. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Ferrer, V.; Bravo-Arrepol, G.; Reyes-Contreras, P.; Elissetche, J.P.; Santos, J.; Fuentealba, C.; Cabrera-Barjas, G. Pretreated Eucalyptus Globulus and Pinus Radiata Barks: Potential Substrates to Improve Seed Germination for a Sustainable Horticulture. Forests 2023, 14, 991. [Google Scholar] [CrossRef]
- Chong, C. Experiences with Wastes and Composts in Nursery Substrates Calvin. HortTechnology 2005, 15, 739. [Google Scholar] [CrossRef]
- Kurki, P.; Nurmi, E.; Haikarainen, I.; Savikurki, R.; Kaseva, J.; Hakala, K.; Valkama, E. Crushed Bark as a Novel Soil Conditioner for Organic Plant Production. Ital. J. Agron. 2021, 16, 1781. [Google Scholar] [CrossRef]
- Surywanshi, G.D.; Leion, H.; Soleimanisalim, A.H. Energy, Exergy, Economic and Exergoeconomic Analyses of Chemical Looping Combustion Plant Using Waste Bark for District Heat and Power Generation with Negative Emissions. Energy Technol. 2023, 12, 2300577. [Google Scholar] [CrossRef]
- Sahupala, P.; Sumbung, F.H. Analysis of Combustion of a Steam Boiler with Chips and Bark as Fuel. Eur. J. Energy Res. 2023, 3, 1–6. [Google Scholar] [CrossRef]
- Wilk, V.; Kitzler, H.; Koppatz, S.; Pfeifer, C.; Hofbauer, H. Gasification of Waste Wood and Bark in a Dual Fluidized Bed Steam Gasifier. Biomass Convers. Biorefinery 2011, 1, 91–97. [Google Scholar] [CrossRef]
- Ahlström, J.M.; Alamia, A.; Larsson, A.; Breitholtz, C.; Harvey, S.; Thunman, H. Bark as Feedstock for Dual Fluidized Bed Gasifiers—Operability, Efficiency, and Economics; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Suwinarti, W.; Amirta, R. Yuliansyah Production of High-Calorie Energy Briquettes from Bark Waste, Plastic and Oil. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018. [Google Scholar] [CrossRef]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; Renault, J.-H. Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, X.; Gao, C.; Ni, L.; Wang, X.; Zhang, W.; Ren, S. Catalytic Hydrogenolysis of Larix Bark Proanthocyanidins in Ionic Liquids Produces UV Blockers with Potential for Use in Cosmetics Meng. RSC Adv. 2021, 11, 30078–30087. [Google Scholar] [CrossRef]
- Potter, C.M.; Jones, D.L. Polyphenolic Profiling of Forestry Waste by UPLC-HDMSE. Processes 2020, 8, 1411. [Google Scholar] [CrossRef]
- Gong, X.; Wang, D.; Zheng, Q.; Liu, L.; Wu, H.; Li, Z.; Hong, H.; Yao, J. Efficient Extraction of Highly Reactive Lignin from Waste Mulberry Branches. Biomass Convers. Biorefinery 2025, 15, 8871–8880. [Google Scholar] [CrossRef]
- Yargic, A.S. Ordered Reticulated-Structured Carbon Foams via Surfactant-Addition into the Polymerization Medium of Bio-Based Polyols. Chem. Eng. Process.—Process Intensif. 2020, 158, 108204. [Google Scholar] [CrossRef]
- Chemetova, C.; Ribeiro, H.; Fabião, A.; Gominho, J. Towards Sustainable Valorisation of Acacia Melanoxylon Biomass: Characterization of Mature and Juvenile Plant Tissues. Environ. Res. 2020, 191, 110090. [Google Scholar] [CrossRef]
- Chen, H.; Chauhan, P.; Yan, N. “Barking” up the Right Tree: Biorefinery from Waste Stream to Cyclic Carbonate with Immobilization of CO2 for Non-Isocyanate Polyurethanes. Green Chem. 2020, 22, 6874–6888. [Google Scholar] [CrossRef]
- Alexandre, S.A.; Granjeiro, P.A.; Silva, J.A.; Gonçalves, D.B. Renewable and Sustainable Biorefinery: A Patent Review. Recent Pat. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Yemele, M.C.N.; Blanchet, P.; Cloutier, A.; Koubaa, A. Effects of Bark Content and Particle Geometry on the Physical and Mechanical Properties of Particleboard Made from Black Spruce and Trembling Aspen Bark. For. Prod. J. 2008, 58, 48–56. [Google Scholar]
- Pedieu, R.; Riedl, B.; Pichette, A. Physical and Mechanical Properties of Panel Based on Outer Bark Particles of White Birch: Mixed Panels with Wood Particles versus Wood Fibres Propiedades Físicas y Mecánicas de Paneles a Base de Partículas de Corteza Externa de Abeto Blanco: Mezcla de Pan. Cienc. Y Tecnol. 2008, 10, 195–206. [Google Scholar] [CrossRef]
- Ngueho Yemele, M.C. Développement de Panneaux de Particules à Base d’Écorce d’Épinette Noire et de Peuplier Faux-Tremble. Ph.D. Thesis, Université Laval, Québec City, QC, Canada, 2008. [Google Scholar]
- Sufra, R.; Latifah, L.; Susilo, N.A.; Adriansyah, E.; Wati, L.A.; Yulia, A.; Syaiful, M.; Viareco, H.; Marhadi, M.; Ghony, M.A.; et al. Pemanfaatan Sisa Kulit Kayu Sebagai Karbon Aktif Dalam Pengolahan Air Lindi Industri Pulp and Paper. J. Civronlit Unbari 2023, 8, 17. [Google Scholar] [CrossRef]
- Deac, T.; Roş, V.; Mariaşiu, F.; Borza, G. Possibilities of Efficient Use of Wood Waste from Silviculture and Wood Industry Posibilităţi de Utilizare Eficientă a Deşeurilor Lemnoase Din Silvicultură Şi Industria de Prelucrare a Lemnului. Res. J. Agric. Sci. 2009, 41, 403–408. [Google Scholar]
- Souza, B.R.; de Moraes, M.D.; Braboza, F.S.; Coneglian, A.; Sette, C.R., Jr. The Presence of Bark in Acacia Mangium Wood Improves Its Energetic Potential. Floresta 2019, 51, 54–60. [Google Scholar] [CrossRef]
- Silva, M.D.; Moreira, R.P.L.; Rosa, A.P.; Borges, A.C. Optimization of Microwave-Assisted Extraction of Tannins and Phenolic Compounds Obtained from the Pequi Tree (Caryocar brasiliense). Chem. Eng. Process.—Process Intensif. 2025, 217, 110462. [Google Scholar] [CrossRef]
- Islam, M.K.; Ratthiwal, J.; Kongparakul, S.; Samart, C. Advances in Oxidative Fractionation and Depolymerization for Sustainable Lignin Valorization. Mol. Catal. 2025, 586, 115436. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. Supercritical Fluids and Ultrasound Assisted Extractions Applied to Spruce Bark Conversion. Environ. Eng. Manag. J. 2015, 14, 615–623. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; Díaz-Reinoso, B.; Torres, M.D.; Domínguez, H. Sequential Extraction of Antioxidants from Paulownia Petioles with Sc-CO2 and with Subcritical Water and Formulation of Hydrogels with the Residual Solids. Food Bioprod. Process. 2021, 130, 195–202. [Google Scholar] [CrossRef]
- Abolins, A.; Ivdre, A.; Volkovs, N.; Makars, R.; Vevere, L.; Paze, A.; Godina, D.; Rizikovs, J. Synthesis and Characterization of Bio-Polyols Synthesized from Various Treated Depolymerized Suberin for Rigid Polyurethane Foams. In Proceedings of the European Biomass Conference and Exhibition Proceedings 2023, Bologna, Italy, 5–9 June 2023; pp. 1085–1088. [Google Scholar]
- Ramazanova, L.; Reimund, L.; Lebedeva, D.; Muangmeesri, S.; Jaworski, A.; Samec, J.S.M. Sequential Fractionation of Spruce Bark in a Continuous Flow-through System. ACS Sustain. Chem. Eng. 2024, 12, 13409–13414. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Muhayyidin, A.H.M.; Ghazali, N.A.; Abu Bakar, N.F.; Ibrahim, W.A.; Sauki, A.; Hassan, Z. Tannin Extraction from Bark of Rhizophora Mucronata Using Soxhlet and Boiling Techniques. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 2525–2530. [Google Scholar] [CrossRef]
- Vazirova, L.Z.; Alakbarova, I.F. Investigation of Various Methods of Extracting Lignin from Tree Bark. Azerbaijan J. Chem. News 2024, 6, 45–53. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Labidi, J. Optimization of Different Extraction Methods to Obtaining Bioactive Compounds from Larix decidua Bark. Chem. Eng. Trans. 2018, 70, 1369–1374. [Google Scholar] [CrossRef]
- Vieito, C.; Fernandes, É.; Velho, M.V.; Pires, P. The Effect of Different Solvents on Extraction Yield, Total Phenolic Content and Antioxidant Activity of Extracts from Pine Bark (Pinus pinaster subsp. atlantica). Chem. Eng. Trans. 2018, 64, 127–132. [Google Scholar] [CrossRef]
- Seabra, I.J.; Dias, A.M.A.; Braga, M.E.M.; De Sousa, H.C. High Pressure Solvent Extraction of Maritime Pine Bark: Study of Fractionation, Solvent Flow Rate and Solvent Composition. J. Supercrit. Fluids 2012, 62, 135–148. [Google Scholar] [CrossRef]
- Tarja, T.; Klaus, N.; Stina, G.; Sami, A.; Miikka, R.; Anna, K. Extraction of Valuable Components from Bark. WO2020084196A1, 30 April 2020. [Google Scholar]
- Mun, S.P. Proanthocyanidin-rich Extract from Pinus radiata Bark: Mild-Alkaline Extraction and Characterization. BioResources 2023, 19, 146. [Google Scholar] [CrossRef]
- Lee, M.; Jong, S.H.; Mun, S.P. Conditions for the Extraction of Polyphenols from Radiata Pine (Pinus radiata) Bark for Bio-Foam Preparation. J. Korean Wood Sci. Technol. 2020, 48, 861–868. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; García-Martínez, N.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Quesada-Medina, J. A Simple Fractionation Method and GPC Analysis of Organosolv Extracts Obtained from Lignocellulosic Materials. Biomass Convers. Biorefinery 2020, 11, 1807–1821. [Google Scholar] [CrossRef]
- Manesh, A.; Hemyeri, R.; Mohapatra, S.; Guenther, J.; Zoborowski, E.; Manesh, M.A. System and Method for Extraction of Chemicals from Lignocellulosic Materials. US 9,365,525, 14 June 2016. [Google Scholar]
- Barbini, S.; Sriranganadane, D.; Orozco, S.E.; Armig Kabrelian, K.K.; Rosenau, T.; Potthast, A. Tools for Bark Biorefineries: Studies toward Improved Characterization of Lipophilic Lignocellulosic Extractives by Combining Supercritical Fluid and Gas Chromatography. ACS Sustain. Chem. Eng. 2021, 9, 1323–1332. [Google Scholar] [CrossRef]
- Strižincová, P.; Ház, A.; Burčová, Z.; Feranc, J.; Kreps, F.; Šurina, I.; Jablonský, M. Spruce Bark-A Source of Polyphenolic Compounds: Optimizing the Operating Conditions of Supercritical Carbon Dioxide Extraction. Molecules 2019, 24, 4049. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Manabe, S.; Nakayama, S.; Nakayama, Y.; Shiono, T. Impregnation of Poly(L-Lactide- Ran - δ -Valerolactone) with Essential Bark Oil Using Supercritical Carbon Dioxide. Sci. Rep. 2019, 9, 16326. [Google Scholar] [CrossRef]
- Fadhlina, A.; Islam Sarker, M.Z.; Ahmed, Q.U.; Jaffri, J.M.; Sheikh, H.I.; Ferdosh, S. Enrichment of Antibacterial Compound from the Stem Bark of Stereospermum fimbriatum Using Supercritical Carbon Dioxide Extraction. Sep. Sci. Technol. 2020, 55, 1656–1666. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Attard, T.M.; Chen, S.S.; Tsang, D.C.W.; Hunt, A.J.; Jérôme, F.; Ok, Y.S.; Poon, C.S. Supercritical Carbon Dioxide Extraction of Value-Added Products and Thermochemical Synthesis of Platform Chemicals from Food Waste. ACS Sustain. Chem. Eng. 2019, 7, 2821–2829. [Google Scholar] [CrossRef]
- Perrett, G. Supercritical Carbon Dioxide Extraction of Agricultural and Food Processing Wastes and Byproducts; ACS Publications: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Botto, E.; Reina, L.; Moyna, G.; Menéndez, P.; Rodríguez, P. Insights into the Hydrolysis of Eucalyptus dunnii bark by Xylanolytic Extracts of Pseudozyma sp. Biomass Convers. Biorefinery 2020, 12, 3249–3256. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-Assisted Extraction. In Green Chemistry Series; RSC Publishing: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Aleman, R.S.; Marcia, J.; Duque-Soto, C.; Lozano-Sánchez, J.; Montero-Fernández, I.; Ruano, J.A.; Hoskin, R.T.; Moncada, M. Effect of Microwave and Ultrasound-Assisted Extraction on the Phytochemical and In Vitro Biological Properties of Willow (Salix alba) Bark Aqueous and Ethanolic Extracts. Plants 2023, 12, 2533. [Google Scholar] [CrossRef] [PubMed]
- Pals, M.; Lauberte, L.; Ponomarenko, J.; Lauberts, M.; Arshanitsa, A. Microwave-Assisted Water Extraction of Aspen (Populus tremula). Plants 2022, 11, 1544. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Labidi, J. Simultaneous Microwave-Ultrasound Assisted Extraction of Bioactive Compounds from Bark. Chem. Eng. Process.—Process Intensif. 2020, 156, 108100. [Google Scholar] [CrossRef]
- Bianchi, S.; Koch, G.; Janzon, R.; Mayer, I.; Saake, B.; Pichelin, F. Hot Water Extraction of Norway Spruce (Picea abies [Karst.]) Bark: Analyses of the Influence of Bark Aging and Process Parameters on the Extract Composition. Holzforschung 2016, 70, 619–631. [Google Scholar] [CrossRef]
- Sumerskiya, I.; Pranovicha, A.; Holmboma, B.; Willför, S. Lignin and Other Aromatic Substances Released from Spruce Wood During Pressurized Hot-Water Extraction, Part 1: Extraction, Fractionation and Physico-Chemical Characterization. J. Wood Chem. Technol. 2015, 35, 387–397. [Google Scholar] [CrossRef]
- Koptelova, E.N.; Kutakova, N.A.; Tretjakov, S.I.; Faleva, A.V. Analysis of Extraction Products and Water-Alkaline Hydrolysis of Technical Birch Bark Under the Action of Microwave Emf. Russ. J. Bioorganic Chem. 2022, 49, 1636–1644. [Google Scholar] [CrossRef]
- Stevanovic, T.; Yoya, G.K. Organosolv Process for the Extraction of Highly Pure Lignin and Products Comprising the Same. WO2016197233A1, 9 June 2016. [Google Scholar]
- Kasangana, P.B.; Bhatta, S.; Stevanovic, T. Effect of Pre-Extraction on Composition of Residual Liquor Obtained from Catalytic Organosolv Pulping of Sugar Maple Bark. Sustain. Chem. 2020, 1, 2. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Thomas, M.; Attard, V.L.B.; Hunt, A.J.; Arshadi, M.; Grams, J. Supercritical Extraction of Biomass?A Green and Sustainable Method to Control the Pyrolysis Product Distribution. ACS Sustain. Chem. Eng. 2021, 9, 5278–5287. [Google Scholar] [CrossRef]
- Farooq, S.; Farooq, S.; Rather, S.A.; Ganaie, T.A. Supercritical CO2 Extraction of Natural Products. In Extraction of Natural Products from Agro-industrial Wastes: A Green and Sustainable Approach; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–90. ISBN 9780128233498. [Google Scholar]
- Jahnavi, P.; Indirabanu, S.; Chellappan, R.D.; Mubeen, M.I.B.K.; Bodapati, A.; Dharmamoorthy, G.; Pandiyan, B.; Reddy, K.T.K. Supercritical Fluid Extraction: A Green and Sustainable Approach for the Isolation of High-Value Compounds from Natural Sources. Asian J. Green Chem. 2025, 9, 605–629. [Google Scholar] [CrossRef]
- Nozari, B.; Kander, R. Supercritical CO2 Technology for Biomass Extraction: Review. Ind. Crops Prod. 2025, 233, 121348. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Plaza, M.; Mendiola, J.A.; Ibáñez, E.; Herrero, M. Subcritical Water Extraction and Neoformation of Antioxidants. Water Extr. Bioact. Compd. Plants Drug Dev. 2017, 109–130. [Google Scholar] [CrossRef]
- Hussain, M.; Ashraf, H.T.; Vasudev, V.; Yasin, S.; Jamil, M.I.; Saleem, M.; Aziz, T.; Al Afroz, E.; Feichao, Z.; Huapeng, Z.; et al. Deep Eutectic Solvents in Biomass Pretreatment for Green Approach: A Comprehensive Review. Polym. Bull. 2025, 82, 10513–10552. [Google Scholar] [CrossRef]
- Yiin, C.L.; Lai, Z.Y.; Chin, B.L.F.; Lock, S.S.M.; Cheah, K.W.; Taylor, M.J.; Al-Gailani, A.; Kolosz, B.W.; Chan, Y.H. Green Pathways for Biomass Transformation: A Holistic Evaluation of Deep Eutectic Solvents (DESs) Through Life Cycle and Techno-Economic Assessment. J. Clean. Prod. 2024, 470, 143248. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Esposito Corcione, C. Efficient and Environmentally Friendly Techniques for Extracting Lignin from Lignocellulose Biomass and Subsequent Uses: A Review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- Granatier, M.; Lê, H.Q.; Ma, Y.; Rissanen, M.; Schlapp-Hackl, I.; Diment, D.; Zaykovskaya, A.; Pokki, J.P.; Balakshin, M.; Louhi-Kultanen, M.; et al. Gamma-Valerolactone Biorefinery: Catalyzed Birch Fractionation and Valorization of Pulping Streams with Solvent Recovery. Heliyon 2023, 9, e17423. [Google Scholar] [CrossRef]
- Khaowdang, S.; Suriyachai, N.; Imman, S.; Kreetachat, N.; Chuetor, S.; Wongcharee, S.; Suwannahong, K.; Nukunudompanich, M.; Kreetachat, T. Valorization of Sugarcane Bagasse in Thailand: An Economic Analysis of Ethanol and Co-Product Recovery via Organosolv Fractionation. Sustain. 2025, 17, 7145. [Google Scholar] [CrossRef]
- Yadav, P.; Korpinen, R.; Räty, T.; Korkalo, P.; Räsänen, K.; Tienaho, J.; Saranpää, P. Life Cycle Assessment of Suberin and Betulin Production from Birch Bark. J. Clean. Prod. 2024, 474, 143570. [Google Scholar] [CrossRef]
- Shi, R.; Guest, J.S. BioSTEAM-LCA: An Integrated Modeling Framework for Agile Life Cycle Assessment of Biorefineries Under Uncertainty. ACS Sustain. Chem. Eng. 2020, 8, 18903–18914. [Google Scholar] [CrossRef]
- Vickram, S.; Infant, S.S.; Balamurugan, B.S.; Jayanthi, P.; Sivasubramanian, M. Techno-Economic and Life Cycle Analysis of Biorefineries: Assessing Sustainability and Scalability in the Bioeconomy. Environ. Qual. Manag. 2025, 34, e70077. [Google Scholar] [CrossRef]
- Meghana, M.; Shastri, Y. Sustainable Valorization of Sugar Industry Waste: Status, Opportunities, and Challenges. Bioresour. Technol. 2020, 303, 122929. [Google Scholar] [CrossRef]
- Duarte, H.; Gomes, V.; Aliaño-González, M.J.; Faleiro, L.; Romano, A.; Medronho, B. Optimization of the Extraction of Polyphenols from Pinus Pinaster Residues Using Deep Eutectic Solvents: A Sustainable Approach. Wood Sci. Technol. 2023, 57, 1175–1196. [Google Scholar] [CrossRef]
- Ma, W.; Tang, M.; Li, S.; Ma, Y.; Ling, M.; Sheng, W. The Effect of Hydrogen Bonding Strength in Natural Deep Eutectic Solvents on the Extraction Efficiency of Polyphenols. Microchem. J. 2025, 208, 112379. [Google Scholar] [CrossRef]
- Wen, C.-M.; Ierapetritou, M. Improving Life Cycle Assessment Consistency for Biomass-Derived Processes: A Case Study on Triacetic Acid Lactone Production with CO2 Recycling. Comput. Chem. Eng. 2025, 201, 109244. [Google Scholar] [CrossRef]
- Gaffey, J.; Collins, M.N.; Styles, D. Review of Methodological Decisions in Life Cycle Assessment (LCA) of Biorefinery Systems Across Feedstock Categories. J. Environ. Manag. 2024, 358, 120813. [Google Scholar] [CrossRef]
- Lima, R.S.; de Azevedo Caldeira-Pires, A.; Cardoso, A.N. Uncertainty Analysis in Life Cycle Assessments Applied to Biorefineries Systems: A Critical Review of the Literature. Process Integr. Optim. Sustain. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Tao, L. Machine Learning-Enabled Techno-Economic Uncertainty Analysis of Sustainable Aviation Fuel Production Pathways. Chem. Eng. J. Adv. 2024, 20, 100650. [Google Scholar] [CrossRef]
- Rahimi, M.; Mashhadimoslem, H.; Vo Thanh, H.; Ranjbar, B.; Safarzadeh Khosrowshahi, M.; Rohani, A.; Elkamel, A. Yield Prediction and Optimization of Biomass-Based Products by Multi-Machine Learning Schemes: Neural, Regression and Function-Based Techniques. Energy 2023, 283, 128546. [Google Scholar] [CrossRef]
- Teuber, L.; Osburg, V.S.; Toporowski, W.; Militz, H.; Krause, A. Wood Polymer Composites and Their Contribution to Cascading Utilisation. J. Clean. Prod. 2016, 110, 9–15. [Google Scholar] [CrossRef]
- Chinenye Divine, D.; Hubert, S.; Epelle, E.I.; Ojo, A.U.; Adeleke, A.A.; Ogbaga, C.C.; Akande, O.; Okoye, P.U.; Giwa, A.; Okolie, J.A. Enhancing Biomass Pyrolysis: Predictive Insights from Process Simulation Integrated with Interpretable Machine Learning Models. Fuel 2024, 366, 131346. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Life cycle assessment: Blazing A Trail for Bioresources Management. Energy Convers. Manag. X 2021, 10, 100063. [Google Scholar] [CrossRef]
- Simões, C.L.; Neto, A.B.P.S.; Rodrigues, A.C.; Ferreira, R.; Simoes, R. Environmental Assessment of Tannin Extraction from Bark Residues for Application in Water Treatment. Biomass 2025, 5, 15. [Google Scholar] [CrossRef]
- Pushpendra; Schonhoff, A.; Füchsl, S.C.; Röder, H.; Zapp, P. Prospective Life Cycle Assessment and Upscaling of An Emerging Biorefinery Process: A Case Study on Methyl Ketone. J. Clean. Prod. 2025, 498, 145208. [Google Scholar] [CrossRef]
- John, H. Process for Fractionated Recovery of Lignin and Cellulose from Bark. US 3,817,826, 18 June 1974. [Google Scholar]
- Radawiec, W.; Gołaszewski, J.; Kalisz, B. Solid Tailings After Supercritical CO2 Extraction of Lignocellulosic Biomass as A Source of Quality Biochar for Energetic Use and As Soil Improvement. J. Agric. Eng. 2023, 54, 1344. [Google Scholar] [CrossRef]
- Şen, A.U.; Simões, R.; Yücedağ, C.; Quilhó, T.; Sousa, V.; Miranda, I.; Fernandes, Â.; Pereira, H. Bark-Based Biorefineries: Anatomical and Chemical Characterization of the Bark of Endemic Quercus Vulcanica of Turkey. Wood Sci. Technol. 2024, 58, 333–355. [Google Scholar] [CrossRef]
- Kuila, A.; Sharma, V. Lignocellulosic Biomass Production and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valorization 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Torre, L. Extraction of Lignocellulosic Materials From Waste Products; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Gil, A. Current Insights into Lignocellulose Related Waste Valorization. Chem. Eng. J. Adv. 2021, 8, 100186. [Google Scholar] [CrossRef]
- Rizikovs, J.; Paze, A.; Godina, D.; Makars, R.; Sosins, G.; Abolins, A. Suberinic Acids as Adhesive in Wood Bio-Based Composites and Polymer Constituents. In Proceedings of the European Biomass Conference and Exhibition Proceedings, Bologna, Italy, 5–8 June 2023; pp. 1027–1032. [Google Scholar]
- Al Bulushi, K.; Attard, T.M.; North, M.; Hunt, A.J. Optimisation and Economic Evaluation of the Supercritical Carbon Dioxide Extraction of Waxes from Waste Date Palm (Phoenix dactylifera) Leaves. J. Clean. Prod. 2018, 186, 988–996. [Google Scholar] [CrossRef]
- Rasi, S.; Kilpeläinen, P.; Rasa, K.; Korpinen, R.; Raitanen, J.-E.; Vainio, M.; Kitunen, V.; Pulkkinen, H.; Jyske, T. Cascade Processing of Softwood Bark with Hot Water Extraction, Pyrolysis and Anaerobic Digestion. Bioresour. Technol. 2019, 292, 121893. [Google Scholar] [CrossRef] [PubMed]
- Aspé, E.; Fernández, K. The Effect of Different Extraction Techniques on Extraction Yield, Total Phenolic, and Anti-Radical Capacity of Extracts from Pinus Radiata Bark. Ind. Crops Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Sidorova, Y.S.; Petrov, N.A.; Zorin, S.N.; Mazo, V.K. Innovative Methods for Extracting Biolactive Compounds from Plant Materials. Vopr. Pitan. 2023, 92, 28–37. [Google Scholar] [CrossRef]
- Karnaouri, A.; Lange, H.; Crestini, C.; Rova, U.; Christakopoulos, P. Chemoenzymatic Fractionation and Characterization of Pretreated Birch Outer Bark. ACS Sustain. Chem. Eng. 2016, 4, 5289–5302. [Google Scholar] [CrossRef]
- Barbini, S.; Jaxel, J.; Karlström, K.; Rosenau, T.; Potthast, A. Multistage Fractionation of Pine Bark by Liquid and Supercritical Carbon Dioxide. Bioresour. Technol. 2021, 341, 125862. [Google Scholar] [CrossRef]
- Dou, J.; Sui, M.; Malinen, K.; Pesonen, T.; Isohanni, T.; Vuorinen, T. Spruce Bark Stilbenes as a Nature-Inspired Sun Blocker for Sunscreens. Green Chem. 2022, 24, 2962–2974. [Google Scholar] [CrossRef]
- Vo, T.P.; Tran, T.Q.D.; Phan, T.H.; Huynh, H.D.; Vo, T.T.Y.; Vo, N.M.K.; Ha, M.P.; Le, T.N.; Nguyen, D.Q. Ultrasonic-Assisted and Enzymatic-Assisted Extraction to Recover Tannins, Flavonoids, and Terpenoids from Used Tea Leaves Using Natural Deep Eutectic Solvents. Int. J. Food Sci. Technol. 2023, 58, 5855–5864. [Google Scholar] [CrossRef]
- Brar, N.K.; Grigsby, W.J.; Hill, S.J.; Raymond, L.; Weber, C.C. Understanding the Effects of Ionic Liquids and Antisolvent Addition on the Extraction and Recovery of Pinus Radiata Bark Components. J. Wood Chem. Technol. 2022, 42, 305–317. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Chmelová, D.; Škulcová, D.; Legerská, B.; Horník, M.; Ondrejovič, M. Ultrasonic-Assisted Extraction of Polyphenols and Antioxidants from Picea Abies Bark. J. Biotechnol. 2020, 314–315, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bachtler, S.; Bart, H.J. Increase the Yield of Bioactive Compounds from Elder Bark and Annatto Seeds Using Ultrasound and Microwave Assisted Extraction Technologies. Food Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Moncada, J.; Cardona, C.A.; Higuita, J.C.; Vélez, J.J.; López-Suarez, F.E. Wood Residue (Pinus patula Bark) as an Alternative Feedstock for Producing Ethanol and Furfural in Colombia: Experimental, Techno-Economic and Environmental Assessments. Chem. Eng. Sci. 2016, 140, 309–318. [Google Scholar] [CrossRef]
- Baquero, G.; Sorolla, S.; Casas, C.; Bacardit, A. Life Cycle Assessment of Polyphenolic Extracts Derived from Pine By-Products. Materials 2025, 18, 1000. [Google Scholar] [CrossRef]
- Morandini, M.; Barbu, M.C.; Váňová, R.; Kain, S.; Tippner, J.; Petutschnigg, A.; Kristak, L.; Kain, G.; Sepperer, T.; Schnabel, T. Valorization of Extracted Bark for Particleboard Production: A Life-Cycle Impact Assessment. Polymers 2025, 17, 925. [Google Scholar] [CrossRef]
- Hytönen, E.; Aaltonen, O. Bioethanol from Spruce Bark—A Concept Study of a Biorefinery Process Integrated into a Finnish Pulp Mill. In Proceedings of the CHISA 2008—18th International Congress of Chemical and Process Engineering, Prague, Czech Republic, 24–28 August 2008. [Google Scholar]
- Marzban, N.; Psarianos, M.; Herrmann, C.; Schulz-Nielsen, L.; Olszewska-Widdrat, A.; Arefi, A.; Pecenka, R.; Grundmann, P.; Schlüter, O.K.; Hoffmann, T.; et al. Smart Integrated Biorefineries in Bioeconomy: A Concept toward Zero-Waste, Emission Reduction, and Self-Sufficient Energy Production. Biofuel Res. J. 2025, 12, 2319–2349. [Google Scholar] [CrossRef]
- Sillero, L.; Marcial, M.; Olaizola, I.; Banales, J.M.; Hernández-Ramos, F.; Erdocia, X.; Morales, A. Sustainable Recovery of Bioactive Compounds from Forest Residues Using Microwave-Assisted Extraction. Sep. Purif. Technol. 2025, 379, 134993. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W. Study on the Process of Microwave-Assisted Ionic Liquid Extraction of Alkaloids from Phellodendron amurense Rupr. For. Eng. 2024, 40, 164–171. [Google Scholar] [CrossRef]
- Bridglall, P.A.; Laing, M.D.; Naidoo, N.; Burgdorf, R.J. A Novel Laboratory Method for the Extraction of Black Wattle (Acacia mearnsii De Wild.) Bark Constituents. J. Am. Leather Chem. Assoc. 2025, 120, 26–41. [Google Scholar] [CrossRef]
- Hofmann, T.; Nebehaj, E.; Albert, L. The High-Performance Liquid Chromatography/Multistage Electrospray Mass Spectrometric Investigation and Extraction Optimization Of Beech (Fagus sylvatica L.) Bark Polyphenols. J. Chromatogr. A 2015, 1393, 96–105. [Google Scholar] [CrossRef]
- Rajesh, K.S.; Tamilarasy, R.S.; Vijayakumar, T.; Subramanian, S.; Subrahmanyam, S.V. Impact of Bark on Fiber Line and Recovery Operations. IPPTA Q. J. Indian Pulp Pap. Tech. Assoc. 2009, 21, 129–133. [Google Scholar]
- Berg, A.; Guzman, F. A Novel Process for Biomass Extraction: The basis for a Pine Bark Biorefinery. In Proceedings of the NWBC 2018—Proceedings of the 8th Nordic Wood Biorefinery Conference, Helsinki, Finland, 23–25 October 2018. [Google Scholar]
- Soto-García, M.; Rosales-Castro, M. Effect of Solvent and Solvent-to-Solid Ratio on the Phenolic Extraction and the Antioxidant Capacity of Extracts from Pinus durangensis and Quercus sideroxyla Bark. Maderas Cienc. y Tecnol. 2016, 18, 701–714. [Google Scholar] [CrossRef]
- Bikoro Bi Athomo, A.; Engozogho Anris, S.P.; Safou Tchiama, R.; Leroyer, L.; Pizzi, A.; Charrier, B. Chemical Analysis and Thermal Stability of African Mahogany (Khaya ivorensis A. Chev) Condensed Tannins. Holzforschung 2020, 74, 683–701. [Google Scholar] [CrossRef]
- Spinelli, S.; Costa, C.; Conte, A.; Porta, N.L.; Padalino, L.; Del Nobile, M.A. Bioactive Compounds from Norway Spruce Bark: Comparison among Sustainable Extraction Techniques for Potential Food Applications. Foods 2019, 8, 524. [Google Scholar] [CrossRef] [PubMed]
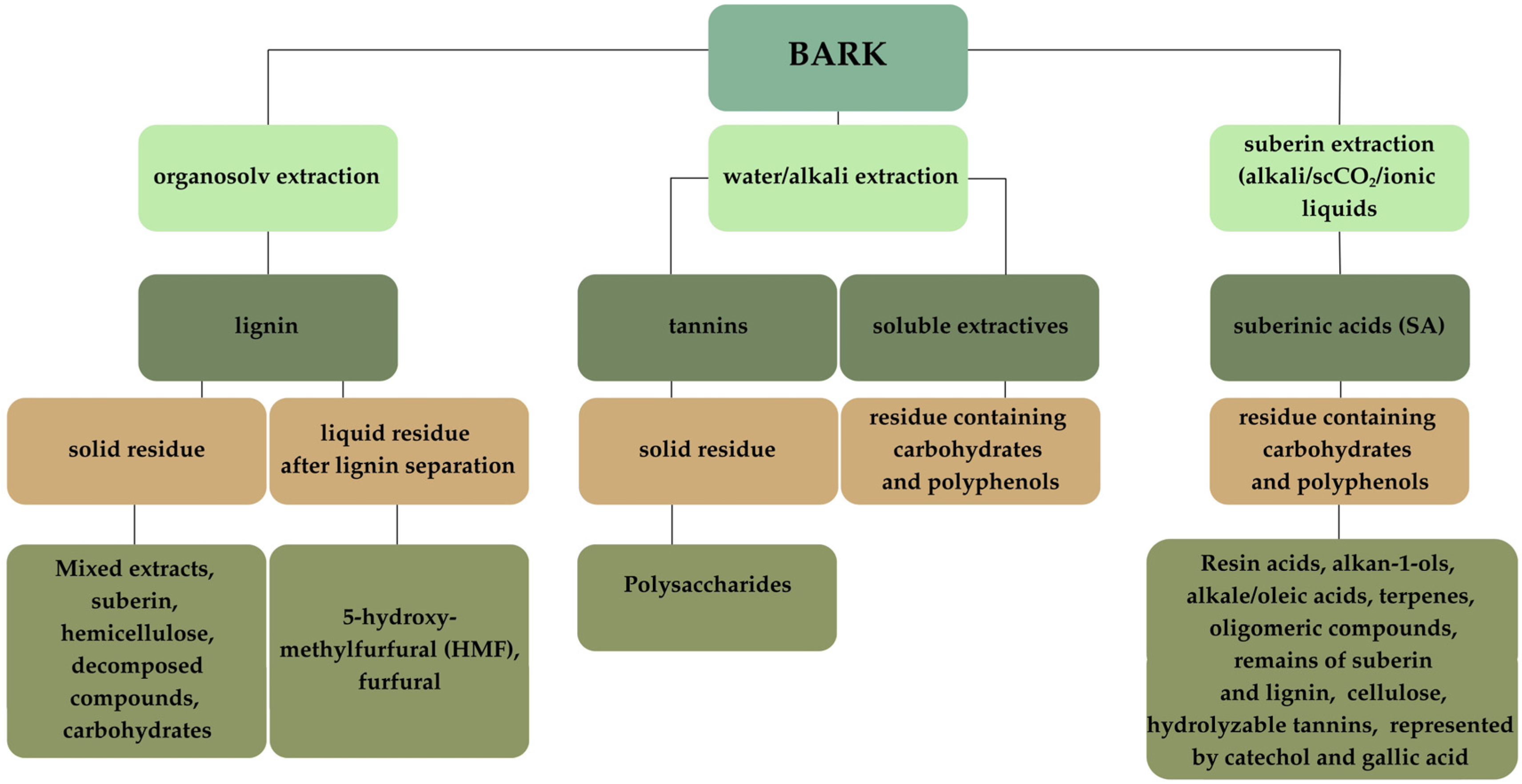
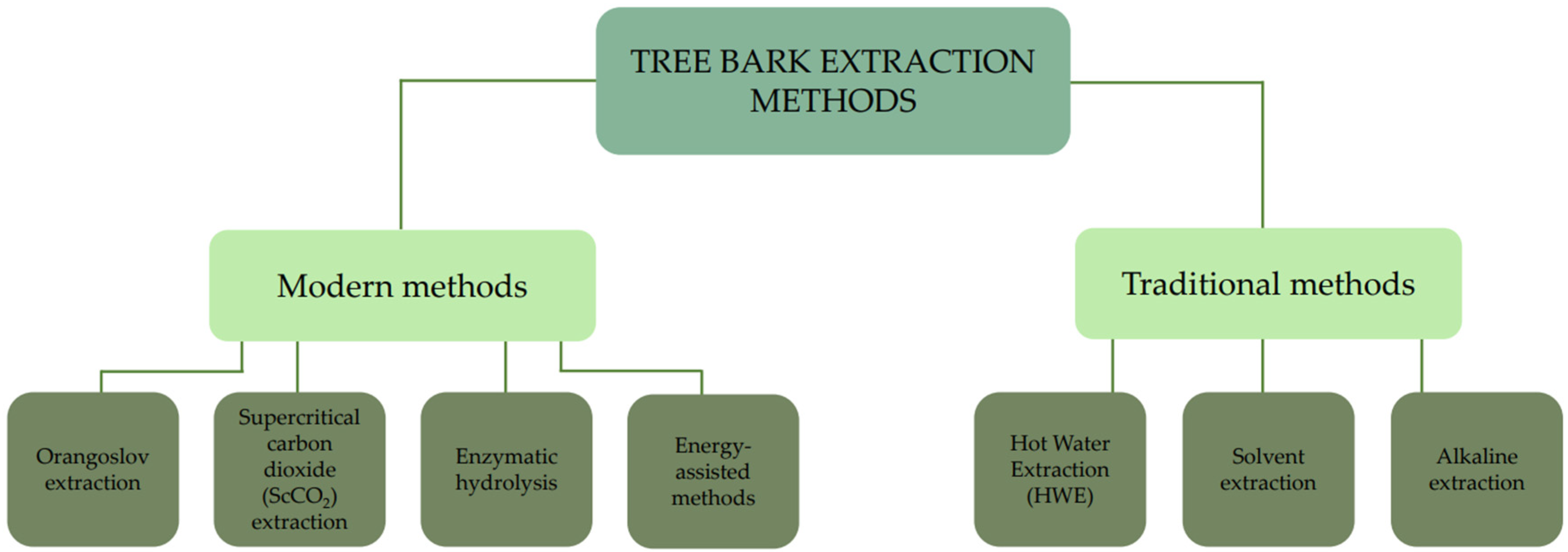
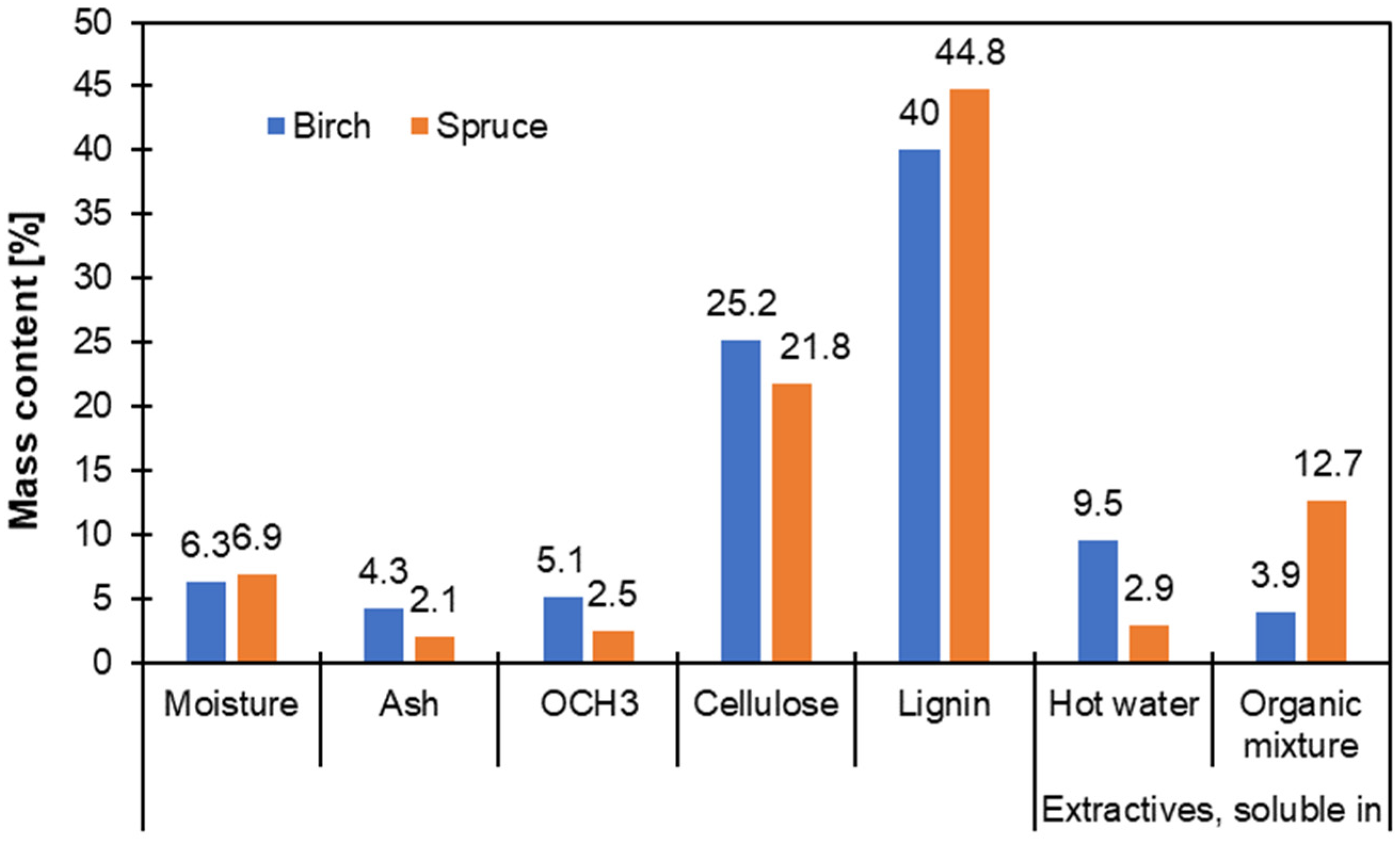
| Aspect | Angiosperms vs. Gymnosperms | Intra- Species Variation | Chemical Composition | Extraction Potential | Environmental/Phylogenetic Influence |
|---|---|---|---|---|---|
| Bark Thickness | Angiosperms: thinner, variable; Gymnosperms: thicker, especially the outer bark [46,53,54] | Varies with stem diameter, fire regime, climate [55,56] | − | Thicker bark often yields more extractives [57,58] | Fire, climate, soil, altitude [59] |
| Microstructure | Vessel presence in angiosperms; tracheids in gymnosperms [60,61] | Cell dimensions, microfibril angle, tissue allocation varies along the stem [62] | − | Microstructure affects mechanical extraction [63] | Genetic and environmental factors [64] |
| Chemical Profile | Species-specific phenolics, triterpenes, sterols [65] | Layer-specific (inner vs. outer bark) [66,67] | Lignin, suberin, extractives [68,69] | Extraction yield depends on compound type and location [70,71] | Age, geography, growth conditions [72,73] |
| Extraction Efficiency | − | − | Varies by method: supercritical CO2, subcritical water, ultrasound [74] | Cosolvent addition, temperature, solvent polarity critical [75,76] | − |
| Knowledge Gaps | Weak phylogenetic signals for thickness; incomplete structure-chemistry correlation [46,77] | High intra-/inter-specific variation [73,77] | Lignin/suberin structure poorly characterized [78,79] | Standardized protocols are lacking [68] | − |
| Group of Compounds | Representative Components | Example Tree Species | Functional Properties | High-Value Applications |
|---|---|---|---|---|
| Polyphenols | Tannins, flavonoids, proanthocyanidins [43,81,82] | Picea abies, Pinus radiata, Betula spp., Abies alba [43,44,45,47] | Antioxidant, antimicrobial, protein-binding, UV-protective properties [47,80] | Natural adhesives, preservatives, nutraceuticals, anti-aging cosmetics, antioxidant extracts [80,81,99] |
| Lignin | Guaiacyl, syringyl and H-type lignin [84,85,86] | Picea abies, Larix spp., Fagus sylvatica, Betula spp. [84,85,86] | Aromaticity, thermal stability, antioxidant properties, polymer network formation [68,86] | Bio-based polymers (epoxy resins, polyurethanes), carbon-fiber precursors, UV-absorbers, composite fillers [84,89,100] |
| Suberin and suberinic fatty acids | ω-hydroxyacids, α,ω-dicarboxylic acids, long-chain fatty alcohols [90,93,96,98] | Betula pendula, cork oak (Quercus suber), Pinus spp. [90,93,94] | Hydrophobicity, chemical resistance, barrier properties, antimicrobial activity [92,96] | Biopolyesters, biodegradable coatings, barrier materials, biosorbents, green composites [90,92,95] |
| Triterpenes | Betulin, betulinic acid, oleanolic acid, ursolic acid [91,101,102] | Betula spp., Pinus spp., Salix spp. [44,45,91] | Antiviral, anti-inflammatory, cytotoxic, antioxidant activity [102,103] | Pharmaceuticals (anticancer, antiviral), dermatological cosmetics, bioactive extracts [102,104] |
| Sterols | β-sitosterol, stigmasterol [91,105] | Pinus nigra, Pinus brutia, Picea spp. [91,101] | Hypocholesterolemic activity, oxidative stability [105,106] | Functional food additives, nutraceuticals, cosmetic stabilizers [105,106,107] |
| Polysaccharides | Cellulose, hemicellulose, pectins [108,109,110] | Pinus spp., Salix spp., Betula spp., Eucalyptus spp. [111,112,113] | Gel-forming capacity, biodegradability, structural reinforcement [108,114] | Hydrogels, bio-polymers, bio-packaging, feedstock for bioethanol, composite additives [108,113,115] |
| Resin and wax components | Resin acids (abietic, dehydroabietic), long-chain alcohols, fatty acids [116,117,118] | Conifer bark (Pinus spp., Picea spp.) [116,118] | Hydrophobicity, adhesive properties, antimicrobial activity [105,106] | Natural coatings, bio-adhesives, surfactants, lubricants, polymer additives [105,107,119] |
| Lipophilic extractives | Phytosterols, long-chain fatty acids and esters [103,116,118] | Pinus spp., Picea spp., Betula spp. [103,118] | Antioxidant, antimicrobial, hydrophobic properties [103,120] | Cosmetics, bio-lubricants, nutraceuticals, green surfactants [104,105,120] |
| Sector | Bark Utilization Strategy | Key Benefits/Properties |
|---|---|---|
| Materials Industry | Production of wood-based boards (e.g., particleboard) | Using natural binders (tannins, polyphenols) to replace synthetic adhesives, resource optimization, sustainable development. [124,125,126,127,128] |
| Production of insulation boards | Better thermal conductivity and heat storage capacity, excellent sound-absorption properties, the possibility of creating panels without using adhesives. [5,97,98,129] | |
| Processing into porous materials | Creating structures with a variety of properties through mechanical foaming. [130] | |
| Farming and Gardening | Use as substrates and soil improvers. | Supports plant growth, improved aeration and drainage, significant increase in wheat yields and quality, potential to increase soil organic carbon content and cation exchange capacity. [23,100,101,104,131,132,133] |
| Energy Sector | Use as biomass for energy production | Contributes to reducing greenhouse gas emissions, high calorific value, and energy efficiency (direct burning). [48,105,108] |
| Chemical Looping Combustion (CLC) | An effective and economical method of utilizing bark from the pulp and paper industry. [134] | |
| Gasification (in two-fluid steam gasifiers) | Allows for the production of synthesis gas (syngas) and subsequent thermal and electrical energy; the efficiency of converting biomass into biomethane can reach up to 65%. [106,107,135,136,137] | |
| Production of high-calorie energy briquettes | Provides an alternative fuel source (when combined with plastics and oil), helps with waste management. [138] | |
| Dietary Supplements, Cosmetics and Pharmaceuticals | Production of cosmetics | Rich in bioactive compounds (polyphenols, flavonoids), strong antioxidant properties, effectively block UV radiation (sunscreens). [99,139,140] |
| Production of dietary supplements | Recognized as potential sources of polyphenols that support health, aligns with the idea of a circular economy. [141] | |
| Bark-derived lignin utilization strategy | Production of lignin-based polymers (e.g., rigid foams) | Bark-derived lignin enables the development of high-performance bio-polymers, renewable adhesive systems and advanced lignin-based materials, benefiting from its elevated phenolic content, reactive hydroxyl groups and suitability for producing bio-polyols [142,143,144,145,146] |
| Manufacturing of bio-based adhesives | ||
| Development of functional materials (e.g., NIPUs) |
| Extraction Method | Main Residue Components | Typical Characteristics/Composition Ranges | Key Remaining Bioactives |
|---|---|---|---|
| Hot Water Extraction (HWE) | Lignocellulosic matrix: cellulose, hemicellulose, lignin [108,109,110] | High ash content (up to 50%); partial hemicellulose solubilization [108,110]; increased structural polysaccharides [113] | Polyphenols and tannins remaining in the insoluble fraction [108,113] |
| Solvent Extraction | Cellulose, lignin, hemicellulose, suberin [43,81,82,91] | Reduced extractives fraction; retention of structural polymers; partial removal of lipophilic compounds [43,81] | Residual tannins, flavonoids and phenolics [43,82] |
| Deep eutectic solvents (DESs) | Cellulose-rich residues, lignin–DES complexes [92,95,96] | Partial delignification; strong DES–lignin interactions; high carbohydrate retention [95,96] | Phenolics and flavonoids trapped in the DES–biomass matrix [92,95] |
| Organosolv Extraction | High-purity lignin, cellulose pulp [84,85,86] | Sulfur-free lignin; improved cellulose accessibility; reduced hemicellulose fraction [85,113] | Polyphenols and low-molecular aromatics retained in residues [84,113] |
| Supercritical CO2 Extraction (ScCO2) | Lignin, cellulose, waxes, long-chain fatty acids [80,105,116] | Limited removal of polar extractives; residues contain waxes and lipophilic compounds [80,116] | Essential oils, triterpenes, phenolics not fully extracted [80,105] |
| Enzymatic extraction/ enzymatic pretreatment | Cellulose, residual lignin, mineral residues [111,112,114] | Enhanced accessibility of polysaccharides; selective removal of target components [112,114] | Polyphenols and oligosaccharides remaining in the solid phase [111,112] |
| Extraction Method | Extraction Medium/Agent | Environmental Impact | Main Products/Applications |
|---|---|---|---|
| Hot Water Extraction (HWE) | Water (100–160 °C, optimal 140 °C) [119,120] | Very low [111] | Non-cellulosic polysaccharides (hemicelluloses, pectins), lignin [108,109,110] |
| Solvent Extraction | Organic solvents (acetone, methanol, ethanol, petroleum ether) [101] | High (toxic organic solvents) [161] | Tannins, lignin, phenolic compounds [101,163,164] |
| Alkaline Extraction | Alkaline solutions (NaOH, KOH) | Moderate [82,165,166,167] | Tannins, proanthocyanidins, botulin [129,130,132] |
| Organosolv Extraction | Organic solvent mixtures (ethanol/water, dioxane/water) with acids [168] | Low (solvent recycling possible) [169] | Lignin, aromatic compounds, biofuels, dyes [89,100,169] |
| Supercritical CO2 Extraction (ScCO2) | Supercritical CO2 (40–100 °C, up to 62 MPa) + co-solvents (ethanol) [139,140,170,171] | Very low [170,172] | Polyphenols, essential oils, antibacterial compounds [140,141,142,145,146,173,174,175] |
| Enzymatic Hydrolysis | Enzymes (cellulase, xylanase, acetoxylanesterase) [147,149] | Very low | Fermentable sugars, biofuels, biopolymers [113,114,176] |
| Energy-assisted Methods (Steam Explosion, Microwave-, Ultrasound-assisted Extraction) | Energy input (steam, microwaves, ultrasound) [150,153] | Low [177] | Phenolic compounds, flavonoids, tannins [151,152,154,178,179,180,181,182,183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasiewicz, J.; Wronka, A.; Kowaluk, G. From Extraction to Valorization: Unlocking the Potential of Bark-Derived Extraction Residues for Sustainable Material Development. Molecules 2025, 30, 4537. https://doi.org/10.3390/molecules30234537
Dasiewicz J, Wronka A, Kowaluk G. From Extraction to Valorization: Unlocking the Potential of Bark-Derived Extraction Residues for Sustainable Material Development. Molecules. 2025; 30(23):4537. https://doi.org/10.3390/molecules30234537
Chicago/Turabian StyleDasiewicz, Julia, Anita Wronka, and Grzegorz Kowaluk. 2025. "From Extraction to Valorization: Unlocking the Potential of Bark-Derived Extraction Residues for Sustainable Material Development" Molecules 30, no. 23: 4537. https://doi.org/10.3390/molecules30234537
APA StyleDasiewicz, J., Wronka, A., & Kowaluk, G. (2025). From Extraction to Valorization: Unlocking the Potential of Bark-Derived Extraction Residues for Sustainable Material Development. Molecules, 30(23), 4537. https://doi.org/10.3390/molecules30234537








