Synthesis and Characterization of Biochar Obtained by Partial Delignification of Waste Biomass
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
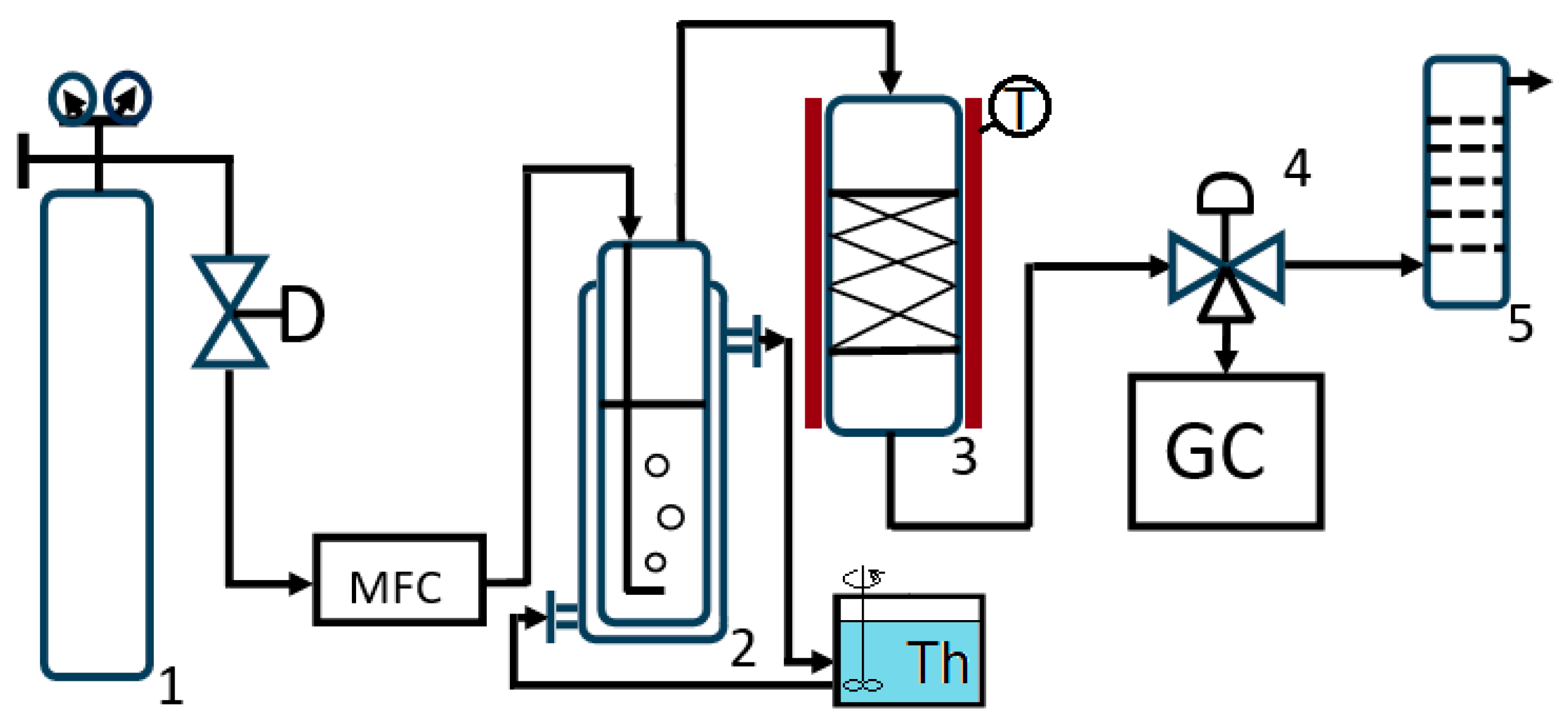
2.2. Synthesis of Adsorbent
2.3. Characterization Methods of Grape Seeds
2.3.1. Determination of Water Content
2.3.2. Determination of Ethanol Extractables
2.3.3. Determination of Lignin Content
2.3.4. Determination of Carbohydrate Content
2.3.5. Determination of Ash Content
2.4. Characterization Methods of Adsorbents
2.4.1. Thermogravimetric Analysis of Adsorbent Precursors
2.4.2. Textural Characteristics of Adsorbent Precursors
2.4.3. Microstructural Morphologies of Adsorbent Precursors
2.4.4. X-Ray Diffraction (XRD) of Adsorbent Precursors
2.4.5. Fourier Transform Infrared (FTIR) of Adsorbent Precursors
2.4.6. Determination of Adsorption Capacity
3. Results and Discussions
3.1. Characterization of Biomass Waste
Analysis by Classes of Chemical Compounds of Biomass Waste Samples
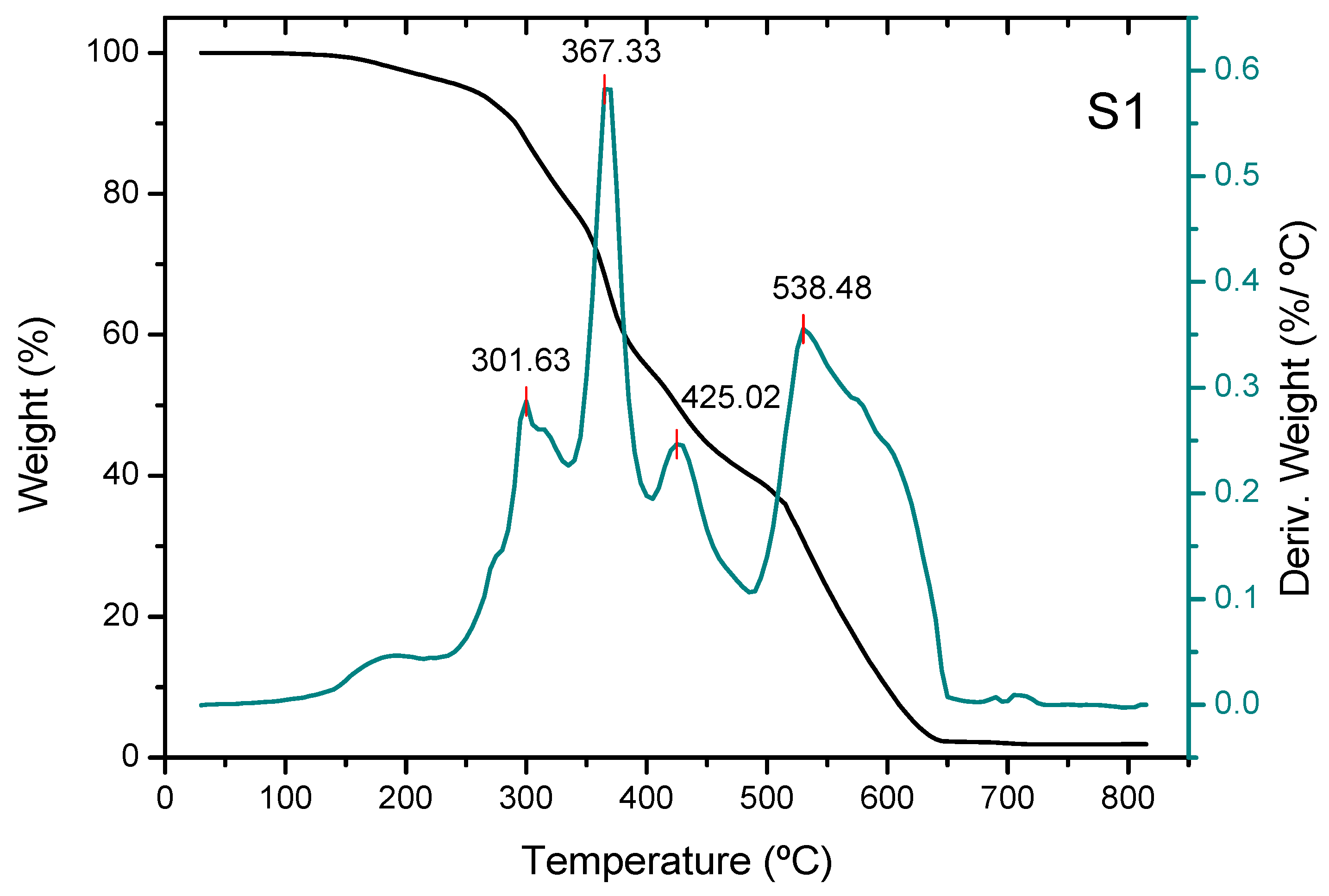
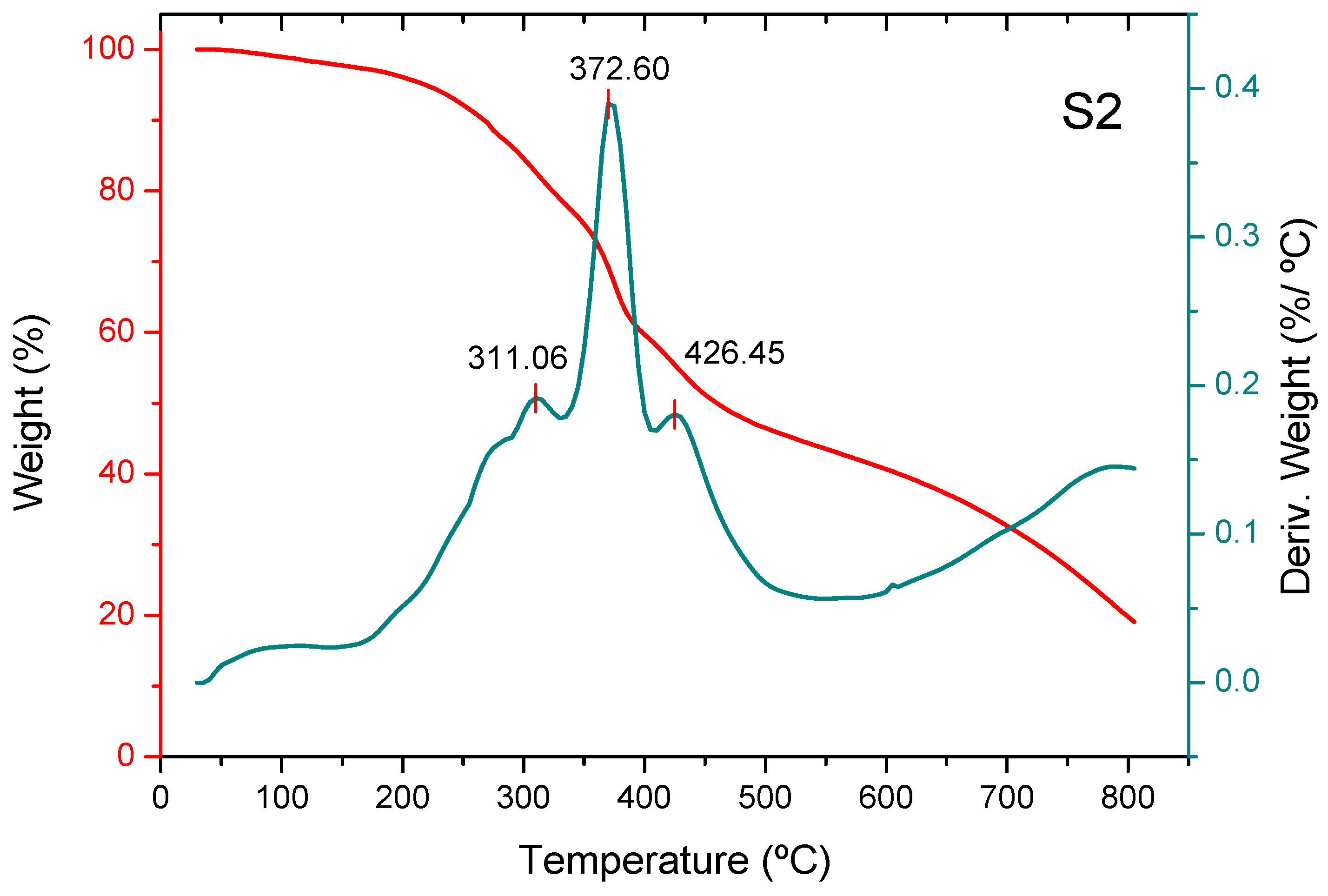
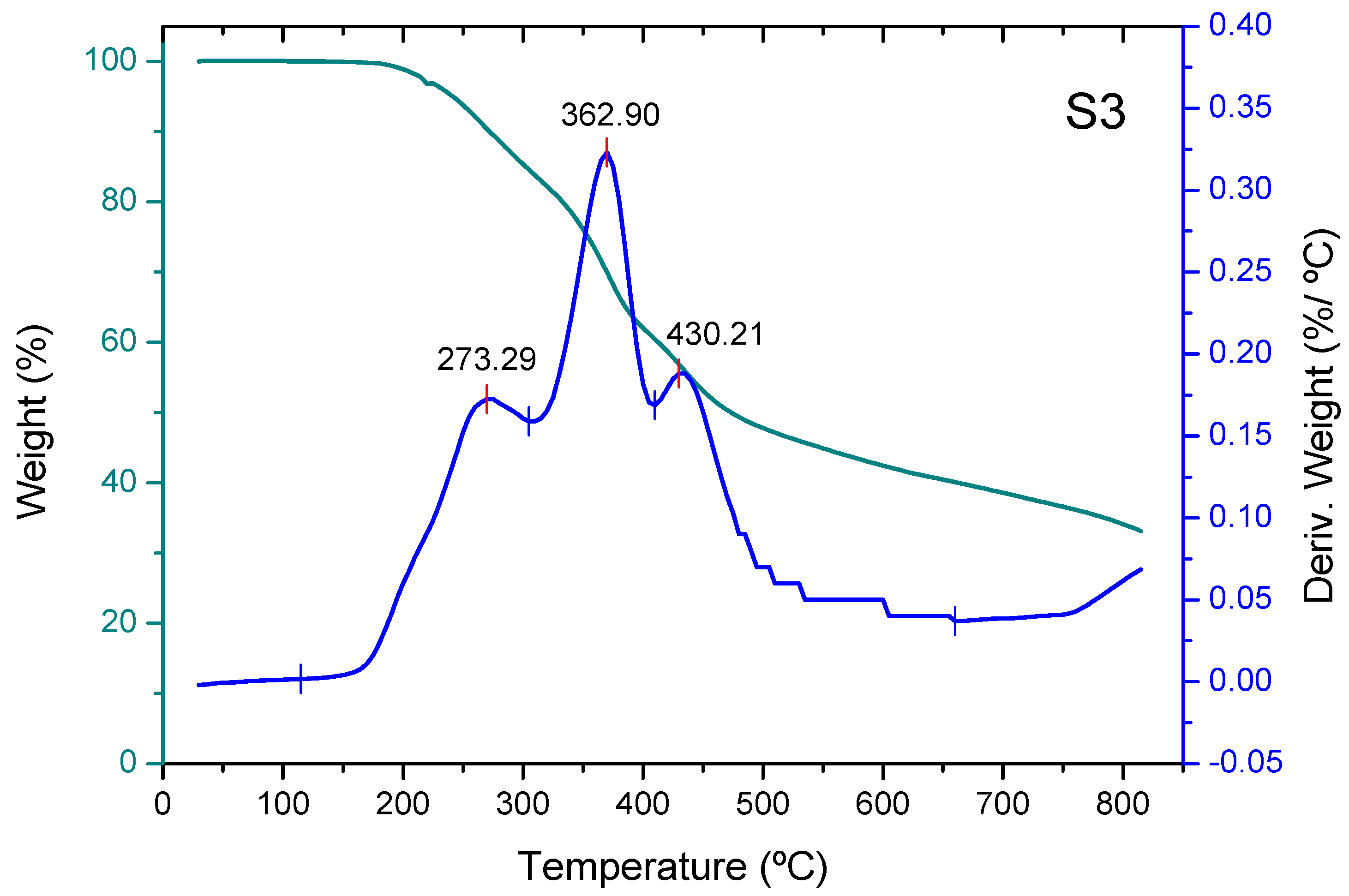
3.2. Thermogravimetric Analysis
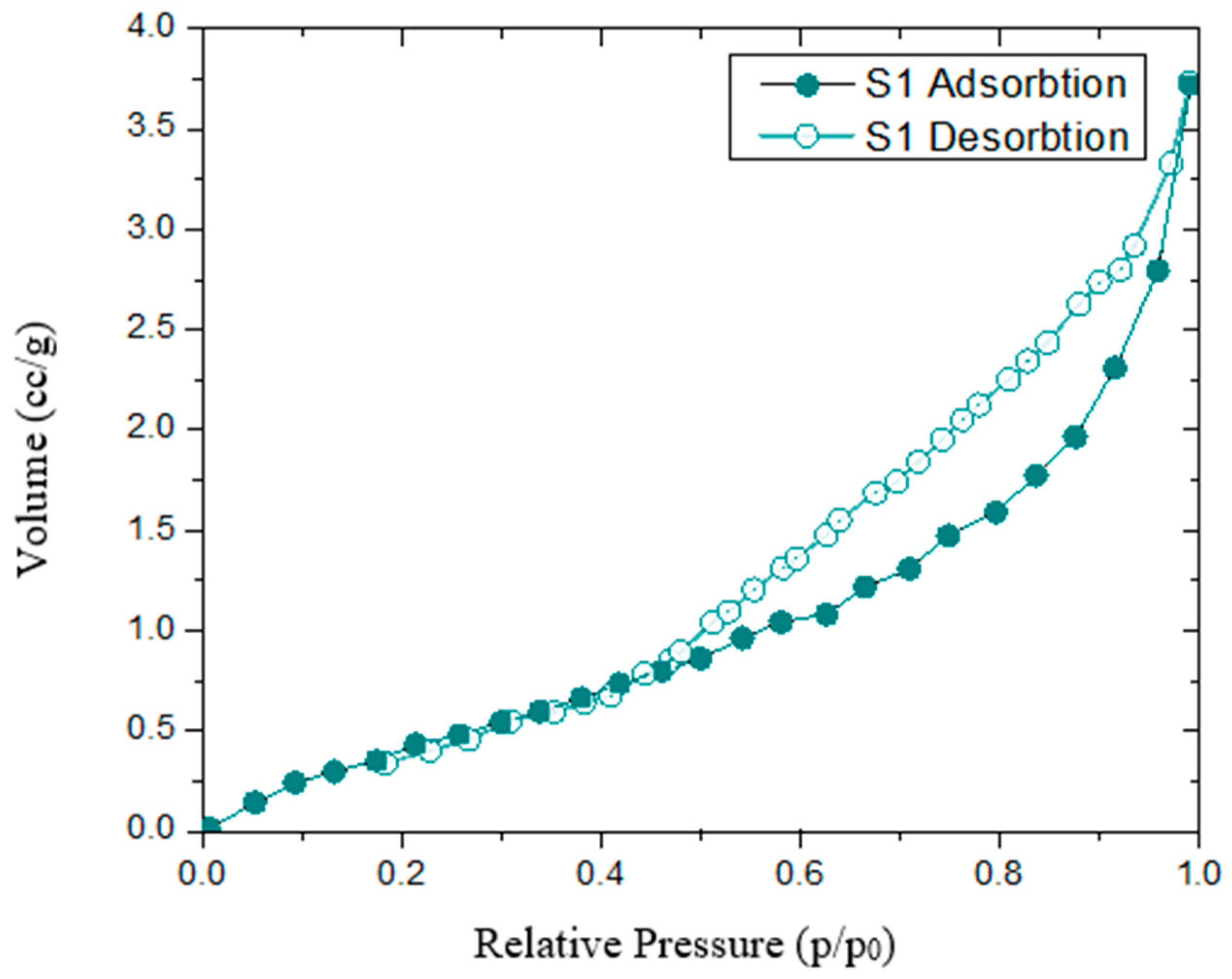
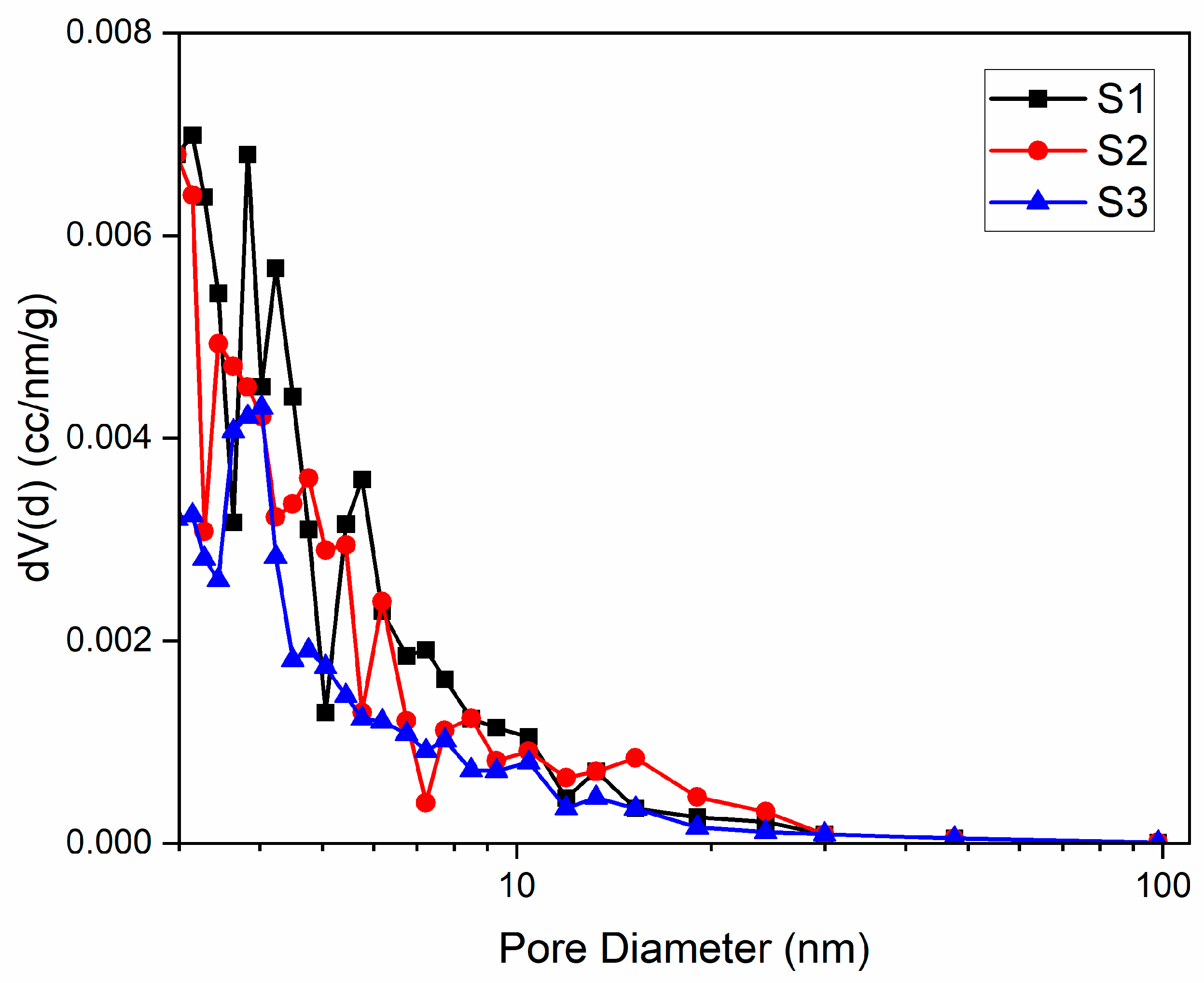
3.3. Textural Characteristics
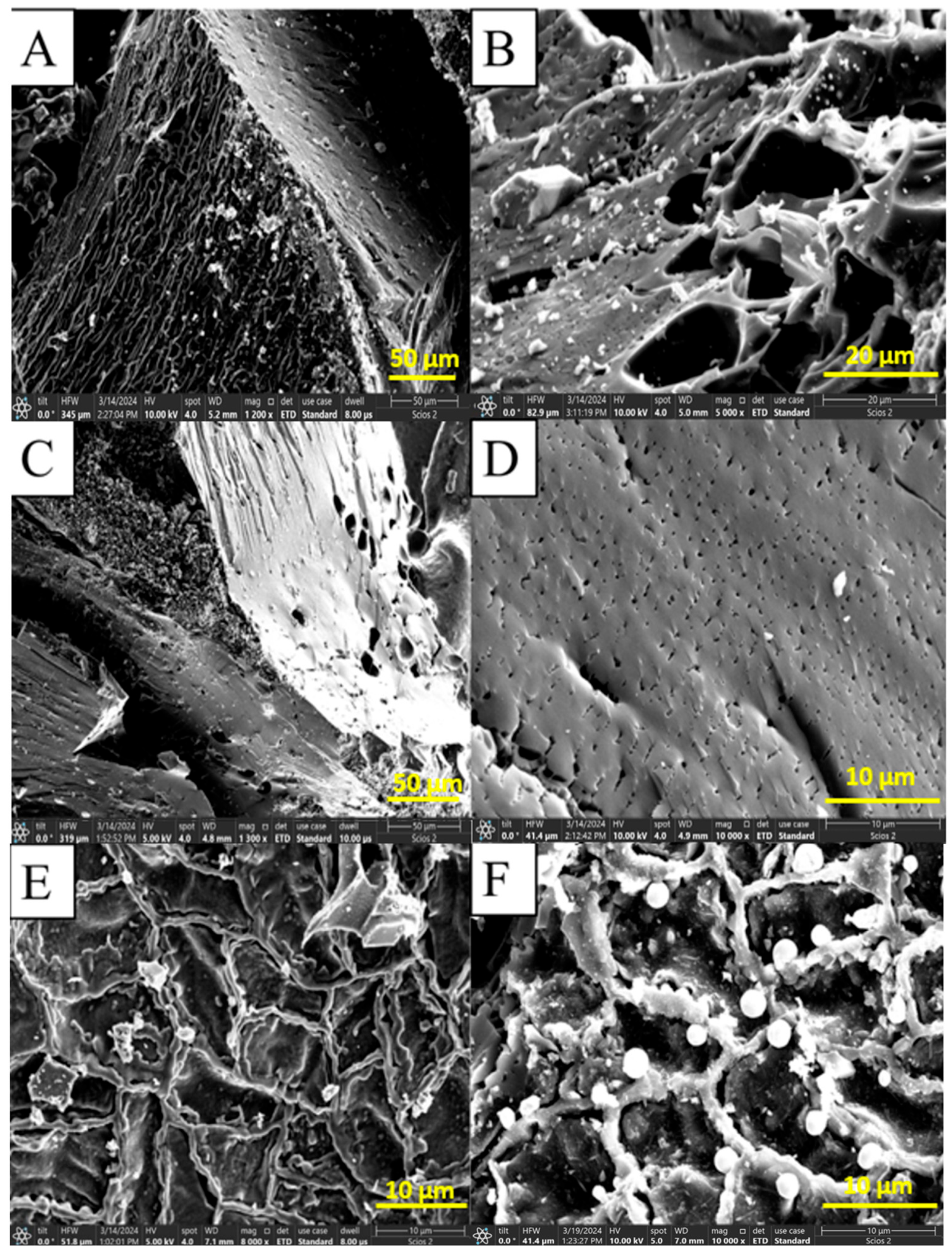
3.4. SEM Analysis
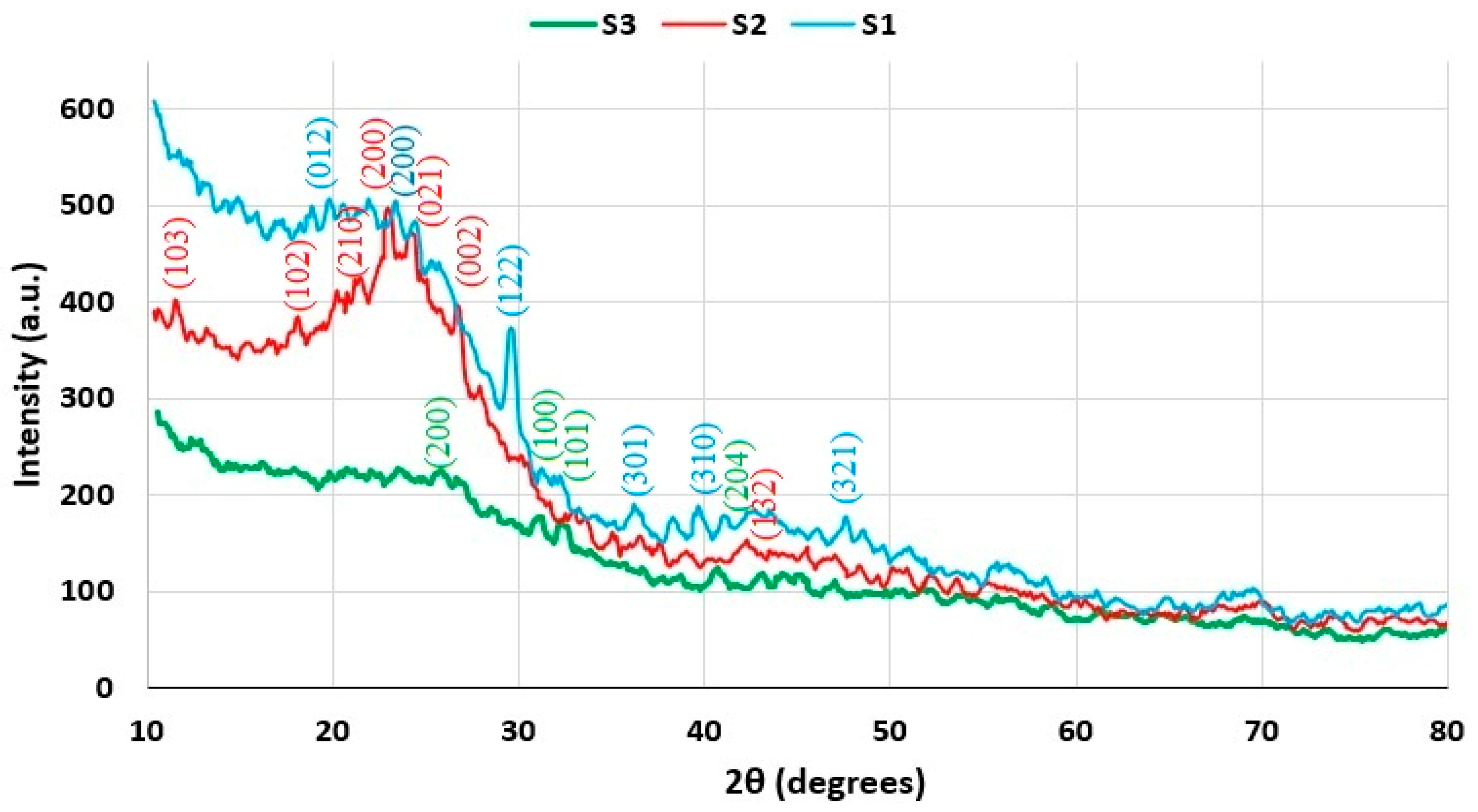
3.5. XRD Analysis
3.6. FTIR Analysis
3.7. Determination of Toluene Adsorption Capacity on Additivated Adsorbents
3.8. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrović, J.; Koprivica, M.; Ercegović, M.; Simić, M.; Dimitrijević, J.; Bugarčić, M.; Trifunović, S. Synthesis and Application of FeMg-Modified Hydrochar for Efficient Removal of Lead Ions from Aqueous Solution. Processes 2025, 13, 2060. [Google Scholar] [CrossRef]
- Sun, K.; Guo, T.; Li, Y.; Wang, W.; Li, Z.; Geng, P.; Fu, P. Rapid pyrolysis of cellulose: Revealing the role of volatile matter and char structure evolution. J. Anal. Appl. Pyrolysis 2024, 182, 106704. [Google Scholar] [CrossRef]
- Jafari, S.; Ghorbani-Shahna, F.; Bahrami, A.; Kazemian, H. Adsorptive removal of toluene and carbon tetrachloride from gas phase using Zeolitic Imidazolate Framework-8: Effects of synthesis method, particle size, and pretreatment of the adsorbent. Microporous Mesoporous Mater. 2018, 268, 58–68. [Google Scholar] [CrossRef]
- Li, Q.E.; Zhang, B.J.; Lyu, S.S.; Qi, Z. Spontaneous Combustion Characteristics of Activated Carbon Modified via Liquid Phase Impregnation during Drying. ACS Omega 2023, 8, 32752–32764. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.-Q.; Hao, Z.; Wang, G.; Li, Y.; Chen, J.-G.; Li, M.; Cheng, J.; Liu, Z.-T. A superhydrophobic hyper-cross-linked polymer synthesized at room temperature used as an efficient adsorbent for volatile organic compounds. RSC Adv. 2016, 6, 97048–97054. [Google Scholar] [CrossRef]
- Lancia, A.; Musmarra, D.; Prisciandaro, D. Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1992; Volume 15. [Google Scholar]
- Rizwan, M.; Leghari, A.; Kumar, A.; Laghari, A.; Mansoor, A.; Nawaz, M.A.; Zhou, X. Controlled hydrothermal carbonization of wood-derived lignin-rich lignocellulose: Redefining pyrolytic pathways to tailored biochar and hydrogen-enriched syngas. J. Anal. Appl. Pyrolysis 2025, 192, 107342. [Google Scholar] [CrossRef]
- Sheng, Y.; Dong, Q.; Ren, Q.; Wang, M. Measurement and prediction of breakthrough curve of low-concentration toluene adsorbed by activated carbon. E3S Web Conf. 2022, 356, 5068. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, D.J.P.; Narula, A.; Chistie, S.M.; Naik, S.U. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Görgülü, A.; Yağli, H.; Koç, Y.; Koç, A.; Baltacioğlu, E. Activated carbon adsorption behaviour of toluene at various temperatures and relative humidity. Environ. Prot. Eng. 2019, 45, 111–126. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Lillo-Rodenas, M.A.; Linares-Solano, A. Factors governing the adsorption of ethanol on spherical activated carbons. Carbon 2015, 83, 240–249. [Google Scholar] [CrossRef]
- Sandoval-Rangel, L.; Ramírez-Murillo, C.J.; Dimas-Rivera, G.L.; De La Rosa, J.R.; Lucio-Ortiz, C.J.; Ahmad, E.; Nigam, K.D.P.; Montesinos-Castellanos, A.; Mendoza, A. Enhancing the quality of products from slow pyrolysis of an agro-industrial biomass waste with natural mineral additives. Ind. Crops Prod. 2024, 216, 118798. [Google Scholar] [CrossRef]
- Xia, S.; Ou, J.; Liang, J.; Chen, S.; Wang, Z.; Zhao, Z.; Zhao, K.; Zheng, A. Synergistic catalysis strategy of Lewis and Brønsted acids for superior aromatic production in biomass pyrolysis. Ind. Crops Prod. 2025, 226, 120710. [Google Scholar] [CrossRef]
- Zhou, K.; Li, L.; Ma, X.; Mo, Y.; Chen, R.; Li, H.; Li, H. Activated carbons modified by magnesium oxide as highly efficient sorbents for acetone. RSC Adv. 2018, 8, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.A.; Mostafa, A.G.; Al-Awadhi, M.; Al-Harwi, W.S.; El-Zeny, A.S. Zinc chloride activated carbon derived from date pits for efficient biosorption of brilliant green: Adsorption characteristics and mechanism study. Appl. Water Sci. 2023, 13, 226. [Google Scholar] [CrossRef]
- Mabrouk, S.; Fizer, M.; Mariychuk, R.; Dhaouadi, H. Grape seeds waste activated carbon as an adsorber of paracetamol drug residue: Experimental and theoretical approaches. J. Mol. Liq. 2024, 408, 125325. [Google Scholar] [CrossRef]
- Ran, B.; Ma, Y.; Tian, H.; Zhu, Y.; Qi, C.; Shang, L. The joint production of green diesel and activated carbon from spent coconut shells (SCS): An energy techno-economic and Life Cycle Assessment case for China. J. Clean. Prod. 2024, 472, 143319. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Osman, A.I.; Farghali, M.; Rashwan, A.K. Life cycle assessment of biochar as a green sorbent for soil remediation. Curr. Opin. Green Sustain. Chem. 2024, 46, 100882. [Google Scholar] [CrossRef]
- Howe-Grant, M.; Kroschwitz, J. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 1996; Volume 20. [Google Scholar]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Carolino, J.M.; Braz, G.S.; Carvalho, J.C.L.; Fagundes, F.G.; Silva, P.A.F.E.; Rocha, I.O.H.; Patrocínio, M.C.; Lima, E.N.; de Lima, R.P.; de Oliveira, M.A.; et al. Assessment of the ecotoxicity of extracts from sugarcane bagasse biochars activated with zinc chloride. Environ. Chem. Ecotoxicol. 2025, 7, 19–26. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Guo, T.; Zhu, S.; Qu, H.; Li, X.; Tian, J. One- and two-step H2O activation of fungus bran for calcium-rich biochar in tar steam reforming. J. Environ. Chem. Eng. 2025, 13, 116147. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Jimenez-Cordero, D.; Heras, F.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J. Porous structure and morphology of granular chars from flash and conventional pyrolysis of grape seeds. Biomass Bioenergy 2013, 54, 123–132. [Google Scholar] [CrossRef]
- Shaabana, A.; Sea, S.M.; Mitanb, N.M.M.; Dimin, M.F. Characterization of biochar derived from rubber wood sawdust through slow pyrolysis on surface porosities and functional groups. Procedia Eng. 2013, 68, 365–371. [Google Scholar] [CrossRef]
- Terrell, L.H. Theory of Physical Adsorption. Adv. Catal. 1952, 4, 211–258. [Google Scholar] [CrossRef]
- Osterrieth, J.W.M.; Rampersad, J.; Madden, D.; Rampal, N.; Skoric, L.; Connolly, B.; Allendorf, M.D.; Stavila, V.; Snider, J.L.; Ameloot, R.; et al. How Reproducible are Surface Areas Calculated from the BET Equation? Adv. Mater. 2022, 34, 2201502. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar] [CrossRef]
- Ahmed, A.; Bakar, M.S.A.; Razzaq, A.; Hidayat, S.; Jamil, F.; Amin, M.N.; Sukri, R.S.; Shah, N.S.; Park, Y.-K. Characterization and Thermal Behavior Study of Biomass from Invasive Acacia mangium Species in Brunei Preceding Thermochemical Conversion. Sustainability 2021, 13, 5249. [Google Scholar] [CrossRef]
- Chaiwong, K.; Kiatsiriroat, T.; Vorayos, N.; Thararax, C. Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenergy 2013, 56, 600–606. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Activated carbon from biomass precursors using phosphoric acid: A review. Heliyon 2022, 8, e11940. [Google Scholar] [CrossRef]
- Fonseca-Bermúdez, O.J.; Giraldo, L.; Sierra-Ramírez, R.; Serafin, J.; Dziejarski, B.; Bonillo, M.G.; Farid, G.; Moreno-Pirajan, J.C. Cashew nut shell biomass: A source for high-performance CO2/CH4 adsorption in activated carbon. J. CO2 Util. 2024, 83, 102799. [Google Scholar] [CrossRef]
- Yakout, S.M.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2011, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Krogh, L.; Griffin, D.W.; Conner, W.C. Hysteresis and Scanning Behavior of Mesoporous Molecular Sieves. Langmuir 2005, 21, 8214–8225. [Google Scholar] [CrossRef] [PubMed]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity, 2nd ed.; Academic Press: Cambridge, MA, USA, 1982; ISBN 978-0-12-300956-2. [Google Scholar]
- Demiral, H.; Demiral, I.; Karabacakoğlu, O.B.; Tümsek, F. Production of activated carbon from olive bagasse by physical activation. Chem. Eng. Res. Des. 2011, 89, 206–213. [Google Scholar] [CrossRef]
- Cui, G.; Wang, X.; Xun, J.; Lou, T. Microwave assisted synthesis and characterization of a ternary flocculant from chitosan, acrylamide and lignin. Int. Biodeterior. Biodegrad. 2017, 123, 269–275. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Gomide, R.A.C.; de Oliveira, A.C.S.; Rodrigues, D.A.C.; de Oliveira, C.R.; de Assis, O.B.G.; Dias, M.V.; Borges, S.V. Development and Characterization of Lignin Microparticles for Physical and Antioxidant Enhancement of Biodegradable Polymers. J. Polym. Environ. 2020, 28, 1326–1334. [Google Scholar] [CrossRef]
- Meng, Y.; Contescu, C.I.; Liu, P.; Wang, S.; Lee, S.-H.; Guo, J.; Young, T.M. Understanding the local structure of disordered carbons from cellulose and lignin. Wood Sci. Technol. 2021, 55, 587–606. [Google Scholar] [CrossRef]
- Gea, S.; Siregar, A.H.; Zaidar, E.; Harahap, M.; Indrawan, D.P.; Perangin-Angin, Y.A. Isolation and Characterisation of Cellulose Nanofibre and Lignin from Oil Palm Empty Fruit Bunches. Materials 2020, 13, 2290. [Google Scholar] [CrossRef]
- Frikha, K.; Limousy, L.; Arif, M.B.; Thevenin, N.; Ruidavets, L.; Zbair, M.; Bennici, S. Exhausted Grape Marc Derived Biochars: Effect of Pyrolysis Temperature on the Yield and Quality of Biochar for Soil Amendment. Sustainability 2021, 13, 11187. [Google Scholar] [CrossRef]
- Mourgkogiannis, N.; Nikolopoulos, I.; Kordouli, E.; Lycourghiotis, A.; Kordulis, C.; Karapanagioti, H.K. The Influence of Biowaste Type on the Physicochemical and Sorptive Characteristics of Corresponding Biochar Used as Sustainable Sorbent. Sustainability 2024, 16, 2889. [Google Scholar] [CrossRef]
- Chu, G.; Wang, W.; Zhao, J.; Zhou, D. Transformation of phosphorus species during phosphoric acid-assisted pyrolysis of lignocellulose. Sci. Total Environ. 2023, 866, 161010. [Google Scholar] [CrossRef] [PubMed]
- Machrouhi, A.; Khiar, H.; Elhalil, A.; Sadiq, M.; Abdennouri, M.; Barka, N. Synthesis, characterization, and photocatalytic degradation of anionic dyes using a novel ZnO/activated carbon composite. Watershed Ecol. Environ. 2023, 5, 80–87. [Google Scholar] [CrossRef]
- Deng, L.; Du, P.; Yu, W.; Yuan, P.; Annabi-Bergaya, F.; Liu, D.; Zhou, J. Novel hierarchically porous allophane/diatomite nanocomposite for benzene adsorption. Appl. Clay Sci. 2019, 168, 155–163. [Google Scholar] [CrossRef]
- Sing, K.S.W. Characterization of porous solids: An introductory survey. In Studies in Surface Science and Catalysis; Characterization of Porous Solids II; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1991; Volume 62, pp. 1–9. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Zhu, J.; Liu, Z. Removal of Toluene by Adsorption/Desorption Using Ultra-stable Y Zeolite. Trans. Tianjin Univ. 2019, 25, 312–321. [Google Scholar] [CrossRef]
- Essa, R.A.; El-Aal, M.A.; Sedky, A.; Zeid, E.F.A.; Amin, S. ZnO NPs-modified biochar derived from banana peels for adsorptive removal of methylene blue from water. J. Mol. Struct. 2025, 1321, 139821. [Google Scholar] [CrossRef]





| Components | Value |
|---|---|
| Moisture, wt.% | 2.16 |
| Ethanol extractables, wt.% | 17.28 |
| Lignin, wt.% | 31.69 |
| Carbohydrates, wt.% | 48.87 |
| Ash, wt.% | 3.61 |
| No. | Adsorbant | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| 1. | S1 | 2.187 | 0.0041 | 6.535 |
| 2. | S2 | 2.778 | 0.0057 | 5.754 |
| 3. | S3 | 2.697 | 0.0054 | 5.833 |
| % Weigh | Unconditioned and Unactivated Biochar | Biochar Treated with Phosphoric Acid | Biochar Treated with Zinc Chloride |
|---|---|---|---|
| C | 92.82% | 79.07% | 94.34% |
| O | - | 14.33% | - |
| Mg | 1.32% | 0.33% | 0.23% |
| P | 3.42% | 6.44% | 0.96% |
| S | 0.08% | - | 0.16% |
| Cl | 0.05% | - | 2.5% |
| Ca | 2.30% | 0.13% | 1.8% |
| Sample | Lignin-Based Biomass,% | Carbonaceous Structures,% | Zinc Oxide,% | Xc % Lignin | Xc % Graphite | Xc % Zinc Oxide |
|---|---|---|---|---|---|---|
| S1 | 100 | - | - | 33.06 | - | - |
| S2 | 69.20 | 30.80 | - | 29.36 | 94.70 | - |
| S3 | 35.33 | 49.07 | 15.61 | 18.09 | 92.67 | 89.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilievici, G.; Sanda, M.; Băjan, M.; Dușescu-Vasile, C.; Onuțu, I.; Brănoiu, G.; Bomboș, D.; Baioun, A.; Borcea, A.F.; Stănică, A.-I. Synthesis and Characterization of Biochar Obtained by Partial Delignification of Waste Biomass. Molecules 2025, 30, 4505. https://doi.org/10.3390/molecules30234505
Vasilievici G, Sanda M, Băjan M, Dușescu-Vasile C, Onuțu I, Brănoiu G, Bomboș D, Baioun A, Borcea AF, Stănică A-I. Synthesis and Characterization of Biochar Obtained by Partial Delignification of Waste Biomass. Molecules. 2025; 30(23):4505. https://doi.org/10.3390/molecules30234505
Chicago/Turabian StyleVasilievici, Gabriel, Mia Sanda, Marian Băjan, Cristina Dușescu-Vasile, Ion Onuțu, Gheorghe Brănoiu, Dorin Bomboș, Abeer Baioun, Anca Florentina Borcea, and Andra-Ioana Stănică. 2025. "Synthesis and Characterization of Biochar Obtained by Partial Delignification of Waste Biomass" Molecules 30, no. 23: 4505. https://doi.org/10.3390/molecules30234505
APA StyleVasilievici, G., Sanda, M., Băjan, M., Dușescu-Vasile, C., Onuțu, I., Brănoiu, G., Bomboș, D., Baioun, A., Borcea, A. F., & Stănică, A.-I. (2025). Synthesis and Characterization of Biochar Obtained by Partial Delignification of Waste Biomass. Molecules, 30(23), 4505. https://doi.org/10.3390/molecules30234505







