Abstract
Amitriptyline (AMT), a widely prescribed antidepressant, and its metabolites have emerged as significant environmental contaminants, posing substantial risks to aquatic organisms and human health. Systematic and in-depth investigations into advanced anode materials, coupled with a profound elucidation of their electrochemical mechanisms, are imperative for the development of efficacious technologies for AMT removal. In this study, a series of amorphous carbon-encapsulated zinc oxide (C@ZnO) modified anodes were systematically synthesized and incorporated into a persulfate-based electrochemical system (CZ-PS) to comprehensively elucidate the catalytic mechanisms and mass transfer efficiencies governing the degradation of AMT via electroperoxidation. Notably, the CZ-PS system achieved a 97.5% degradation for 5.0 mg/L AMT within 120 min under optimized conditions (200 C@ZnO electrode, pH 7.0, current density 20 mA/cm2, PS concentration 0.5 mM), significantly outperforming the single PS system (37.8%) or the pure electrocatalytic system. Quenching experiments and EPR analysis confirmed hydroxyl radicals (•OH) and sulfate radicals (SO4•−) as the dominant reactive species. Both acidic and neutral pH conditions were demonstrated to favorably enhance the electrocatalytic degradation efficiency by improving adsorption performance and inhibiting •OH decomposition. The system retained >90% degradation efficiency after 5 electrode cycles. Three degradation pathways and 13 intermediates were identified via UPLC–MS/MS analysis, including side-chain demethylation and oxidative ring-opening of the seven-membered ring to form aldehyde/carboxylic acid compounds, ultimately mineralizing into CO2 and H2O. It demonstrates strong engineering potential and provides a green, high-efficiency strategy for antibiotic wastewater treatment.
1. Introduction
Antibiotics in pharmaceuticals and personal care products (PPCPs) have garnered significant attention due to their persistence, bioaccumulative potential, and ecotoxicity [1,2,3]. The antibiotic wastewater primarily originates from hospitals, pharmaceutical manufacturers, and livestock farms [4,5,6]. The release of large quantities of antibiotics into the environment inevitably exerts negative impacts on non-target organisms within ecosystems. Thus, the effective elimination of antibiotics from aquatic environments has emerged as a critical research focus. Amitriptyline (AMT), a tricyclic antidepressant, is clinically used for treating enuresis, migraines, and diabetic peripheral neuropathic pain [7,8]. AMT is frequently detected in environmental matrices, with concentrations exceeding 500 ng L−1 in certain regions [9]. Consequently, developing effective technologies for treating AMT and other antibiotics and PPCPs in wastewater is of significant importance.
To enhance the removal efficiency of AMT from aqueous environments, several emerging strategies have been developed and optimized in recent research endeavors, including biodegradation, metal oxide oxidation, advanced oxidation processes (AOPs), electrocatalytic oxidation, photocatalysis, and metal/bimetallic reduction [10,11,12,13,14,15,16]. For instance, Tee et al. [11] successfully synthesized boron-doped three-dimensional (3D) graphene composite materials, achieving outstanding adsorption performance on AMT removal through optimized surface functionalization and porous architecture design. Concurrently, Finčur et al. [16] reported a novel heterogeneous photocatalytic system utilizing TiO2/WO3 composite coatings, which exhibited enhanced charge separation efficiency and visible light responsiveness to improved oxidative degradation kinetics of AMT pollutants. Although these methods demonstrate strong degradation capabilities, the toxicity of primary intermediates and byproducts from AMT degradation remains largely unknown. Carbon materials (e.g., carbon nanotubes, graphene, and amorphous carbon) with high electrical conductivity and light absorption intensity have attracted extensive attention in recent years.
Zinc oxide nanoparticles (ZnO), as a promising n-type semiconductor material, exhibit a zeta potential of 16 mV in colloidal particles and approximately 27 clusters in ZnO nanoparticles [17,18,19]. This charge-driven aggregation enables feasible coagulation–sedimentation for organic contaminants. Furthermore, anchoring ZnO onto carbon matrices (e.g., reduced graphene oxide) to form heterostructures effectively mitigates nanoparticle agglomeration by reducing electrostatic repulsion while enhancing interfacial charge transfer efficiency [20,21]. In electrocatalytic systems, carbon materials (e.g., biochar) act as highly conductive scaffolds that promote expedited electron shuttling through their interconnected three-dimensional networks, thereby endowing the system with enhanced catalytic efficacy and stability under electrochemical conditions [22]. Recently, oxidation processes based on persulfates (e.g., peroxymonosulfate HSO5−/PMS, peroxydisulfate S2O82−/PDS) have been widely applied in water pollution treatment. Serving as stable, low-cost, and safe oxidant carriers, they form Fenton-like systems with transition metals for pollutant degradation [23,24,25]. However, practical limitations in natural water matrices often reduce their efficiency. Thus, integrating complementary systems to enhance performance while reducing energy consumption has become research priority [26,27].
In this study, a novel series of amorphous C@ZnO composites were synthesized via a simple impregnation–calcination method and integrated into a customized anode configuration within the CZ-PS electrocatalytic system for AMT degradation. The morphology, composition, optical properties, and stability of C@ZnO were characterized. Batch experiments were conducted to elucidate the effects of PS dosage, pH, and initial AMT concentration on the degradation performance. Mechanistic investigations were further deepened through selective radical scavenging and EPR spectroscopy, confirmed ROS-mediated mineralization. Notably, intermediate studies elucidated the degradation pathways and mechanisms of AMT in the electrocatalytically activated PS system. This work pioneers an innovative paradigm for the rational design of high-efficiency electrocatalysts, holding significant promise for advanced antibiotic remediation in practical wastewater treatment scenarios.
2. Results and Discussion
2.1. Structural Characteristics of C@ZnO Materials
The surface morphology and elemental composition of the material samples were characterized by SEM, EDS, and TEM, as shown in Figure 1. The C@ZnO samples exhibited a grayish-black color and a columnar structure with a rough surface, closely resembling the morphology of pristine ZnO nanoparticles (Figure 1a). This observation confirmed the formation of a composite structure in which amorphous carbon encapsulates ZnO. TEM images revealed tightly bound ZnO particles within the carbon matrix. High-resolution TEM analysis of C@ZnO (Figure 1b,c) showed an interplanar spacing of 0.282 nm, corresponding to the (100) plane of ZnO. According to previous studies, the (0001) basal plane of ZnO possesses high surface energy in a polarized state, potentially influencing its interaction with the carbon coating [28]. EDS analysis and elemental mapping (Figure 1d–f) of 200 C@ZnO demonstrated homogeneous distribution of C, O, and Zn elements across the surface, indicating successful integration of the composite components.
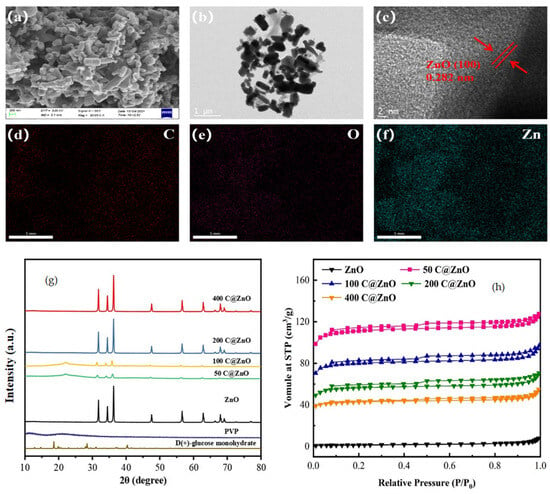
Figure 1.
Characterization of C@ZnO (a) SEM, (b,c) TEM, (e) HRTEM, and (d–f) EDS images, (g) XRD pattern of C@ZnO, and (h) the N2 adsorption–desorption isotherm of ZnO and C@ZnO.
X-ray diffraction (XRD) patterns (Figure 1g) verified the crystalline phase of C@ZnO composites. Characteristic peaks corresponding to the (100), (002), (101), (102), (110), (103), (200), (112), and (201) planes of ZnO were observed at 31.93°, 34.68°, 36.44°, 47.74°, 56.75°, 63.0°, 66.5°, 68.06°, and 69.3°, respectively, consistent with the standard ZnO reference (JCPDS No. 36-1451). Increasing ZnO content enhanced diffraction peak intensity. The absence of PVP/glucose diffraction peaks indicated phase transformation during synthesis, with residual peaks potentially serving as background signals [21]. BET/BJH analysis of N2 adsorption–desorption isotherms (Figure 1h) confirmed Type IV isotherms (IUPAC classification) for all samples, signifying mesoporous capillary condensation systems.
Hysteresis loops exhibited H4-type behavior, indicating low pore structure regularity without distinct saturation adsorption. As summarized in Table 1, specific surface area decreased sequentially: 443.44 m2 g−1 (50 C@ZnO) > 319.45 m2 g−1 (100 C@ZnO) > 224.30 m2 g−1 (200 C@ZnO) > 169.20 m2 g−1 (400 C@ZnO) > 4.04 m2 g−1 (pristine ZnO); the amorphous carbon coating dramatically increased specific surface area (e.g., 4.04 → 224.30 m2 g−1 for pristine ZnO vs. 200 C@ZnO). This enhanced porosity and surface area directly correlated with superior adsorption capacity compared to pristine ZnO.

Table 1.
BET of Pristine ZnO, 50 C@ZnO, 100 C@ZnO, 200 C@ZnO, and 400 C@ZnO.
2.2. Surface Structure of C@ZnO Materials
The elemental composition of the material sample 200 C@ZnO was characterized by XPS, and the results are shown in Figure 2. As shown in Figure 2a, the 200 C@ZnO sample revealed the presence of Zn, O, and C as major elements. The high-resolution C 1s spectrum (Figure 2b) showed peaks at 284.80 eV (C-C), 285.63 eV (C-O), and 289.18 eV (O-C=O), indicating the formation of carbon bonds [29]. The O 1s spectrum (Figure 2c) exhibited peaks at 531.25 eV (Zn-O) and 532.89 eV (C-O), attributed to lattice oxygen in ZnO and surface hydroxyl/chemisorbed oxygen, respectively [30]. The high-resolution XPS spectrum of Zn 2p for the sample (Figure 2d) exhibited two characteristic peaks at 1022.4 eV (Zn 2p3/2) and 1045.4 eV (Zn 2p1/2), consistent with the binding energies of Zn-O bonds in ZnO. The ~23 eV separation between these peaks, arising from spin-orbit coupling, matched the characteristic Zn 2p doublet spacing reported in previous studies, confirming the presence of ZnO in its crystalline phase [31].
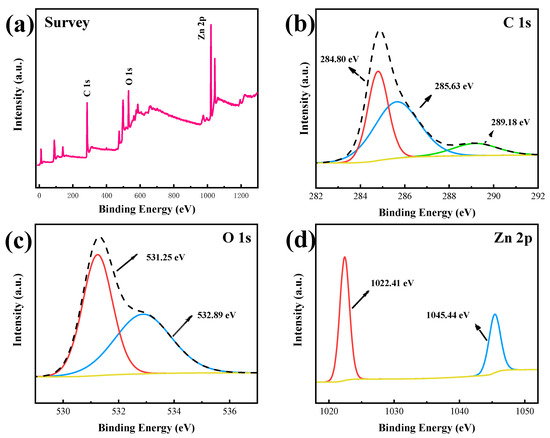
Figure 2.
XPS spectra of 200 C@ZnO: (a) Survey, (b) C1 s, (c) O1 s, and (d) Zn 2p.
Raman spectroscopy was employed to characterize the crystal structure of the sample under ambient conditions, with results shown in Figure 3. The Raman spectrum of 200 C@ZnO (Figure 3a) exhibited two characteristic peaks at 332 cm−1 and 438 cm−1, assignable to the Zn-O stretching modes, consistent with XRD data. Additionally, peaks at 1340 cm−1 (D band) and 1590 cm−1 (G band) were observed, indicative of carbonaceous components (e.g., graphene-like structures or disordered carbon). According to previous reports, ZnO exhibits Raman-active optical phonons of A1 + E1 + 2E2 symmetry, with A1 and E1 modes capable of splitting into transverse optical (TO) and longitudinal optical (LO) branches, respectively [32]. The presence of Zn-O peaks and carbon-related bands collectively confirms the composite nature of the material. As depicted in Figure 3b, the Raman spectrum of pristine ZnO exhibits characteristic peaks at 332 cm−1 and 438 cm−1, corresponding to the Zn-O stretching modes of the B4 phase, which confirm its hexagonal wurtzite structure [28]. In contrast, the C@ZnO spectrum lacks the derivative peaks associated with PVP and glucose (Figure 3c,d), indicating that these precursor materials underwent complete transformation during synthesis, preventing their spectral signatures from being detected. The additional bands observed in C@ZnO likely arise from the amorphous carbon layer formed on the surface.
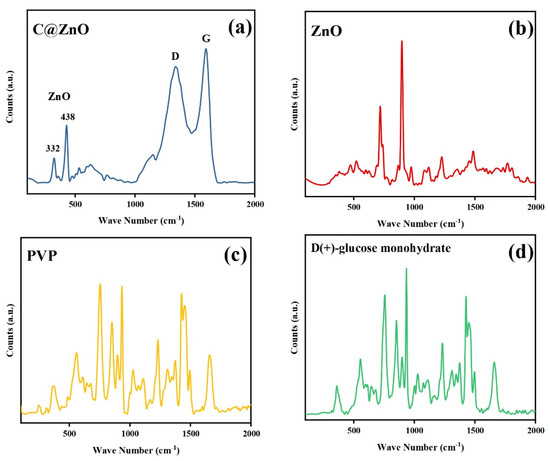
Figure 3.
Raman spectra of 200 C@ZnO (a), ZnO (b) and the synthetic raw: polyvinylpyrrolidone (c), D(+)-Glucose monohydrate (d).
2.3. Degradation Performance and Optimization
To investigate the degradation efficiency of AMT in different electrocatalytic oxidation systems, 5 mg/L AMT was degraded for 120 min using persulfate (PS) alone, the electrochemical (EC) system alone, and the C@ZnO anode (CZ-PS) system. As shown in Figure 4a, the electrode materials used in the CZ-PS system were 50 C@ZnO, 100 C@ZnO, 200 C@ZnO, and 400 C@ZnO. As the ZnO concentration increased from 50 mg to 200 mg, the AMT removal rate in the CZ-PS system significantly improved (78.9%, 83.4%, and 97.4%, respectively), demonstrating the crucial role of ZnO in the system. However, when the ZnO concentration reached 400 mg, the degradation efficiency slightly decreased, likely due to the blocking of the semiconductor’s active sites, which hindered the generation of free radicals [33,34].
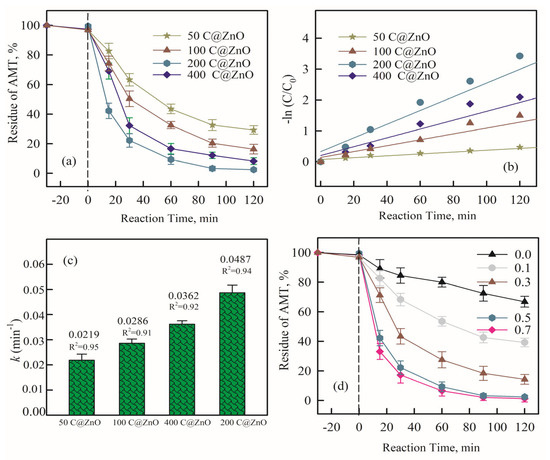
Figure 4.
The impact of initial ZnO concentration (mg of ZnO) in C@ZnO material on AMT removal (a), the pseudo-first-order rate constant of AMT removal by different C@ZnO material in AMT removal (b,c), and initial PS concentration (d, mM) on AMT removal in 200 C@ZnO material system after 120 min. CNa2SO4 = 5 mM, current density = 20 mA/cm2, pH = 7.0, and CAMT = 5 mg/L. Data points for AMT residue were means ± standard deviations (n = 3).
The first-order kinetic reaction model of different systems to the degradation of AMT is shown in Figure 4b. The first-order kinetic reaction model was also improved in the same order [35]: ln (C/C0) = −kt. Additionally, the half-lives (t1/2) were calculated by: t1/2 = ln2/k. Obviously, the catalytic efficiency was enhanced with the addition of ZnO concentration, and the corresponding k values increased from 0.0219 min−1 to 0.0489 min−1. This significant improvement is owing to a better decrease in SO4•−, •OH, and 1O2 generation.
As shown in Figure 4b, compared to the EC system using only the C@ZnO anode, the CZ-PS system exhibited a significantly enhanced AMT removal rate. Within 120 min, the system containing only PS degraded 37.8% of AMT. The degradation performance of the electrocatalytic system with the 200 C@ZnO anode increased markedly. As the PS concentration increased from 0.1 mM to 0.7 mM, the removal rate rose from 60.2% to 100%. This can be attributed to the direct oxidation of AMT on the C@ZnO anode and the oxidation by reactive species (ROS) generated via hydrolysis [34]. Since the generated reactive species adsorb onto the anode, the removal rate in the CZ-PS system increased to 97.5% within 120 min after adding PS. Notably, elevated PMS concentrations may result in suppressed pollutant removal efficiency, attributed to the scavenging of generated ROS [36,37]. This phenomenon indicates a significant synergistic effect when PS is combined with electrochemical oxidation.
2.4. Influence of Reaction Conditions
Figure 5a illustrates the effect of different initial AMT concentrations on the degradation process (CZ-PS system). The AMT removal rate slightly increased as the initial AMT concentration decreased. After 120 min of degradation, the removal rate was 97.4% at an initial concentration of 14.42 μM, while it reached 98.1% and 99.5% at concentrations of 9.16 μM and 3.67 μM, respectively. The removal rate improved as the initial AMT concentration decreased from 14.42 μM to 3.67 μM, and at an initial concentration of 1.83 μM, AMT was almost completely removed (near 100%). Clearly, a higher pollutant concentration corresponds to a lower removal rate. This phenomenon may be attributed to the generation of more intermediates at higher concentrations [14]. These intermediates diffuse and adsorb onto the anode surface, competing with the pollutant for active sites and Reactive oxygen species (ROS) [38,39], thereby reducing the pollutant removal efficiency.
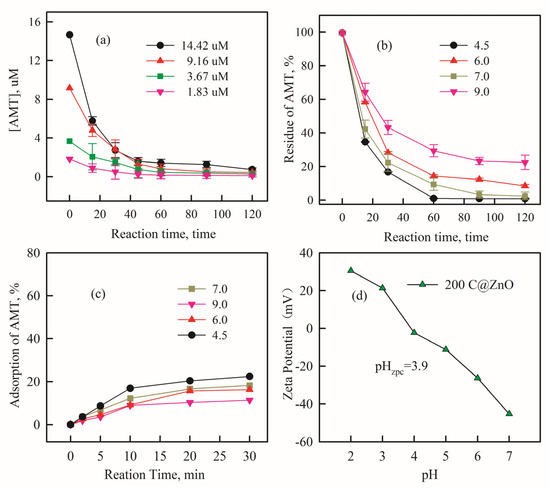
Figure 5.
The impact of initial AMT concentration (a) and initial pH (b) on AMT removal in system after 120 min; the sorption properties of AMT change with the different pH values after 30 min (c); and Zeta potential of 200 C@ZnO material under various pH conditions (d). CNa2SO4 = 5 mM, current density = 20 mA/cm2, and CPS = 0.5 mM.
The initial pH of the solution significantly impacts the degradation process because H+ participates in numerous free radical chain reactions [40]. Experiments investigated the effect of initial pH (ranging from 4.5 to 9) on the electrocatalytic degradation of AMT using the 200 C@ZnO anode. As shown in Figure 5b, the AMT removal rate was higher under acidic conditions compared to alkaline conditions. Furthermore, the removal rate reached its maximum at pH 4.5, indicating that acidic conditions favor antibiotic degradation. As can be seen from Figure 5c, it demonstrates that during the adsorption process, pH values ranging from acidic to neutral significantly facilitate the adsorption of amitriptyline onto the catalyst surface, achieving adsorption efficiencies of approximately 10–20%. When the solution is alkaline, electron transfer at the C@ZnO anode surface is impeded, reducing electron generation [41]. Conversely, under acidic conditions, the presence of H+ inhibits the decomposition of ·OH into oxygen (OH + H2O → O2 + 3H+ + 3e−) [42], thereby enhancing the utilization efficiency of ·OH and increasing pollutant removal efficiency. Based on the Zeta potential measurements in Figure 5d, the zero charge point (ZPC) of the C@ZnO anode was determined to be 3.9. At elevated pH values, the electrode surface develops a pronounced negative potential, which enhances the electrostatic repulsion toward persulfate and sulfate ions. Consequently, this hampers the adsorption of pollutants onto the electrode, resulting in diminished removal efficiency. At lower pH values, the N lone pair electrons of the amine group undergo protonation, forming a positively charged ammonium moiety. This protonation significantly hampers electron donation from the amino group, thereby impeding electron transfer processes. Conversely, during deprotonation, the recovery of the amine group’s electron-donating capacity facilitates redox reactions, enabling more efficient degradation of pollutants [43]. Additionally, the energy consumption for AMT degradation was relatively lower at pH 7. Hence, pH 7 was selected for subsequent degradation application studies.
Electron paramagnetic resonance (EPR) spectroscopy was employed to characterize the reactive oxygen species (ROS) generated by the CZ-PS system. As shown in Figure 6a, distinct signals corresponding to DMPO-SO4•− and DMPO-•OH radicals were detected, indicating the formation of sulfate (SO4•−) and hydroxyl radicals (•OH), respectively. Furthermore, Figure 6b revealed the characteristic signals of O2•−, while a 1:1:1 triplet signal attributed to TEMP-1O2 was observed in Figure 6c. Notably, the intensities of all ROS signals exhibited a time-dependent increase between 5 and 10 min (Figure 6d), suggesting continuous generation of SO4•−, •OH, O2•−, and 1O2 within the system. These findings collectively demonstrate the efficacy of this system as a sustainable ROS generator, paving the way for its application in PPCPs removal experiments.
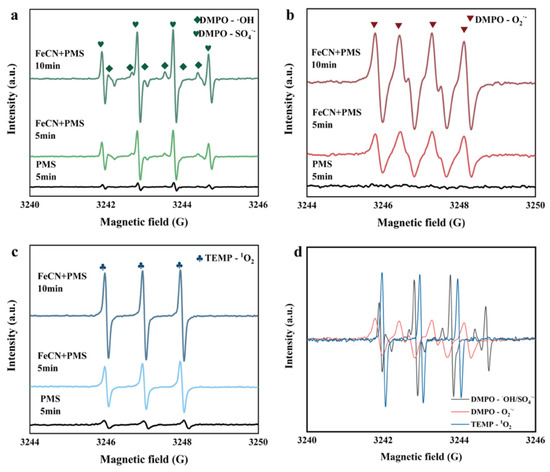
Figure 6.
The EPR spectrum of SO4•− and •OH (a), O2•− (b), 1O2 (c), The intensity increase of ROS from 5 to 10 min (d).
To investigate the stability and reusability of the electrode, the same piece of C@ZnO anode was used to degrade AMT for 5 consecutive cycles under identical operating conditions. As shown in Figure 7, the AMT removal rate using the C@ZnO anode remained above 90% throughout all cycles. The degradation efficiency showed almost no reduction after five reuses, demonstrating the excellent electrocatalytic activity and stability of the C@ZnO anode.
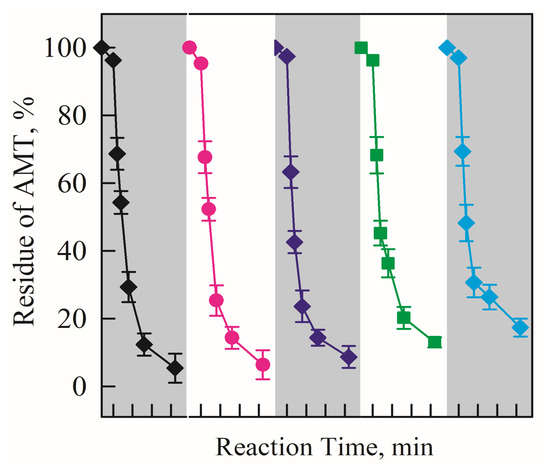
Figure 7.
AMT was degraded over five consecutive cycles using the electrocatalytic system with the 200 C@ZnO anode.
2.5. Degradation Pathway and Reaction Mechanism
In the CZ-PS system, the generation pathways of O2•−, •OH, SO4•−, and 1O2 are depicted in Figure 8 and Figure 9 as follows. In persulfate-mediated reactions, the disruption of -O-O- bonds in persulfate can lead to the formation of free radicals such as •OH and sulfate SO4•− [40]. The continuous redox cycling between two valence states of Zn ions on the surface enables the potential generation of 1O2 via 2 distinct mechanistic pathways: either through direct oxidation and subsequent recombination of O2•−, or through the oxidative transformation of SO4•− [41].
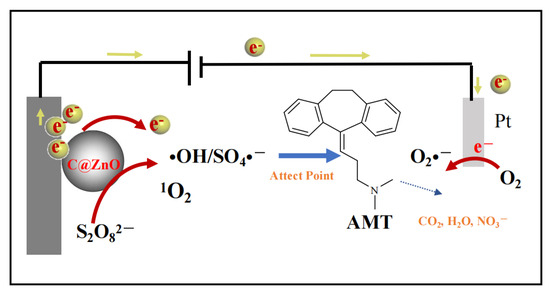
Figure 8.
Proposed schematic illustration of the mechanism for degradation of AMT in the CZ-PS system.
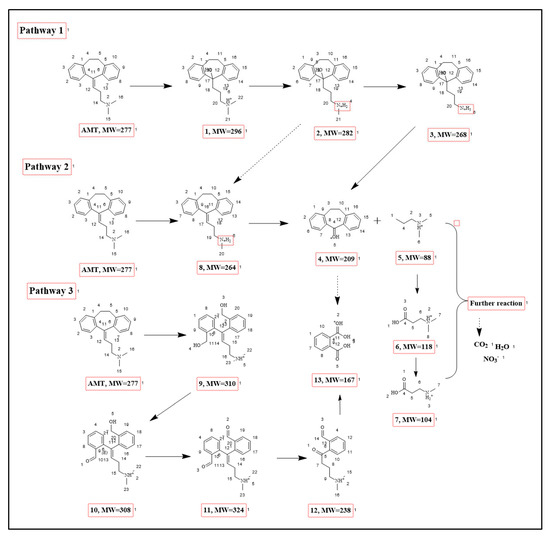
Figure 9.
Proposed Degradation Pathway of AMT in the CZ-PS System.
To further reveal the primary transformation products formed during AMT degradation in the C@ZnO anode/PS system, UPLC–MS/MS was employed for their identification. As can be seen from Figure 9, the degradation of AMT primarily proceeds through three distinct pathways, involving a total of 13 intermediates. Sequential demethylation and hydroxylation reactions initially modify the AMT molecule, yielding intermediates Product 1, Product 2, and Product 3 [42]. Subsequently, the dimethylamine moiety in Product 3 undergoes further demethylation and oxidative transformations, generating Products 4 and 5 [44]. Finally, Product 13 is formed via the cleavage of side chains from both Product 4 and Product 5, completing the degradation cascade. Then, Product 6 and Product 7 are formed via continuous ring-opening and hydroxylation reactions attacked by ROS; the seven-membered ring of the AMT molecule is opened through oxidation and hydroxylation, generating intermediate Product 9 [45]. The alcoholic hydroxyl group in Product 9 is continuously oxidized to an aldehyde, forming Product 10 and Product 11. Product 11 can also react with ·OH and sulfate radicals SO4•− to yield Product 12. Subsequently, Product 11 and Product 12 undergo aldehyde oxidation and alcoholic hydroxyl oxidation reactions, respectively, forming Product 13, where the aldehyde is further converted into a carboxyl group; Product 6 and Product 7 are also obtained through the cleavage of the side chain from Product 3. These aromatic intermediates are then further oxidized and mineralized into small molecules (such as NO3−, H2O, and CO2) via continuous ring-opening and hydroxylation reactions attacked by ROS.
3. Materials and Methods
3.1. Chemicals and Reagents
Amitriptyline (AMT, 98%), zinc oxide (ZnO, 99%), and polyvinylpyrrolidone ((C6H9NO)n, K23-27) were purchased from Aladdin (Shanghai, China) and Macklin (Shanghai, China), respectively. D(+)-Glucose monohydrate (C6H12O6·H2O, 98%), ethanol (C2H6O, ≥99.7%), potassium chloride (KCl, ≥99.5%), and nitric acid (HNO3, 65.0–68.0%) were all obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were of reagent grade or higher purity and were used without further purification. All aqueous solutions used in the experiments were prepared using deionized water.
3.2. Synthesis of C@ZnO Catalysts
Carbon-encapsulated zinc oxide nanocomposites (C@ZnO) were synthesized using a hydrothermal–calcination method [21]. Specifically, 0.5, 1.0, 2.0, and 4.0 g of ZnO were mixed with equal amounts of PVP in 300 mL of ultrapure water. Then, 19.8 g of glucose was added to the above suspension. After 24 h of stirring, the suspension was hydrothermally treated at 180 °C for 4 h, washed with ethanol, and dried at 60 °C. The resulting powder was calcined under N2 at 600 °C (10 °C/min ramp, 4 h hold), yielding dark grey solid powder. This powder was ground and sieved through a 60-mesh nylon sieve to ensure uniform particle size. Through this process, amorphous carbon-encapsulated ZnO composites were obtained, with ZnO masses of 50 mg, 100 mg, 200 mg, and 400 mg incorporated during synthesis. These were designated as 50 C@ZnO, 100 C@ZnO, 200 C@ZnO, and 400 C@ZnO, respectively.
3.3. Electrode Preparation and Screening
The C@ZnO-modified anode was fabricated via spin-coating of active material powder onto a dopant layer prepared on fluorine-doped tin oxide (FTO) glass. Prior to modification, the FTO substrates (50 mm × 40 mm × 2 mm) were successively rinsed with acetone and deionized water to remove surface impurities and oxide films. Following a 15-min ultrasonic cleaning step, 30 mg of C@ZnO powder was dispersed in a 1.5 mL mixture of ethanol and naphthol (1:1 v/v), subjected to ultrasonic treatment for 30 min to ensure homogeneous suspension. The resultant colloidal dispersion was uniformly coated onto the FTO substrate under infrared irradiation and subsequently dried overnight in a vacuum oven at 60 °C, yielding the C@ZnO-modified electrode.
3.4. Screening Experiments for Modified Electrodes
Electrocatalytic oxidation experiments were performed under the following conditions: current density of 20 mA/cm2, electrode spacing of 20 mm, initial AMT concentration of 5 mg/L, and 5 mM sodium sulfate added as the supporting electrolyte. Through these screening experiments, the modified C@ZnO electrode exhibiting the best electrocatalytic activity was identified. Before powering, the suspension was magnetically stirred for 30 min in dark in order to obtain a homogeneous mixture. This selected electrode was then used in subsequent experiments to investigate the factors influencing AMT removal from water. Using the screened modified electrode as the anode and Pt as the cathodes, with 5 mM Na2SO4 as the supporting electrolyte and an electrode spacing of 20 mm, the effects of different initial AMT concentrations, different current densities, and different initial pH values on the electrocatalytic oxidation removal efficiency of AMT from water were examined. Samples were taken every 30 min during the 120-min reaction period. To evaluate stability and reusability in experiments (e.g., sensor performance, catalyst efficiency, material durability), the Relative Standard Deviation (RSD) is essential for quantifying precision and consistency [46]. Here’s why and how it’s used:
RSD (%) = (Standard Deviation (SD)/Mean) × 100%
In these conditions, the treated samples were incubated at 25 ± 1 °C. At designated time intervals, triplicate samples (one centrifuge tube for each sample) were withdrawn for the total AMT residue analysis. All of the experiments were treated in triplicate. Data points for AMT residue were means ± standard deviations (n = 3).
3.5. Analytical Methods
The spectral information of ROS was recorded on an electron paramagnetic resonance spectrometer (EPR, JE-FA 300, Tokyo, Japan) using 50 mM DMPO as spin capture agents. The concentration of AMT in reaction samples was determined using reversed-phase high-performance liquid chromatography (HPLC) coupled with a variable wavelength ultraviolet detector (Jasco, Tokyo, Japan, λ = 205 nm) and a Spheri-5 C18 separation column. The isocratic mobile phase consisted of 65% (v/v) acetonitrile and 35% (v/v) water, delivered at a flow rate of 1.0 mL/min. Degradation products were analyzed using UPLC–MS/MS instrumentation (Xevo TQ-S, Waters, Milford, MA, USA), with an Agilent 6460 triple quadrupole system equipped with a Zorbax XDBC8 chromatographic column (2.1 mm × 150 mm × 3.5 μm) and operated in positive electrospray ionization mode (ESI+; m/z 50–600). The LC–MS mobile phase comprised 65% (v/v) acetonitrile and 35% (v/v) water, flowing at 1.0 mL/min.
4. Conclusions
This study developed an electrocatalytic persulfate (PS) activation system using carbon-coated ZnO (C@ZnO) electrodes, enabling 97.5% degradation of 5 mg/L amitriptyline (AMT) in 120 min under optimized conditions (200 C@ZnO anode, pH 7.0, 20 mA/cm2, 0.5 mM PS). Material characterization revealed that the amorphous carbon layer enhanced surface area (443.44 m2/g) and porosity, facilitating charge transfer and radical generation. EPR and quenching experiments showed that both acidic and neutral pH conditions were demonstrated to favorably enhance the electrocatalytic degradation efficiency by improving adsorption performance and inhibiting •OH decomposition, with •OH and SO4•− as predominant reactive species. UPLC–MS/MS analysis identified three AMT degradation pathways involving 13 intermediates, and sustained radical attacks achieved complete mineralization to CO2, H2O, and inorganic ions. The system efficiently degraded AMT under near-neutral pH (7.0) while avoiding toxic dealkylated byproducts. The electrode maintained >90% efficiency after five reuse cycles, providing a viable electrocatalytic strategy for antibiotic wastewater treatment and laying a foundation for advanced industrial purification technologies.
Author Contributions
Conceptualization, Y.Y.; Methodology, T.W. and F.H.; Software, C.L. and Y.H.; Validation, Y.Y.; Formal analysis, T.W.; Investigation, C.L.; Resources, F.H. and Y.H.; Data curation, Y.Y.; Writing—original draft, T.W. and J.D.; Writing—review & editing, F.H.; Visualization, C.L.; Supervision, F.H.; Project administration, J.D.; Funding acquisition, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (Grant No. 2023C03149).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, R.; Yang, S.; An, Y.; An, Y.W.; Wang, Y.Q.; Lei, Y.; Song, L.Y. Antibiotics and antibiotic resistance genes in landfills: A review. Sci. Total Environ. 2022, 806, 150647. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhang, Y.Y.; Li, B.C.; Yuan, X.; Men, C.; Zuo, J.E. Antibiotic intermediates and antibiotics synergistically promote the development of multiple antibiotic resistance in antibiotic production wastewater. J. Hazard. Mater. 2024, 479, 135601. [Google Scholar] [CrossRef]
- Yuan, X.; Lv, Z.Q.; Zhang, Z.Y.; Han, Y.; Liu, Z.Q.; Zhang, H.J. A review of antibiotics, antibiotic resistant bacteria, and resistance genes in aquaculture: Occurrence, contamination, and transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Jiang, G.Y.; Wang, C.; Ma, J.B.; Chen, H. Effects of antibiotics consumption on the behavior of airborne antibiotic resistance genes in chicken farms. J. Hazard. Mater. 2022, 437, 129288. [Google Scholar] [CrossRef]
- Čižman, M.; Kastrin, T.; Beović, B.; Mahnic, A.; Bajec, T. The impact of national activities on antibiotic consumption in hospitals and different departments over a 14-Year period. Antibiotics 2024, 13, 498. [Google Scholar] [CrossRef]
- Sun, S.J.; Wang, Q.; Wang, N.; Yang, S.J.; Qi, H. High-risk antibiotics positively correlated with antibiotic resistance genes in five typical urban wastewater. J. Environ. Chem. Eng. 2023, 342, 118296. [Google Scholar] [CrossRef]
- Pantke, S.; Steinberg, J.H.; Weber, L.K.H.; Fricke, T.C.; Carvalheira Arnaut Pombeiro Stein, I.; Oprita, G.; Herzog, C.; Leffler, A. A high concentrations of the antidepressant amitriptyline activate and desensitize the capsaicin receptor TRPV1. Pharmaceuticals 2025, 18, 560. [Google Scholar] [CrossRef]
- Lampl, C.; Versijpt, J.; Amin, F.M.; Deligianni, C.I.; Gil-Gouveia, R.; Jassal, T.; MaassenVanDenBrink, A.; Ornello, R.; Paungarttner, J.; Sanchez-del-Rio, M.; et al. European Headache Federation (EHF) critical re-appraisal and meta-analysis of oral drugs in migraine prevention—Part 1: Amitriptyline. J. Headache Pain 2023, 24, 39. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Visible light photocatalytic degradation of amitriptyline using cobalt doped titanate nanowires: Kinetics and characterization of transformation products. J. Environ. Chem. Eng. 2020, 8, 103585. [Google Scholar] [CrossRef]
- Madej, M.; Fendrych, K.; Porada, R.; Flacha, M.; Kochana, J.; Baś, B. Application of Fe(III)-exchanged clinoptilolite/graphite nanocomposite for electrochemical sensing of amitriptyline. Microchem. J. 2021, 160, 105648. [Google Scholar] [CrossRef]
- Tee, W.T.; Loh, N.Y.L.; Hiew, B.Y.Z.; Show, P.L.; Hanson, S.; Gan, S.Y.; Lee, L.Y. Evaluation of adsorption performance and mechanisms of a highly effective 3D boron-doped graphene composite for amitriptyline pharmaceutical removal. J. Environ. Manage. 2023, 344, 118363. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Liu, Y.P.; Zhang, L.; Xie, P.C.; Wang, Z.P.; Zhou, A.J.; Fang, Z.; Ma, J. Efficient degradation of imipramine by iron oxychloride-activated peroxymonosulfate process. J. Hazard. Mater. 2018, 353, 18–25. [Google Scholar] [CrossRef]
- Wang, Z.Y.; He, J.G.; Yu, D.H.; Tang, B.; Zheng, Y.S.; Qiu, W. N-doped coralline Co9S8−xNx for inducing Amitriptyline decontamination in electro-fenton Process: Degradation scheme elucidation, nitrogen activating catalyst delocalized electron and enhancing 2-electron oxygen reduction reaction mechanism investigation. Chem. Eng. J. 2023, 457, 141171. [Google Scholar] [CrossRef]
- Chen, F.; Wu, X.L.; Yang, L.; Chen, C.F.; Lin, H.J.; Chen, J.R. Efficient degradation and mineralization of antibiotics via heterogeneous activation of peroxymonosulfate by using graphene supported single-atom Cu catalyst. Chem. Eng. J. 2020, 394, 124904. [Google Scholar] [CrossRef]
- Zhang, H.J.; He, Y.Y.; He, M.F.; Yang, Q.Y.; Ding, G.Y.; Mo, Y.S.; Liu, Z.Q.; Gao, P.P. Construction of cubic CaTiO3 perovskite modified by highly-dispersed cobalt for efficient catalytic degradation of psychoactive pharmaceuticals. J. Hazard. Mater. 2023, 459, 132191. [Google Scholar] [CrossRef] [PubMed]
- Finčur, N.L.; Grujić-Brojčin, M.; Šćepanović, M.J.; Četojević-Simin, D.D.; Maletić, S.P.; Stojadinović, S.; Abramović, B.F. UV-driven removal of tricyclic antidepressive drug amitriptyline using TiO2 and TiO2/WO3 coatings. React. Kinet. Mech. Cat. 2021, 132, 1193–1209. [Google Scholar] [CrossRef]
- Cai, W.W.; Yang, H.; Guo, X.Z. Preparation, characterization and infrared emissivity of ZnO nanorod/mica composites. Chin. J. Inorg. Chem. 2014, 30, 229–234. [Google Scholar]
- Zhang, P.; Xu, X.Y.; Chen, Y.P.; Xiao, M.Q.; Feng, B.; Tian, K.X.; Chen, Y.H.; Dai, Y.Z. Protein corona between nanoparticles and bacterial proteins in activated sludge: Characterization and effect on nanoparticle aggregation. Bioresour. Technol. 2018, 250, 10–16. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, X.Z.; Wu, D.X.; Yu, B.Z.; Lu, S.H.; Wang, J.J.; Ding, J.F. A novel strategy for efficient capture of intact harmful algal cells using Zinc oxide modified carbon nitride composites. Algal Res. 2023, 69, 102932. [Google Scholar] [CrossRef]
- Roy, N.; Kannabiran, K.; Mukherjee, A. Integrated adsorption and photocatalytic degradation based removal of ciprofloxacin and sulfamethoxazole antibiotics using Fe@rGO-ZnO nanocomposite in aqueous systems. Chemosphere 2023, 333, 138912. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, X.Z.; Yu, B.Z.; Wang, J.J.; Lu, S.H.; Zhong, Y.C.; Ding, J.F. Fabrication of amorphous carbon-based zinc oxide for efficient capture of intact Microcystis aeruginosa: Lysis in sedimentation process. J. Environ. Chem. Eng. 2022, 10, 108793. [Google Scholar] [CrossRef]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Hu, L.M.; Li, L.T.; Zheng, Q.Z.; Xin, Y.J.; Zhang, G.S. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Preethi; Shanmugavel, S.P.; Kumar, G.; Yogalakshmi, K.N.; Gunasekaran, M.; Banu, J.R. Recent progress in mineralization of emerging contaminants by advanced oxidation process: A review. Environ. Pollut. 2024, 341, 122842. [Google Scholar] [CrossRef]
- Milh, H.; Yu, X.; Cabooter, D.; Dewil, R. Degradation of ciprofloxacin using UV-based advanced removal processes: Comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci. Total Environ. 2021, 764, 144510. [Google Scholar] [CrossRef]
- Ansari, A.T.A.; Mukherji, S.; Mukherji, S. Enhanced visible-light photocatalysis of a senary mixture of antibiotics using a low-dose of TiO2–ZnO nanocomposite. Environ. Res. 2025, 282, 122083. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.C.; Zhang, L.; Chen, J.H.; Ding, J.Q.; Wan, Y.; Wang, S.L.; Wang, Z.P.; Zhou, A.J.; Ma, J. Enhanced degradation of organic contaminants by zero-valent iron/sulfite process under simulated sunlight irradiation. Water Res. 2019, 149, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Seo, H.; Kim, K.; Lee, J.; Kim, J.-H. Facile synthesis and electrochemical properties of carbon-coated ZnO nanotubes for high-rate lithium storage. Ceram. Int. 2018, 44, 18222–18226. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Ma, C.; Zhu, Z.; Song, X.H.; Wang, H.Q.; Huo, P.W.; Li, X.Y. Local surface plasma resonance effect enhanced Z-scheme ZnO/Au/g-C3N4 film photocatalyst for reduction of CO2 to CO. Appl. Catal. B—Environ. 2021, 283, 119638. [Google Scholar] [CrossRef]
- Lang, J.H.; Wang, J.Y.; Zhang, Q.; Li, X.Y.; Han, Q.; Wei, M.B.; Sui, Y.R.; Wang, D.D.; Yang, J.H. Chemical precipitation synthesis and significant enhancement in photocatalytic activity of Ce-doped ZnO nanoparticles. Ceram. Int. 2016, 42, 14175–14181. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, X.; Zhu, B.Y.; Wang, J.S.; Lan, H.X.; Chen, X.B. Synthesis of porous ZnS, ZnO and ZnS/ZnO nanosheets and their photocatalytic properties. RSC Adv. 2017, 7, 30956–30962. [Google Scholar] [CrossRef]
- Decremps, F.; Pellicer-Porres, J.; Saitta, A.M.; Chervin, J.C.; Polian, A. High-pressure raman spectroscopy study of wurtzite ZnO. Phy. Rev. B 2002, 65, 092101. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Kim, D.S.; Chinh, N.D.; Park, J.S.; Jeong, Y.J.; Piao, J.J.; Kim, D.; Lee, D.W. N-/S- dual doped C@ZnO: An excellent material for highly selective and responsive NO2 sensing at ambient temperatures. Chem. Eng. J. 2021, 421, 127740. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.F.; Li, X.; Zhang, J.; Wang, M.; Che, R.C. MOF-derived yolk-shell Ni@C@ZnO schottky contact structure for enhanced microwave absorption. Chem. Eng. J. 2020, 383, 123099. [Google Scholar] [CrossRef]
- Goncearenco, E.; Morjan, I.P.; Fleaca, C.; Dutu, E.; Criveanu, A.; Viespe, C.; Galca, A.C.; Maraloiu, A.V.; Stan, M.S.; Fort, C.I.; et al. The influence of SnO2 and noble metals on the properties of TiO2 for environmental sustainability. Sustainability 2024, 16, 2904. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, J.W.; Qiao, X.L.; Wang, D.G.; Cai, X.Y. Performance of nano-Co3O4/peroxymonosulfate system: Kinetics and mechanism study using Acid Orange 7 as a model compound. Appl. Catal. B—Environ. 2008, 80, 116–121. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.Z.; Li, X.Z.; He, M.F.; Li, Y.; Ding, J.F.; Zhong, Y.C.; Zhang, H.J. Selective production of singlet oxygen for harmful cyanobacteria inactivation and cyanotoxins degradation: Efficiency and mechanisms. J. Hazard. Mater. 2023, 441, 129940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Yu, B.Z.; Li, X.Z.; Li, Y.; Zhong, Y.C.; Ding, J.F. Inactivation of Microcystis aeruginosa by peroxydisulfate activated with single-atomic iron catalysis: Efficiency and mechanisms. J. Environ. Chem. Eng. 2022, 10, 108310. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. A comparative study of electrocoagulation, electrochemical fenton, electro-fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability improvement. J. Environ. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Guan, J.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.C.; Chen, L.; Yan, L.L.; Cheng, T.; Wang, X.L.; Zhang, Y. Singlet oxygendominated transformation of oxytetracycline by peroxymonosulfate with CoAlLDH modified hierarchical porous ceramics: Toxicity assessment. Chem. Eng. J. 2022, 436, 135199. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, J.F.; Liu, L.; Lu, X.Y.; Deng, J.W.; Pozdnyakov, I.P.; Zuo, Y.G. Photosensitized degradation of amitriptyline and its active metabolite nortriptyline in aqueous fulvic acid solution. J. Environ. Qual. 2017, 46, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, J.F.; Yin, L.F.; Huang, J.X.; Hou, L.A. Electrochemical degradation of fluoxetine on nanotube array intercalated anode with enhanced electronic transport and hydroxyl radical production. Chem. Eng. J. 2018, 346, 662–671. [Google Scholar] [CrossRef]
- Zhan, M.M.; Hong, Y.; Fang, Z.; Qiu, D.P. Visible light-driven photocatalytic degradation of Microcystin-LR by Bi2WO6/Reduced graphene oxide heterojunctions: Mechanistic insight, DFT calculation and degradation pathways. Chemosphere 2023, 321, 138105. [Google Scholar] [CrossRef]
- Fort, C.I.; Ortiz, R.; Contet, L.C.; Danciu, V.; Popescu, I.C.; Gorton, L. Carbon aerogel as electrode material for improved direct electron transfer in biosensors incorporating cellobiose dehydrogenase. Electroanalysis 2016, 10, 219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).