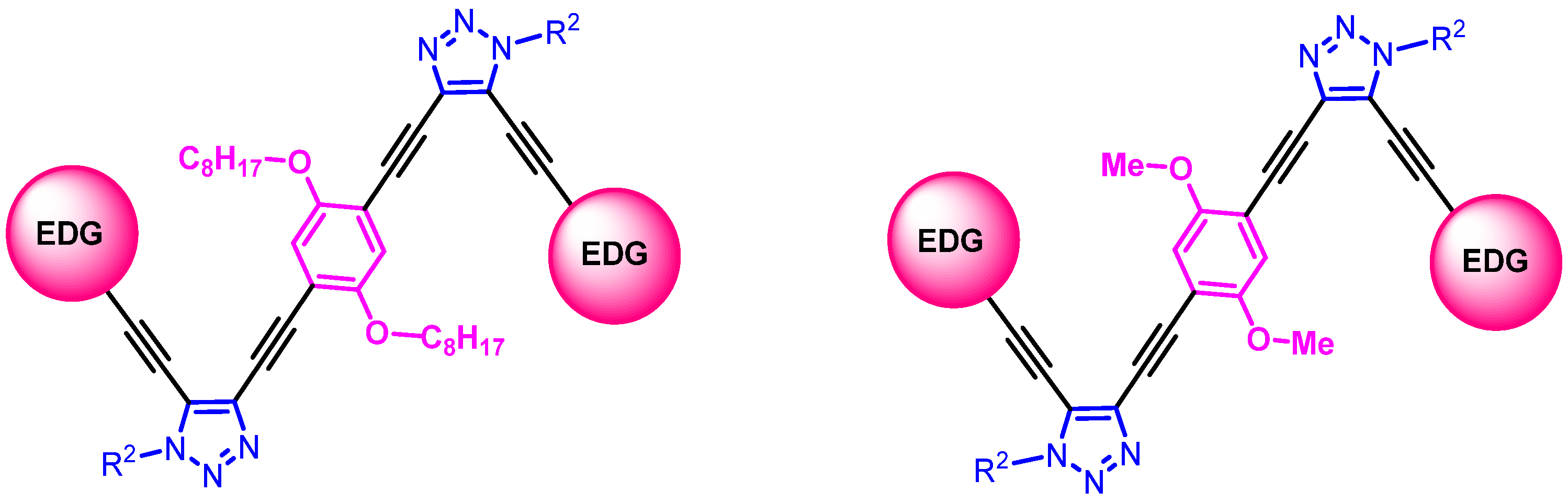
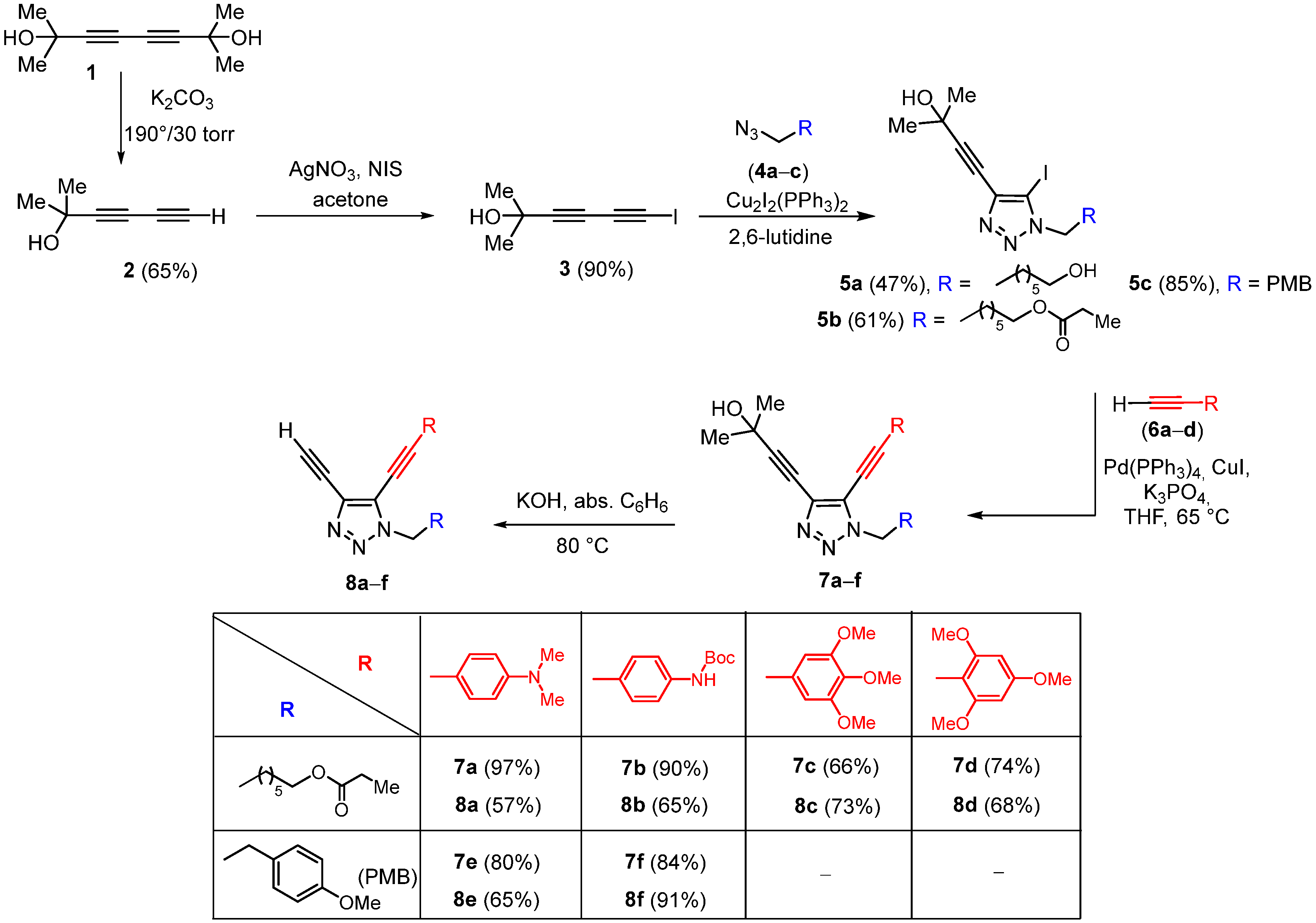
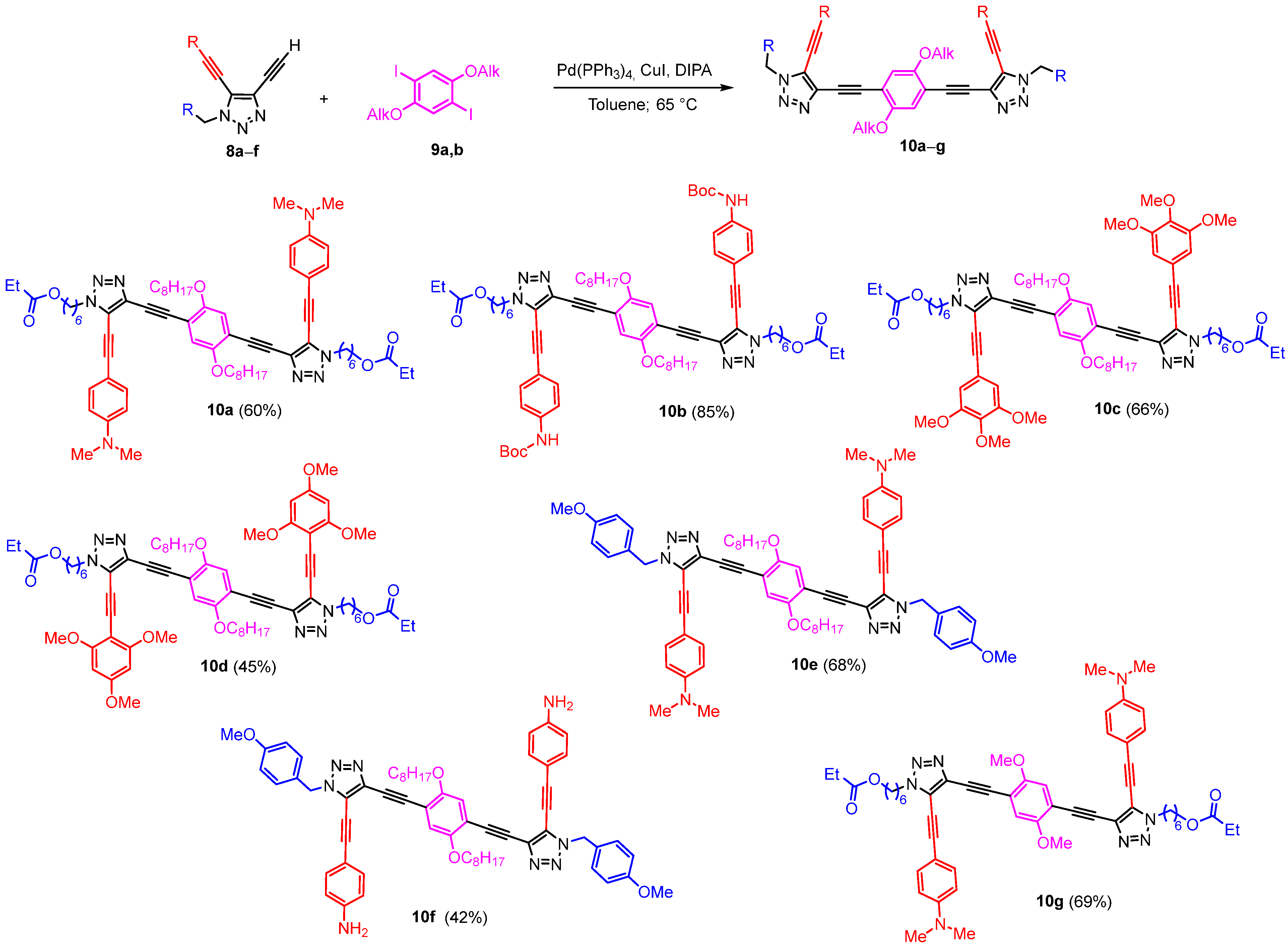
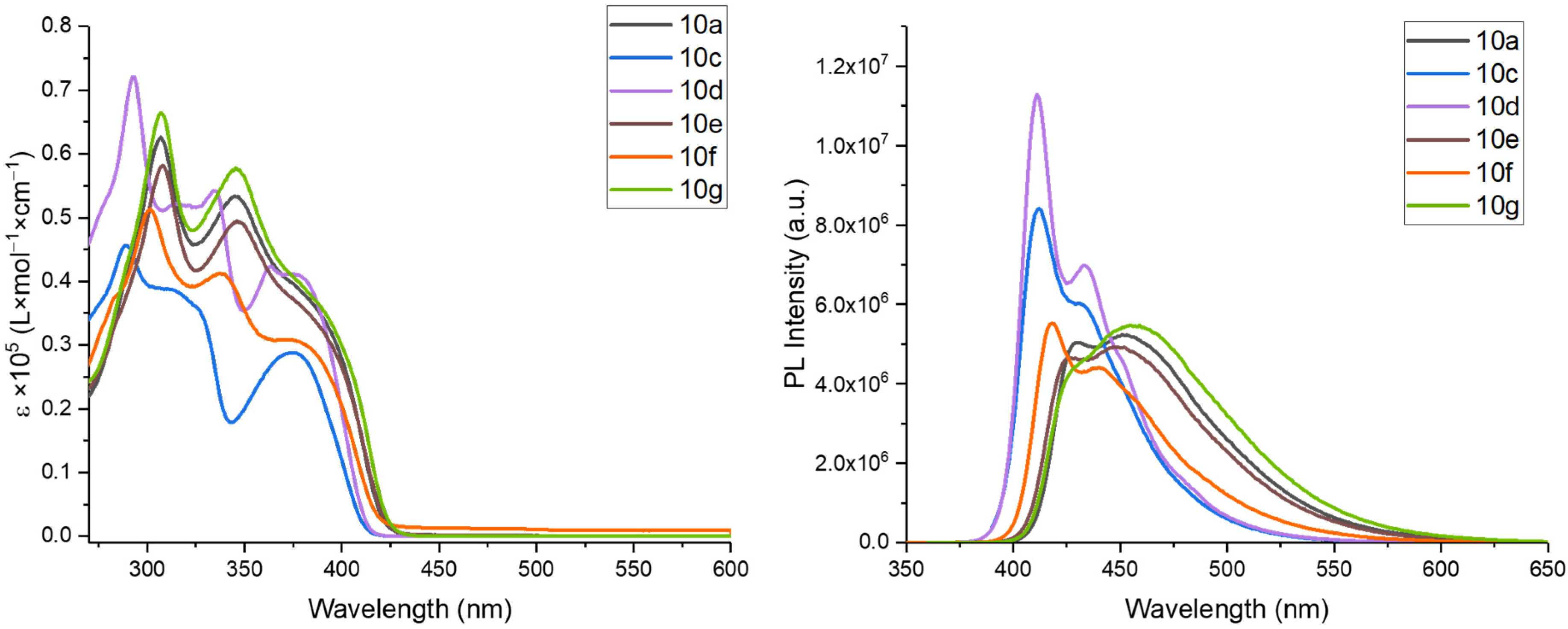
3.2. Synthetic Methods and Analytic Data of Compounds
3.2.1. General Procure for the CuAAC
To an azide (1.00 equiv.) in a vial were consistently added 1-iodobuta-1,3-diyne (1.00 equiv.), [Cu2I2(PPh2)]2 (1.5–5 mol%) and 2,6-lutidine (4–8 mol%). The thick resulting mixture was vigorously stirred overnight at room temperature or 40 °C (TLC control). Then the reaction mixture was diluted with CH2Cl2, and the resulting solution was washed with a saturated aqueous solution of NH4Cl. The mixture was extracted, and the combined organic layers were dried over Na2SO4, filtered, and concentrated. The residue was purified by column chromatography on silica gel.
6-(4-(3-Hydroxy-3-methylbut-1-yn-1-yl)-5-iodo-1H-1,2,3-triazol-1-yl)hexan-1-ol (5a) was prepared in accordance with the general procedure from 6-iodo-2-methylhexa-3,5-diyn-2-ol 3 (100.0 mg, 0.43 mmol), 6-azidohexan-1-ol 4a (85.6.0 mg, 0.60 mmol), [Cu2I2(PPh2)]2 (64.2 mg, 0.086 mmol, 20 mol%) and 2,6-lutidine (3.7 mg, 0.034 mmol, 8 mol%). The reaction mixture was stirred for 30 h at 40 °C. The crude product was purified by column chromatography (eluent:hexane/acetone = 2:1) to afford brown viscous oil (76 mg, 47%). 1H NMR (400 MHz, CDCl3) δ 4.37 (t, J = 7.2 Hz, 2H), 3.17 (t, J = 6.9 Hz, 2H), 2.17 (s, 1H), 1.92 (p, J = 7.3 Hz, 2H), 1.81 (p, J = 7.0 Hz, 2H), 1.65 (s, 6H), 1.50–1.40 (m, 2H), 1.40–1.30 (m, 1H).
6-(4-(3-Hydroxy-3-methylbut-1-yn-1-yl)-5-iodo-1H-1,2,3-triazol-1-yl)hexyl propionate (5b) was prepared in accordance with the general procedure from 6-iodo-2-methylhexa-3,5-diyn-2-ol 3 (300 mg, 1.28 mmol), 6-azidohexyl propionate 4b (383 mg, 1.79 mmol), [Cu2I2(PPh2)]2 (96.3 mg, 0.064 mmol, 5 mol%) and 2,6-lutidine (11 mg, 0.10 mmol, 8 mol%). The reaction mixture was stirred overnight at 40 °C. The crude product was purified by column chromatography (eluent:hexane/acetone = 3:1) to afford a brown, viscous oil (350 mg, 61%). 1H NMR (CDCl3, 400 MHz) δ 4.35 (t, J = 7.2 Hz, 2H), 4.05 (t, J = 6.6 Hz, 2H), 2.36–2.26 (m, 2H), 1.97–1.84 (m, 2H), 1.45–1.30 (m, 4H), 1.13 (t, J = 7.6 Hz, 3H). 13C NMR (CDCl3, 101 MHz) 174.7, 137.6, 99.8, 84.1, 65.7, 64.2, 51.2, 31.3, 29.7, 28.5, 27.7, 26.1, 25.5, 9.3. HRMS ESI: [M + Na]+ calcd. for C16H24IN3NaO3+: 456.0755; found: 456.0759.
4-(5-Iodo-1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)-2-methylbut-3-yn-2-ol (5c) was prepared in accordance with the general procedure from 6-iodo to 2-methylhexa-3,5-diyn-2-ol 3 (200 mg, 0.85 mmol), [Cu2I2(PPh3)2]2 (32.1 mg, 0.021 mmol, 5 mol%), 2,6-lutidine (3.66 mg, 0.034 mmol, 4 mol%) and 1-(azidomethyl)-4-methoxybenzene 4c (153 mg, 0.94 mmol). The reaction mixture was stirred overnight at RT. The crude product was purified by column chromatography (eluent:hexane/EtOAc = 3:1) to afford a white solid (290 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 7.25–7.19 (m, 2H), 6.90–6.81 (m, 2H), 5.50 (s, 2H), 3.78 (s, 3H), 1.62 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 160.0, 138.1, 129.6, 126.0, 114.4, 99.9, 84.1, 71.8, 65.7, 55.5, 54.4, 31.3. HSMS ESI: [M + H]+ calcd for C15H16IN3O2+ 398.0360; found: 398.0367.
3.2.2. General Procedure for the Sonogashira Cross-Coupling
A mixture of 5-iodo-1H-1,2,3-triazole 5b or 5c (1.0 equiv.), Pd(PPh3)4 (5 mol%), CuI (10 mol%), and K3PO4 (1.1 equiv.) were placed in a vial. The vial was sealed, evacuated, and refilled with argon (three cycles). Tetrahydrofuran (1 mL) was added, and the mixture was stirred at room temperature for 10 min. The respective alkyne 6a–d (1.2 equiv.) was then introduced, and the reaction vial was immersed in a pre-heated aluminum block at 65 °C. The mixture was stirred for 5–6 h, with the reaction progress monitored by TLC. After cooling to ambient temperature, the mixture was filtered through a short pad of silica gel, eluting with CH2Cl2 (3 × 10 mL). The combined filtrates were concentrated under reduced pressure, and the crude residue was purified by column chromatography on silica gel.
6-(5-((4-(Dimethylamino)phenyl)ethynyl)-4-(3-hydroxy-3-methylbut-1-yn-1-yl)-1H-1,2,3-triazol-1-yl)hexyl propionate (7a) was prepared in accordance with the general procedure from 5-iodo-1H-1,2,3-triazole 5b (80 mg, 0.19 mmol), 4-ethynyl-N,N-dimethylaniline 6a (29.5 mg, 0.2 mmol), Pd(PPh3)4 (10.7 mg, 0.0092 mmol), CuI (3.5 mg, 0.019 mmol) and K3PO4 (43 mg, 0.2 mmol) with a reaction time of 6 h. The crude product was purified by column chromatography (eluent: hexane/acetone = 2:1) to afford a brown, viscous oil (81 mg, 97%). 1H NMR (400 MHz, CDCl3) δ 7.46–7.36 (m, 2H, Ar), 6.72–6.64 (m, 2H, Ar), 4.41 (t, J = 7.0 Hz, 2H, CH2), 4.05 (t, J = 6.5 Hz, 2H, CH2), 3.04 (s, 6H, NMe2), 2.31 (q, J = 7.6 Hz, 2H, CH2), 2.04–1.94 (m, 2H, CH2), 1.66 (s, 6H, C(CH3)2), 1.65–1.58 (m, 2H, CH2), 1.45–1.35 (m, 4H, 2CH2), 1.13 (t, J = 7.6 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 151.1, 133.1, 132.3, 124.9, 111.8, 107.3, 104.8, 99.7, 71.8, 71.5, 65.7, 64.2, 49.3, 40.2, 31.3, 29.6, 28.6, 27.7, 26.2, 25.5, 9.2; HSMS ESI: [M + H]+ calc for C26H34N4O3+ 451.2704; found: 451.2695.
6-(5-((4-((tert-Butoxycarbonyl)amino)phenyl)ethynyl)-4-(3-hydroxy-3-methylbut-1-yn-1-yl)-1H-1,2,3-triazol-1-yl)hexyl propionate (7b) was prepared in accordance with the general procedure from 5-iodo-1H-1,2,3-triazole 5b (100 mg, 0.23 mmol), alkyne 6b (55.2 mg, 0.25 mmol), Pd(PPh3)4 (13.3 mg, 0.0115 mmol), CuI (4.4 mg, 0.023 mmol) and K3PO4 (53.9 mg, 0.25 mmol) with a reaction time of 6 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1) to afford a brown viscous oil (109 mg, 90%). 1H NMR (400 MHz, CDCl3) δ 7.49–7.39 (m, 4H, Ar), 6.75 (m, 1H, NH), 4.40 (t, J = 7.0 Hz, 2H, CH2), 4.02 (t, J = 6.5 Hz, 2H, CH2), 2.29 (q, J = 7.6 Hz, 2H, CH2), 2.03–1.92 (m, 2H, CH2), 1.64 (s, 6H, C(CH3)2), 1.62–1.53 (m, 2H, CH2), 1.52 (s, 9H, Boc), 1.45–1.32 (m, 4H, 2CH2), 1.11 (t, J = 7.6 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 152.4, 140.2, 133.1, 132.8, 124.1, 118.3, 115.1, 103.1, 100.1, 81.4, 72.6, 71.6, 65.7, 64.2, 49.5, 31.4, 29.6, 28.6, 28.4, 27.7, 26.2, 25.5, 9.3. HSMS ESI: [M + Na]+ calcd. for C29H38N4O5Na+ 545.2734; found: 545.2732.
6-(4-(3-Hydroxy-3-methylbut-1-yn-1-yl)-5-((3,4,5-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazol-1-yl)hexyl propionate (7c) was prepared in accordance with the general procedure from 5-iodo-1H-1,2,3-triazole 5b (150 mg, 0.35 mmol), 5-ethynyl-1,2,3-trimethoxybenzene 6c (73.2 mg, 0.38 mmol), Pd(PPh3)4 (20 mg, 0.017 mmol), CuI (6.6 mg, 0.035 mmol) and K3PO4 (80.8 mg, 0.38 mmol) with a reaction time of 6 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 1:1) to afford a white solid (114 mg, 66%). 1H NMR (400 MHz, CDCl3) δ 6.76 (s, 2H, Ar), 4.41 (t, J = 7.0 Hz, 2H), 4.02 (t, J = 6.6 Hz, 2H), 3.90–3.85 (m, 9H, 3OMe), 2.28 (q, J = 7.6 Hz, 2H), 2.01–1.97 (m, J = 7.2 Hz, 2H), 1.64 (s, 6H), 1.62–1.53 (m, 2H), 1.42–1.34 (m, 4H), 1.10 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 174.7, 153.4, 140.4, 133.4, 123.9, 116.0, 109.2, 103.1, 100.2, 72.3, 71.5, 65.7, 64.1, 61.2, 56.4, 49.5, 31.3, 29.6, 28.5, 27.7, 26.2, 25.5, 9.2. HSMS ESI: [M + H]+ calc for C27H35N3O6Na+ 520.2418; found: 520.2415.
6-(4-(3-Hydroxy-3-methylbut-1-yn-1-yl)-5-((2,4,6-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazol-1-yl)hexyl propionate (7d) was prepared in accordance with the general procedure from 5-iodo-1H-1,2,3-triazole 5b (150 mg, 0.35 mmol), 2-ethynyl-1,3,5-trimethoxybenzene 6d (73.2 mg, 0.38 mmol), Pd(PPh3)4 (20 mg, 0.017 mmol), CuI (6.6 mg, 0.035 mmol) and K3PO4 (80.8 mg, 0.38 mmol) with a reaction time of 6 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1) to afford a white solid (128 mg, 74%). 1H NMR (400 MHz, CDCl3) δ 6.11 (s, 2H, Ar), 4.43 (t, J = 7.0 Hz, 2H), 4.01 (t, J = 6.6 Hz, 2H), 3.87 (s, 6H, 2OMe), 3.84 (s, 3H, OMe), 2.38 (s, 1H, OH), 2.28 (q, J = 7.6 Hz, 2H), 2.08–1.91 (m, 2H), 1.63 (s, 6H), 1.63–1.52 (m, 2H), 1.43–1.29 (m, 4H), 1.10 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 174.7, 163.2, 162.7, 131.8, 125.3, 99.6, 96.9, 93.0, 90.7, 80.1, 72.1, 65.7, 64.3, 56.2, 55.7, 49.3, 31.3, 29.5, 28.6, 27.7, 26.2, 25.5, 9.2. HSMS ESI: [M + Na]+ calcd. for C27H35N3O6Na+ 520.2418; found: 520.2413.
4-(5-((4-(Dimethylamino)phenyl)ethynyl)-1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)-2-methylbut-3-yn-2-ol (7e) was prepared in accordance with the general procedure from 5-iodo-1H-1,2,3-triazole 5c (100 mg, 0.25 mmol), 4-ethynyl-N,N-dimethylaniline 6a (43.9 mg, 0.30 mmol), Pd(PPh3)4 (14.6 mg, 0.013 mmol), CuI (4.8 mg, 0.025 mmol) and K3PO4 (58.8 mg, 0.28 mmol) with a reaction time of 5 h. The crude product was purified by column chromatography (eluent: hexane/acetone = 2:1) to afford a brown solid (80 mg, 77%). 1H NMR (400 MHz, CDCl3) δ 7.44–7.37 (m, 2H, Ar), 7.37–7.30 (m, 2H, Ar), 6.95–6.83 (m, 2H, Ar), 6.73–6.65 (m, 2H, Ar), 5.52 (s, 2H, CH2), 3.80 (s, 3H, OMe), 3.05 (s, 6H, NMe2), 2.31 (s, 1H, OH), 1.65 (s, 6H, C(CH3)2). 13C NMR (101 MHz, CDCl3) δ 159.9, 151.1, 133.1, 132.7, 129.9, 126.8, 124.7, 114.7, 111.8, 107.5, 105.2, 99.8, 71.9, 71.8, 65.7, 55.4, 52.8, 40.2, 31.4. HSMS ESI: [M + H]+ calcd. for C25H27N4O2+ 415.2129; found: 415.2122.
tert-Butyl (4-((4-(3-hydroxy-3-methylbut-1-yn-1-yl)-1-(4-methoxybenzyl)-1H-1,2,3-triazol-5-yl)ethynyl)phenyl)carbamate (7f) was prepared in accordance with the general procedure from 5-iodo-1H-1,2,3-triazole 5c (100 mg, 0.25 mmol), tert-butyl (4-ethynylphenyl)carbamate 6b (65.4 mg, 0.30 mmol), Pd(PPh3)4 (14.6 mg, 0.013 mmol), CuI (4.8 mg, 0.025 mmol) and K3PO4 (58.8 mg, 0.28 mmol) with a reaction time of 5 h. The crude product was purified by column chromatography (eluent: hexane/acetone = 2:1) to afford a yellow solid. (103 mg, 84%). 1H NMR (400 MHz, CDCl3) δ 7.42 (s, 4H, Ar), 7.35–7.27 (m, 2H, Ar), 6.91–6.84 (m, 2H, Ar), 6.75 (br.s, 1H, NH), 5.51 (s, 2H, CH2), 3.77 (s, 3H, OMe), 1.62 (s, 6H, C(CH3)2), 1.52 (s, 9H, Boc). 13C NMR (101 MHz, CDCl3) δ 160.0, 152.4, 140.2, 132.8, 129.8, 126.5, 124.0, 118.3, 115.2, 114.4, 103.4, 100.1, 81.4, 72.9, 71.6, 65.7, 55.5, 53.0, 31.3, 28.4. HSMS ESI: [M + H]+ calcd. for C28H31N4O4+ 487.2340; found: 487.2336.
3.2.3. General Procedure for the Synthesis of Terminal 4-Ethynyl-1,2,3-Triazoles 8a–f by the Retro-Favorskii Reaction
A round-bottom oven-dried flask equipped with a magnetic stirring bar was charged with a solution of corresponding alcohol 7a–f (1.00 equiv.) in dry benzene (2.0 mL) through the septum via syringe. Argon was bubbled through the solution for 10 min, and then well-ground anhydrous KOH (2.0 equiv. for 7c,d,f, 3.0 equiv. for 7a, and 4.0 equiv. for compound 7b,e) was added in the stream of Ar. A reflux condenser was equipped, and the flask with the resulting mixture was heated on an oil bath (bath temperature 75 °C). After completion of the reaction (TLC control), the reaction mixture was cooled, and a precipitate was filtered through a short pad of silica gel eluting with benzene. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography on silica gel using EtOAc/hexane as the eluent.
6-(5-((4-(Dimethylamino)phenyl)ethynyl)-4-ethynyl-1H-1,2,3-triazol-1-yl)hexyl propionate (8a) was prepared in accordance with the general procedure from 7a (70 mg, 0.12 mmol) and KOH (20 mg, 0.36 mmol). The crude product was purified by column chromatography (eluent: hexane/acetone = 3:1), which afforded a brown viscous oil (35 mg, 57% yield). 1H NMR (400 MHz, CDCl3) δ 7.48–7.40 (m, 2H, Ar), 6.73–6.64 (m, 2H, Ar), 4.41 (t, J = 7.1 Hz, 2H, CH2), 4.04 (t, J = 6.6 Hz, 2H, CH2), 3.41 (s, 1H, CH), 3.02 (s, 6H, NMe2), 2.30 (q, J = 7.5 Hz, 2H, CH2), 2.05–1.91 (m, 2H, CH2), 1.67–1.58 (m, 2H, CH2), 1.46–1.36 (m, 4H, 2CH2), 1.12 (t, J = 7.5 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 151.2, 133.2, 131.7, 125.6, 111.8, 107.2, 105.0, 83.2, 73.3, 71.3, 64.3, 49.3, 40.2, 29.6, 28.6, 27.7, 26.2, 25.6, 9.3. HSMS ESI: [M + H]+ calc C23H29N4O2+ 393.2285; found: 393.2286.
6-(5-((4-((tert-Butoxycarbonyl)amino)phenyl)ethynyl)-4-ethynyl-1H-1,2,3-triazol-1-yl)hexyl propionate (8b) was prepared in accordance with the general procedure from 7b (100 mg, 0.19 mmol) and KOH (43 mg, 0.77 mmol). The crude product was purified by column chromatography (eluent: hexane/EtOAc = 3:1), which afforded a white solid (58 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 7.52–7.39 (m, 4H, Ar), 6.70 (s, 1H, NH), 4.42 (t, J = 7.0 Hz, 2H, CH2), 4.03 (t, J = 6.6 Hz, 2H, CH2), 3.42 (s, 1H, CH), 2.30 (q, J = 7.6 Hz, 2H, CH2), 2.06–1.93 (m, 2H, CH2), 1.67–1.56 (m, 2H, CH2), 1.52 (s, 9H, Boc), 1.47–1.32 (m, 4H, CH2), 1.12 (t, J = 7.6 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 152.4, 140.3, 132.9, 132.4, 124.9, 118.2, 115.0, 103.2, 83.5, 81.4, 73.0, 72.3, 64.2, 49.5, 29.7, 28.6, 28.3, 27.7, 26.2, 25.6, 9.3. HSMS ESI: [M + Na]+ calcd. for C26H32N4O4Na+ 487.2316; found: 487.2307.
6-(4-Ethynyl-5-((3,4,5-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazol-1-yl)hexyl propionate (8c) was prepared in accordance with the general procedure from 7c (50 mg, 0.10 mmol) and KOH (11.3 mg, 0.20 mmol). The crude product was purified by column chromatography (eluent: hexane/EtOAc = 3:1) to afford a white solid (32 mg, 73%). 1H NMR (400 MHz, CDCl3) δ 6.77 (s, 2H, Ar), 4.42 (t, J = 7.0 Hz, 2H, CH2), 4.03 (t, J = 6.6 Hz, 2H, CH2), 3.90–3.87 (m, 9H, 3OMe), 3.43 (s, 1H, CH), 2.33–2.25 (m, 2H, CH2), 2.03–1.95 (m, 2H, CH2), 1.67–1.56 (m, 2H, CH2), 1.43–1.35 (m, 4H, 2CH2), 1.13–1.07 (m, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 174.6, 153.4, 140.5, 132.6, 124.6, 115.8, 109.4, 103.1, 83.7, 72.9, 72.0, 64.1, 61.2, 56.4, 49.5, 29.6, 28.6, 27.7, 26.2, 25.5, 9.2. HSMS ESI: [M + Na]+ calcd. for C24H29N3O5Na+ 462.1999; found: 462.1996.
6-(4-Ethynyl-5-((2,4,6-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazol-1-yl)hexyl propionate (8d) was prepared in accordance with the general procedure from 7d (50 mg, 0.10 mmol) and KOH (11.3 mg, 0.20 mmol). The crude product was purified by column chromatography (eluent: hexane/EtOAc = 3:1) to afford a white solid (30 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 6.11 (s, 2H, Ar), 4.44 (t, J = 7.1 Hz, 2H, CH2), 4.01 (t, J = 6.6 Hz, 2H, CH2), 3.87 (s, 6H, 2OMe), 3.84 (s, 3H, OMe), 3.40 (s, 1H, CH), 2.28 (q, J = 7.6 Hz, 2H, CH2), 2.05–1.95 (m, 2H, CH2), 1.65–1.54 (m, 2H, CH2), 1.42–1.34 (m, 4H, 2CH2), 1.10 (t, J = 7.6 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 174.6, 163.3, 162.8, 131.3, 125.8, 97.1, 92.8, 90.7, 83.0, 79.7, 73.3, 64.2, 56.2, 55.7, 49.3, 29.5, 28.6, 27.7, 26.2, 25.5, 9.2. HSMS ESI: [M + Na]+ calcd. for C24H29N3O5Na+ 462.1999; found: 462.1993.
4-((4-Ethynyl-1-(4-methoxybenzyl)-1H-1,2,3-triazol-5-yl)ethynyl)-N,N-dimethylaniline (8e) was prepared in accordance with the general procedure from 7e (50 mg, 0.12 mmol) and KOH (27 mg, 0.48 mmol). The crude product was purified by column chromatography (eluent: hexane/acetone = 3:1) to afford a slightly yellow solid (28 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 8.5 Hz, 2H, Ar), 7.33 (d, J = 8.3 Hz, 2H, Ar), 6.86 (d, J = 8.3 Hz, 2H, Ar), 6.66 (d, J = 8.5 Hz, 2H, Ar), 5.50 (s, 2H, CH2), 3.78 (s, 3H, OMe), 3.39 (s, 1H, CH), 3.02 (s, 6H, NMe2). 13C NMR (101 MHz, CDCl3) δ 159.9, 151.2, 133.2, 132.0, 129.9, 126.7, 125.4, 114.3, 111.7, 107.2, 105.3, 83.3, 73.2, 71.5, 55.4, 52.7, 40.2. HSMS ESI: [M + H]+ calcd. for C22H21N4O+ 357.1710; found: 357.1712.
tert-Butyl (4-((4-ethynyl-1-(4-methoxybenzyl)-1H-1,2,3-triazol-5-yl)ethynyl)phenyl)carbamate (8f) was prepared in accordance with the general procedure from 7f (50 mg, 0.10 mmol) and KOH (11.5 mg, 0.21 mmol). The crude product was purified by column chromatography (eluent: hexane/EtOAc = 3:1) to afford a white solid (40 mg, 91%). 1H NMR (400 MHz, CDCl3) δ 7.53–7.40 (m, 4H, Ar), 7.34–7.27 (m, 2H, Ar), 6.88–6.82 (m, 2H, Ar), 6.75 (s, 1H, NH), 5.52 (s, 2H, CH2), 3.77 (s, 3H, OMe), 3.40 (s, 1H, CH), 1.52 (s, 9H, Boc). 13C NMR (101 MHz, CDCl3) δ 160.0, 152.4, 140.3, 132.9, 132.7, 129.9, 126.4, 124.7, 118.2, 115.0, 114.4, 103.5, 83.6, 81.4, 72.9, 72.5, 55.4, 53.0, 28.4. HSMS ESI: [M + Na]+ calcd. for C25H24N4O3Na+ 451.1741; found: 451.1738.
3.2.4. General Procedure for the Synthesis of Trz-OAEs 10 by Sonogashira Cross-Coupling
Dietynyltriazole 8a–f (1 equiv.), 1,4-diiodo-2,5-bis(alkyloxy)benzene 9a,b (0.5 equiv.), CuI (3 mol%) and Pd(PPh3)4 (3 mol%) were placed in a vial. The vial was sealed, and the mixture was evacuated and flushed with argon several times. Diisopropilamine (DIPA) (40 equiv.) and dry toluene (0.03M) were added. The vial with the reaction mixture was placed in a pre-heated aluminum block at 40 °C and stirred at this temperature for 2–4 h (TLC control). After completion of the reaction, the reaction mixture was cooled, poured into a saturated aqueous solution of NH4Cl, and extracted with DCM. The combined organic layers were washed with a saturated solution of NH4Cl, two times with EDTA, and two times with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure to give the crude product, which was purified by column chromatography on silica gel.
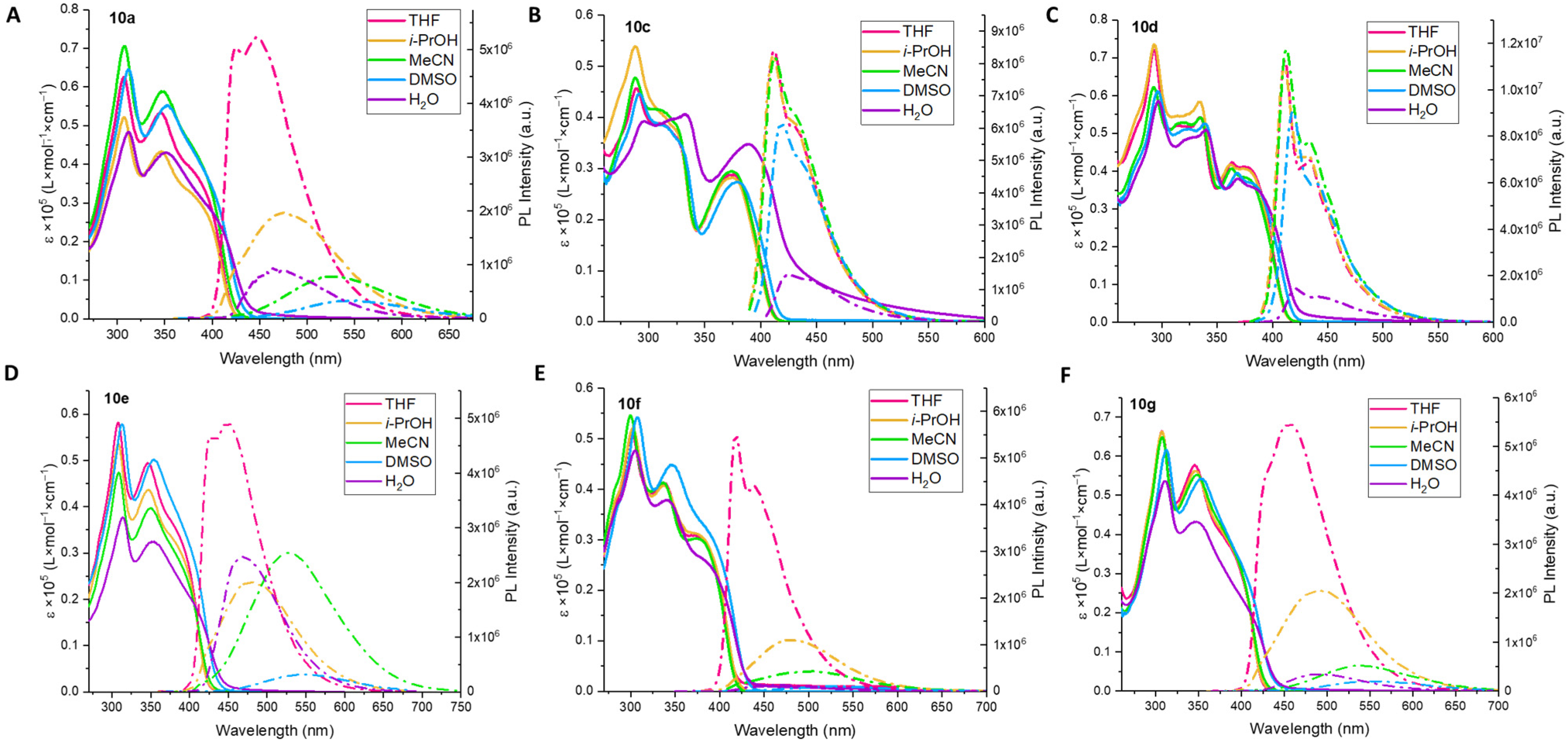
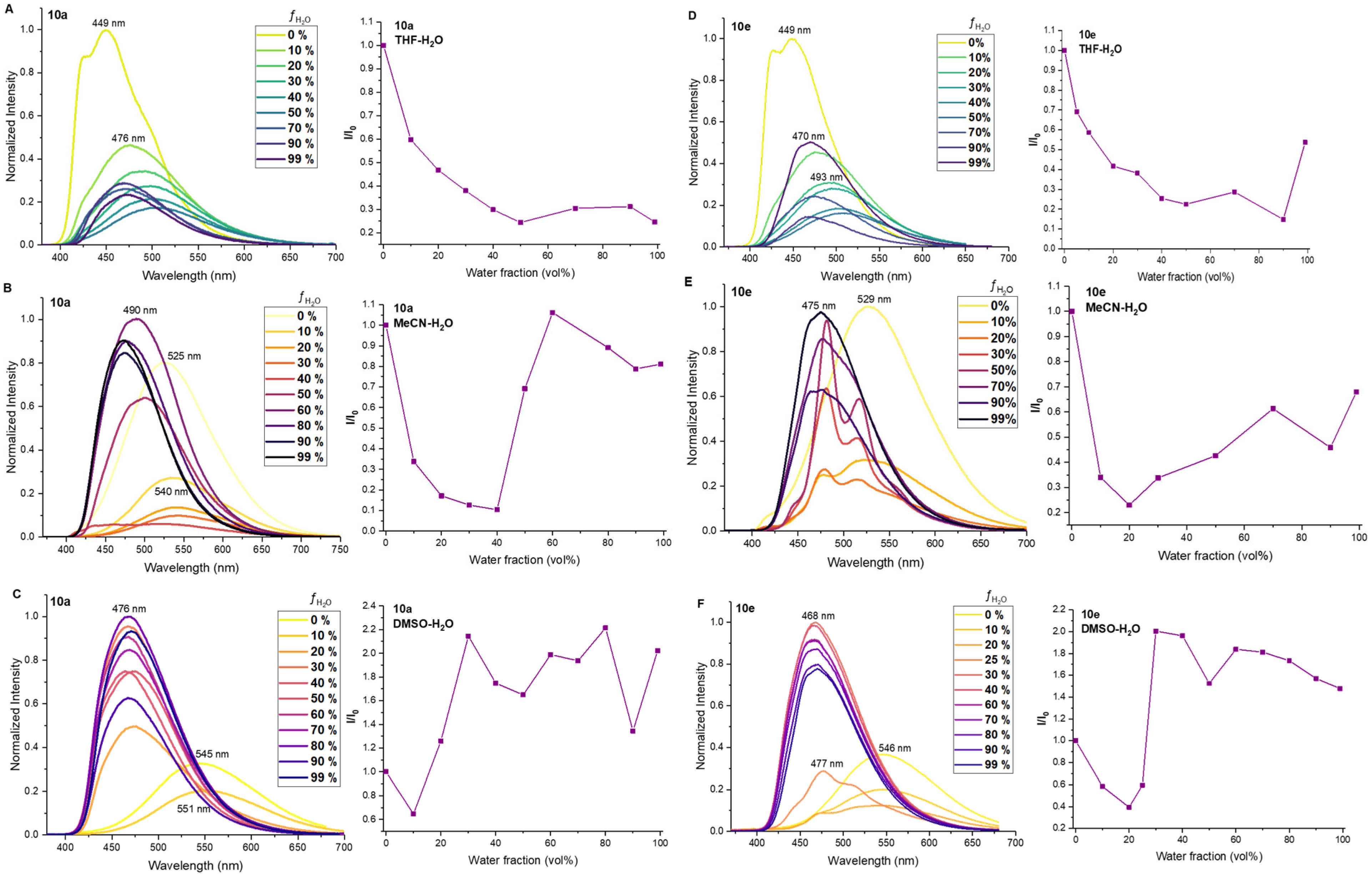
(((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((4-(dimethylamino)phenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexane-6,1-diyl) dipropionate (10a) was synthesized in accordance with the general procedure from alkyne 8a (35 mg, 0.0892 mmol), 1,4-diiodobenzene 9a (26.1 mg, 0.0446 mmol), Pd(PPh3)4 (3.1 mg, 0.0027 mmol), CuI (0.5 mg, 0.0027 mmol) and DIPA (0.500 mL, 3.57 mmol) with a reaction time of 2 h. The crude product was purified by recrystallization from acetonitrile/water (50:1) to afford 10a as a yellow solid (30 mg, 60%). 1H NMR (400 MHz, CDCl3) δ 7.46–7.37 (m, 4H, Ar), 7.08 (s, 2H, Ar), 6.69–6.61 (m, 4H, Ar), 4.43 (t, J = 7.0 Hz, 4H, 2CH2), 4.04 (t, J = 6.5 Hz, 4H, 2CH2), 3.98 (t, J = 6.8 Hz, 4H, 2CH2), 3.02 (s, 12H, 2NMe2), 2.30 (q, J = 7.6 Hz, 4H, 2CH2), 2.06–1.94 (m, 4H, 2CH2), 1.74 (p, J = 7.0 Hz, 4H, 2CH2), 1.66–1.58 (m, 4H, 2CH2), 1.48–1.31 (m, 12H, 6CH2), 1.28–1.17 (m, 14H, 7CH2), 1.12 (t, J = 7.6 Hz, 6H, 2CH3), 0.83 (t, J = 6.8 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 153.8, 151.1, 133.2, 133.0, 124.7, 117.7, 114.0, 111.7, 107.5, 105.0, 91.6, 84.2, 71.8, 70.1, 64.3, 49.3, 40.2, 31.9, 29.7, 29.38, 29.37, 29.3, 28.6, 27.7, 26.2, 26.0, 25.6, 22.8, 14.2, 9.3. HSMS ESI: [M + H]+ calcd. for C68H91N8O6+ 1115.7056; found: 1115.7057.
(((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((4-((tert-butoxycarbonyl)amino)phenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexane-6,1-diyl) dipropionate (10b) was synthesized in accordance with the general procedure from alkyne 8b (58 mg, 0.125 mmol), 1,4-diiodobenzene 9a (36.6 mg, 0.0624 mmol), Pd(PPh3)4 (4.3 mg, 0.0038 mmol), CuI (0.7 mg, 0.0038 mmol) and DIPA (0.700 mL, 5 mmol) with a reaction time of 2 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1→1:1) to afford a yellow solid (67 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 7.52–7.46 (m, 4H, Ar), 7.47–7.39 (m, 4H, Ar), 7.07 (s, 2H, Ar), 6.66 (s, 2H, 2 NH), 4.44 (t, J = 7.0 Hz, 4H, 2CH2), 4.04 (t, J = 6.6 Hz, 4H, 2CH2), 3.966 (t, J = 6.8 Hz, 4H, 2CH2), 2.30 (q, J = 7.6 Hz, 4H, 2CH2), 2.05–1.95 (m, 4H, 2CH2), 1.71 (p, J = 6.8 Hz, 4H, 2CH2), 1.66–1.56 (m, 4H, 2CH2), 1.55 (s, 18H, 2Boc), 1.45–1.31 (m, 14H, 2CH2), 1.29–1.15 (m, 14H, 2CH2), 1.12 (t, J = 7.6 Hz, 6H, 2CH3), 0.83 (t, J = 6.8 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 153.8, 152.3, 140.2, 133.7, 132.9, 123.9, 118.1, 117.7, 115.3, 114.0, 103.1, 91.9, 83.9, 81.4, 72.9, 70.0, 64.2, 49.5, 31.9, 29.7, 29.4, 29.3, 28.6, 28.4, 27.7, 26.3, 26.0, 25.6, 22.8, 14.2, 9.3. HSMS ESI: [M + Na]+ calcd. for C74H98N8O10Na+ 1281.7298; found: 1281.7304.
(((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((3,4,5-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexane-6,1-diyl) dipropionate (10c) was synthesized in accordance with the general procedure from alkyne 8c (45 mg, 0.103 mmol), 1,4-diiodobenzene 9a (30 mg, 0.0512 mmol), Pd(PPh3)4 (3.6 mg, 0.0031 mmol), CuI (0.6 mg, 0.0031 mmol) and DIPA (0.574 mL, 4.1 mmol) with a reaction time of 2 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1→1:1) to afford a white solid (41 mg, 66%). 1H NMR (400 MHz, CDCl3) δ 7.07 (s, 2H, Ar), 6.79 (s, 4H, Ar), 4.45 (t, J = 7.0 Hz, 4H, 2CH2), 4.04 (t, J = 6.6 Hz, 4H, 2CH2), 3.96 (t, J = 6.7 Hz, 4H, 2CH2), 3.89 (s, 6H, 2OMe), 3.87 (s, 12H, 4OMe), 2.29 (q, J = 7.6 Hz, 4H, 2CH2), 2.07–1.96 (m, 4H, 2CH2), 1.75–1.57 (m, 8H, 4CH2), 1.47–1.28 (m, 12H, 6CH2), 1.24–1.15 (m, 14H, 7CH2), 1.11 (t, J = 7.6 Hz, 6H, 2CH3), 0.83 (t, J = 6.9 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 154.0, 153.4, 140.5, 133.9, 123.7, 118.0, 116.1, 114.2, 109.4, 103.2, 91.8, 83.9, 72.6, 70.3, 64.2, 61.2, 56.4, 49.5, 31.9, 29.7, 29.3, 29.3, 29.3, 28.6, 27.7, 26.3, 26.0, 25.6, 22.7, 14.2, 9.3. HSMS ESI: [M + H]+ calcd. for C70H92N6O12Na+ 1231.6665; found: 1231.6664.
(((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((2,4,6-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexane-6,1-diyl) dipropionate (10d) was synthesized in accordance with the general procedure from alkyne 8d (67 mg, 0.152 mmol), 1,4-diiodobenzene 9a (44.7 mg, 0.0762 mmol), Pd(PPh3)4 (5.3 mg, 0.0046 mmol), CuI (0.9 mg, 0.0046 mmol), DIPA (0.855 mL, 6.1 mmol) in dry toluene (3.0 mL) with a reaction time of 2 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 1:1→1:2) to afford a white solid (41 mg, 45%). 1H NMR (400 MHz, CDCl3) δ 7.08 (s, 2H, Ar), 6.11 (s, 4H, Ar), 4.46 (t, J = 7.0 Hz, 4H, 2CH2), 4.03 (t, J = 6.6 Hz, 4H, 2CH2), 3.97 (t, J = 6.7 Hz, 4H, 2CH2), 3.87–3.80 (m, 6H, 2OMe), 3.83 (s, 12H, 4OMe), 2.29 (q, J = 7.6 Hz, 4H, 2CH2), 2.08–1.95 (m, 4H, 2CH2), 1.72 (p, J = 6.9 Hz, 4H, 2CH2), 1.66–1.56 (m, 4H, 2CH2), 1.44–1.30 (m, 12H, 6CH2), 1.27–1.16 (m, 14H, 7CH2), 1.12 (t, J = 7.6 Hz, 6H, 2CH3), 0.86 (t, J = 6.9 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 163.2, 162.8, 154.0, 132.6, 125.1, 118.5, 114.5, 97.0, 93.2, 91.4, 90.7, 84.3, 80.3, 70.5, 64.3, 56.2, 55.7, 49.3, 32.0, 29.5, 29.4, 29.34, 29.30, 28.6, 27.7, 26.2, 26.0, 25.6, 22.8, 14.2, 9.3. HSMS ESI: [M + Na]+ calcd. for C70H92N6O12Na+ 1231.6665; found: 1231.6671.
4,4′-((((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(1-(4-methoxybenzyl)-1H-1,2,3-triazole-4,5-diyl))bis(ethyne-2,1-diyl))bis(N,N-dimethylaniline) (10e) was synthesized in accordance with the general procedure from alkyne 8e (35 mg, 0.098 mmol), 1,4-diiodobenzene 9a (28.8 mg, 0.0491 mmol), Pd(PPh3)4 (3.4 mg, 0.003 mmol), CuI (0.6 mg, 0.003 mmol), DIPA (0.550 mL, 3.93 mmol) in dry toluene 1.6 mL with a reaction time of 2 h. The crude product was purified by recrystallization from acetonitrile, to give a yellow solid (35 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 7.43–7.37 (m, 4H, Ar), 7.37–7.30 (m, 4H, Ar), 7.05 (s, 2H, Ar), 6.91–6.82 (m, 4H, Ar), 6.69–6.61 (m, 4H, Ar), 5.53 (s, 4H, 2CH2), 3.96 (t, J = 6.8 Hz, 4H, 2CH2), 3.78 (s, 6H, 2OMe), 3.02 (s, 12H, 2NMe2), 1.72 (p, J = 7.0 Hz, 4H, 2CH2), 1.40–1.29 (m, 4H, 2CH2), 1.27–1.12 (m, 16H, 8CH2), 0.83 (t, J = 6.8 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 159.9, 153.8, 151.1, 133.3, 133.2, 129.9, 126.9, 124.5, 117.8, 114.3, 114.1, 111.7, 107.6, 105.3, 91.7, 84.1, 72.1, 70.1, 55.4, 52.7, 40.2, 31.9, 29.4, 29.3, 26.0, 22.8, 14.2. HSMS ESI: [M + H]+ calcd. for C66H75N8O4+ 1043.5906; found: 1043.5911.
Di-tert-butyl (((((2,5-bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(1-(4-methoxybenzyl)-1H-1,2,3-triazole-4,5-diyl))bis(ethyne-2,1-diyl))bis(4,1-phenylene))dicarbamate (10′f) was synthesized in accordance with the general procedure from alkyne 8f (33 mg, 0.077 mmol), 1,4-diiodobenzene 9a (22.6 mg, 0.0385 mmol), Pd(PPh3)4 (2.7 mg, 0.0023 mmol), CuI (0.4 mg, 0.0023 mmol), DIPA (0.432 mL, 3.08 mmol) in dry toluene 1.5 mL with a reaction time of 2 h. After removal of the solvent under reduced pressure, the product obtained was a yellow powder (41 mg, 90%), which we used without additional purification in the next step.
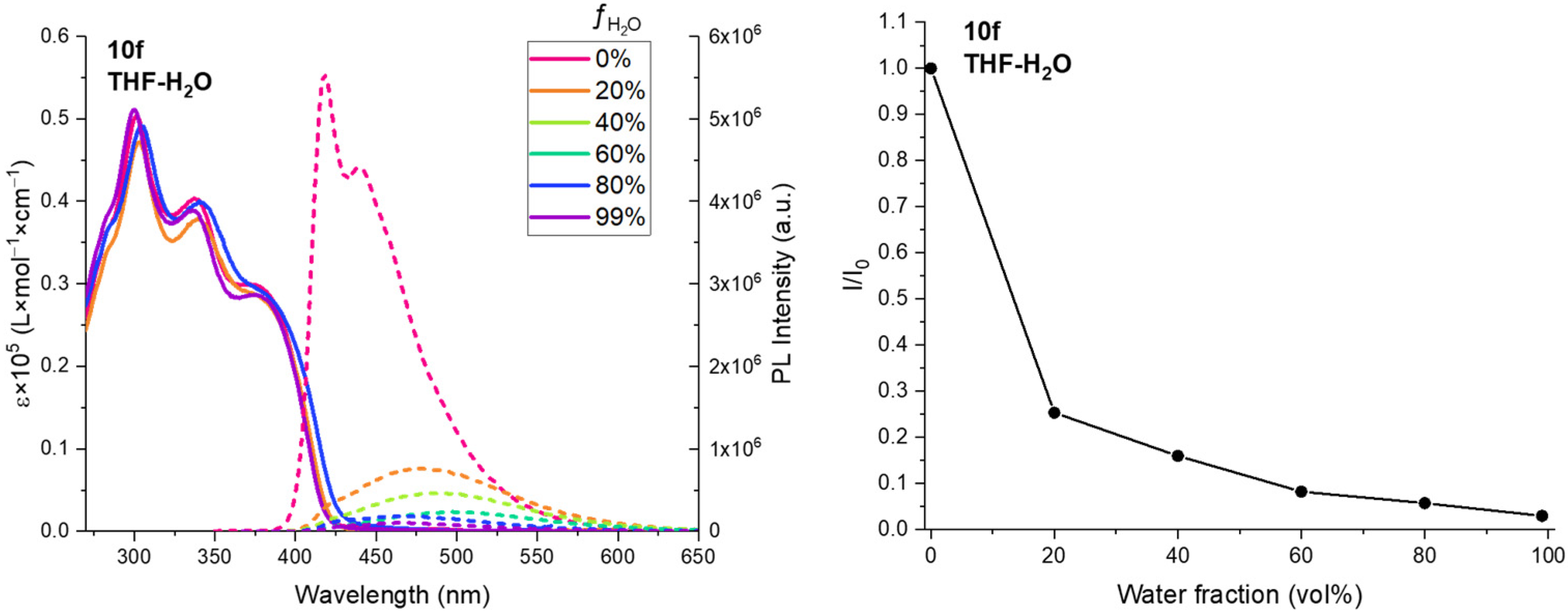
4,4′-((((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(1-(4-methoxybenzyl)-1H-1,2,3-triazole-4,5-diyl))bis(ethyne-2,1-diyl))dianiline (10f). Compound 10′f (40 mg, 0.0337 mmol) was dissolved in dry DCM (1 mL), and TFA (0.150 mL, 2.0 mmol) was added. The reaction mixture was stirred at room temperature for 3 h (TLC control) and then quenched by Na2CO3 (3 mL). The resulting mixture was extracted with DCM (3 × 5 mL). The combined organic layer was washed with brine (5 mL), dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 1:1→1:2) to afford a white solid (14 mg, 42%). 1H NMR (400 MHz, DMSO-d6) δ 7.35–7.28 (m, 4H, Ar), 7.28–7.22 (m, 4H, Ar), 7.19 (s, 2H, Ar), 6.98–6.92 (m, 4H, Ar), 6.63–6.55 (m, 4H, Ar), 5.83 (s, 4H), 5.61 (s, 4H, 2CH2), 4.02 (t, J = 6.4 Hz, 4H, 2CH2), 3.73 (s, 6H, 2OMe), 1.63 (p, J = 6.8 Hz, 4H, 2CH2), 1.40–1.29 (m, 4H, 2CH2), 1.24–1.07 (m, 16H, 8CH2), 0.75 (t, J = 6.6 Hz, 6H, 2CH3). 13C NMR (101 MHz, DMSO-d6) δ 159.3, 153.1, 151.0, 133.1, 131.4, 129.5, 127.0, 124.2, 117.0, 114.2, 113.5, 113.0, 105.9, 105.5, 91.1, 84.0, 70.9, 69.1, 55.1, 52.0, 31.1, 28.6, 28.5, 25.3, 22.0, 13.9. HSMS ESI: [M + H]+ calcd. for C62H67N8O4+ 987.5280; found: 987.5296.
(((2,5-Dimethoxy-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((4-(dimethylamino)phenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexane-6,1-diyl) dipropionate (10g) was synthesized in accordance with the general procedure from alkyne 8g (58 mg, 0.148 mmol), 1,4-diiodo-2,5-dimethoxybenzene 9b (28.8 mg, 0.0739 mmol), Pd(PPh3)4 (5.1 mg, 0.0044 mmol), CuI (0.8 mg, 0.0044 mmol), DIPA (0.828 mL, 5.9 mmol) in dry toluene 2.9 mL with a reaction time of 2 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1) to afford a white solid (47 mg, 69%). 1H NMR (400 MHz, CDCl3) δ 7.47–7.39 (m, 4H, Ar), 7.08 (s, 2H, Ar), 6.70–6.62 (m, 4H, Ar), 4.43 (t, J = 7.0 Hz, 4H, 2CH2), 4.04 (t, J = 6.5 Hz, 4H, 2CH2), 3.84 (s, 6H, 2OMe), 3.02 (s, 12H, 2NMe2), 2.30 (q, J = 7.6 Hz, 4H, 2CH2), 2.06–1.94 (m, 4H, 2CH2), 1.68–1.56 (m, 4H, 2CH2), 1.44–1.36 (m, 8H, 4CH2), 1.12 (t, J = 7.5 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 174.7, 154.2, 151.1, 133.2, 133.0, 125.0, 115.9, 113.4, 111.7, 107.5, 105.2, 91.5, 84.5, 71.8, 64.3, 56.7, 49.3, 40.2, 29.7, 28.6, 27.7, 26.2, 25.6, 9.3. HSMS ESI: [M + Na]+ calcd. for C54H62N8O6Na+ 941.4685; found: 941.4698.
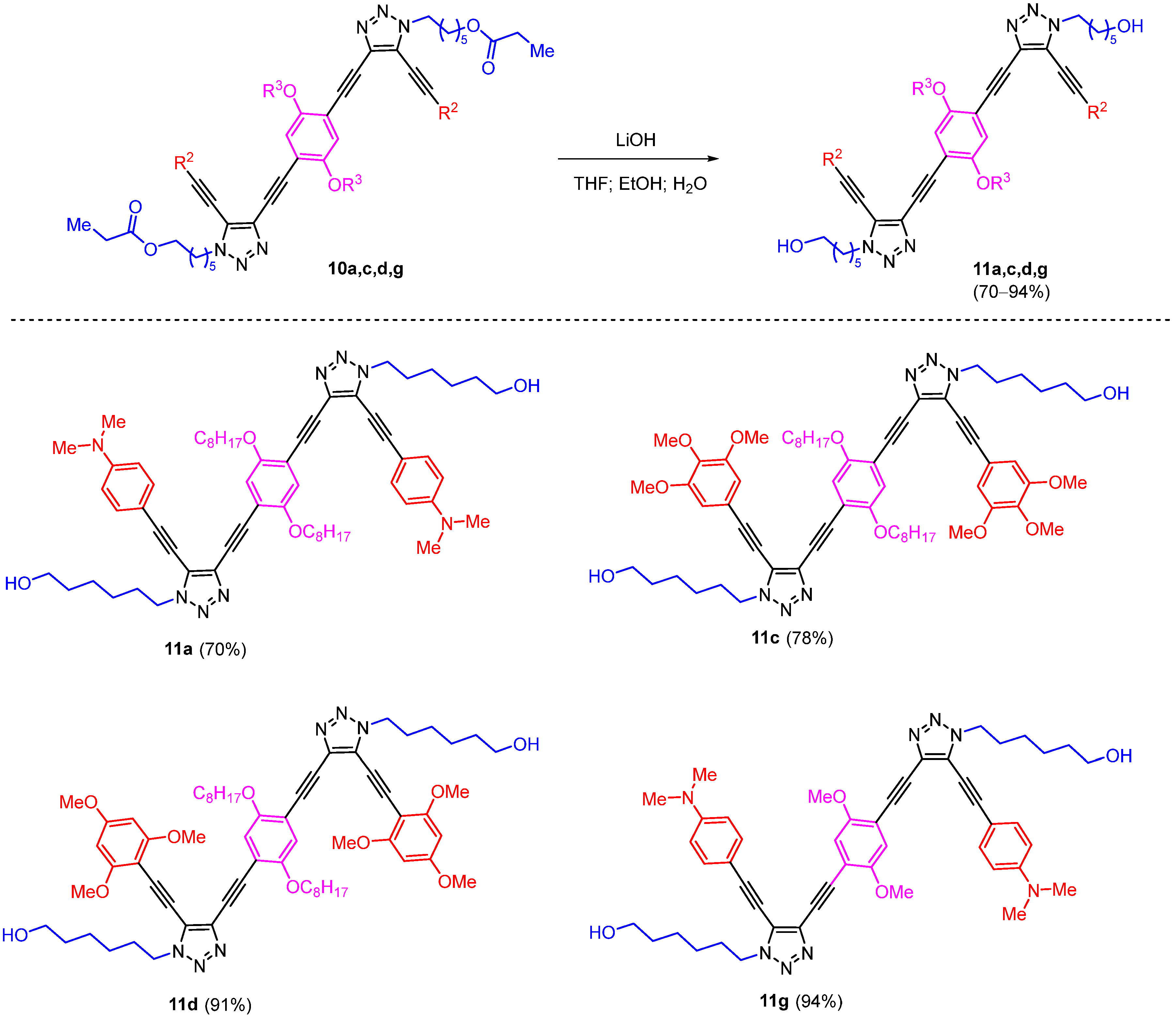
3.2.5. General Procure for the Synthesis of Trz-OAEs 11
To a stirred solution of triazoles 10 (1 equiv.) in 5 mL of THF/H2O/EtOH (3:1:1) an aqueous solution of LiOH × H2O (20 equiv., 2M) was added, and the reaction mixture was stirred at room temperature for 4 h; the reaction mixture was poured into a saturated aqueous solution of NH4Cl and extracted with DCM. The combined extracts were washed with brine (1 × 10 mL) and dried with Na2SO4, and concentrated under reduced pressure to give the crude product. The formed Trz-OAEs 11 were then dissolved in THF (1 mL) and precipitated in cold water. The precipitate was filtered, washed with water, and dried under vacuum.
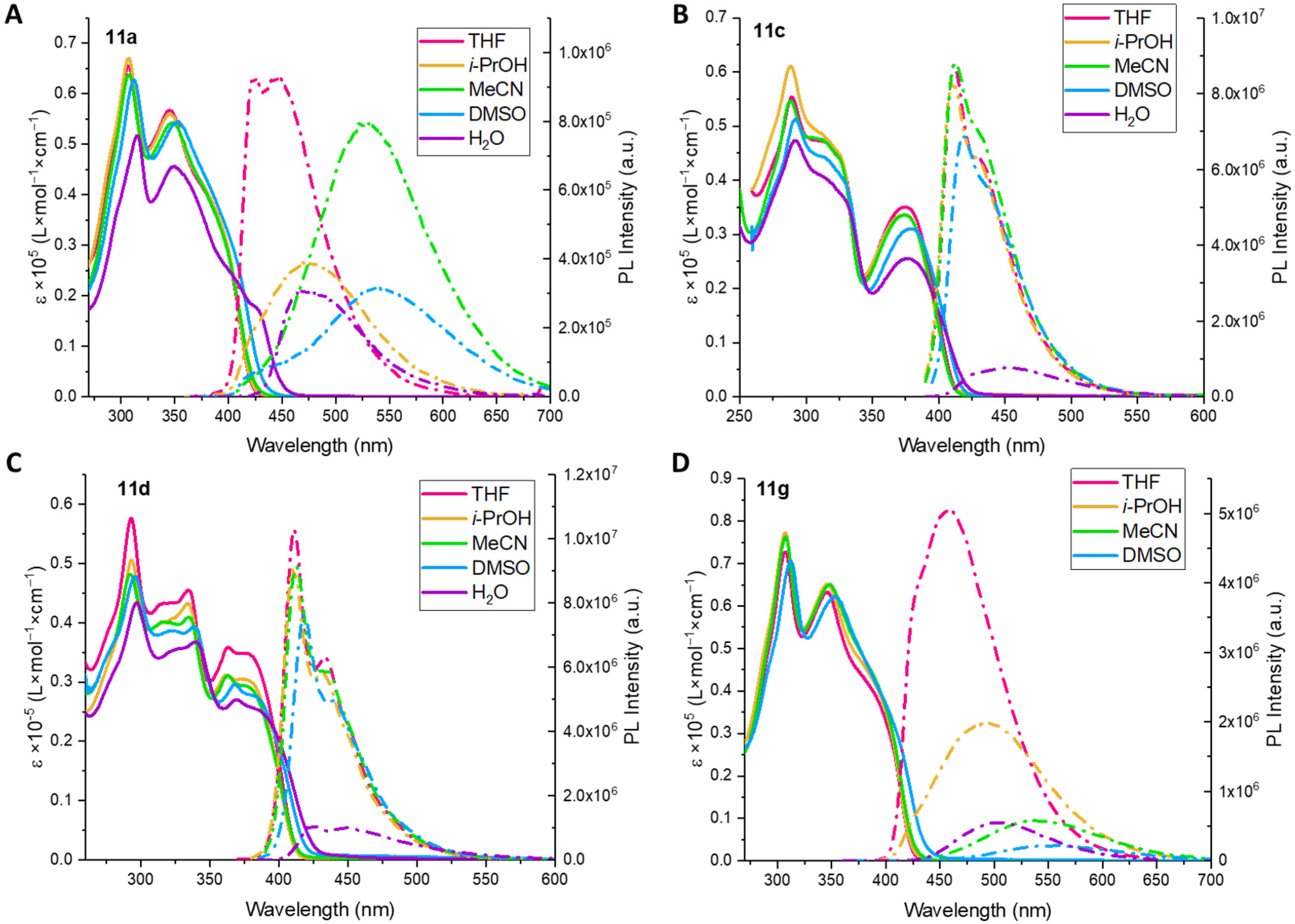
6,6′-(((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((4-(dimethylamino)phenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexan-1-ol) (11a) was prepared in accordance with the general procedure from 10a (75 mg, 68.2 mmol), LiOH × H2O (0.682 mL, 2M) in THF/H2O/EtOH (2.4 mL/0.8 mL/0.8 mL). The final product 11a was obtained as a yellow solid (47 mg, 70%). 1H NMR (400 MHz, CDCl3) δ 7.46–7.36 (m, 4H, Ar), 7.08 (s, 2H, Ar), 6.69–6.64 (m, 4H, Ar), 4.43 (t, J = 7.0 Hz, 4H, 2CH2), 3.98 (t, J = 6.8 Hz, 4H, 2CH2), 3.61 (t, J = 6.5 Hz, 4H, 2CH2), 3.01 (s, 12H, 2NMe2), 2.00 (p, J = 7.0 Hz, 4H, 2CH2), 1.74 (p, J = 7.0 Hz, 4H, 2CH2), 1.62–1.51 (m, 4H, 2CH2), 1.49–1.31 (m, 12H, 6CH2), 1.23–1.14 (m, 14H, 7CH2), 0.83 (t, J = 6.8 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 153.7, 151.1, 133.2, 133.0, 124.7, 117.7, 114.0, 111.7, 107.5, 104.9, 91.6, 84.2, 71.8, 70.1, 62.8, 49.3, 40.2, 32.6, 31.9, 29.8, 29.7, 29.38, 29.36, 29.3, 26.3, 26.0, 25.2, 22.8, 14.2. HSMS ESI: [M + H]+ calcd. for C62H83N8O4+ 1003.6532; found: 1003.6526.
6,6′-(((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((3,4,5-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexan-1-ol) (11c) was prepared in accordance with general procedure from 10c (35 mg, 28.9 mmol), LiOH × H2O (0.289 mL, 2M) in THF/H2O/EtOH (1.2 mL/0.4 mL/0.4 mL). The final product 11c was obtained as a white solid (25 mg, 78%). 1H NMR (400 MHz, CDCl3) δ 7.07 (s, 2H, Ar), 6.79 (s, 4H, Ar), 4.45 (t, J = 7.0 Hz, 4H, 2CH2), 3.96 (t, J = 6.7 Hz, 4H, 2CH2), 3.88 (s, 6H, OMe), 3.86 (s, 12H, 2OMe), 3.62 (t, J = 6.5 Hz, 4H, 2CH2), 2.02 (p, J = 7.1 Hz, 4H, 2CH2), 1.75–1.63 (m, 4H, 2CH2), 1.56 (p, J = 6.7 Hz, 4H, 2CH2), 1.48–1.30 (m, 12H, 6CH2), 1.21–1.14 (m, 14H, 7CH2), 0.82 (t, J = 6.9 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 154.0, 153.4, 140.5, 133.8, 123.7, 117.9, 116.1, 114.2, 109.4, 103.1, 91.8, 83.9, 72.6, 70.3, 62.7, 61.2, 56.4, 49.6, 32.5, 31.9, 29.8, 29.7, 29.30, 29.28, 29.25, 26.3, 25.9, 25.3, 22.7, 14.2. HSMS ESI: [M + Na]+ calcd. for C64H84N6O10Na+ 1119.6141; found: 1119.6132.
6,6′-(((2,5-Bis(octyloxy)-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((2,4,6-trimethoxyphenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexan-1-ol) (11d) was prepared in accordance with general procedure from 10d (35 mg, 28.9 mmol), LiOH × H2O (0.289 mL, 2M) in THF/H2O/EtOH (1.2 mL/0.4 mL/0.4 mL). The final product 11d was obtained as a white solid (28 mg, 91%). 1H NMR (400 MHz, CDCl3) δ 7.07 (s, 2H, Ar), 6.10 (s, 4H, Ar), 4.47 (t, J = 7.1 Hz, 4H, 2CH2), 3.97 (t, J = 6.7 Hz, 4H, 2CH2), 3.84 (s, 6H, 2OMe), 3.85 (s, 12H, 4OMe), 3.60 (t, J = 6.4 Hz, 4H, 2CH2), 2.02 (p, J = 7.0 Hz, 4H, 2CH2), 1.78–1.66 (m, 4H, 2CH2), 1.61–1.50 (m, 4H, 2CH2), 1.47–1.33 (m, 12H, 6CH2), 1.20–1.15 (m, 14H, 7CH2), 0.83 (t, J = 6.9 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 163.2, 162.8, 154.0, 132.6, 125.1, 118.5, 114.5, 97.1, 93.2, 91.4, 90.7, 84.3, 80.3, 70.5, 62.8, 56.2, 55.6, 49.3, 32.6, 31.9, 29.6, 29.4, 29.33, 29.29, 26.2, 26.0, 25.2, 22.8, 14.2. HSMS ESI: [M + Na]+ calcd. for C64H84N6O10Na+ 1119.6141; found: 1119.6131.
6,6′-(((2,5-Dimethoxy-1,4-phenylene)bis(ethyne-2,1-diyl))bis(5-((4-(dimethylamino)phenyl)ethynyl)-1H-1,2,3-triazole-4,1-diyl))bis(hexan-1-ol) (11g) was prepared in accordance with general procedure from 10g (40 mg, 43.5 mmol), LiOH × H2O (0.435 mL, 2M) in THF/H2O/EtOH (1.5 mL/0.5 mL/0.5 mL). The final product, 11g, was obtained as a yellow solid (33 mg, 94%). 1H NMR (400 MHz, CDCl3) δ 7.48–7.43 (m, 4H, Ar), 7.08 (s, 2H, Ar), 6.70–6.62 (m, 4H, Ar), 4.44 (t, J = 7.0 Hz, 4H, 2CH2), 3.84 (s, 6H, 2OMe), 3.62 (t, J = 6.4 Hz, 4H, 2CH2), 3.02 (s, 12H, 2NMe2), 2.01 (p, J = 7.1 Hz, 4H, 2CH2), 1.57 (p, J = 6.6 Hz, 4H, 2CH2), 1.48–1.34 (m, 8H, 2CH2). 13C NMR (101 MHz, CDCl3) δ 154.3, 151.1, 133.2, 133.0, 125.0, 116.0, 113.4, 111.8, 107.5, 105.2, 91.5, 84.5, 71.9, 62.8, 56.7, 49.4, 40.2, 32.6, 29.7, 26.2, 25.2. HSMS ESI: [M + H]+ calcd. for C48H55N8O4+ 807.4341; found: 807.4352.