Electrically Controlled Structures in Cholesteric Droplets with Planar Anchoring
Abstract
1. Introduction
2. Results
2.1. Initial State
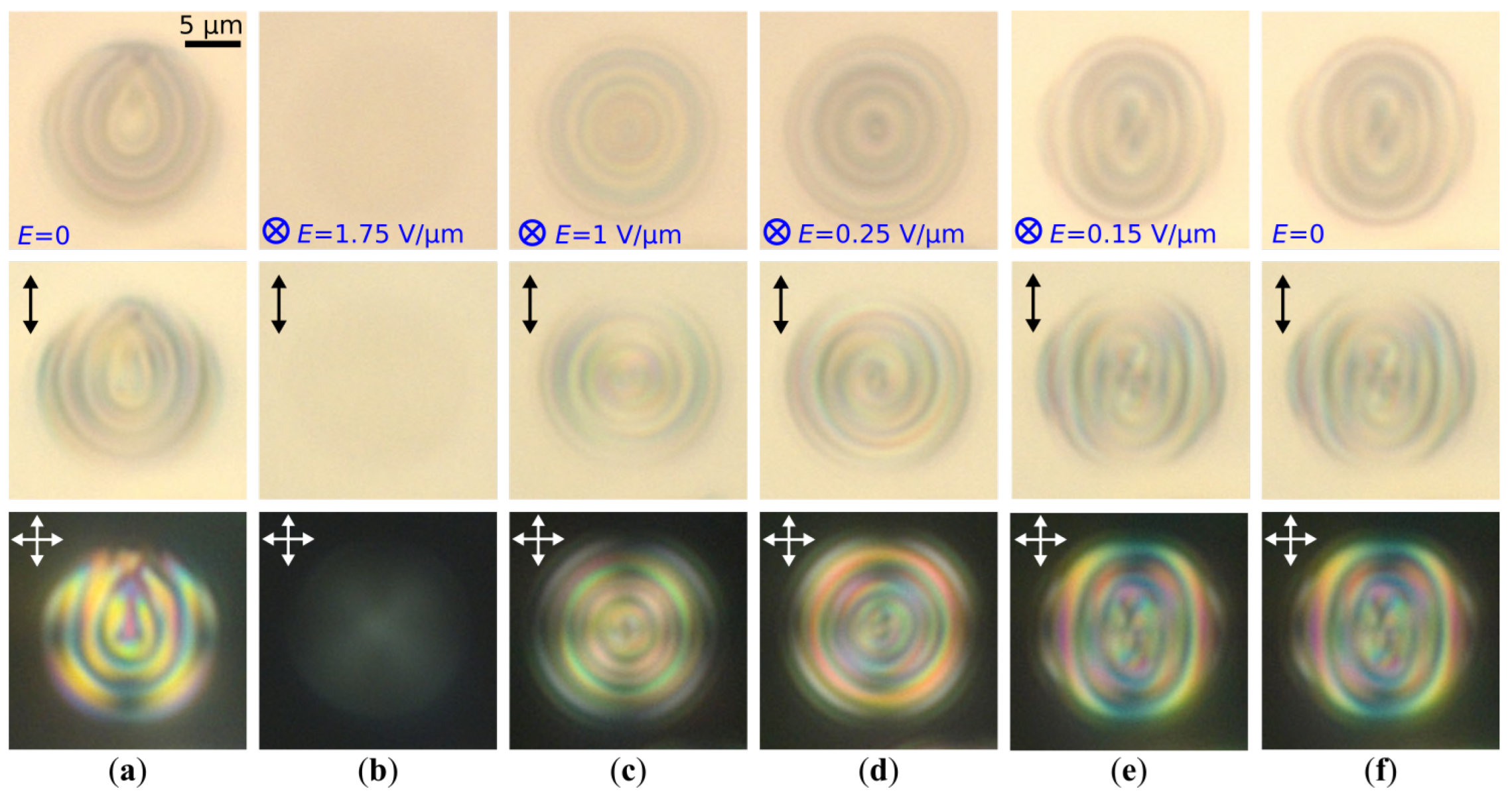
2.2. Structure Transformation at One-Step Voltage-Off Mode
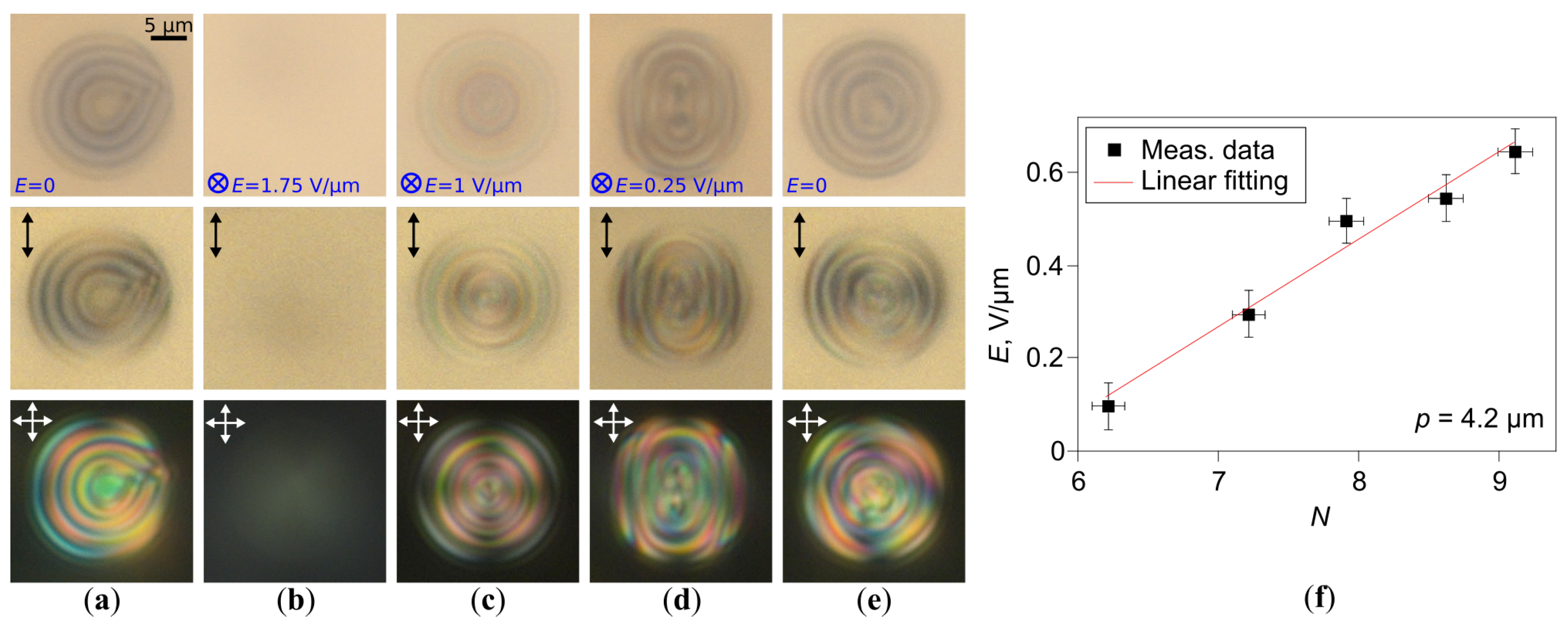
2.3. Structure Transformation at Multi-Step Voltage-Off Mode
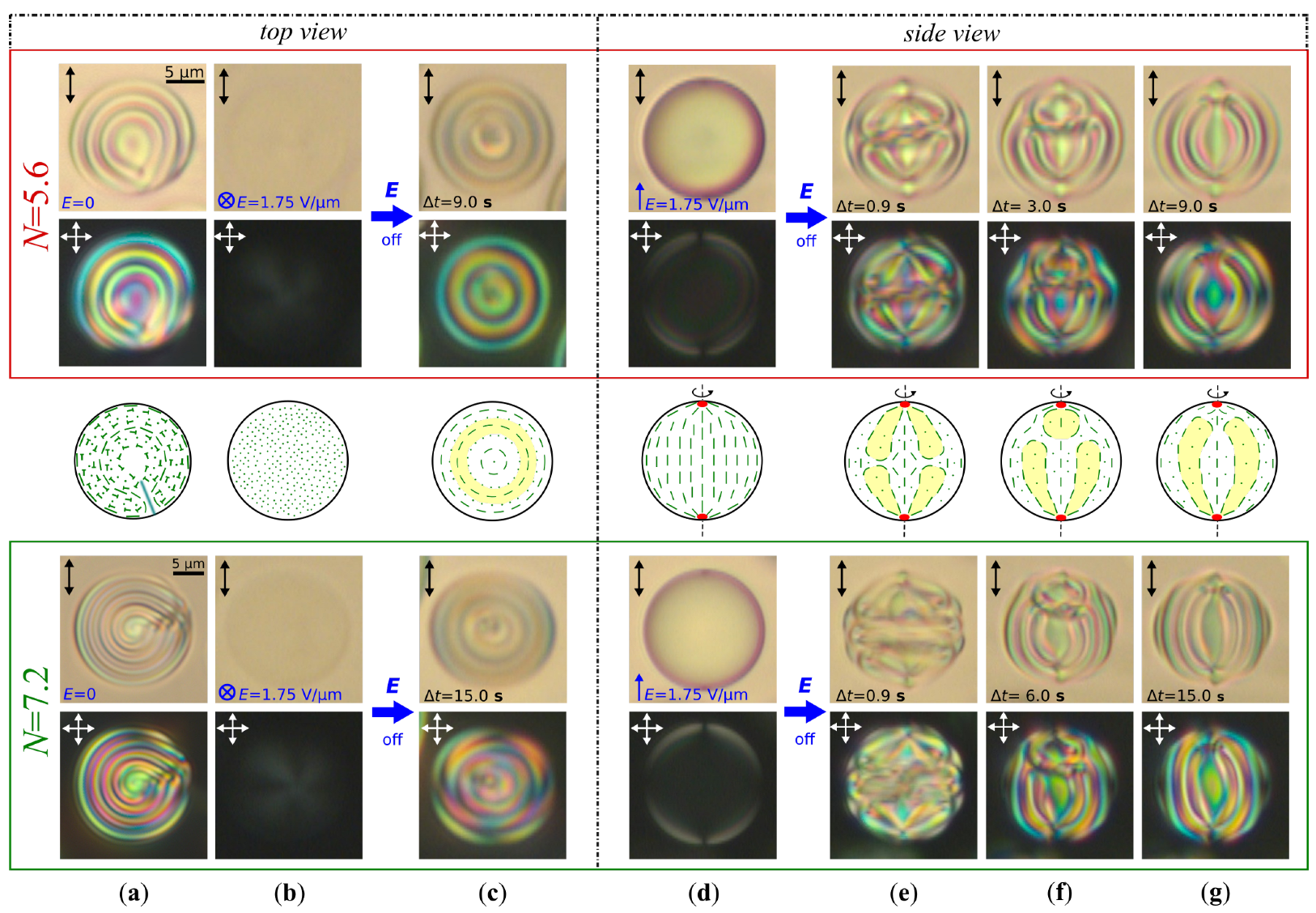
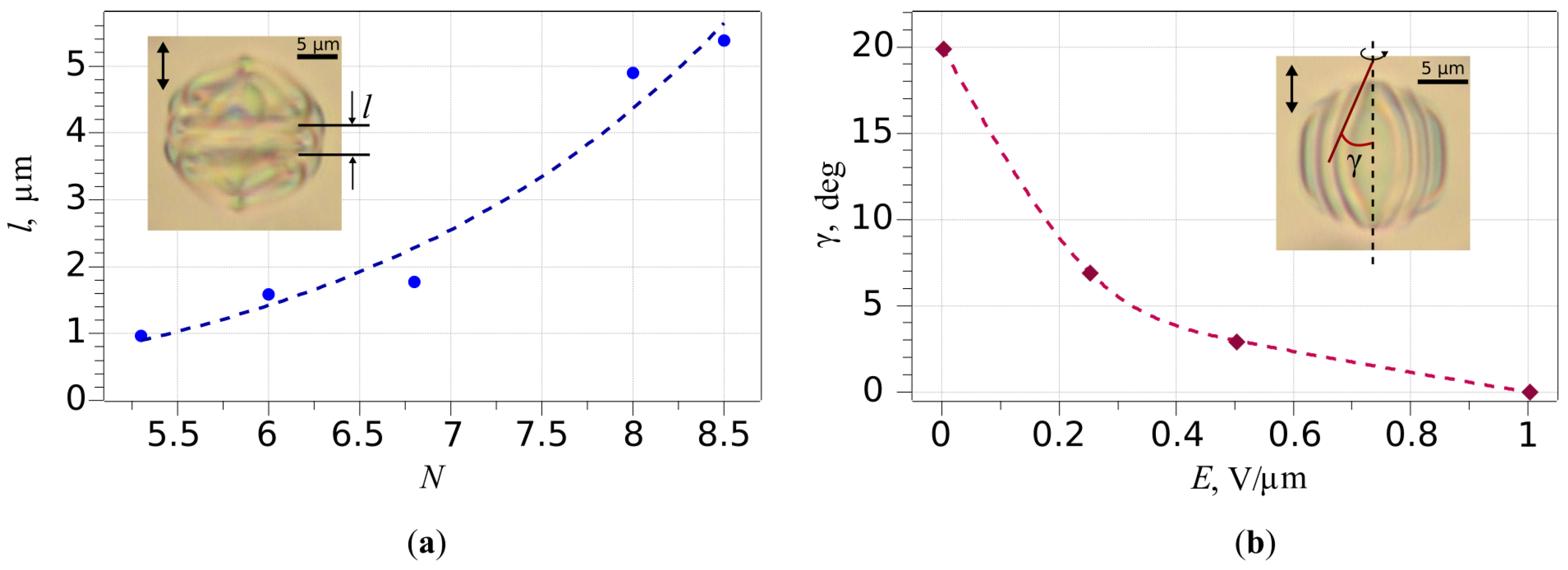
2.3.1. Bipolar Structure
2.3.2. Planar Bipolar Structure
3. Discussion
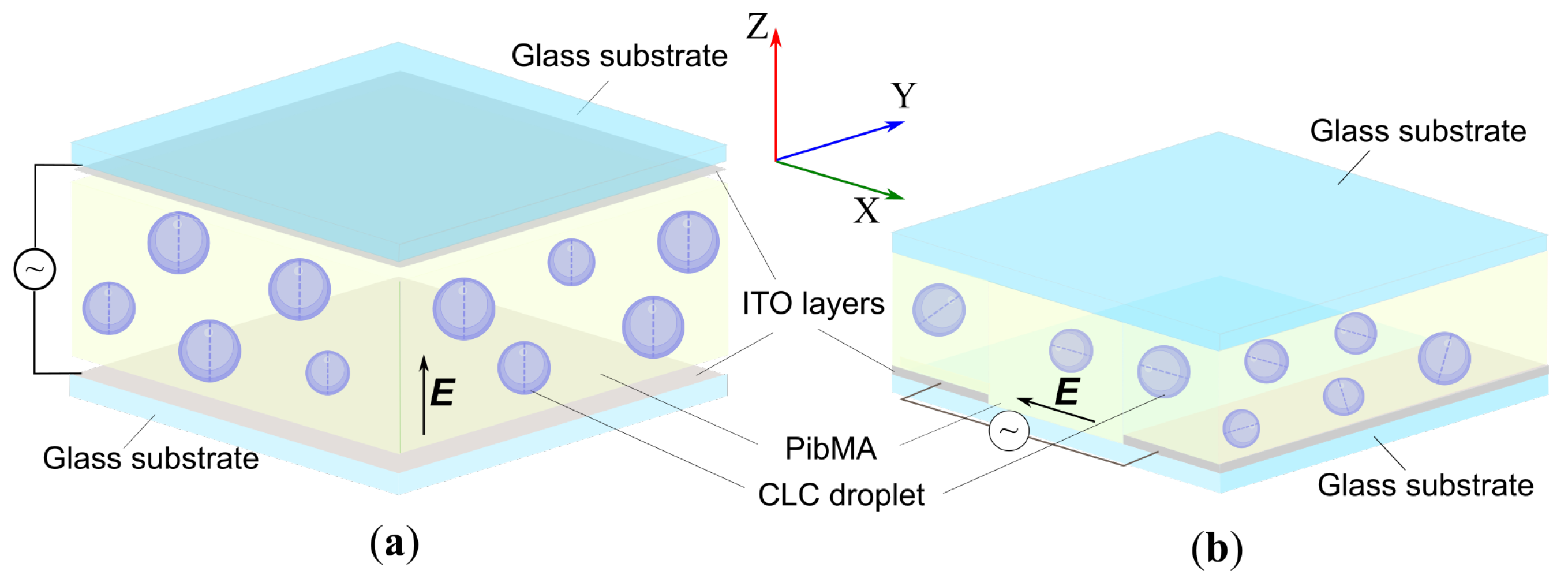
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Posnjak, G.; Čopar, S.; Muševič, I. Hidden topological constellations and polyvalent charges in chiral nematic droplets. Nat. Commun. 2017, 8, 14594. [Google Scholar] [CrossRef]
- Bouligand, Y.; Livolant, F. The organization of cholesteric spherulites. J. Phys. 1984, 45, 1899–1923. [Google Scholar] [CrossRef]
- Krakhalev, M.N.; Rudyak, V.Y.; Prishchepa, O.O.; Gardymova, A.P.; Emelyanenko, A.V.; Liu, J.H.; Zyryanov, V.Y. Orientational structures in cholesteric droplets with homeotropic surface anchoring. Soft Matter 2019, 15, 5554–5561. [Google Scholar] [CrossRef]
- Lee, H.G.; Munir, S.; Park, S.Y. Cholesteric liquid crystal droplets for biosensors. ACS Appl. Mater. Interfaces 2016, 8, 26407–26417. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Park, S.Y. pH-responsive cholesteric liquid crystal double emulsion droplets prepared by microfluidics. Sens. Actuators B Chem. 2017, 241, 636–643. [Google Scholar] [CrossRef]
- Gollapelli, B.; Tatipamula, A.K.; Dewanjee, S.; Pathinti, R.S.; Vallamkondu, J. Detection of bile acids using optical biosensors based on cholesteric liquid crystal droplets. J. Mater. Chem. C 2021, 9, 13991–14002. [Google Scholar] [CrossRef]
- Noh, J.; Liang, H.L.; Drevensek-Olenik, I.; Lagerwall, J.P. Tuneable multicoloured patterns from photonic cross-communication between cholesteric liquid crystal droplets. J. Mater. Chem. C 2014, 2, 806–810. [Google Scholar] [CrossRef]
- Humar, M.; Muševič, I. 3D microlasers from self-assembled cholesteric liquid-crystal microdroplets. Opt. Express 2010, 18, 26995–27003. [Google Scholar] [CrossRef]
- Petriashvili, G.; Japaridze, K.; Devadze, L.; Zurabishvili, C.; Sepashvili, N.; Ponjavidze, N.; De Santo, M.P.; Matranga, M.A.; Hamdi, R.; Ciuchi, F.; et al. Paper like cholesteric interferential mirror. Opt. Express 2013, 21, 20821–20830. [Google Scholar] [CrossRef]
- Yang, C.; Wu, B.; Ruan, J.; Zhao, P.; Shan, J.; Zhang, R.; Yoon, D.K.; Chen, D.; Liu, K. Mechanochromic responses of cholesteric liquid crystal droplets with nanoscale periodic helical structures showing reversible and tunable structural color. ACS Appl. Polym. Mater. 2021, 4, 463–468. [Google Scholar] [CrossRef]
- Geng, Y.; Noh, J.; Drevensek-Olenik, I.; Rupp, R.; Lenzini, G.; Lagerwall, J.P. High-fidelity spherical cholesteric liquid crystal Bragg reflectors generating unclonable patterns for secure authentication. Sci. Rep. 2016, 6, 26840. [Google Scholar] [CrossRef] [PubMed]
- Agha, H.; Geng, Y.; Ma, X.; Avşar, D.I.; Kizhakidathazhath, R.; Zhang, Y.S.; Tourani, A.; Bavle, H.; Sanchez-Lopez, J.L.; Voos, H.; et al. Unclonable human-invisible machine vision markers leveraging the omnidirectional chiral Bragg diffraction of cholesteric spherical reflectors. Light. Sci. Appl. 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Drzaic, P.S. Liquid Crystal Dispersions; World Scientific: Singapore, 1995. [Google Scholar]
- Bronnikov, S.; Kostromin, S.; Zuev, V. Polymer-dispersed liquid crystals: Progress in preparation, investigation, and application. J. Macromol. Sci. Part B 2013, 52, 1718–1735. [Google Scholar] [CrossRef]
- Saeed, M.H.; Zhang, S.; Cao, Y.; Zhou, L.; Hu, J.; Muhammad, I.; Xiao, J.; Zhang, L.; Yang, H. Recent advances in the polymer dispersed liquid crystal composite and its applications. Molecules 2020, 25, 5510. [Google Scholar] [CrossRef]
- De Santo, M.P.; Spina, L.; Ciuchi, F. Chiral chromonics confined in spherical geometries. Appl. Sci. 2023, 13, 4507. [Google Scholar] [CrossRef]
- Kitzerow, H.S.; Crooker, P. Electric field effects on the droplet structure in polymer dispersed cholesteric liquid crystals. Liq. Cryst. 1993, 13, 31–43. [Google Scholar] [CrossRef]
- Crooker, P.; Yang, D. Polymer-dispersed chiral liquid crystal color display. Appl. Phys. Lett. 1990, 57, 2529–2531. [Google Scholar] [CrossRef]
- Gardymova, A.P.; Krakhalev, M.N.; Zyryanov, V.Y.; Gruzdenko, A.A.; Alekseev, A.A.; Rudyak, V.Y. Polymer dispersed cholesteric liquid crystals with a toroidal director configuration under an electric field. Polymers 2021, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Balenko, N.; Shibaev, V.; Bobrovsky, A. Polymer dispersed cholesteric liquid crystals with combined photo-and mechanochromic response. J. Mol. Liq. 2024, 401, 124637. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Sagisaka, T.; Matsushima, T.; Ubukata, T.; Yokoyama, Y. Chiral, thermally irreversible and quasi-stealth photochromic dopant to control selective reflection wavelength of cholesteric liquid crystal. ChemPhysChem 2020, 21, 1375–1383. [Google Scholar]
- Oh, H.S.; Kim, K.J.; Lee, J.; Kim, J.B.; Ku, K.H. Unveiling the structural influence of nematic mesogens on customizable temperature and spectral responses. J. Colloid Interface Sci. 2025, 677, 250–258. [Google Scholar] [CrossRef]
- Xu, F.; Crooker, P. Chiral nematic droplets with parallel surface anchoring. Phys. Rev. E 1997, 56, 6853. [Google Scholar] [CrossRef]
- Gardymova, A.P.; Krakhalev, M.N.; Zyryanov, V.Y. Optical textures and orientational structures in cholesteric droplets with conical boundary conditions. Molecules 2020, 25, 1740. [Google Scholar] [CrossRef]
- Hernández, R.; Provenzano, C.; Mazzulla, A.; Pagliusi, P.; Viola, M.; Cipparrone, G. Cholesteric solid spherical microparticles: Chiral optomechanics and microphotonics. Liq. Cryst. Rev. 2016, 4, 59–79. [Google Scholar] [CrossRef]
- Pierron, J.; Tournier-Lasserve, V.; Sopena, P.; Boudet, A.; Sixou, P.; Mitov, M. Three-dimensional microstructure of a polymer-dispersed liquid crystal observed by transmission electron microscopy. J. Phys. II 1995, 5, 1635–1647. [Google Scholar] [CrossRef]
- Zhou, Y.; Bukusoglu, E.; Martínez-González, J.A.; Rahimi, M.; Roberts, T.F.; Zhang, R.; Wang, X.; Abbott, N.L.; De Pablo, J.J. Structural transitions in cholesteric liquid crystal droplets. ACS Nano 2016, 10, 6484–6490. [Google Scholar] [CrossRef] [PubMed]
- Seč, D.; Porenta, T.; Ravnik, M.; Žumer, S. Geometrical frustration of chiral ordering in cholesteric droplets. Soft Matter 2012, 8, 11982–11988. [Google Scholar] [CrossRef]
- Pollard, J.; Posnjak, G.; Čopar, S.; Muševič, I.; Alexander, G.P. Point defects, topological chirality, and singularity theory in cholesteric liquid-crystal droplets. Phys. Rev. X 2019, 9, 021004. [Google Scholar] [CrossRef]
- Seč, D.; Čopar, S.; Žumer, S. Topological zoo of free-standing knots in confined chiral nematic fluids. Nat. Commun. 2014, 5, 3057. [Google Scholar] [CrossRef]
- Gardymova, A.P.; Krakhalev, M.N.; Rudyak, V.Y.; Barbashov, V.A.; Zyryanov, V.Y. Polymer-Dispersed Cholesteric Liquid Crystal under Homeotropic Anchoring: Electrically Induced Structures with λ 1/2-Disclination. Polymers 2022, 14, 1454. [Google Scholar] [CrossRef]
- Sleczkowski, P.; Zhou, Y.; Iamsaard, S.; De Pablo, J.J.; Katsonis, N.; Lacaze, E. Light-activated helical inversion in cholesteric liquid crystal microdroplets. Proc. Natl. Acad. Sci. USA 2018, 115, 4334–4339. [Google Scholar] [CrossRef]
- Orlova, T.; Asshoff, S.J.; Yamaguchi, T.; Katsonis, N.; Brasselet, E. Creation and manipulation of topological states in chiral nematic microspheres. Nat. Commun. 2015, 6, 7603. [Google Scholar] [CrossRef]
- Yoshioka, J.; Ito, Y.; Fukao, K. Morphogenesis of a chiral liquid crystalline droplet with topological reconnection and Lehmann rotation. Sci. Rep. 2024, 14, 7597. [Google Scholar] [CrossRef] [PubMed]
- Krakhalev, M.N.; Gardymova, A.P.; Rudyak, V.Y.; Barbashov, V.A.; Zyryanov, V.Y. Electrically induced transformation of cholesteric droplets under homeotropic boundary conditions. J. Mol. Liq. 2023, 385, 122379. [Google Scholar] [CrossRef]
- Swisher, R. The cholesteric-nematic transition in droplets subjected to electric fields. Liq. Cryst. 1999, 26, 57–62. [Google Scholar] [CrossRef]
- Kurik, M.V.; Lavrentovich, O. Defects in liquid crystals: Homotopy theory and experimental studies. Sov. Phys. Uspekhi 1988, 31, 196. [Google Scholar] [CrossRef]
- Cipparrone, G.; Mazzulla, A.; Pane, A.; Hernandez, R.J.; Bartolino, R. Chiral self-assembled solid microspheres: A novel multifunctional microphotonic device. Adv. Mater. 2011, 23, 5773. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.J.; Mazzulla, A.; Pane, A.; Volke-Sepúlveda, K.; Cipparrone, G. Attractive-repulsive dynamics on light-responsive chiral microparticles induced by polarized tweezers. Lab A Chip 2013, 13, 459–467. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prishchepa, O.O.; Krakhalev, M.N.; Gardymova, A.P. Electrically Controlled Structures in Cholesteric Droplets with Planar Anchoring. Molecules 2025, 30, 4482. https://doi.org/10.3390/molecules30224482
Prishchepa OO, Krakhalev MN, Gardymova AP. Electrically Controlled Structures in Cholesteric Droplets with Planar Anchoring. Molecules. 2025; 30(22):4482. https://doi.org/10.3390/molecules30224482
Chicago/Turabian StylePrishchepa, Oxana O., Mikhail N. Krakhalev, and Anna P. Gardymova. 2025. "Electrically Controlled Structures in Cholesteric Droplets with Planar Anchoring" Molecules 30, no. 22: 4482. https://doi.org/10.3390/molecules30224482
APA StylePrishchepa, O. O., Krakhalev, M. N., & Gardymova, A. P. (2025). Electrically Controlled Structures in Cholesteric Droplets with Planar Anchoring. Molecules, 30(22), 4482. https://doi.org/10.3390/molecules30224482








