Abstract
Two new cytochalasans (1–2), along with eight known ones (3–10) were isolated from sponge-derived Aspergillus sp. SCSIO 41044. The planar structures of 1–2 were elucidated through extensive spectroscopic analyses and their absolute configurations determined by modified Mosher’s methods. Biological evaluation revealed that 5, 7, and 8 showed potent cytotoxicity against small cell lung cancers H446 and H1048, with IC50 values ranging from 0.0441 to 1.61 μM.
1. Introduction
Cancer remains a major public health issue in China, with lung cancer ranking first in terms of both new cancer cases and cancer deaths in 2022 [1]. Also, according to global cancer survey data, lung cancer was the most commonly diagnosed cancer worldwide in 2022 [2]. Small cell lung cancer accounts for approximately 15% of all lung cancer cases. It is characterized by an extremely high rate of cell proliferation, a strong tendency to metastasize at an early stage, and an unfavorable prognosis. Despite extensive research on the mechanisms and drugs of small cell lung cancer, there is still a lack of small molecule drugs for treating small cell lung cancer in clinical practice [3,4]. Cytochalasans, the original meaning of which refers to the ability to disrupt actin filaments in cells, represent a category of fungal metabolites boasting diverse biological activities. These compounds always feature a unique tricyclic core structure fused to various aromatic or heteroaromatic rings, with the core consisting of an 11-, 13-, or 14-membered macrocyclic ring of polyketide origin [5,6]. Cytochalasans have demonstrated cytotoxic effects, and they are capable of inhibiting the growth and proliferation of cancer cells. Owing to this critical property, cytochalasans have attracted considerable attention in scientific research, particularly in the effort to develop them into potential anticancer agents [7,8,9]. Numerous structurally unique and biologically active secondary metabolites have been isolated from marine sources, with sponge-derived fungi being one of the most prolific [10,11]. In our ongoing investigations aimed at identifying chemically diverse secondary metabolites with anti-tumor activities, two new cytochalasans (1–2) and eight known ones (3–10) (Figure 1) were isolated from a liquid culture of sponge-derived Aspergillus sp. SCSIO 41044 under the guidance of Global Natural Products Social (GNPS) Molecular Networking analysis (Figure 2). Herein, we report the isolation, structural characterization, and anti-tumor activities of these cytochalasans.
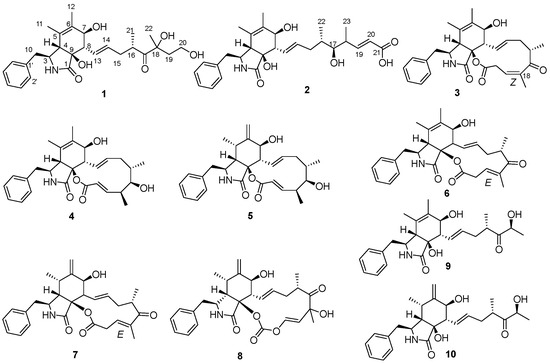
Figure 1.
Cytochalasans (1–10) from sponge-derived Aspergillus sp. SCSIO 41044.
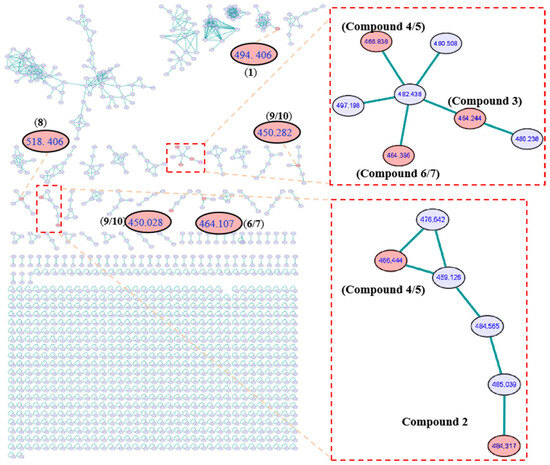
Figure 2.
GNPS molecular network obtained through HR-ESI-MS/MS analysis of extracts from Aspergillus sp. SCSIO 41044 and clusters corresponding to cytochalasans observed in this molecular network.
2. Results and Discussion
Cytochalasin Z29 (1) was obtained as white powder, and was established as C27H37NO6 with ten degrees of unsaturation by a protonated molecule at m/z 472.2697 [M + H]+ in the HRESIMS spectrum and its 13C NMR data. Its 1H NMR spectrum (Table 1) showed five aromatic protons [δH 7.21–7.34, m, 5H], two sp2 methines [δH 5.65 (dd, J = 15.5, 9.5 Hz, CH-13), 5.31 (dd, J = 15.5, 7.0 Hz, CH-14)], one oxygenated sp3 methine [δH 3.62 (d, J = 7.0 Hz, CH-7)], one oxygenated sp3 methylene [δH 3.37–3.50 (m, CH2-20)], four methyls [δH 1.39 (s, CH3-11), 1.61 (s, CH3-12), 0.96 (d, J = 7.0 Hz, CH3-21), 1.18 (s, CH3-22)], and five active hydrogen protons [δH 7.80 (s, NH-2), 4.46 (t, J = 5.0 Hz, OH-3), 4.61 (d, J = 7.0 Hz, OH-7), 5.18 (s, OH-9), 5.08 (OH-17)]. Correspondingly, the 13C NMR and HSQC spectra (Table 1) displayed 27 carbons including two carbonyls (δC 218.6, 174.8), three sp2 nonprotonated carbons (δC 137.8, 131.3, 125.0), two oxygenated sp3 nonprotonated carbons (δC 77.9, 75.9), seven sp2 methines, five sp3 methines including one oxygenated methine (δC 70.7), four sp3 methylenes including one oxygenated methylene (δC 56.8), and four methyls (δC 25.8, 17.4, 16.8, 15.9).

Table 1.
NMR data for compounds 1–2 (500/125 MHz, TMS, δ ppm) in DMSO-d6.
Part of the NMR data of 1 showed similarity to those of cytochalasin Z13 (9) [12] which suggested 1 was a cytochalasin. The obvious differences were two more methylenes (CH2-19 and CH2-20) and one more oxygenated nonprotonated carbon (C-18) in 1 than in 9, while oxygenated methine at C-18 in 9 was absent in the spectrum for 1. The differences suggested 1 was a derivative of 9 with a 1-hydroxyethyl instead of a proton at C-18. The above suggestion was confirmed by correlations of H2-19/H2-20/OH-20 in the 1H-1H COSY spectrum and HMBC correlations from H2-19 to C-17, C-18, and C-22, from H3-22 to C-17, C-18, and C-19, from OH-18 to C-18, C-19, and C-22, and from OH-20 to C-19 (Figure 3). The planar structure of 1 was further established by its 1H-1H COSY spectrum and HMBC correlations (Figure 3).
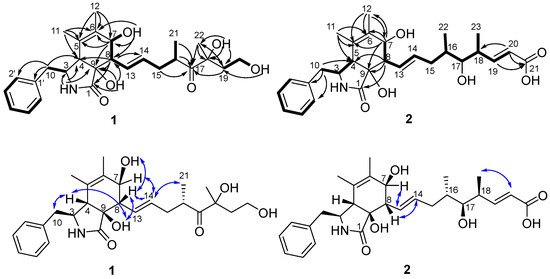
Figure 3.
Key 1H-1H COSY (-), HMBC (→), and NOESY (↔) correlations of compounds 1–2.
The relative configuration of 1 was identified by analysis of the coupling constant data and NOESY spectra. The coupling constant of H-13 and H-14 is 15.5 Hz and the cross peak of H-8 and H-14 in NOESY spectrum indicated an E geometry of Δ13. The NOESY correlations of H2-10/H-4/OH-9/H-8/OH-7 indicated the same relative stereochemistry of the 3-benzyl-isoindol-1-one skeleton as the cytochalasans reported before [13,14,15]. The absolute configuration of C-7 was established as 7S by modified Mosher’s method [16,17] (Figure 4).
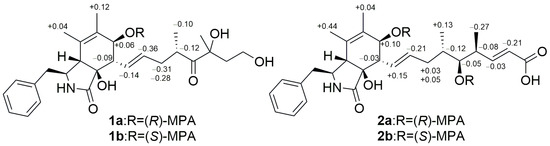
Figure 4.
Δδ value [Δδ (in ppm) = δR–δS] obtained for (R)-MPA and (S)-MPA esters of compounds 1 and 2.
The molecular formula of cytochalasin Z30 (2) was assigned as C28H37NO6 with 11 degrees of unsaturation based on its HRESIMS spectrum and 13C NMR data. Its 1H NMR spectrum (Table 1) showed five aromatic protons [δH 7.20–7.34, m, 5H], four sp2 methines [δH 6.88 (dd, J = 15.5, 8.0 Hz, CH-19), 5.73 (d, J = 15.5 Hz, CH-20), 5.61 (dd, J = 15.5, 9.0 Hz, CH-13), 5.37 (dd, J = 15.5, 7.0 Hz, CH-14)], two oxygenated sp3 methines [δH 3.63 (brd, J = 6.5 Hz, CH-7), 3.13–3.18 (m, CH-17)], four methyls, and one active hydrogen proton [δH 7.77 (s, NH-2)]. Correspondingly, the 13C NMR and HSQC spectra (Table 1) displayed 28 carbons, including two carbonyls (δC 174.8, 167.8), three sp2 nonprotonated carbons (δC 137.8, 131.3, 125.0), one oxygenated sp3 nonprotonated carbon (δC 76.0), nine sp2 methines, seven sp3 methines including two oxygenated methines (δC 75.9, 70.9), two sp3 methylenes, and four methyls (δC 17.4, 17.0, 16.0, 13.7). Its NMR data closely resembled those of the first new cytochalasin reported by Miao et al. from Aspergillus flavipes RD-13 [15] except for the absence of methoxyl group at C-21 in compound 2, which was further established by its 1H-1H COSY and HMBC correlations (Figure 3).
The coupling constants of H-13 and H-14, as well as of H-19 and H-20, are all 15.5 Hz, indicating the E geometry of Δ13 and Δ19, which was also supported by its NOESY correlations of H-8/H-14 and H-20/H-18. The NOESY cross peak of H-7/H-13 suggested that OH-7 and H-8 were in the same side. The signals of hydroxyl protons at C-7 and C-9 were not detected, and the signals of H-4 and H-8 overlapped in 1H NMR of 2. The 1D NMR chemical shifts in C-1 to C-12 of 2 are nearly the same as those of 1, with no more than 0.2 ppm offset (Table 1), which suggests that 2 also has same relative stereochemistry of the 3-benzyl-isoindol-1-one skeleton as 1. The absolute configurations of C-7 and C-17 were established as 7S and 17S by Mosher’s modified method [16,17] (Figure 4).
The configuration of C-16 in the macrocyclic ring moieties was established as S in all cytochalasans isolated so far [7,18,19]. Open-chain cytochalasans are presumably biosynthesized from those cytochalasans with a 12-memberedmacrocyclic ring [13,20]. On this basis, we tentatively concluded that the two new cytochalasans (1–2) have an S-configuration at C-16. Therefore, the absolute configurations two new cytochalasans (1–2), except that of C-18, have been assigned as shown in Figure 1.
By comparing the 1H and 13C NMR and specific rotation data with the previous literature, seven known compounds were identified: 10-phenyl-[12]-cytochalasins Z8 (4) [18], 10-phenyl-[12]-cytochalasin Z7 (5) [18], cytochalasin Z17 (6) [20], 10-phenyl-[12]-cytochalasin Z16 (7) [14], Δ6,12-isomer of 5,6-dehydro-7-hydroxy-cytochalasin E (8) [21], cytochalasin Z13 (9) [13], and cytochalasin Z11 (10) [13]. It is worth mentioning the configuration of Δ18 for compound 3; its 1D NMR data were similar to those of cytochalasin Z17 (6) reported by Lin et al. [20], except for C-17(δC 211.1) and C-23 (δC 19.0) in 3 with obvious shifts downfield, as well as C-19 (δC 124.3) and C-20 (δC 32.1) in 3 with obvious shifts upfield in comparison with those in 6 (Table S1), while the COSY and HMBC correlations of 3 (Figure S33) suggested the same planar structure with 6. The differences in chemical shifts may cause by the different configuration of Δ18 [22], which was also established as Z based on the cross peak of H-19 and H3-23 in NOESY spectrum. However, cytochalasin Z17 bearing a Z configuration at C-18 has been reported by Zhang et al. [14], and upon careful reading of this article, it was discovered that the name and structure of cytochalasin Z17, which in the article is described as the Z configuration at C-18, are inconsistent with the E configuration shown by its X-ray crystallographic drawing. Thus, compound 3 was identified as 18Z-cytochalasin Z17.
All isolated compounds (1–10) were assessed for their cytotoxicity against the small cell lung cancer cell lines H446 and H1048. As a result, compounds 3–8 exhibited varying degrees of inhibitory effect, among which compound 5, 7, and 8 showed significant cytotoxicity with IC50 values ranging from 0.044 to 1.61 μM (Figure 5). Structurally, compounds 3–8 all possess a 12-/13-membered macrocyclic fragment, while compounds 5, 7, and 8 all have a double bond between C-6 and C-12. Thus, the 12-/13-membered macrocyclic fragment and the double bond between C-6 and C-12 may be essential for the cytotoxic activity of cytochalasans. Compounds 1–10 were also tested for their antimicrobial activities against three plant-pathogenic fungi (Alternaria alternate, Curvularia australiensis, and Rhizoctonia solani), as well as three human pathogenic bacteria (Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Exiguobacterium profundum). But none of those compounds showed observable activity with 50 μg per 6 mm disk.
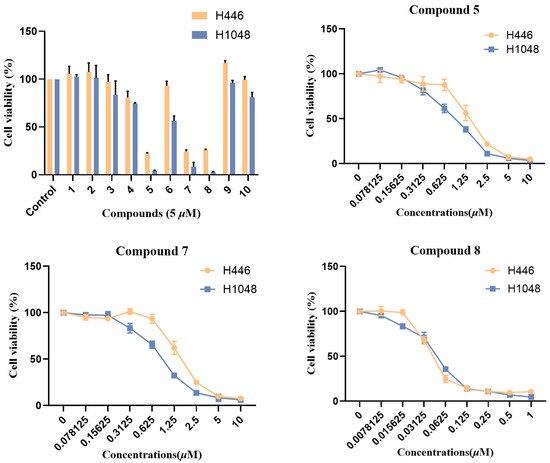
Figure 5.
Cytotoxic activities of isolated compounds (1–10).
3. Materials and Methods
3.1. General Experimental Procedures
HRESIMS spectra were recorded on a Bruker maXis Q-TOF mass spectrometer in positive ion mode. One-dimensional and two-dimensional NMR spectra were measured on a Bruker AV 500 or 700 MHz NMR spectrometer with TMS as an internal standard. Optical rotations were measured using an MCP-500 Polarimeter (Anton, Graz, Austria). ECD and UV spectra were measured with a circular dichroism spectrometer (Applied Photophysics). MPLC was carried on SepaBean machine with YMC ODS-A (12 nm, S-50 μm YMC). HPLC was carried on Hitachi primaide with YMC ODS SERIES (YMC-Pack ODS-A, YMC Co., Ltd., Kyoto, Japan, 250 × 10 mm I.D., S-5 μm, 12 nm). Chromatographic pure organic solvents were purchased from DWSCI HiPurSolv Co., Ltd. Wuhan, China. (S)-α-methoxy-α-phenylacetic acid (MPA) and (R)-MPA were purchased from J&K Scientific, Beijing, China. The analytical reagents (EtOAc and MeOH) were purchased from Jindongtianzheng Fine Chemical Reagent Factory, Tianjin, China. The deuterated reagents were purchased from Qingdao Tenglong Microwave Technology Co., Ltd., Qingdao, China.
3.2. Fungal Material
The fungal strain SCSIO 41044 was obtained from the sponge collected from Yitong Shoal, Hainan Province, China. The producing strain was stored on MB agar (malt extract 15 g, sea salt 10 g, agar 16 g, H2O 1 L, and pH 7.4–7.8) slants at 4 °C and deposited at the Guangdong Key Laboratory of Marine Materia Medica, South China Sea Institute of Oceanology. The ITS1-5.8S-ITS2 sequence region (541 base pairs, accession number PX239440) of strain SCSIO 41044 was amplified by PCR. The Web BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 9 October 2025) analysis against the GenBank database revealed that its DNA sequence shared 99% similarity with Aspergillus urmiensis. Thus, the strain was identified as Aspergillus sp.
3.3. Fermentation, Extraction, and Isolation
The mass fermentation of this fungus was carried out in 19.8 L liquid medium (10 g soluble starch, 1 g tryptone, 20 g sea salt, and 1 L tap water), which was divided into 66 flasks, with each containing 300 mL, at 25 °C under static conditions. After 30 days, the cultures were soaked in EtOAc (500 mL/flask), and the mycelia were cut into small pieces and sonicated for 20 min. The EtOAc solution was concentrated under reduced pressure to gain a crude extract (9.3 g).
The crude extract was subjected to reversed-phase C-18 MPLC eluted with MeOH/H2O (10:90–100:0, v/v) and separated into seven fractions (Fr-1–Fr-7). Fr-6 (2.8 g) was applied to reversed-phase C-18 MPLC again eluted with MeOH/H2O (30:70–100:0, v/v) to obtain five sub-fractions (Fr-6-1–Fr-6-6). Fr-6-1 was subjected to semipreparative HPLC (32% CH3CN/H2O, 2 mL/min) to gain 10 (9.5 mg, tR = 42.0 min) and 1 (5.7 mg, tR = 45.0 min). Fr-6-2 was subjected to semipreparative HPLC (72% CH3OH/H2O, 2 mL/min) to obtain 2 (6.4 mg, tR = 16.5 min). Fr-6-3 was subjected to semipreparative HPLC (60% CH3OH/H2O, 2 mL/min) to afford 8 (12.6 mg, tR = 23.5 min) and 9 (13.7 mg, tR = 19.5 min). Fr-6-5 was subjected to semipreparative HPLC (72% CH3OH/H2O, 2 mL/min) to gain 3 (14.1 mg, tR = 17.0 min) and 6 (38.2 mg, tR = 18.0 min). Fr-7 (1.1 g) was subjected to semipreparative HPLC (2 mL/min) to obtain 7 (12.8 mg, 58% CH3CN/H2O, tR = 15.2 min), 5 (8.0 mg, 75% CH3OH/H2O, tR = 13.8 min) and 4 (22.1 mg, 75% CH3OH/H2O, tR = 14.5 min).
3.4. Structural Characterizations of 1, 2 and 3
Cytochalasin Z29 (1): white powder; [α]20D + 54.8 (c 0.10, MeOH); UV (MeOH) λmax (logε) 200 (3.62) nm; ECD (0.35 mM, MeOH) λmax (Δε) 202 (34.75), 219 (0.11), 222 (2.44) and 236 (−0.61) nm; 1H and 13C NMR data, Table 1; HRESIMS m/z 472.2697 [M + H]+ (calcd for C27H38NO6, 472.2694).
Cytochalasin Z30 (2): white powder; [α]20D + 47.4 (c 0.10, MeOH); UV (MeOH) λmax (logε) 200 (3.56) nm; ECD (0.68 mM, MeOH) λmax (Δε) 202 (20.09), 218 (−0.87), 222 (1.12) and 236 (−0.36) nm; 1H and 13C NMR data, Table 1; HRESIMS m/z 484.2692 [M + H]+ (calcd for C28H38NO6, 484.2694).
18Z-cytochalasin Z17 (3): white powder; [α]20D + 81.4 (c 0.10, MeOH); UV (MeOH) λmax (logε) 200 (3.39), and 239 (2.44) nm; ECD (0.71 mM, MeOH) λmax (Δε) 200 (25.50), and 233 (−4.47) nm; 1H and 13C NMR data, Table S1; HRESIMS m/z 464.2442 [M + H]+ (calcd for C28H34NO5, 464.2431).
3.5. Preparation of the (S)- and (R)-MPA Esters of Cytochalasins Z29 and Z30 Using the Modified Mosher’s Method
Cytochalasins Z29 (1) (1.0 mg) and a small mixing rotor were added into a round-bottom flask (5 mL) and dried in a vacuum desiccator for 2 h. Then, dimethylaminopyridine (0.1 mg), dicyclohexylcarbodiimide (0.1 mg), (R)-MPA (0.69 mg), and 500 μL of CD2Cl2 were added in sequence and the reaction mixture was stirred for 16 h at room temperature. Then the crude product was purified by semipreparative HPLC (80% CH3OH/H2O, 2.2 mL/min, 10.5 min) to yield the (R)-MPA ester 1a (0.7 mg): white powder; 1H NMR (DMSO-d6, 700 MHz) 5.19 (brd, J = 7.0 Hz, H-7), 2.31 (t, J = 9.1 Hz, H-8), 1.32 (brs, H3-11), 1.24 (s, H3-12), 5.75 (dd, J = 15.4, 9.1 Hz, H-13), 4.94 (ddd, J = 19.6, 9.1, 4.9 Hz, H-14), 1.87 (m, H-15a), 1.56 (dt, J = 13.3, 9.1 Hz, H-15b), 3.12 (brdd, J = 14.0, 7.0 Hz, H-16), 0.88 (s, H3-21); ESIMS: m/z 790.3 [M + Na]+. By the same procedure, the (S)-MPA ester 1b (0.8 mg): white powder; 1H NMR (DMSO-d6, 700 MHz) 5.13 (brd, J = 8.4 Hz, H-7), 2.40 (t, J = 8.4 Hz, H-8), 1.18 (brs, H3-11), 1.12 (s, H3-12), 5.89 (dd, J = 15.4, 9.8 Hz, H-13), 5.30 (ddd, J = 15.4, 7.7, 6.3 Hz, H-14), 2.18 (m, H-15a), 1.84 (dt, J = 14.0, 8.4 Hz, H-15b), 3.24 (brdd, J = 14.0, 7.0 Hz, H-16), 0.98 (s, H3-21); ESIMS: m/z 790.3 [M + Na]+ was obtained from the reaction of 1 (1.0 mg) with (S)-MPA (0.69 mg).
Cytochalasins Z30 (2) (1.0 mg) and a small mixing rotor were added into a round-bottom flask (5 mL) and dried in a vacuum desiccator for 2 h. Then, dimethylaminopyridine (0.15 mg), dicyclohexylcarbodiimide (0.26 mg), (R)-MPA (2.75 mg), and 500 μL of CD2Cl2 were added in sequence and the reaction mixture was stirred for 16 h at room temperature. Then, the crude product was purified by semipreparative HPLC (80% CH3OH/H2O with 0.04% formic acid, 2.2 mL/min, 23.5 min) to yield the (R)-MPA ester 2a (0.9 mg): white powder; 1H NMR (DMSO-d6, 500 MHz) 5.17 (brd, J = 8.0 Hz, H-7), 2.35 (m, H-8), 1.33 (s, H3-11), 1.26 (s, H3-12), 5.74 (dd, J = 15.5, 9.5 Hz, H-13), 4.98 (dt, J = 15.5, 6.5 Hz, H-14), 1.67 (m, H-15a), 1.31 (m, H-15b), 1.55 (m, H-16), 4.66 (t, J = 6.0 Hz, H-17), 2.54 (m, H-18), 6.46 (dd, J = 15.5, 8.5 Hz, H-19), 5.56 (d, J = 15.0 Hz, H2-20), 0.67 (d, J = 6.5 Hz, H3-22), 0.67 (d, J = 6.5 Hz, H3-23); ESIMS: m/z 778.3 [M − H]−. By the same procedure, the (S)-MPA ester 2b (0.9 mg): white powder; 1H NMR (DMSO-d6, 500 MHz) 5.07 (brd, J = 8.0 Hz, H-7), 2.38 (m, H-8), 0.89 (s, H3-11), 1.22 (s, H3-12), 5.59 (dd, J = 15.5, 10.0 Hz, H-13), 5.19 (dt, J = 15.0, 6.5 Hz, H-14), 1.64 (m, H-15a), 1.26 (m, H-15b), 1.67 (m, H-16), 4.71 (dd, J = 7.0, 4.5 Hz, H-17), 2.62 (m, H-18), 6.49 (m, H-19), 5.77 (d, J = 15.5 Hz, H2-20), 0.54 (d, J = 6.5 Hz, H3-22), 0.94 (d, J = 6.5 Hz, H3-23); ESIMS: m/z 778.2 [M − H]− was obtained from the reaction of 2 (1.0 mg) with (S)-MPA (2.75 mg).
3.6. Cytotoxicity Assay
H446 and H1048 cells were purchased from the American Type Culture Collection (ATCC). Cells were seeded in 96-well plates at 5000–8000 cells per well, with each well containing a total volume of 100 μL of media. After 24 h, compounds were serially diluted and added 50 μL to the cells per well. Following four days of incubation. CCK-8 reagents (Selleck chemicals, America) were added, and the 96-well plates were incubated at 37 °C in 5% CO2 incubators for 1–2 h. The absorbance at 450 nm was measured using a microplate reader (Promega) according to the manufacturer’s instructions. The final results were expressed as percentages relative to the vehicle-treated cell group, which was defined as 100% [23,24].
4. Conclusions
In summary, under the guidance of GNPS molecular network analysis two new cytochalasans (1–2), along with eight known ones (3–10) were isolated from sponge-derived Aspergillus sp. SCSIO 41044. The modified Mosher’s method was employed to determine the absolute configurations of compounds 1 and 2. Compounds 5, 7, and 8 showed potent cytotoxicity against small cell lung cancers H446 and H1048, with IC50 values ranging from 0.044 to 1.61 μM. Analysis of the structure–activity relationship reveals that the 12-/13-membered macrocyclic fragment and the double bond between C-6 and C-12 may be essential for the cytotoxic activity of cytochalasans. The present study enriches the structural diversity of cytochalasans, and may lay a foundation for the discovery of anti-tumor marine drugs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30224483/s1. Figures S1–S32: 1D and 2D NMR, HRESIMS/ESIMS spectra of 1–3 and Mosher esters. Figure S33: Key 2D NMR correlations. Table S1: NMR data for compounds 3 and 6, and ITS1-5.8S-ITS2 sequences of Aspergillus sp. SCSIO 41044.
Author Contributions
Conceptualization, Y.L.; data curation, X.P., I.W. and Q.C.; writing—original draft preparation, X.P.; writing—review and editing, J.W. (Junfeng Wang) and Y.L.; formal analysis, X.Z. and B.Y.; bioactive test, I.W. and H.W.; funding acquisition, Y.L., J.W. (Junjian Wang), and J.W. (Junfeng Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. U23A20528, 42376124), Guangzhou Science and Technology Project (No. 2024B03J0001), National Key R&D Program of China (No. 2024YFC2815900), Guangdong MEPP Funds (No. GDNRC [2024]28), Guangdong Basic and Applied Basic Research Foundation (No. 2022B1515130008), Key-Area Research and Development Program of Guangdong Province (No. 2023B1111050008), Hainan Provincial Natural Science Foundation of China (No. 823CXTD393), and the National Animal Collection Resource Center, China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Materials.
Acknowledgments
The authors sincerely thank all team members for their diligent efforts in sample collection. Special gratitude is extended to Wei Jiang from the Institute of Oceanology, Chinese Academy of Sciences, and Zhiyun Chen from SCSIO, Chinese Academy of Sciences. The authors are grateful to X.H. Zheng, A.J. Sun, Y. Zhang, and X. Ma at SCSIO for analytic data recording. We also thank the data archive support from the National Earth System Data Center, National Science & Technology Infrastructure of China (http://www.geodata.cn).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Pineros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liang, W.; Hu, Y.; Zhang, X.; Yu, P.; Cai, M.; Xie, D.; Zhou, Q.; Zhou, X.; Liu, Y.; et al. Metabolism characterization and toxicity of N-hydap, a marine candidate drug for lung cancer therapy by LC-MS method. Nat. Prod. Bioprospect. 2024, 14, 33. [Google Scholar]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef]
- Tammam, M.A.; Pereira, F.; Skellam, E.; Bidula, S.; Ganesan, A.; El-Demerdash, A. The cytochalasans: Potent fungal natural products with application from bench to bedside. Nat. Prod. Rep. 2025, 42, 788–841. [Google Scholar] [CrossRef]
- Ma, K.L.; Dong, S.H.; Li, H.Y.; Wei, W.J.; Tu, Y.Q.; Gao, K. Cytochalasins from Xylaria sp. CFL5, an endophytic fungus of Cephalotaxus fortunei. Nat. Prod. Bioprospect. 2021, 11, 87–98. [Google Scholar] [CrossRef]
- Hu, X.Y.; Wang, C.Y.; Li, X.M.; Yang, S.Q.; Li, X.; Wang, B.G.; Si, S.Y.; Meng, L.H. Cytochalasin Derivatives from the endozoic Curvularia verruculosa CS-129, a fungus isolated from the deep-sea squat lobster Shinkaia crosnieri living in the cold seep environment. J. Nat. Prod. 2021, 84, 3122–3130. [Google Scholar] [CrossRef]
- Tian, C.; Feng, Y.; Zhang, H.; Mao, X.; Zhu, X.; Wang, X.; Hou, C.; Han, X.; Yang, H.; Liu, J. Discovery of highly oxygenated cytochalasans with antiproliferative activity from an endophytic fungus Boeremia exigua. Bioorg. Chem. 2025, 156, 108198. [Google Scholar] [CrossRef]
- Cheng, M.M.; Tang, X.L.; Sun, Y.T.; Song, D.Y.; Cheng, Y.J.; Liu, H.; Li, P.L.; Li, G.Q. Biological and chemical diversity of marine sponge-derived microorganisms over the last two decades from 1998 to 2017. Molecules 2020, 25, 853. [Google Scholar] [CrossRef]
- Liang, J.; She, J.; Fu, J.; Wang, J.; Ye, Y.; Yang, B.; Liu, Y.; Zhou, X.; Tao, H. Advances in natural products from the marine-sponge-associated microorganisms with antimicrobial activity in the last decade. Mar. Drugs 2023, 21, 236. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Gulder, T.A.M.; Tsuruta, H.; Mühlbacher, J.; Maksimenka, K.; Steffens, S.; Schaumann, K.; Stöhr, R.; Wiese, J.; et al. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar] [CrossRef]
- Liu, R.; Lin, Z.; Zhu, T.; Fang, Y.; Gu, Q.; Zhu, W. Novel open-chain cytochalsins from the marine-derived fungus Spicaria elegans. J. Nat. Prod. 2008, 71, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Zhang, J.; Hu, S.; Zhang, Z.J.; Zhu, C.J.; Ng, S.W.; Tan, R.X. Ardeemins and cytochalasins from Aspergillus terreus residing in Artemisia annua. Planta Med. 2010, 76, 1616–1621. [Google Scholar] [CrossRef]
- Miao, S.; Liu, M.; Qi, S.; Wu, Y.; Sun, K.; Zhang, Z.; Zhu, K.; Cai, G.; Gong, K. Cytochalasins from coastal saline soil-derived fungus Aspergillus flavipes RD-13 and their cytotoxicities. J. Antibiot. 2022, 75, 410–414. [Google Scholar] [CrossRef]
- Seco, J.M.; Quinoa, E.; Riguera, R. The assignment of absolute configuration by NMR. Chem. Rev. 2004, 104, 17–117. [Google Scholar] [CrossRef]
- Guo, X.; Meng, Q.; Niu, S.; Liu, J.; Guo, X.; Sun, Z.; Liu, D.; Gu, Y.; Huang, J.; Fan, A.; et al. Epigenetic manipulation to trigger production of guaiane-type sesquiterpenes from a marine-derived Spiromastix sp. fungus with antineuroinflammatory effects. J. Nat. Prod. 2021, 84, 1993–2003. [Google Scholar] [CrossRef]
- Liu, R.; Gu, Q.; Zhu, W.; Cui, C.; Fa, G.; Fang, Y.; Zhu, T.; Liu, H. 10-Phenyl-[12]-cytochalasins Z7,Z8, and Z9 from the marine-derived fungus Spicaria elegans. J. Nat. Prod. 2006, 69, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.L.; Li, X.M.; Wang, Y.R.; Meng, L.H.; Wang, B.G. Antibacterial and cytotoxic methylthioether-containing cytochalasins from Chaetomium globosum AS-506, an endozoic fungus associated with deep-sea sponge of Magellan Seamounts. J. Agric. Food Chem. 2025, 73, 1319–1330. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhang, G.J.; Zhu, T.J.; Liu, R.; Wei, H.J.; Gu, Q.Q. Bioactive cytochalasins from Aspergillus flavipes, an endophytic fungus associated with the mangrove plant Acanthus ilicifolius. Helv. Chim. Acta 2009, 92, 1538–1544. [Google Scholar] [CrossRef]
- Takamatsu, S.; Zhang, Q.; Schrader, K.K.; Elsohly, H.N.; Walker, L.A. Characterization of mycotypha metabolites found to be inhibitors of cell adhesion molecules. J. Antibiot. 2002, 55, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Xu, W.; Zhang, X.; Li, Q.; Du, N.N.; Liu, D.F.; Yao, G.D.; Lin, B.; Song, S.J.; Huang, X.X. HSQC-based small molecule accurate recognition technology discovery of diverse cytotoxic sesquiterpenoids from Elephantopus tomentosus L. and structural revision of molephantins A and B. Phytochemistry 2023, 206, 113562. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Zhang, J.; Wang, Q.; Wu, X.; Huang, W.; Wang, Q.; Cai, G.; Wang, H.; Ou, T.; et al. Therapeutic targeting RORgamma with natural product N-hydroxyapiosporamide for small cell lung cancer by reprogramming neuroendocrine fate. Pharmacol. Res. 2022, 178, 106160. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ma, S.; Pang, X.; Cong, M.; Liu, Q.; Han, F.; Wang, J.; Feng, W.; Liu, Y.; Wang, J. Cytotoxic pyridine alkaloids from a marine-derived fungus Arthrinium arundinis exhibiting apoptosis-inducing activities against small cell lung cancer. Phytochemistry 2023, 213, 113765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).