The Crystal Chemistry and Topology of Modular Structures. III. 2D and 3D Zeolites Containing Tetrahedral Layers with the Apophyllite-Type Topology
Abstract
1. Introduction
2. Polymorphism of Tetrahedral Layers
2.1. Stoichiometry of Single-Layer Tetrahedral Anions
2.2. Formula Generation Function
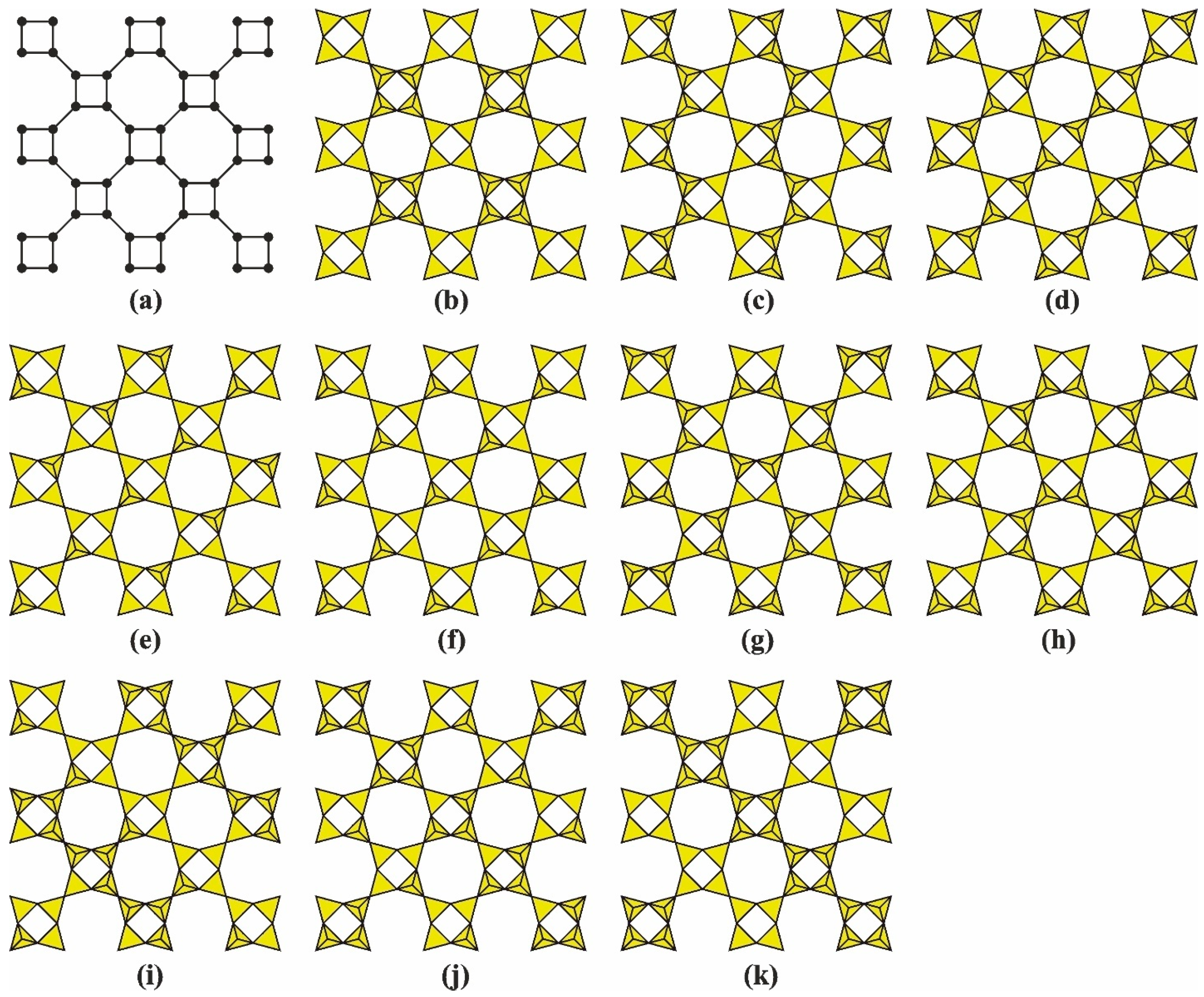
3. Nets of the Apophyllite-Type: Topology and Structural Isomerism
3.1. Topology and Structural Isomerism of the Single-Layer Apophyllite-Type Net
| Mineral/Compound | Symmetry of the Layer | The Ud-Matrix Description of the Layer | References |
|---|---|---|---|
| Apophyllite-related compounds | P4/nmm | [111] | |
| Gillespite-group minerals | [112] | ||
| KSr4[Si4O6(OH)4]2(OH)9 (AES-18) | [113] | ||
| Cavansite | Pmna | [73] | |
| Na2(VO)(Si4O10)(H2O)4 (VSH-13Na) | [104] | ||
| Seidite-(Ce) * | [108] | ||
| Shlykovite | Pm | [109] | |
| Cryptophyllite | [109] | ||
| KNa3(UO2)2(Si4O10)(H2O)4 (KNARSI) | P2/m | [110] | |
| Na4(UO2)2(Si4O10)(H2O)4 (NARSI) | [114] | ||
| KNa3(UO2)2(Si4O10)(H2O)4 (USH-1) | [115] | ||
| Mountainite | Pma2 | [101] | |
| Carletonite * | P4/nbm | [102] | |
| Cs2(VO)(Si4O10)(H2O)x (VSH-12Cs) | Pmc21 | [104] | |
| Li2(VO)(Si4O10)(H2O)x (VSH-12LiX) | [104] | ||
| K2Sb(Si4O10)(OH) | P21/m | [105] | |
| KEu2(Si4O10)F | Pmma | [106] |
3.2. Structural Isomerism as a Factor in Combining Layers into Multilayer Polyanions
4. Condensation of Apophyllite-Type Layers
4.1. Multilayer Polyanions Based on Shlykovite- and Mountainite-Type Layers
- By reflection across a plane, preserving the overall polar motif of the shlykovite layer, with the double-layer symmetry becoming Pmm2.
- Via a twofold rotation axis perpendicular to the existing mirror plane of the original layer, rendering the double layer nonpolar with P2/m symmetry (Figure 3b).
4.2. Silicates with Triple-Layered Apophyllite-Type Complexes: The Günterblassite Group
4.3. Transition from 2D to 3D Frameworks: Apophyllite-Type Net in Zeolite Architectures
4.3.1. Topological Characteristics of Framework Structures
4.3.2. Hierarchical Building Units
- Primary Building Units (PBUs): Vertex-linked TO4 tetrahedra.
- Secondary Building Units (SBUs): Finite/infinite subunits (e.g., the 4 = 2 chain in EDI/THO/NAT frameworks [123]).
- Compositional Building Units (CBUs): Recurrent clusters revealing structural homology.
4.3.3. Main Types of Zeolite Frameworks Based on Apophyllite Nets
4.4. Multilayer Polyanions Based on Seidite-Type Layers
- Sequential layer-by-layer stacking of seidite layers (with possible preservation and/or alternation of stacking types, leading to different polytypes).
- Connection via an “additional” tetrahedron, analogous to the “günterblassite” mechanism of combining double-layer rhodesite-type layers.
- A combination of mechanisms 1 and 2, leading to hybrid structures.
4.5. Frameworks Based on Apophyllite-Type Layers
4.6. Isolated Tetrahedral Layers and Frameworks Containing Apophyllite-Type Nets
4.6.1. Double Layer Based on Carletonite-Type Layers and the Topology of a Hypothetical Framework
4.6.2. Frameworks Constructed by Combining Paracelsian-Type Layers
4.6.3. Frameworks Constructed by Combining Phillipsite-Type Layers and Their Variants
4.6.4. Frameworks Constructed by Combining Merlinoite-Type Layers
4.6.5. Frameworks Constructed by Combining Cavansite-Type Layers and Hybrid Frameworks with Alternating Cavansite- and Pentagonite-Type Layers
4.6.6. Influence of Dcc- and Nsc-Type CBUs on the Topology of Frameworks Containing Apophyllite-Type Nets
5. Polysomatism in Structures with Apophyllite-Type Nets
5.1. Polysomatic Series
- Type of stereoisomer;
- Number of layers in the tetrahedral anion.
5.1.1. The Edingtonite Polysomatic Series
5.1.2. The Montesommaite Polysomatic Series and Other Hypothetical Series Based on the Condensation of Seidite-Type Layers
5.1.3. The ACP-1 Polysomatic Series
5.2. Complexity of Framework Structures
6. Crystal Chemistry of Compounds in the Edingtonite Polysomatic Series
6.1. Mechanisms of Combining Layered Tetrahedral Polyanions
6.2. Single-Layered Members: Shlykovite and Cryptophyllite
6.3. Double-Layer Silicates: The Rhodesite-Related Minerals
6.3.1. The Rhodesite Structural Family
6.3.2. The Delhayelite Structural Family
→ K4Ca2[AlSi7O17(O2−x(OH)x)][(H2O)2−x(OH)x]Cl + 2Na+ + 2F−
→ KCa2[AlSi7O17(OH)2]∙(6 − x)H2O + 3K+ + Cl−.
6.3.3. Related Titanosilicates
2D-Titanosilicates: Jonesite
1D-Titanosilicates: Yuksporite
6.3.4. Triple-Layer Silicates: The Günterblassite-Related Minerals
6.3.5. Framework Silicates: The Edingtonite Subgroup
Natural and Synthetic Compounds with EDI-Type Frameworks
Natural and Synthetic Compounds with THO-Type Frameworks
- The ratio of Si and Al is very stable, and the Si/Al ratio typically deviates only slightly from unity.
- The Na: (Ca+Sr) ratio of ~0.5 also fluctuates little.
- There is a strictly inverse relationship between the contents of Ca and Sr, and the Ca/Sr ratio varies widely: The strontium content ranges from trace to 19.4% SrO (Sr/Ca = 0.00–3.45), recorded in thomsonite-Sr from Mount Rasvumchorr in Khibiny, with no significant gaps observed in this isomorphic series.
- Impurities of other cations (K, Ba, Mg, Fe) are insignificant.
7. Polysomatic Series of Natrolite, Acp-1, and Montesommite
7.1. Polysomatic Series of Mountainite
7.1.1. Single-Layer Silicates: Mountainite
7.1.2. Framework Silicates: The Natrolite Subgroup
→ Na6[Al6Si9O30]·nH2O + 2Ca(CO3)
7.2. Polysomatic Series of ACP-1
7.2.1. Single-Layer Silicates: Apophyllite Group
7.2.2. Framework Silicates: ACP-1
7.3. Polysomatic Series of Montesommaite
7.3.1. Double-Layer Silicates: Seidite-(Ce)
7.3.2. Framework Silicates: The Montesommaite Subgroup
7.3.3. Hypothetical Structures of Single-Layer and Multilayer Members of the Montesommaite Polysomatic Series
8. Properties of Multilayer Silicates (Natural Analogs of 2D Zeolites)
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Liebau, F. Ordered Microporous and Mesoporous Materials with Inorganic Hosts: Definitions of Terms, Formula Notation, and Systematic Classification. Microporous Mesoporous Mater. 2003, 58, 15–72. [Google Scholar] [CrossRef]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of Structural and Compositional Characteristics of Ordered Microporous and Mesoporous Materials with Inorganic Hosts. Microporous Mesoporous Mater. 2003, 58, 3–13. [Google Scholar] [CrossRef]
- Masters, A.F.; Maschmeyer, T. Zeolites—From Curiosity to Cornerstone. Microporous Mesoporous Mater. 2011, 142, 423–438. [Google Scholar] [CrossRef]
- Suib, S.L.; Přech, J.; Čejka, J.; Kuwahara, Y.; Mori, K.; Yamashita, H. Some Novel Porous Materials for Selective Catalytic Oxidations. Mater. Today 2020, 32, 244–259. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Wilson, K.; Lee, A.F. Hierarchical Porous Materials: Catalytic Applications. Chem. Soc. Rev. 2013, 42, 3876–3893. [Google Scholar] [CrossRef]
- Perego, C.; Millini, R. Porous Materials in Catalysis: Challenges for Mesoporous Materials. Chem. Soc. Rev. 2013, 42, 3956–3976. [Google Scholar] [CrossRef]
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesoporous Materials as Gas Sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef]
- Walcarius, A. Mesoporous Materials and Electrochemistry. Chem. Soc. Rev. 2013, 42, 4098. [Google Scholar] [CrossRef]
- Coasne, B.; Galarneau, A.; Pellenq, R.J.M.; Di Renzo, F. Adsorption, Intrusion and Freezing in Porous Silica: The View from the Nanoscale. Chem. Soc. Rev. 2013, 42, 4141. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore Size Determination in Modified Micro- and Mesoporous Materials. Pitfalls and Limitations in Gas Adsorption Data Analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical Zeolites: Enhanced Utilisation of Microporous Crystals in Catalysis by Advances in Materials Design. Chem. Soc. Rev. 2008, 37, 2530. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.S.O.; de Pietre, M.K.; Pastore, H.O. Lamellar Zeolites: An Oxymoron? RSC Adv. 2013, 3, 2084–2111. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Kim, W.; Nair, S. Membranes from Nanoporous 1D and 2D Materials: A Review of Opportunities, Developments, and Challenges. Chem. Eng. Sci. 2013, 104, 908–924. [Google Scholar] [CrossRef]
- Tsapatsis, M. 2-dimensional Zeolites. AIChE J. 2014, 60, 2374–2381. [Google Scholar] [CrossRef]
- Shamzhy, M.; Gil, B.; Opanasenko, M.; Roth, W.J.; Čejka, J. MWW and MFI Frameworks as Model Layered Zeolites: Structures, Transformations, Properties, and Activity. ACS Catal. 2021, 11, 2366–2396. [Google Scholar] [CrossRef]
- Opanasenko, M.V.; Roth, W.J.; Čejka, J. Two-Dimensional Zeolites in Catalysis: Current Status and Perspectives. Catal. Sci. Technol. 2016, 6, 2467–2484. [Google Scholar] [CrossRef]
- Sardar, M.; Maharana, M.; Manna, M.; Sen, S. 2D Zeolites. In Layered 2D Advanced Materials and Their Allied Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 193–210. [Google Scholar]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-Modified Zeolites: Preparation, Characterization, and Applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef]
- de Pietre, M.K.; Freitas, J.C.C. Fundamental Studies on Zeolite–Adsorbate Interactions: Designing a Better Aluminosilicate Adsorbent for Pollutants’ Removal. Environ. Earth Sci. 2022, 81, 17. [Google Scholar] [CrossRef]
- Marler, B.; Gies, H. Hydrous Layer Silicates as Precursors for Zeolites Obtained through Topotactic Condensation: A Review. Eur. J. Mineral. 2012, 24, 405–428. [Google Scholar] [CrossRef]
- Ikeda, T.; Akiyama, Y.; Oumi, Y.; Kawai, A.; Mizukami, F. The Topotactic Conversion of a Novel Layered Silicate into a New Framework Zeolite. Angew. Chem. Int. Ed. 2004, 43, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.K.; Chu, P. Composition of Synthetic Porous Crystalline Material, Its Synthesis and Use 1990. U.S. Patent No. 4,954,325, 4 September 1990. [Google Scholar]
- Fabbiani, M.; Morsli, A.; Confalonieri, G.; Cacciaguerra, T.; Fajula, F.; Haines, J.; Bengueddach, A.; Arletti, R.; Di Renzo, F. On the Chemical Condensation of the Layers of Zeolite Precursor MCM-22(P). Microporous Mesoporous Mater. 2022, 332, 111678. [Google Scholar] [CrossRef]
- Leonowicz, M.E.; Lawton, J.A.; Lawton, S.L.; Rubin, M.K. MCM-22: A Molecular Sieve with Two Independent Multidimensional Channel Systems. Science 1994, 264, 1910–1913. [Google Scholar] [CrossRef]
- Marler, B.; Ströter, N.; Gies, H. The Structure of the New Pure Silica Zeolite RUB-24, Si32O64, Obtained by Topotactic Condensation of the Intercalated Layer Silicate RUB-18. Microporous Mesoporous Mater. 2005, 83, 201–211. [Google Scholar] [CrossRef]
- Wang, Y.X.; Gies, H.; Marler, B.; Müller, U. Synthesis and Crystal Structure of Zeolite RUB-41 Obtained as Calcination Product of a Layered Precursor: A Systematic Approach to a New Synthesis Route. Chem. Mater. 2005, 17, 43–49. [Google Scholar] [CrossRef]
- Schreyeck, L.; Caullet, P.; Mougenel, J.C.; Guth, J.L.; Marler, B. PREFER: A New Layered (Alumino) Silicate Precursor of FER-Type Zeolite. Microporous Mater. 1996, 6, 259–271. [Google Scholar] [CrossRef]
- Li, J.; Gao, Z.R.; Lin, Q.-F.; Liu, C.; Gao, F.; Lin, C.; Zhang, S.; Deng, H.; Mayoral, A.; Fan, W.; et al. A 3D Extra-Large-Pore Zeolite Enabled by 1D-to-3D Topotactic Condensation of a Chain Silicate. Science 2023, 379, 283–287. [Google Scholar] [CrossRef]
- Tang, X.; Altundal, O.F.; Daeyaert, F.; Liu, Z.; Sastre, G. Computational Insights on the Role of Structure-Directing Agents (SDAs) in the Synthesis of Zeolites. Chem. Soc. Rev. 2025, 54, 7067–7092. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of New Zeolite Structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Kasneryk, V.; Shamzhy, M.; Opanasenko, M.; Wheatley, P.S.; Morris, S.A.; Russell, S.E.; Mayoral, A.; Trachta, M.; Čejka, J.; Morris, R.E. Expansion of the ADOR Strategy for the Synthesis of Zeolites: The Synthesis of IPC-12 from Zeolite UOV. Angew. Chem. Int. Ed. 2017, 56, 4324–4327. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Marszalek, B.; Eliášová, P. Layer like Porous Materials with Hierarchical Structure. Chem. Soc. Rev. 2016, 45, 3400–3438. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Marszalek, B. Comprehensive System Integrating 3D and 2D Zeolite Structures with Recent New Types of Layered Geometries. Catal. Today 2014, 227, 9–14. [Google Scholar] [CrossRef]
- Schulman, E.; Wu, W.; Liu, D. Two-Dimensional Zeolite Materials: Structural and Acidity Properties. Materials 2020, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.C.; Katsoulis, D.E.; Kenney, M.E. Synthesis and Characterization of Organosilicon Sheet and Tube Polymers. Chem. Mater. 2001, 13, 4269–4277. [Google Scholar] [CrossRef]
- Chen, C.; Yebassa, D.; Raghavan, D. Synthesis, Characterization, and Mechanical Properties Evaluation of Thermally Stable Apophyllite Vinyl Ester Nanocomposites. Polym. Adv. Technol. 2007, 18, 574–581. [Google Scholar] [CrossRef]
- Specht, K.M.; Jackson, M.; Sunkel, B.; Boucher, M.A. Synthesis of a Functionalized Sheet Silicate Derived from Apophyllite and Further Modification by Hydrosilylation. Appl. Clay Sci. 2010, 47, 212–216. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium–Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Aksenov, S.M.; Chukanov, N.V. Crystal Structure of Günterblassite, a New Mineral with a Triple Tetrahedral Layer. Dokl. Chem. 2012, 442, 57–62. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Rastsvetaeva, R.K.; Aksenov, S.M.; Pekov, I.V.; Zubkova, N.V.; Britvin, S.N.; Belakovskiy, D.I.; Schüller, W.; Ternes, B. Günterblassite, (K,Ca)3-XFe[(Si,Al)13O25(OH,O)4]·7H2O, a New Mineral: The First Phyllosilicate with Triple Tetrahedral Layer. Geol. Ore Depos. 2012, 54, 656–662. [Google Scholar] [CrossRef]
- Krivovichev, S. Topology of Microporous Structures. Rev. Mineral. Geochem. 2005, 57, 17–68. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V. Infrared Spectroscopy as a Tool for the Analysis of Framework Topology and Extra-Framework Components in Microporous Cancrinite- and Sodalite-Related Aluminosilicates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 287, 121993. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K. Structural Chemistry, IR Spectroscopy, Properties, and Genesis of Natural and Synthetic Microporous Cancrinite- and Sodalite-Related Materials: A Review. Microporous Mesoporous Mater. 2021, 323, 111098. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M. Structural Features, Chemical Diversity, and Physical Properties of Microporous Sodalite-Type Materials: A Review. Int. J. Mol. Sci. 2024, 25, 10218. [Google Scholar] [CrossRef]
- Topnikova, A.P.; Eremina, T.A.; Belokoneva, E.L.; Dimitrova, O.V.; Volkov, A.S.; Aksenov, S.M. Synthesis, Crystal Structure and Topological Features of Microporous “Anti-Zeolite” Yb3(BO3)(OH)6∙2.1H2O, a New Cubic Borate with Isolated BO3 Groups. Microporous Mesoporous Mater. 2020, 300, 110147. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Mackley, S.A.; Deyneko, D.V.; Taroev, V.K.; Tauson, V.L.; Rastsvetaeva, R.K.; Burns, P.C. Crystal Chemistry of Compounds with Lanthanide Based Microporous Heteropolyhedral Frameworks: Synthesis, Crystal Structures, and Luminescence Properties of Novel Potassium Cerium and Erbium Silicates. Microporous Mesoporous Mater. 2019, 284, 25–35. [Google Scholar] [CrossRef]
- Chong, S.; Aksenov, S.M.; Dal Bo, F.; Perry, S.N.; Dimakopoulou, F.; Burns, P.C. Framework Polymorphism and Modular Crystal Structures of Uranyl Vanadates of Divalent Cations: Synthesis and Characterization of M (UO2)(V2O7) (M = Ca, Sr) and Sr3(UO2)(V2O7)2. Z. Für Anorg. Allg. Chem. 2019, 645, 981–987. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Charkin, D.O.; Banaru, A.M.; Banaru, D.A.; Volkov, S.N.; Deineko, D.V.; Kuznetsov, A.N.; Rastsvetaeva, R.K.; Chukanov, N.V.; Shkurskii, B.B.; et al. Modularity, Polytypism, Topology, and Complexity of Crystal Structures of Inorganic Compounds (Review). J. Struct. Chem. 2023, 64, 1797–2028. [Google Scholar] [CrossRef]
- Belov, N.V. Essays on Structural Mineralogy; Nedra: Moscow, Russia, 1976. (In Russian) [Google Scholar]
- Liebau, F. Structural Chemistry of Silicates; Springer: Berlin/Heidelberg, Germany, 1985; ISBN 978-3-642-50078-7. [Google Scholar]
- Pushcharovsky, D.Y. Structural Mineralogy of Silicates and Their Structural Analogs; Nedra: Moscow, Russia, 1986. [Google Scholar]
- Hawthorne, F.C. Generating Functions for Stoichiometry and Structure of Single- and Double-Layer Sheet-Silicates. Mineral. Mag. 2015, 79, 1675–1709. [Google Scholar] [CrossRef]
- Day, M.C.; Hawthorne, F.C. A Structure Hierarchy for Silicate Minerals: Chain, Ribbon, and Tube Silicates. Mineral. Mag. 2020, 84, 165–244. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Uvarova, Y.A.; Sokolova, E. A Structure Hierarchy for Silicate Minerals: Sheet Silicates. Mineral. Mag. 2019, 83, 3–55. [Google Scholar] [CrossRef]
- Polyakov, V.B.; Ariskin, A.A. Simulation of the Composition and Proportions of Anions in Polymerized Silicate Melts. Glass Phys. Chem. 2008, 34, 50–62. [Google Scholar] [CrossRef]
- Fraser, D.G. Thermodynamic Properties of Silicate Melts. In Thermodynamics in Geology; Springer: Dordrecht, The Netherlands, 1977; pp. 301–325. [Google Scholar]
- Bokii, G.B. Systematics of Natural Silicates; VINITI Crystal Chemistry: Moscow, Russia, 1997; Volume 31. [Google Scholar]
- Sandomirskiy, P.A.; Belov, N.V. Crystal Chemistry of Mixed Anionic Radicals; Nauka: Moscow, Russia, 1984. [Google Scholar]
- Pushcharovsky, D.Y.; Zubkova, N.V.; Pekov, I.V. Structural Chemistry of Silicates: New Discoveries and Ideas. Struct. Chem. 2016, 27, 1593–1603. [Google Scholar] [CrossRef]
- Krivovichev, S.V. High-Pressure Silicates: Crystal Chemistry and Systematics. Zapiski Rossiiskogo Mineral. Obs. 2021, 150, 1–78. [Google Scholar] [CrossRef]
- Christensen, K.E. Design of Open-Framework Germanates. Crystallogr. Rev. 2010, 16, 91–104. [Google Scholar] [CrossRef]
- Fleet, M.E. Rock-Forming Minerals. Volume 3A: Sheet Silicates: Micas, 2nd ed.; Deer, W.A., Howie, R.A., Zussman, J., Eds.; Geological Society of London: London, UK, 2004. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals. 3B. Layered Silicates Excluding Micas and Clay Minerals, 2nd ed.; The Geological Society: London, UK, 2009. [Google Scholar]
- Wilson, M.J. Rock-Forming Minerals, Vol. 3c, Sheet Silicates–Clay Minerals, 2nd ed.; The Geological Society: London, UK, 2013. [Google Scholar]
- Chukhrov, F.V. Minerals. Vol. IV. Issue 2; Chukhrov, F.V., Ed.; Nauka: Moscow, Russia, 1992. [Google Scholar]
- Hawthorne, F.C. Graphical Enumeration of Polyhedral Clusters. Acta Crystallogr. A 1983, 39, 724–736. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Combinatorial Topology of Salts of Inorganic Oxoacids: Zero-, One- and Two-Dimensional Units with Corner-Sharing between Coordination Polyhedra. Crystallogr. Rev. 2004, 10, 185–232. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural Crystallography of Inorganic Oxysalts; Oxford University Press: Oxford, UK, 2009; ISBN 9780199213207. [Google Scholar]
- Krivovichev, S.V. Structure Description, Interpretation and Classification in Mineralogical Crystallography. Crystallogr. Rev. 2017, 23, 2–71. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Jonsson, E.; Aksenov, S.M.; Britvin, S.N.; Rastsvetaeva, R.K.; Belakovskiy, D.I.; Van, K.V. Roymillerite, Pb24Mg9Si9AlO28)(SiO4)(BO3)(CO3)10(OH)14O4, a New Mineral: Mineralogical Characterization and Crystal Chemistry. Phys. Chem. Miner. 2017, 44, 685–699. [Google Scholar] [CrossRef]
- Kolitsch, U.; Merlino, S.; Holtstam, D. Molybdophyllite: Crystal Chemistry, Crystal Structure, OD Character and Modular Relationships with Britvinite. Mineral. Mag. 2012, 76, 493–516. [Google Scholar] [CrossRef]
- Evans, H.T., Jr. The Crystal Structures of Cavansite and Pentagonite. Am. Mineral. 1973, 58, 412–424. [Google Scholar]
- Solov’ev, M.V.; Rastsvetaeva, R.K.; Pushcharovsky, D.Y. Refined Crystal Structure of Kavansite. Crystallogr. Rep. 1993, 38, 274–275. [Google Scholar]
- Shumyatskaya, N.G.; Voronkov, A.A.; Pyatenko, Y.A. Sazhinite, Na2Ce[Si6O14(OH)]NH2O: A New Representative of the Dalyite Family in Crystal Chemistry. Sov. Phys. Crystallogr. 1980, 25, 419–423. [Google Scholar]
- Pushcharovsky, D.Y.; Kabalov, K.; Zubkova, N.V.; Schneider, J.; Sapozhnikov, A.N. Powder Rietveld Refinement of Armstrongite, CaZr[Si6O15]·3H2O. Z. Kristallogr. Cryst. Mater. 2000, 215, 757–761. [Google Scholar] [CrossRef]
- Fleet, S.G. The Crystal Structure of Dalyite. Z. Für Krist. 1965, 121, 349–368. [Google Scholar] [CrossRef][Green Version]
- Karpov, O.G.; Pushcharovsky, D.Y.; Pobedimskaya, E.A.; Burshtein, I.F.; Belov, N.V. Crystal Structure of Rare Earth Silicate NaNd[Si6O13(OH)2] x nH2O. Sov. Physics. Dokl. 1977, 236, 593–596. [Google Scholar]
- Pushcharovsky, D.Y.; Karpov, O.G.; Pobedimskaya, E.A.; Belov, N.V. The Crystal Structure of K3NdSi6O15. Sov. Physics. Dokl. 1977, 234, 1323–1325. [Google Scholar]
- Rastsvetaeva, R.K.; Aksenov, S.M.; Taroev, V.K. Crystal Structures of Endotaxic Phases in Europium Potassium Silicate Having a Pellyite Unit Cell. Crystallogr. Rep. 2010, 55, 1041–1049. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Aksenov, S.M. New Phases of K, Eu-Silicate in the Family of Compounds with the Orthorhombic Pellyite-like Unit Cell. Bulg. Chem. Commun. 2011, 43, 308–315. [Google Scholar]
- Topnikova, A.; Belokoneva, E.; Dimitrova, O.; Volkov, A.; Deyneko, D. Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction. Minerals 2021, 11, 395. [Google Scholar] [CrossRef]
- Cadoni, M.; Cheah, Y.L.; Ferraris, G. New RE Microporous Heteropolyhedral Silicates Containing 41516182 Tetrahedral Sheets. Acta Crystallogr. B 2010, 66, 158–164. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Actinyl Compounds with Hexavalent Elements (S, Cr, Se, Mo)—Structural Diversity, Nanoscale Chemistry, and Cellular Automata Modeling. Eur. J. Inorg. Chem. 2010, 2010, 2594–2603. [Google Scholar] [CrossRef]
- Gorelova, L.A.; Bubnova, R.S.; Krivovichev, S.V.; Krzhizhanovskaya, M.G.; Filatov, S.K. Thermal Expansion and Structural Complexity of Ba Silicates with Tetrahedrally Coordinated Si Atoms. J. Solid State Chem. 2016, 235, 76–84. [Google Scholar] [CrossRef]
- Moore, P.B. Laueite, Pseudolaueite, Stewartite and Metavauxite; a Study in Combinatorial Polymorphism. Neues Jahrb. Fuer Mineral. Abh. 1975, 123, 148–159. [Google Scholar]
- Rastsvetaeva, R.K.; Aksenov, S.M. Crystal Chemistry of Silicates with Three-Layer TOT and HOH Modules of Layered, Chainlike, and Mixed Types. Crystallogr. Rep. 2011, 56, 910–934. [Google Scholar] [CrossRef]
- Hawthorne, F.C. Bond Topology and Structure-Generating Functions: Graph-Theoretic Prediction of Chemical Composition and Structure in Polysomatic T–O–T (Biopyribole) and H–O–H Structures. Mineral. Mag. 2012, 76, 1053–1080. [Google Scholar] [CrossRef]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, Face-, Point-, Schläfli-, and Delaney-Symbols in Nets, Polyhedra and Tilings: Recommended Terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef]
- Smith, J.V. Enumeration of 4-Connected 3-Dimensional Nets and Classification of Framework Silicates, II, Perpendicular and near-Perpendicular Linkages from 4.82, 3.122 and 4.6.12 Nets. Am. Mineral. 1978, 63, 960–969. [Google Scholar]
- Bačík, P.; Miyawaki, R.; Atencio, D.; Cámara, F.; Fridrichová, J. Nomenclature of the Gadolinite Supergroup. Eur. J. Mineral. 2017, 29, 1067–1082. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K.; Kristiansen, R.; Pekov, I.V.; Belakovskiy, D.I.; Van, K.V.; Bychkova, Y.V.; Britvin, S.N. Crystal Structure of the OH-Dominant Gadolinite-(Y) Analogue (Y,Ca)2(Fe,□)Be2Si2O8(OH,O)2 from Heftetjern Pegmatite, Norway. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 899–906. [Google Scholar] [CrossRef]
- Huminicki, D.M.C.; Hawthorne, F.C. The Crystal Chemistry of the Phosphate Minerals. Rev. Mineral. Geochem. 2002, 48, 123–253. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Huminicki, D.M.C. The Crystal Chemistry of Beryllium. Rev. Mineral. Geochem. 2002, 50, 333–403. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Burns, P.C.; Grice, J.D. The Crystal Chemistry of Boron. Rev. Mineral. Geochem. 1996, 33, 41–116. [Google Scholar] [CrossRef]
- Piret, P.; Deliens, M.; Piret-Meunier, J. Occurrence and Crystal Structure of Kipushite, a New Copper-Zinc Phosphate from Kipushi, Zaire. Can. Mineral. 1985, 23, 35–42. [Google Scholar]
- Ghose, S.; Leo, S.R.; Wan, C.N. Structural Chemistry of Copper and Zinc Minerals: Part I. Veszelyite, (Cu, Zn)2ZnPO4(OH)3∙2H2O: A Novel Type of Sheet Structure and Crystal Chemistry of Copper-Zinc Substitution. Am. Mineral. 1974, 59, 573–581. [Google Scholar]
- Peacor, D.R.; Dunn, P.J.; Ramik, R.A.; Sturman, B.D.; Zeihen, L.G. Philipsburgite, a New Copper Zinc Arsenate Hydrate Related to Kipushite, from Montana. Can. Mineral. 1985, 23, 255–258. [Google Scholar]
- Siidra, O.I.; Nazarchuk, E.V.; Lukina, E.A.; Zaitsev, A.N.; Shilovskikh, V.V. Belousovite, KZn(SO4)Cl, a New Sulfate Mineral from the Tolbachik Volcano with Apophyllite Sheet-Topology. Mineral. Mag. 2018, 82, 1079–1088. [Google Scholar] [CrossRef]
- Bosson, B. The Crystal Structures of RbZnSO4Cl and TlZnSO4Cl. Acta Crystallogr. B 1976, 32, 2044–2047. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Pekov, I.V.; Pushcharovsky, D.Y.; Chukanov, N.V. The Crystal Structure and Refined Formula of Mountainite, KNa2Ca2[Si8O19(OH)]·6 H2O. Z. Für Krist. 2009, 224, 389–396. [Google Scholar] [CrossRef]
- Chao, G.Y. The Crystal Structure of Carletonite, KNa4Ca4Si8O18(CO3)4F0.5(OH)0.5H2O. Am. Mineral. 1972, 57, 765–778. [Google Scholar]
- Kaneva, E.; Radomskaya, T.; Suvorova, L.; Sterkhova, I.; Mitichkin, M. Crystal Chemistry of Fluorcarletonite, a New Mineral from the Murun Alkaline Complex (Russia). Eur. J. Mineral. 2020, 32, 137–146. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Jacobson, A.J. Open-Framework and Microporous Vanadium Silicates. J. Am. Chem. Soc. 2002, 124, 7812–7820. [Google Scholar] [CrossRef] [PubMed]
- Balko, V.P.; Bakakin, V.V.; Gatilov, Y.V.; Tseitlin, M.N.; Kurbanov, K.M. Crystal Structure of Dicalciumantimony (III) Hydroxotetrasilicate K2Sb(OH) (Si4O10) = K4Sb2(OH)2(Si8O20)2∞. Dokl. Akad. Nauk. SSSR 1985, 283, 616–620. [Google Scholar]
- Jacobsen, H.; Meyer, G. Synthesis, Crystal Growth and Structure of KEu2[Si4O10]F. Z. Kristallogr. Cryst. Mater. 1994, 209, 348–350. [Google Scholar] [CrossRef]
- Pechar, F. An X-ray diffraction refinement of the crystal structure of natural apophyllite. Cryst. Res. Technol. 1987, 22, 1041–1046. [Google Scholar] [CrossRef]
- Ferraris, G.; Belluso, E.; Gula, A.; Soboleva, S.V.; Khomyakov, A.P. The Crystal Structure of Seidite-(Ce), Na4(Ce,Sr)2 (O,OH,F)4·5H2O, A Modular Microporous Titanosilicate of the Rhodesite Group. Can. Mineral. 2003, 41, 1183–1192. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Filinchuk, Y.E.; Pekov, I.V.; Pushcharovsky, D.Y.; Gobechiya, E.R. Crystal Structures of Shlykovite and Cryptophyllite: Comparative Crystal Chemistry of Phyllosilicate Minerals of the Mountainite Family. Eur. J. Mineral. 2010, 22, 547–555. [Google Scholar] [CrossRef]
- Burns, P.C.; Olson, R.A.; Finch, R.J.; Hanchar, J.M.; Thibault, Y. KNa3(UO2)2(Si4O10)2(H2O)4, a New Compound Formed during Vapor Hydration of an Actinide-Bearing Borosilicate Waste Glass. J. Nucl. Mater. 2000, 278, 290–300. [Google Scholar] [CrossRef]
- Bartl, H.; Pfeifer, G. Neutronenbeugungsanalyse Des Apophyllit KCa4(Si4O10)2(F/OH)(H2O)8. Neues Jahrb. Für Mineral.—Monatshefte 1976, 58–65. [Google Scholar]
- Hazen, R.M.; Burnham, C.V. The Crystal Structure of Gillespite I and II: A Structure Determination at High Pressure. Am. Mineral. 1974, 59, 1166–1174. [Google Scholar]
- Ikeda, T.; Ideta, C.; Yamamoto, K. Ab-Initio Structure Determination of Novel Strontium-Containing Layered Silicate AES-18 Synthesized Using Mechanochemical Reaction. Z. Kristallogr. Cryst. Mater. 2013, 228, 173–179. [Google Scholar] [CrossRef]
- Li, Y.; Burns, P.C. The Structures of Two Sodium Uranyl Compounds Relevant to Nuclear Waste Disposal. J. Nucl. Mater. 2001, 299, 219–226. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Liu, L.; Jacobson, A.J. The Novel Open-Framework Uranium Silicates Na2(UO2)(Si4O10)·2.1H2O (USH-1) and RbNa(UO2)(Si2O6)·H2O (USH-3). J. Mater. Chem. 2002, 12, 406–410. [Google Scholar] [CrossRef]
- Hesse, K.-F.; Liebau, F.; Merlino, S. Crystal Structure of Rhodesite, HK1−xNax+2yCa2−y{ LB,3,22∞}[Si8O19]·(6–z)H2O, from Three Localities and Its Relation to Other Silicates with Dreier Double Layers. Z. Für Krist. 1992, 199, 25–48. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Filinchuk, Y.E.; Chukanov, N.V.; Zadov, A.E.; Pushcharovsky, D.Y.; Gobechiya, E.R. Shlykovite KCa[Si4O9(OH)]·3H2O and Cryptophyllite K2Ca[Si4O10]·5H2O, New Mineral Species from the Khibiny Alkaline Pluton, Kola Peninsula, Russia. Geol. Ore Depos. 2010, 52, 767–777. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Topnikova, A.P.; Dimitrova, O.V.; Volkov, A.S. KNa2Tm[Si8O19] · 4H2O, a New Layered Silicate Related to Rhodezite-Shlykovite-Delhayelite-Umbrianite-Guenterblassite-Hilleshaymite: Topology-Symmetry Analysis of the OD-Family and Structure Prediction. Crystallogr. Rep. 2014, 59, 513–522. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ikeda, T.; Ideta, C. Synthesis and Crystal Structure Analysis of a Novel Strontosilicate AES-19 Having Two Dimensional Eight-Membered Ring Micropores. Microporous Mesoporous Mater. 2013, 172, 13–19. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Pekov, I.V.; Zubkova, N.V.; Khomyakov, A.P.; Stoppa, F.; Pushcharovsky, D.Y. Umbrianite, K7Na2Ca2[Al3Si10O29]F2Cl2, a New Mineral Species from Melilitolite of the Pian Di Celle Volcano, Umbria, Italy. Eur. J. Mineral. 2013, 25, 655–669. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Zubkova, N.V.; Pekov, I.V.; Belakovskiy, D.I.; Schüller, W.; Ternes, B.; Blass, G.; Pushcharovsky, D.Y. Hillesheimite, (K,Ca,□)2(Mg,Fe,Ca,□)2[(Si,Al)13O23(OH)6](OH)·8H2O, a New Phyllosilicate Mineral of the Günterblassite Group. Geol. Ore Depos. 2013, 55, 549–557. [Google Scholar] [CrossRef]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended Nomenclature for Zeolite Minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Mineral. Mag. 1998, 62, 533–571. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780444530646. [Google Scholar]
- Galli, E. Crystal Structure Refinement of Edingtonite. Acta Crystallogr. B 1976, 32, 1623–1627. [Google Scholar] [CrossRef]
- Malinovskii, Y.A.; Belov, N.V. Crystal Structure of Kalborsite. Dokl. Acad. Nauk. SSSR 1980, 252, 611–615. [Google Scholar]
- Pekov, I.V.; Chukanov, N.V. New Data on Kalborsite. Zap. Vseross. Mineral. Obs. 1996, 125, 55–59. [Google Scholar]
- Bosi, F.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC)—Newsletter 85. Eur. J. Mineral. 2025, 37, 337–342. [Google Scholar] [CrossRef]
- McCusker, L.B. IUPAC Nomenclature for Ordered Microporous and Mesoporous Materials and Its Application to Non-Zeolite Microporous Mineral Phases. Rev. Mineral. Geochem. 2005, 57, 1–16. [Google Scholar] [CrossRef]
- Foster, M.D.; Rivin, I.; Treacy, M.M.J.; Delgado Friedrichs, O. A Geometric Solution to the Largest-Free-Sphere Problem in Zeolite Frameworks. Microporous Mesoporous Mater. 2006, 90, 32–38. [Google Scholar] [CrossRef]
- Gatta, G.D.; Rotiroti, N.; McIntyre, G.J.; Guastoni, A.; Nestola, F. New Insights into the Crystal Chemistry of Epididymite and Eudidymite from Malosa, Malawi: A Single-Crystal Neutron Diffraction Study. Am. Mineral. 2008, 93, 1158–1165. [Google Scholar] [CrossRef]
- Rudolf, P.R.; Saldarriaga-Molina, C.; Clearfield, A. Preparation and X-Ray Powder Structure Solution of a Novel Aluminum Phosphate Molecular Sieve, (AlPO4)3.Cntdot.(CH3)4NOH. J. Phys. Chem. 1986, 90, 6122–6125. [Google Scholar] [CrossRef]
- Kongshaug, K.O.; Fjellvåg, H.; Lillerud, K.P. Synthesis and Characterization of the Open-Framework Magnesium Aluminophosphate UiO-28. J. Mater. Chem. 2001, 11, 1242–1247. [Google Scholar] [CrossRef]
- Chen, J.; Natarajan, S.; Thomas, J.M.; Jones, R.H.; Hursthouse, M.B. A Novel Open-Framework Cobalt Phosphate Containing a Tetrahedrally Coordinated Cobalt(II) Center: CoPO4·0.5C2H10N2. Angew. Chem. Int. Ed. Engl. 1994, 33, 639–640. [Google Scholar] [CrossRef]
- Rouse, R.C.; Dunn, P.J.; Grice, J.D.; Schenker, J.L.; Higgins, J.B. Montesommaite, (K,Na)9Al9Si23O64∙10H2O, a New Zeolite Related to Merlinoite and the Gismondite Group. Am. Mineral. 1990, 75, 1415–1420. [Google Scholar]
- van Koningsveld, H. Compendium of Zeolite Framework Types: Building Schemes and Type Characteristics; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Petersen, O.V.; Giester, G.; Brandstatter, F.; Niedermayr, G. Nabesite, Na2BeSi4O10·4H2O, A New Mineral Species from the Ilimaussaq Alkaline Complex, South Greenland. Can. Mineral. 2002, 40, 173–181. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Friis, H.; Lieb, A.; Finch, A.A.; Weller, M.T. Combined Single-Crystal X-Ray and Neutron Powder Diffraction Structure Analysis Exemplified through Full Structure Determinations of Framework and Layer Beryllate Minerals. Am. Mineral. 2010, 95, 519–526. [Google Scholar] [CrossRef]
- Merlino, S. Lovdarite, K4Na12(Be8Si28O72)·18H2O, a Zeolite-like Mineral: Structural Features and OD Character. Eur. J. Mineral. 1990, 2, 809–818. [Google Scholar] [CrossRef]
- Annen, M.J.; Davis, M.E.; Higgins, J.B.; Schlenker, J.L. VPI-7: The First Zincosilicate Molecular Sieve Containing Three-Membered T-Atom Rings. J. Chem. Soc. Chem. Commun. 1991, 1175–1176. [Google Scholar] [CrossRef]
- Röhrig, C.; Gies, H. A New Zincosilicate Zeolite with Nine-Ring Channels. Angew. Chem. Int. Ed. Engl. 1995, 34, 63–65. [Google Scholar] [CrossRef]
- Feng, P.; Bu, X.; Stucky, G.D. Hydrothermal Syntheses and Structural Characterization of Zeolite Analogue Compounds Based on Cobalt Phosphate. Nature 1997, 388, 735–741. [Google Scholar] [CrossRef]
- Marler, B.; Grosskreuz, I. Synthesis and Crystal Structures of Two Crystalline Silicic Acids: Hydrated H-Apophyllite, H16Si16O40·8–10H2O and H-Carletonite, H32Si64O144. Crystals 2024, 14, 326. [Google Scholar] [CrossRef]
- Chiari, G.; Gazzoni, G.; Craig, J.R.; Gibbs, G.V.; Louisnathan, S.J. Two Independent Refinements of the Structure of Paracelsian, BaAl2Si2O8. Am. Mineral. 1985, 70, 969–974. [Google Scholar]
- Krivovichev, S.V. Feldspar Polymorphs: Diversity, Complexity, Stability. Zapiski Rossiisjogo Mineral. Obs. 2020, 149, 16–66. [Google Scholar] [CrossRef]
- Phillips, M.W.; Gibbs, G.V.; Ribbe, P.H. The Crystal Structure of Danburite: A Comparison with Anorthite, Albite, Reedmergnerite. Am. Mineral. 1974, 59, 79–85. [Google Scholar]
- Kimata, M. Crystal Structure of KBSi3O8 Isostructural with Danburite. Mineral. Mag. 1993, 57, 157–164. [Google Scholar] [CrossRef]
- Logar, N.Z.; Mrak, M.; Kaučič, V.; Golobič, A. Syntheses and Structures of Two Ammonium Zinc Gallophosphates: Analcime and Paracelsian Analogs. J. Solid State Chem. 2001, 156, 480–486. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, S.; Shi, Y. Further Examples of the P-O-P Connection in Borophosphates: Synthesis and Characterization of Li2Cs2B2P4O15, LiK2BP2O8, and Li3M2BP4O14 (M=K, Rb). Chem.—A Eur. J. 2012, 18, 12046–12051. [Google Scholar] [CrossRef]
- Dal Bo, F.; Hatert, F.; Baijot, M. Crystal Chemistry of Synthetic M2+Be2P2O8(M2+ = Ca, Sr, Pb, Ba) Beryllophosphates. Can. Mineral. 2014, 52, 337–350. [Google Scholar] [CrossRef]
- Mrose, M.E. Hurlbutite, CaBe2(PO4)2, a New Mineral. Am. Mineral. 1952, 37, 931–940. [Google Scholar]
- Fischer, K.F.; Schramm, V. Crystal Structure of Gismondite, a Detailed Refinement; ACS Publications: Washington, DC, USA, 1974; pp. 250–258. [Google Scholar]
- Skrzyńska, K.; Cametti, G.; Galuskina, I.O.; Vapnik, Y.; Galuskin, E.V. Gismondine-Sr, Sr4(Al8Si8O32)·9H2O, a New Strontium Dominant, Orthorhombic Zeolite of the Gismondine Series from the Hatrurim Complex, Israel. Am. Mineral. 2023, 108, 249–258. [Google Scholar] [CrossRef]
- Braithwaite, R.S.W.; Dyer, A.; Lamb, R.P.H.; Wilson, J.I. Gismondine-Ba, a Zeolite from the Weathering of Slag. J. Russell Soc. 2001, 7, 83–85. [Google Scholar]
- Zubkova, N.V.; Chukanov, N.V.; Pekov, I.V.; Schäfer, C.; Ksenofontov, D.A.; Skrzyńska, K.; Pushcharovsky, D.Y. Crystal Chemistry of the High Hydrous Analogue of Gismondine-Sr and the Role of Water in the Extra-Framework Cations Ordering. Z. Kristallogr. Cryst. Mater. 2025, 240, 77–85. [Google Scholar] [CrossRef]
- Steinfink, H. The Crystal Structure of the Zeolite, Phillipsite. Acta Crystallogr. 1962, 15, 644–651. [Google Scholar] [CrossRef]
- Rinaldi, R.; Pluth, J.J.; Smith, J.V. Zeolites of the Phillipsite Family. Refinement of the Crystal Structures of Phillipsite and Harmotome. Acta Crystallogr. B 1974, 30, 2426–2433. [Google Scholar] [CrossRef]
- Gatta, G.D.; Cappelletti, P.; Rotiroti, N.; Slebodnick, C.; Rinaldi, R. New Insights into the Crystal Structure and Crystal Chemistry of the Zeolite Phillipsite. Am. Mineral. 2009, 94, 190–199. [Google Scholar] [CrossRef]
- Galli, E.; Ghittoni, A.G.L. Crystal Chemistry of the Phillipsites. Am. Mineral. 1972, 57, 1125–1145. [Google Scholar]
- Lengauer, C.L.; Kolitsch, U.; Tillmanns, E. Florkeite, K3Ca2Na[Al8Si8O32]·12H2O, a New Phillipsite-Type Zeolite from the Bellerberg, East Eifel Volcanic Area, Germany. Eur. J. Mineral. 2009, 21, 901–913. [Google Scholar] [CrossRef]
- Atanassova, R.; Vassileva, R.D.; Kadiyski, M.; Zlatev, Z. Crystallographic, Chemical and Structural Characteristics of Harmotome from Zlatolist, Eastern Rhodopes, Bulgaria. Bulg. Chem. Commun. 2012, 44, 7–16. [Google Scholar]
- Barrett, P.A.; Sankar, G.; Stephenson, R.; Catlow, C.R.A.; Thomas, J.M.; Jones, R.H.; Teat, S.J. A New Addition to the Phillipsite Family of Molecular Sieves: A Divalent Metal-Ion-Framework Substituted Microporous Aluminophosphate (DAF-8). Solid State Sci. 2006, 8, 337–341. [Google Scholar] [CrossRef]
- Sokolova, E.; Hawthorne, F.C.; Ball, N.A.; Mitchell, R.H.; Della Ventura, G. Vlasovite, Na2Zr(Si4O11), from the Kipawa Alkaline Complex, Quebec, Canada: Crystal-Structure Refinement and Infrared Spectroscopy. Can. Mineral. 2006, 44, 1349–1356. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. The Ionothermal Synthesis of Cobalt Aluminophosphate Zeolite Frameworks. J. Am. Chem. Soc. 2006, 128, 2204–2205. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Reutova, O.V.; Dimitrova, O.V.; Volkov, A.S. Germanosilicate Cs2In2[(Si2.1Ge0.9)2O15](OH)2 · H2O with a New Corrugated Tetrahedral Layer: Topological Symmetry-Based Prediction of Anionic Radicals. Crystallogr. Rep. 2020, 65, 566–572. [Google Scholar] [CrossRef]
- McCusker, L.B.; Brunner, G.O.; Ojo, A.F. The Synthesis, Structure Determination and Rietveld Refinement of the Magnesium Aluminophosphate MAPO-39. Acta Crystallogr. A Found. Adv. 1990, 46, C59. [Google Scholar]
- Passaglia, E.; Pongiluppi, D.; Rinaldi, R. Merlinoite, a New Mineral of the Zeolite Group. Neues Jahrb. Für Mineral.—Monatshefte 1977, 1977, 355–364. [Google Scholar]
- Walter, F. Weinebeneite, CaBe3(PO4)2(OH)2 ∙ 4H2O, a New Mineral Species: Mineral Data and Crystal Structure. Eur. J. Mineral. 1992, 4, 1275–1284. [Google Scholar] [CrossRef]
- Staples, L.W.; Evans, H.T., Jr.; Lindsay, J.R. Cavansite and Pentagonite, New Dimorphous Calcium Vanadium Silicate Minerals from Oregon. Am. Mineral. 1973, 58, 405–411. [Google Scholar]
- Ishida, N.; Kimata, M.; Nishida, N.; Hatta, T.; Shimizu, M.; Akasaka, T. Polymorphic Relation between Cavansite and Pentagonite: Genetic Implications of Oxonium Ion in Cavansite. J. Mineral. Petrol. Sci. 2009, 104, 241–252. [Google Scholar] [CrossRef]
- Keller, E. Synthesis, Structures of AlPO4-C and AlPO4-D, and Their Topotactic Transformation. Solid State Ion. 1990, 43, 93–102. [Google Scholar] [CrossRef]
- Boruntea, C.-R.; Vennestrøm, P.N.R.; Lundegaard, L.F. K-Paracelsian (KAlSi3O8·H2O) and Identification of a Simple Building Scheme of Dense Double-Crankshaft Zeolite Topologies. IUCrJ 2019, 6, 66–71. [Google Scholar] [CrossRef]
- Sokolova, E.; Hawthorne, F.C. The Crystal Chemistry of the Scapolite-Group Minerals. I. Crystal Structure and Long-Range Order. Can. Mineral. 2008, 46, 1527–1554. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.; Kadiyski, M.; Nikolova, R. Further on the Choice of Space Group for Scapolite Group Members and Genetic Considerations about the Si-Al Ordering in Their Framework Construction. Minerals 2024, 14, 556. [Google Scholar] [CrossRef]
- Shendrik, R.; Chukanov, N.V.; Bogdanov, A.; Myasnikova, A.; Pankrushina, E.; Zolotarev, A.A.; Babkina, A.; Popova, E.; Vigasina, M.F.; Aksenov, S.M.; et al. Nature of Scapolite Color: Ab Initio Calculations, Spectroscopy, and Structural Study. Minerals 2024, 14, 937. [Google Scholar] [CrossRef]
- Lima-de-Faria, J.; Hellner, E.; Liebau, F.; Makovicky, E.; Parthé, E. Nomenclature of Inorganic Structure Types. Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types. Acta Crystallogr. A 1990, 46, 1–11. [Google Scholar] [CrossRef]
- Veblen, D.R. Polysomatism and Polysomatic Series: A Review and Applications. Am. Mineral. 1991, 76, 801–826. [Google Scholar]
- Ferraris, G.; Makovicky, E.; Merlino, S. Crystallography of Modular Materials; Oxford University Press: Oxford, UK, 2008; ISBN 9780191712111. [Google Scholar]
- Aksenov, S.M. The Crystal Chemistry and Topology of Modular Structures. I. Selenites of Rare-Earth Elements. Struct. Chem. 2025. [Google Scholar] [CrossRef]
- Ferraris, G.; Gula, A. Polysomatic Aspects of Microporous Minerals—Heterophyllosilicates, Palysepioles and Rhodesite-Related Structures. Rev. Mineral. Geochem. 2005, 57, 69–104. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I.V.; Kuporev, I.V.; et al. Structural and Chemical Complexity of Minerals: An Update. Mineral. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural Complexity of Minerals: Information Storage and Processing in the Mineral World. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Banaru, D.A.; Aksenov, S.M.; Banaru, A.M.; Oganov, A.R. Mutual Correlations of Complexity Indices of the Crystal Structure for the Series of Mercury-Containing Minerals. Z. Kristallogr. Cryst. Mater. 2024, 239, 207–215. [Google Scholar] [CrossRef]
- Banaru, D.A.; Aksenov, S.M. Combinatorial and Algorithmic Complexity of Crystal Structures. Lithosphere 2024, 24, 240–253. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural Complexity and Configurational Entropy of Crystals. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 274–276. [Google Scholar] [CrossRef]
- Ferraris, G.; Ivaldi, G. Structural Features of Micas. Rev. Mineral. Geochem. 2002, 46, 117–153. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Aksenov, S.M. The Crystal Chemistry of Lamprophyllite-Related Minerals: A Review. Eur. J. Mineral. 2016, 28, 915–930. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Topnikova, A.P.; Aksenov, S.M. Topolology-Symmetry Law of Structure of Natural Titanosilicate Micas and Related Heterophyllosilicates Based on the Extended OD Theory: Structure Prediction. Crystallogr. Rep. 2015, 60, 1–15. [Google Scholar] [CrossRef]
- Cannillo, E.; Rossi, G.; Ungaretti, L. The Crystal Structure of Macdonaldite. Atti Della Accad. Naz. Dei Lincei Ser. 8 1968, 45, 399–414. [Google Scholar]
- Ghose, S.; Sen Gupta, P.K.; Campana, C.F. Symmetry and Crystal Structure of Monteregianite, K2Na4Y2Si16O38 10H2O, a Double-Sheet Silicate with Zeolitic Properties. Am. Mineral. 1987, 72, 365–374. [Google Scholar]
- Gore, T.E.; McDonald, A.M. Melansonite, (Na,□)□2KZrSi8O19·5H2O, a New Member of the Rhodesite Group, from Mont Saint-Hilaire, Québec, Canada: Characterization, Crystal-Structure Determination, and Origin. Can. J. Mineral. Petrol. 2023, 61, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Lykova, I.; Rowe, R.; Poirier, G.; Friis, H.; Barnes, S. Natromelansonite, Na 3 Zr[Si 7 AlO 19 ]⋅4–5H 2 O, a New Member of the Rhodesite Mero-Plesiotype Series from Mont Saint-Hilaire, Quebec, Canada. Mineral. Mag. 2024, 88, 195–202. [Google Scholar] [CrossRef]
- Rocha, J.; Ferreira, P.; Lin, Z.; Brandão, P.; Ferreira, A.; Pedrosa de Jesus, J.D. Synthesis and Structural Characterization of Microporous Yttrium and Calcium Silicates. J. Phys. Chem. B 1998, 102, 4739–4744. [Google Scholar] [CrossRef]
- Rocha, J.; Ferreira, P.; Carlos, L.D.; Ferreira, A. The First Microporous Framework Cerium Silicate. Angew. Chem. 2000, 39, 3276–3279. [Google Scholar] [CrossRef]
- Ananias, D.; Ferreira, A.; Rocha, J.; Ferreira, P.; Rainho, J.P.; Morais, C.; Carlos, L.D. Novel Microporous Europium and Terbium Silicates. J. Am. Chem. Soc. 2001, 123, 5735–5742. [Google Scholar] [CrossRef]
- Ananias, D.; Rainho, J.P.; Ferreira, A.; Rocha, J.; Carlos, L.D. The First Examples of X-Ray Phosphors, and C-Band Infrared Emitters Based on Microporous Lanthanide Silicates. J. Alloys Compd. 2004, 374, 219–222. [Google Scholar] [CrossRef]
- Munhá, R.F.; Ananias, D.; Paz, F.A.A.; Rocha, J. Synthesis and Structure of New Microporous Nd(III) Silicates of the Rhodesite Group. Z. Kristallogr. Cryst. Mater. 2015, 230, 353–362. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Ferreira, A.; Rainho, J.P.; Ananias, D.; Lin, Z. Novel Microporous and Layered Luminescent Lanthanide Silicates. Mater. Sci. Forum 2004, 455, 527–531. [Google Scholar] [CrossRef]
- Zanardi, S.; Bellussi, G.; Carati, A.; Cruciani, G.; Millini, R.; Rizzo, C. EMS-6, a Novel Microporous Gadoliniumsilicate with Monteregianite Structure: Synthesis, Crystal Structure and Thermal Behavior. Microporous Mesoporous Mater. 2010, 134, 115–123. [Google Scholar] [CrossRef]
- Jordá, J.L.; Prokic, S.; McCusker, L.B.; Baerlocher, C.; Xue, C.F.; Dong, J. Synthesis and Structure Analysis of the Potassium Calcium Silicate CAS-1. Application of a Texture Approach to Structure Solution Using Data Collected in Transmission Mode. Comptes Rendus Chim. 2005, 8, 331–339. [Google Scholar] [CrossRef]
- Cadoni, M.; Ferraris, G. Two New Members of the Rhodesite Mero-Plesiotype Series Close to Delhayelite and Hydrodelhayelite: Synthesis and Crystal Structure. Eur. J. Mineral. 2009, 21, 485–493. [Google Scholar] [CrossRef]
- Cadoni, M.; Ferraris, G. Two New Silicate Structures Based on a Rhodesite-Type Heteropolyhedral Microporous Framework. Acta Crystallogr. B 2010, 66, 151–157. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Nair, S.; Vogt, T.; Dickinson, L.C.; Tsapatsis, M. A Highly Crystalline Layered Silicate with Three-Dimensionally Microporous Layers. Nat. Mater. 2003, 2, 53–58. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Chukanov, N.V.; Sharygin, V.V.; Pushcharovsky, D.Y. Crystal Chemistry of Delhayelite and Hydrodelhayelite. Dokl. Earth Sci. 2009, 428, 1216–1221. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Chukanov, N.V.; Zadov, A.E.; Pushcharovsky, D.Y. Fivegite K4Ca2[AlSi7O17(O2−x OHx)][(H2O)2−x OH]Cl: A New Mineral Species from the Khibiny Alkaline Pluton of the Kola Peninsula in Russia. Geol. Ore Depos. 2011, 53, 591–603. [Google Scholar] [CrossRef]
- Ragimov, K.G.; Chiragov, M.I.; Mamedov, K.S.; Dorfman, M.D. Crystal Structure of Hydrodelhayelite, KH2Ca(Si,Al)8O19 H2O. Daklady Akad. Nauk AzSSR 1980, 36, 49–51. [Google Scholar]
- Zubkova, N.V.; Chukanov, N.V.; Pekov, I.V.; Turchkova, A.G.; Lykova, I.S.; Schüller, W.; Ternes, B.; Pushcharovsky, D.Y. Crystal Chemistry of a Ba-Dominant Analogue of Hydrodelhayelite and Natural Ion-Exchange Transformations in Double- and Triple-Layer Phyllosilicates in Post-Volcanic Systems of the Eifel Region, Germany. Mineral. Petrol. 2016, 110, 885–893. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Pekov, I.V.; Chukanov, N.V.; Lisitsin, D.V.; Rabadanov, M.K.; Pushcharovsky, D.Y. New Data on Megacyclite. New Data Miner. 2007, 42, 81–92. [Google Scholar]
- Pushcharovsky, D.Y.; Zubkova, N.V.; Pekov, I.V. Structural and Mineralogical School of N.V. Belov at Moscow State University: New Data on Silicates Obtained at the Department of Crystallography and Crystal Chemistry. Crystallogr. Rep. 2011, 56, 986–993. [Google Scholar] [CrossRef]
- Cadoni, M.; Ferraris, G. Minerals as Materials—Silicate Sheets Based on Mixed Rings as Modules to Build Heteropolyhedral Microporous Frameworks. In Minerals as Advanced Materials II; Springer: Berlin/Heidelberg, Germany, 2011; pp. 153–162. [Google Scholar]
- Mountain, E.D. Rhodesite, a New Mineral from the Bultfontein Mine, Kimberley. Mineral. Mag. J. Mineral. Soc. 1957, 31, 607–610. [Google Scholar] [CrossRef]
- Sheppard, R.A.; Gude, A.J. Rhodesite from Trinity County, California. Am. Mineral. 1969, 54, 251–255. [Google Scholar]
- Jacob, H. Rhodesit Im Basalt Des Zeilbergs Bei Maroldsweisach. Aufschlus 1976, 27, 57–58. [Google Scholar]
- Hesse, K.-F. Verfeinerung Der Struktur Des Rhodesits, H2K2Ca4Si16O38∙10H2O. Z. Fuer Krist. 1987, 178, 98–99. [Google Scholar]
- Exel, R. Die Mineralien Und Erzlagerstätten Österreichs; R. Exel: Wien, Austria, 1993. [Google Scholar]
- Stoppa, F.; Sharygin, V.V.; Cundari, A. New Mineral Data from the Kamafugitecarbonatite Association: The Melilitolite from Pian Di Celle, Italy. Mineral. Petrol. 1997, 61, 27–45. [Google Scholar] [CrossRef]
- Gard, J.A.; Taylor, H.F.W.; Chalmers, R.A. An Investigation of Two New Minerals: Rhodesite and Mountainite. Mineral. Mag. J. Mineral. Soc. 1957, 31, 611–623. [Google Scholar] [CrossRef]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of Structural and Compositional Characteristics of Ordered Microporous and Mesoporous Materials with Inorganic Hosts(IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 381–394. [Google Scholar] [CrossRef]
- Dorfman, M.D. New Data on the Mineralogy of Yukspor in the Khibiny Tundra. In Questions of Geology and Mineralogy of the Kola Peninsula; Izd-vo Akademii nauk SSSR: Moscow, Russia, 1958; pp. 146–164. [Google Scholar]
- Sahama, T.G.; Hytönen, K. Delhayelite, a New Silicate from the Belgian Congo. Mineral. Mag. J. Mineral. Soc. 1959, 32, 6–9. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Belova, E.N.; Neronova, N.N. Delhayelite from Khibiny. Trans. Mineral. Mus. USSR Acad. Sci. 1961, 12, 191–195. [Google Scholar]
- Cannillo, E.; Rossi, G.; Ungaretti, L. The Crystal Structure of Delhayelite. Rend. Della Soc. Ital. Mineral. Petrol. 1970, 26, 63–75. [Google Scholar]
- Chiragov, M.I.; Mamedov, K.S. Crystal Structure of Delhayelite Ca2Na4K3[(Si,Al)8O19](F,Cl)2. Mineral. Collect. Lviv. Univ. 1974, 28, 3–7. [Google Scholar]
- Dawson, J.B. Peralkaline Nephelinite-Natrocarbonatite Relationships at Oldoinyo Lengai, Tanzania. J. Petrol. 1998, 39, 2077–2094. [Google Scholar] [CrossRef]
- Sokolova, M.N.; Smolyaninova, N.N.; Golovanova, T.I.; Chukanov, N.V.; Dmitrieva, M.T. On Delhayelite Crystals from Rischorrites of the Rasvumchorr Plateau (Khibiny Massif). New Data Miner. 2005, 40, 115–118. [Google Scholar]
- Sharygin, V.V.; Kamenetsky, V.S.; Zaitsev, A.N.; Kamenetsky, M.B. Silicate–Natrocarbonatite Liquid Immiscibility in 1917 Eruption Combeite–Wollastonite Nephelinite, Oldoinyo Lengai Volcano, Tanzania: Melt Inclusion Study. Lithos 2012, 152, 23–39. [Google Scholar] [CrossRef]
- Andersen, T.; Elburg, M.A.; Erambert, M. Extreme Peralkalinity in Delhayelite- and Andremeyerite-Bearing Nephelinite from Nyiragongo Volcano, East African Rift. Lithos 2014, 206, 164–178. [Google Scholar] [CrossRef]
- Khomyakov, A.P. Mineralogy of Hyperagpaitic Alkaline Rocks; Claredon Press: Oxford, UK/New York, NY, USA, 1995. [Google Scholar]
- Dorfman, M.D.; Chiragov, M.I. Hydrodelhayelite, a Product of Hypergene Alteration of Delhayelite. New Data Miner. 1979, 28, 172–175. [Google Scholar]
- Aksenov, S.M.; Ryanskaya, A.D.; Shchapova, Y.V.; Chukanov, N.V.; Vladykin, N.V.; Votyakov, S.L.; Rastsvetaeva, R.K. Crystal Chemistry of Lamprophyllite-Group Minerals from the Murun Alkaline Complex (Russia) and Pegmatites of Rocky Boy and Gordon Butte (USA): Single Crystal X-Ray Diffraction and Raman Spectroscopy Study. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2021, 77, 287–298. [Google Scholar] [CrossRef]
- Lykova, I.S.; Pekov, I.V.; Chukanov, N.V.; Yapaskurt, V.O.; Varlamov, D.A.; Zolotarev, A.A., Jr. Ion Exchange in Lomonosovite, Murmaniteand Gunterblassite: Experimental Data. In Proceedings of the International Conference “Minerals as Advanced Materials III”, Kirovsk, Russia, 23–30 June 2013; pp. 11–12. [Google Scholar]
- Sokolova, E.; Cámara, F.; Hawthorne, F.C.; Ciriotti, M.E. The Astrophyllite Supergroup: Nomenclature and Classification. Mineral. Mag. 2017, 81, 143–153. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Yamnova, N.A.; Chukanov, N.V.; Kabanova, N.A.; Kobeleva, E.A.; Deyneko, D.V.; Krivovichev, S.V. Theoretical Analysis of Cation-Migration Paths in Microporous Heterophyllosilicates with Astrophyllite and Veblenite Type Structures. J. Struct. Chem. 2022, 63, 293–301. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V.; Chervonnaya, N.A.; Varlamov, D.A.; Ermolaeva, V.N.; Britvin, S.N. Ion Exchange Properties of Natural Titanium Silicate Caryochroite (Na,Sr)3{(Fe,Mg)2+10(OH)6[TiO(Si6O17)(OH)0.5]2}·8H2O with a 1D System of Parallel Wide Channels: Experimental Study and Theoretical Analysis of the Topochemical Mechanisms. Microporous Mesoporous Mater. 2021, 312, 110776. [Google Scholar] [CrossRef]
- Yamnova, N.A.; Aksenov, S.M. New Data on Astrophyllite Supergroup Minerals: Crystall Chemical Mechanisms of the Formation of Triclinic and Monoclinic Structures. Crystallogr. Rep. 2021, 66, 1169–1184. [Google Scholar] [CrossRef]
- Sokolova, E.; Cámara, F. The Seidozerite Supergroup of TS-Block Minerals: Nomenclature and Classification, with Change of the Following Names: Rinkite to Rinkite-(Ce), Mosandrite to Mosandrite-(Ce), Hainite to Hainite-(Y) and Innelite-1T to Innelite-1A. Mineral. Mag. 2017, 81, 1457–1484. [Google Scholar] [CrossRef]
- Wise, W.S.; Pabst, A.; Hinthorne, J.R. Jonesite, a New Mineral from the Benitoite Gem Mine, San Benito County, California. Mineral. Rec. 1977, 8, 453–456. [Google Scholar]
- Krivovichev, S.V.; Armbruster, T. The Crystal Structure of Jonesite, Ba2(K, Na) [Ti2(Si5Al)O18(H2O)](H2O)n: A First Example of Titanosilicate with Porous Double Layers. Am. Mineral. 2004, 89, 314–318. [Google Scholar] [CrossRef]
- Cámara, F.; Sokolova, E.; Abdu, Y.A.; Pautov, L.A. From Structure Topology To Chemical Composition. XIX. Titanium Silicates: Revision of the Crystal Structure and Chemical Formula of Bafertisite, Ba2Fe2+4 Ti2(Si2O7)2O2(OH)2F2, A Group-II TS-Block Mineral. Can. Mineral. 2016, 54, 49–63. [Google Scholar] [CrossRef]
- Fersman, A.E. Mineralogy of the Khibina and Lovozero Tundras. Trans. North. Sci. Econ. Exped. 1923, 16, 16–73. [Google Scholar]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Armbruster, T.; Döbelin, N.; Pattison, P.; Weber, H.-P.; Depmeier, W. Porous Titanosilicate Nanorods in the Structure of Yuksporite, (Sr,Ba)2K4(Ca,Na)14(□,Mn,Fe) {(Ti,Nb)4(O,OH)4[Si6O17]2[Si2O7]3}(H2O,OH)n, Resolved Using Synchrotron Radiation. Am. Mineral. 2004, 89, 1561–1565. [Google Scholar] [CrossRef][Green Version]
- Rozhdestvenskaya, I.V.; Krivovichev, S.V. Tubular Chains in the Structures of Natural and Synthetic Silicates. Crystallogr. Rep. 2011, 56, 1007–1018. [Google Scholar] [CrossRef]
- Dal Bo, F.; Friis, H.; Mills, S.J. Nomenclature of Wöhlerite-Group Minerals. Mineral. Mag. 2022, 86, 661–676. [Google Scholar] [CrossRef]
- Perchiazzi, N.; McDonald, A.M.; Gault, R.A.; Johnsen, O.; Merlino, S. The Crystal Structure of Normandite and Its crystal-chemical Relationships with Lavenite. Can. Mineral. 2000, 38, 641–648. [Google Scholar] [CrossRef][Green Version]
- Annehed, H.; Falth, L.; Raade, G. The Crystal Structure of Janhaugite, a Sorosilicate of the Cuspidine Family. Neues Jahrb. Für Mineral.—Monatshefte 1985, 7–18. [Google Scholar]
- Hejny, C.; Armbruster, T. Polytypism in Xonotlite Ca6Si6O17(OH)2. Z. Kristallogr. Cryst. Mater. 2001, 216, 396–408. [Google Scholar] [CrossRef]
- Pekov, I.V.; Turchkova, A.G.; Lovskaya, E.V.; Chukanov, N.V. Zeolites Alkaline Massfis; EKOST: Moscow, Russia, 2004. [Google Scholar]
- Mazzi, F.; Galli, E.; Gottardi, G. Crystal Structure Refinement of Two Tetragonal Edingtonites. Neues Jahrb. Für Mineral. —Monatshefte 1984, 373–382. [Google Scholar]
- Gatta, G.D.; Ballaran, T.B.; ComodI, P.; Zanazzi, P.F. Isothermal Equation of State and Compressional Behavior of Tetragonal Edingtonite. Am. Mineral. 2004, 89, 633–639. [Google Scholar] [CrossRef]
- Nadezhina, T.N.; Pobedimskaya, E.A.; Khomyakov, A.P. The Crystal Structure of Tetragonal Edingtonite from Khibiny. Mineral. J. 1984, 6, 56–63. [Google Scholar]
- Gatta, G.D.; Boffa Ballaran, T. New Insight into the Crystal Structure of Orthorhombic Edingtonite: Evidence for a Split Ba Site. Mineral. Mag. 2004, 68, 167–175. [Google Scholar] [CrossRef]
- Kvick, Å.; Smith, J.V. A Neutron Diffraction Study of the Zeolite Edingtonite. J. Chem. Phys. 1983, 79, 2356–2362. [Google Scholar] [CrossRef]
- Gatta, G.D.; Ballaran, T.B.; Comodi, P.; Zanazzi, P.F. Comparative Compressibility and Equation of State of Orthorhombic and Tetragonal Edingtonite. Phys. Chem. Miner. 2004, 31, 288–298. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Men’shikov, Y.P.; Yakovenchuk, V.N. Barium Zeolites of Khibiny Alkaline Massif. Zap. Vseross. Mineral. Obs. 1992, 121, 54–61. [Google Scholar]
- Grice, J.D.; Gault, R.A.; Ansell, H.G. Edingtonite; the First Two Canadian Occurrences. Can. Mineral. 1984, 22, 253–258. [Google Scholar]
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Berlin/Heidelberg, Germany, 1985; Volume 18, ISBN 978-3-642-46520-8. [Google Scholar]
- Ståhl, K.; Hanson, J.C. An in Situ Study of the Edingtonite Dehydration Process from X-Ray Synchrotron Powder Diffraction. Eur. J. Mineral. 1998, 10, 221–228. [Google Scholar] [CrossRef]
- Goryainov, S.V.; Kurnosov, A.V.; Miroshnichenko, Y.M.; Smirnov, M.B.; Kabanov, I.S. Low-Temperature Anomalies of Infrared Band Intensities and High-Pressure Behavior of Edingtonite. Microporous Mesoporous Mater. 2003, 61, 283–289. [Google Scholar] [CrossRef]
- Lee, Y.; Hriljac, J.A.; Studer, A.; Vogt, T. Anisotropic Compression of Edingtonite and Thomsonite to 6 GPa at Room Temperature. Phys. Chem. Miner. 2004, 31, 22–27. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Sandomirskaya, S.M.; Malinovsky, Y.A. Kalborsite, K6BAl4Si6O20 (OH)4Cl, a New Mineral. Dokl. Akad. Nauk. USSR 1980, 252, 1465–1468. [Google Scholar]
- Ghobarkar, H.; Schäf, O. Hydrothermal Synthesis and Morphology of Thomsonite and Edingtonite. Cryst. Res. Technol. 1997, 32, 653–657. [Google Scholar] [CrossRef]
- Baerlocher, C.; Barker, R.M. The Crystal Structure of Synthetic Zeolite F. Z. Kristallogr. Cryst. Mater. 1974, 140, 10–26. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.J.; Parise, J.B. Synthesis and Crystal Structures of Gallium- and Germanium-Variants of the Fibrous Zeolites with the NAT, EDI and THO Structure Types. Microporous Mesoporous Mater. 2000, 34, 255–271. [Google Scholar] [CrossRef]
- Gatta, G.D.; Tabacchi, G.; Fois, E.; Lee, Y. Behaviour at High Pressure of Rb7NaGa8Si12O40·3H2O (a Zeolite with EDI Topology): A Combined Experimental–Computational Study. Phys. Chem. Miner. 2016, 43, 209–216. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, T.; Bu, X. Arsenate Zeolite Analogues with 11 Topological Types. J. Am. Chem. Soc. 2001, 123, 8608–8609. [Google Scholar] [CrossRef]
- Matsumoto, T.; Miyazaki, T.; Goto, Y. Synthesis and Characterization of Li-Type EDI Zeolite. J. Eur. Ceram. Soc. 2006, 26, 455–458. [Google Scholar] [CrossRef]
- Bu, X.; Gier, T.E.; Feng, P.; Stucky, G.D. Cobalt Phosphate Based Zeolite Structures with the Edingtonite Framework Topology. Chem. Mater. 1998, 10, 2546–2551. [Google Scholar] [CrossRef]
- Harrison, W.T.A. [H3N(CH2)3NH3]0.5[ZnPO4], an Organically Templated Zincophosphate Analogue of the Aluminosilicate Zeolite Edingtonite. Acta Crystallogr. Sect. Struct. Rep. Online 2001, 57, m248–m250. [Google Scholar] [CrossRef]
- Pekov, I.V.; Lovskaya, E.V.; Turchkova, A.G.; Chukanov, N.V.; Zadov, A.E.; Rastsvetaeva, R.K.; Kononkova, N.N. Thomsonite-Sr (Sr,Ca)2Na[Al2Si5O20]·6–7H2O, a New Zeolite Mineral from Khibiny Massif (Kola Peninsula) and Thomsonite-Ca—Thomsonite-Sr an Isomorphous Series. Zap. Vseross. Mineral. Obs. 2001, 130, 46–55. [Google Scholar]
- Alberti, A.; Vezzalini, G.; Tazzoli, V. Thomsonite: A Detailed Refinement with Cross Checking by Crystal Energy Calculations. Zeolites 1981, 1, 91–97. [Google Scholar] [CrossRef]
- Ståhl, K.; Kvick, Å.; Smith, J.V. Thomsonite, a Neutron Diffraction Study at 13 K. Acta Crystallogr. C 1990, 46, 1370–1373. [Google Scholar] [CrossRef]
- Ross, M.; Flohr, M.J.K.; Ross, D.R. Crystalline Solution Series and Order-Disorder within the Natrolite Mineral Group. Am. Mineral. 1992, 77, 685–703. [Google Scholar]
- Gatta, G.D.; Kahlenberg, V.; Kaindl, R.; Rotiroti, N.; Cappelletti, P.; de’ Gennaro, M. Crystal Structure and Low-Temperature Behavior of “Disordered” Thomsonite. Am. Mineral. 2010, 95, 495–502. [Google Scholar] [CrossRef]
- Giordani, M.; Skrzyńska, K.; Cametti, G. Stepwise Dehydration of Thomsonite (THO) with Disordered Si/Al Distribution: A New Partially Hydrated Phase. Microporous Mesoporous Mater. 2022, 346, 112308. [Google Scholar] [CrossRef]
- Ke, Y.; He, G.; Li, J.; Zhang, Y.; Lu, S. A New Mixed Divalent Metal Phosphate with Zeolite Thomsonite Framework Topology. New J. Chem. 2001, 25, 1627–1630. [Google Scholar] [CrossRef]
- Neeraj, S.; Natarajan, S. A Three-Dimensional Zeolitic Zinc Phosphate, [C8N5H28][Zn5(PO4)5]H2O, with Thomsonite Structure. J. Phys. Chem. Solids 2001, 62, 1499–1505. [Google Scholar] [CrossRef]
- Ng, H.Y.; Harrison, W.T.A. (CH3CH[NH3]CH2NH3)1/2·ZnPO4, a Zincophosphate Analogue of Aluminosilicate Zeolite Thomsonite. Microporous Mesoporous Mater. 2001, 50, 187–194. [Google Scholar] [CrossRef]
- Kuznicki, S.M.; Bell, V.A.; Nair, S.; Hillhouse, H.W.; Jacubinas, R.M.; Braunbarth, C.M.; Toby, B.H.; Tsapatsis, M. A Titanosilicate Molecular Sieve with Adjustable Pores for Size-Selective Adsorption of Molecules. Nature 2001, 412, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Chukanov, N.V.; Rastsvetaeva, R.K.; Zubkova, N.V.; Vigasina, M.F.; Pekov, I.V.; Zolotarev, A.A.; Mikhailova, J.A.; Aksenov, S.M. Spectroscopic Characterization of Extra-framework Hydrated Proton Complexes with the Extremely Strong Hydrogen Bonds in Microporous Silicate Minerals. J. Raman Spectrosc. 2024, 55, 581–597. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Vigasina, M.F.; Rastsvetaeva, R.K.; Aksenov, S.M.; Mikhailova, J.A.; Pekov, I.V. The Evidence of Hydrated Proton in Eudialyte-group Minerals Based on Raman Spectroscopy Data. J. Raman Spectrosc. 2022, 53, 1188–1203. [Google Scholar] [CrossRef]
- Vaitieva, Y.A.; Chukanov, N.V.; Vigasina, M.F.; Varlamov, D.A.; Volkov, S.N.; Rastsvetaeva, R.K.; Aksenov, S.M. Structural Features of Hydrated Representatives of the Labuntsovite Group: Refinement of Crystal Structures of Intermediate Members of the Tsepinite-Na–“Tsepinite-Ba”–Tsepinite-K Solid Solution. J. Struct. Chem. 2024, 65, 1357–1370. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Bakakin, V.V.; Fursenko, B.A.; Belitsky, I.A.; Joswig, W.; Radaelli, P.G. Structural Evolution of Natrolite during Over-Hydration: A High-Pressure Neutron Diffraction Study. Eur. J. Mineral. 2005, 17, 305–314. [Google Scholar] [CrossRef]
- Colligan, M.; Lee, Y.; Vogt, T.; Celestian, A.J.; Parise, J.B.; Marshall, W.G.; Hriljac, J.A. High-Pressure Neutron Diffraction Study of Superhydrated Natrolite. J. Phys. Chem. B 2005, 109, 18223–18225. [Google Scholar] [CrossRef]
- Pauling, L. The Structure of Some Sodium and Calcium Aluminosilicates. Proc. Natl. Acad. Sci. USA 1930, 16, 453–459. [Google Scholar] [CrossRef]
- Taylor, W.H. The Nature and Properties of Aluminosilicate Framework Structures. Proc. R. Soc. London Ser. A Contain. Pap. A Math. Phys. Character 1934, 145, 80–103. [Google Scholar] [CrossRef]
- Armbruster, T.; Gunter, M.E. Crystal Structures of Natural Zeolites. Rev. Mineral. Geochem. 2001, 45, 1–67. [Google Scholar] [CrossRef]
- Chao, G.Y. Paranatrolite a New Zeolite from Mont St-Hilaire, Quebec. Can. Mineral. 1980, 18, 85–88. [Google Scholar]
- Seryotkin, Y.V.; Bakakin, V.V.; Belitsky, I.A. The Crystal Structure of Paranatrolite. Eur. J. Mineral. 2004, 16, 545–550. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Bakakin, V.V. Crystal Structure of K-Substituted Gonnardite: Separation of Local Water–Cation Assemblages. Phys. Chem. Miner. 2014, 41, 173–180. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Dement’ev, S.N.; Ancharov, A.I. The Influence of Extraframework Cations on the Behavior of K-Exchanged Gonnardite at High Pressure. J. Struct. Chem. 2016, 57, 1386–1391. [Google Scholar] [CrossRef]
- Passaglia, E.; Sheppard, R.A. The Crystal Chemistry of Zeolites. Rev. Mineral. Geochem. 2001, 45, 69–116. [Google Scholar] [CrossRef]
- Popova, V.I.; Kasatkin, A.V.; Popov, V.A.; Nikandrov, V.A.; Makagonov, E.P.; Kuznetsov, A.M.; Škoda, R. Zeolites in pegmatites and late veinlets of the Vishnevogorsky alkaline-carbonatite complex (South Urals). Mineralogia. 2020, 6, 3–16. [Google Scholar] [CrossRef]
- Alberti, A.; Pongilappi, D.; Vezzalini, G. The Crystal Chemistry of Natrolite, Mesolite and Scolecite. Neues Jahrb. Fur Mineral. —Abh. 1982, 143, 231–248. [Google Scholar]
- Stuckenschmidt, E.; Joswig, W.; Baur, W.H.; Hofmeister, W. Scolecite, Part I: Refinement of High-Order Data, Separation of Internal and External Vibrational Amplitudes from Displacement Parameters. Phys. Chem. Miner. 1997, 24, 403–410. [Google Scholar] [CrossRef]
- Kvick, Å.; Ståhl, K.; Smith, J.V. A neutron diffraction study of the bonding of zeolitic water in scolecite at 20 K. Z. Kristallogr. Cryst. Mater. 1985, 171, 141–154. [Google Scholar]
- Moiseev, M.M.; Chukanov, N.V. Mineralogy of Alkaline Pegmatites and Hydrothermalites of the Kovdor Massif. New Data Miner. 2006, 41, 56–70. [Google Scholar]
- Spiridonov, E.M. Forming of Natrolite after Mesolite as a Result of the Ion Exchange Mechanism in Metabasalts (Spilites) of Karadag in the Mountain Crimea. Zapiski Rossiiskogo Mineral. Obs. 2021, 150, 98–113. [Google Scholar] [CrossRef]
- Artioli, G.; Smith, J.V.; Pluth, J.J. X-Ray Structure Refinement of Mesolite. Acta Crystallogr. C 1986, 42, 937–942. [Google Scholar] [CrossRef]
- Sulikowski, B. Solid-State 29Si and 27Al NMR Studies of Natural Mesolite. Microporous Mesoporous Mater. 2015, 206, 144–149. [Google Scholar] [CrossRef]
- Battiston, T.; Comboni, D.; Lotti, P.; Chrappan-Soldavini, B.; Fabelo, O.; Canadillas-Delgado, L.; Garbarino, G.; Liermann, H.-P.; Gatta, G.D. Mesolite, |Na2Ca2(H2O)8|[Al6Si9O30]: Crystal Structure Reinvestigation and Pressure-Mediated Crystal-Fluid Interaction. Microporous Mesoporous Mater. 2025, 393, 113643. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Mineral Species; Springer Geochemistry/Mineralogy; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7127-7. [Google Scholar]
- Wang, H.W.; Bish, D.L.; Ma, H. PH2O-Dependent Structural Phase Transitions in the Zeolite Mesolite: Real- and Reciprocal-Space Crystal Structure Refinements. Am. Mineral. 2010, 95, 686–698. [Google Scholar] [CrossRef]
- Wang, H.-W.; Bish, D.L. Infrared Spectroscopic Characterization of Dehydration and Accompanying Phase Transition Behaviors in NAT-Topology Zeolites. Phys. Chem. Miner. 2012, 39, 277–293. [Google Scholar] [CrossRef]
- Wilson, W.E.; Dunn, P.J. Nomenclature Revisions in the Apophyllite Group: Hydroxyapophyllite, Apophyllite, Fluorapophyllite. Mineral. Rec. 1978, 9, 95–98. [Google Scholar]
- Hatert, F.; Mills, S.J.; Pasero, M.; Williams, P.A. CNMNC Guidelines for the Use of Suffixes and Prefixes in Mineral Nomenclature, and for the Preservation of Historical Names. Eur. J. Mineral. 2013, 25, 113–115. [Google Scholar] [CrossRef]
- Dunn, P.J.; Rouse, R.C.; Norberg, J.A. Hydroxyapophyllite, a New Mineral, and a Redefinition of the Apophyllite Group. Part 1. Description, Occurrences, and Nomenclature. Am. Mineral. 1977, 62, 196–202. [Google Scholar]
- Marriner, G.F.; Tarney, J.; Langford, J.I. Apophyllite Group: Effects of Chemical Substitutions on Dehydration Behaviour, Recrystallization Products and Cell Parameters. Mineral. Mag. 1990, 54, 567–577. [Google Scholar] [CrossRef]
- Colville, A.A.; Anderson, C.P.; Black, P.M. Refinement of the Crystal Structure of Apophyllite I. X-Ray Diffraction and Physical Properties. Am. Mineral. 1971, 56, 1222–1233. [Google Scholar]
- Miura, Y.; Kato, T.; Rucklidge, J.C.; Matsueda, H. Natroapophyllite, a New Orthorhombic Sodium Analog of Apophyllite II. Crystal Structure. Am. Mineral. 1981, 66, 410–423. [Google Scholar]
- Agakhanov, A.A.; Pautov, L.A.; Kasatkin, A.V.; Karpenko, V.Y.; Sokolova, E.; Day, M.C.; Hawthorne, F.C.; Muftakhov, V.A.; Pekov, I.V.; Cámara, F.; et al. Fluorapophyllite-(Cs), CsCa4(Si8O20)F(H2O)8, a New Apophyllite-Group Mineral from the Darai-Pioz Massif, Tien-Shan, Northern Tajikistan. Can. Mineral. 2019, 57, 965–971. [Google Scholar] [CrossRef]
- Števko, M.; Sejkora, J.; Plášil, J.; Dolníček, Z.; Škoda, R. Fluorapophyllite-(NH4), NH4Ca4(Si8O20)F8H2O, a New Member of the Apophyllite Group from the Vechec Quarry, Eastern Slovakia. Mineral. Mag. 2020, 84, 533–539. [Google Scholar] [CrossRef]
- Yang, H.; Gu, X.; Scott, M.M. Hydroxymcglassonite-(K), KSr4Si8O20(OH)·8H2O, the First Sr-Bearing Member of the Apophyllite Group, from the Wessels Mine, Kalahari Manganese Field, South Africa. Am. Mineral. 2022, 107, 1818–1822. [Google Scholar] [CrossRef]
- Taylor, W.H.; Naray-Szabo, S. The Structure of Apophyllite. Z. Kristallogr. Cryst. Mater. 1931, 77, 146–158. [Google Scholar] [CrossRef]
- Ståhl, K. A Neutron Powder Diffraction Study of Partially Dehydrated Fluorapophyllite, KCa4Si8O20F.6.9H2O. Eur. J. Mineral. 1993, 5, 845–850. [Google Scholar] [CrossRef]
- Chao, G.Y. The Refinement of the Crystal Structure of Apophyllite. II Determination of the Hydrogen Positions by X-Ray Diffraction. Am. Mineral. 1971, 56, 1234–1242. [Google Scholar]
- Ståhl, K.; Kvick, Å.; Ghose, S. A Neutron Diffraction and Thermogravimetric Study of the Hydrogen Bonding and Dehydration Behaviour in Fluorapophyllite, KCa4(Si8O20)F.8H2O, and Its Partially Dehydrated Form. Acta Crystallogr. B 1987, 43, 517–523. [Google Scholar] [CrossRef]
- Burzo, E. Apophyllite-Type Silicates. In Phyllosilicates; Springer: Berlin/Heidelberg, Germany, 2007; pp. 16–29. [Google Scholar]
- Seryotkin, Y.V. Structure Evolution of Hydroxyapophyllite-(K) under High Pressure. Phys. Chem. Miner. 2024, 51, 3. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Ignatov, M.A. Structure Evolution of Fluorapophyllite-(K) under High Pressure. High Press. Res. 2023, 43, 279–292. [Google Scholar] [CrossRef]
- Wang, B.; Xiang, M.; Xiao, W. Pressure-Induced Phase Changes in Natural Fluorapophyllite-(K) Studied by Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 325, 125125. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A. Structures of Some Tetragonal Sheet Silicates. Acta Crystallogr. 1959, 12, 733–739. [Google Scholar] [CrossRef]
- Knight, K.S.; Henderson, C.M.B.C. Structural Variations in the Wesselsiteeffenbergerite (Sr1−xBaxCuSi4O10) Solid Solution. Eur. J. Mineral. 2010, 22, 411–423. [Google Scholar] [CrossRef]
- Miletich, R.; Allan, D.R.; Angel, R.J. The Synthetic Cr2+ Silicates BaCrSi4O10; the Missing Links in the Gillespite-Type ABSi4O10 Series. Am. Mineral. 1997, 82, 697–707. [Google Scholar] [CrossRef]
- Knight, K.S.; Henderson, C.M.B. Structural Basis for the Anomalous Low-Temperature Thermal Expansion Behaviour of the Gillespite-Structured Phase Ba0.5Sr0.5CuSi4O10. Eur. J. Mineral. 2007, 19, 189–200. [Google Scholar] [CrossRef]
- Mazzi, F.; Pabst, A. Reexamination of Cuprorivaite. Am. Mineral. 1962, 47, 409–411. [Google Scholar]
- Giester, G.; Rieck, B. Wesselsite, SrCu[Si4O10], a Further New Gillespite-Group Mineral from the Kalahari Manganese Field, South Africa. Mineral. Mag. 1996, 60, 795–798. [Google Scholar] [CrossRef]
- Giester, G.; Rieck, B. Effenbergerite, BaCu[Si4O10], a New Mineral from the Kalahari Manganese Field, South Africa: Description and Crystal Structure. Mineral. Mag. 1994, 58, 663–670. [Google Scholar] [CrossRef]
- FitzHugh, E.W.; Zycherman, L.A. An Early Man-Made Blue Pigment from China—Barium Copper Silicate. Stud. Conserv. 1983, 28, 15–23. [Google Scholar] [CrossRef]
- Jaksch, H.; Seipel, W.; Weiner, K.L.; Goresy, A. El Egyptian Blue? Cuprorivaite a Window to Ancient Egyptian Technology. Naturwissenschaften 1983, 70, 525–535. [Google Scholar] [CrossRef]
- Berke, H. The Invention of Blue and Purple Pigments in Ancient Times. Chem. Soc. Rev. 2007, 36, 15–30. [Google Scholar] [CrossRef]
- Kendrick, E.; Kirk, C.J.; Dann, S.E. Structure and Colour Properties in the Egyptian Blue Family, M1−xM′xCuSi4O10, as a Function of M, M′ Where M, M′=Ca, Sr and Ba. Dye. Pigment. 2007, 73, 13–18. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Painting Conditioned by Chemistry: The Case of Egyptian and Ultramarine Blue Pigments. ChemTexts 2025, 11, 9. [Google Scholar] [CrossRef]
- Rajaramanan, T.; Keykhaei, M.; Gourji, F.H.; Ravirajan, P.; Senthilnanthanan, M.; Frette, Ø.; Velauthapillai, D. Eco-Friendly Egyptian Blue (CaCuSi4O10) Dye for Luminescent Solar Concentrator Applications. Mater. Adv. 2023, 4, 4344–4348. [Google Scholar] [CrossRef]
- Song, X.; Lei, W.; Wang, F.; Chen, T.; Ta, S.; Fu, Z.; Lu, W. Phase Evolution, Crystal Structure, and Microwave Dielectric Properties of Gillespite-type Ceramics. J. Am. Ceram. Soc. 2021, 104, 1740–1749. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Ma, M.; Liu, F.; Qi, Z.-M.; Li, Y. Structure and Microwave Dielectric Properties of Gillespite-Type ACuSi4O10 (A = Ca, Sr, Ba) Ceramics and Quantitative Prediction of the Q × f Value via Machine Learning. ACS Appl. Mater. Interfaces 2021, 13, 17817–17826. [Google Scholar] [CrossRef]
- Knight, K.S.; Marshall, W.G.; Henderson, C.M.B.; Chamberlain, A.A. Equation of State and a High-Pressure Structural Phase Transition in the Gillespite-Structured Phase Ba0.5Sr0.5CuSi4O10. Eur. J. Mineral. 2014, 25, 909–917. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Ferraris, G.; Belluso, E.; Britvin, S.N.; Nechelyustov, G.N.; Soboleva, S.V. Seidite-(Ce), Na4SrCeTiSi8O22F 5H2O—A New Mineral with Zeolite Properties. Zap. Vseross. Mineral. Obs. 1998, 127, 94–100. [Google Scholar]
- Tripathi, A.; Parise, J.B. Hydrothermal Synthesis and Structural Characterization of the Aluminogermanate Analogues of JBW, Montesommaite, Analcime and Paracelsian. Microporous Mesoporous Mater. 2002, 52, 65–78. [Google Scholar] [CrossRef]
- Chiragov, M.I. Study of Cation-Exchanged Forms of Delhayelite and a New Synthetic Zeolite. In Problems of Mineralogy and Geochemistry of Ore and Non-Metallic Deposits of Azerbaijan; Azerbaijan State University: Baku, Azerbaijan, 1982; pp. 24–33. [Google Scholar]
- Turchkova, A.G.; Pekov, I.V.; Lykova, I.S.; Chukanov, N.V.; Yapaskurt, V.O. Delhayelite: Ion Leaching and Ion Exchange. In Minerals as Advanced Materials II; Springer: Berlin/Heidelberg, Germany, 2011; pp. 221–228. [Google Scholar]
- Chukanov, N.V.; Chervonnaya, N.A.; Kazheva, O.N.; Ermolaeva, V.N.; Varlamov, D.A.; Van, K.V. Ion Exchange Properties of Günterblassite and Gmelinite, Prototypes of Microporous Materials for Water Purification. Russ. J. Appl. Chem. 2020, 93, 595–602. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K.; Aksenov, S.M.; Zadov, A.E.; Van, K.V.; Blass, G.; Schüller, W.; Ternes, B. Lileyite, Ba2(Na,Fe,Ca)3MgTi2(Si2O7)2O2F2, a New Lamprophyllite-Group Mineral from the Eifel Volcanic Area, Germany. Eur. J. Mineral. 2012, 24, 181–188. [Google Scholar] [CrossRef]
- Rocha, J.; Lin, Z. Microporous Mixed Octahedral-Pentahedral-Tetrahedral Framework Silicates. Rev. Mineral. Geochem. 2005, 57, 173–201. [Google Scholar] [CrossRef]
- Rocha, J.; Ananias, D.; Paz, F.A.A. Photoluminescent Zeolite-Type Lanthanide Silicates. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 87–110. [Google Scholar]
- Richardson, I.G. The Calcium Silicate Hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- De Ceukelaire, L. The Determination of the Most Common Crystalline Alkali-Silica Reaction Product. Mater. Struct. 1991, 24, 169–171. [Google Scholar] [CrossRef]
- Pollmann, H. Calcium Aluminate Cements—Raw Materials, Differences, Hydration and Properties. Rev. Mineral. Geochem. 2012, 74, 1–82. [Google Scholar] [CrossRef]
- Lima, S.; Dias, A.S.; Lin, Z.; Brandão, P.; Ferreira, P.; Pillinger, M.; Rocha, J.; Calvino-Casilda, V.; Valente, A.A. Isomerization of D-Glucose to d-Fructose over Metallosilicate Solid Bases. Appl. Catal. A Gen. 2008, 339, 21–27. [Google Scholar] [CrossRef]
- Grutzeck, M.W.; Marks, J.A. Synthesis of Double-Layer Silicates from Recycled Glass Cullet: A New Type of Chemical Adsorbent. Environ. Sci. Technol. 1999, 33, 312–317. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S.; Bucknall, D.G.; Koros, W.J.; Nair, S. Nanoporous Layered Silicate AMH-3/Cellulose Acetate Nanocomposite Membranes for Gas Separations. J. Memb. Sci. 2013, 441, 129–136. [Google Scholar] [CrossRef]
- Choi, S.; Coronas, J.; Jordan, E.; Oh, W.; Nair, S.; Onorato, F.; Shantz, D.F.; Tsapatsis, M. Layered Silicates by Swelling of AMH-3 and Nanocomposite Membranes. Angew. Chem. Int. Ed. 2008, 47, 552–555. [Google Scholar] [CrossRef]
- Choi, S.; Coronas, J.; Lai, Z.; Yust, D.; Onorato, F.; Tsapatsis, M. Fabrication and Gas Separation Properties of Polybenzimidazole (PBI)/Nanoporous Silicates Hybrid Membranes. J. Memb. Sci. 2008, 316, 145–152. [Google Scholar] [CrossRef]
- Choi, S.; Coronas, J.; Sheffel, J.A.; Jordan, E.; Oh, W.; Nair, S.; Shantz, D.F.; Tsapatsis, M. Layered Silicate by Proton Exchange and Swelling of AMH-3. Microporous Mesoporous Mater. 2008, 115, 75–84. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, J.-D.; Kwak, S.-Y. Nafion-Based Composite Membrane with a Permselective Layered Silicate Layer for Vanadium Redox Flow Battery. Electrochem. Commun. 2014, 38, 68–70. [Google Scholar] [CrossRef]
- Konduri, S.; Nair, S. A Computational Study of Gas Molecule Transport in a Polymer/Nanoporous Layered Silicate Nanocomposite Membrane Material. J. Phys. Chem. C 2007, 111, 2017–2024. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent Multifunctional Lanthanides-Based Metal–Organic Frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef]
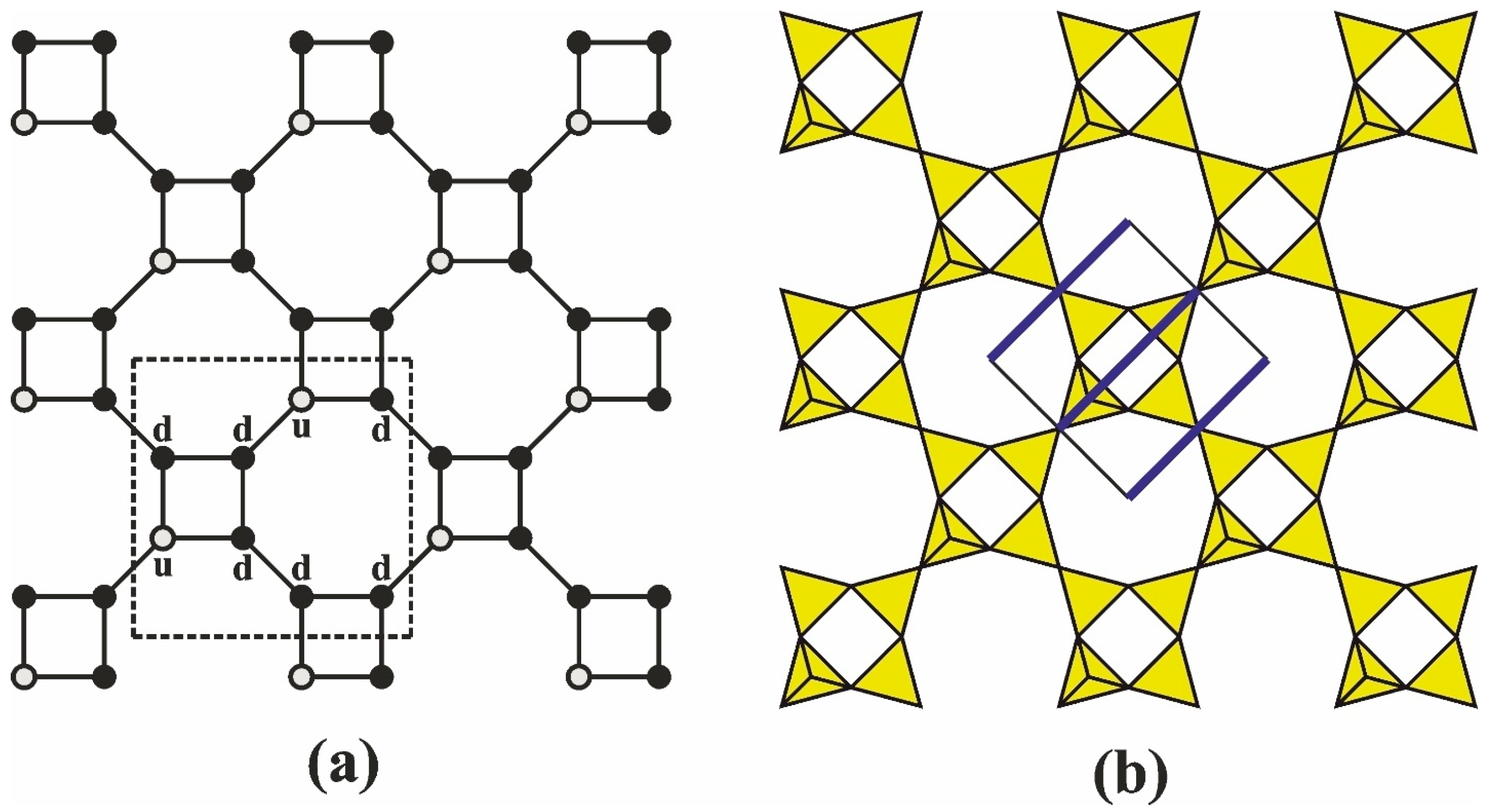
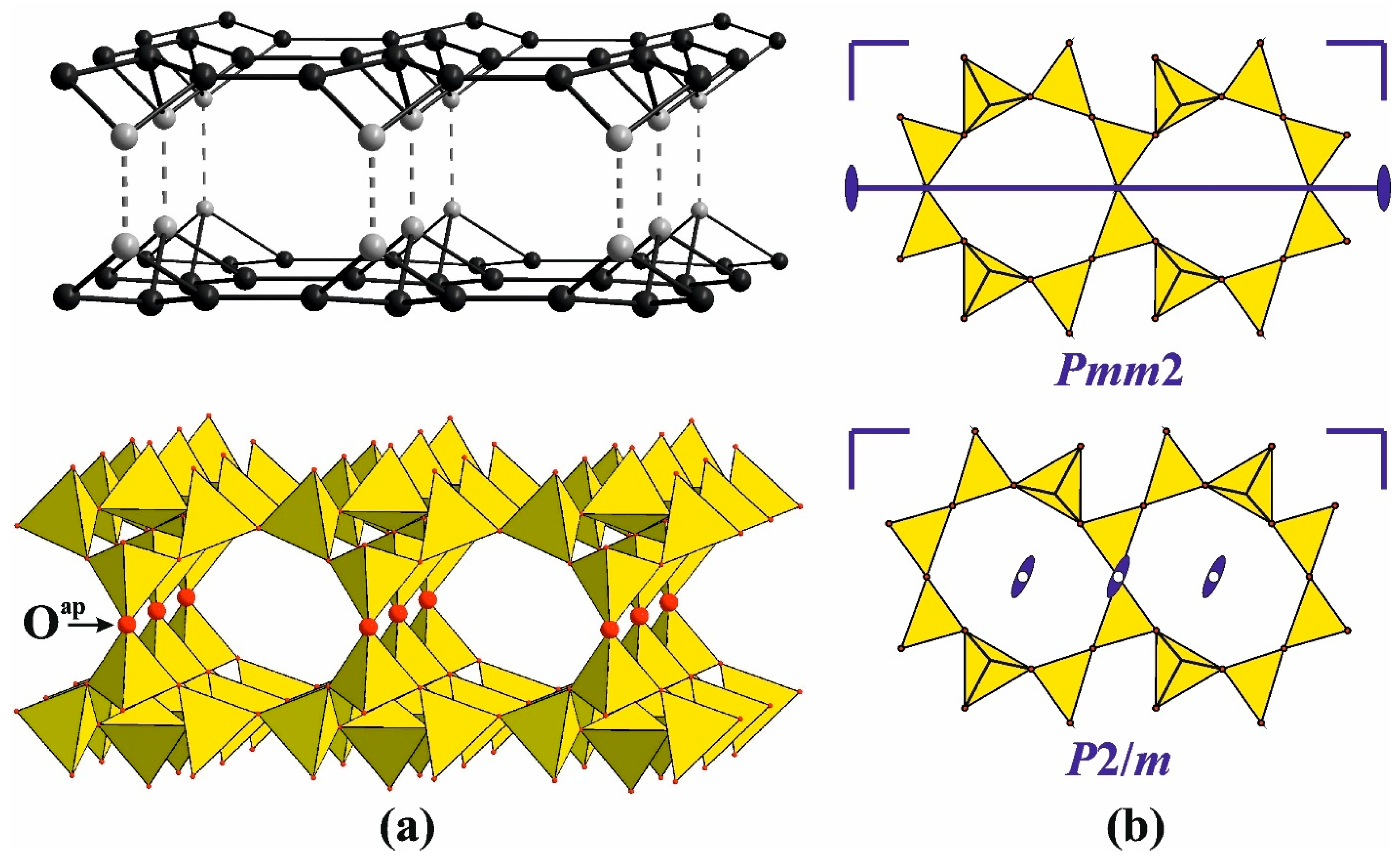
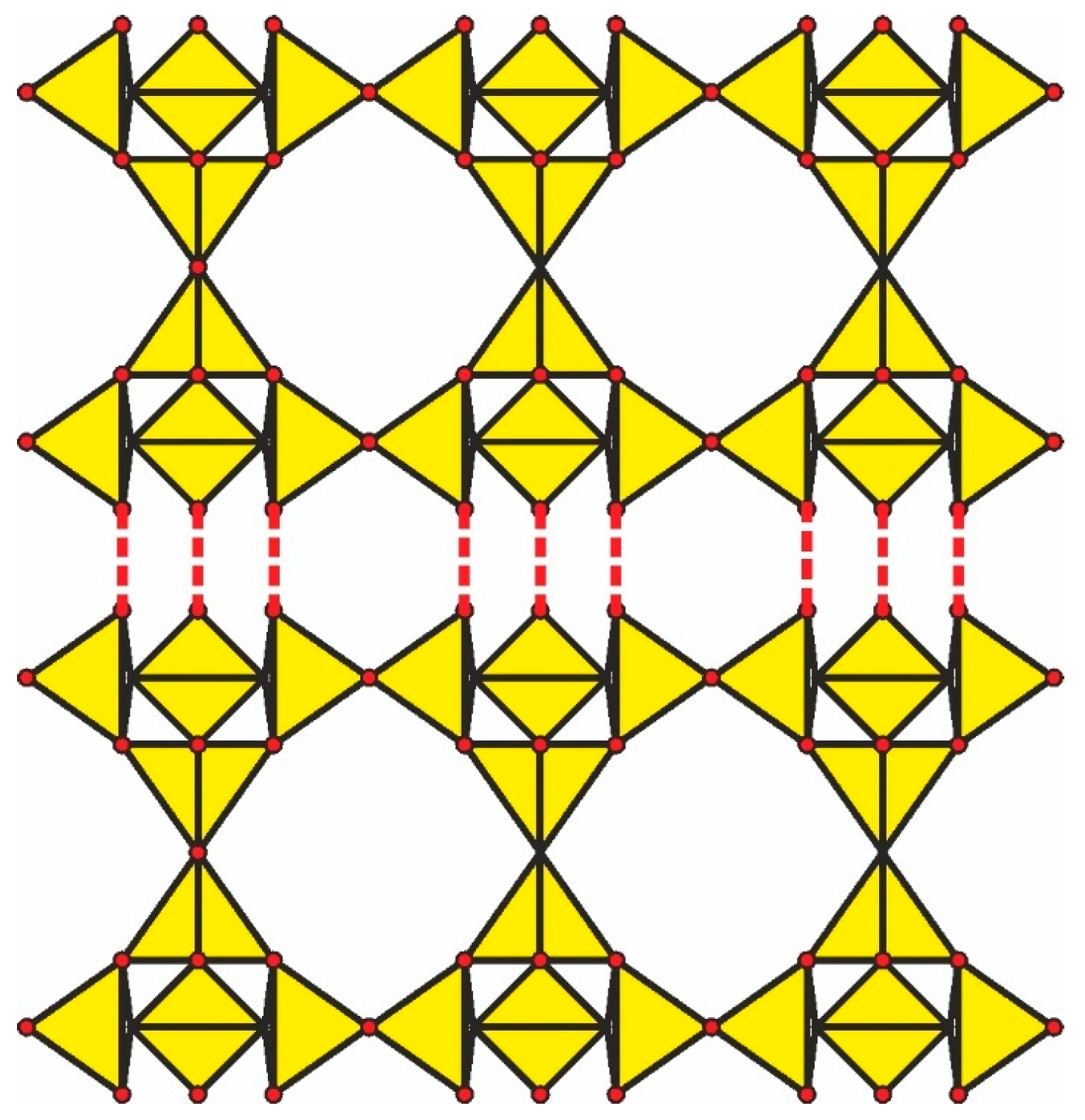

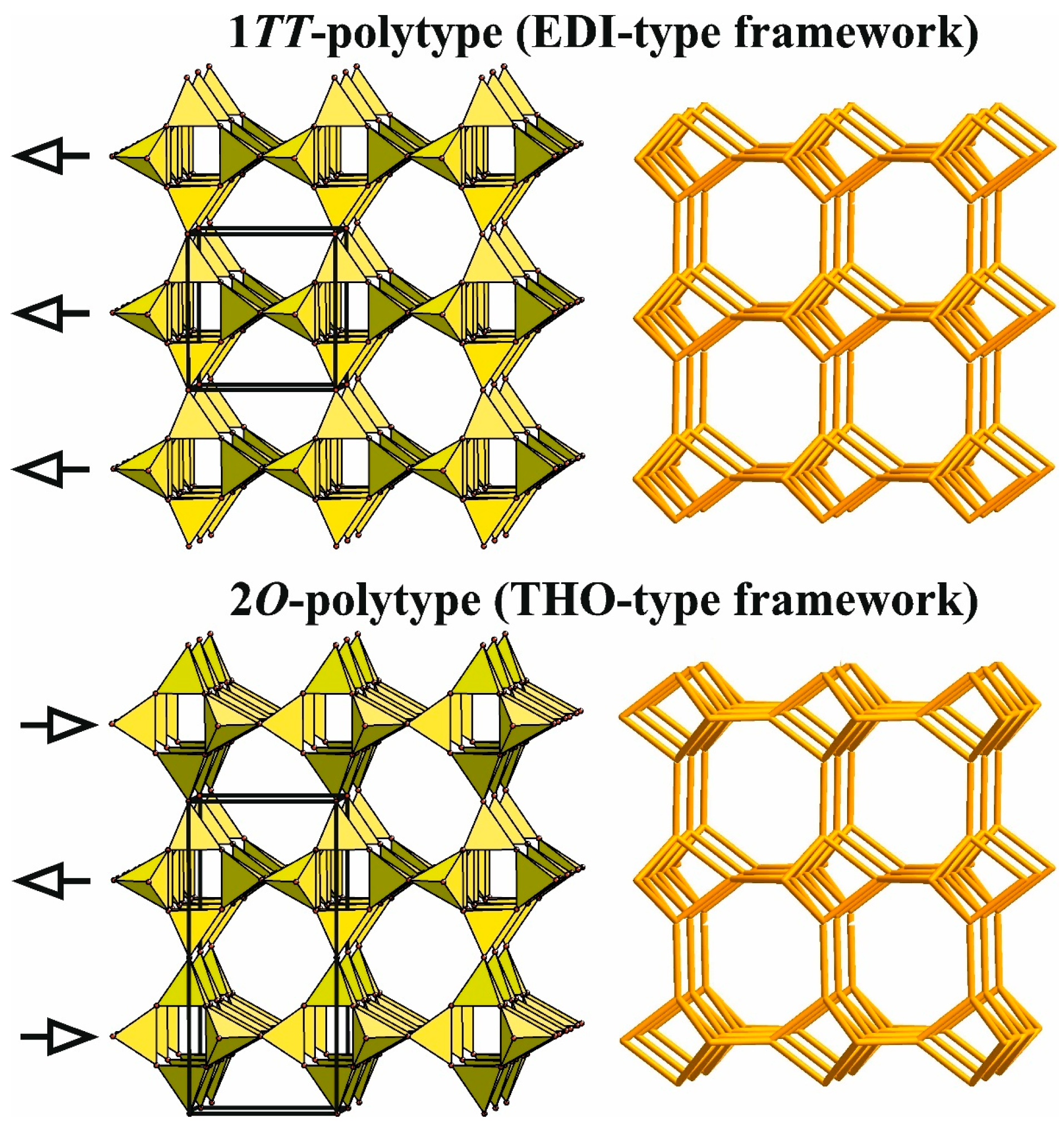
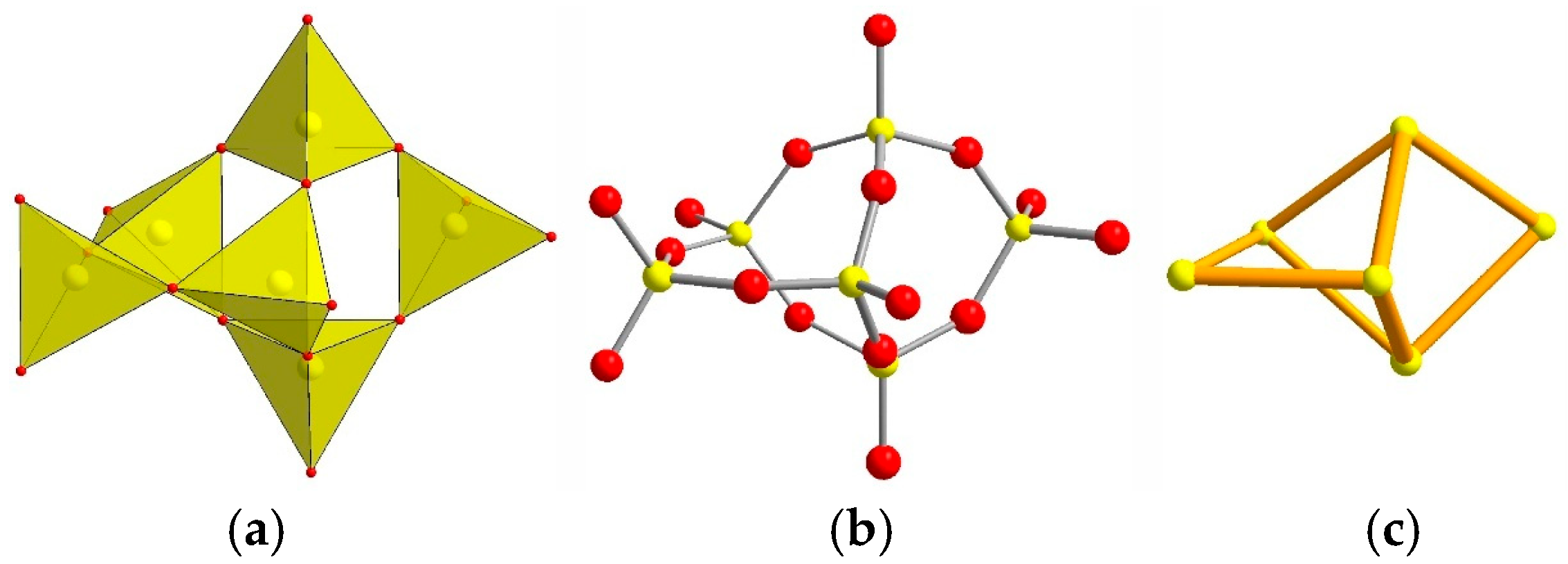
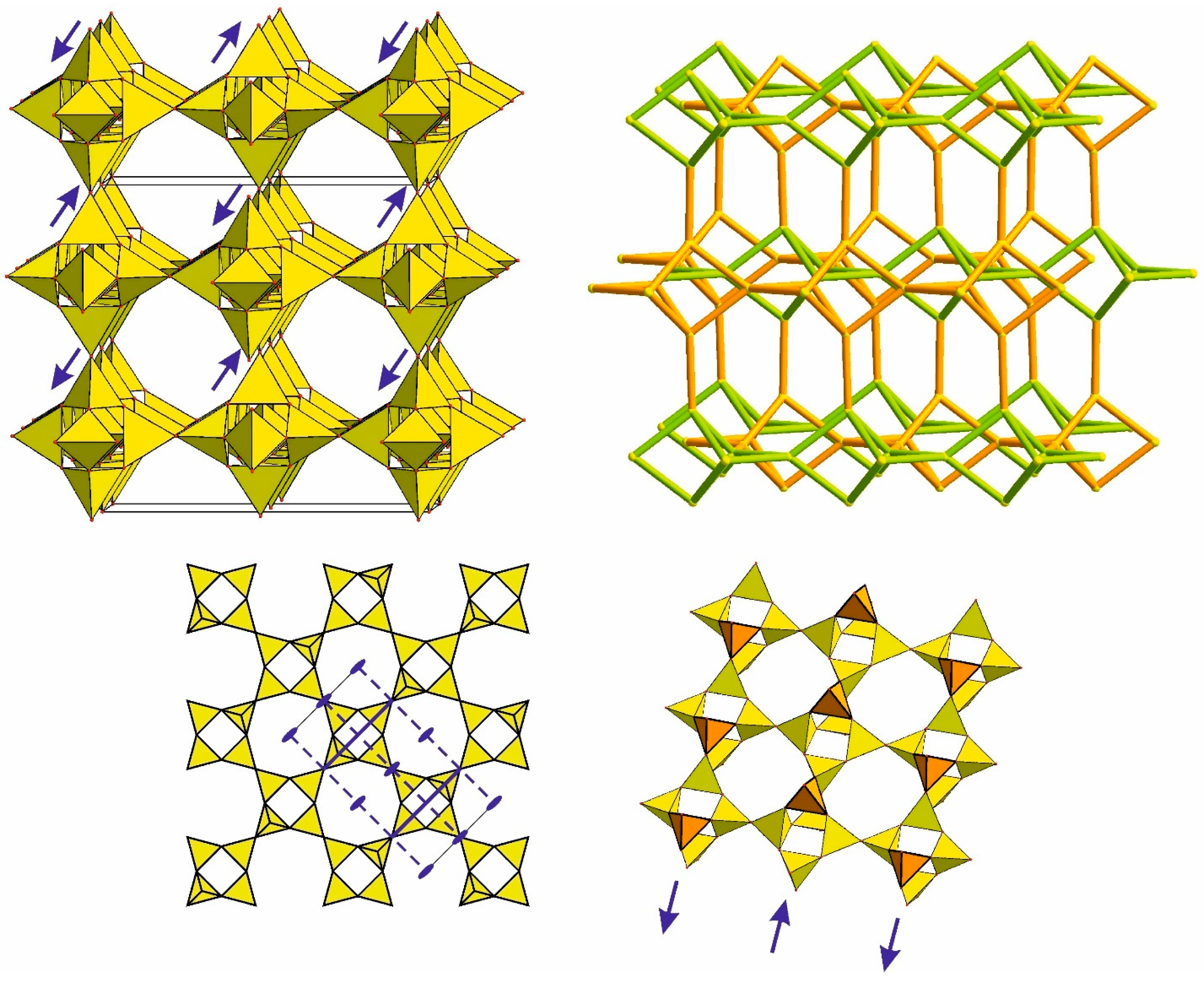
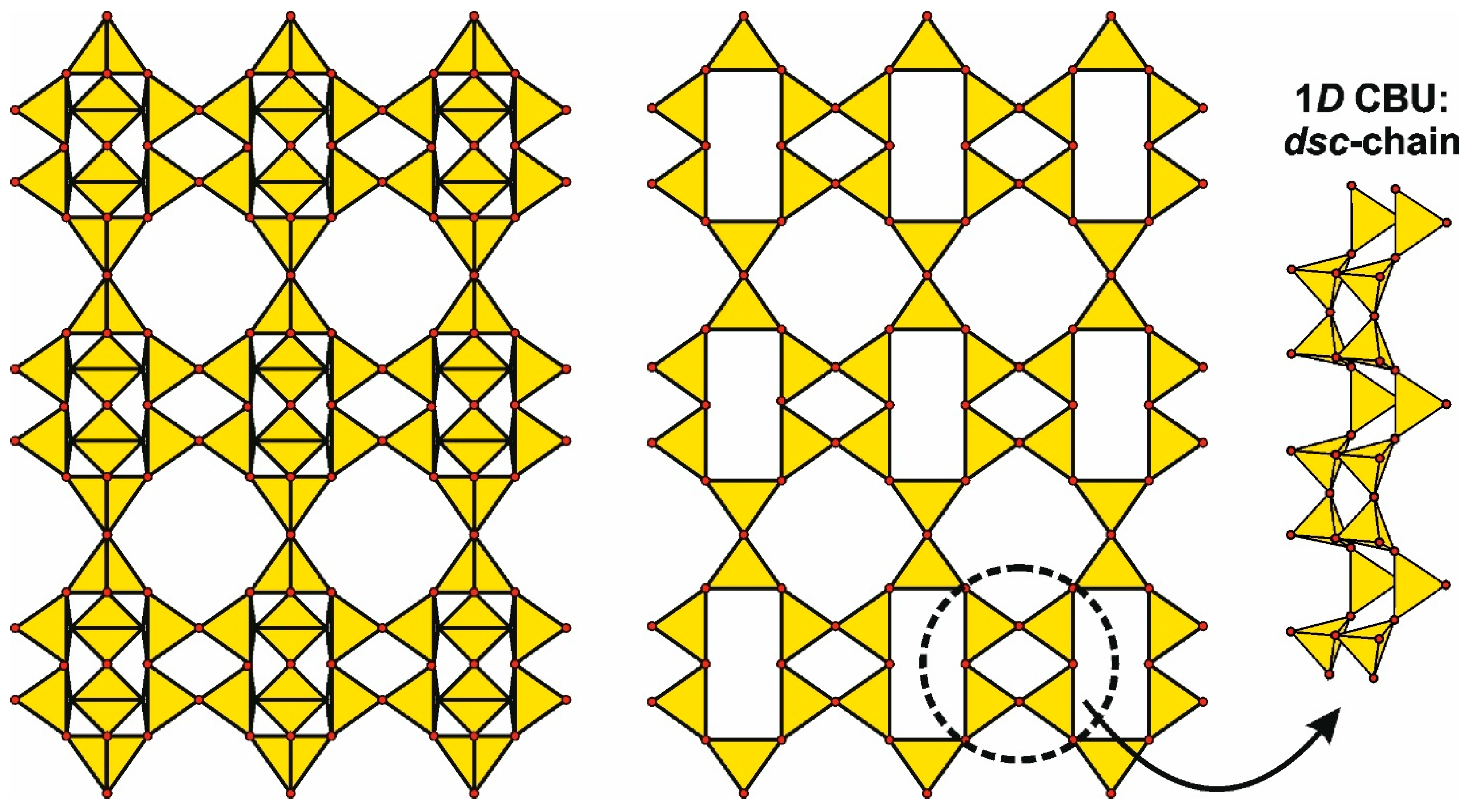
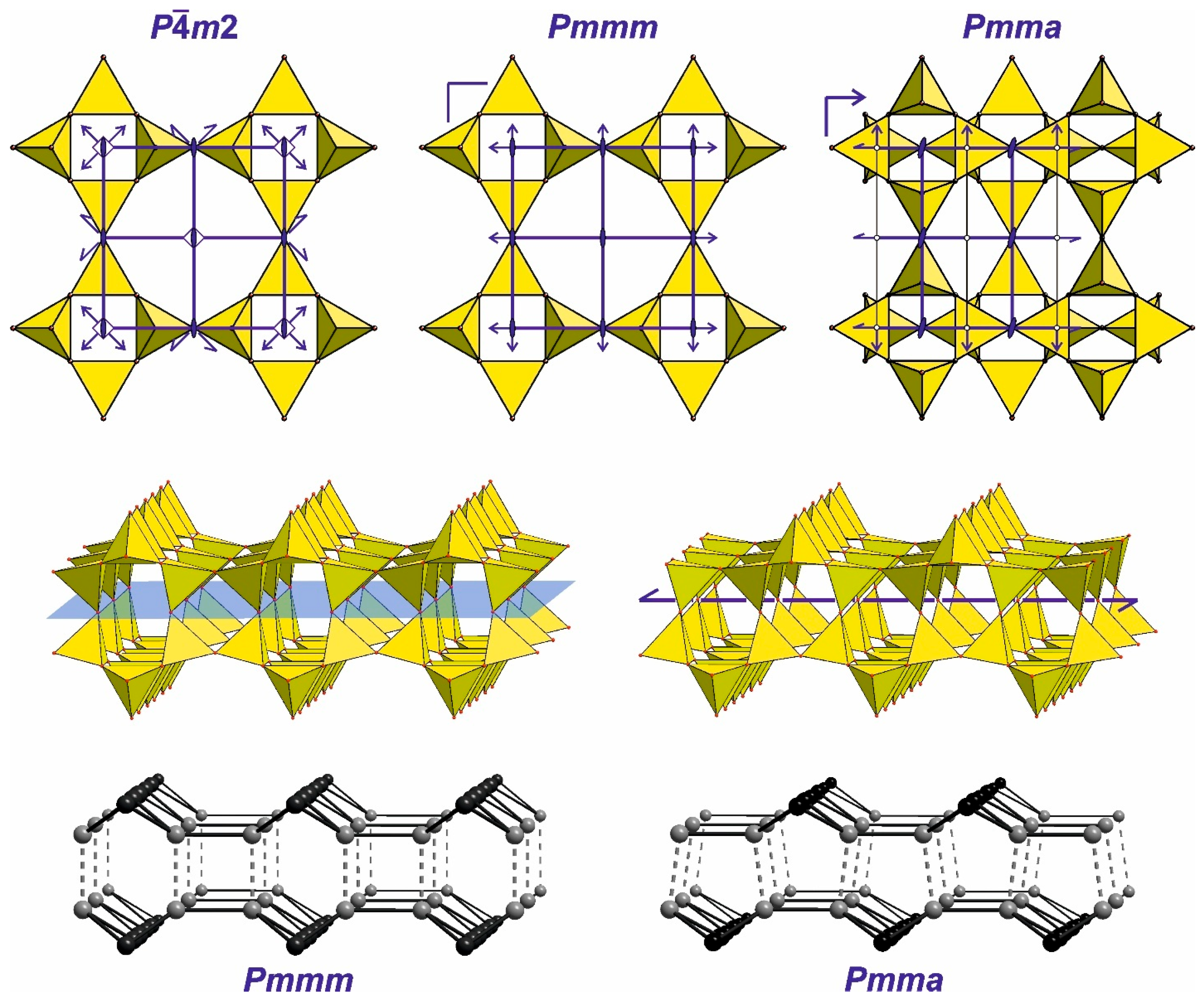
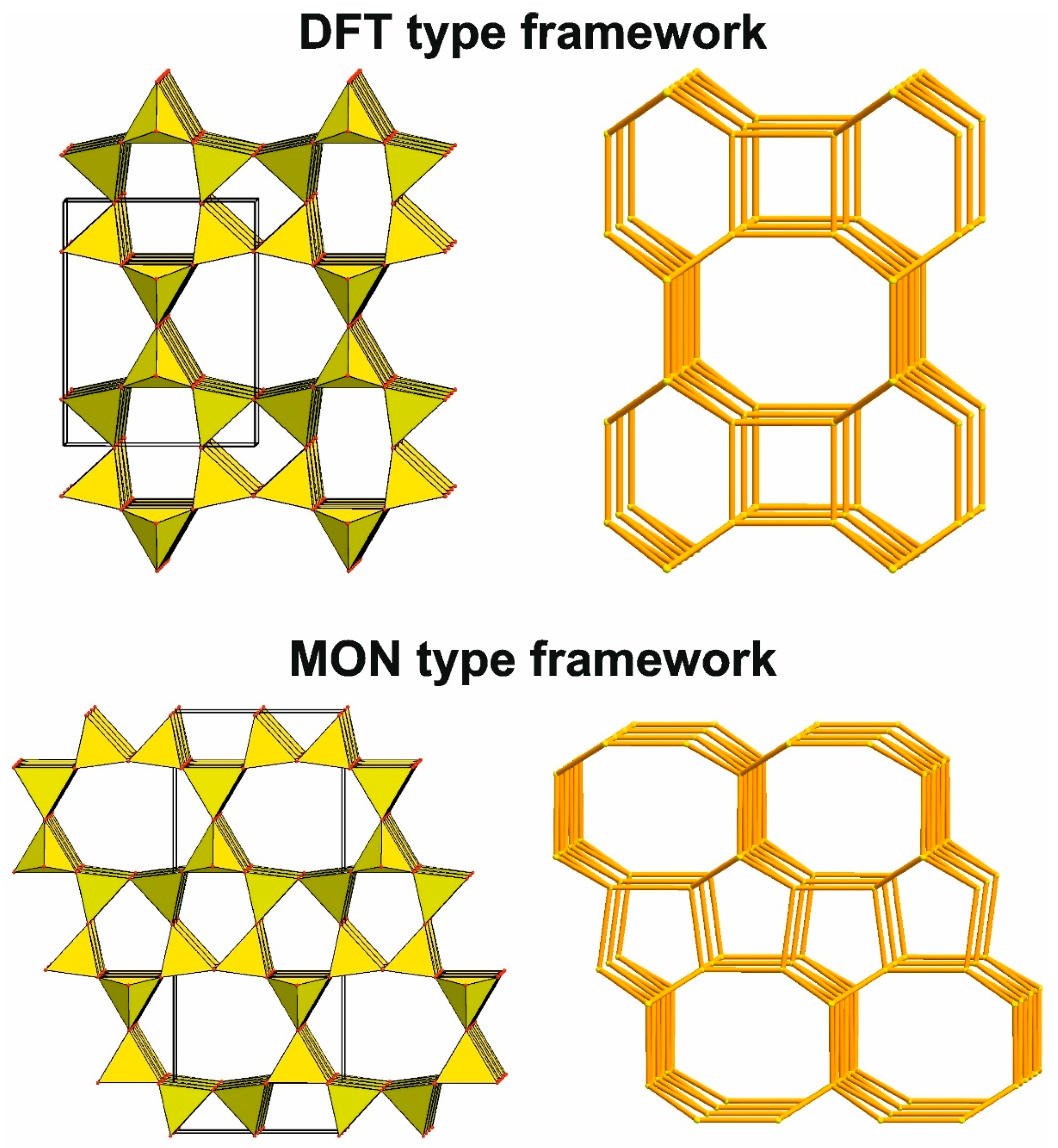
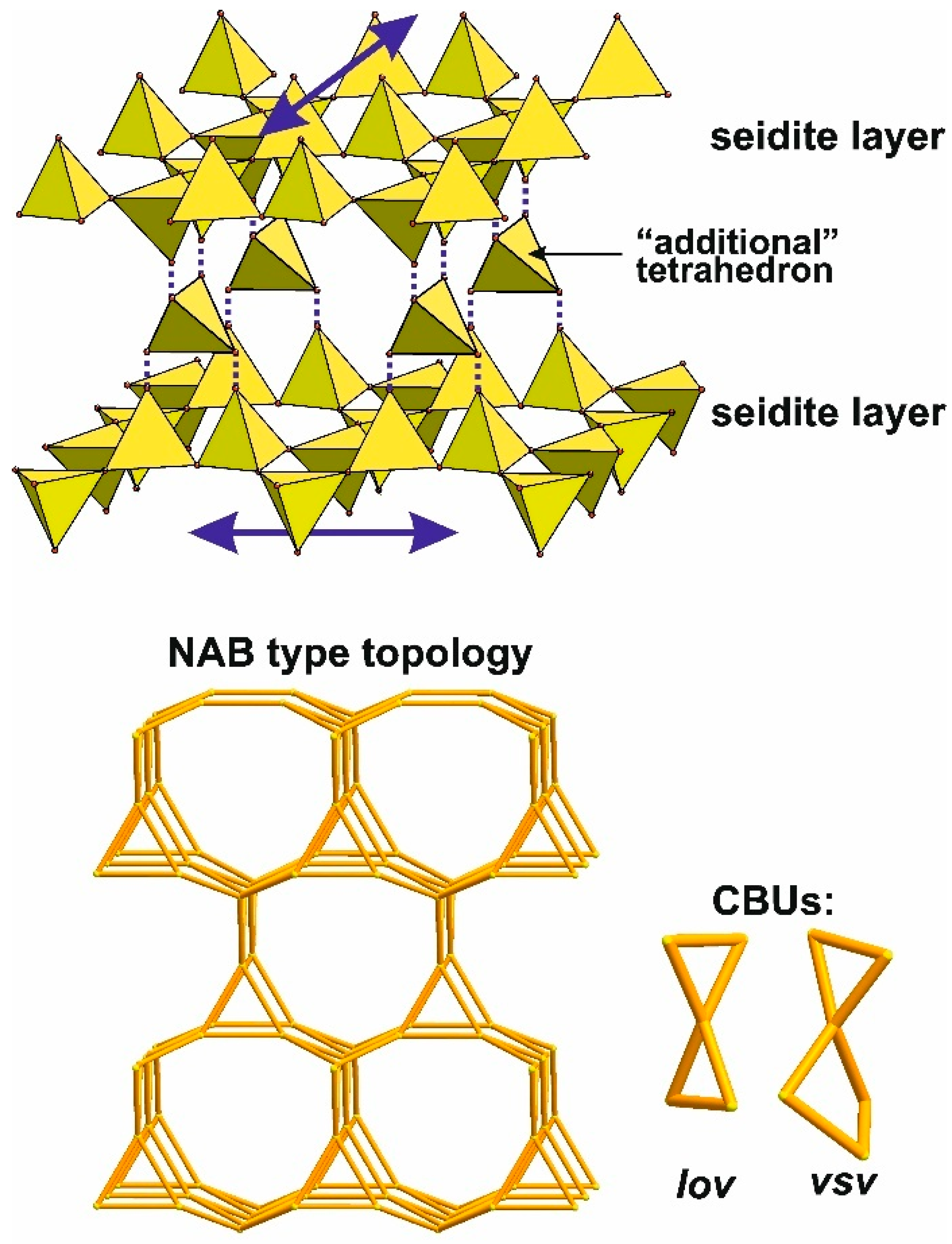
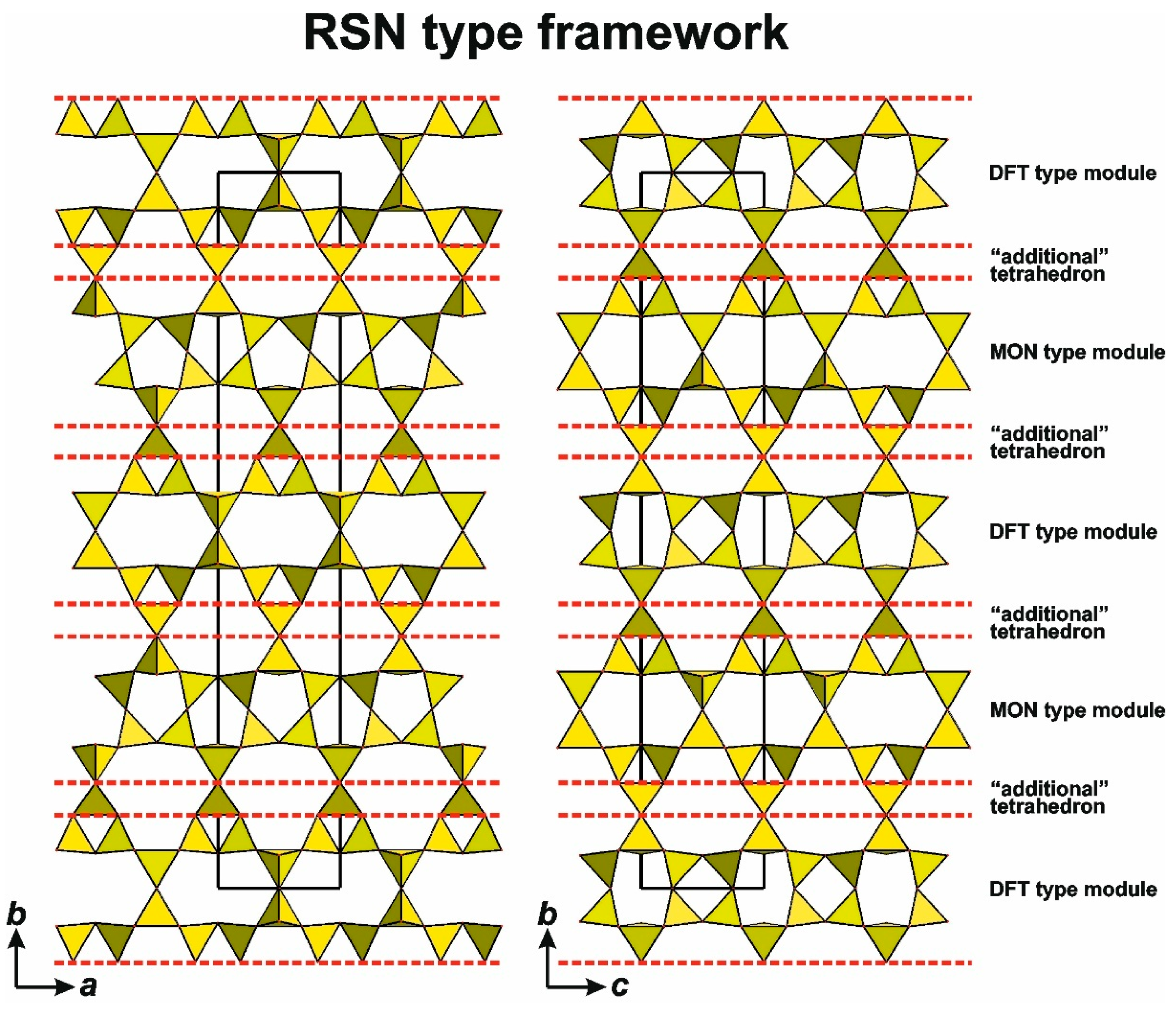
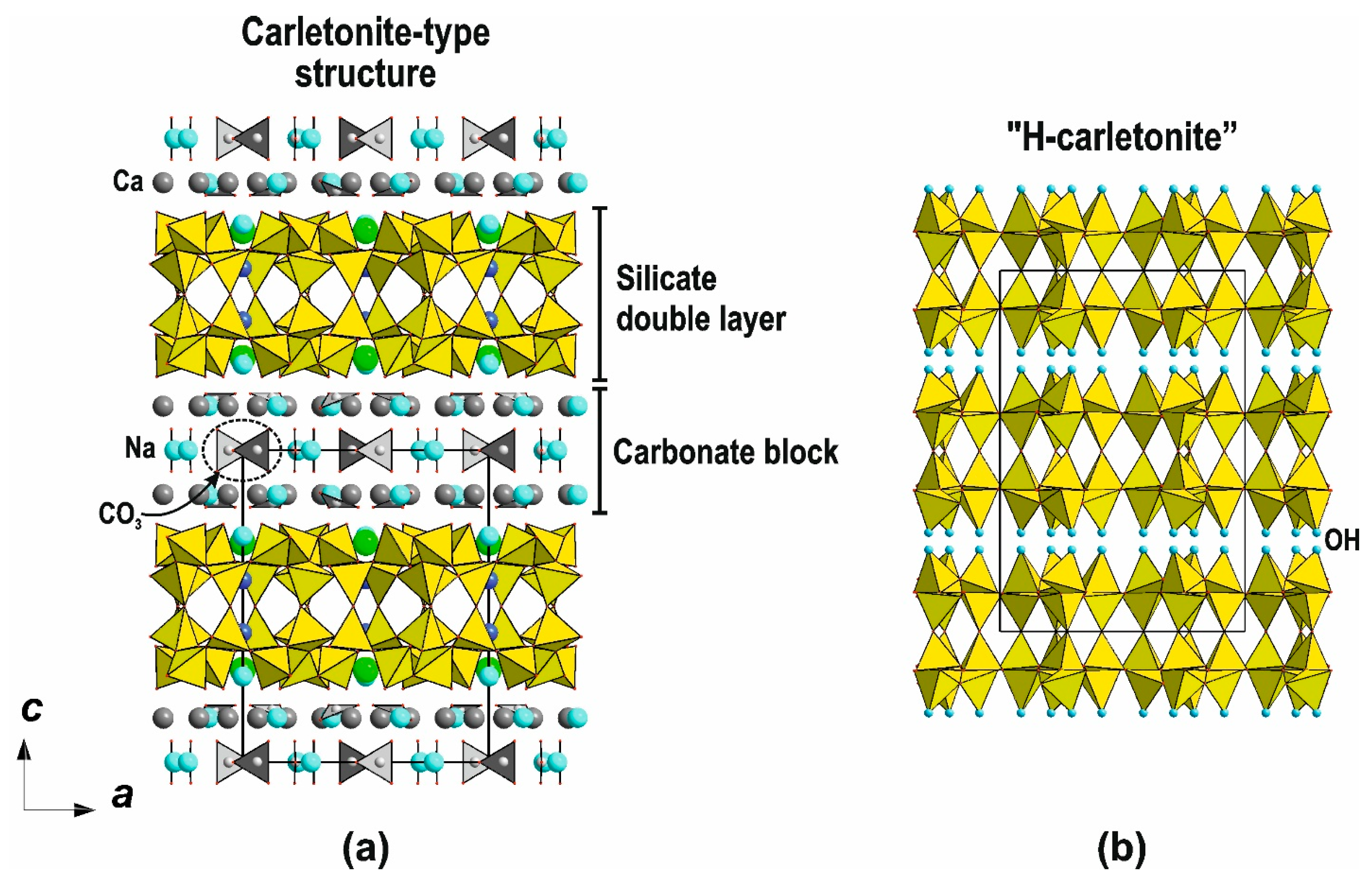
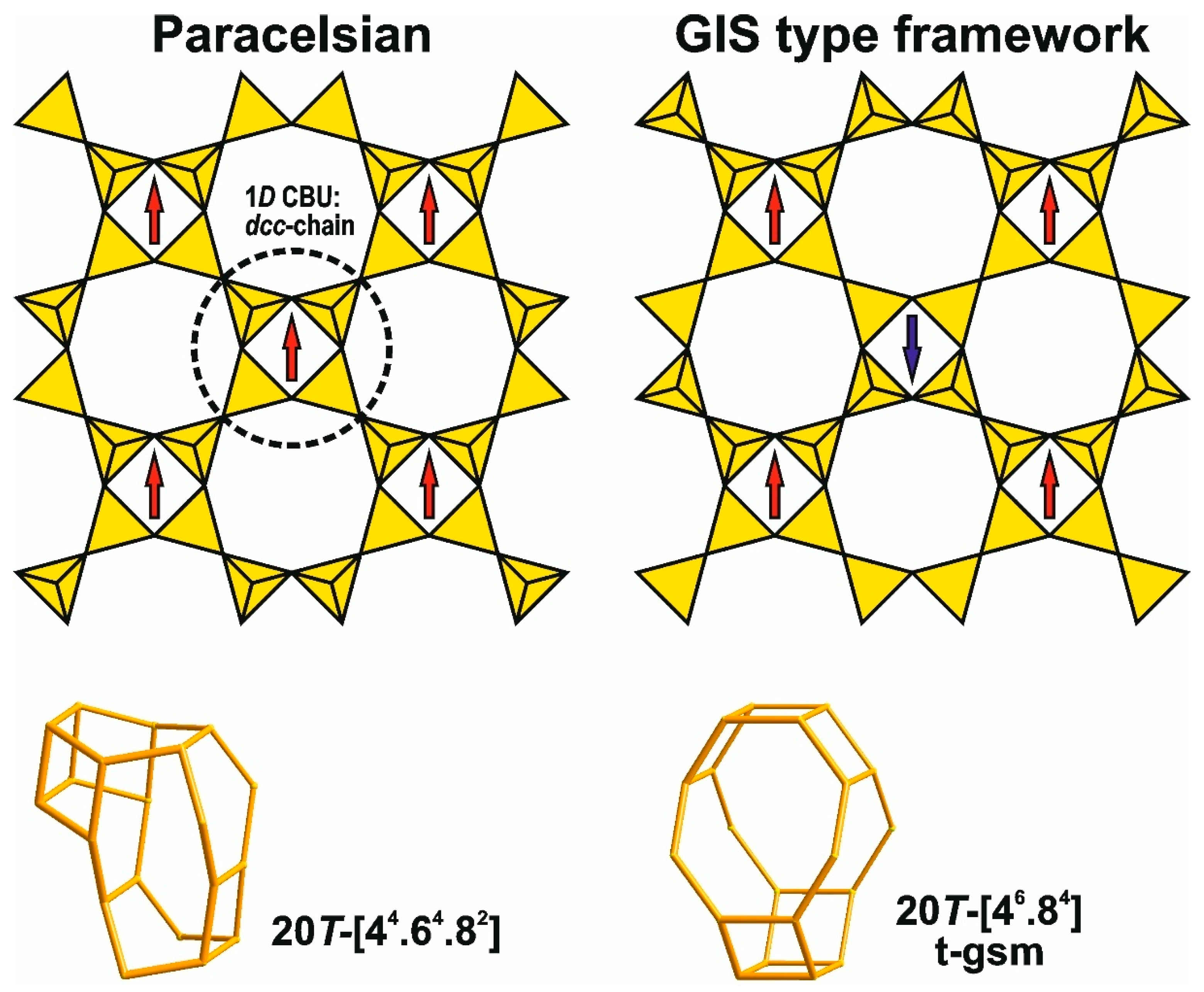
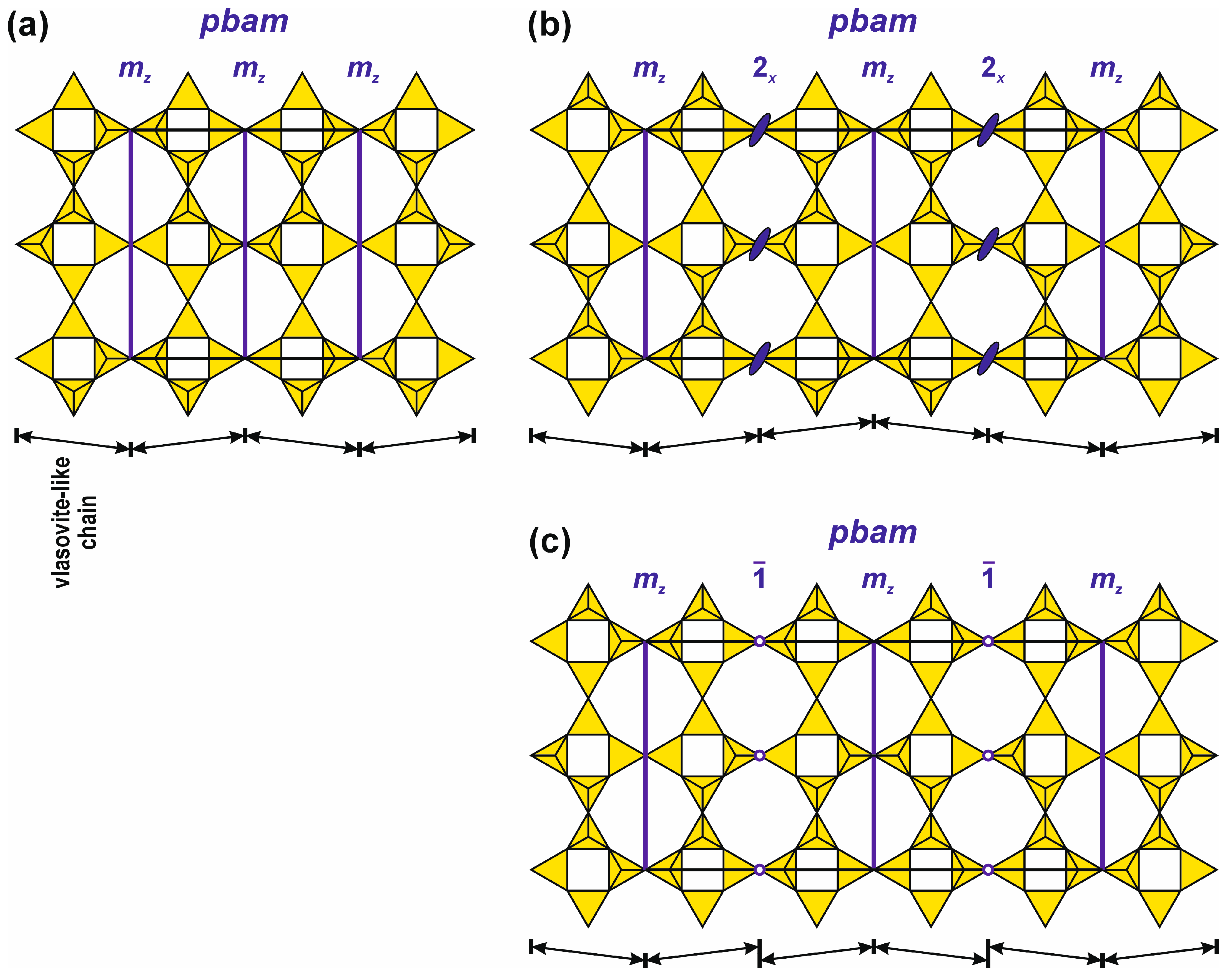
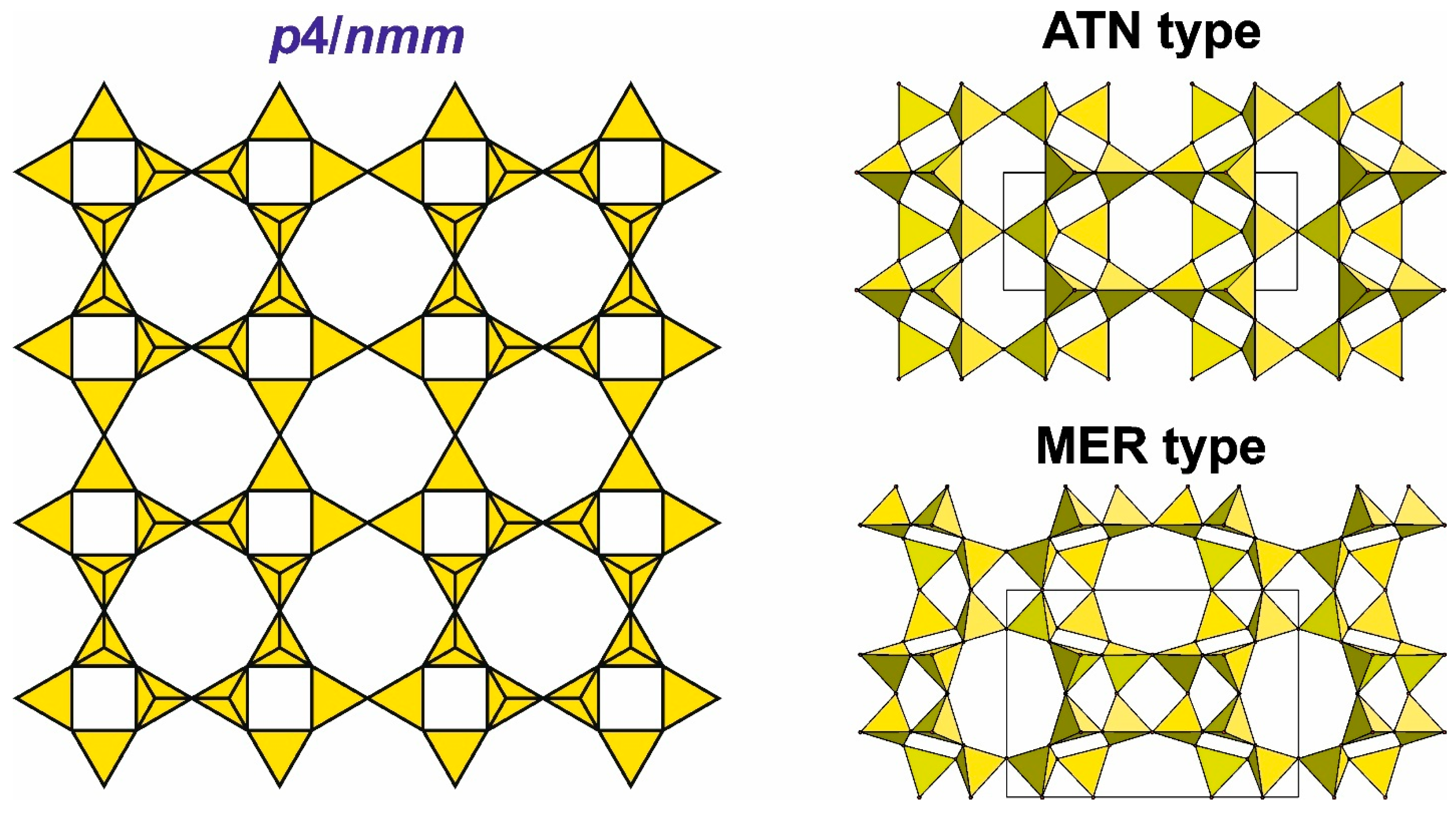
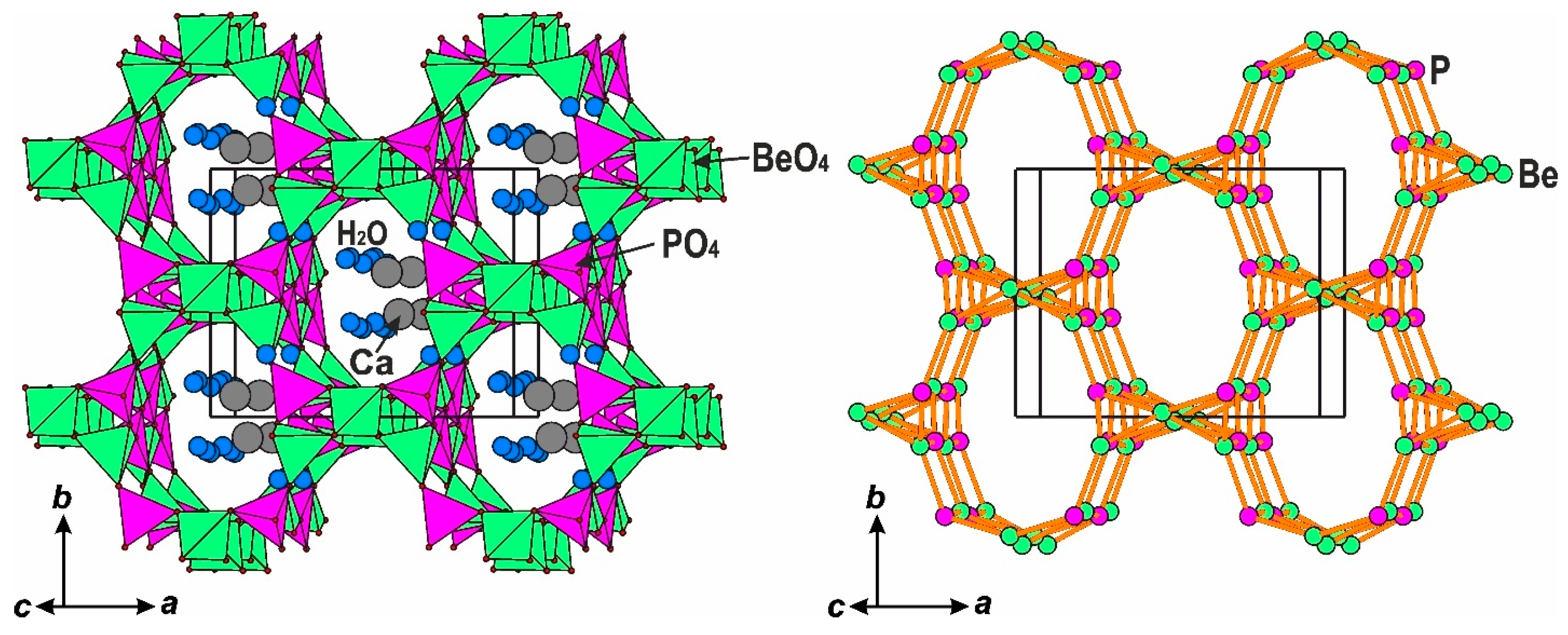

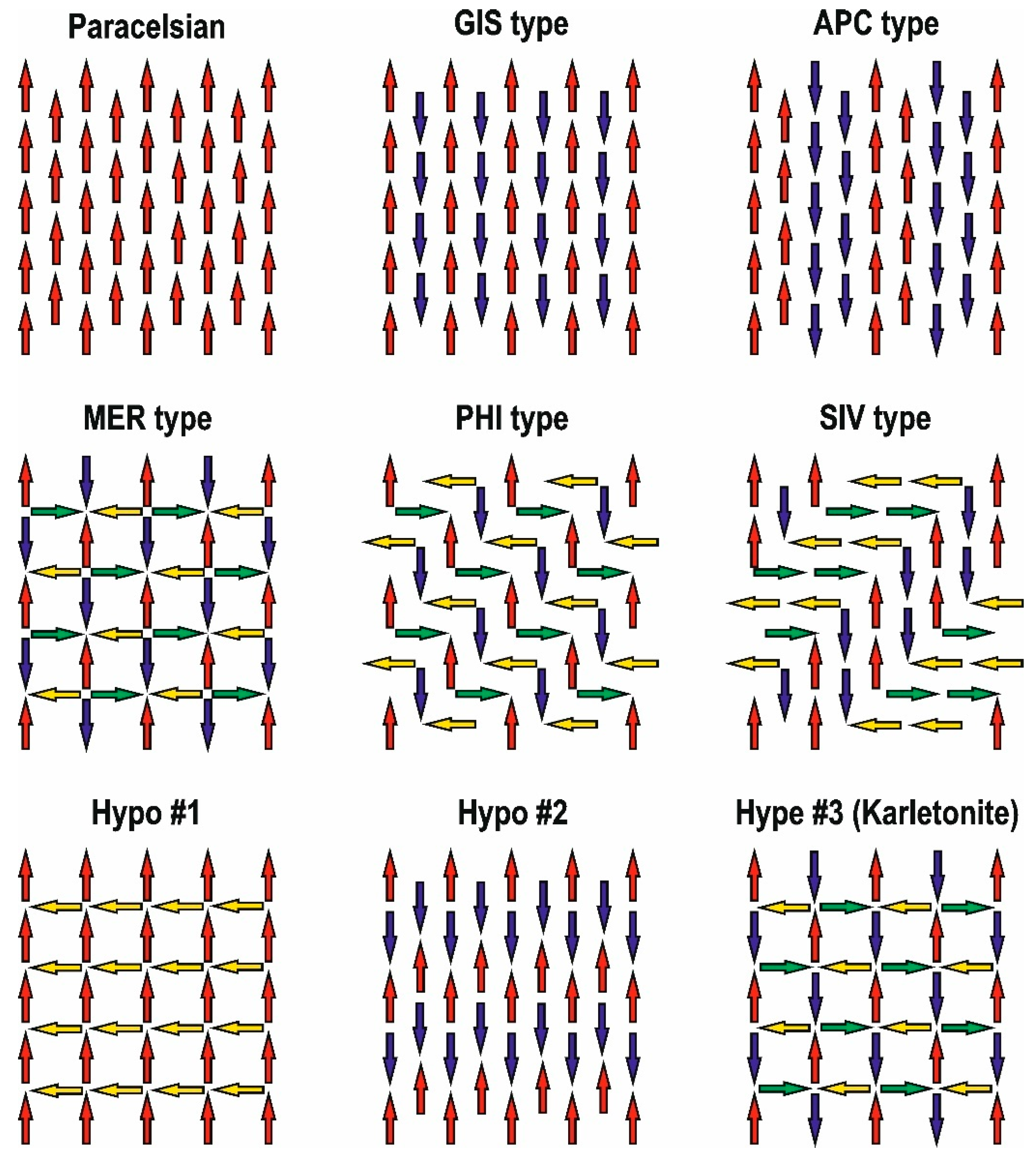


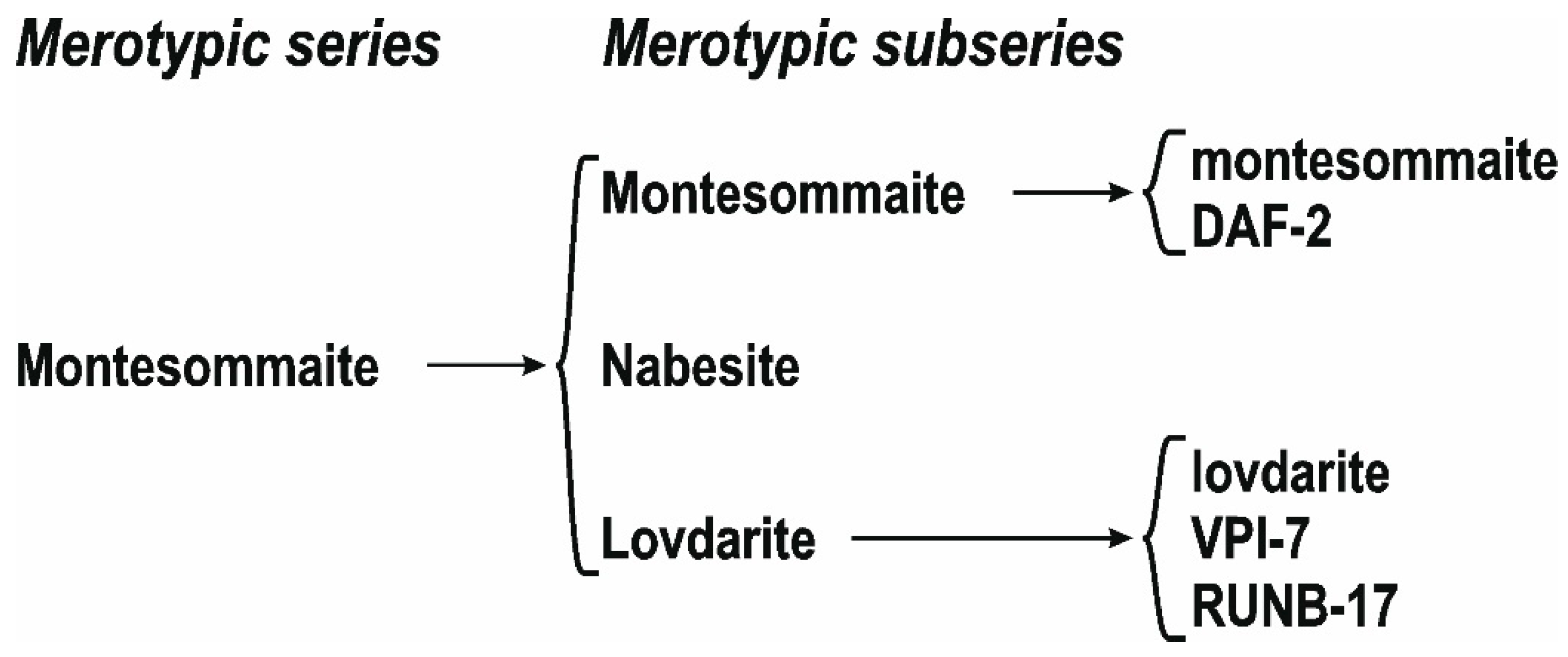
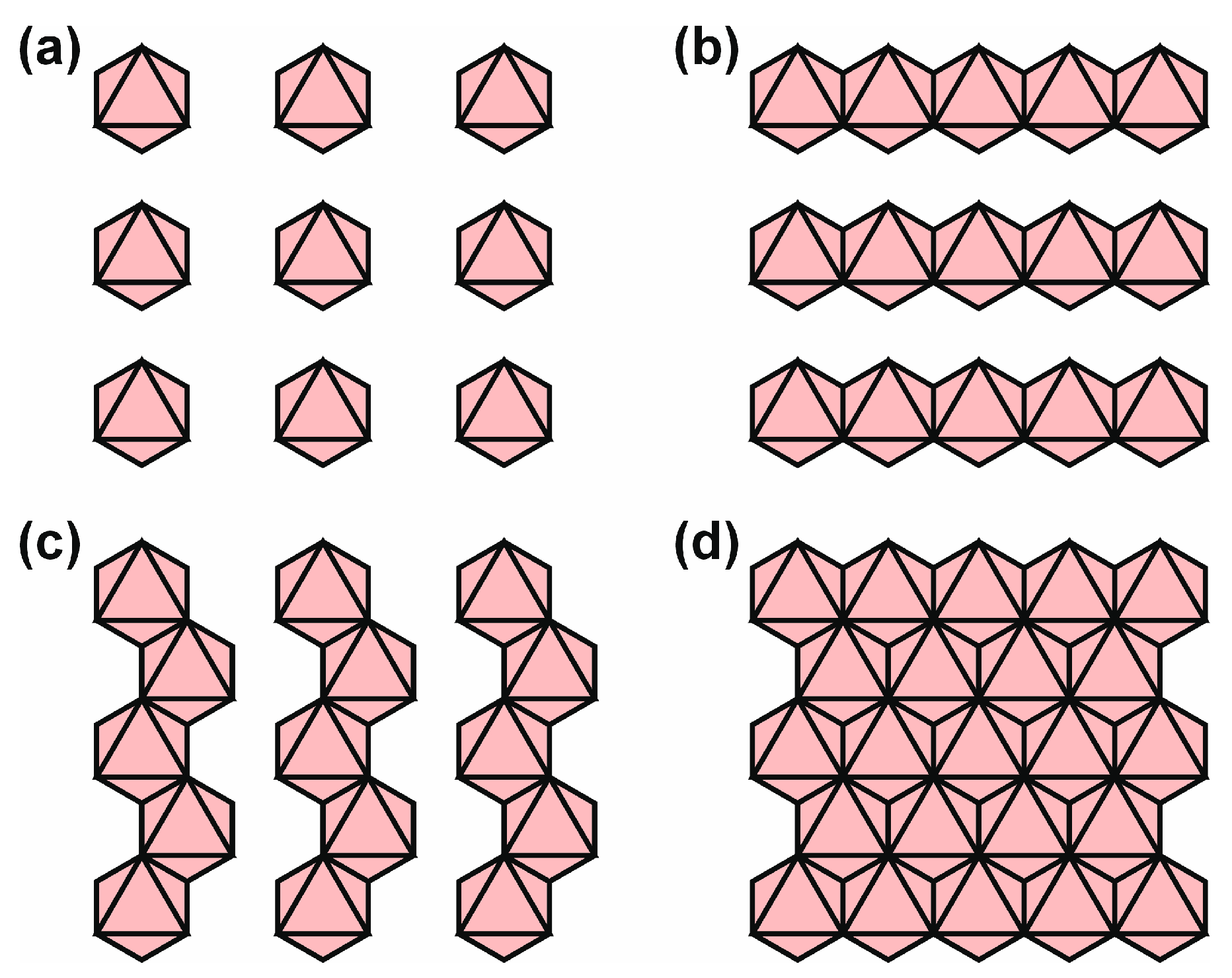

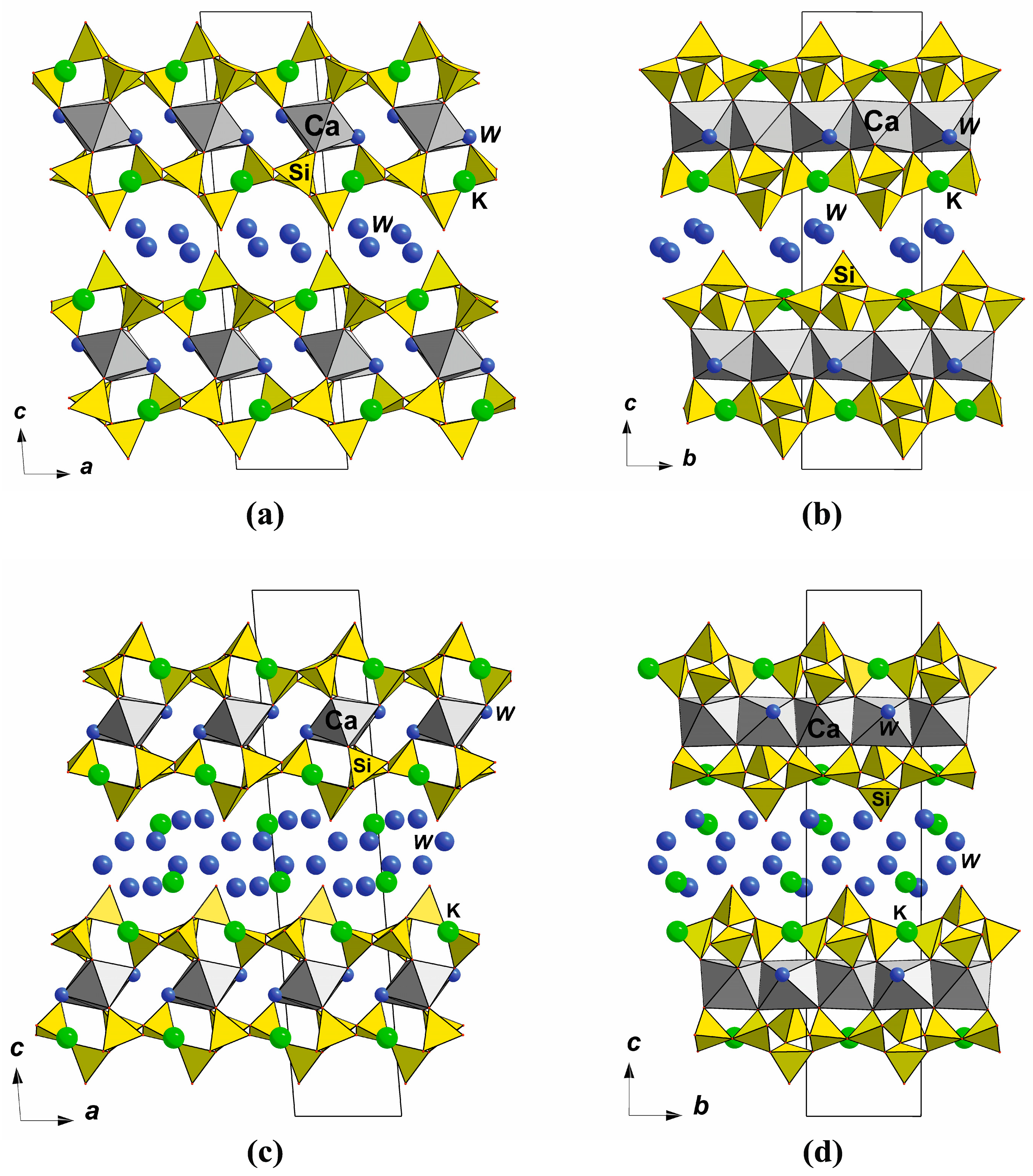
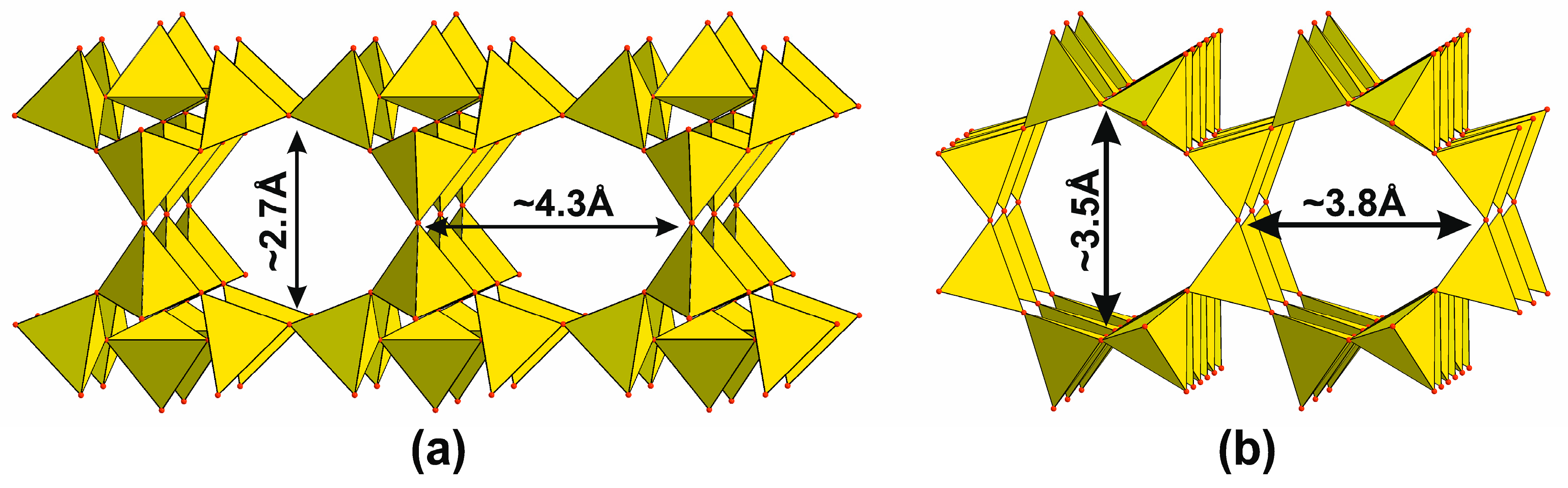
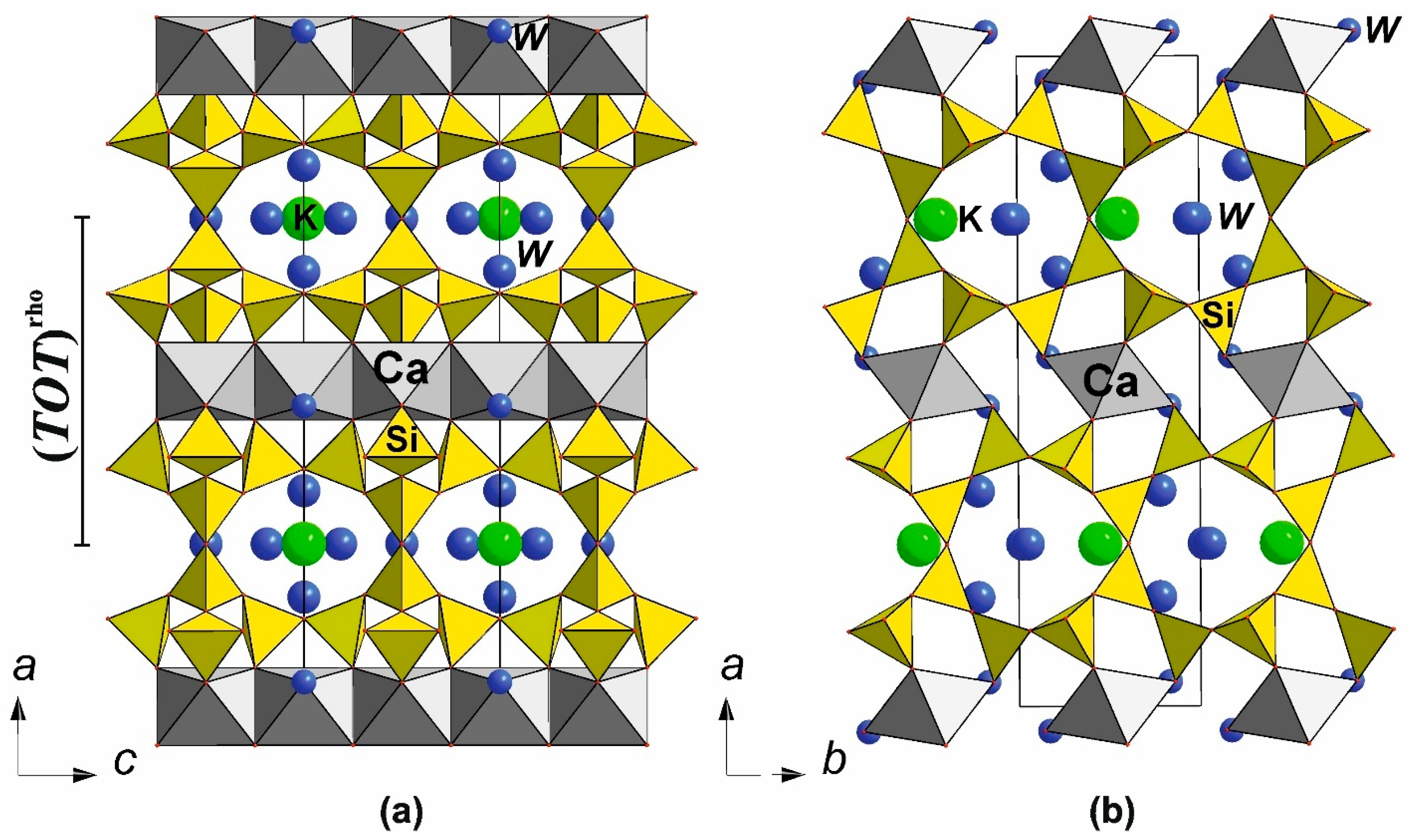
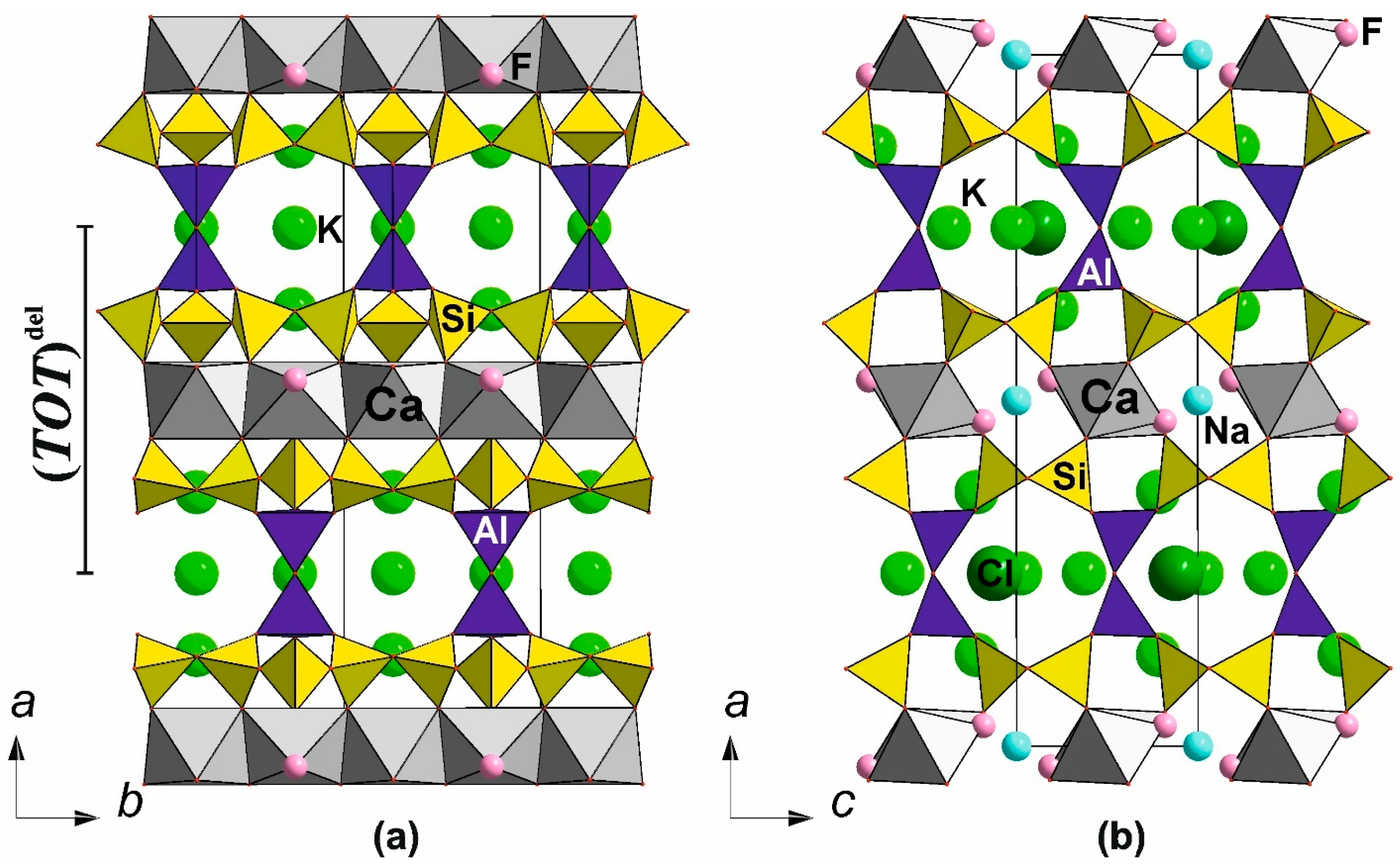

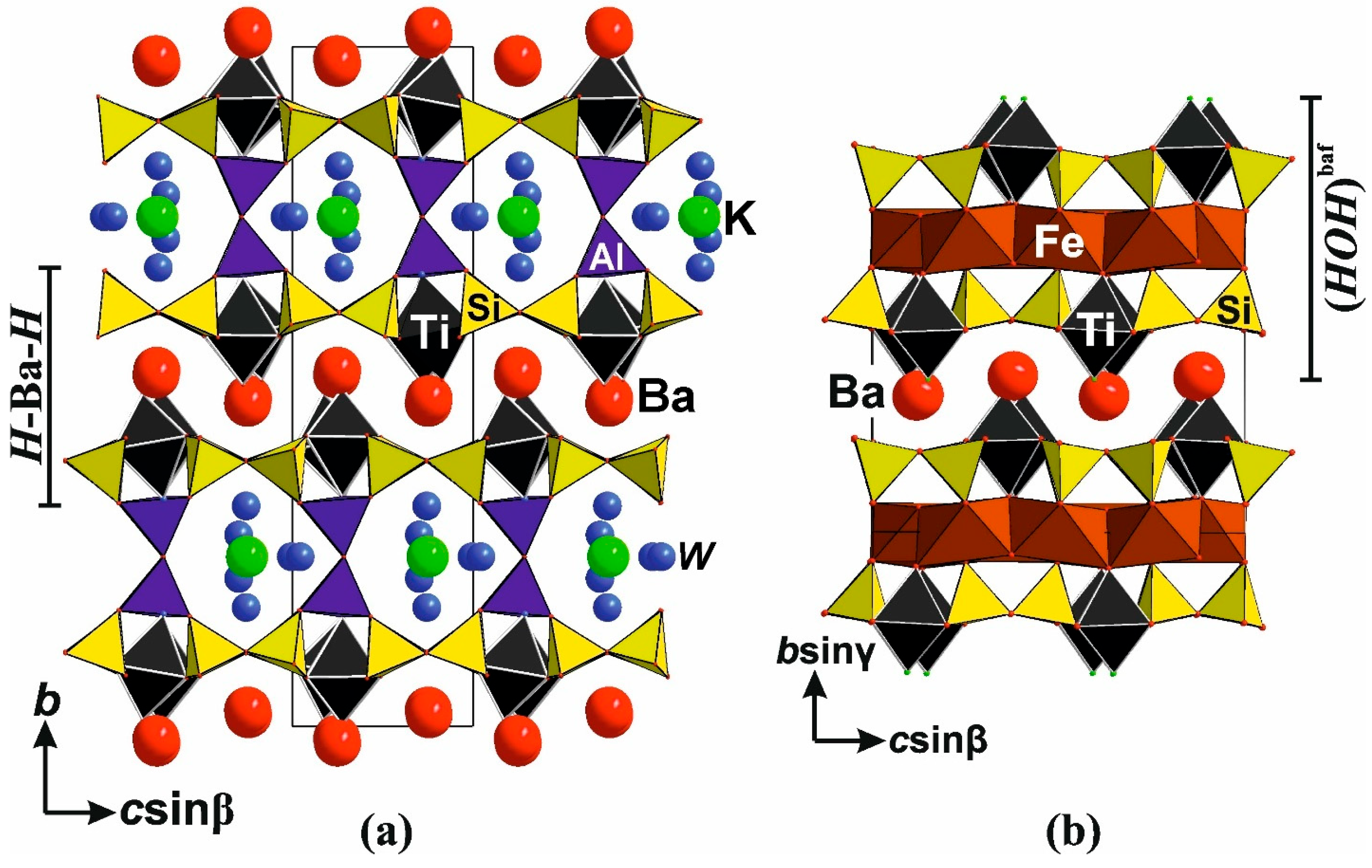
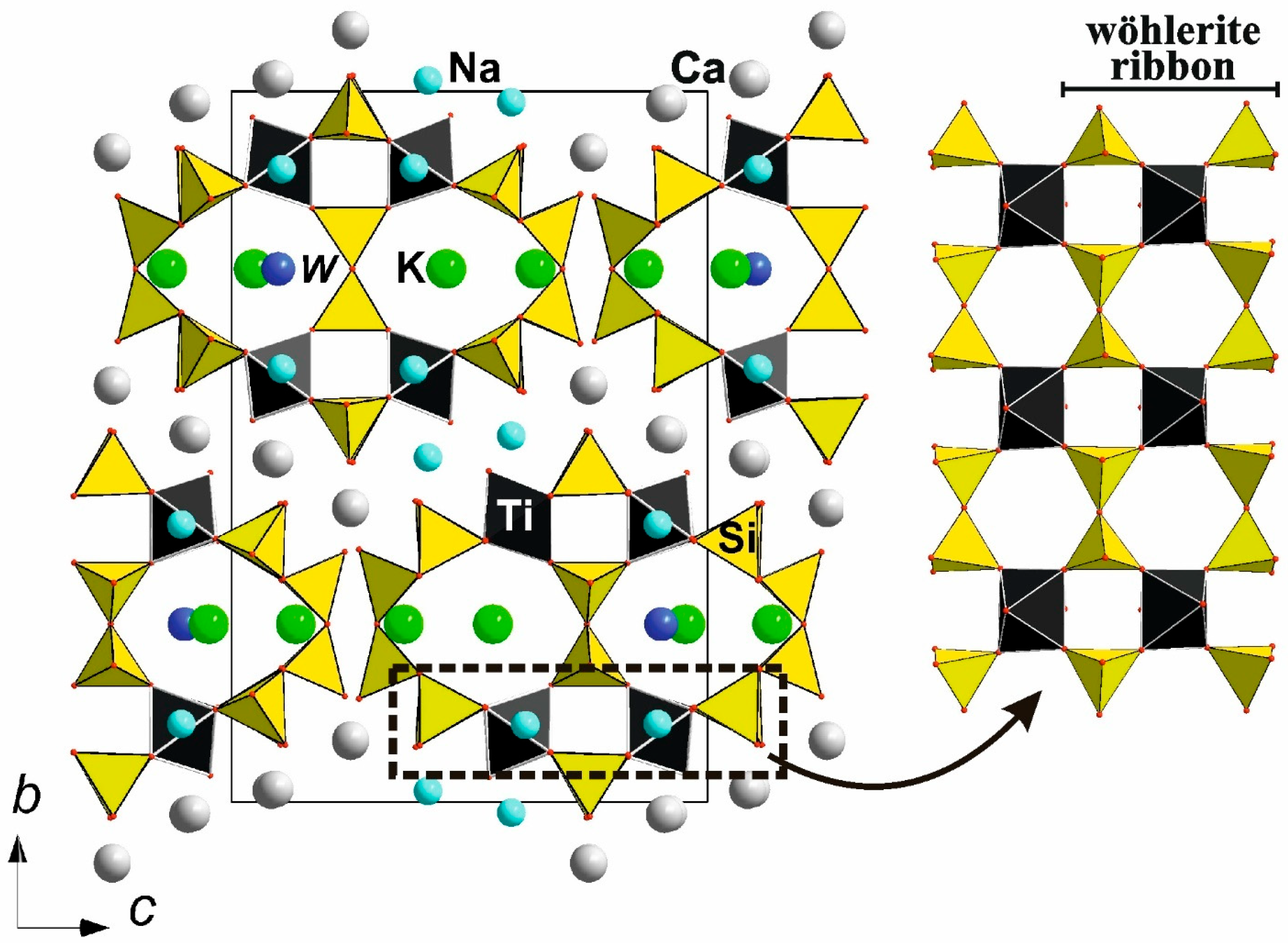
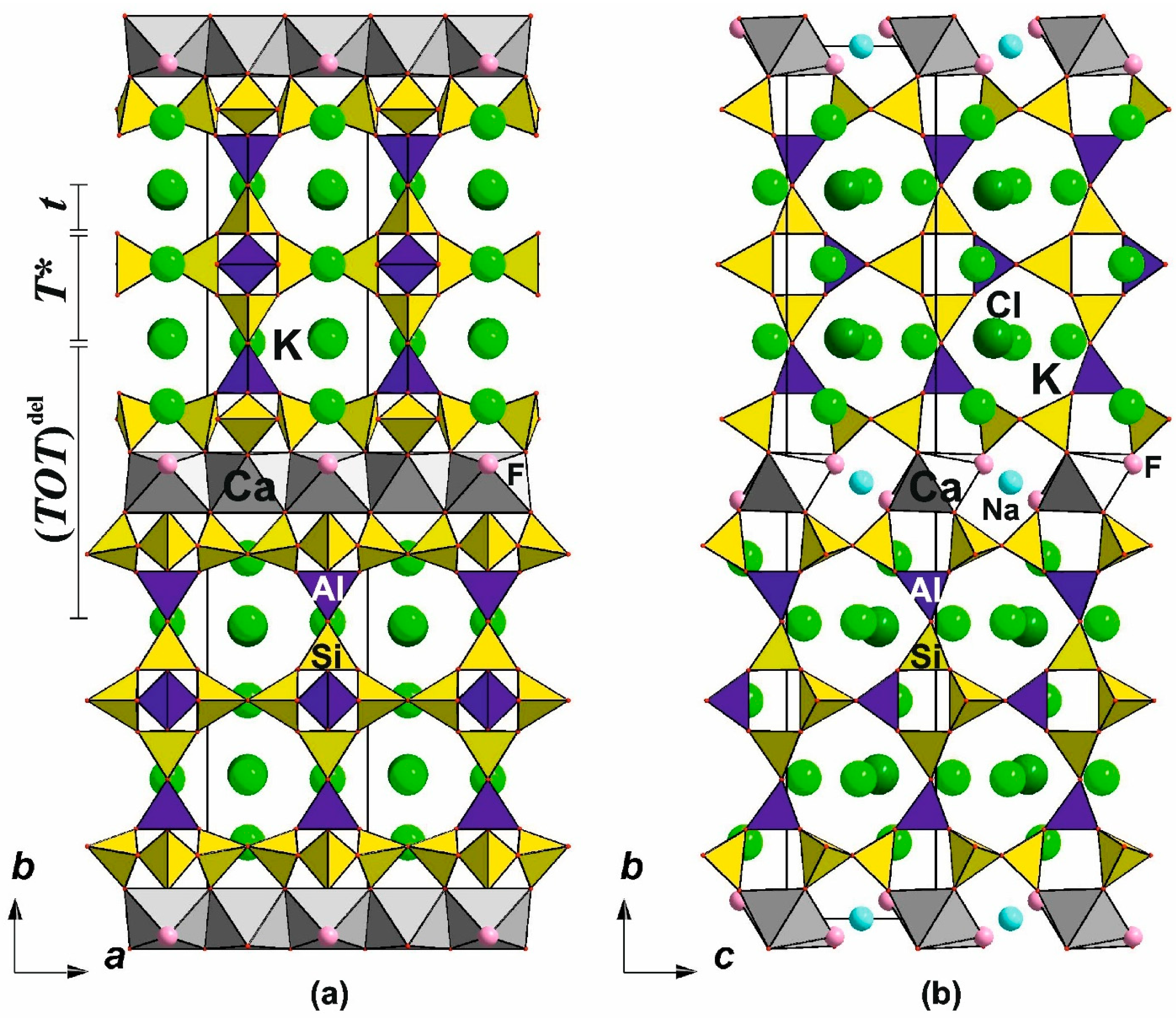
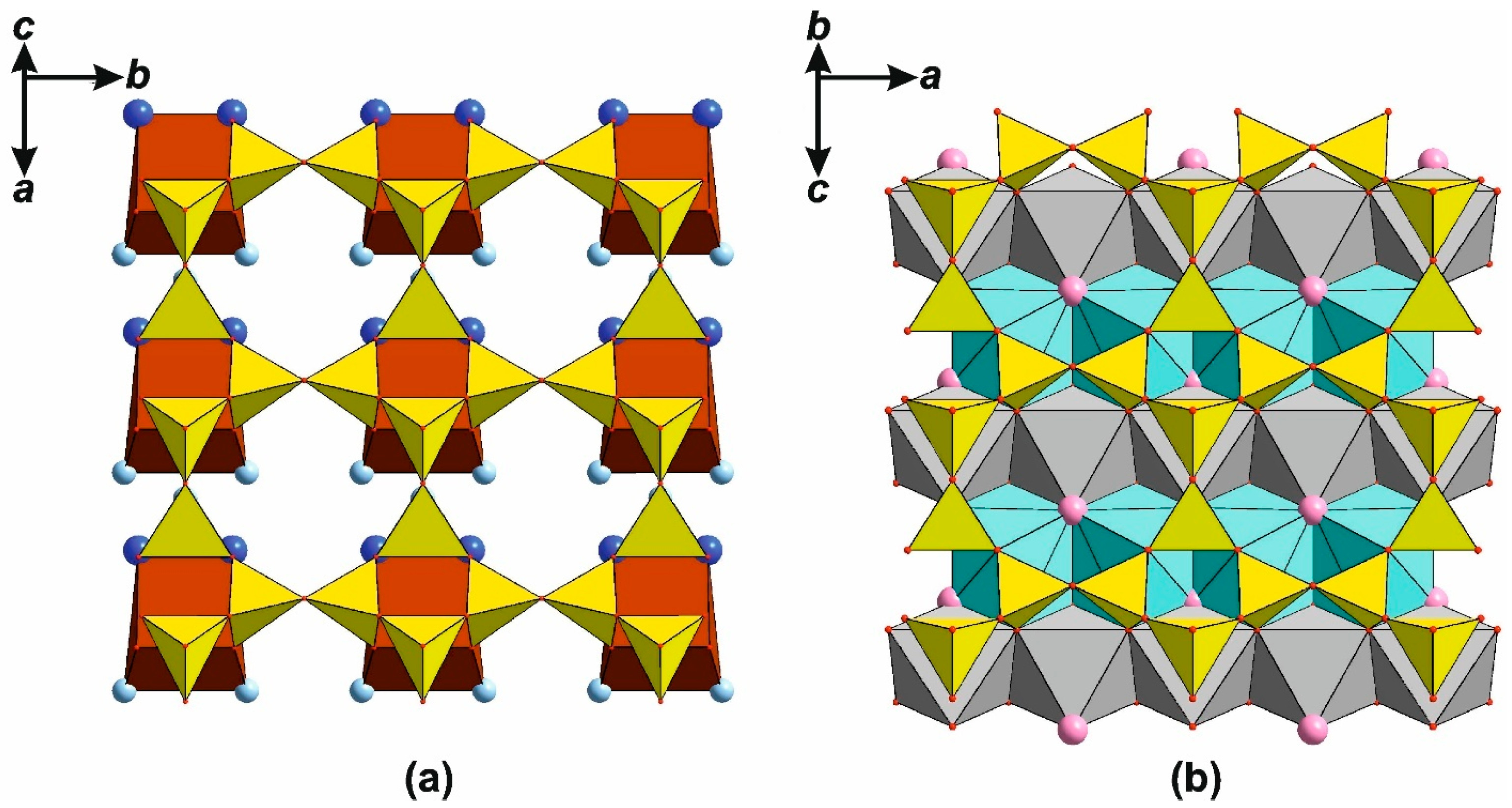

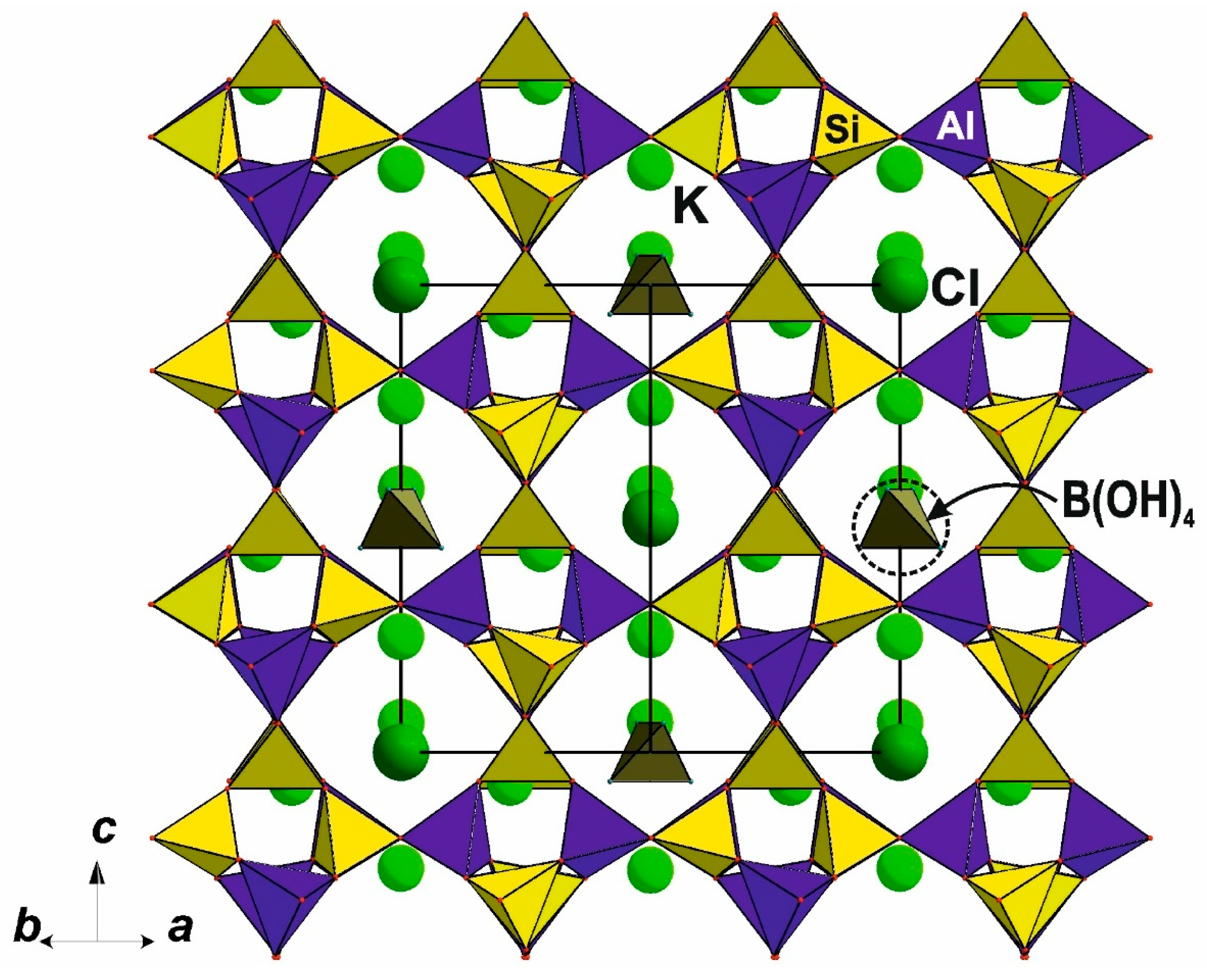
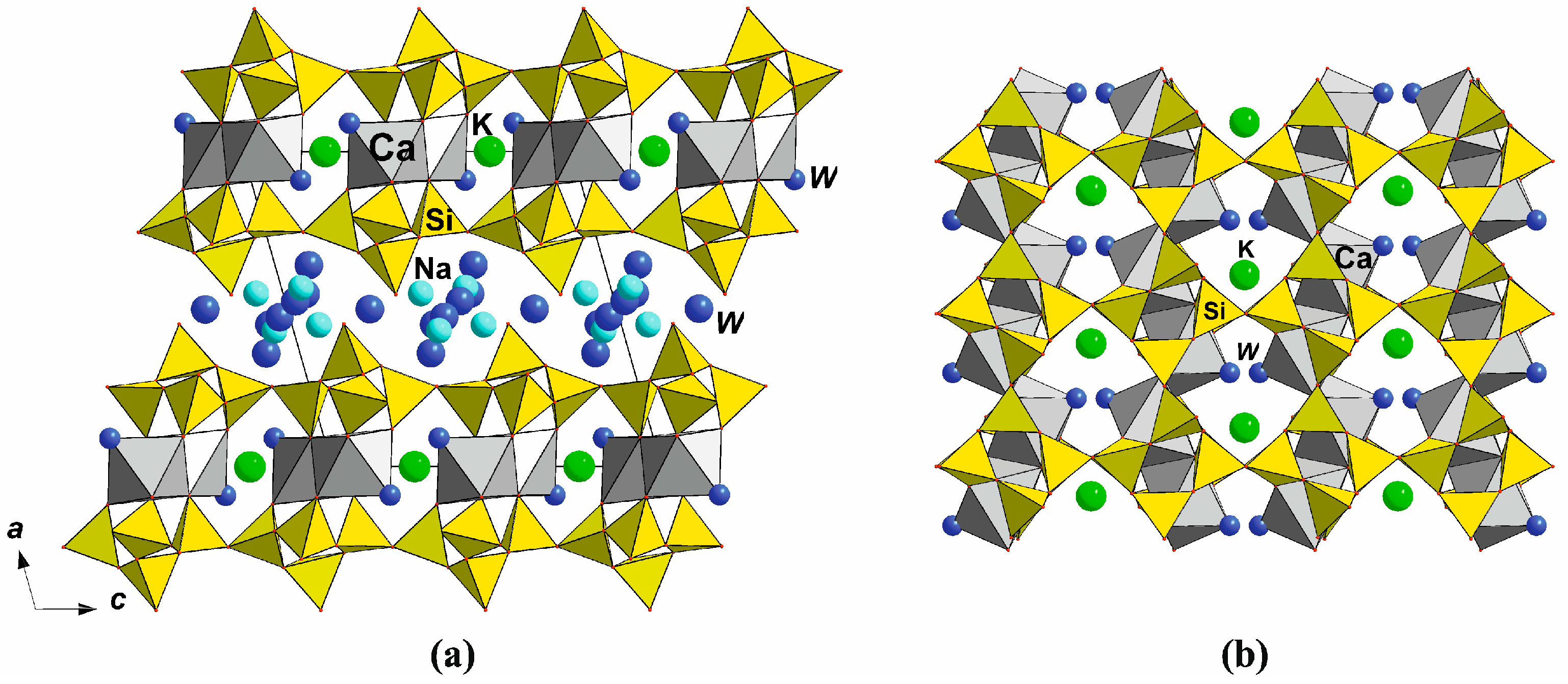

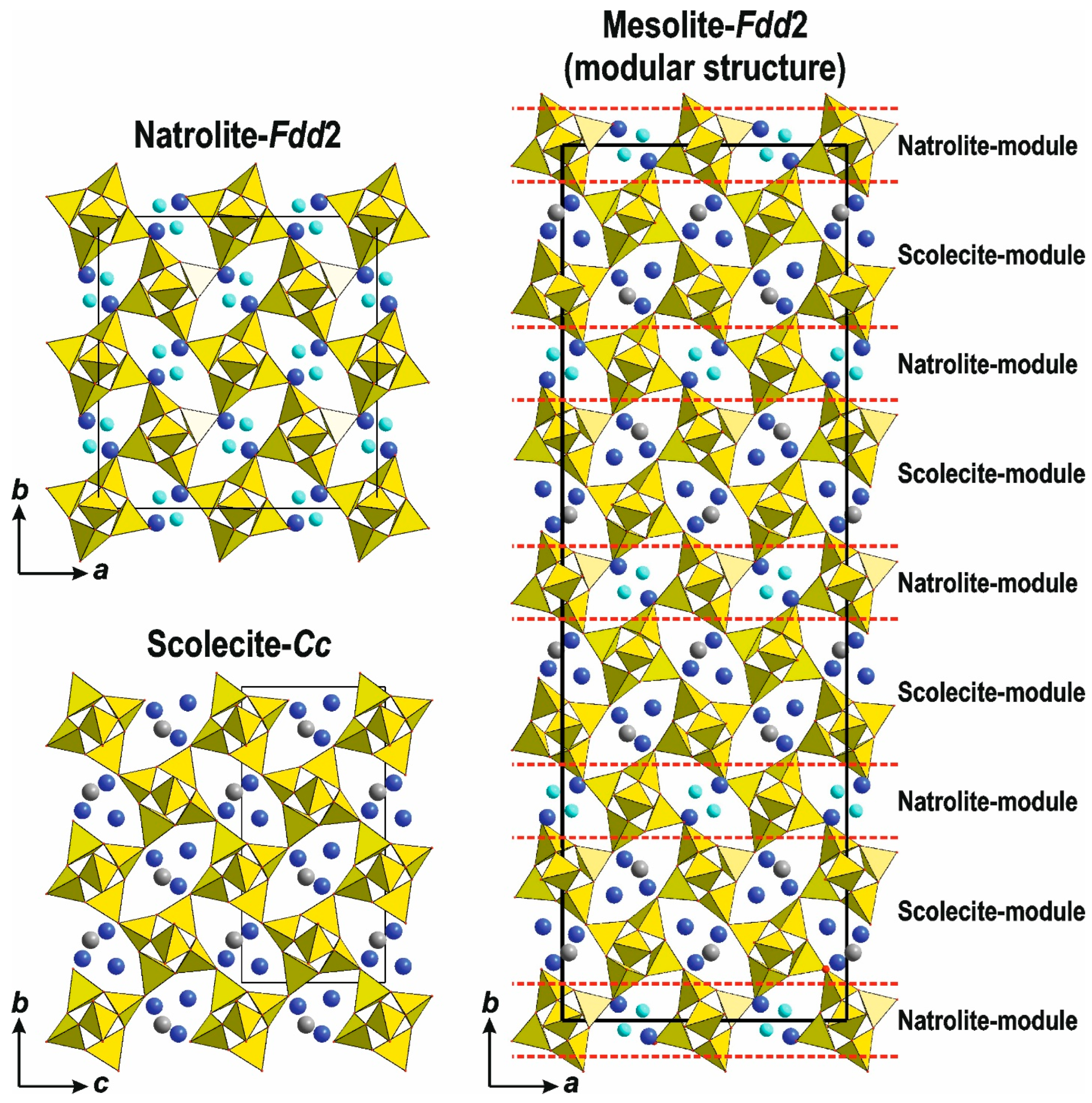
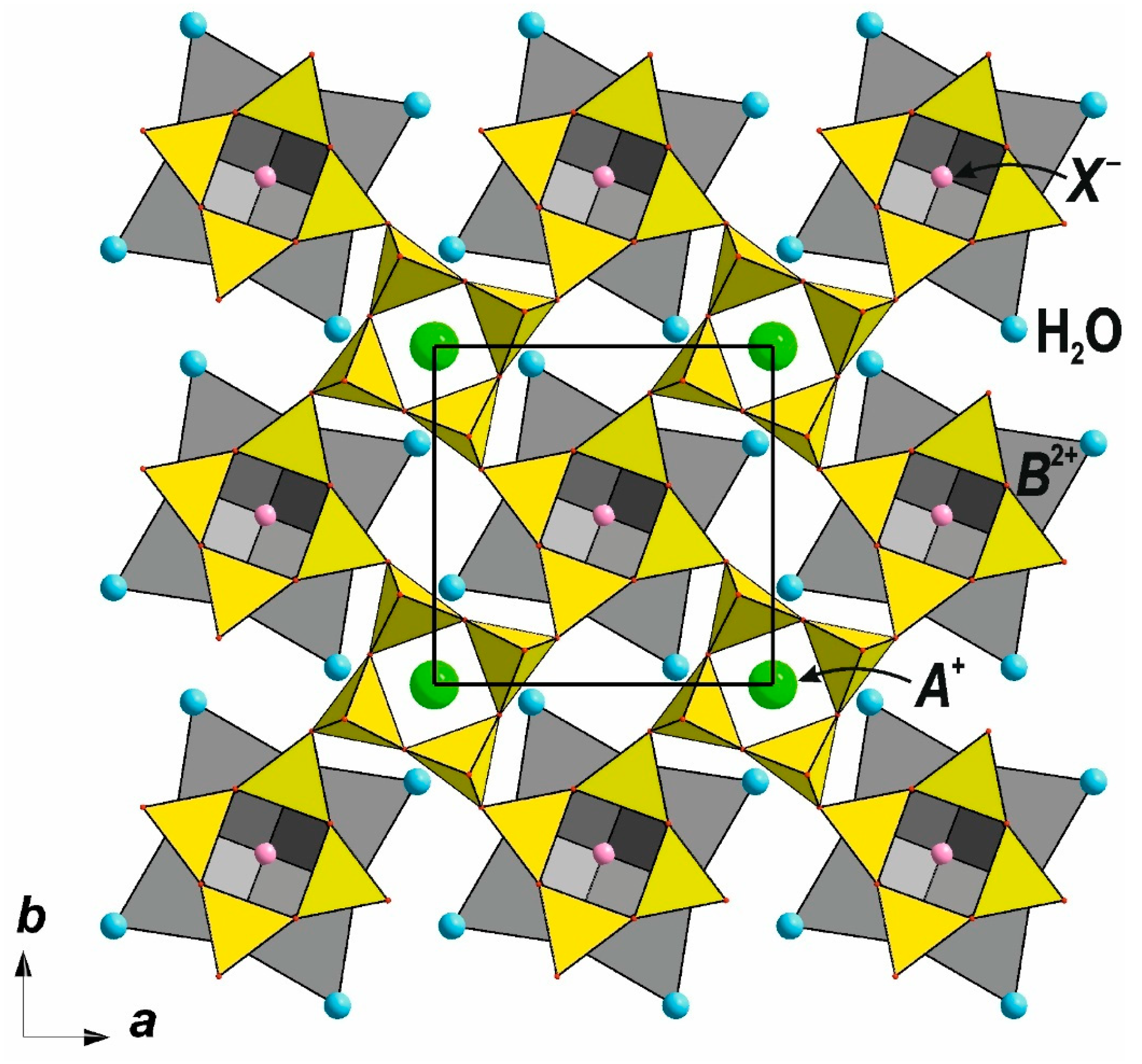
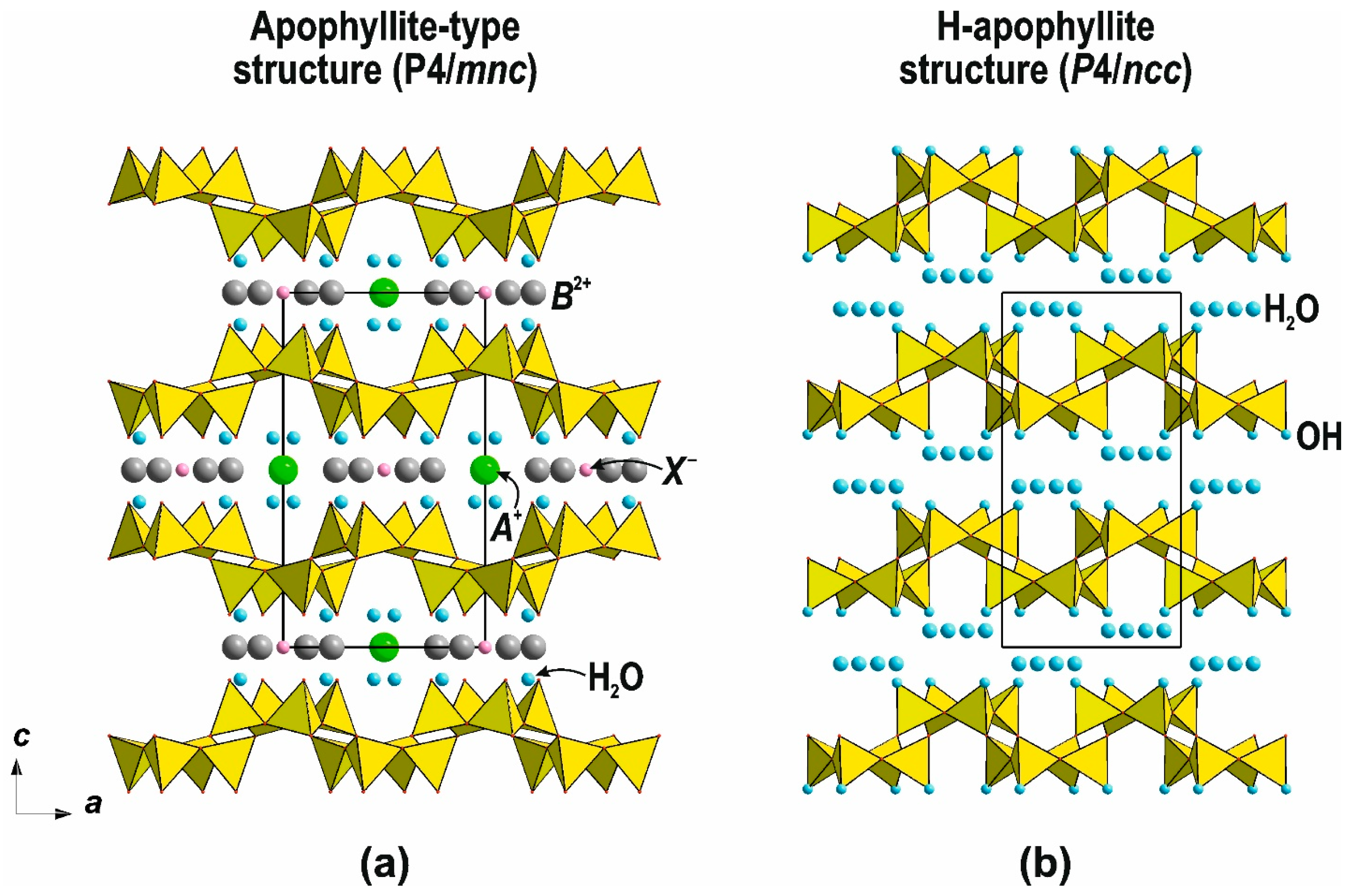
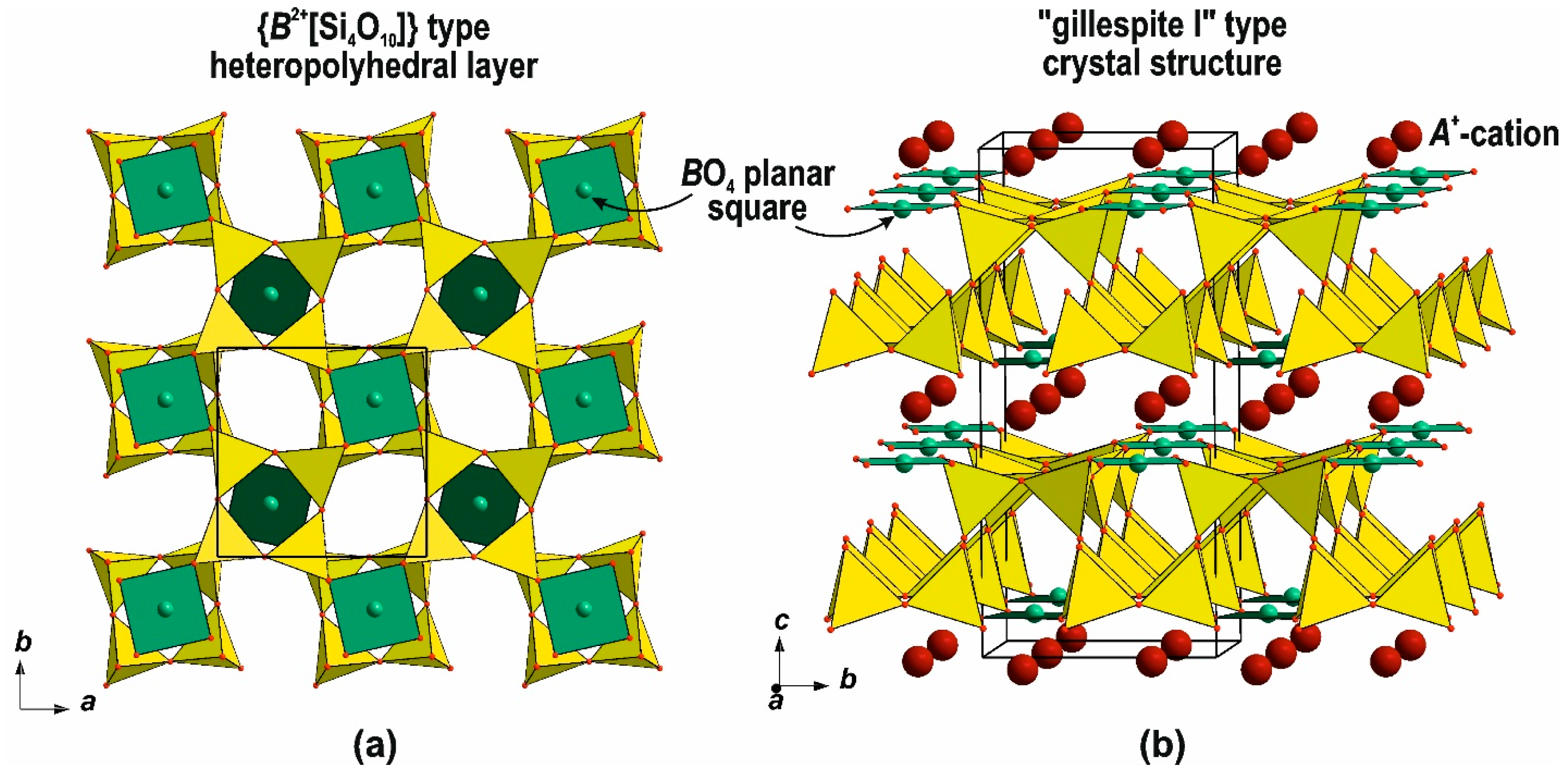
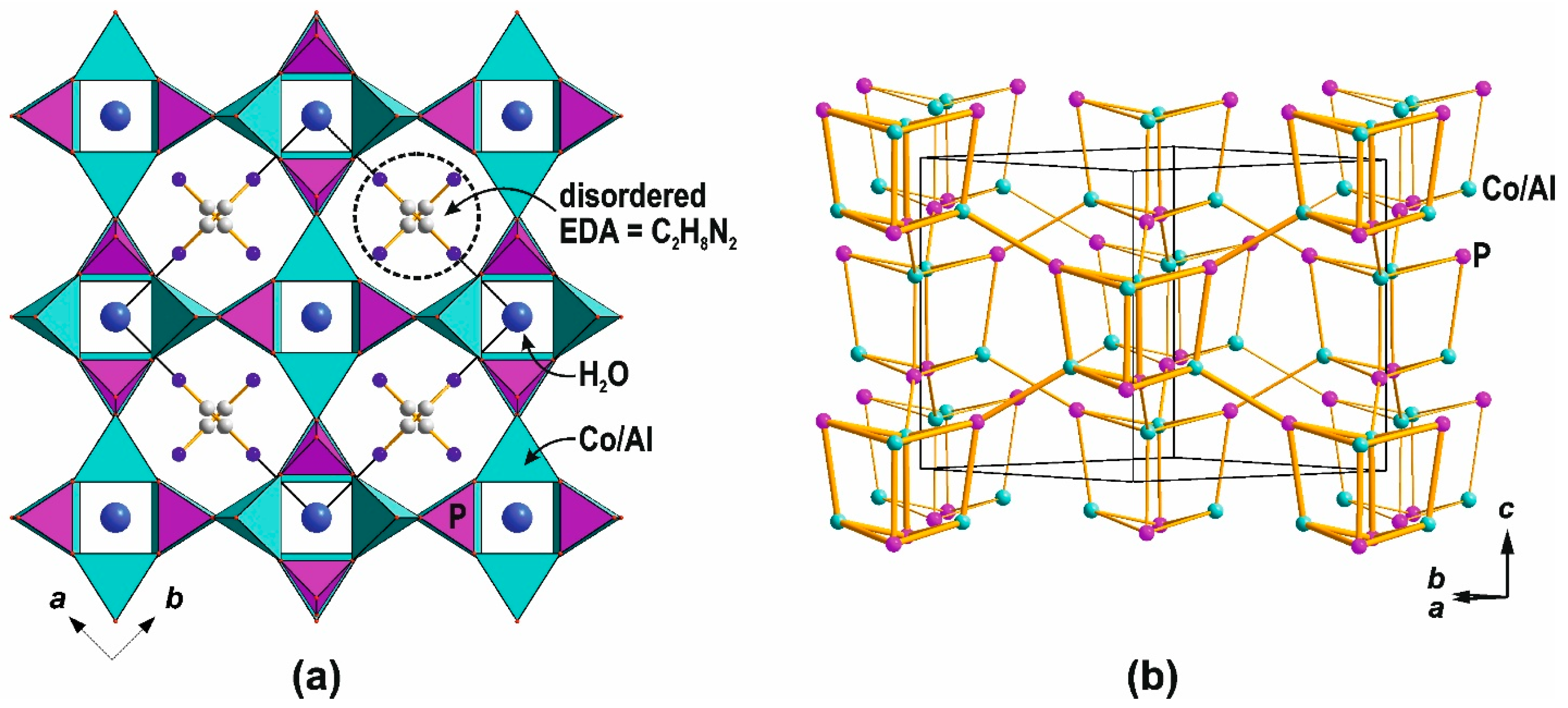

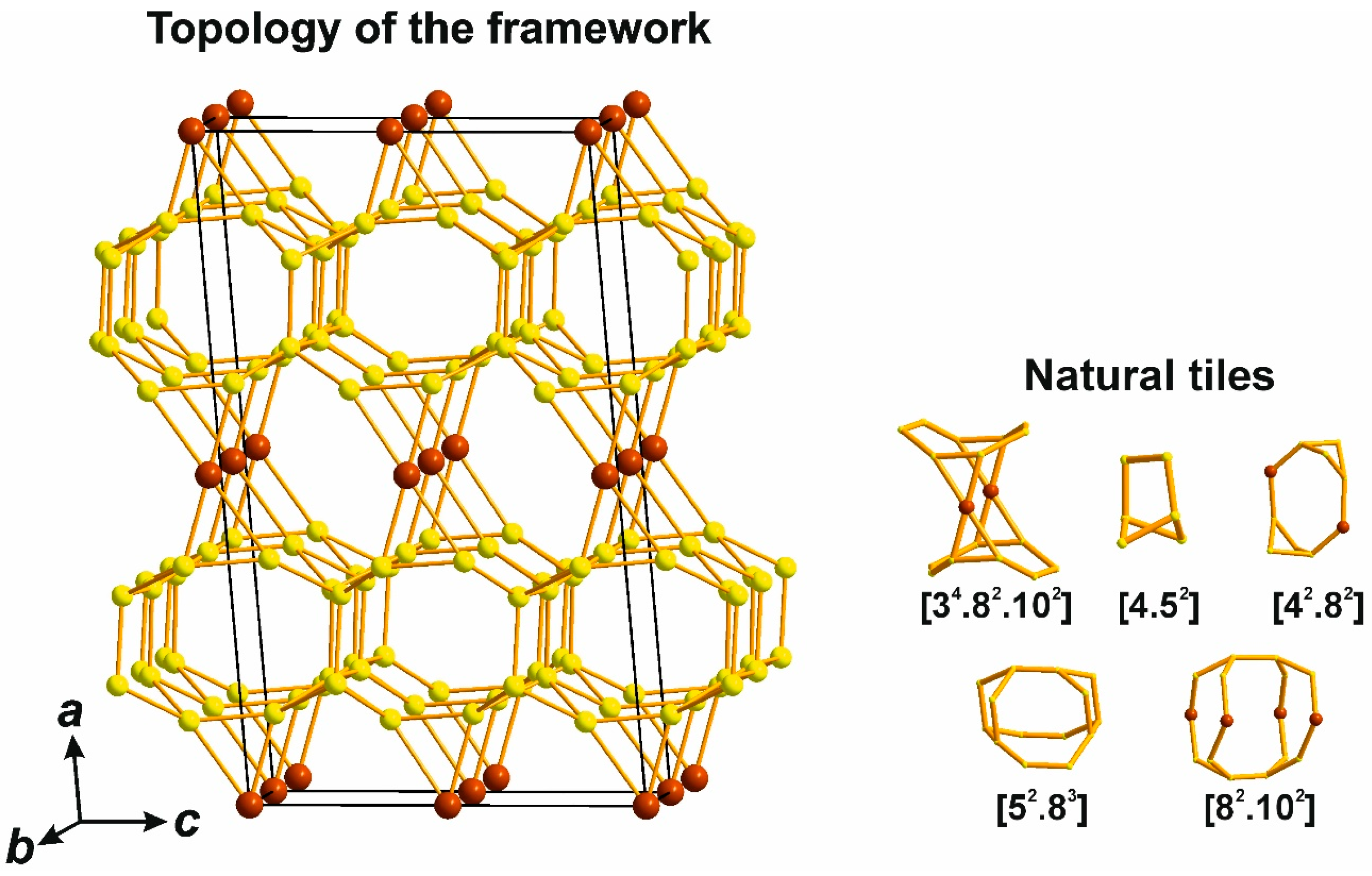

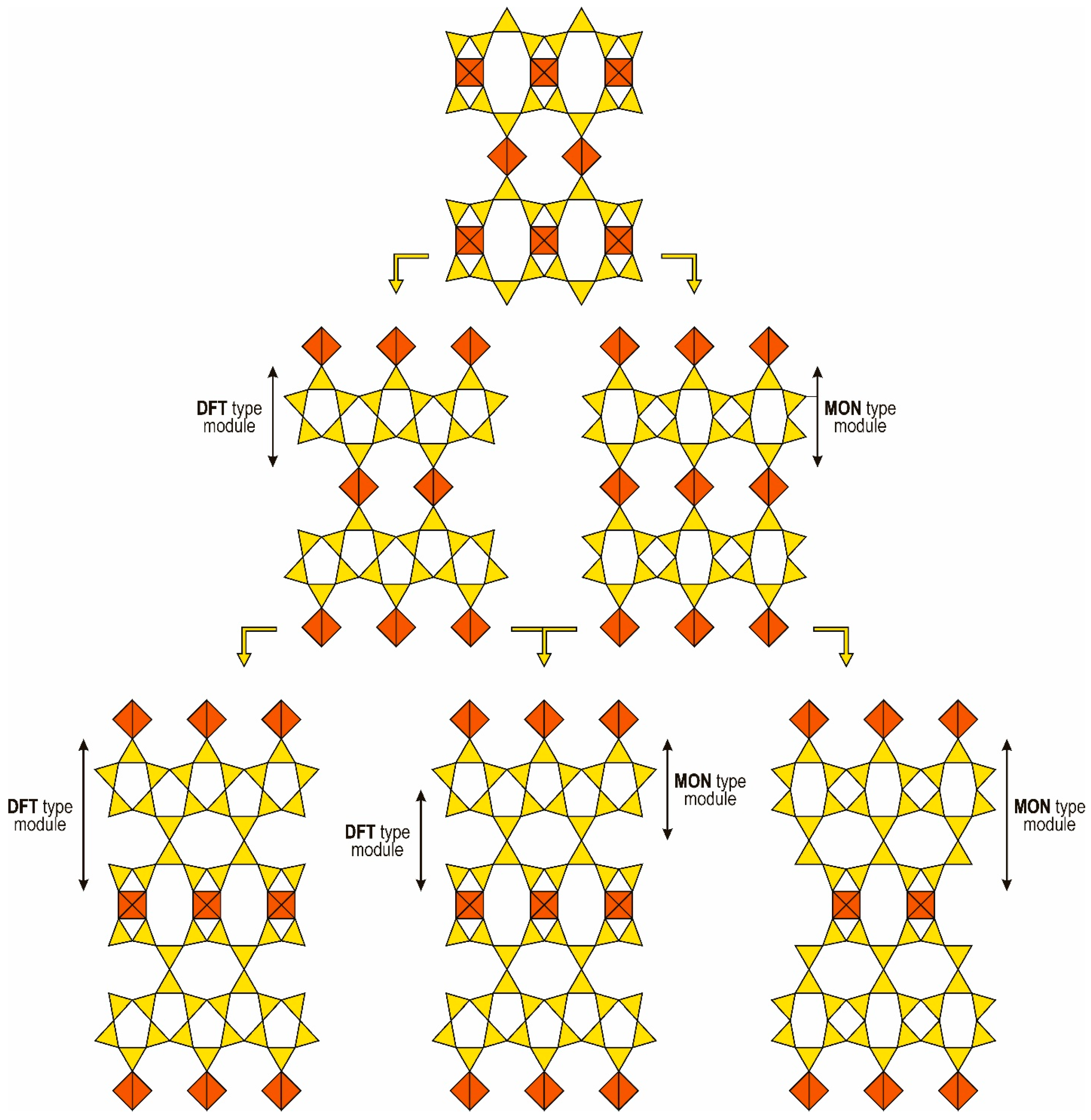
| Face Symbol | [43] | [42.82] | [42.82] |
| Figure |  |  | 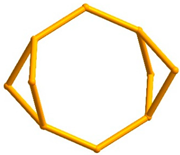 |
| (V, E, F) | (5, 6, 3) | (10, 12, 4) | (10, 12, 4) |
| Symmetry | mm2 | mm2 | 2/m |
| Tile label | t-kzd | t-kdt | t-kds |
| Face symbol | [86] | [86] | [8.92] |
| Figure | 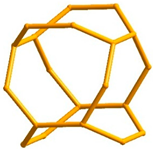 |  | 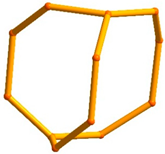 |
| (V, E, F) | (20, 24, 6) | (20, 24, 6) | (10, 13, 3) |
| Symmetry | .2/m. | .2. | |
| Tile label | t-krq | t-krr | t-nat |
| Framework Type | Mineral/ Compound | Idealized Unit Cell Parameters (Å); V (Å3); sp. gr.* | DP/da/db/dc (Å) ** | FD *** | CBU | Tiles | Complexity Parameters | ||
|---|---|---|---|---|---|---|---|---|---|
| v (Atoms) | IG (Bit/Atom) | IG,total (Bit/Unit Cell) | |||||||
| Shlykovite-type stereoisomer | |||||||||
| EDI | Edingtonite (1TT-polytype) | a = 6.926 c = 6.410 V = 307.5 P-4m2 | 5.72/ 3.2/ 3.2/ 3.44 | 16.3 | 6T-nat | t-kdt t-krq t-kzd | 15 | 2.174 | 32.603 |
| THO | Thomsonite (2O-polytype) | a = 14.000 b = 7.000 c = 6.482 V = 635.2 Pmma | 5.15/ 3.26/ 3.0/ 3.69 | 15.7 | 6T-nat | t-kds t-kdt t-krr t-kzd | 30 | 2.840 | 85.207 |
| Mountainite-type stereoisomer | |||||||||
| NAT | Natrolite | a = 13.850 c = 6.420 V = 1231.5 I41/amd | 4.52/ 2.99/ 2.99/ 4.38 | 16.2 | 6T-nat | t-kds t-kzd t-nat | 30 | 2.174 | 65.207 |
| Seidite-type stereoisomer | |||||||||
| DFT | DAF-2 (2TT-polytype) | a = 7.075 c = 9.023 V = 451.7 P42/mmc | 5.1/ 3.07/ 3.07/ 3.65 | 17.7 | nsc-chain | t-kaa t-lov t-ste | 24 | 1.918 | 46.039 |
| MON | Montesomaite (4TT-polytype) | a = 7.135 c = 17.809 V = 906.6 I41/amd | 4.24/ 3.54/ 3.54/ 3.54 | 17.6 | – | t-euo t-kaj | 24 | 1.918 | 46.039 |
| NAB | Nabesite | a = 7.184 c = 12.043 V = 621.4 | 4.31/ 3.57/ 3.57/ 3.57 | 16.1 | 5T-lov 6T-vsv | t-nab-1 t-nab-2 | 15 | 2.174 | 32.603 |
| LOV | Lovdarite | a = 7.163 c = 20.876 V = 1071.1 P42/mmc | 5.15/ 3.78/ 3.78/ 3.77 | 16.8 | 5T-lov 6T-vsv | t-kaa t-lov t-nab-1 t-nab-2 t-ste | 54 | 2.755 | 148.764 |
| VSV | VPI-7 | a = 7.156 c = 41.842 V = 2142.7 I41/amd | 4.31/ 3.77/ 3.77/ 2.9 | 16.8 | 5T-lov 6T-vsv | t-euo t-kaj t-nab-1 t-nab-2 | 54 | 2.755 | 148.764 |
| RSN | RUB-17 | a = 7.156 b = 41.910 c = 7.160 V = 2147.5 Cmmm | 5.15/ 3.77/ 2.9/ 3.77 | 16.8 | 5T-lov 6T-vsv | t-euo t-kaa t-kaj t-lov t-nab-1 t-nab-2 t-ste | 54 | 4.014 | 216.764 |
| Apophyllite-type stereoisomer | |||||||||
| ACO | ACP-1 | a = 9.905 V = 971.8 Im-3m | 4.58/ 3.56/ 3.56/ 3.56 | 16.5 | 8T-d4r | t-cub t-ste | 24 | 1.459 | 35.020 |
| Paracelsian-type stereoisomer | |||||||||
| GIS | Gismondine | a = 9.801 c = 10.158 I41/amd | 4.97/ 3.32/ 3.32/ 3.32 | 16.4 | 20T-gis | t-gsm | 24 | 1.585 | 38.039 |
| Phillipsite-type stereoisomer | |||||||||
| PHI | Phillipsite | a = 9.890 b = 14.064 c = 14.046 Cmcm | 5.4/ 3.69/ 3.11/ 3.31 | 16.4 | 24T-phi | t-phi t-oto | 48 | 2.918 | 140.078 |
| SIV | SIZ-7 | a = 9.8768 b = 14.0754 c = 28.1314 Cmcm | 5.38/ 3.73/ 3.31/ 3.37 | 16.4 | 20T-gis 24T-phi | t-gsm t-phi t-oto | 96 | 3.835 | 368.156 |
| Merlinoite-type stereoisomer | |||||||||
| ATN | MAPO-39 | a = 13.071 c = 5.256 I4/mmm | 5.91/ 1.94/ 1.94/ 4.11 | 17.8 | 24T-atn | t-kaa t-ocn | 24 | 1.918 | 46.039 |
| MER | Merlinoite | a = 14.012 c = 9.954 I4/mmm | 6.65/ 3.12/ 3.12/ 4.2 | 16.4 | 16T-d8r (16T pau) | t-opg t-pau t-ste | 48 | 2.252 | 108.078 |
| Mineral/Compound | Chemical Formula | Sp. Gr. (Z = 2) | Unit Cell Parameters | References | ||
|---|---|---|---|---|---|---|
| a, Å α, ° | b, Å β, ° | c, Å γ, ° | ||||
| Single layer (n = 1) | ||||||
| Shlykovite | K2Na2[Si8O18(OH)2]·6H2O | P2/c | 6.4897 | 6.9969 94.597 | 26.714 | [109] |
| Cryptophyllite | K4Ca2[Si8O20]·10H2O | P21/n | 6.4934 | 6.9919 94.680 | 32.087 | [109] |
| Double layer (n = 2) | ||||||
| Rhodesite series | ||||||
| Rhodesite | KCa2[Si8O18(OH)]·6H2O | Pmam | 23.416 | 6.555 | 7.050 | [116] |
| Makdonaldite | BaCa4[Si8O18(OH)]2·10H2O | Cmcm | 14.081 | 13.109 | 23.560 | [188] |
| Monteregianite-(Y) | KNa2Y[Si8O19]·5H2O | P21/n | 9.512 | 23.956 93.85 | 9.617 | [189] |
| Melansonite | (Na,□)□2KZr[Si8O19]·5H2O | Pmma | 24.063 | 6.982 | 6.526 | [190] |
| Natromelansonite | Na3Zr[Si7AlO19]⋅4–5H2O | P21/m | 6.516 | 24.061 90.453 | 6.976 | [191] |
| AV-1 | KNa2Y[Si8O19]·5H2O | P21/n | 9.595 | 23.956 93.850 | 9.583 | [192] |
| AV-2 | KCa2[Si8O18(OH)]·6H2O | - | 23.797 | 7.031 | 6.598 | [192,193] |
| AV-5 | KNa2Ce[Si8O19]·5H2O | P21/n | 9.6906 | 24.055 93.683 | 9.5759 | [193] |
| Eu-AV-9 | KNa2Eu[Si8O19]·5H2O | C2/m * | 23.973 | 14.040 90.352 | 6.5655 | [194] |
| Tb-AV-9 | KNa2Tb[Si8O19]·5H2O | C2/m * | 23.945 | 14.019 90.288 | 6.5542 | [194] |
| Er-AV-9 | KNa2Er[Si8O19]·5H2O | C2/m * | 23.951 | 14.013 | 6.550 | [195] |
| Nd-AV-9 | KNa2Nd[Si8O19]·5H2O | Pmma | 23.902 | 6.998 | 6.547 | [196,197] |
| Nd-AV-9 | KNa2Nd[Si8O19]·2.8H2O | Pmma | 23.923 | 6.995 | 6.547 | [196] |
| Gd-AV-9 | KNa2Gd[Si8O19]·5H2O | - | - | - | - | [197] |
| EMS-6 | K4Na8Gd4[Si32O76]·17H2O | P21/n | 9.6595 | 23.9862 93.757 | 9.5275 | [198] |
| CAS-1 | K2Ca2[Si8O19]·4H2O | C2 | 24.158 | 7.0160 95.19 | 6.4816 | [199] |
| AES-19 | K8Sr8[Si32O76]·16H2O | Pnna * | 13.9368 | 23.4754 | 6.7631 | [119] |
| TR03 | KNaCa2[Si8O19]·5H2O | Pn21m | 6.585 | 23.776 | 7.025 | [200] |
| TR04 | KNa3Sr[Si8O19]·4.3H2O | P21/m | 6.6599 | 23.7225 91.81 | 7.0225 | [200] |
| TR09 | Na2Sr2[Si8O19]·4H2O | C2/c | 22.7681 | 6.9352 92.578 | 13.5789 | [201] |
| TR10 | Na4Sr[Si8O19]·4H2O | P2/c | 22.5102 | 7.0292 92.538 | 13.3140 | [201] |
| Phase I | KNa2Tm[Si8O19]·4H2O | P2/m ** | 6.5315 | 6.9935 90.383 | 11.9430 | [118] |
| AMH-3 | Na2Sr2[Si8O19]·4H2O | C2/c | 22.783 | 6.9395 92.5935 | 13.581 | [202] |
| Delhayelite series | ||||||
| Delhayelite | K4Na2Ca2[AlSi7O19]F2Cl | Pmmn | 24.579 | 7.0575 | 6.5811 | [203] |
| Fivegite | K4Ca2[AlSi7O17(O2−x,OHx)] [(H2O)2−xOHx]Cl | Pm21n | 24.335 | 7.0375 | 6.5400 | [204] |
| Hydrodelhayelite | KCa2[AlSi7O17(OH)2]·(6−x)H2O | Pn21m | 6.648 | 23.846 | 7.073 | [205] |
| Ba-analog of delhayelite | Ba0.5Ca0.5[AlSi7O17(OH)2]·6H2O | Pmmn | 23.953 | 7.052 | 6.606 | [206] |
| Triple layer (n = 3) | ||||||
| Günterblassite | (K,Ca)3−xFe[(Si,Al)13O25(OH,O)4]·7H2O | Pm21n | 6.970 | 37.216 | 6.528 | [40,121] |
| Umbrianite | K7Na2Ca2[Al3Si10O29]F2Cl2 | Pmmn | 7.062 | 38.420 | 6.574 | [120] |
| Hillesheimite | (K,Ca,□)2(Mg,Fe,Ca,□) [(Si,Al)13O23(OH)6]OH·8H2O | Pmmn | 6.979 | 37.182 | 6.530 | [121] |
| Component | Content (wt.%) | |
|---|---|---|
| Günterblassite | “Günterblassite-Ba” | |
| Na2O | 0.40 | 0 |
| K2O | 5.18 | 2.85 |
| MgO | 0.58 | 0.81 |
| CaO | 3.58 | 2.98 |
| BaO | 4.07 | 8.84 |
| FeO | 3.06 | 3.45 |
| Al2O3 | 13.98 | 13.56 |
| SiO2 | 52.94 | 53.59 |
| H2O | 15.2 | No data |
| Total | 98.99 | 86.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenov, S.M.; Chukanov, N.V.; Rastsvetaeva, R.K.; Pushcharovsky, D.Y.; Deyneko, D.V.; Kalashnikova, G.O.; Tananaev, I.G.; Burns, P.C. The Crystal Chemistry and Topology of Modular Structures. III. 2D and 3D Zeolites Containing Tetrahedral Layers with the Apophyllite-Type Topology. Molecules 2025, 30, 4477. https://doi.org/10.3390/molecules30224477
Aksenov SM, Chukanov NV, Rastsvetaeva RK, Pushcharovsky DY, Deyneko DV, Kalashnikova GO, Tananaev IG, Burns PC. The Crystal Chemistry and Topology of Modular Structures. III. 2D and 3D Zeolites Containing Tetrahedral Layers with the Apophyllite-Type Topology. Molecules. 2025; 30(22):4477. https://doi.org/10.3390/molecules30224477
Chicago/Turabian StyleAksenov, Sergey M., Nikita V. Chukanov, Ramiza K. Rastsvetaeva, Dmitry Yu. Pushcharovsky, Dina V. Deyneko, Galina O. Kalashnikova, Ivan G. Tananaev, and Peter C. Burns. 2025. "The Crystal Chemistry and Topology of Modular Structures. III. 2D and 3D Zeolites Containing Tetrahedral Layers with the Apophyllite-Type Topology" Molecules 30, no. 22: 4477. https://doi.org/10.3390/molecules30224477
APA StyleAksenov, S. M., Chukanov, N. V., Rastsvetaeva, R. K., Pushcharovsky, D. Y., Deyneko, D. V., Kalashnikova, G. O., Tananaev, I. G., & Burns, P. C. (2025). The Crystal Chemistry and Topology of Modular Structures. III. 2D and 3D Zeolites Containing Tetrahedral Layers with the Apophyllite-Type Topology. Molecules, 30(22), 4477. https://doi.org/10.3390/molecules30224477










