Robust All-Solid-State Batteries with Sodium Ion Electrolyte, Aluminum and Additive Manufacturing Inconel 625 Electrodes
Abstract
1. Introduction
| Cell Configuration | Electrolyte | Open-Circuit Voltage/Voltage Drift Behavior | Cycle Stability/Duration | Ref. |
|---|---|---|---|---|
| Anode-less half-cell: Li//Cu | Nb-LLZO | Initial OCV: ~0 V Voltage Drift: The initial Li nucleation shows a sharp voltage drop to -65 mV, followed by a stable growth plateau at around -45 mV, with a gradual polarization increase during cycling due to void formation and dead Li. | Stability: The cells demonstrated cycling stability over hundreds of hours (≈180 h until short circuit for the 50 nm Cu configuration) at current densities of 0.05–0.1 mA.cm−2. Coulombic efficiency stabilized in the range of 75–90% in later cycles. Capacity: A fixed areal capacity of 0.1 mAh.cm−2 was used for stripping/plating cycles. The performance and failure mechanisms were highly dependent on the current collector thickness, influencing the achievable cycle life before short-circuiting. | [58] |
| Anode-less half-cell: Li//stainless steel current collector | LPSCl | Initial OCV: ~0 V Voltage Drift: A sharp voltage drops to ~0.25 V occurs during initial lithium nucleation, followed by a stable plating plateau at ~0.05 V. Increasing stack pressure from 2 to 10 MPa reduces initial polarization and extends the stable voltage plateau, enabling higher plating capacities before failure. | Stability: The failure mechanism shifts with stack pressure: at low pressure (2–5 MPa), failure is caused by irregular Li plating; at high pressure (20 MPa), failure is dominated by mechanical fracture induced at surface notches of the solid electrolyte. Capacity: The maximum areal capacity before short circuit is highly dependent on stack pressure, increasing from 1.24 mAh.cm−2 at 2 MPa to a maximum of 4.56 mAh.cm−2 at 10 MPa, before dropping significantly to 2.44 mAh·cm−2 at 20 MPa due to pressure induced fracture. | [59] |
| Full cell Zn//ZnI2 | DCHE—Dual-Confinement Hydrogel Electrolyte | Initial OCV: ~1.3 V Voltage Drift: Minimal voltage drift with 93.5% capacity retention after 48 h open-circuit stand. | Stability: The cell demonstrated exceptional long-term stability over 6000 h (2500 cycles), maintaining high operational integrity throughout the test. Capacity: The cell achieved an outstanding capacity retention of 88.9% when cycled at a current density of 100 mA.g−1. | [60] |
| Full cell Na//ZnCl2 | Dual-phase (β″—Al2O3 solid electrolyte + NaAlCl4 molten salt catholyte) | Initial OCV: ~1.92 V–2.13 V (dependent on operating temperature). Voltage Drift: Stable charge/discharge plateaus observed over 56 cycles at 280 °C with minimal polarization growth. Performance degradation and increased polarization were significantly more rapid at 240 °C due to the absence of beneficial liquid-phase formation. | Stability: Stable performance attributed to liquid-phase formation (NaCl-ZnCl2) that suppresses Zn and NaCl particle growth. Cells operated at 240 °C showed significantly faster degradation due to solid-state reactions only. Capacity: ~65 mAh (cycling between 48 and 90% SOC) and ~110 mAh (deep cycling between 20 and 90% SOC) at 280 °C. active cell area: 3 cm2 | [61] |
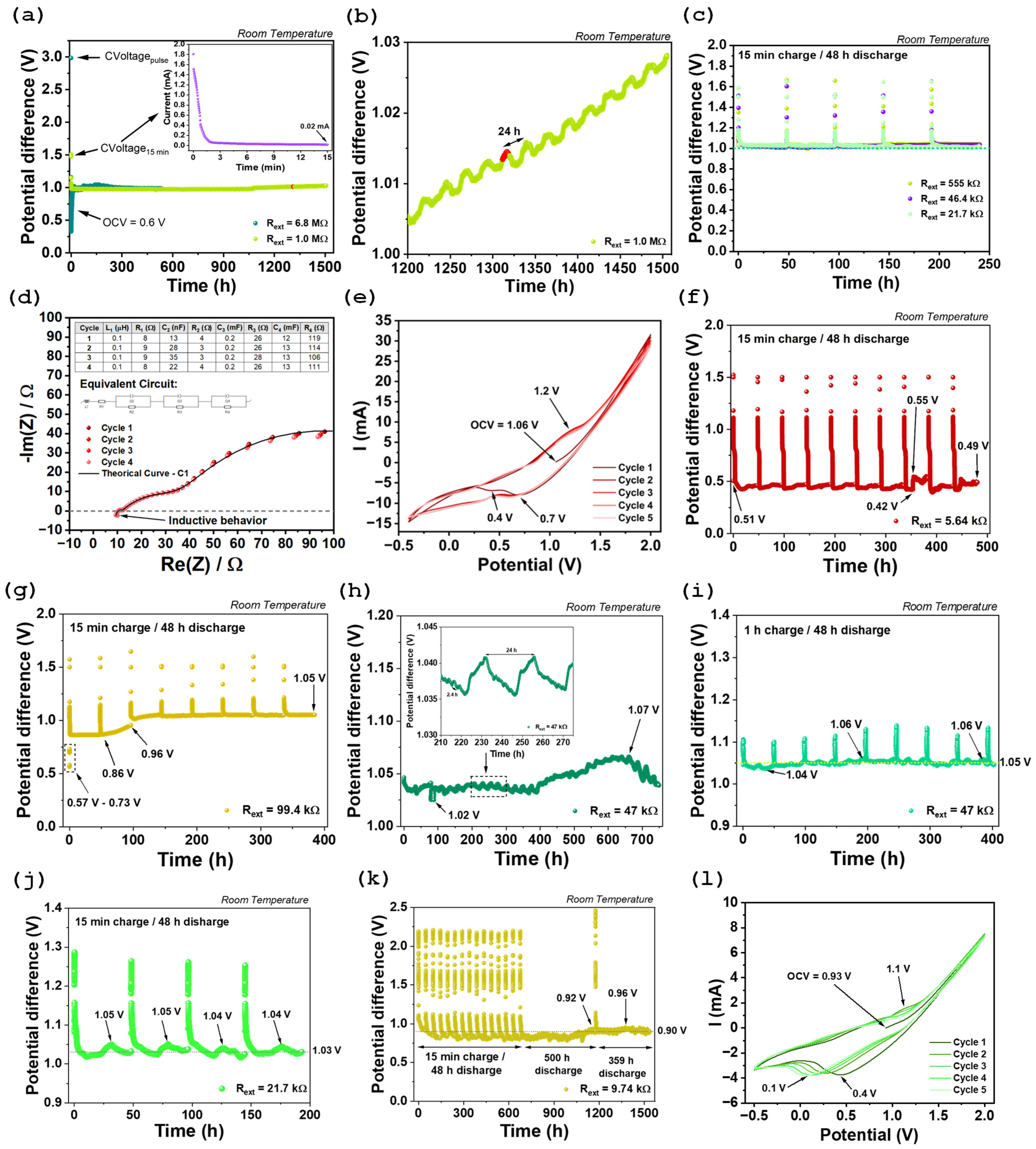
| Electrodeless Cell (Al//Inconel 625) | Na2.99Ba0.005ClO The capacity of the cell is solely dependent on the sodium concentration on the electrolyte | Initial OCV: ~1.06 V (after assembly). Voltage Drift: Stable discharge plateau at ~1.1 V observed for months under different resistances; after one year, OCV decayed to ~0.93 V. Self-charging events observed during discharge (e.g., voltage increased from 0.79 V to 0.94 V under 9.74 kΩ load). | Stability: Cell remained functional for over one year under continuous and sequential discharge through various external resistors, demonstrating exceptional long-term stability. Inconel 625 showed no significant degradation; aluminum exhibited superficial mechanical degradation due to residual moisture. Capacity: Discharge capacity of 35 mAh (~2.3 mAh.cm−2) under 9.47 kΩ load. | This work |
2. Results and Discussion
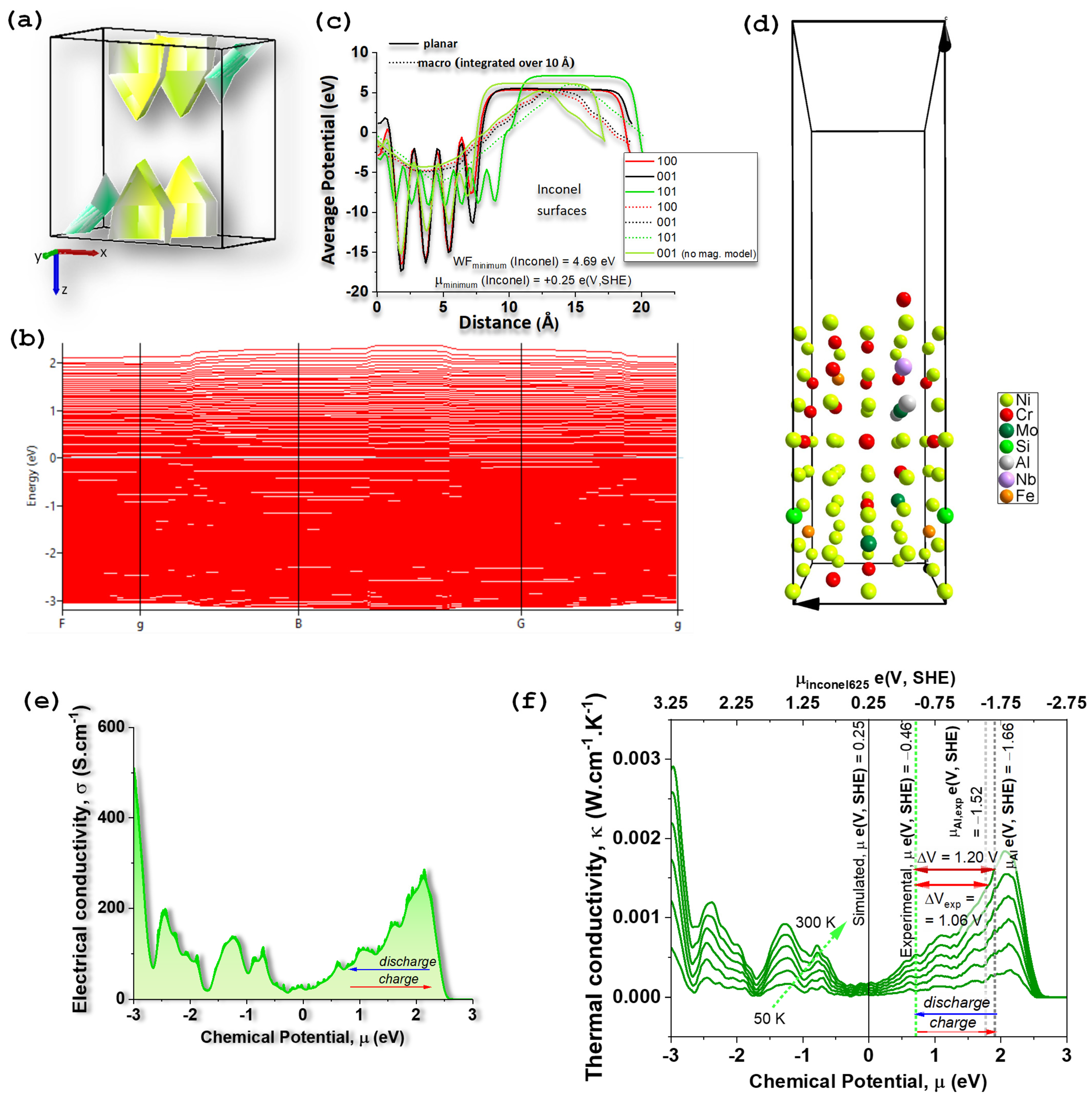
2.1. Characterization of Inconel 625 Surface by SKP
2.2. First Principles Simulations for INCONEL625: Electrical, Thermal, and Potential Properties
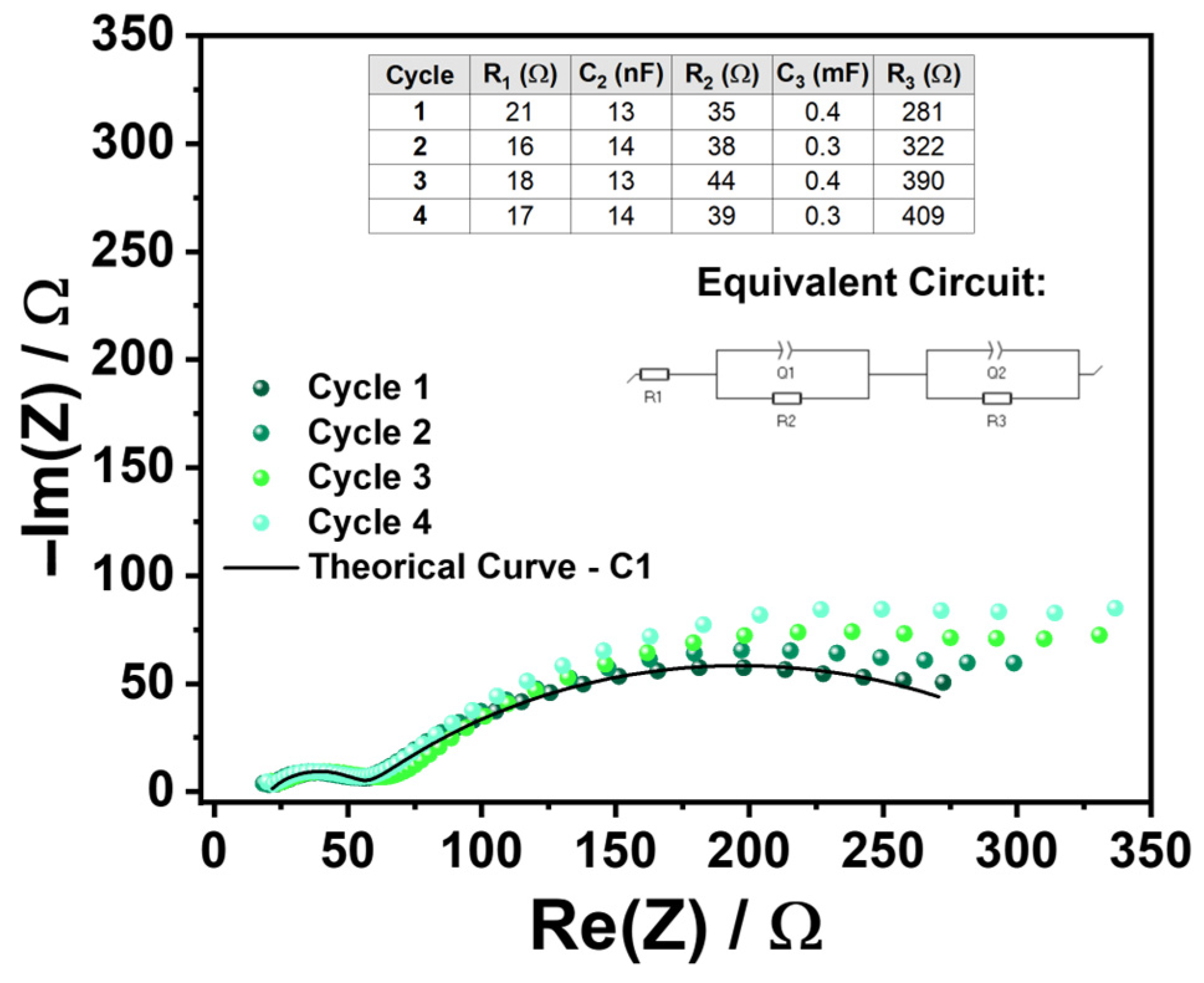
2.3. Electrochemical Performance of the Device
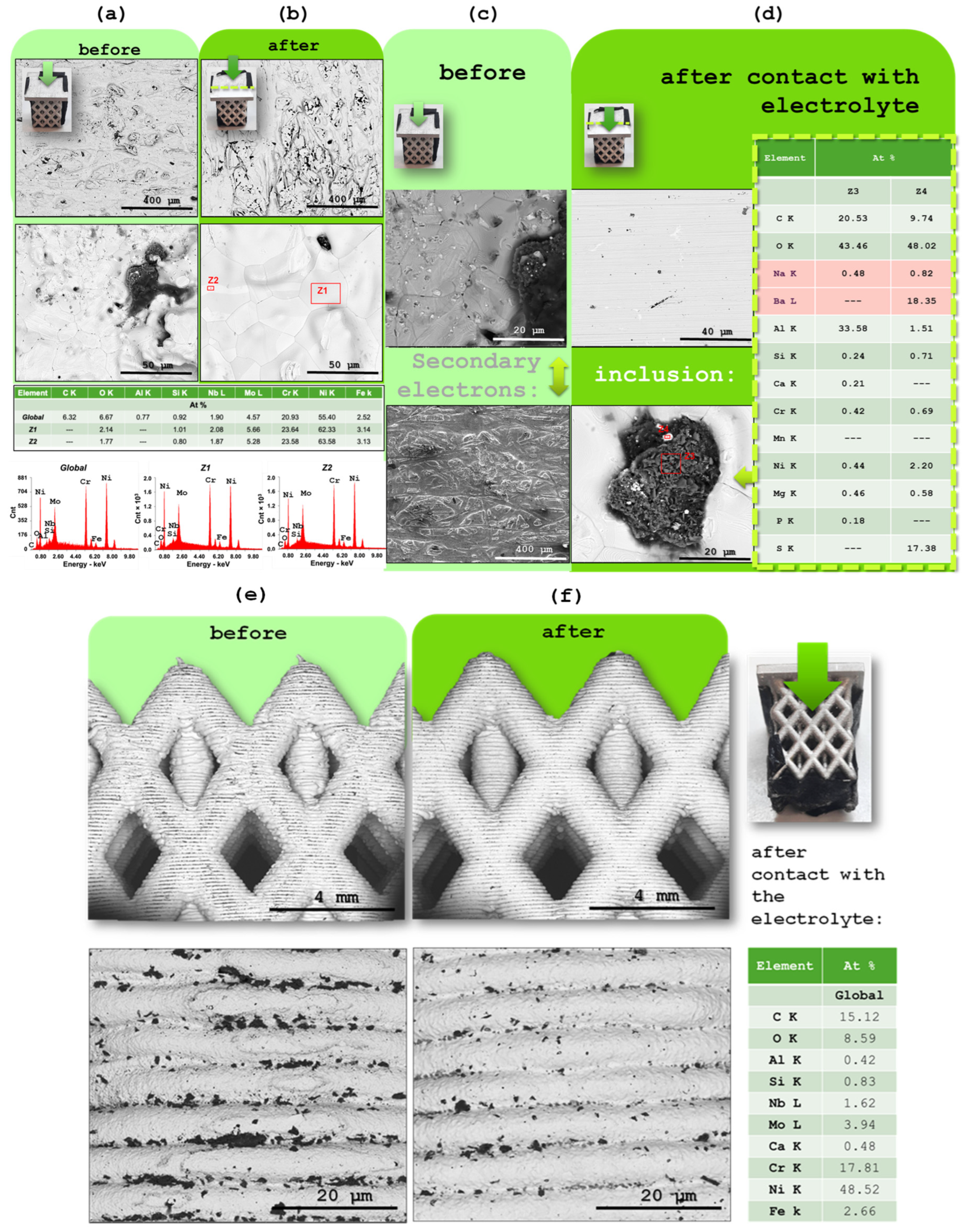
2.4. SKP Evaluation of Inconel 625 Surface After Electrolyte Contact
2.5. SEM/EDX Characterization
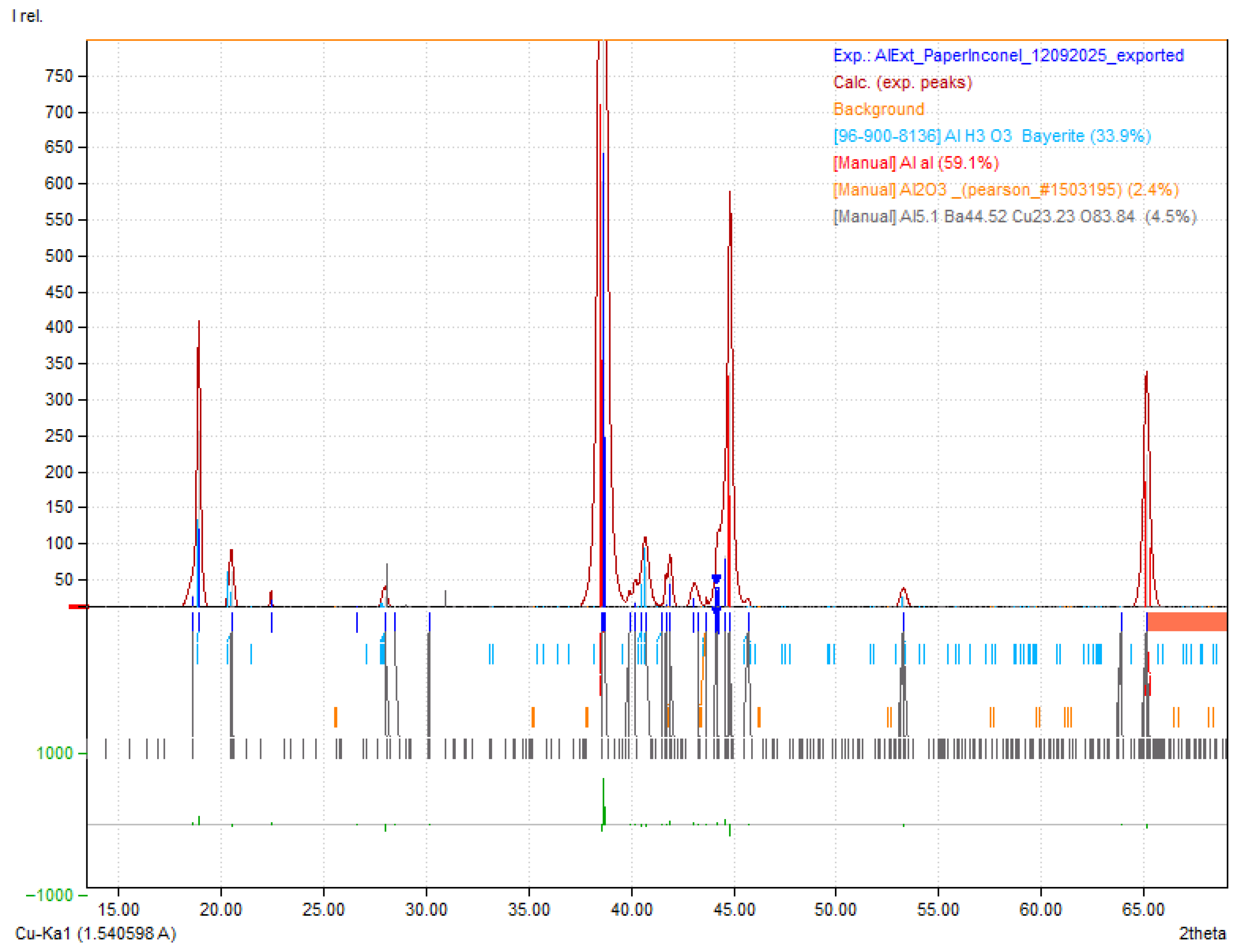
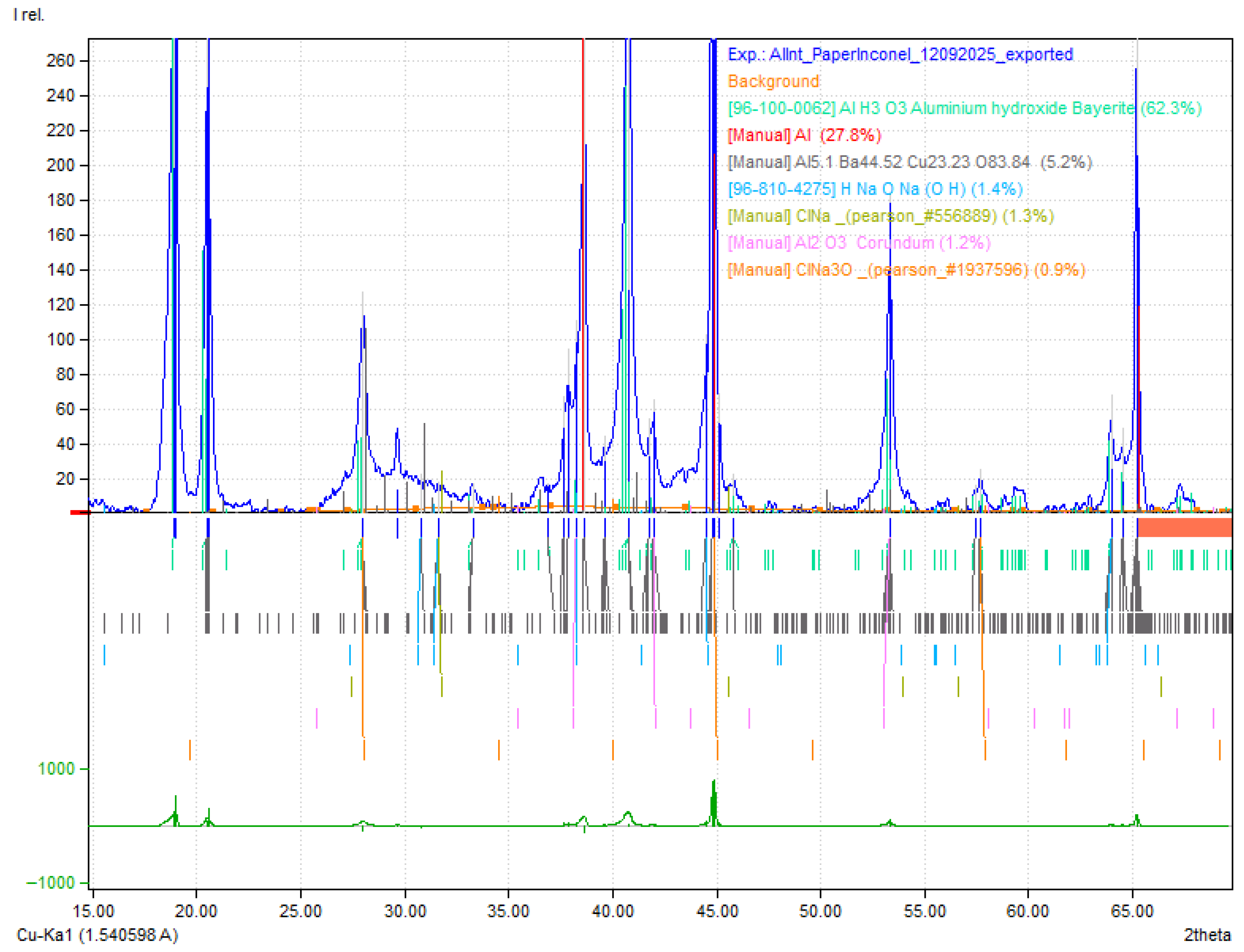
2.6. XRD Characterization of the Aluminum
3. Materials and Methods
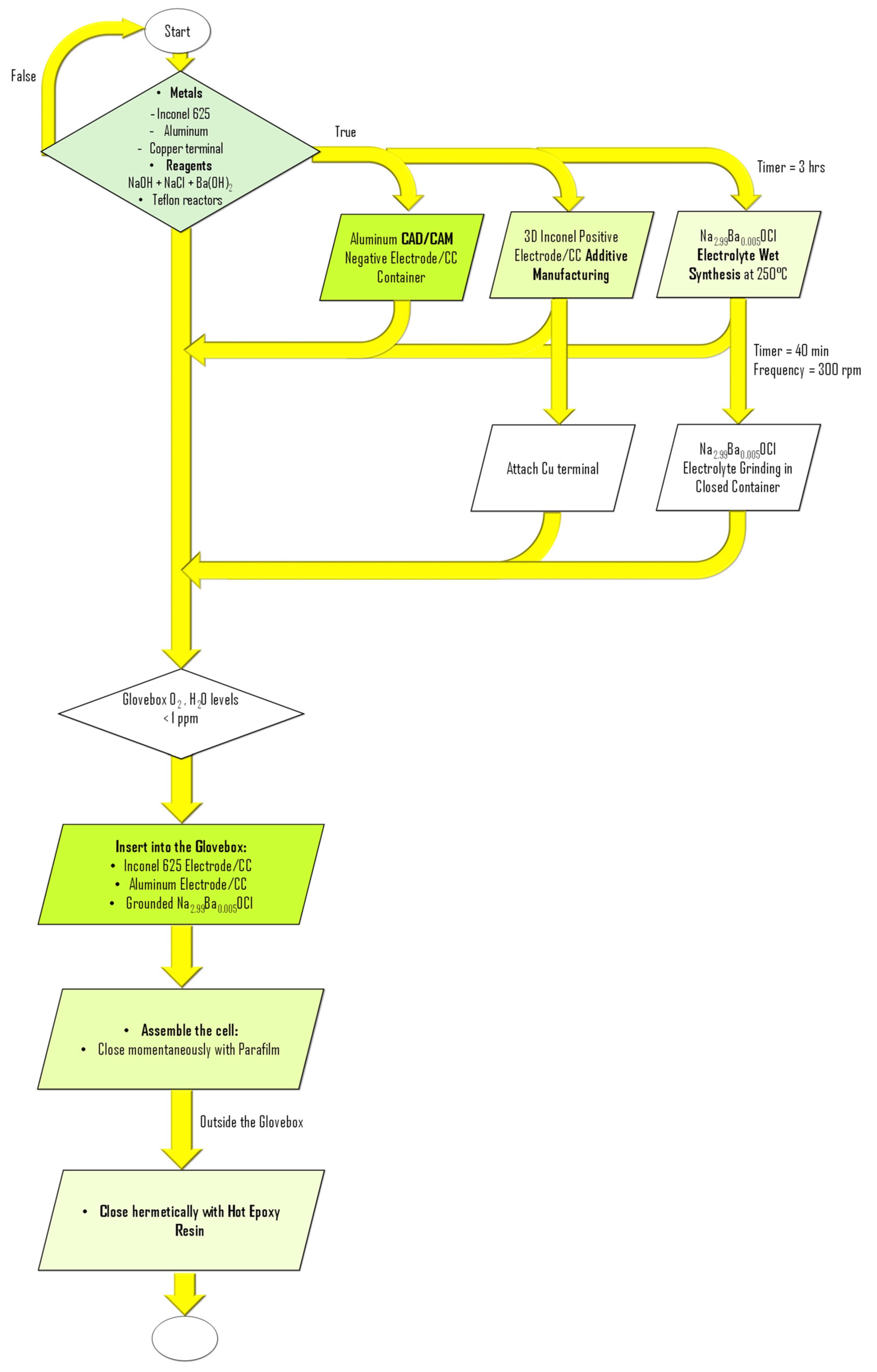
3.1. Inconel 625 and Aluminum Container
3.2. Solid-State Electrolyte: Na2.99Ba0.005OCl
3.3. Battery Cell Assembly
3.4. Scanning Kelvin Probe (SKP)
3.5. Electrochemical Performance Measurements
3.6. SEM/EDX Analysis and Samples Preparation
3.7. X-Ray Diffraction (XRD) Analyses
3.8. First Principles Simulations for INCONEL 625
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Summary of the 64 Discharge Cycles of the Device (6477 h in Total) After a 15 min Constant Voltage Charge (Except for 6.8 MΩ Resistor) A = 15 cm2 | |||||
| External Resistors (kΩ) | Cycle | Discharge Time (h) | Average Discharge Potential (V) | Average Discharge Current (mA) | Discharge Capacity (mA.h) |
| 6800 | 1st | 532 | 1.00 | 0.0001 | 0.078 |
| 1000 | 2nd | 1505 | 0.98 | 0.001 | 1.484 |
| 555 | 3rd | 48 | 1.03 | 0.002 | 0.089 |
| 4th | 48 | 1.03 | 0.002 | 0.089 | |
| 5th | 48 | 1.03 | 0.002 | 0.089 | |
| 6th | 48 | 1.04 | 0.002 | 0.090 | |
| 7th | 48 | 1.04 | 0.002 | 0.090 | |
| 46.4 | 8th | 48 | 1.02 | 0.022 | 1.057 |
| 9th | 48 | 1.02 | 0.022 | 1.051 | |
| 10th | 48 | 1.02 | 0.022 | 1.058 | |
| 11th | 48 | 1.03 | 0.022 | 1.066 | |
| 12th | 48 | 1.04 | 0.022 | 1.072 | |
| 21.7 | 13th | 48 | 1.03 | 0.048 | 2.287 |
| 14th | 48 | 1.03 | 0.047 | 2.277 | |
| 15th | 48 | 1.02 | 0.047 | 2.267 | |
| 16th | 48 | 1.02 | 0.047 | 2.256 | |
| 17th | 48 | 1.02 | 0.047 | 2.253 | |
| 5.64 | 18th | 48 | 0.47 | 0.084 | 4.019 |
| 19th | 48 | 0.48 | 0.085 | 4.064 | |
| 20th | 48 | 0.47 | 0.084 | 4.038 | |
| 21st | 48 | 0.48 | 0.084 | 4.051 | |
| 22nd | 48 | 0.48 | 0.085 | 4.065 | |
| 23rd | 48 | 0.47 | 0.084 | 4.013 | |
| 24th | 48 | 0.46 | 0.082 | 3.956 | |
| 25th | 48 | 0.51 | 0.090 | 4.317 | |
| 26th | 48 | 0.46 | 0.082 | 3.918 | |
| 27th | 48 | 0.49 | 0.086 | 4.149 | |
| 99.4 | 28th | 48 | 0.86 | 0.009 | 0.417 |
| 29th | 48 | 0.90 | 0.009 | 0.435 | |
| 30th | 48 | 1.04 | 0.010 | 0.501 | |
| 31st | 48 | 1.04 | 0.011 | 0.504 | |
| 32nd | 48 | 1.05 | 0.011 | 0.507 | |
| 33rd | 48 | 1.05 | 0.011 | 0.508 | |
| 34th | 48 | 1.05 | 0.011 | 0.509 | |
| 35th | 48 | 1.05 | 0.011 | 0.509 | |
| 47.0 | 36th | 749 | 1.05 | 0.022 | 16.641 |
| 37th | 48 | 1.04 | 0.022 | 1.063 | |
| 38th | 48 | 1.05 | 0.022 | 1.068 | |
| 39th | 48 | 1.05 | 0.022 | 1.073 | |
| 40th | 48 | 1.05 | 0.022 | 1.075 | |
| 41st | 48 | 1.05 | 0.022 | 1.077 | |
| 42nd | 48 | 1.05 | 0.022 | 1.076 | |
| 43rd | 48 | 1.05 | 0.022 | 1.071 | |
| 44th | 48 | 1.05 | 0.022 | 1.075 | |
| 21.7 | 45th | 48 | 1.03 | 0.048 | 2.287 |
| 46th | 48 | 1.04 | 0.048 | 2.300 | |
| 47th | 48 | 1.03 | 0.048 | 2.291 | |
| 48th | 48 | 1.04 | 0.048 | 2.290 | |
| 9.74 | 49th | 48 | 0.96 | 0.099 | 4.738 |
| 50th | 48 | 0.90 | 0.092 | 4.411 | |
| 51st | 48 | 0.86 | 0.088 | 4.224 | |
| 52nd | 48 | 0.88 | 0.090 | 4.331 | |
| 53rd | 48 | 0.89 | 0.091 | 4.386 | |
| 54th | 48 | 0.85 | 0.088 | 4.211 | |
| 55th | 48 | 0.86 | 0.089 | 4.250 | |
| 56th | 48 | 0.86 | 0.088 | 4.226 | |
| 57th | 48 | 0.86 | 0.088 | 4.237 | |
| 58th | 48 | 0.84 | 0.086 | 4.134 | |
| 59th | 48 | 0.87 | 0.089 | 4.270 | |
| 60th | 48 | 0.86 | 0.088 | 4.239 | |
| 61st | 48 | 0.84 | 0.087 | 4.164 | |
| 62nd | 48 | 0.85 | 0.087 | 4.179 | |
| 63rd | 500 | 0.84 | 0.087 | 43.342 | |
| 64th | 359 | 0.91 | 0.094 | 33.546 | |
References
- Meinhold, R.; Wagner, C.; Dhar, B.K. Digital Sustainability and Eco-environmental Sustainability: A Review of Emerging Technologies, Resource Challenges, and Policy Implications. Sustain. Dev. 2025, 33, 2323–2338. [Google Scholar] [CrossRef]
- Blanco, C.F.; Behrens, P.; Vijver, M.; Peijnenburg, W.; Quik, J.; Cucurachi, S. A Framework for Guiding Safe and Sustainable-by-design Innovation. J. Ind. Ecol. 2025, 29, 47–65. [Google Scholar] [CrossRef]
- Timilsina, R.R.; Zhang, J.; Rahut, D.B.; Patradool, K.; Sonobe, T. Global Drive toward Net-Zero Emissions and Sustainability via Electric Vehicles: An Integrative Critical Review. Energy Ecol. Env. 2025, 10, 125–144. [Google Scholar] [CrossRef]
- Ndhlovu, E.; Mhlanga, D.; Duri, B. Decarbonising Urban Transport: An Overview of Electric Vehicles, Public Transport, and Sustainable Infrastructure in Achieving Net-Zero Emissions. Discov. Glob. Soc. 2025, 3, 53. [Google Scholar] [CrossRef]
- Szumska, E.M. Comprehensive Review of Life Cycle Assessment Methodologies for Passenger Vehicles. J. Sustain. Dev. Transp. Logist. 2024, 9, 53–71. [Google Scholar] [CrossRef]
- Baars, J.; Cerdas, F.; Heidrich, O. An Integrated Model to Conduct Multi-Criteria Technology Assessments: The Case of Electric Vehicle Batteries. Environ. Sci. Technol. 2023, 57, 5056–5067. [Google Scholar] [CrossRef]
- Wang, H.; Pan, Y.; Liu, X.; Cao, Y.; Liu, Y.; Zhang, X.; Mao, Y.; Liu, B. Criteria and Design Guidance for Lithium-Ion Battery Safety from a Material Perspective. J. Mater. Chem. A Mater. 2022, 10, 6538–6550. [Google Scholar] [CrossRef]
- Granvik, P.; Hanski, J.; Lähdesmäki, S.; Jokilaakso, A.; Huttunen-Saarivirta, E. Critical Raw Materials for Green Transition: Key Parameters and Feasibility Index for Sufficiency. Resour. Conserv. Recycl. 2025, 218, 108197. [Google Scholar] [CrossRef]
- Lehtimäki, H.; Karhu, M.; Kotilainen, J.M.; Sairinen, R.; Jokilaakso, A.; Lassi, U.; Huttunen-Saarivirta, E. Sustainability of the Use of Critical Raw Materials in Electric Vehicle Batteries: A Transdisciplinary Review. Environ. Chall. 2024, 16, 100966. [Google Scholar] [CrossRef]
- Lyu, W.; Fu, H.; Rao, A.M.; Lu, Z.; Yu, X.; Lin, Y.; Zhou, J.; Lu, B. Permeable Void-Free Interface for All-Solid-State Alkali-Ion Polymer Batteries. Sci. Adv. 2024, 10, eadr9602. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Li, C.; Tian, F.; Qiao, Z.; Lei, D.; Wang, C. Facilitating Uniform Lithium-Ion Transport via Polymer-Assisted Formation of Unique Interfaces to Achieve a Stable 4.7 V Li Metal Battery. Natl. Sci. Rev. 2025, 12, nwaf182. [Google Scholar] [CrossRef]
- Jing, M.; Yang, H.; Han, C.; Chen, F.; Yuan, W.; Ju, B.; Tu, F.; Shen, X.; Qin, S. Improving Room-Temperature Electrochemical Performance of Solid-State Lithium Battery by Using Electrospun La2Zr2O7 Fibers-Filled Composite Solid Electrolyte. Ceram. Int. 2019, 45, 18614–18622. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, M.; Yang, H.; Liu, Q.; Chen, F.; Yuan, W.; Liu, M.; Ji, Y.; Shen, X. Highly Efficient Interface Modification between Poly(Propylene Carbonate)-Based Solid Electrolytes and a Lithium Anode by Facile Graphite Coating. ACS Sustain. Chem. Eng. 2020, 8, 17106–17115. [Google Scholar] [CrossRef]
- Yang, T.; Luo, D.; Liu, Y.; Yu, A.; Chen, Z. Anode-Free Sodium Metal Batteries as Rising Stars for Lithium-Ion Alternatives. iScience 2023, 26, 105982. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H. Sulfide-Based Anode-Free Solid-State Batteries: Key Challenges and Emerging Solutions. ACS Energy Lett. 2025, 10, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.C.; Gomes, B.M.; Vale, A.B.; Braga, M.H. In-Series All-Solid-State Anode-Less Cells. J. Energy Storage 2024, 102, 113983. [Google Scholar] [CrossRef]
- Baptista, M.C.; Gomes, B.M.; Capela, D.; Ferreira, M.F.S.; Guimarães, D.; Silva, N.A.; Jorge, P.A.S.; Silva, J.J.; Braga, M.H. Conditioning Solid-State Anode-Less Cells for the Next Generation of Batteries. Batteries 2023, 9, 402. [Google Scholar] [CrossRef]
- Tang, K.; Tian, L.; Zhang, Y.; Xu, Z.J. Anode-Free Lithium Metal Batteries: A Promising Flexible Energy Storage System. J. Mater. Chem. A Mater. 2024, 12, 16268–16292. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, K.; Krishnamurthy, N. A Review on Conventional to Bipolar Design of Anode Less All Solid-State Batteries. Energy Adv. 2024, 3, 1222–1237. [Google Scholar] [CrossRef]
- Duan, X.; Sun, J.; Shi, L.; Dong, S.; Cui, G. Exploring the Active Lithium Loss in Anode-Free Lithium Metal Batteries: Mechanisms, Challenges, and Strategies. Interdiscip. Mater. 2025, 4, 217–234. [Google Scholar] [CrossRef]
- Pal, S.; Zhang, X.; Babu, B.; Lin, X.; Wang, J.; Vlad, A. Materials, Electrodes and Electrolytes Advances for next-Generation Lithium-Based Anode-Free Batteries. Oxf. Open Mater. Sci. 2022, 2, itac005. [Google Scholar] [CrossRef]
- Lal, M.S.; Albertus, P.; Noked, M. Anode-Less Sulfide-Based All-Solid-State Batteries: Interfacial Challenges, Material Strategies, and Future Prospects. Small 2025, e10624. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Chen, W.; Tour, J.M. What Can Be Expected from “Anode-Free” Lithium Metal Batteries? Adv. Energy Sustain. Res. 2021, 2, 2000110. [Google Scholar] [CrossRef]
- Yanagihara, S.; Huebner, J.; Huang, Z.; Inoishi, A.; Akamatsu, H.; Hayashi, K.; Ohno, S. Compatibility of Halide Electrolytes in Solid-State Li–S Battery Cathodes. Chem. Mater. 2024, 37, 109–118. [Google Scholar] [CrossRef]
- Molaiyan, P.; Bhattacharyya, S.; dos Reis, G.S.; Sliz, R.; Paolella, A.; Lassi, U. Towards Greener Batteries: Sustainable Components and Materials for next-Generation Batteries. Green. Chem. 2024, 26, 7508–7531. [Google Scholar] [CrossRef]
- Titirici, M.; Baird, S.G.; Sparks, T.D.; Yang, S.M.; Brandt-Talbot, A.; Hosseinaei, O.; Harper, D.P.; Parker, R.M.; Vignolini, S.; Berglund, L.A. The Sustainable Materials Roadmap. J. Phys. Mater. 2022, 5, 032001. [Google Scholar] [CrossRef]
- Panagiotopoulou, V.C.; Stavropoulos, P.; Chryssolouris, G. A Critical Review on the Environmental Impact of Manufacturing: A Holistic Perspective. Int. J. Adv. Manuf. Technol. 2022, 118, 603–625. [Google Scholar] [CrossRef]
- Gilich, J.; Meschut, G.; Schiemann, T.; Purrio, M. Towards an Efficient Life Cycle Assessment of Production Processes for Mechanical Fastening Elements. Int. J. Life Cycle Assess. 2025, 1–22. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Mahapatra, S.D.; Mandal, N.K. Advancements and Challenges in Additive Manufacturing: A Comprehensive Review. Eng. Res. Express 2024, 6, 012505. [Google Scholar] [CrossRef]
- Patel, S.; Liu, Y.; Siddique, Z.; Ghamarian, I. Metal Additive Manufacturing: Principles and Applications. J. Manuf. Process 2024, 131, 1179–1201. [Google Scholar] [CrossRef]
- Segovia-Guerrero, L.; Baladés, N.; Gallardo-Galán, J.J.; Gil-Mena, A.J.; Sales, D.L. Additive vs. Subtractive Manufacturing: A Comparative Life Cycle and Cost Analyses of Steel Mill Spare Parts. J. Manuf. Mater. Process. 2025, 9, 138. [Google Scholar] [CrossRef]
- Tan, C.; Li, R.; Su, J.; Du, D.; Du, Y.; Attard, B.; Chew, Y.; Zhang, H.; Lavernia, E.J.; Fautrelle, Y. Review on Field Assisted Metal Additive Manufacturing. Int. J. Mach. Tools Manuf. 2023, 189, 104032. [Google Scholar] [CrossRef]
- Vafadar, A.; Guzzomi, F.; Rassau, A.; Hayward, K. Advances in Metal Additive Manufacturing: A Review of Common Processes, Industrial Applications, and Current Challenges. Appl. Sci. 2021, 11, 1213. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Zhang, Y.; Bose, S. Recent Developments in Metal Additive Manufacturing. Curr. Opin. Chem. Eng. 2020, 28, 96–104. [Google Scholar] [CrossRef]
- Costa, J.; Sequeiros, E.; Vieira, M.T.; Vieira, M. Additive Manufacturing: Material Extrusion of Metallic Parts. U. Porto J. Eng. 2021, 7, 53–69. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Fenollosa-Artés, F.; Minguella-Canela, J. Three-Dimensional Printing of Metallic Parts by Means of Fused Filament Fabrication (FFF). Metals 2024, 14, 1291. [Google Scholar] [CrossRef]
- Costa, J.M.; Sequeiros, E.W.; Vieira, M.F. Fused Filament Fabrication for Metallic Materials: A Brief Review. Materials 2023, 16, 7505. [Google Scholar] [CrossRef]
- Jacob, J.; Pejak Simunec, D.; Kandjani, A.E.Z.; Trinchi, A.; Sola, A. A Review of Fused Filament Fabrication of Metal Parts (Metal FFF): Current Developments and Future Challenges. Technologies 2024, 12, 267. [Google Scholar] [CrossRef]
- Gebisa, A.W.; Lemu, H.G. Design for Manufacturing to Design for Additive Manufacturing: Analysis of Implications for Design Optimality and Product Sustainability. Procedia Manuf. 2017, 13, 724–731. [Google Scholar] [CrossRef]
- Costa, J.M.; Sequeiros, E.W.; Figueiredo, D.; Reis, A.R.; Vieira, M.F. Optimizing Metal AM Potential through DfAM: Design, Performance, and Industrial Impact. In Additive Manufacturing-Present and Sustainable Future, Materials and Applications; IntechOpen: London, UK, 2024; Volume 11. [Google Scholar]
- Khan, N.; Riccio, A. A Systematic Review of Design for Additive Manufacturing of Aerospace Lattice Structures: Current Trends and Future Directions. Prog. Aerosp. Sci. 2024, 149, 101021. [Google Scholar] [CrossRef]
- Liu, F.; Chen, M.; Liu, S.; Xiang, Z.; Huang, S.; Lim, E.G.; Zhang, S. Stress-Driven Generative Design and Numerical Assessment of Customized Additive Manufactured Lattice Structures. Mater. Des. 2024, 241, 112956. [Google Scholar] [CrossRef]
- Arefin, N.; Moni, H.-E.; Espinosa, D.; Cong, W.; Zeng, M. Multi-Material Additive Manufacturing of Energy Storage and Conversion Devices: Recent Progress and Future Prospects. Appl. Phys. Rev. 2025, 12, 011330. [Google Scholar] [CrossRef]
- Jia, L.; Zhu, J.; Zhang, X.; Guo, B.; Du, Y.; Zhuang, X. Li–Solid Electrolyte Interfaces/Interphases in All-Solid-State Li Batteries. Electrochem. Energy Rev. 2024, 7, 12. [Google Scholar] [CrossRef]
- Li, W.; Bao, Z.; Du, Q.; Xu, Y.; Jiao, K. Open-Source CFD Elucidating Mechanism of 3D Pillar Electrode in Improving All-Solid-State Battery Performance. Adv. Sci. 2022, 9, 2105454. [Google Scholar] [CrossRef]
- De, P.; Pumera, M. Aqueous Multivalent Metal-ion Batteries: Toward 3D-printed Architectures. Small 2024, 20, 2404227. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bucci, G.; Brady, N.W.; Cross, N.R.; Ehlinger, V.M.; Lin, T.Y.; Salazar de Troya, M.; Tortorelli, D.; Worsley, M.A.; Roy, T. Topology Optimization for the Full-Cell Design of Porous Electrodes in Electrochemical Energy Storage Devices. Struct. Multidiscip. Optim. 2024, 67, 188. [Google Scholar] [CrossRef]
- Hariharan, M.K.; Anderson, A.; Raghavan, K.; Nithya, S. Hot Corrosion Behaviour of Hastelloy X and Inconel 625 in an Aggressive Environment for Superalloys for High-Temperature Energy Applications. Appl. Nanosci. 2023, 13, 3359–3368. [Google Scholar] [CrossRef]
- Condruz, M.R.; Matache, G.; Paraschiv, A.; Badea, T.; Badilita, V. High Temperature Oxidation Behavior of Selective Laser Melting Manufactured IN 625. Metals 2020, 10, 668. [Google Scholar] [CrossRef]
- Kumar, M.P.; Manikandan, M. Insights into the Surface Behavior of Inconel 617 and Inconel 625 Material in Molten Salt. Mater. Lett. 2023, 333, 133679. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Zhang, G.; Xue, H.; Cui, M.; Wang, W.; Liu, D. Hot Corrosion Behavior of Inconel 625 in Na2SO4 and V2O5 Molten Salt System. Metals 2023, 13, 1069. [Google Scholar] [CrossRef]
- Ferraresi, R.; Avanzini, A.; Cecchel, S.; Petrogalli, C.; Cornacchia, G. Microstructural, Mechanical, and Tribological Evolution under Different Heat Treatment Conditions of Inconel 625 Alloy Fabricated by Selective Laser Melting. Adv. Eng. Mater. 2022, 24, 2100966. [Google Scholar] [CrossRef]
- Rubino, F.; Merino, D.; Munez, C.; Poza, P. Evaluating the Corrosion Resistance of Inconel 625 Coatings, Processed by Compact Plasma Spray, for Applications in Concentrating Solar Power Plants. Key Eng. Mater. 2022, 926, 1736–1745. [Google Scholar] [CrossRef]
- Rademacher, L.; Häcker, J.; Blázquez, J.A.; Nojabaee, M.; Friedrich, K.A. Corrosion Study of Current Collectors for Magnesium Batteries. Batter. Supercaps 2025, 8, e202400392. [Google Scholar] [CrossRef]
- Braga, M.H. Energy Harnessing and Storage from Surface Switching with a Ferroelectric Electrolyte. Chem. Commun. 2024, 60, 5395–5398. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Baptista, M.; Khalifa, H.; Araújo, A.; Maia, B.A.; Souto, M.; Braga, M.H. Giant Polarization in Quasi-Adiabatic Ferroelectric Na+ Electrolyte for Solid-State Energy Harvesting and Storage. Adv. Funct. Mater. 2023, 33, 2212344. [Google Scholar] [CrossRef]
- Freitas, Â.; Baptista, M.C.; Braga, M.H. Sustainable Solid-State Sodium-Ion Batteries Featuring Ferroelectric Electrolytes. Int. J. Mol. Sci. 2024, 25, 12694. [Google Scholar] [CrossRef]
- Rafique, A.; Fallarino, L.; Accardo, G.; Pesce, A.; Bonilla, F.; Zhang, Y.; Lanceros-Mendez, S.; Casas-Cabanas, M.; López-Aranguren, P. Interfacial Analysis of In-Situ Anode Formation in Solid-State Batteries with Nanometric Current Collector. Chem. Eng. J. 2025, 509, 160956. [Google Scholar] [CrossRef]
- Park, S.H.; Ayyaswamy, A.; Gjerde, J.; Andrews, W.B.; Vishnugopi, B.S.; Drakopoulos, M.; Vo, N.T.; Zhong, Z.; Thornton, K.; Mukherjee, P.P.; et al. Filament-Induced Failure in Lithium-Reservoir-Free Solid-State Batteries. ACS Energy Lett. 2025, 10, 1174–1182. [Google Scholar] [CrossRef]
- Huang, X.; Pan, T.; Zhang, B.; Wang, J.; Hu, T.; Duan, A.; Luo, S.; Zhao, B.; Li, M.; Lin, Y.; et al. Functionally Segregated Ion Regulation Enables Dual Confinement Effect for Highly Stable Zinc-Iodine Batteries. Adv. Mater. 2025, 37, 2500500. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. A Novel Low-Cost Sodium–Zinc Chloride Battery. Energy Env. Sci. 2013, 6, 1837–1843. [Google Scholar] [CrossRef]
- Braga, M.H.; Grundish, N.S.; Murchison, A.J.; Goodenough, J.B. Thermodynamic Considerations of Same-Metal Electrodes in an Asymmetric Cell. Mater. Theory 2019, 3, 1. [Google Scholar] [CrossRef]
- Trasa’tti, S. The Absolute Electrode Potential: An Explanatory Note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Guerreiro, A.N.; Baptista, M.C.; Maia, B.A.; Braga, M.H. Interfacial Chemistry with ZnO: In Operando Work Functions in Hetero Cells. ACS Appl. Energy Mater. 2022, 5, 9811–9822. [Google Scholar] [CrossRef]
- Prior, T.; Figueira, J.; Freitas, Â.; Carvalho, D.; Gomes, B.M.; Baptista, M.C.; Lebre, H.; Martins, R.; Pereira, L.; Pinto, J.V.; et al. Surface Morphology and Electrochemical Behavior of Microstructured Cu Electrodes in All-Solid-State Sodium Batteries. Molecules 2025, 30, 3493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, G.; Mei, D.; Zhao, C.; Li, L.; Wang, Y. Stimuli-Responsive Actuators in Water Environment: A Review and Future Research Agenda. Int. J. Extrem. Manuf. 2024, 7, 022013. [Google Scholar] [CrossRef]
- Wieland, F.; Sokolov, A.P.; Böhmer, R.; Gainaru, C. Transient Nonlinear Response of Dynamically Decoupled Ionic Conductors. Phys. Rev. Lett. 2018, 121, 064503. [Google Scholar] [CrossRef]
- Danzi, F.; Camanho, P.P.; Braga, M.H. An All-Solid-State Coaxial Structural Battery Using Sodium-Based Electrolyte. Molecules 2021, 26, 5226. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- MedeA, Version 3.6; Materials Design, Inc.: San Diego, CA, USA, 2022.
- Home|Materials Design Inc. Available online: https://www.materialsdesign.com/ (accessed on 5 August 2025).



| Element: | Al Container | Inconel + Cu Tab | Na2.99Ba0.005OCl | Total Device |
| Mass (g): | 46.871 | 19.454 | 27.878 | 94.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, M.C.; Vale, A.B.; Costa, J.M.; Braga, M.H. Robust All-Solid-State Batteries with Sodium Ion Electrolyte, Aluminum and Additive Manufacturing Inconel 625 Electrodes. Molecules 2025, 30, 4465. https://doi.org/10.3390/molecules30224465
Baptista MC, Vale AB, Costa JM, Braga MH. Robust All-Solid-State Batteries with Sodium Ion Electrolyte, Aluminum and Additive Manufacturing Inconel 625 Electrodes. Molecules. 2025; 30(22):4465. https://doi.org/10.3390/molecules30224465
Chicago/Turabian StyleBaptista, Manuela C., Antonio B. Vale, Jose M. Costa, and Maria Helena Braga. 2025. "Robust All-Solid-State Batteries with Sodium Ion Electrolyte, Aluminum and Additive Manufacturing Inconel 625 Electrodes" Molecules 30, no. 22: 4465. https://doi.org/10.3390/molecules30224465
APA StyleBaptista, M. C., Vale, A. B., Costa, J. M., & Braga, M. H. (2025). Robust All-Solid-State Batteries with Sodium Ion Electrolyte, Aluminum and Additive Manufacturing Inconel 625 Electrodes. Molecules, 30(22), 4465. https://doi.org/10.3390/molecules30224465







