Paramagnetic Agents for SE DNP: Synthesis and ESR Characterization of New Lipophilic Derivatives of Finland Trityl
Abstract
1. Introduction
2. Results and Discussion
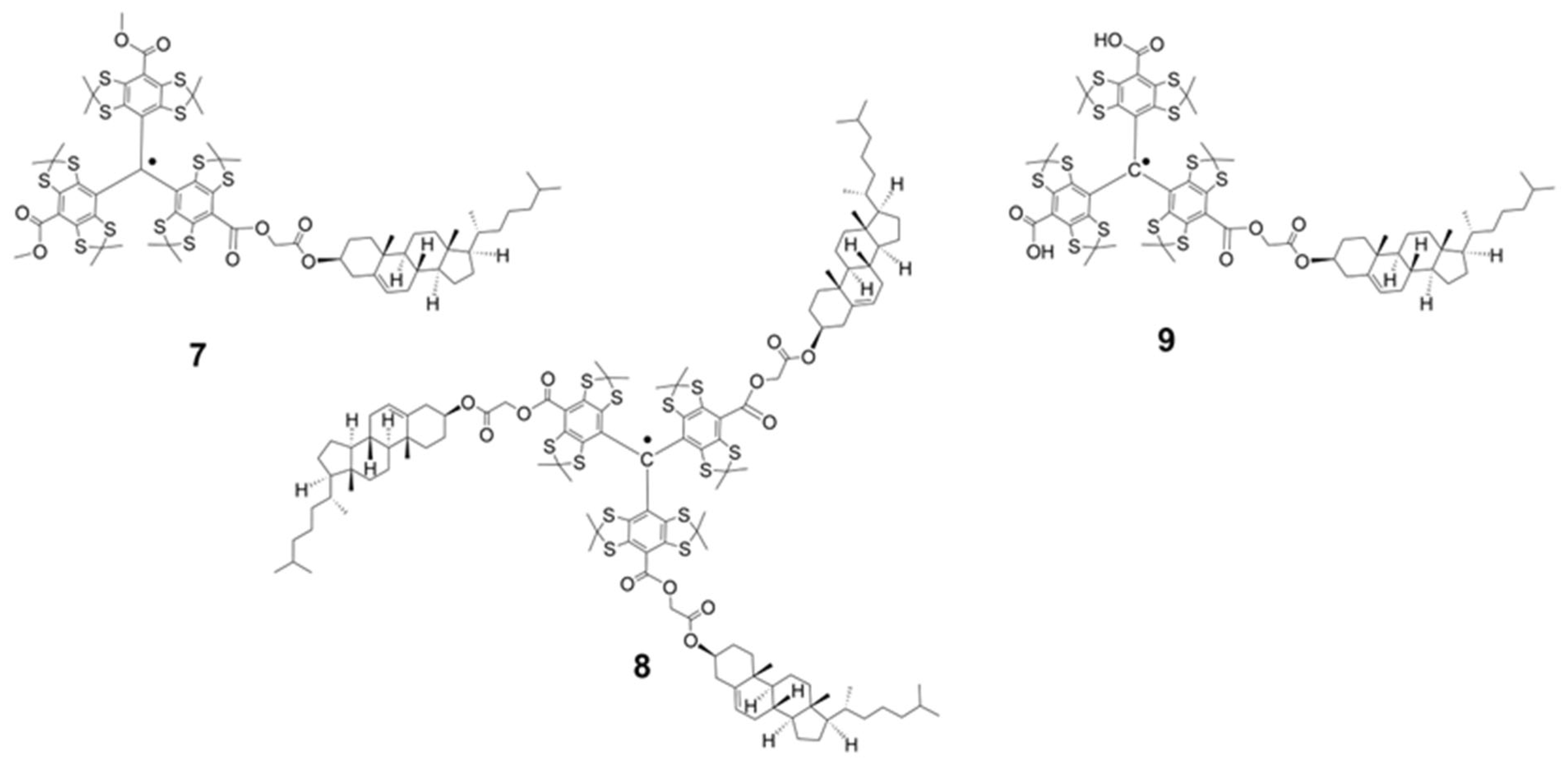
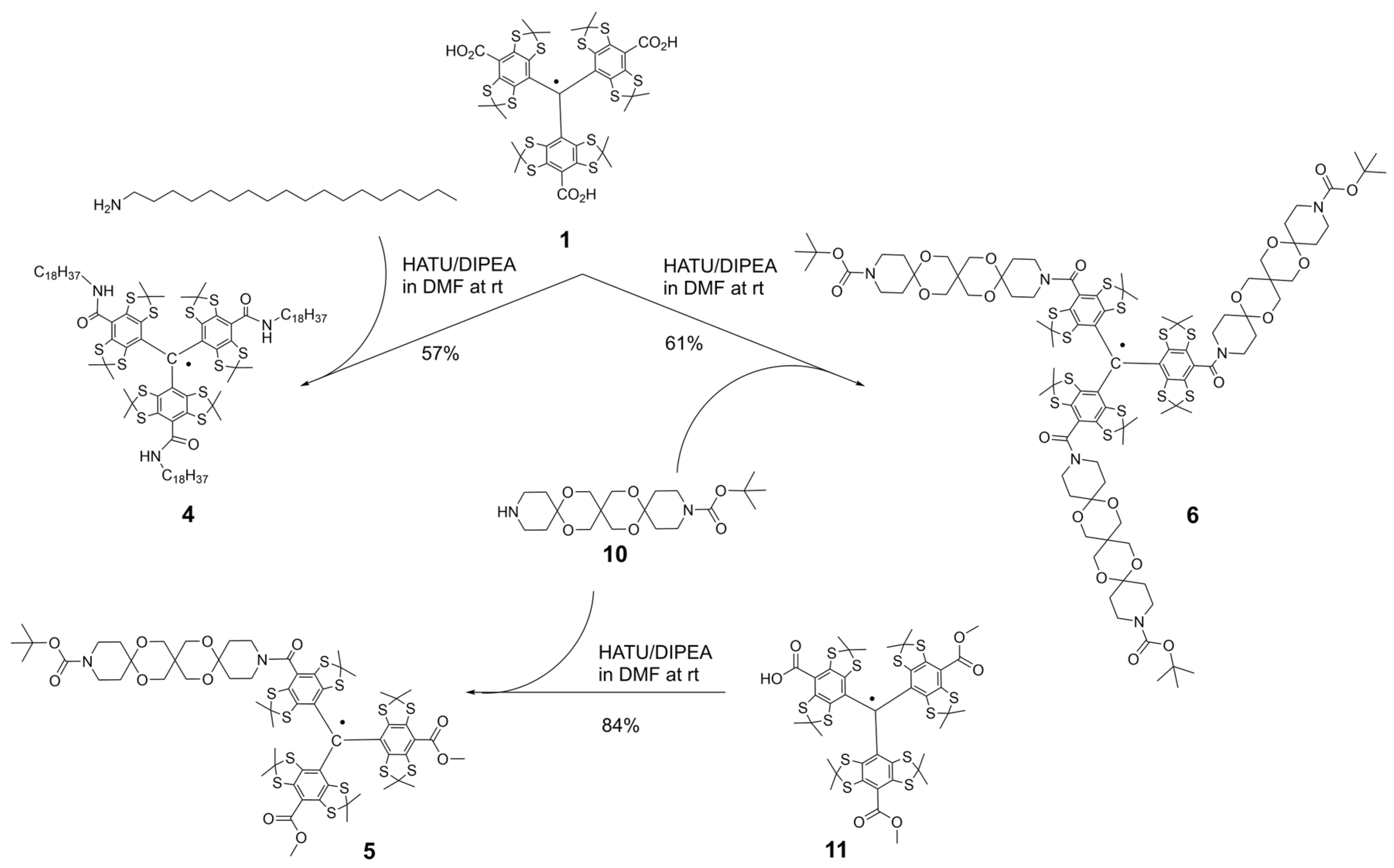
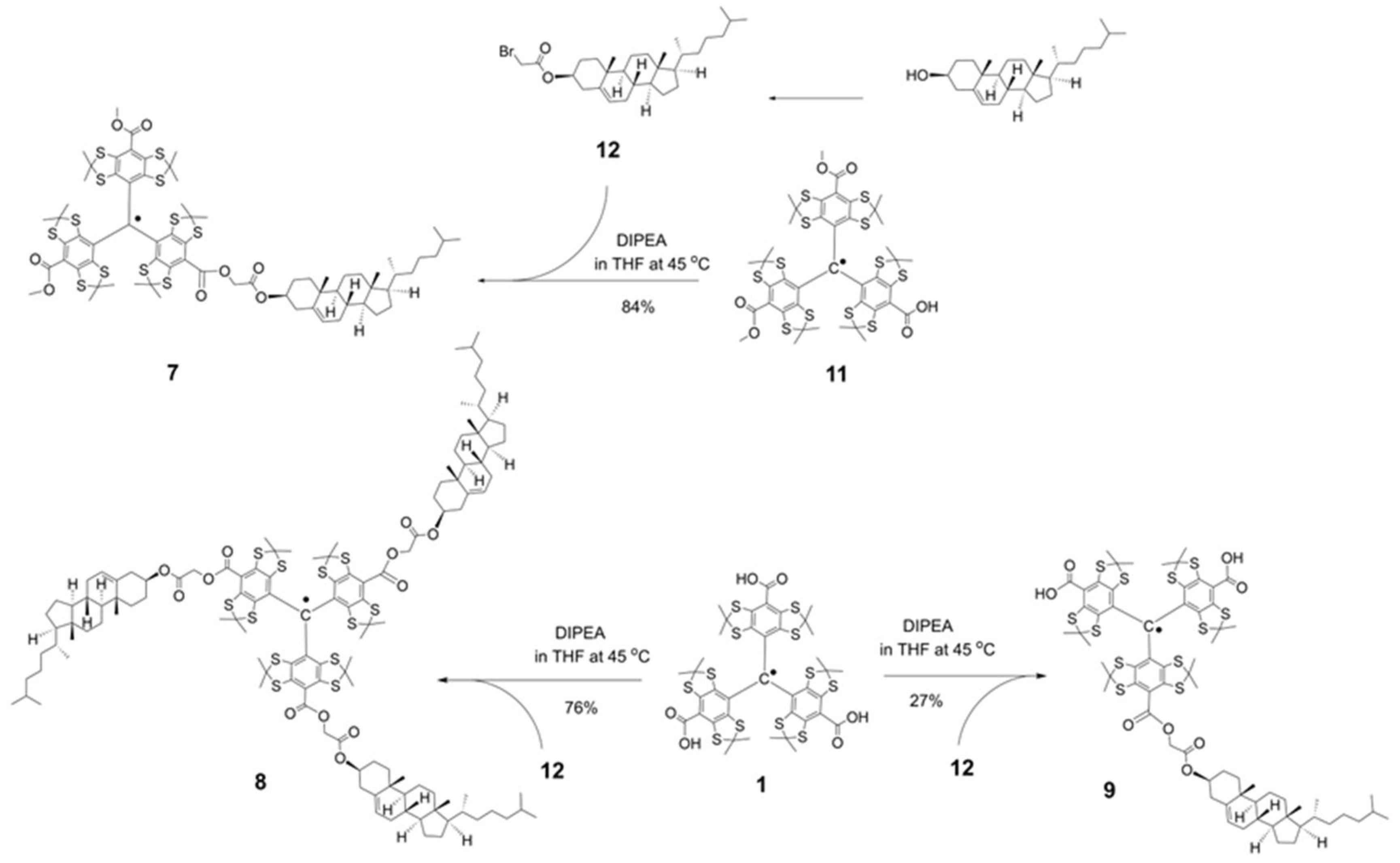
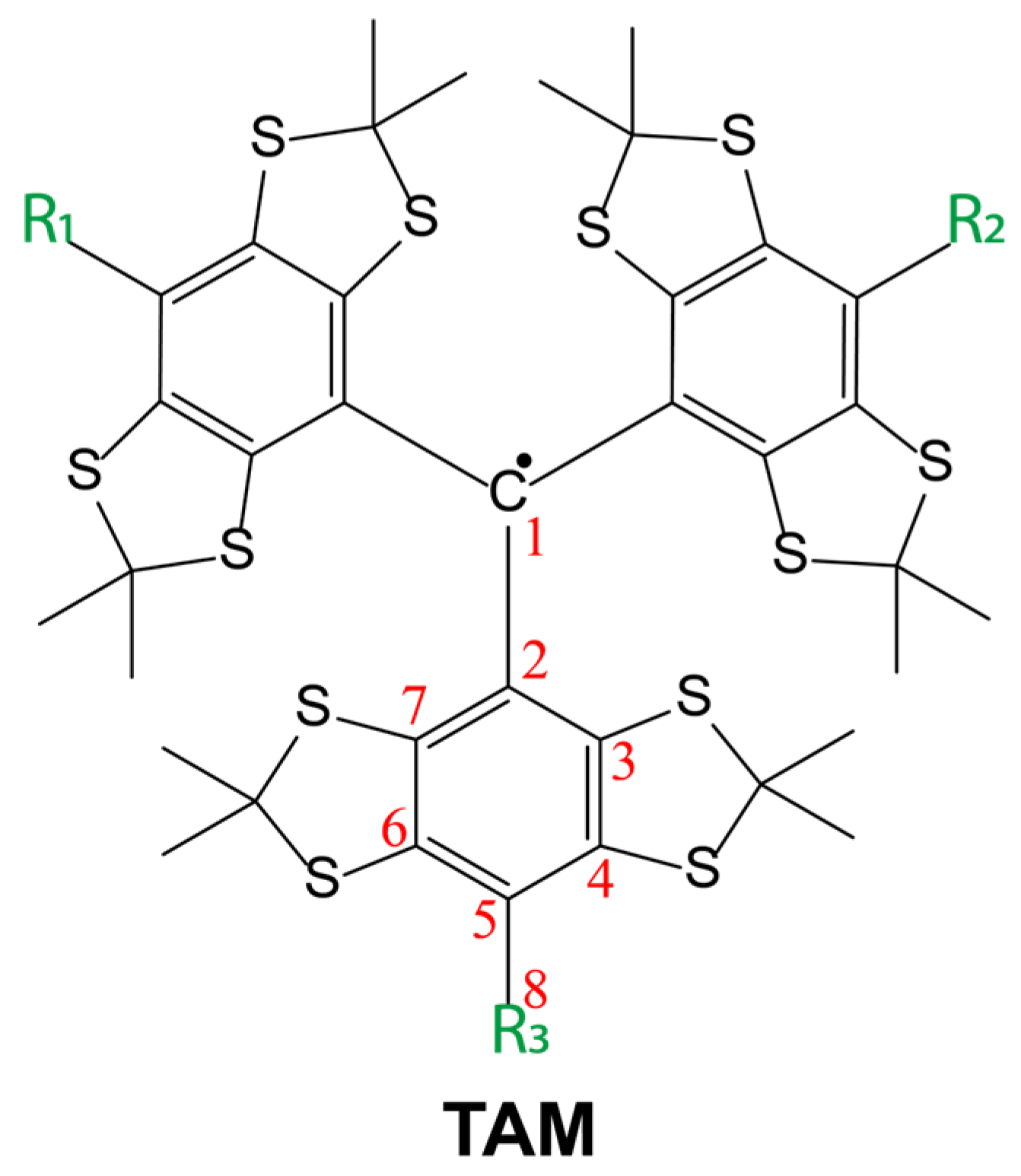
2.1. Synthesis of the Trityl Radicals
2.2. CW-EPR Studies of the Trityl Radicals
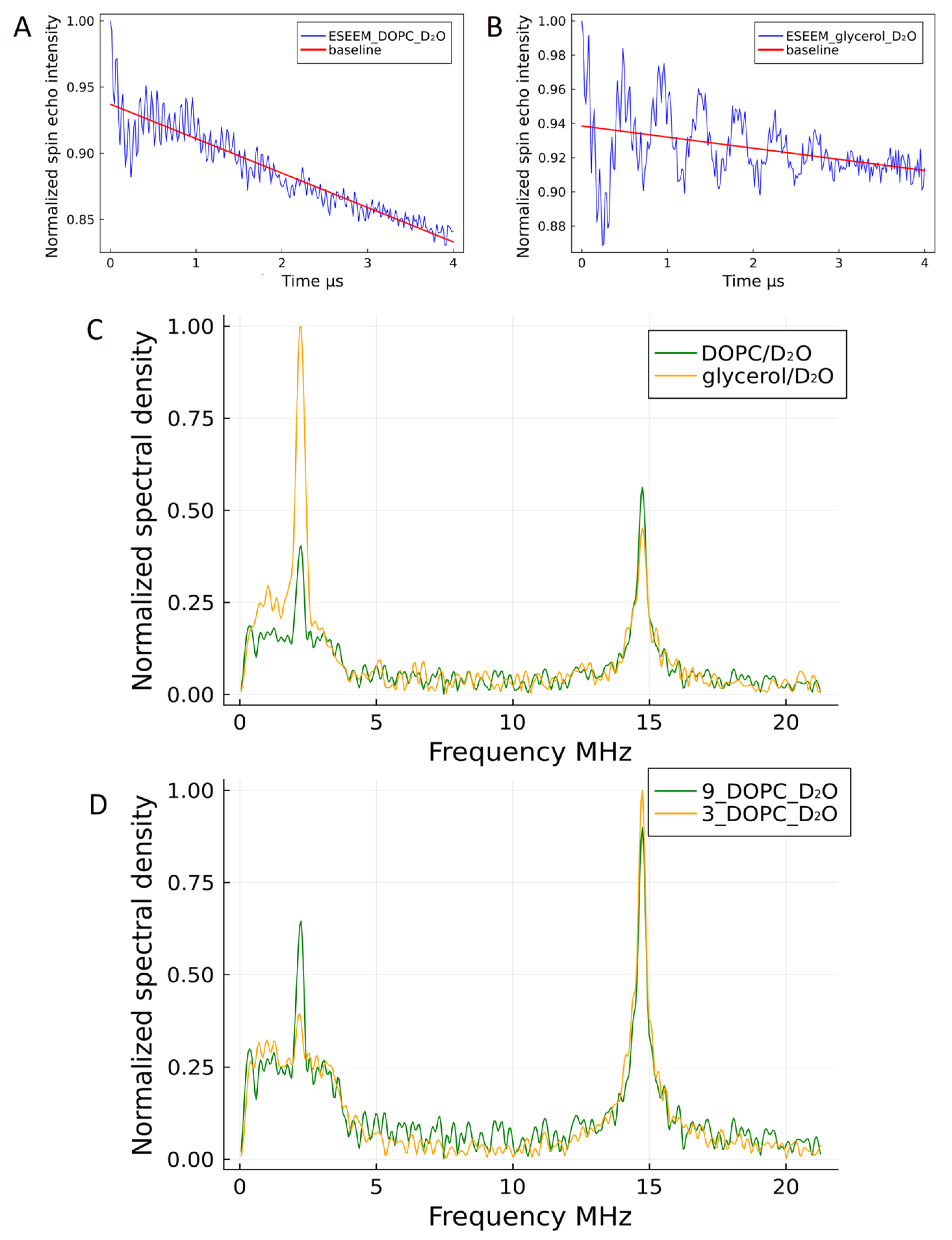
2.3. Pulse EPR, ED-EPR, and ESEEM Studies of the Trityl Radical
3. Materials and Methods
3.1. Synthesis of TAM Radicals 3–9
3.2. Sample Preparation for EPR Measurements
3.3. CW-EPR Spectroscopy
3.4. Spectral Simulation
3.5. Pulse EPR Measurements and Analysis
3.6. Preparation of the Liposomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAM | Triarylmethyl radicals |
| DNP | Dynamic nuclear polarization |
| SE DNP | Solid-effect DNP mechanism |
| ESEEM | Electron spin echo envelope modulations |
| HFI | Hyperfine interaction |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| BDPA | 1,3-bis(diphenylene)-2-phenylallyl |
| DIPEA | Diisopropylethylamine |
| HATU | Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium |
References
- Chen, S.; Zhang, L.; Li, S.; Yuan, Y.; Jiang, B.; Jiang, Z.; Zhang, X.; Zhou, X.; Liu, M. Detecting biomarkers by dynamic nuclear polarization enhanced magnetic resonance. Natl. Sci. Rev. 2024, 11, nwae228. [Google Scholar] [CrossRef]
- Hyodo, F.; Eto, H.; Naganuma, T.; Koyasu, N.; Elhelaly, A.E.; Noda, Y.; Kato, H.; Murata, M.; Akahoshi, T.; Hashizume, M. In vivo dynamic nuclear polarization magnetic resonance imaging for the evaluation of redox-related diseases and theranostics. Antioxid. Redox Signal. 2022, 36, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Gutte, H.; Hansen, A.E.; Johannesen, H.H.; Clemmensen, A.E.; Ardenkjær-Larsen, J.H.; Nielsen, C.H.; Kjær, A. The use of dynamic nuclear polarization 13C-pyruvate MRS in cancer. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 548. [Google Scholar] [PubMed]
- Kragelj, J.; Ghosh, R.; Xiao, Y.; Dumarieh, R.; Lagasca, D.; Krishna, S.; Frederick, K.K. Spatially resolved DNP-assisted NMR illuminates the conformational ensemble of α-synuclein in intact viable cells. Proc. Natl. Acad. Sci. USA 2025, 122, e2500367122. [Google Scholar] [CrossRef]
- Beriashvili, D.; Zhou, J.; Liu, Y.; Folkers, G.E.; Baldus, M. Cellular applications of DNP solid-State NMR–state of the art and a look to the future. Chem. A Eur. J. 2024, 30, e202400323. [Google Scholar] [CrossRef] [PubMed]
- Overall, S.A.; Barnes, A.B. Biomolecular perturbations in in-cell dynamic nuclear polarization experiments. Front. Mol. Biosci. 2021, 8, 743829. [Google Scholar] [CrossRef]
- Aladin, V.; Sreemantula, A.K.; Biedenbänder, T.; Marchanka, A.; Corzilius, B. Specific Signal Enhancement on an RNA—Protein Interface by Dynamic Nuclear Polarization. Chem. A Eur. J. 2023, 29, e202203443. [Google Scholar] [CrossRef]
- Parzy, E.; Boudries, D.; Jacoutot, S.; Albalat, M.; Vanthuyne, N.; Franconi, J.M.; Mellet, P.; Thiaudiere, E.; Audran, G.; Marque, S.R.A. Enzymatic activity monitoring through dynamic nuclear polarization in Earth magnetic field. J. Magn. Reson. 2021, 333, 107095. [Google Scholar] [CrossRef]
- Chakraborty, A.; Deligey, F.; Quach, J.; Mentink-Vigier, F.; Wang, P.; Wang, T. Biomolecular complex viewed by dynamic nuclear polarization solid-state NMR spectroscopy. Biochem. Soc. Trans. 2020, 48, 1089–1099. [Google Scholar] [CrossRef]
- Moroz, I.B.; Leskes, M. Dynamic nuclear polarization solid-state NMR spectroscopy for materials research. Annu. Rev. Mater. Res. 2022, 52, 25–55. [Google Scholar] [CrossRef]
- Liao, W.-C.; Ghaffari, B.; Gordon, C.P.; Xu, J.; Copéret, C. Dynamic nuclear polarization surface enhanced NMR spectroscopy (DNP SENS): Principles, protocols, and practice. Curr. Opin. Colloid Interface Sci. 2018, 33, 63–71. [Google Scholar] [CrossRef]
- Rossini, A.J.; Zagdoun, A.; Lelli, M.; Canivet, J.; Aguado, S.; Ouari, O.; Tordo, P.; Rosay, M.; Maas, W.E.; Copéret, C. Dynamic nuclear polarization enhanced solid-state NMR spectroscopy of functionalized metal–organic frameworks. Angew. Chem. Int. Ed. 2012, 51, 123–127. [Google Scholar] [CrossRef]
- Biedenbänder, T.; Aladin, V.; Saeidpour, S.; Corzilius, B.R. Dynamic nuclear polarization for sensitivity enhancement in biomolecular solid-state NMR. Chem. Rev. 2022, 122, 9738–9794. [Google Scholar] [CrossRef]
- Dey, A.; Charrier, B.; Lemaitre, K.; Ribay, V.; Eshchenko, D.; Schnell, M.; Melzi, R.; Stern, Q.; Cousin, S.F.; Kempf, J.G. Fine optimization of a dissolution-DNP experimental setting for 13 C NMR of metabolic samples. Magn. Reson. Discuss. 2022, 2022, 1–27. [Google Scholar]
- Jannin, S.; Dumez, J.-N.; Giraudeau, P.; Kurzbach, D. Application and methodology of dissolution dynamic nuclear polarization in physical, chemical and biological contexts. J. Magn. Reson. 2019, 305, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jakdetchai, O.; Denysenkov, V.; Becker-Baldus, J.; Dutagaci, B.; Prisner, T.F.; Glaubitz, C. Dynamic nuclear polarization-enhanced NMR on aligned lipid bilayers at ambient temperature. J. Am. Chem. Soc. 2014, 136, 15533–15536. [Google Scholar] [CrossRef] [PubMed]
- Kuzhelev, A.A.; Dai, D.; Denysenkov, V.; Prisner, T.F. Solid-like dynamic nuclear polarization observed in the fluid phase of lipid bilayers at 9.4 T. J. Am. Chem. Soc. 2022, 144, 1164–1168. [Google Scholar] [CrossRef]
- Kuzhelev, A.A.; Denysenkov, V.; Ahmad, I.M.; Rogozhnikova, O.Y.; Trukhin, D.V.; Bagryanskaya, E.G.; Tormyshev, V.M.; Sigurdsson, S.T.; Prisner, T.F. Solid-effect dynamic nuclear polarization in viscous liquids at 9.4 T using narrow-line polarizing agents. J. Am. Chem. Soc. 2023, 145, 10268–10274. [Google Scholar] [CrossRef]
- Dai, D.; Denysenkov, V.; Bagryanskaya, E.G.; Tormyshev, V.M.; Prisner, T.F.; Kuzhelev, A.A. 13C Hyperpolarization of Viscous Liquids by Transfer of Solid-Effect 1H Dynamic Nuclear Polarization at High Magnetic Field. J. Phys. Chem. Lett. 2023, 14, 7059–7064. [Google Scholar] [CrossRef]
- Kuzhelev, A. 31P Dynamic Nuclear Polarization through the Solid Effect: Study of Biomolecules in Aqueous Solutions at 9.4 T. Anal. Chem. 2025, 97, 14890–14893. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Diaz, S.; Poncelet, M.; Driesschaert, B.; Barth, E.; Kotecha, M.; Epel, B.; Eaton, G.R.; Biller, J.R. Toward a nanoencapsulated EPR imaging agent for clinical use. Mol. Imaging Biol. 2024, 26, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, M.; Poncelet, M.; Perini, J.A.; Kupec, J.T.; Dietz, M.J.; Driesschaert, B.; Khramtsov, V.V. EPR Viscometric Measurements Using a 13C-Labeled Triarylmethyl Radical in Protein-Based Biotherapeutics and Human Synovial Fluids. Appl. Magn. Reson. 2023, 54, 779–791. [Google Scholar] [CrossRef]
- Shaw, M.A.; Poncelet, M.; Viswakarma, N.; Vallerini, G.P.; Hameed, S.; Gluth, T.D.; Geldenhuys, W.J.; Hoblitzell, E.H.; Eubank, T.D.; Epel, B. SOX71, A biocompatible succinyl derivative of the triarylmethyl radical OX071 for in vivo quantitative oxygen mapping using electron paramagnetic resonance. Mol. Imaging Biol. 2024, 26, 542–552. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, B.-B.; Leng, Y.; Wang, X.-W.; Li, S.; Deng, P.; Ma, Y.; Song, Y.; Su, X.-C.; Yang, Y. SNAr-Based Labeling of Proteins with Trityl Radicals Enables High-Precision, High-Sensitivity, and Long-Range Distance Measurement. Anal. Chem. 2025, 97, 9256–9264. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhou, J.; Yang, L.; Chang, Q.; Li, S.-Y.; Rockenbauer, A.; Song, Y.; Liu, Y. Simultaneous quantitation of persulfides, biothiols, and hydrogen sulfide through sulfur exchange reaction with trityl spin probes. J. Am. Chem. Soc. 2024, 146, 30422–30433. [Google Scholar] [CrossRef]
- Li, S.; Deng, P.; Chang, Q.; Feng, M.; Shang, Y.; Song, Y.; Liu, Y. In Situ Generation and High Bioresistance of Trityl—based Semiquinone Methide Radicals Under Anaerobic Conditions in Cellular Systems. Chem. A Eur. J. 2024, 30, e202400985. [Google Scholar] [CrossRef]
- Feng, Y.; Tan, X.; Shi, Z.; Villamena, F.A.; Zweier, J.L.; Song, Y.; Liu, Y. Trityl quinodimethane derivatives as unimolecular triple-function extracellular EPR probes for redox, pH, and oxygen. Anal. Chem. 2023, 95, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, L.; Tan, X.; Rockenbauer, A.; Song, Y.; Liu, Y. Synthesis and Redox Properties of Water-Soluble Asymmetric Trityl Radicals. J. Org. Chem. 2021, 86, 8351–8364. [Google Scholar] [CrossRef]
- Halbritter, T.; Harrabi, R.; Paul, S.; van Tol, J.; Lee, D.; Hediger, S.; Sigurdsson, S.T.; Mentink-Vigier, F.; De Paëpe, G. PyrroTriPol: A semi-rigid trityl-nitroxide for high field dynamic nuclear polarization. Chem. Sci. 2023, 14, 3852–3864. [Google Scholar] [CrossRef]
- Zhai, W.; Lucini Paioni, A.; Cai, X.; Narasimhan, S.; Medeiros-Silva, J.o.; Zhang, W.; Rockenbauer, A.; Weingarth, M.; Song, Y.; Baldus, M. Postmodification via thiol-click chemistry yields hydrophilic trityl-nitroxide biradicals for biomolecular high-field dynamic nuclear polarization. J. Phys. Chem. B 2020, 124, 9047–9060. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Marque, S.R.A.; Rogozhnikova, O.Y.; Tormyshev, V.M.; Troitskaya, T.I.; Bagryanskaya, E.G. Radical polymerization of radical—labeled monomers: The triarylmethyl—based radical monomer as an example. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2656–2664. [Google Scholar] [CrossRef]
- Kuzhelev, A.A.; Trukhin, D.V.; Krumkacheva, O.A.; Strizhakov, R.K.; Rogozhnikova, O.Y.; Troitskaya, T.I.; Fedin, M.V.; Tormyshev, V.M.; Bagryanskaya, E.G. Room-temperature electron spin relaxation of triarylmethyl radicals at the X-and Q-bands. J. Phys. Chem. B 2015, 119, 13630–13640. [Google Scholar] [CrossRef]
- Bretschneider, M.; Spindler, P.E.; Rogozhnikova, O.Y.; Trukhin, D.V.; Endeward, B.; Kuzhelev, A.A.; Bagryanskaya, E.; Tormyshev, V.M.; Prisner, T.F. Multiquantum counting of trityl radicals. J. Phys. Chem. Lett. 2020, 11, 6286–6290. [Google Scholar] [CrossRef] [PubMed]
- Trukhin, D.V.; Rogozhnikova, O.Y.; Troitskaya, T.I.; Vasiliev, V.G.; Bowman, M.K.; Tormyshev, V.M. Facile and high-yielding synthesis of TAM Biradicals and Monofunctional TAM radicals. Synlett 2016, 27, 893–899. [Google Scholar] [PubMed]
- Kuzhelev, A.A.; Tormyshev, V.M.; Rogozhnikova, O.Y.; Trukhin, D.V.; Troitskaya, T.I.; Strizhakov, R.K.; Krumkacheva, O.A.; Fedin, M.V.; Bagryanskaya, E.G. Triarylmethyl radicals: An EPR study of 13C hyperfine coupling constants. Z. Phys. Chem. 2017, 231, 777–794. [Google Scholar] [CrossRef]
- Olivero, J.J.; Longbothum, R.L. Empirical fits to the Voigt line width: A brief review. J. Quant. Spectrosc. Radiat. Transf. 1977, 17, 233–236. [Google Scholar] [CrossRef]
- Kielkopf, J.F. New approximation to the Voigt function with applications to spectral-line profile analysis. J. Opt. Soc. Am. 1973, 63, 987–995. [Google Scholar] [CrossRef]
- Joseph, B.; Tormyshev, V.M.; Rogozhnikova, O.Y.; Akhmetzyanov, D.; Bagryanskaya, E.G.; Prisner, T.F. Selective High—Resolution Detection of Membrane Protein–Ligand Interaction in Native Membranes Using Trityl–Nitroxide PELDOR. Angew. Chem. Int. Ed. 2016, 55, 11538–11542. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Maryasov, A.G.; Rogozhnikova, O.Y.; Trukhin, D.V.; Tormyshev, V.M.; Bowman, M.K. Electron spin dynamics and spin–lattice relaxation of trityl radicals in frozen solutions. Phys. Chem. Chem. Phys. 2016, 18, 24954–24965. [Google Scholar] [CrossRef]
- Yong, L.; Harbridge, J.; Quine, R.W.; Rinard, G.A.; Eaton, S.S.; Eaton, G.R.; Mailer, C.; Barth, E.; Halpern, H.J. Electron spin relaxation of triarylmethyl radicals in fluid solution. J. Magn. Reson. 2001, 152, 156–161. [Google Scholar] [CrossRef]
- Fielding, A.J.; Carl, P.J.; Eaton, G.R.; Eaton, S.S. Multifrequency EPR of four triarylmethyl radicals. Appl. Magn. Reson. 2005, 28, 231–238. [Google Scholar] [CrossRef]
- Owenius, R.; Eaton, G.R.; Eaton, S.S. Frequency (250 MHz to 9.2 GHz) and viscosity dependence of electron spin relaxation of triarylmethyl radicals at room temperature. J. Magn. Reson. 2005, 172, 168–175. [Google Scholar] [CrossRef]
- Eaton, S.S.; Eaton, G.R. Relaxation Mechanisms. eMagRes 2016, 5, 1543–1556. [Google Scholar]
- Zhou, Y.; Bowler, B.E.; Eaton, G.R.; Eaton, S.S. Electron spin lattice relaxation rates for S = 12 molecular species in glassy matrices or magnetically dilute solids at temperatures between 10 and 300 K. J. Magn. Reson. 1999, 139, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kathirvelu, V.; Eaton, G.R.; Eaton, S.S. Impact of chlorine substitution on spin–lattice relaxation of triarylmethyl and 1, 4-benzosemiquinone radicals in glass-forming solvents between 25 and 295 K. Appl. Magn. Reson. 2010, 37, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Dikanov, S.A.; Tsvetkov, Y. Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Milov, A.D.; Samoilova, R.I.; Shubin, A.A.; Grishin, Y.A.; Dzuba, S.A. ESEEM measurements of local water concentration in D2O-containing spin-labeled systems. Appl. Magn. Reson. 2008, 35, 73–94. [Google Scholar] [CrossRef]
- Syryamina, V.N.; Maryasov, A.G.; Bowman, M.K.; Dzuba, S.A. Electron spin echo envelope modulation of molecular motions of deuterium nuclei. J. Magn. Reson. 2015, 261, 169–174. [Google Scholar] [CrossRef]
- Erilov, D.A.; Bartucci, R.; Guzzi, R.; Shubin, A.A.; Maryasov, A.G.; Marsh, D.; Dzuba, S.A.; Sportelli, L. Water concentration profiles in membranes measured by ESEEM of spin-labeled lipids. J. Phys. Chem. B 2005, 109, 12003–12013. [Google Scholar] [CrossRef]
- Rogozhnikova, O.Y.; Vasiliev, V.G.; Troitskaya, T.I.; Trukhin, D.V.; Mikhalina, T.V.; Halpern, H.J.; Tormyshev, V.M. Generation of Trityl Radicals by Nucleophilic Quenching of Tris (2, 3, 5, 6-tetrathiaaryl) methyl Cations and Practical and Convenient Large—Scale Synthesis of Persistent Tris (4-carboxy-2, 3, 5, 6-tetrathiaaryl) methyl Radical. Eur. J. Org. Chem. 2013, 2013, 3347–3355. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin: Simulating cw ESR spectra. ESR Spectrosc. Membr. Biophys. 2007, 27, 299–321. [Google Scholar]
- Isaev, N.P.; Melnikov, A.R.; Lomanovich, K.A.; Dugin, M.V.; Ivanov, M.Y.; Polovyanenko, D.N.; Veber, S.L.; Bowman, M.K.; Bagryanskaya, E.G. A broadband pulse EPR spectrometer for high-throughput measurements in the X-band. J. Magn. Reson. Open 2023, 14, 100092. [Google Scholar] [CrossRef]
- Moldovan, O.; Lameiras, P.; Nagy, I.; Opruta, T.; Popa, F.; Antheaume, C.; Ramondenc, Y.; Darabantu, M. Stereochemistry of six-membered spiranes arising from the first use of a diaza-trispiro-heneicosane motif in the synthesis of a G-1 dendritic melamine. Tetrahedron 2013, 69, 2199–2213. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Todorova, D.I.; Bankova, V.S.; Milkova, T.S. Synthesis of steryl esters of phenolic acids by a heterogeneous Wittig reaction. J. Nat. Prod. 1995, 58, 280–283. [Google Scholar] [CrossRef]


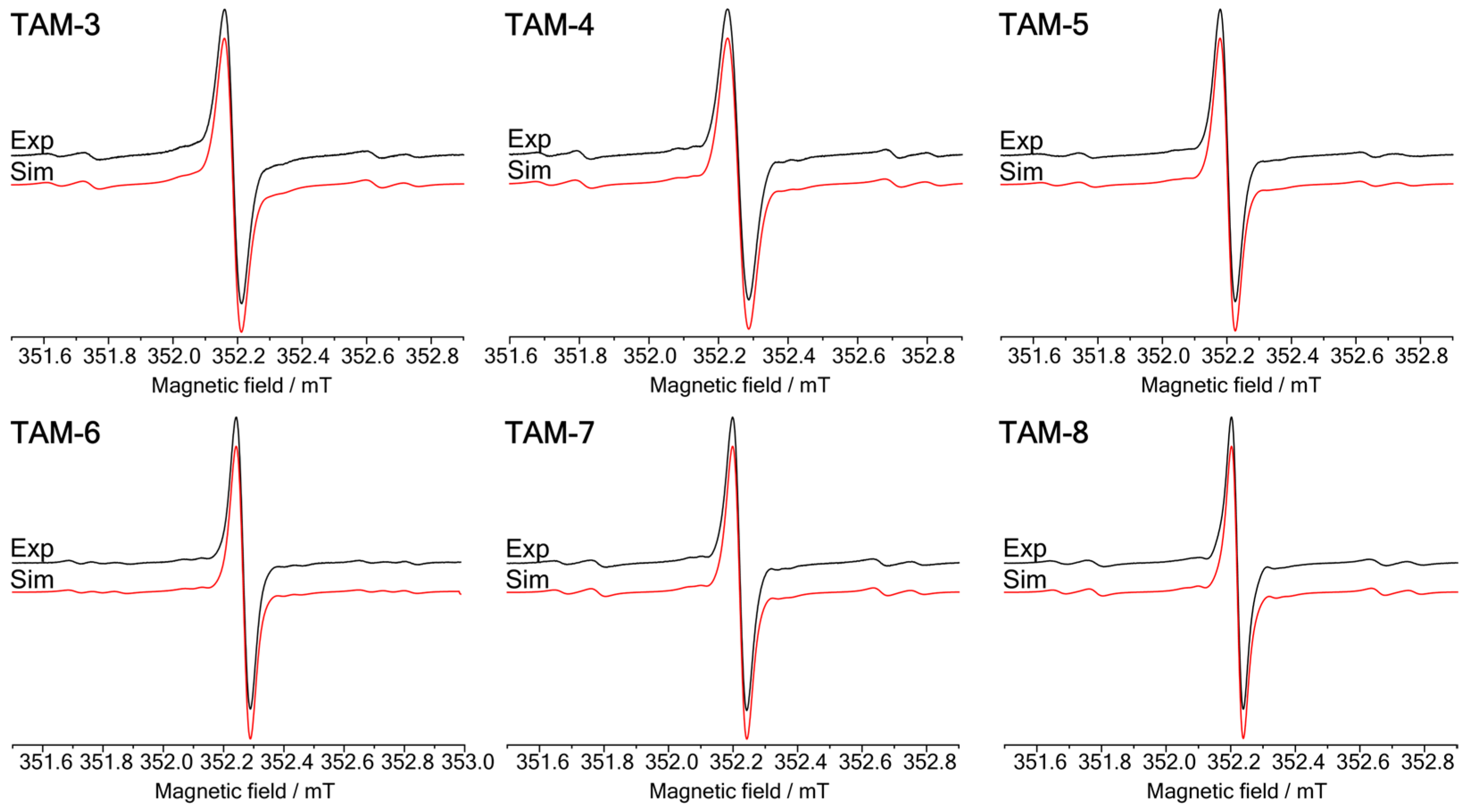

| a1/mT | a2/mT | a3/mT | a7/mT | a4,6/mT | a5/mT | a8/mT | |
|---|---|---|---|---|---|---|---|
| TAM 3 | 2.37 | 1.1 | 0.88 | 0.88 | 0.29 | 0.21 | 0.06 |
| TAM 4 | 2.38 | 1.12 | 0.89 | 0.89 | 0.32 | 0.23 | 0.05 |
| TAM 5 | 2.35 | 1.1 | 0.87 | 0.87 | 0.31 | 0.22 | 0.06 |
| TAM 6 | 2.35 | 1.11 | 0.82 | 0.97 | 0.36 | 0.24 | 0.04 |
| TAM 7 | 2.36 | 1.1 | 0.88 | 0.88 | 0.29 | 0.21 | 0.05 |
| TAM 8 | 2.38 | 1.1 | 0.88 | 0.88 | 0.3 | 0.21 | 0.06 |
| Compound | Line Width (Gaussian)/mT | Line Width (Lorentzian)/mT | Line Width (Voight)/mT |
|---|---|---|---|
| TAM 3 | 0.042 | 0.035 | 0.064 |
| TAM 4 | 0.059 | 0.030 | 0.077 |
| TAM 5 | 0.041 | 0.035 | 0.063 |
| TAM 6 | 0.043 | 0.023 | 0.057 |
| TAM 7 | 0.038 | 0.002 | 0.039 |
| TAM 8 | 0.024 | 0.004 | 0.026 |
| TAM 3 *,1 | 0.043 | 0.030 | 0.061 |
| TAM 3 *,2 | 0.117 | 0.192 | 0.250 |
| TAM 9 * | 0.003 | 0.116 | 0.116 |
| D | H | |||
|---|---|---|---|---|
| Max | Intensity | Max | Intensity | |
| TAM 9 1 | 1.00 | 7.7 ± 0.5 | 0.45 ± 0.05 | 3.2 ± 0.5 |
| TAM 9 2 | 0.40 ± 0.05 | 2.8 ± 0.5 | 0.56 ± 0.05 | 3.8 ± 0.5 |
| TAM 3 1 | 1.00 | 6.3 ± 0.5 | 0.10 ± 0.05 | 0.70 |
| TAM 3 2 | 0.08 ± 0.05 | 0.7 ± 0.5 | 0.19 ± 0.05 | 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tormyshev, V.M.; Kuznetsov, D.A.; Raizvikh, A.E.; Rogozhnikova, O.Y.; Troitskaya, T.I.; Bagryanskaya, E.G. Paramagnetic Agents for SE DNP: Synthesis and ESR Characterization of New Lipophilic Derivatives of Finland Trityl. Molecules 2025, 30, 4463. https://doi.org/10.3390/molecules30224463
Tormyshev VM, Kuznetsov DA, Raizvikh AE, Rogozhnikova OY, Troitskaya TI, Bagryanskaya EG. Paramagnetic Agents for SE DNP: Synthesis and ESR Characterization of New Lipophilic Derivatives of Finland Trityl. Molecules. 2025; 30(22):4463. https://doi.org/10.3390/molecules30224463
Chicago/Turabian StyleTormyshev, Victor M., Danil A. Kuznetsov, Arthur E. Raizvikh, Olga Yu. Rogozhnikova, Tatiana I. Troitskaya, and Elena G. Bagryanskaya. 2025. "Paramagnetic Agents for SE DNP: Synthesis and ESR Characterization of New Lipophilic Derivatives of Finland Trityl" Molecules 30, no. 22: 4463. https://doi.org/10.3390/molecules30224463
APA StyleTormyshev, V. M., Kuznetsov, D. A., Raizvikh, A. E., Rogozhnikova, O. Y., Troitskaya, T. I., & Bagryanskaya, E. G. (2025). Paramagnetic Agents for SE DNP: Synthesis and ESR Characterization of New Lipophilic Derivatives of Finland Trityl. Molecules, 30(22), 4463. https://doi.org/10.3390/molecules30224463








