Fractionation of Saffron (Crocus sativus L.) Extract by Solid-Phase Extraction and Subsequent Encapsulation in Liposomes Prepared by Reverse-Phase Evaporation
Abstract
1. Introduction
2. Results
2.1. Extraction and SPE Fractionation
2.2. Characterization of Saffron Extract
2.3. Liposomes Characterization and Entrapment Efficiency
3. Materials and Methods
3.1. Materials
3.2. Extraction and SPE Purification
3.3. HPLC Analysis
3.4. Liposome Preparation
3.5. Liposome Dimensions, Stability, and Morphology
3.6. Encapsulation Efficiency (EE)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RPE | reverse-phase evaporation |
| SPE | solid-phase extraction |
| DoE | Design of Experiments |
| UN-PC | unsaturated lipid matrix extracted by soy |
| SAT-PC | saturated lipid matrix extracted by soy |
| EE | encapsulation efficiency |
| ANOVA | analysis of variance |
| HPLC | high-performance liquid chromatography |
| DAD | diode array detector |
| NTA | Nanoparticle Tracking Analysis |
| TEM | Transmission Electron Microscopy |
References
- Van Calsteren, M.R.; Bissonnette, M.C.; Cormier, F.; Dufresne, C.; Yves LeBlanc, J.C.; Perreault, D.; Roewer, I. Spectroscopic Characterization of Crocetin Derivates from Crocus sativus L. and Gardenia jasminoides. J. Agric. Food Chem. 1997, 45, 1055–1061. [Google Scholar] [CrossRef]
- Li, N.; Lin, G.; Kwan, Y.-W.; Min, Z.-D. Simultaneous Quantification of Five Major Biologically Active Ingredients of Saffron by High-Performance Liquid Chromatography. J. Chromatogr. A 1999, 849, 349–355. [Google Scholar] [CrossRef]
- Pfister, S.; Meyer, P.; Steck, A.; Pfander, H. Isolation and Structure Elucidation of Carotenoid-Glycosyl Esters in Gardenia Fruits (Gardenia jasminoides Ellis) and Saffron (Crocus sativus L.). J. Agric. Food Chem. 1996, 44, 2612–2615. [Google Scholar] [CrossRef]
- Cusano, E.; Consonni, R.; Petrakis, E.A.; Astraka, K.; Cagliani, L.R.; Polissiou, M.G. Integrated Analytical Methodology to Investigate Bioactive Compounds in Crocus sativus L. Flowers. Phytochem. Anal. 2018, 29, 476–486. [Google Scholar] [CrossRef]
- Rödel, W.; Petrizka, M. Analysis of Volatile Components of Saffron. J. High Resolut. Chromatogr. 1991, 14, 771–774. [Google Scholar] [CrossRef]
- Karami, Z.; Jafari, S.M.; Duangmal, K. Crocins. In Handbook of Food Bioactive Ingredients: Properties and Applications; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 791–817. ISBN 978-3-031-28109-9. [Google Scholar]
- Finley, J.W.; Gao, S. A Perspective on Crocus sativus L. (Saffron) Constituent Crocin: A Potent Water-Soluble Antioxidant and Potential Therapy for Alzheimer’s Disease. J. Agric. Food Chem. 2017, 65, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht-Jam, I.; Khademi, M.; Nosrati, M.; Eslami, S.; Foroutan-Tanha, M.; Sahebkar, A.; Tavalaie, S.; Ghayour-Mobarhan, M.; Ferns, G.; Hadizadeh, F.; et al. Effect of Crocin Extracted from Saffron on Pro-Oxidant–Anti-Oxidant Balance in Subjects with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial. Eur. J. Integr. Med. 2016, 8, 307–312. [Google Scholar] [CrossRef]
- Oruc, S.; Gönül, Y.; Tunay, K.; Oruc, O.A.; Bozkurt, M.F.; Karavelioğlu, E.; Bağcıoğlu, E.; Coşkun, K.S.; Celik, S. The Antioxidant and Antiapoptotic Effects of Crocin Pretreatment on Global Cerebral Ischemia Reperfusion Injury Induced by Four Vessels Occlusion in Rats. Life Sci. 2016, 154, 79–86. [Google Scholar] [CrossRef]
- Tsolaki, M.; Karathanasi, E.; Lazarou, I.; Dovas, K.; Verykouki, E.; Karakostas, A.; Georgiadis, K.; Tsolaki, A.; Adam, K.; Kompatsiaris, I.; et al. Efficacy and Safety of Crocus sativus L. in Patients with Mild Cognitive Impairment: One Year Single-Blind Randomized, with Parallel Groups, Clinical Trial. J. Alzheimer’s Dis. 2016, 54, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical Applications of Saffron (Crocus sativus) and Its Constituents: A Review. Drug Res. 2015, 65, 287. [Google Scholar] [CrossRef]
- Mousavi, B.; Bathaie, S.Z.; Fadai, F.; Ashtari, Z.; Alibeigi, N.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Heidarzadeh, H. Safety Evaluation of Saffron Stigma (Crocus sativus L.) Aqueous Extract and Crocin in Patients with Schizophrenia. Avicenna J. Phytomed. 2015, 5, 413–419. [Google Scholar]
- Pitsikas, N. The Effect of Crocus sativus L. and Its Constituents on Memory: Basic Studies and Clinical Applications. Evidence-Based Complement. Altern. Med. 2015, 2015, 926284. [Google Scholar] [CrossRef]
- Marrone, G.; Urciuoli, S.; Di Lauro, M.; Cornali, K.; Montalto, G.; Masci, C.; Vanni, G.; Tesauro, M.; Vignolini, P.; Noce, A. Saffron (Crocus Sativus L.) and Its By-Products: Healthy Effects in Internal Medicine. Nutrients 2024, 16, 2319. [Google Scholar] [CrossRef]
- Mir, R.A.; Tyagi, A.; Hussain, S.J.; Almalki, M.A.; Zeyad, M.T.; Deshmukh, R.; Ali, S. Saffron, a Potential Bridge between Nutrition and Disease Therapeutics: Global Health Challenges and Therapeutic Opportunities. Plants 2024, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Moratalla-López, N.; Sánchez, A.M.; Campayo, A.; Salinas, M.R.; Alonso, G.L. Effect of Different Storage Conditions on the Stability of the Polyphenolic Content in Bio-Residues Obtained from Saffron Spice Production. Acta Hortic. 2017, 1184, 159–164. [Google Scholar] [CrossRef]
- Morimoto, S.; Umezaki, Y.; Shoyama, Y.; Saito, H.; Nishi, K.; Irino, N. Post-Harvest Degradation of Carotenoids Glucose Esters in Saffron. Planta Med. 1994, 60, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Tsimidou, M.; Biliaderis, C.G. Kinetic Studies of Saffron (Crocus sativus L.) Quality Deterioration. J. Agric. Food Chem. 1997, 45, 2890–2898. [Google Scholar] [CrossRef]
- Alonso, G.L.; Salinas, M.R.; Sánchez, M.A.; Garijo, J. Técnicas Culturales, Métodos de Deshidratación y Formas de Conservación En La Producción Del Azafrán En España. Agric. Vergel 1998, 169, 357–370. [Google Scholar]
- Ruggieri, F.; Maggi, M.A.; Rossi, M.; Consonni, R. Comprehensive Extraction and Chemical Characterization of Bioactive Compounds in Tepals of Crocus sativus L. Molecules 2023, 28, 5976. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, J.; Hong, Y.; Jiao, L.; Li, S.; Sun, R.; Xie, Y.; Yan, C.; Aa, J.; Wang, G. Orally Administered Crocin Protects Against Cerebral Ischemia/Reperfusion Injury Through the Metabolic Transformation of Crocetin by Gut Microbiota. Front. Pharmacol. 2019, 10, 440. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Du, P.; Fu, J. Pharmacokinetic Properties of Crocin (Crocetin digentiobiose Ester) Following Oral Administration in Rats. Phytomedicine 2007, 14, 633–636. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z.; Callaghan, Y.C.O.; Galvin, K.; Brien, N.M.O. Changes in Total and Individual Crocetin Esters upon in Vitro Gastrointestinal Digestion of Sa Ff Ron Aqueous Extracts. J. Agric. Food Chem. 2013, 61, 5318–5327. [Google Scholar] [CrossRef]
- Saroglu, O.; Bekiroglu, H.; Karadag, A. Chapter 5—Encapsulation of Saffron Bioactive Compounds. In Saffron; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 183–220. ISBN 978-0-12-821219-6. [Google Scholar]
- Lee, M.-K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Orally Administered Liposomal Formulations for Colon Targeted Drug Delivery. Front. Pharmacol. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally Administered Crocetin and Crocins Are Absorbed into Blood Plasma as Crocetin and Its Glucuronide Conjugates in Mice. J. Agric. Food Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Investigation by Response Surface Methodology of Extraction of Caffeine, Gallic Acid and Selected Catechins from Tea Using Water-Ethanol Mixtures. Food Anal. Methods 2016, 9, 2773–2779. [Google Scholar] [CrossRef]
- Leardi, R. Experimental Design in Chemistry: A Tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and Biological Properties of the World’s Most Expensive Spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical Composition and Neuroprotective Activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef]
- Wehrle, N.; Tran, L.M.; Zheng, A.; Pissay, R.; Park, Y.C. Effect of Solvent and Cholesterol on Liposome Production by the Reverse-Phase Evaporation (RPE) Method. Langmuir 2024, 40, 23521–23528. [Google Scholar] [CrossRef]
- Sum, A.K.; Faller, R.; Pablo, J.J. De Molecular Simulation Study of Phospholipid Bilayers and Insights of the Interactions with Disaccharides. Biophys. J. 2003, 85, 2830–2844. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Sethi, A.; Swanson, B.I.; Goldstein, B.; Gnanakaran, S. Taste of Sugar at the Membrane: Thermodynamics and Kinetics of the Interaction of a Disaccharide with Lipid Bilayers. Biophys. J. 2013, 104, 622–632. [Google Scholar] [CrossRef]
- Tamai, N.; Kamiya, M.; Kiriyama, N.; Goto, M.; Fukada, K.; Matsuki, H. Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine. Membranes 2024, 14, 258. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Biancolillo, A.; Maggi, M.A.; De Martino, A.; Marini, F.; Ruggieri, F.; D’Archivio, A.A. Authentication of PDO Saffron of L’Aquila (Crocus sativus L.) by HPLC-DAD Coupled with a Discriminant Multi-Way Approach. Food Control 2020, 110, 107022. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Di Donato, F.; Foschi, M.; Maggi, M.A.; Ruggieri, F. Uhplc Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters. Molecules 2018, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Suchareau, M.; Bordes, A.; Lemée, L. Improved Quantification Method of Crocins in Saffron Extract Using HPLC-DAD after Qualification by HPLC-DAD-MS. Food Chem. 2021, 362, 130199. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Loukidi, B.; Mir, M.A.; Assadpour, E.; Boudghene-Stambouli, M.; Kharazmi, M.S.; Jafari, S.M. Bioactivity and Applications of Saffron Floral Bio-Residues (Tepals): A Natural by-Product for the Food, Pharmaceutical, and Cosmetic Industries. Crit. Rev. Food Sci. Nutr. 2024, 64, 8399–8413. [Google Scholar] [CrossRef]

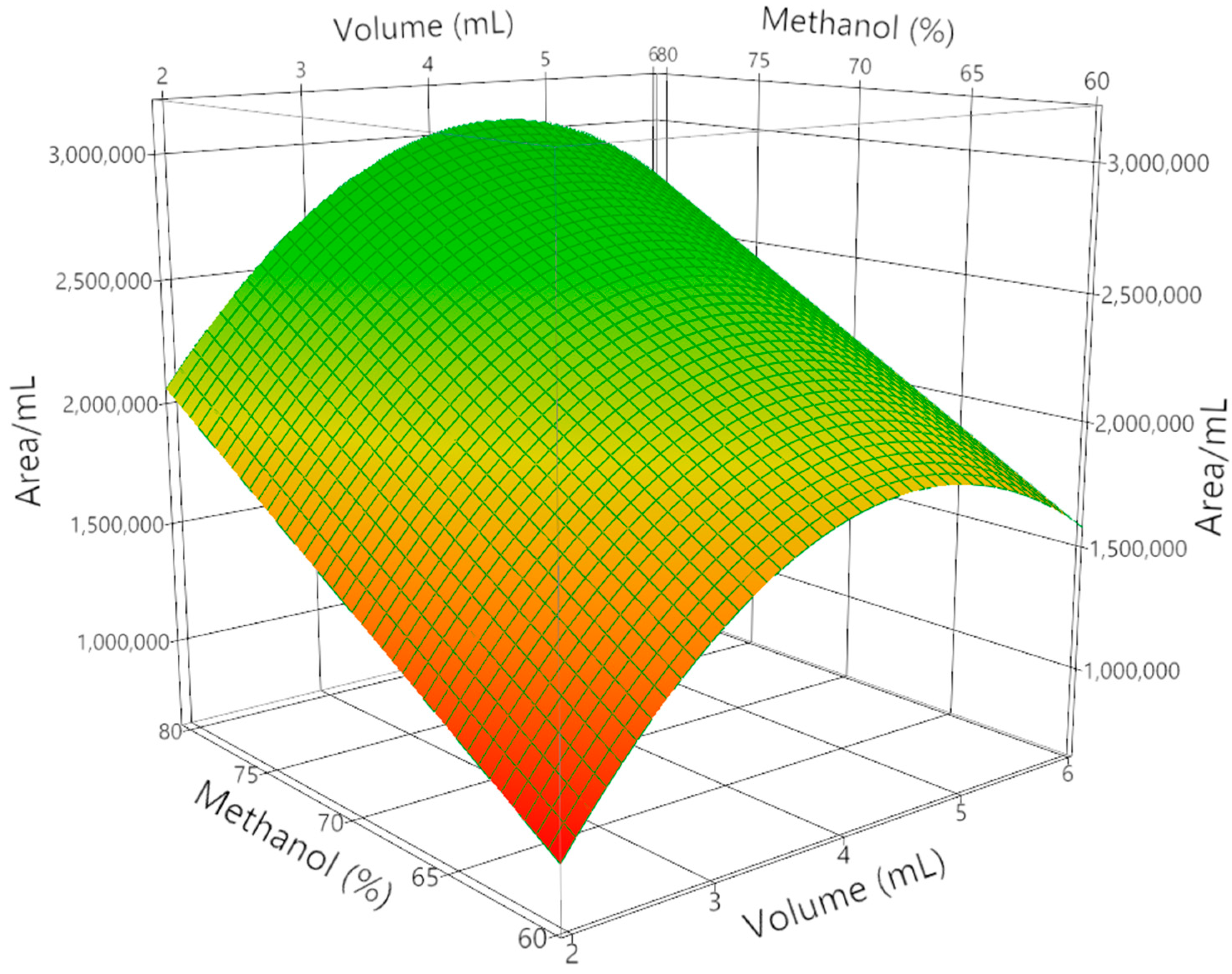

| Parameters | Value ± SD | R2 | Adj-R2 | ||
|---|---|---|---|---|---|
| intercept | 2.41 × 106 ± 0.11 × 106 | ||||
| * X1 | 3.2 × 105 ± 1.0 × 105 | ||||
| * X2 | 5.6 × 105 ± 1.0 × 105 | 0.883 | 0.834 | ||
| * X12 | -5.4 × 105 ± 1.5 × 105 | ||||
| variation source | sum of squares | degrees of freedom | mean square | F-value | p-value |
| lack of fit | 4.1 × 1011 | 5 | 8.3 × 1010 | 5.4001 | 0.1636 |
| pure error | 3.1 × 1010 | 2 | 1.5 × 1010 | ||
| model | 3.4 × 1012 | 3 | 1.1 × 1012 | 17.7387 | 0.0012 |
| residual | 4.5 × 1011 | 7 | 6.4 × 1010 |
| Run | Volume [mL] | CH3OH [%] | X1 | X2 | Area Crocin/Volume [AU/mL] × 103 |
|---|---|---|---|---|---|
| 1 | 2 | 60 | −1 | −1 | 1453 |
| 2 | 2 | 70 | −1 | 0 | 1950 |
| 3 | 2 | 80 | −1 | 1 | 2250 |
| 4 | 4 | 60 | 0 | −1 | 2250 |
| 5 | 4 | 70 | 0 | 0 | 3091 |
| 6 | 4 | 80 | 0 | 1 | 3284 |
| 7 | 6 | 60 | 1 | −1 | 1750 |
| 8 | 6 | 70 | 1 | 0 | 1928 |
| 9 | 6 | 80 | 1 | 1 | 2226 |
| 10 * | 4 | 70 | 0 | 0 | 3091 |
| 11 * | 4 | 70 | 0 | 0 | 3064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggieri, F.; Maggi, M.A.; Commito, F.; Badia, F.; Giansanti, L. Fractionation of Saffron (Crocus sativus L.) Extract by Solid-Phase Extraction and Subsequent Encapsulation in Liposomes Prepared by Reverse-Phase Evaporation. Molecules 2025, 30, 4408. https://doi.org/10.3390/molecules30224408
Ruggieri F, Maggi MA, Commito F, Badia F, Giansanti L. Fractionation of Saffron (Crocus sativus L.) Extract by Solid-Phase Extraction and Subsequent Encapsulation in Liposomes Prepared by Reverse-Phase Evaporation. Molecules. 2025; 30(22):4408. https://doi.org/10.3390/molecules30224408
Chicago/Turabian StyleRuggieri, Fabrizio, Maria Anna Maggi, Francesca Commito, Federica Badia, and Luisa Giansanti. 2025. "Fractionation of Saffron (Crocus sativus L.) Extract by Solid-Phase Extraction and Subsequent Encapsulation in Liposomes Prepared by Reverse-Phase Evaporation" Molecules 30, no. 22: 4408. https://doi.org/10.3390/molecules30224408
APA StyleRuggieri, F., Maggi, M. A., Commito, F., Badia, F., & Giansanti, L. (2025). Fractionation of Saffron (Crocus sativus L.) Extract by Solid-Phase Extraction and Subsequent Encapsulation in Liposomes Prepared by Reverse-Phase Evaporation. Molecules, 30(22), 4408. https://doi.org/10.3390/molecules30224408








