Exploring the Scope of Functionalized N-Acylneuraminic Acid β-Methyl Glycosides as Inhibitors of Neisseria meningitidis CMP-Sialic Acid Synthetase
Abstract
1. Introduction
2. Results and Discussion
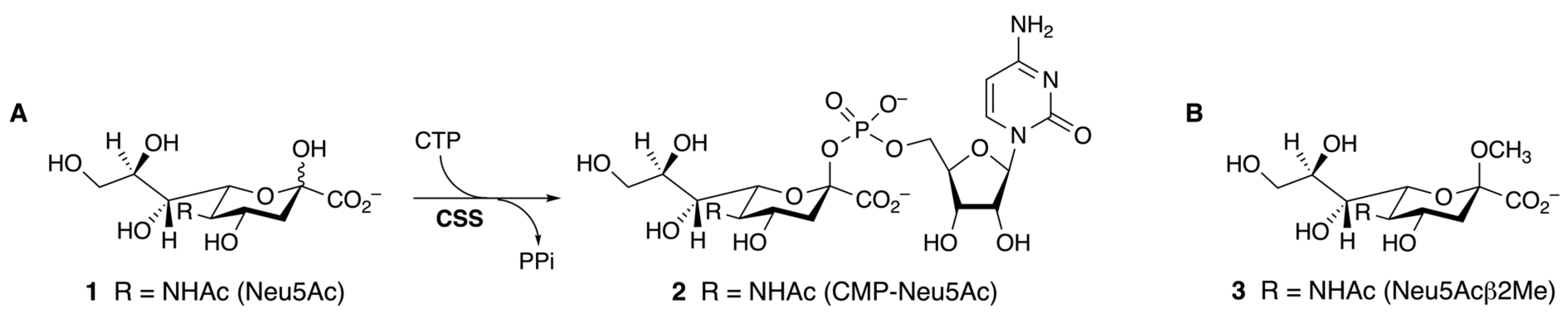
2.1. Structure Analysis of NmB CSS
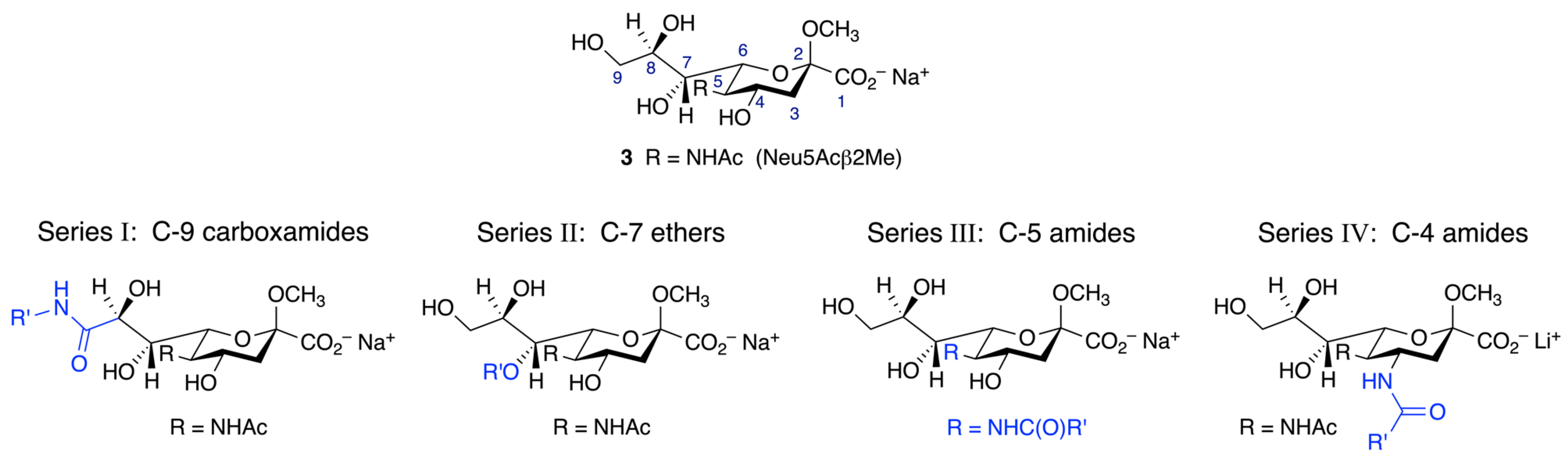
2.2. Synthesis of Functionalized Neu5Acβ2Me Derivatives
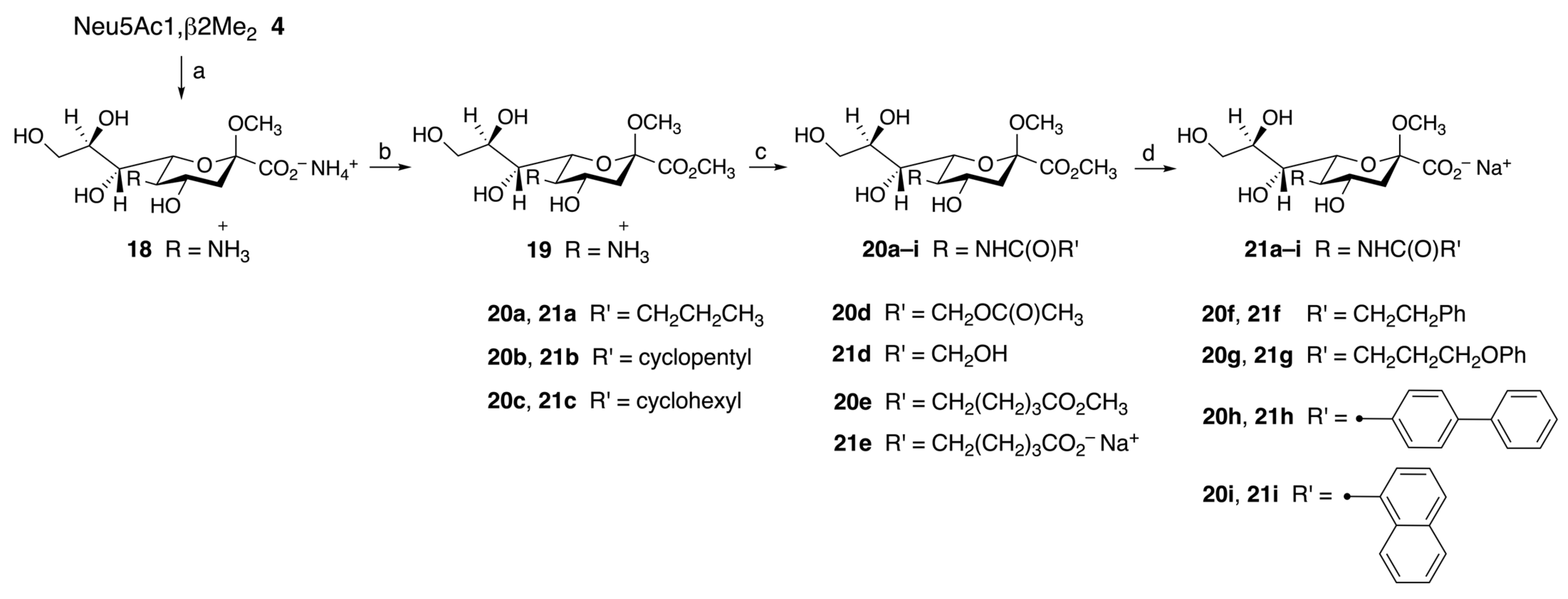
2.2.1. Synthesis of C-9 Functionalized Neu5Acβ2Me Derivatives (Series I)
2.2.2. Synthesis of C-7 Functionalized Neu5Acβ2Me Derivatives (Series II)
2.2.3. Synthesis of C-5 Functionalized Neuβ2Me Derivatives (Series III)
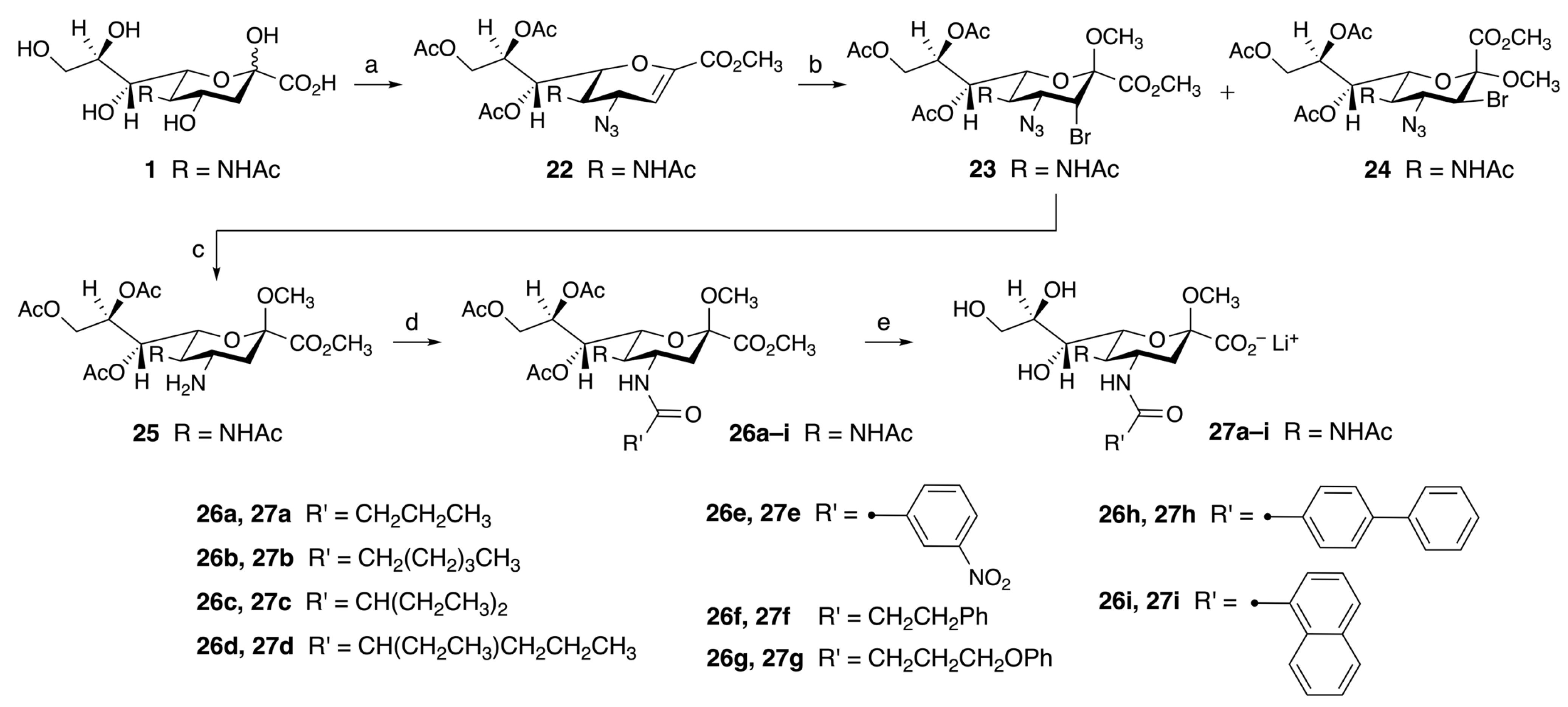
2.2.4. Synthesis of C-4 Functionalized Neu5Acβ2Me Derivatives (Series IV)
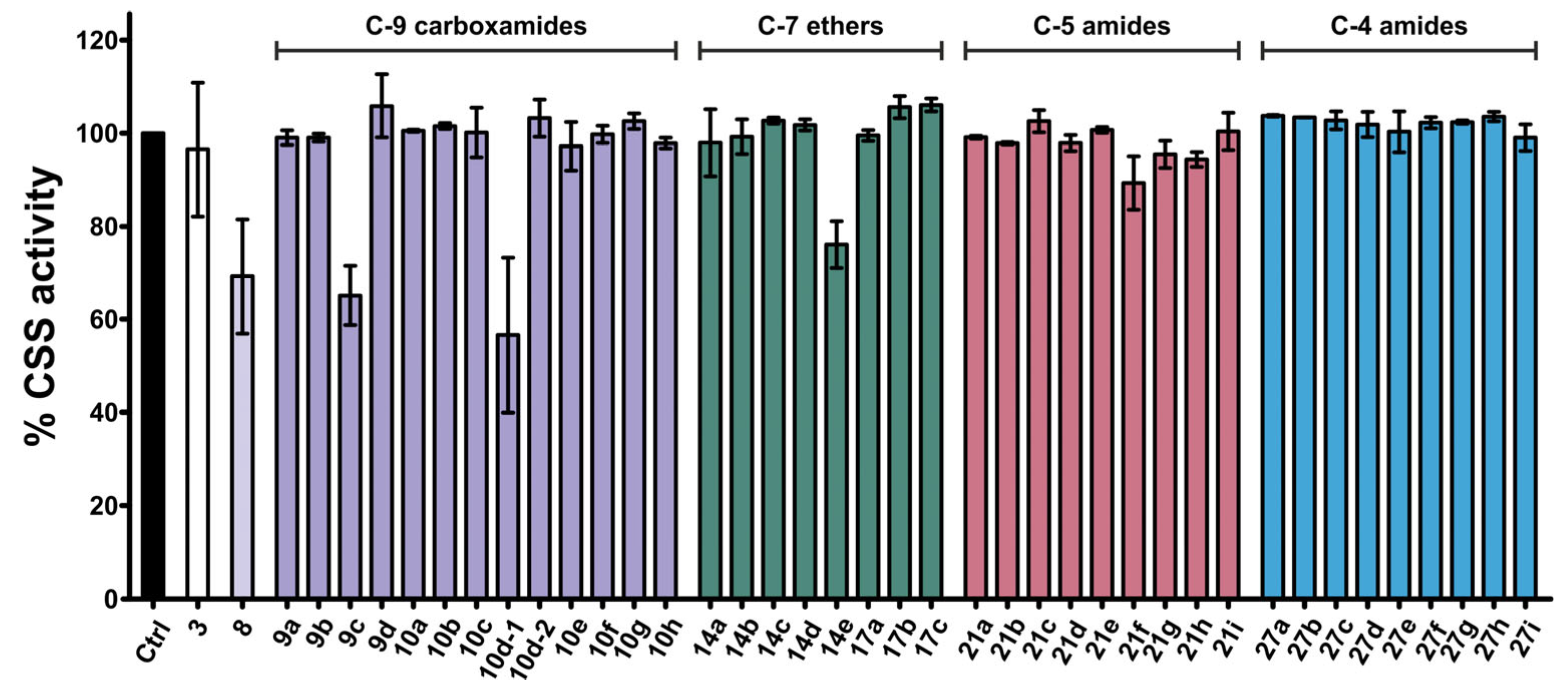
2.3. Screening of Functionalized Neu5Acβ2Me Derivatives for Inhibition of NmB CSS
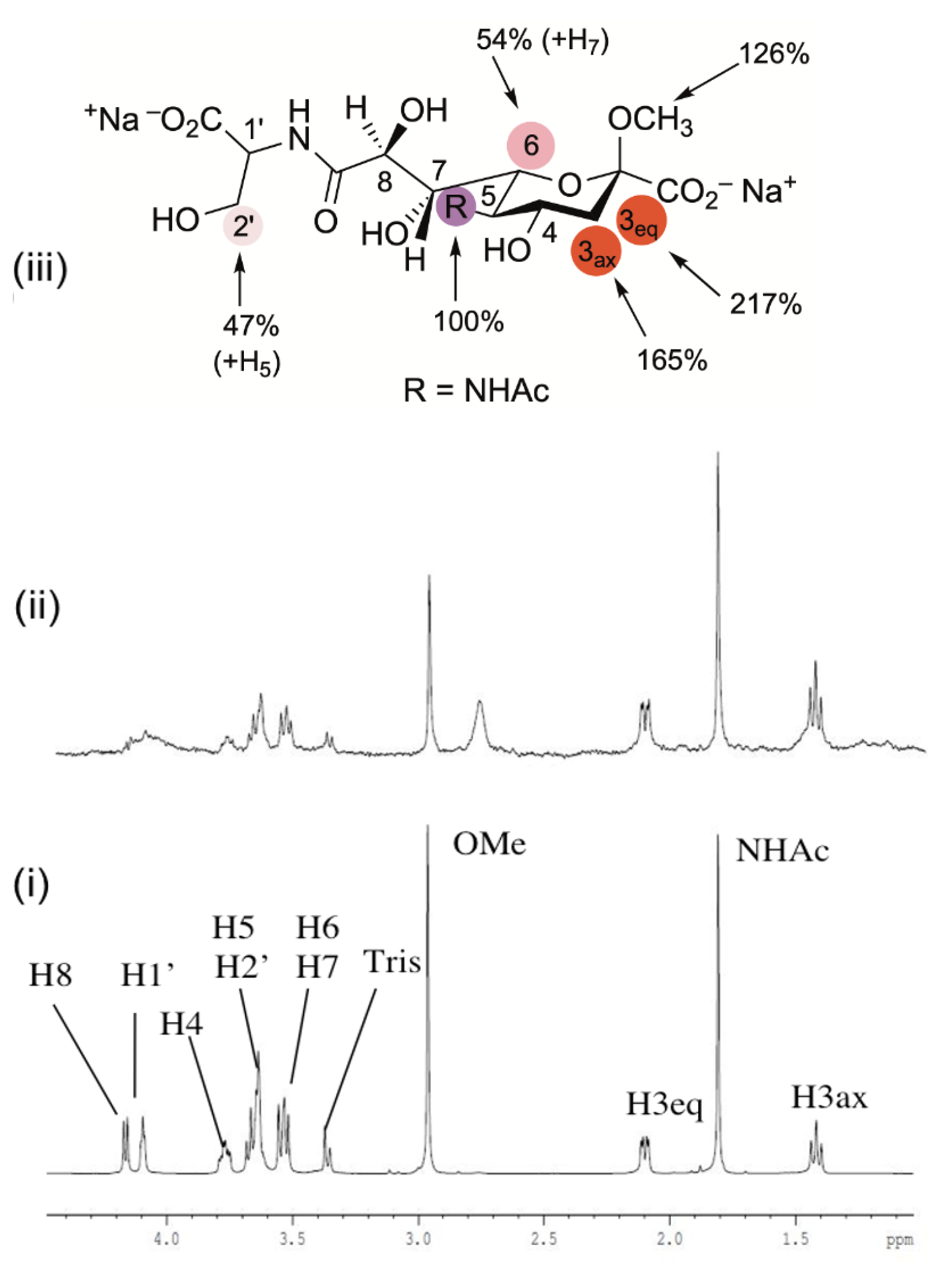
2.4. Interaction of Neu5Acβ2Me C-9 Serine Carboxamide 10d-1 with NmB CSS as Determined by STD NMR
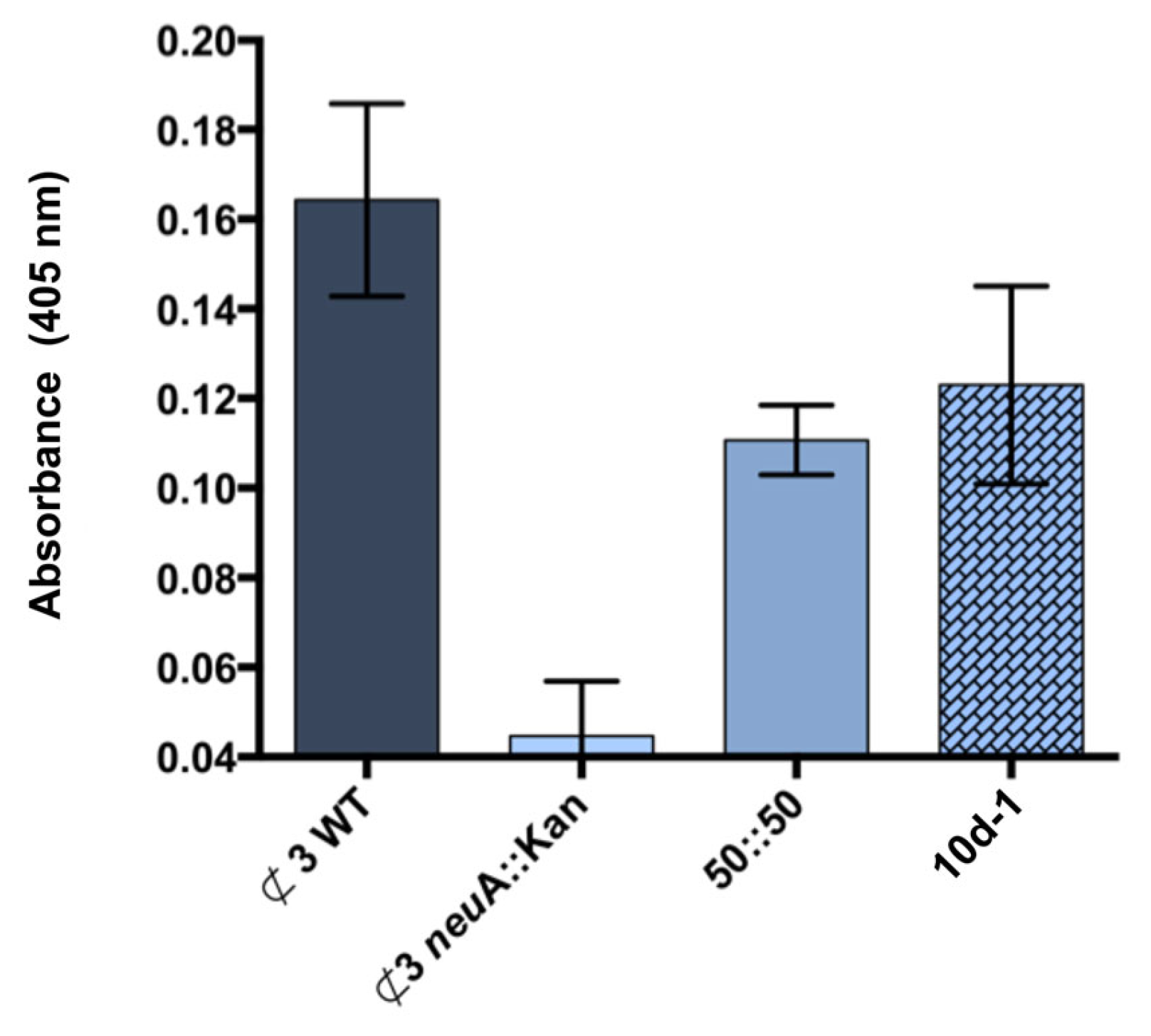
2.5. Neu5Acβ2Me C-9 Serine Carboxamide 10d-1 as a Modulator of NmB LOS Sialylation
3. Materials and Methods
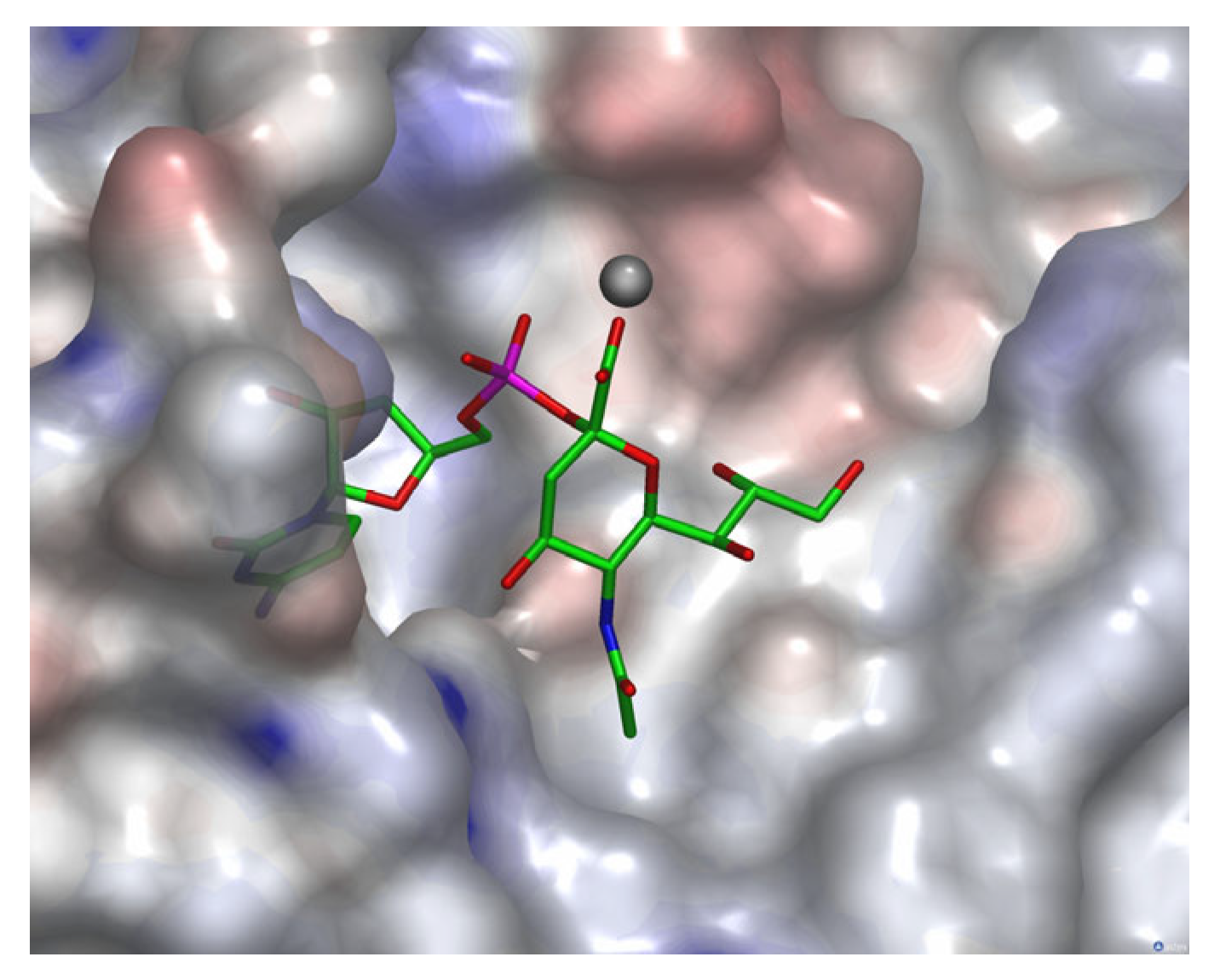
3.1. Modelling of Neu5Ac 3 in the Active Site of NmB CSS
3.2. Chemical Synthesis—Materials and Methods
3.3. Protein Expression and Purification (For Screening Assay and STD NMR)
3.4. In Vitro Inhibition Assay and Kinetic Characterization
3.5. STD NMR Spectroscopy
3.6. ELISA-Based Assay to Monitor Sialylation of NmB LOS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSS | CMP-sialic acid synthetase |
| LOS | lipooligosaccharide |
| NmB | Neisseria meningitidis serotype B |
| STD | Saturation Transfer Difference (STD) NMR |
References
- Harrison, L.H.; Trotter, C.L.; Ramsay, M.E. Global epidemiology of meningococcal disease. Vaccine 2009, 27 (Suppl. 2), B51–B63. [Google Scholar] [CrossRef]
- Dull, P.M.; McIntosh, E.D. Meningococcal vaccine development—From glycoconjugates against MenACWY to proteins against MenB—Potential for broad protection against meningococcal disease. Vaccine 2012, 30, B18–B25. [Google Scholar] [CrossRef]
- Berti, F.; Romano, M.R.; Micoli, F.; Adamo, R. Carbohydrate based meningococcal vaccines: Past and present overview. Glycoconj. J. 2021, 38, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Mukherjee, M.M.; Ghosh, R. Recent advances on the syntheses of oligosaccharides and the corresponding glycoconjugates towards development of vaccine candidates against invasive Neisseria meningitidis strains. Carbohydr. Res. 2025, 555, 109545. [Google Scholar] [CrossRef] [PubMed]
- Serruto, D.; Bottomley, M.J.; Ram, S.; Giuliani, M.M.; Rappuoli, R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine 2012, 30, B87–B97. [Google Scholar] [CrossRef]
- Finne, J.; Bitter-Suermann, D.; Goridis, C.; Finne, U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J. Immunol. 1987, 138, 4402–4407. [Google Scholar] [CrossRef]
- Häyrinen, J.; Jennings, H.; Raff, H.V.; Rougon, G.; Hanai, N.; Gerardy-Schahn, R.; Finne, J. Antibodies to polysialic acid and its N-propyl derivative: Binding properties and interaction with human embryonal brain glycopeptides. J. Infect. Dis. 1995, 171, 1481–1490. [Google Scholar] [CrossRef]
- Granoff, D.M.; Bartoloni, A.; Ricci, S.; Gallo, E.; Rosa, D.; Ravenscroft, N.; Guarnieri, V.; Seid, R.C.; Shan, A.; Usinger, W.R.; et al. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J. Immunol. 1998, 160, 5028–5036. [Google Scholar] [CrossRef] [PubMed]
- Frosch, M.; Weisgerber, C.; Meyer, T.F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 1989, 86, 1669–1673. [Google Scholar] [CrossRef]
- Mandrell, R.E.; Kim, J.J.; John, C.M.; Gibson, B.W.; Sugai, J.V.; Apicella, M.A.; Griffiss, J.M.; Yamasaki, R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J. Bacteriol. 1991, 173, 2823–2832. [Google Scholar] [CrossRef]
- Schauer, R.; Kamerling, J.P. Exploration of the sialic acid world. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–213. [Google Scholar] [CrossRef] [PubMed]
- Vogel, U.; Hammerschmidt, S.; Frosch, M. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med. Microbiol. Immunol. 1996, 185, 81–87. [Google Scholar] [CrossRef]
- Virji, M.; Makepeace, K.; Peak, I.R.A.; Ferguson, D.J.P.; Jennings, M.P.; Moxon, E.R. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: Mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 1995, 18, 741–754. [Google Scholar] [CrossRef]
- Unkmeir, A.; Kämmerer, U.; Stade, A.; Hübner, C.; Haller, S.; Kolb-Mäurer, A.; Frosch, M.; Dietrich, G. Lipooligosaccharide and polysaccharide capsule: Virulence factors of Neisseria meningitidis that determine meningococcal interaction with human dendritic cells. Infect. Immun. 2002, 70, 2454–2462. [Google Scholar] [CrossRef]
- Kurzai, O.; Schmitt, C.; Claus, H.; Vogel, U.; Frosch, M.; Kolb-Mäurer, A. Carbohydrate composition of meningococcal lipopolysaccharide modulates the interaction of Neisseria meningitidis with human dendritic cells. Cell Microbiol. 2005, 7, 1319–1334. [Google Scholar] [CrossRef]
- Jarvis, G.A.; Vedros, N.A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 1987, 55, 174–180. [Google Scholar] [CrossRef]
- Jarvis, G.A. Recognition and control of neisserial infection by antibody and complement. Trends Microbiol. 1995, 3, 198–201. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Hilse, R.; van Putten, J.P.; Gerardy-Schahn, R.; Unkmeir, A.; Frosch, M. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 1996, 15, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kahler, C.M.; Martin, L.E.; Shih, G.C.; Rahman, M.M.; Carlson, R.W.; Stephens, D.S. The (alpha 2–>8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 1998, 66, 5939–5947. [Google Scholar] [CrossRef]
- Warren, L.; Blacklow, R.S. The biosynthesis of cytidine 5′-monophospho-N-acetylneuraminic acid by an enzyme from Neisseria meningitidis. J. Biol. Chem. 1962, 237, 3527–3534. [Google Scholar] [CrossRef] [PubMed]
- Roseman, S. Enzymatic synthesis of cytidine 5′-mono-phospho-sialic acids. Proc. Natl. Acad. Sci. USA 1962, 48, 437–441. [Google Scholar] [CrossRef]
- Kean, E.L. Sialic acid activation. Glycobiology 1991, 1, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.; Holbein, B.E. Physiology of sialic acid capsular polysaccharide synthesis in serogroup B Neisseria meningitidis. J. Bacteriol. 1983, 154, 728–736. [Google Scholar] [CrossRef]
- Münster-Kühnel, A.K.; Tiralongo, J.; Krapp, S.; Weinhold, B.; Ritz-sedlacek, V.; Jacob, U.; Gerardy-Schahn, R. Structure and function of vertebrate CMP-sialic acid synthetase. Glycobiology 2004, 14, 43R–51R. [Google Scholar] [CrossRef]
- Severi, E.; Hood, D.W.; Thomas, G.H. Sialic acid utilization by bacterial pathogens. Microbiology 2007, 153, 2817–2822. [Google Scholar] [CrossRef]
- Abeln, M.; Borst, K.M.; Cajic, S.; Thiesler, H.; Kats, E.; Albers, I.; Kuhn, M.; Kaever, V.; Rapp, E.; Münster-Kühnel, A.; et al. Sialylation is dispensable for early murine embryonic development in vitro. ChemBioChem 2017, 18, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Schmid, W.; Christian, R.; Zbiral, E. Synthesis of both epimeric 2-deoxy-N-acetylneuraminic acids and their behaviour towards CMP-sialate synthetase—A comparison with 2-β-methylketoside of N-acetylneuraminic acid. Tetrahedron Lett. 1988, 29, 3643–3646. [Google Scholar] [CrossRef]
- Petrie, C.R.I.; Sharma, M.; Simmons, O.D.; Korytnyk, W. Synthesis of analogs of N-acetylneuraminic acid and their effect on CMP-sialate synthase. Carbohydr. Res. 1989, 186, 326–334. [Google Scholar] [CrossRef]
- Knorst, M.; Fessner, W.-D. CMP-Sialate synthetase from Neisseria meningitidis—Overexpression and application to the synthesis of oligosaccharides containing modified sialic acids. Adv. Synth. Catal. 2001, 343, 698–710. [Google Scholar] [CrossRef]
- Yu, H.; Yu, H.; Karpel, R.; Chen, X. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: Comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg. Med. Chem. 2004, 12, 6427–6435. [Google Scholar] [CrossRef]
- He, N.; Yi, D.; Fessner, W.-D. Flexibility of substrate binding of cytosine-5′-monophosphate-N-acetylneuraminate synthetase (CMP-Sialate Synthetase) from Neisseria meningitidis: An enabling catalyst for the synthesis of neo-sialoconjugates. Adv. Synth. Catal. 2011, 353, 2384–2398. [Google Scholar] [CrossRef]
- Yi, D.; He, N.; Kickstein, M.; Metzner, J.; Weiß, M.; Berry, A.; Fessner, W.-D. Engineering of a cytidine 5′-monophosphate-sialic acid synthetase for improved tolerance to functional sialic acids. Adv. Synth. Catal. 2013, 355, 3597–3612. [Google Scholar] [CrossRef]
- Khedri, Z.; Li, Y.; Muthana, S.; Muthana, M.M.; Hsiao, C.-W.; Yu, H.; Chen, X. Chemoenzymatic synthesis of sialosides containing C7-modified sialic acids and their application in sialidase substrate specificity studies. Carbohydr. Res. 2014, 389, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Morley, T.J.; Withers, S.G. Chemoenzymatic synthesis and enzymatic analysis of 8-modified cytidine monophosphate-sialic acid and sialyl lactose derivatives. J. Am. Chem. Soc. 2010, 132, 9430–9437. [Google Scholar] [CrossRef] [PubMed]
- Rauvolfova, J.; Venot, A.; Boons, G.-J. Chemo-enzymatic synthesis of C-9 acetylated sialosides. Carbohydr. Res. 2008, 343, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Mertsch, A.; Poschenrieder, S.; Fessner, W.-D. Semi-synthetic sialic acid probes for challenging the substrate promiscuity of enzymes in the sialoconjugation pathway. Adv. Synth. Catal. 2020, 362, 5485–5495. [Google Scholar] [CrossRef]
- Mizanur, R.M.; Pohl, N.L. Bacterial CMP-sialic acid synthetases: Production, properties, and applications. Appl. Microbiol. Biotechnol. 2008, 80, 757–765. [Google Scholar] [CrossRef]
- Yu, H.; Chokhawala, H.A.; Huang, S.; Chen, X. One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat. Protoc. 2006, 1, 2485–2492. [Google Scholar] [CrossRef]
- Malekan, H.; Fung, G.; Thon, V.; Khedri, Z.; Yu, H.; Qu, J.; Li, Y.; Ding, L.; Lam, K.S.; Chen, X. One-pot multi-enzyme (OPME) chemoenzymatic synthesis of sialyl-Tn-MUC1 and sialyl-T-MUC1 glycopeptides containing natural or non-natural sialic acid. Bioorg. Med. Chem. 2013, 21, 4778–4785. [Google Scholar] [CrossRef]
- Hartmann, M.; Christian, R.; Zbiral, E. Structural variations of N-acetylneuraminic acid, 14. Synthesis of the β-methyl ketosides of 4-oxo-, 7-oxo-, and 8-oxo-N-acetylneuraminic acid and the corresponding 7,7- and 8,8-dimethoxy derivatives their behaviour towards CMP-sialate synthase. Liebigs Ann. Chem. 1990, 83–91. [Google Scholar] [CrossRef]
- Hartmann, M.; Christian, R.; Zbiral, E. Structural variations of N-acetylneuraminic acid. Part 19. Synthesis of both epimeric pairs of the 4-C-methyl- and 4-deoxy-4-C-methyl- as well as of the β-methylketoside of 4-deoxy-4-C-methylene-N-acetylneuraminic acid. Behavior towards CMP-sialate synthase. Monatsh. Chem. 1991, 122, 111–125. [Google Scholar] [CrossRef]
- Wong, J.H.; Sahni, U.; Li, Y.; Chen, X.; Gervay-Hague, J. Synthesis of sulfone-based nucleotide isosteres: Identification of CMP-sialic acid synthetase inhibitors. Org. Biomol. Chem. 2009, 7, 27–29. [Google Scholar] [CrossRef]
- Horsfall, L.E.; Nelson, A.; Berry, A. Identification and characterization of important residues in the catalytic mechanism of CMP-Neu5Ac synthetase from Neisseria meningitidis. FEBS J. 2010, 277, 2779–2790. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.M.; McArthur, J.B.; Li, Y.; Yu, H.; Chen, X.; Fisher, A.J. Catalytic cycle of Neisseria meningitidis CMP-sialic acid synthetase illustrated by high-resolution protein crystallography. Biochemistry 2020, 59, 3157–3168. [Google Scholar] [CrossRef] [PubMed]
- Krapp, S.; Münster-Kühnel, A.K.; Kaiser, J.T.; Huber, R.; Tiralongo, J.; Gerardy-Schahn, R.; Jacob, U. The crystal structure of murine CMP-5-N-acetylneuraminic acid synthetase. J. Mol. Biol. 2003, 334, 625–637. [Google Scholar] [CrossRef]
- Haselhorst, T.; Münster-Kühnel, A.K.; Stolz, A.; Oschlies, M.; Tiralongo, J.; Kitajima, K.; Gerardy-Schahn, R.; von Itzstein, M. Probing a CMP-Kdn synthetase by 1H, 31P, and STD NMR spectroscopy. Biochem. Biophy. Res. Commun. 2005, 327, 565–570. [Google Scholar] [CrossRef]
- Sellmeier, M.; Weinhold, B.; Münster-Kühnel, A. CMP-Sialic acid synthetase: The point of constriction in the sialylation pathway. In SialoGlyco Chemistry and Biology I: Biosynthesis, Structural Diversity and Sialoglycopathologies; Gerardy-Schahn, R., Delannoy, P., von Itzstein, M., Eds.; Springer: Berlin, Heidelberg, 2015; pp. 139–167. [Google Scholar]
- Jen, F.E.-C.; Ketterer, M.R.; Semchenko, E.A.; Day, C.J.; Seib, K.L.; Apicella, M.A.; Jennings, M.P. The Lst sialyltransferase of Neisseria gonorrhoeae can transfer keto-deoxyoctanoate as the terminal sugar of lipooligosaccharide: A glyco-achilles heel that provides a new strategy for vaccines to prevent Gonorrhea. mBio 2021, 12, e03666-20. [Google Scholar] [CrossRef] [PubMed]
- Mosimann, S.C.; Gilbert, M.; Dombroswki, D.; To, R.; Wakarchuk, W.; Strynadka, N.C.J. Structure of a sialic acid-activating synthetase, CMP-acylneuraminate synthetase in the presence and absence of CDP. J. Biol. Chem. 2001, 276, 8190–8196. [Google Scholar] [CrossRef]
- Hartshorn, M.J. AstexViewer: A visualisation aid for structure-based drug design. J. Comp. Aided Mol. Des. 2002, 16, 871–881. [Google Scholar] [CrossRef]
- Yuan, Y.; Bleile, D.W.; Wen, X.; Sanders, D.A.R.; Itoh, K.; Liu, H.-w.; Pinto, B.M. Investigation of binding of UDP-Galf and UDP-[3-F]Galf to UDPgalactopyranose mutase by STD-NMR spectroscopy, molecular dynamics, and CORCEMA-ST calculations. J. Am. Chem. Soc. 2008, 130, 3157–3168. [Google Scholar] [CrossRef]
- Winger, M.; von Itzstein, M. Exposing the flexibility of human parainfluenza virus hemagglutinin-neuraminidase. J. Am. Chem. Soc. 2012, 134, 18447–18452. [Google Scholar] [CrossRef]
- Bose, S.; Purkait, D.; Joseph, D.; Nayak, V.; Subramanian, R. Structural and functional characterization of CMP-N-acetylneuraminate synthetase from Vibrio cholerae. Acta Crystallogr. D Struct. Biol. 2019, 75, 564–577. [Google Scholar] [CrossRef]
- Zbiral, E.; Schreiner, E.; Christian, R. Synthesis of the 4-acetamido-4-deoxy analogue of N-acetylneuraminic acid and its behaviour towards CMP-sialate synthase. Carbohydr. Res. 1989, 194, c15–c18. [Google Scholar] [CrossRef]
- Kuhn, R.; Lutz, P.; MacDonald, D.L. Syntheses anomerer sialinsaure-methylketoside. Chem. Ber. 1966, 99, 611–617. [Google Scholar] [CrossRef]
- Chopra, P.; Thomson, R.J.; Grice, I.D.; von Itzstein, M. Rapid and clean microwave-assisted synthesis of N-acetylneuraminic acid methyl ester and its β-methyl glycoside. Tetrahedron Lett. 2012, 53, 6254–6256. [Google Scholar] [CrossRef]
- Byramova, N.E.; Tuzikov, A.B.; Bovin, N.V. A simple procedure for the synthesis of the methyl and benzyl glycosides of Neu5Ac and 4-epi-Neu5Ac. Conversion of the benzyl and methyl glycosides of Neu5Ac into N-trifluoroacetylneuraminic acid benzyl glycosides. Carbohydr. Res. 1992, 237, 161–175. [Google Scholar] [CrossRef]
- Czarniecki, M.F.; Thornton, E.R. Carbon-13 nuclear magnetic resonance spin-lattice relaxation in the N-acylneuraminic acids. Probes for internal dynamics and conformational analysis. J. Am. Chem. Soc. 1977, 99, 8273. [Google Scholar] [CrossRef]
- Kiefel, M.J.; Chopra, P.; Madge, P.D.; Szyczew, A.; Thomson, R.J.; Grice, I.D.; von Itzstein, M. Synthesis of C-9 oxidised N-acetylneuraminic acid derivatives as biological probes. Tetrahedron Lett. 2011, 52, 98–100. [Google Scholar] [CrossRef]
- Ramamoorthy, P.S.; Gervay, J. Solution phase synthesis of amide-linked N-acetyl neuraminic acid, α-amino acid, and sugar amino acid conjugates. J. Org. Chem. 1997, 62, 7801–7805. [Google Scholar] [CrossRef]
- Gregar, T.Q.; Gervay-Hague, J. Synthesis of oligomers derived from amide-linked neuraminic acid analogues. J. Org. Chem. 2003, 69, 1001–1009. [Google Scholar] [CrossRef]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef]
- Honda, T.; Masuda, T.; Yoshida, S.; Arai, M.; Kaneko, S.; Yamashita, M. Synthesis and anti-Influenza virus activity of 7-O-alkylated derivatives related to zanamivir. Bioorg. Med. Chem. Lett. 2002, 12, 1925–1928. [Google Scholar] [CrossRef]
- Masuda, T.; Yoshida, S.; Arai, M.; Kaneko, S.; Yamashita, M.; Honda, T. Synthesis and anti-influenza evaluation of polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives. Chem. Pharm. Bull. 2003, 51, 1386–1398. [Google Scholar] [CrossRef]
- Masuda, T.; Shibuya, S.; Arai, M.; Yoshida, S.; Tomozawa, T.; Ohno, A.; Yamashita, M.; Honda, T. Synthesis and anti-influenza evaluation of orally active bicyclic ether derivatives related to zanamivir. Bioorg. Med. Chem. Lett. 2003, 13, 669–673. [Google Scholar] [CrossRef]
- Brandstetter, H.H.; Zbiral, E. Strukturelle Abwandlungen an N-Acetylneuraminsäure, 2 [Transformations with N-acetylneuraminic acid. 2]. Liebigs Ann. Chem. 1983, 2055–2065. [Google Scholar] [CrossRef]
- Zbiral, E.; Phadtare, S.; Schmid, W. Strukturelle Abwandlungen an N-Acetylneuraminsäure, 6. Synthesen von 7-Oxo- und 8-Oxo-N-acetylneuraminsäure-Derivaten. Liebigs Ann. Chem. 1987, 39–43. [Google Scholar] [CrossRef]
- Sato, K.; Ikeda, K.; Suzuki, T.; Aoyama, S.; Maki, N.; Suzuki, Y.; Sato, M. Synthesis of 4-O-[3-(aryl)prop-2-ynyl]-Neu5Ac2en and its 4-epi-analogs modified at C-4 by Sonogashira coupling reaction. Tetrahedron 2007, 63, 7571–7581. [Google Scholar] [CrossRef]
- Isecke, R.; Brossmer, R. Synthesis of 5-N- and 9-N-thioacylated sialic acids. Tetrahedron 1994, 50, 7445–7460. [Google Scholar] [CrossRef]
- Roy, R.; Pon, R.A. Efficient synthesis of α(2-8)-linked N-acetyl and N-glycolylneuraminic acid disaccharides from colominic acid. Glycoconj. J. 1990, 7, 3–12. [Google Scholar] [CrossRef]
- Chopra, P.; Madge, P.D.; Thomson, R.J.; Grice, I.D.; von Itzstein, M. Microwave-assisted synthesis of N-glycolylneuraminic acid derivatives. Tetrahedron Lett. 2013, 54, 5558–5561. [Google Scholar] [CrossRef]
- Sparks, M.A.; Williams, K.W.; Lukacs, C.; Schrell, A.; Priebe, G.; Spaltenstein, A.; Whitesides, G.M. Synthesis of potential inhibitors of hemagglutination by influenza virus: Chemoenzymic preparation of N-5 analogs of N-acetylneuraminic acid. Tetrahedron 1993, 49, 1–12. [Google Scholar] [CrossRef]
- Schreiner, E.; Zbiral, E.; Kleineidam, R.G.; Schauer, R. Structural variations on N-acetylneuraminic acid, 20. Synthesis of some 2,3-didehydro-2-deoxysialic acids structurally varied at C-4 and their behavior towards sialidase from Vibrio cholerae. Liebigs Ann. Chem. 1991, 129–134. [Google Scholar] [CrossRef]
- von Itzstein, M.; Jin, B.; Wu, W.Y.; Chandler, M. A convenient method for the introduction of nitrogen and sulfur at C-4 on a sialic acid analog. Carbohydr. Res. 1993, 244, 181–185. [Google Scholar] [CrossRef]
- Chandler, M.; Bamford, M.J.; Conroy, R.; Lamont, B.; Patel, B.; Patel, V.K.; Steeples, I.P.; Storer, R.; Weir, N.G.; Wright, M.; et al. Synthesis of the potent influenza neuraminidase inhibitor 4-guanidino-Neu5Ac2en. X-Ray molecular structure of 5-acetamido-4-amino-2,6-anhydro-3,4,5-trideoxy-d-erythro-l-gluco-nononic acid. J. Chem. Soc. Perkin Trans. 1 1995, 1173–1180. [Google Scholar] [CrossRef]
- Okamoto, K.; Kondo, T.; Goto, T. Functionalization of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid methyl ester. Bull. Chem. Soc. Jpn. 1987, 60, 631–636. [Google Scholar] [CrossRef]
- Ciccotosto, S.; von Itzstein, M. Synthesis of methyl 5-acetamido-3,4,5-trideoxy-4-guanidinyl-d-glycero-d-galacto-2-nonulopyranosidonic acid (4-deoxy-4-guanidino-Neu5Acα2Me). Tetrahedron Lett. 1995, 36, 5405–5408. [Google Scholar] [CrossRef]
- Warren, L. The thiobarbituric acid assay of sialic acids. J. Biol. Chem. 1959, 234, 1971–1975. [Google Scholar] [CrossRef]
- Blat, Y. Non-competitive inhibition by active site binders. Chem. Biol. Drug Des. 2010, 75, 535–540. [Google Scholar] [CrossRef]
- Pesaresi, A. Mixed and non-competitive enzyme inhibition: Underlying mechanisms and mechanistic irrelevance of the formal two-site model. J. Enzym. Inhib. Med. Chem. 2023, 38, 2245168. [Google Scholar] [CrossRef]
- Mayer, M.; Meyer, B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 1999, 38, 1784–1788. [Google Scholar] [CrossRef]
- Meyer, B.; Peters, T. NMR spectroscopy techniques for screening and identifying ligand binding to protein receptors. Angew. Chem. Int. Ed. Engl. 2003, 42, 864–890. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P. The Development of Carbohydrate-Based Chemical Probes of CMP-N-Acetylneuraminate Synthetase. Ph.D. Thesis, Griffith Univeristy, Gold Coast, Australia, 2013. Available online: http://hdl.handle.net/10072/366420 (accessed on 14 July 2025).
- Alexander, H.E. The haemophilus group. In Bacterial and Mycotic Infections of Man, 4th ed.; Dubos, R.J., Hirsch, J.G., Eds.; Pitman Medical Publishing Co.: London, UK, 1965; pp. 724–741. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Power, P.M.; Roddam, L.F.; Dieckelmann, M.; Srikhanta, Y.N.; Tan, Y.C.; Berrington, A.W.; Jennings, M.P. Genetic characterization of pilin glycosylation in Neisseria meningitidis. Microbiology 2000, 146, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Steichen, C.T.; Shao, J.Q.; Ketterer, M.R.; Apicella, M.A. Gonococcal Cervicitis: A role for biofilm in pathogenesis. J. Infect. Dis. 2008, 198, 1856–1861. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, P.; Führing, J.; Ng, P.; Haselhorst, T.; Dyason, J.C.; Rose, F.J.; Thomson, R.J.; Gerardy-Schahn, R.; Grice, I.D.; Jennings, M.P.; et al. Exploring the Scope of Functionalized N-Acylneuraminic Acid β-Methyl Glycosides as Inhibitors of Neisseria meningitidis CMP-Sialic Acid Synthetase. Molecules 2025, 30, 4329. https://doi.org/10.3390/molecules30224329
Chopra P, Führing J, Ng P, Haselhorst T, Dyason JC, Rose FJ, Thomson RJ, Gerardy-Schahn R, Grice ID, Jennings MP, et al. Exploring the Scope of Functionalized N-Acylneuraminic Acid β-Methyl Glycosides as Inhibitors of Neisseria meningitidis CMP-Sialic Acid Synthetase. Molecules. 2025; 30(22):4329. https://doi.org/10.3390/molecules30224329
Chicago/Turabian StyleChopra, Pradeep, Jana Führing, Preston Ng, Thomas Haselhorst, Jeffrey C. Dyason, Faith J. Rose, Robin J. Thomson, Rita Gerardy-Schahn, I. Darren Grice, Michael P. Jennings, and et al. 2025. "Exploring the Scope of Functionalized N-Acylneuraminic Acid β-Methyl Glycosides as Inhibitors of Neisseria meningitidis CMP-Sialic Acid Synthetase" Molecules 30, no. 22: 4329. https://doi.org/10.3390/molecules30224329
APA StyleChopra, P., Führing, J., Ng, P., Haselhorst, T., Dyason, J. C., Rose, F. J., Thomson, R. J., Gerardy-Schahn, R., Grice, I. D., Jennings, M. P., Münster-Kühnel, A. K., & von Itzstein, M. (2025). Exploring the Scope of Functionalized N-Acylneuraminic Acid β-Methyl Glycosides as Inhibitors of Neisseria meningitidis CMP-Sialic Acid Synthetase. Molecules, 30(22), 4329. https://doi.org/10.3390/molecules30224329







