Zinc β-Diketonates with Donor-Acceptor Ligands: Synthesis and Comprehensive Structural, Thermal, and Photophysical Characterization
Abstract
1. Introduction
2. Results and Discussion
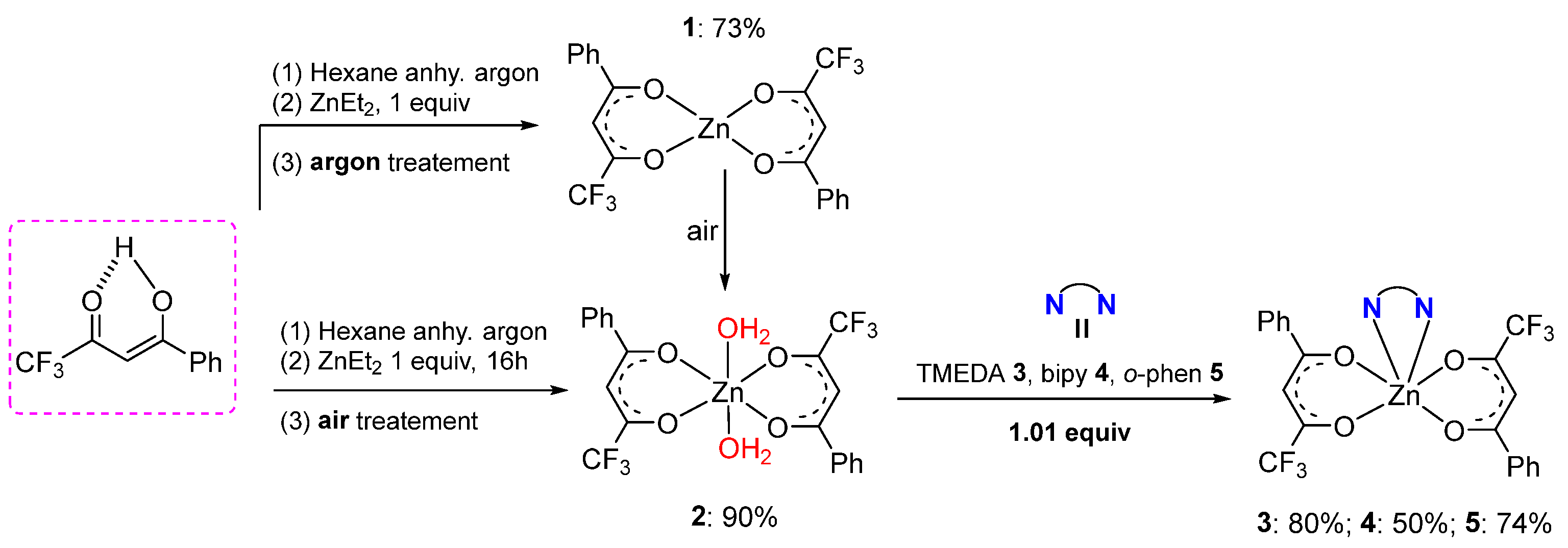
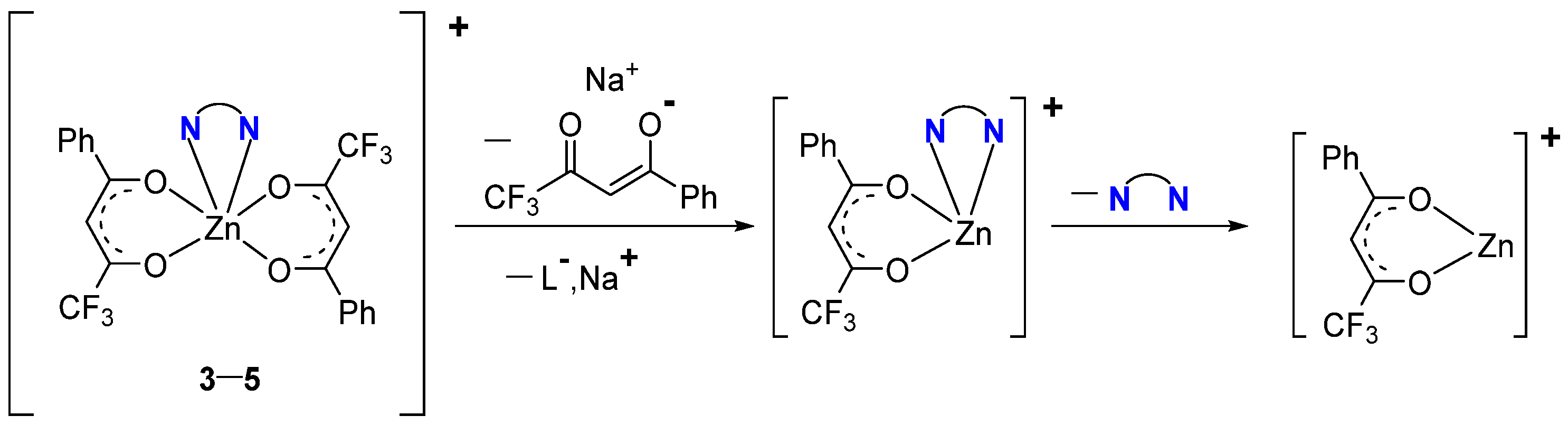
2.1. Synthesis
2.2. Mass Spectrometry: Electro-Spray Ionization (ESI)
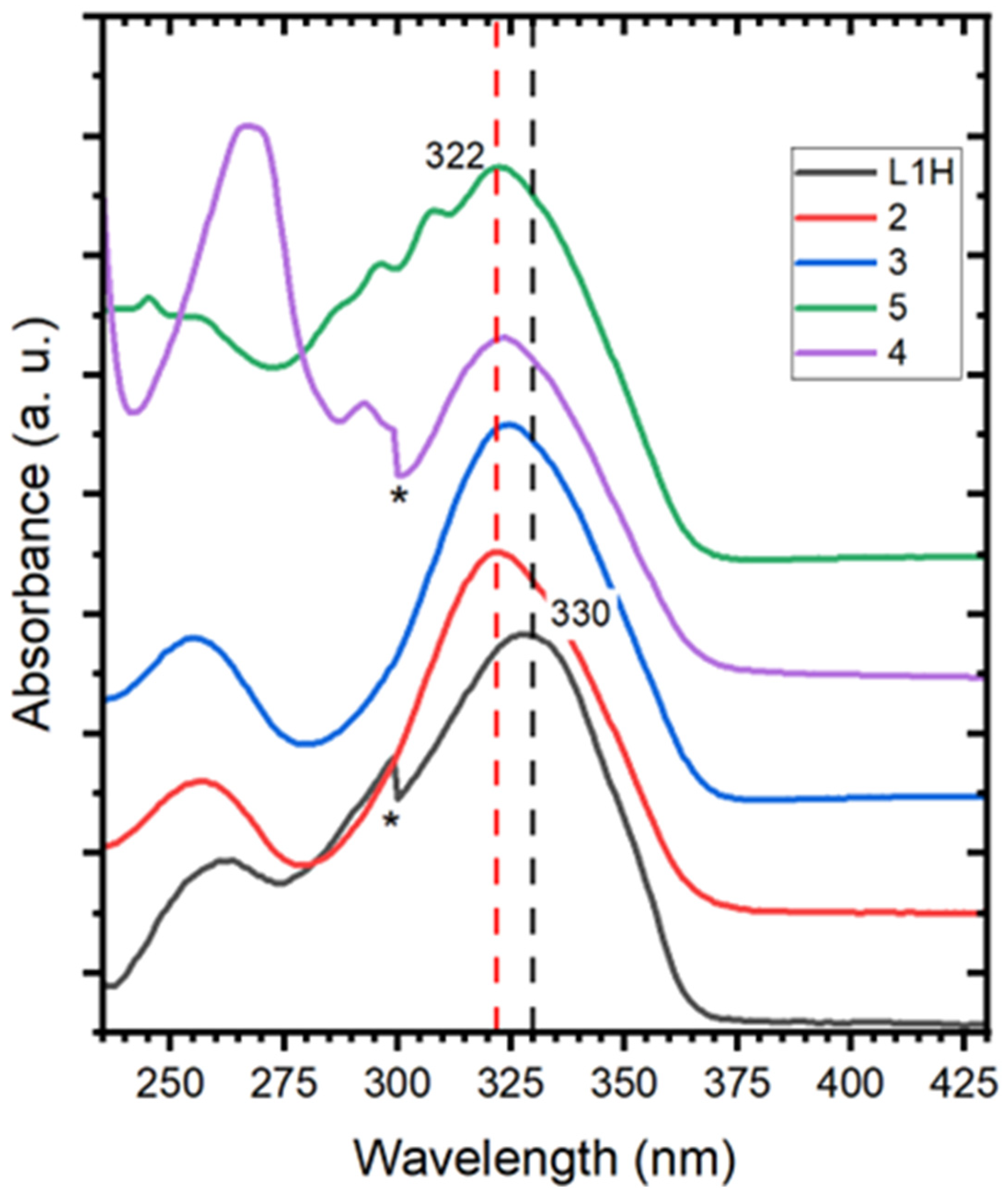
2.3. NMR and ATR-FTIR Spectroscopy
2.4. Single-Crystal X-Ray Diffraction
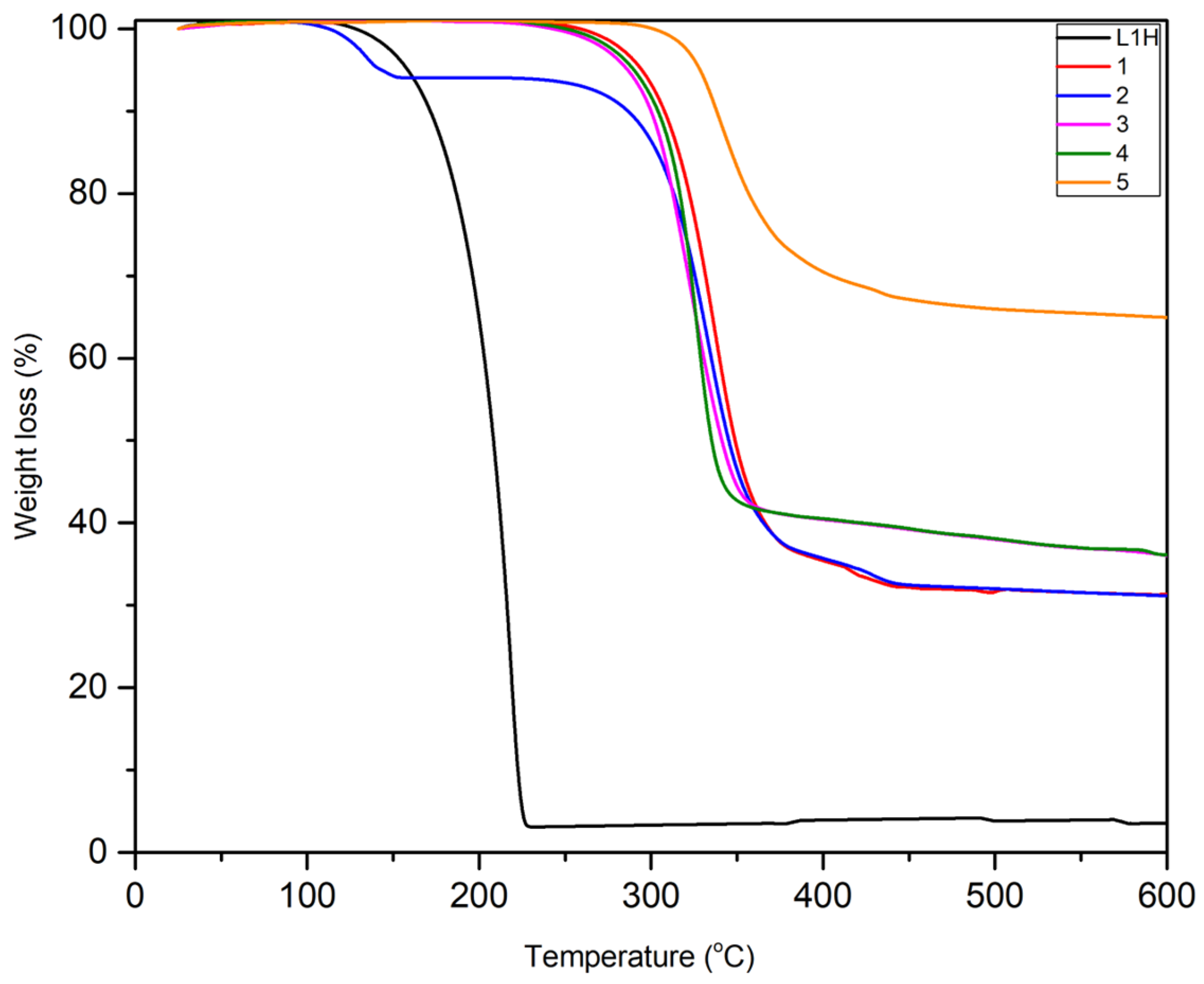
2.5. Thermal Analysis
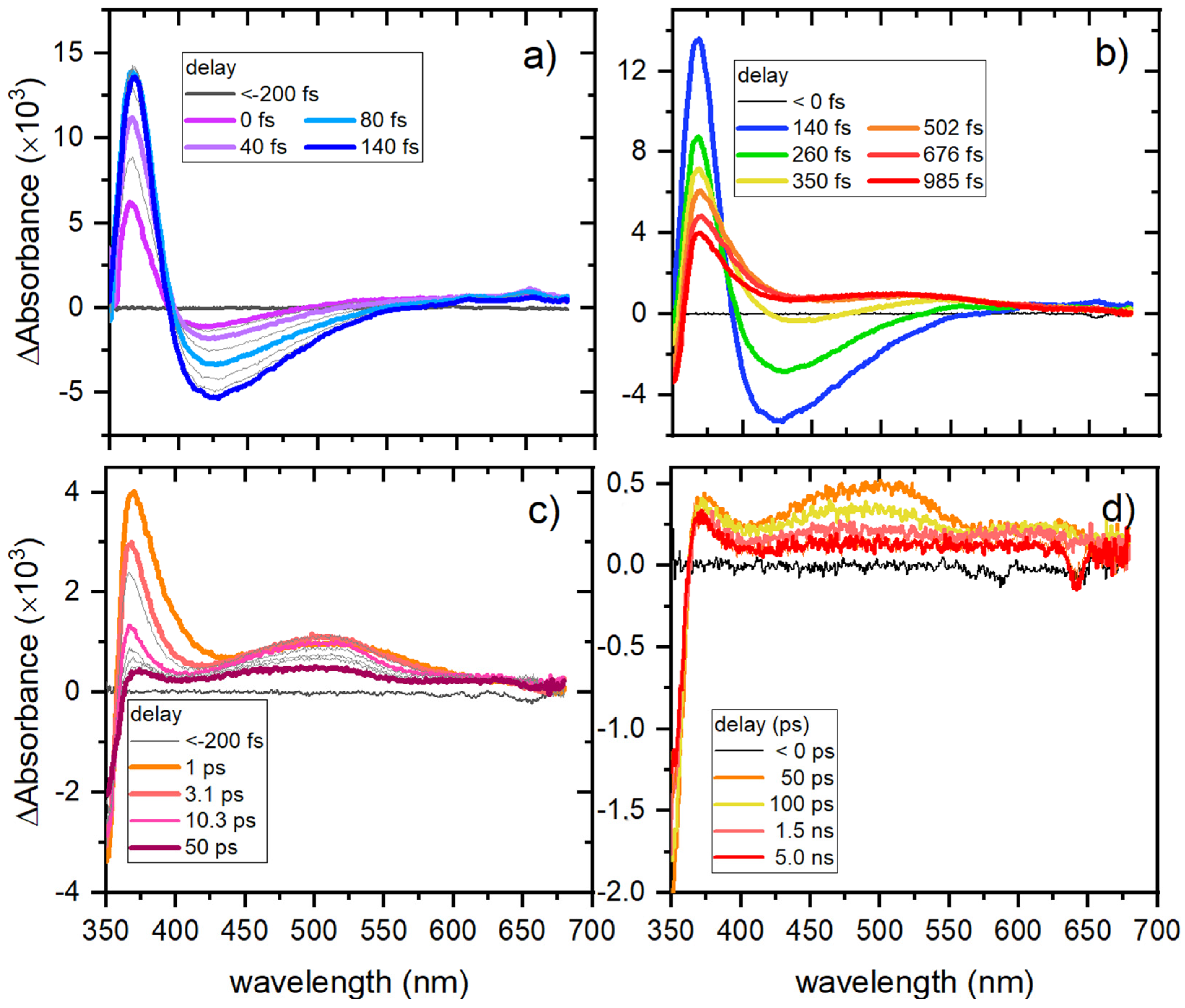
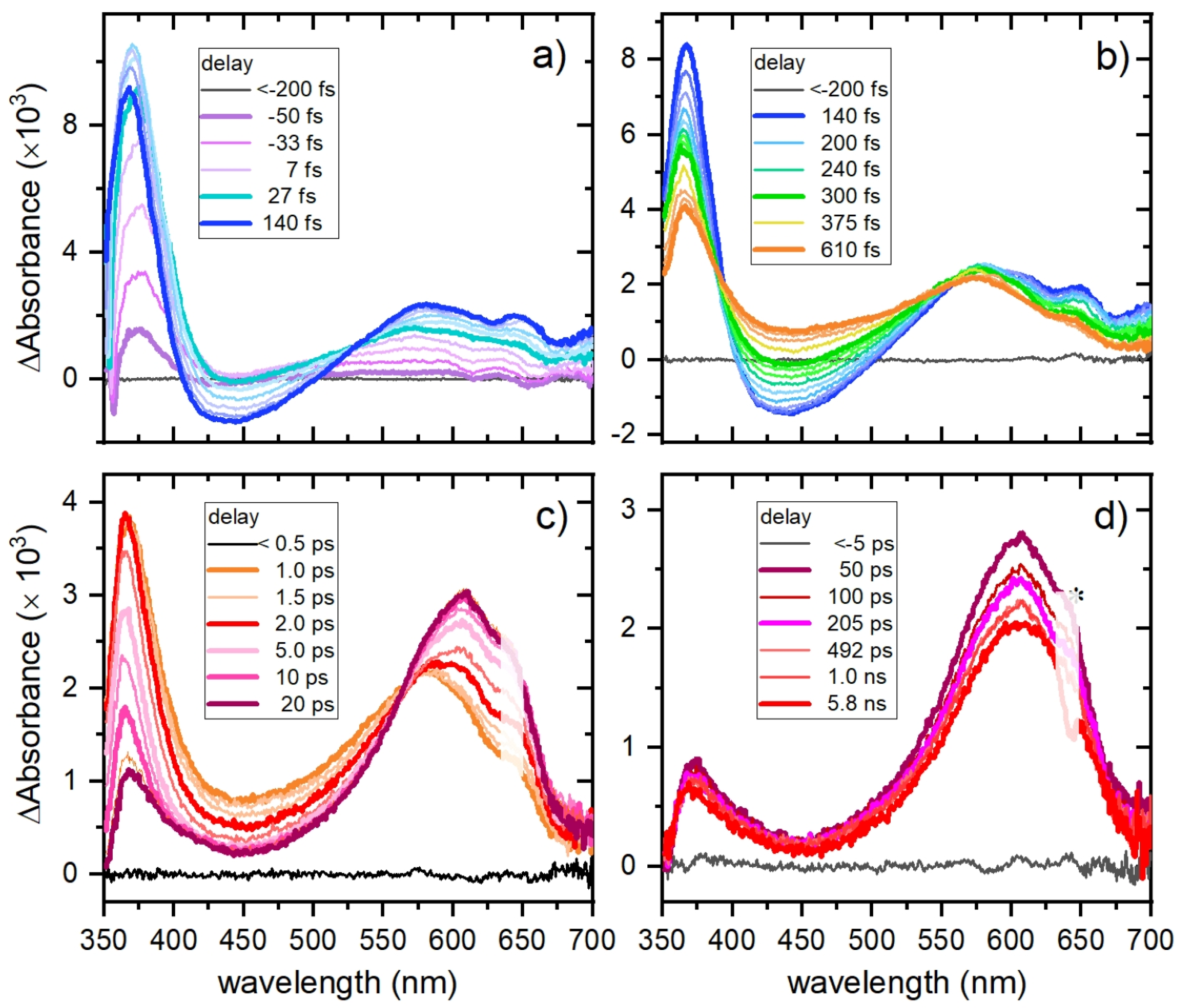
2.6. Femtosecond Transient Absorption Spectroscopy (TAS)
3. Materials and Methods
- General procedure for the synthesis of the complexes 1–5
- [Zn(L1)2]m (1)
- [Zn(L1)2(H2O)2] (2)
- [Zn(L1)2(TMEDA)] (3)
- [Zn(L1)2(bipy)] (4)
- [Zn(L1)2(o-phen)] (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESI | Electro-Spray Ionization |
| UV-Vis | Ultraviolet-Visible Spectroscopy |
| TGA | Thermogravimetric Analysis |
| ZnO | Zinc Oxide |
| TMEDA | N,N,N’,N’-Tetramethylethylenediamine |
| bipy | 2,2′-Bipyridine |
| o-phen | 1,10-Phenanthroline |
| CIF | Crystallographic Information File |
| LMCT | Ligand-to-Metal Charge Transfer |
| ESA | Excited-State Absorption |
| EtOH | Ethanol |
| CH2Cl2 | Dichloromethane |
| CHCl3 | Chloroform |
| THF | Tetrahydrofuran |
| CH3CN | Acetonitrile |
| H2O | Water |
| ZnEt2 | Diethylzinc |
| L1H | 4,4,4-Trifluoro-1-phenylbutane-1,3-dione |
| LB | Lewis Base |
| DBM | Dibenzoylmethane |
References
- Music, S.; Saric, A.; Popovic, S. Formation of nanosize ZnO particles by thermal decomposition of zinc acetylacetonate monohydrate. Ceram. Int. 2010, 36, 1117–1123. [Google Scholar] [CrossRef]
- Klotzsche, M.; Barreca, D.; Bigiani, L.; Seraglia, R.; Gasparotto, A.; Vanin, L.; Jandl, C.; Pöthig, A.; Roverso, M.; Bogialli, S.; et al. Facile preparation of a cobalt diamine diketonate adduct as a potential vapor phase precursor for Co3O4 films. Dalton Trans. 2021, 50, 10374–10382. [Google Scholar] [CrossRef] [PubMed]
- Promdet, P.; Niiranen, P.; Lagerkvist, S.; Lundin, D.; Pedersen, H. Self-limiting deposition of copper from copper beta-diketonates and plasma electrons. J. Vac. Sci. Technol. A 2025, 43, 040402. [Google Scholar] [CrossRef]
- Condorelli, G.G.; Malandrino, G.; Fragalà, I. Engineering of molecular architectures of β-diketonate precursors toward new advanced materials. Coord. Chem. Rev. 2007, 251, 1931–1950. [Google Scholar] [CrossRef]
- Stienen, C.; Grahl, J.; Wolper, C.; Schulz, S.; Bendt, G. Fluorinated β-diketonate complexes M(tfac)2(TMEDA) (M = Fe, Ni, Cu, Zn) as precursors for the MOCVD growth of metal and metal oxide thin films. RSC Adv. 2022, 12, 22974–22983. [Google Scholar] [CrossRef] [PubMed]
- Bijou, D.; Cornier, T.; Mishra, S.; Merzoud, L.; Chermette, H.; Jeanneau, E.; Maudez, W.; Benvenuti, G.; Daniele, S. Synthesis and Thermal Behavior of Heteroleptic γ-Substituted Acetylacetonate-Alkoxides of Titanium. Eur. J. Inorg. Chem. 2021, 20, 1976–1983. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, D.; Yang, M.; Wang, Y.; Zhou, F.; Zhou, H. β-Diketonate ligands for photophysical applications. Asian J. Chem. 2013, 25, 6900–6906. [Google Scholar] [CrossRef]
- Kashiwaba, Y.; Katahira, F.; Haga, K.; Sekiguchi, T.; Watanabe, H. Heteroepitaxial growth of ZnO thin films by atmospheric pressure CVD method. J. Cryst. Growth 2000, 221, 431–434. [Google Scholar] [CrossRef]
- Sato, H.; Minami, T.; Miyata, T.; Takata, S.; Ishii, M. Transparent conducting ZnO thin films prepared on low temperature substrates by chemical vapour deposition using Zn(C5H7O2)2. Thin Solid Films 1994, 246, 65–70. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, S.; Xu, Y.; Long, J.; Jiao, B.; Gao, H.; Fan, X.; Liu, Y.; Deng, L.; Xiong, W. Glycerol-assisted grain modulation in femtosecond-laser-induced photochemical synthesis of patterned ZnO nanomaterials. Light Adv. Manuf. 2025, 6, 43–51. [Google Scholar] [CrossRef]
- Tran, V.T.; Wei, Y.; Yang, H.; Zhan, Z.; Du, H. All-inkjet-printed flexible ZnO micro photodetector for a wearable UV monitoring device. Nanotechnology 2017, 28, 095204. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.; Yu, J.; Mummery, P. Advances in micro/nano fabrication for functional materials. Fusion Eng. Des. 2013, 88, 2453–2456. [Google Scholar] [CrossRef]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. Organic–inorganic hybrid microstructures for additive manufacturing. RSC Adv. 2020, 10, 42838–42859. [Google Scholar] [CrossRef]
- Ramelan, A.H.; Wahyuningsih, S.; Munawaroh, H.; Narayan, R. Fabrication of micro/nano devices using multiphoton precursors. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012008. [Google Scholar] [CrossRef]
- Hervé, M.; Boyer, A.; Brédy, R.; Compagnon, I.; Lépine, F. Ultrafast dynamics in molecular ions following UV and XUV excitation: A perspective. Adv. Phys. X 2022, 7, 2123283. [Google Scholar] [CrossRef]
- Braicovich, L.; Ghiringhelli, G.; Tagliaferri, A.; van der Laan, G.; Annese, E.; Brookes, N.B. Femtosecond dynamics in ferromagnetic metals investigated with soft X-ray resonant emission. Phys. Rev. Lett. 2005, 95, 267402. [Google Scholar] [CrossRef] [PubMed]
- Salassa, L.; Garino, C.; Salassa, G.; Gobetto, R.; Nervi, C. Structural analysis of Zn β-diketonates. J. Am. Chem. Soc. 2008, 130, 9590–9597. [Google Scholar] [CrossRef] [PubMed]
- Záliš, S.; Busby, M.; Kotrba, T.; Matousek, P.; Towrie, M.; Vlček, A. Coordination geometries of heteroleptic complexes. Inorg. Chem. 2004, 43, 1723–1734. [Google Scholar] [CrossRef]
- Zakrzewski, J.; Delaire, J.A.; Daniel, C.; Cote-Bruand, I. Structural versatility of metal–β-diketonates. New J. Chem. 2004, 28, 1514–1521. [Google Scholar] [CrossRef]
- Buono-Core, G.E.; Cabello, G.; Klahn, A.H.; Del Río, R.; Hill, R.H. Characterization of Pure ZnO Thin Films Prepared by a Direct Photochemical Method. J. Non-Cryst. Solids 2006, 352, 4088–4092. [Google Scholar] [CrossRef]
- Fauteux, C.; Longtin, R.; Pegna, J.; Therriault, D. Fast Synthesis of ZnO Nanostructures by Laser-Induced Decomposition of Zinc Acetylacetonate. Inorg. Chem. 2007, 46, 11036–11047. [Google Scholar] [CrossRef]
- Shi, P.; Jiang, Q.; Zhao, X.; Zhang, Q.; Tian, Y. Femtosecond laser deposition of ZnO. Dalton Trans. 2015, 44, 8041–8048. [Google Scholar] [CrossRef]
- Ushida, T.; Higashiyama, K.; Hirabayashi, I.; Tanaka, S. Excimer Laser-Assisted Chemical Vapor Deposition of Metal-Oxide Thin Film from β-Diketone Complexes. Jpn. J. Appl. Phys. 1991, 30, L35–L38. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Loh, X.Q.; Alshammari, A.S.; Naji, A.M.; Cabrera, H.; Binzowaimil, A.M.; Aldaghri, O.A.; Ibnaouf, K.H. Investigating the Role of Temperature in Laser Assisted Chemical Bath Deposition for ZnO Growth for Photodetector Application. Photonics 2023, 10, 910. [Google Scholar] [CrossRef]
- Yang, L.; Hu, H.; Scholz, A.; Feist, F.; Cadilha Marques, G.; Kraus, S.; Bojanowski, N.M.; Blasco, E.; Barner-Kowollik, C.; Aghassi-Hagmann, J.; et al. Micro/nano printing of 3D ZnO structures. Nat. Commun. 2023, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, R.; Peeters, D.; Rogalla, D.; Becker, H.-W.; Rechmann, J.; Henke, S.; Winter, M.; Devi, A. Systematic molecular engineering of Zn-ketoiminates for application as precursors in atomic layer depositions of zinc oxide. Dalton Trans. 2016, 45, 19012–19024. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Acosta, I.; Baker, J.; Cordes, W.; Pulay, P. Calculated and Experimental Geometries and Infrared Spectra of Metal Tris-Acetylacetonates: Vibrational Spectroscopy as a Probe of Molecular Structure for Ionic Complexes. Part I. J. Phys. Chem. A 2001, 105, 238–244. [Google Scholar] [CrossRef]
- Nakamoto, K.; McCarthy, P.J.; Martell, A.E. Infrared Spectra of Metal Chelate Compounds. III. Infrared Spectra of Acetylacetonates of Trivalent Metals. J. Am. Chem. Soc. 1961, 83, 1272–1276. [Google Scholar] [CrossRef]
- Brahma, S.; Shivashankar, S.A. Zinc acetylacetonate hydrate adducted with nitrogen donor ligands: Synthesis, spectroscopic characterization, and thermal analysis. J. Mol. Struct. 2015, 1101, 41–49. [Google Scholar] [CrossRef]
- Hemaa, M.K.; Karthik, C.S.; Mahesha; Pampa, K.J.; Mallu, P.; Lokanath, N.K. 4,4,4-Trifluoro-1-phenylbutane-1,3-dione metal [Cu(II) and Ni(II)] complexes as a superlative antibacterial agent against MRSA: Synthesis, structural quantum-chemical and molecular docking studies. J. Mol. Struct. 2021, 1243, 130774. [Google Scholar] [CrossRef]
- Halz, J.H.; Heiser, C.; Wagner, C.; Merzweiler, K. Syntheses and crystal structures of three [M(acac)2(TMEDA)] complexes (M = Mn, Fe and Zn). Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Yan, H.; Wang, A.; Yang, Y.; Stern, C.L.; Metz, A.W.; Jin, S.; Wang, L.; Marks, T.J.; Ireland, J.R.; et al. MOCVD-derived highly transparent, conductive zinc- and tin-doped indium oxide thin films: Precursor synthesis, metastable phase film growth and characterization, and application as anodes in polymer light-emitting diodes. J. Am. Chem. Soc. 2005, 127, 5613–5624. [Google Scholar] [CrossRef]
- Grassie, N. Polymer Degradation and Char Formation. In The Chemistry and Technology of Polymer Degradation and Stabilization; Elsevier: London, UK, 1995; pp. 45–67. [Google Scholar]
- Wang, J.; Lei, Y.; Guo, Y.; Wang, J.; Ma, J. Investigation of different photochemical reactions of avobenzone derivatives by ultrafast transient absorption spectroscopy. Photochem. Photobiol. Sci. 2019, 18, 3000–3007. [Google Scholar] [CrossRef]
- Verma, P.; Koch, F.; Steinbacher, A.; Nuernberger, P.; Brixner, T. Ultrafast UV-Induced Photoisomerization of Intramolecularly H-Bonded Symmetric β-Diketones. J. Am. Chem. Soc. 2014, 136, 14981–14989. [Google Scholar] [CrossRef]
- Verma, P.K.; Steinbacher, A.; Koch, F.; Nuernberger, P.; Brixner, T. Monitoring ultrafast intramolecular proton transfer processes in an unsymmetric β-diketone. Phys. Chem. Chem. Phys. 2015, 17, 8459–8466. [Google Scholar] [CrossRef]
- Perrinet, Q.; Ghosh, A.C.; Canivet, J.; Wisser, F.; Roland, T.; De Waele, V. Unexpected Interference of the Triethanolamine Sacrificial Electron Donor with the Excited States of Molecular and Heterogenized Rhodium Bipyridine Photocatalysts Revealed by Femtosecond Transient Absorption Spectroscopy. J. Phys. Chem. C 2025, 129, 1313–1326. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2024. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71 Pt 1, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71 Pt 1, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
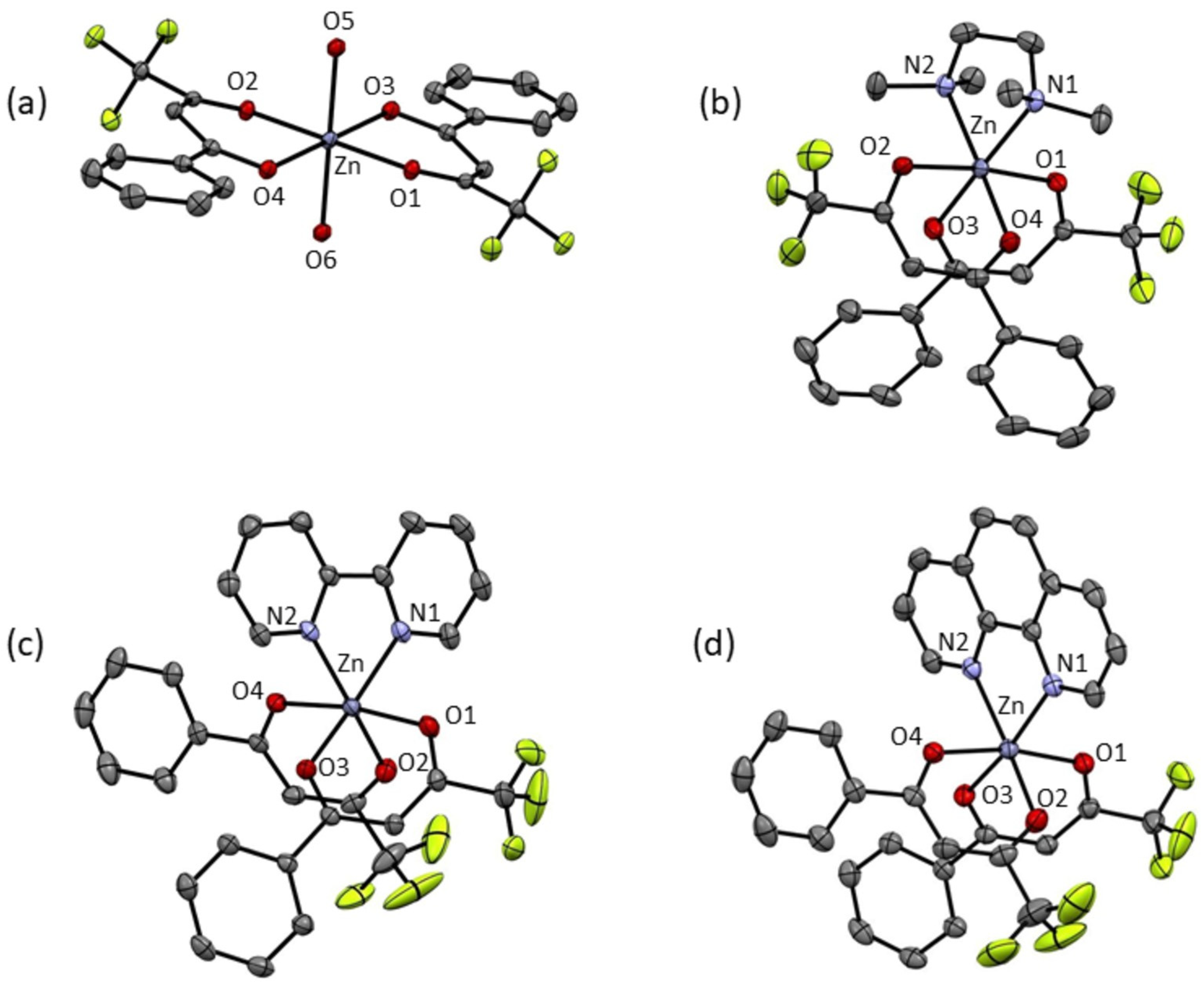
| Å | 2 | 3 | 4 | 5 |
| Zn-O1 | 2.0534 (10) | 2.0661 (15) | 2.1059 (12) | 2.0714 (16) |
| Zn-O2 | 2.0534 (10) | 2.0661 (15) | 2.0579 (13) | 2.0712 (17) |
| Zn-O3 | 2.0953 (10) | 2.0856 (17) | 2.0686 (12) | 2.0667 (15) |
| Zn-O4 | 2.0953 (10) | 2.0856 (17) | 2.1251 (11) | 2.1330 (15) |
| Zn-O5 | 2.1197 (11) | - | - | - |
| Zn-O6 | 2.1197 (11) | - | - | - |
| Zn-N1 | - | 2.1981 (19) | 2.1190 (14) | 2.1369 (18) |
| Zn-N2 | - | 2.1981 (19) | 2.1228 (14) | 2.1476 (19) |
| o | 2 | 3 | 4 | 5 |
| O1-Zn-O2 | 180.00 (5) | 175.53 (7) | 86.16 (5) | 84.83 (6) |
| O3-Zn-O4 | 180.00 (5) | 92.25 (10) | 91.38 (5) | 90.33 (6) |
| O3-Zn-N2 | - | 92.38 (7) | 92.69 (5) | 90.55 (7) |
| O4-Zn-N1 | - | 92.38 (7) | 84.61 (5) | 94.38 (6) |
| Complex | Theoretical % of Residues for ZnO | Theoretical % of Residues for ZnF2 | Experimental % Residues at 600 °C |
|---|---|---|---|
| 1 | 16 | 21 | 32 |
| 2 | 15 | 19 | 32 |
| 3 | 13 | 17 | 38 |
| 4 | 12 | 16 | 46 |
| 5 | 12 | 15 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daher, A.; Choudhari, M.; Roland, T.; De Waele, V.; Daniele, S. Zinc β-Diketonates with Donor-Acceptor Ligands: Synthesis and Comprehensive Structural, Thermal, and Photophysical Characterization. Molecules 2025, 30, 4325. https://doi.org/10.3390/molecules30224325
Daher A, Choudhari M, Roland T, De Waele V, Daniele S. Zinc β-Diketonates with Donor-Acceptor Ligands: Synthesis and Comprehensive Structural, Thermal, and Photophysical Characterization. Molecules. 2025; 30(22):4325. https://doi.org/10.3390/molecules30224325
Chicago/Turabian StyleDaher, Ahmad, Manjiri Choudhari, Thomas Roland, Vincent De Waele, and Stéphane Daniele. 2025. "Zinc β-Diketonates with Donor-Acceptor Ligands: Synthesis and Comprehensive Structural, Thermal, and Photophysical Characterization" Molecules 30, no. 22: 4325. https://doi.org/10.3390/molecules30224325
APA StyleDaher, A., Choudhari, M., Roland, T., De Waele, V., & Daniele, S. (2025). Zinc β-Diketonates with Donor-Acceptor Ligands: Synthesis and Comprehensive Structural, Thermal, and Photophysical Characterization. Molecules, 30(22), 4325. https://doi.org/10.3390/molecules30224325









