Exploring the Structural Design, Antibacterial Activity, and Molecular Docking of Newly Synthesized Zn(II) Complexes with NNO-Donor Carbazate Ligands
Abstract
1. Introduction
2. Results and Discussion
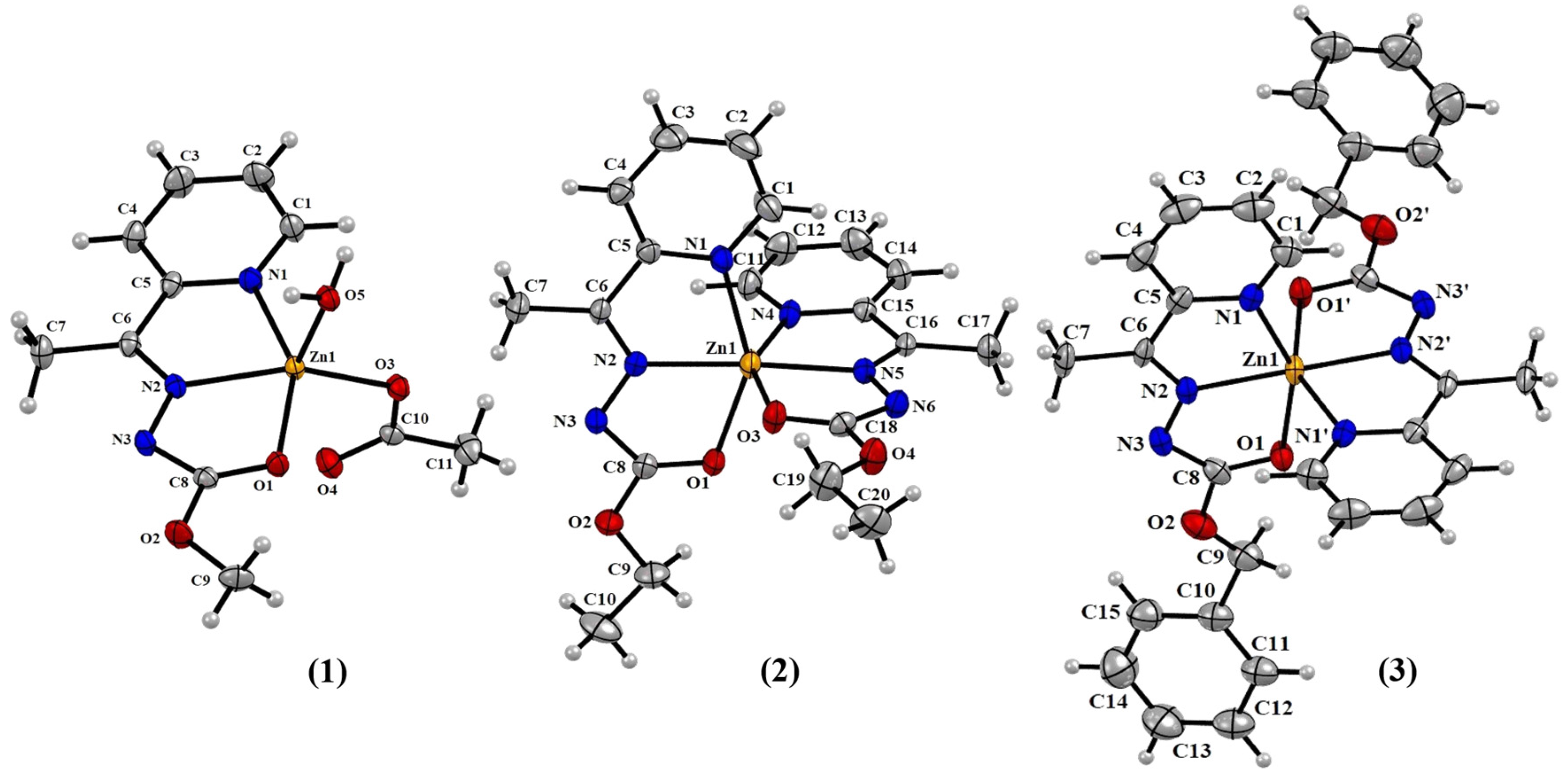
2.1. Structural Analyses
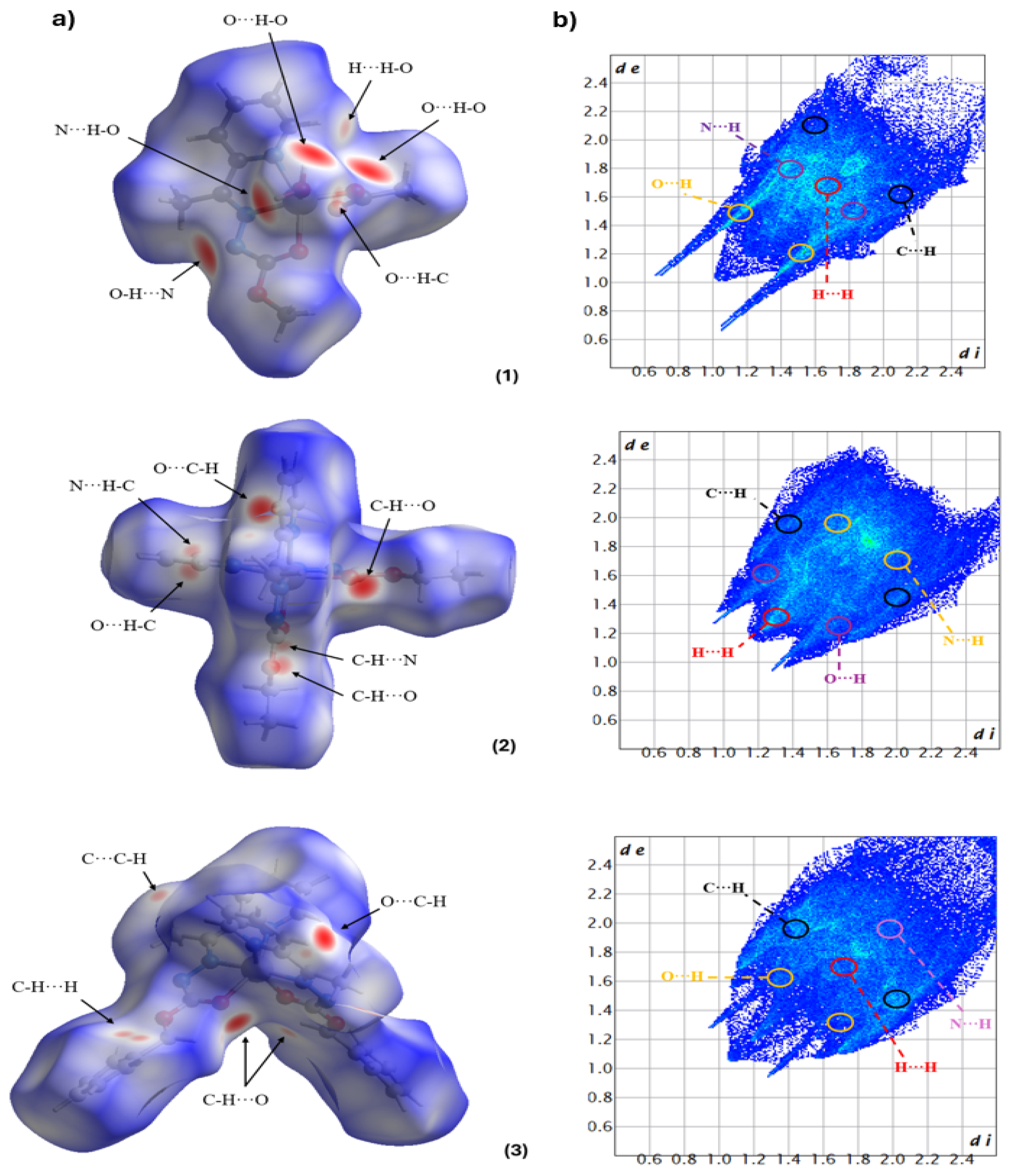
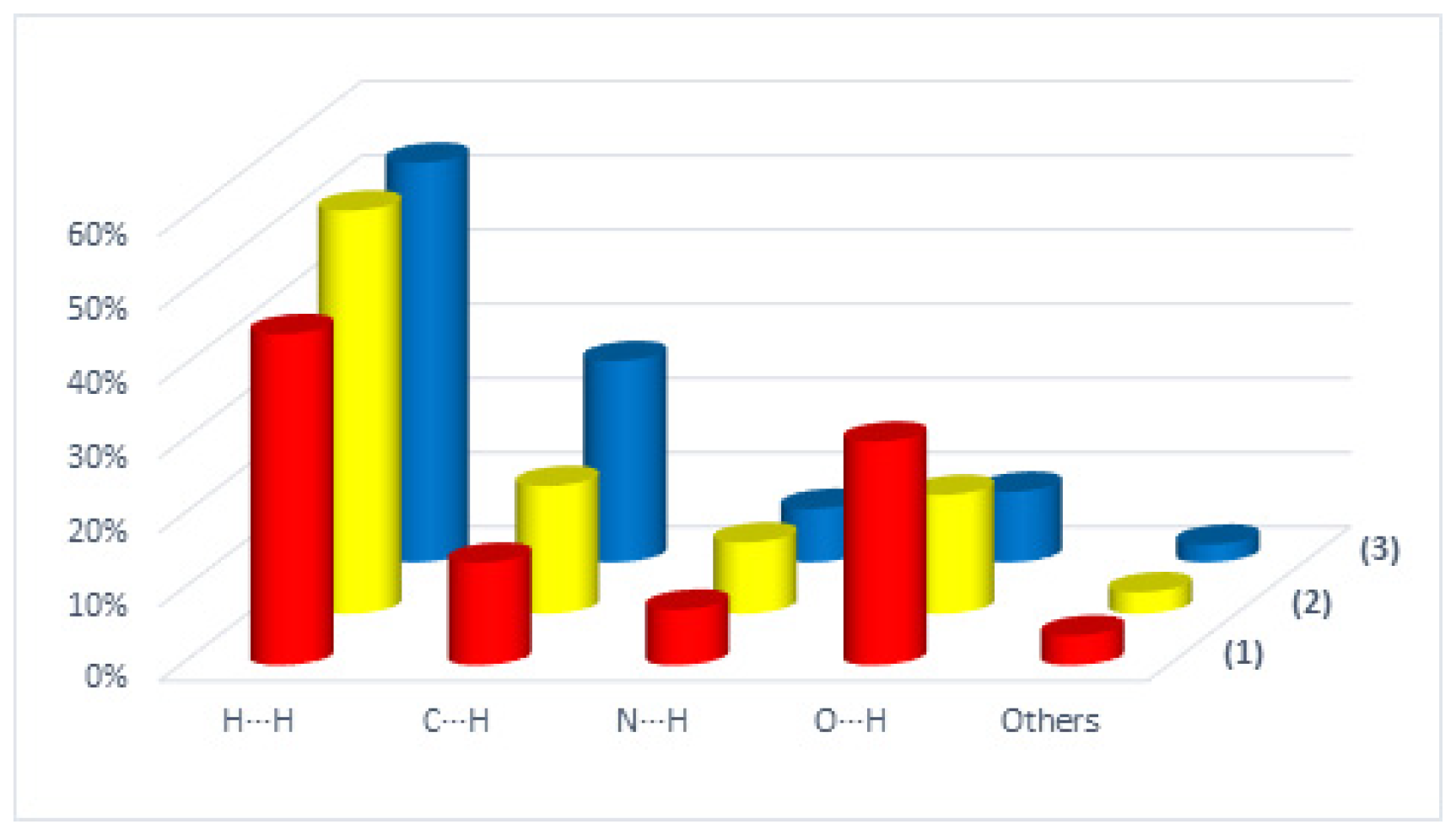
2.2. Hirshfeld Surface
2.3. Infrared Spectra
2.4. Electronic Spectra
2.5. H NMR Spectra
2.6. Antibacterial Activity
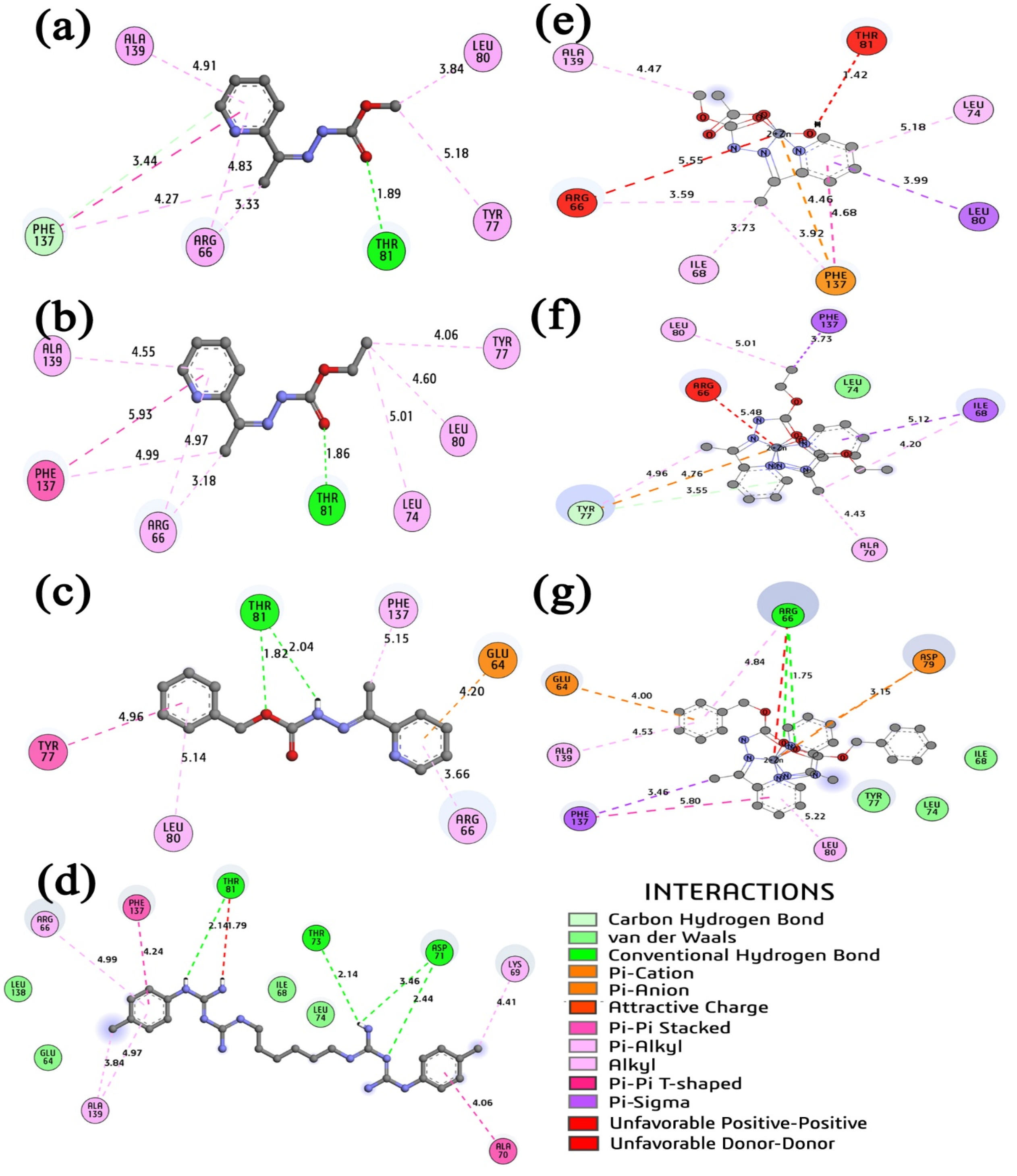
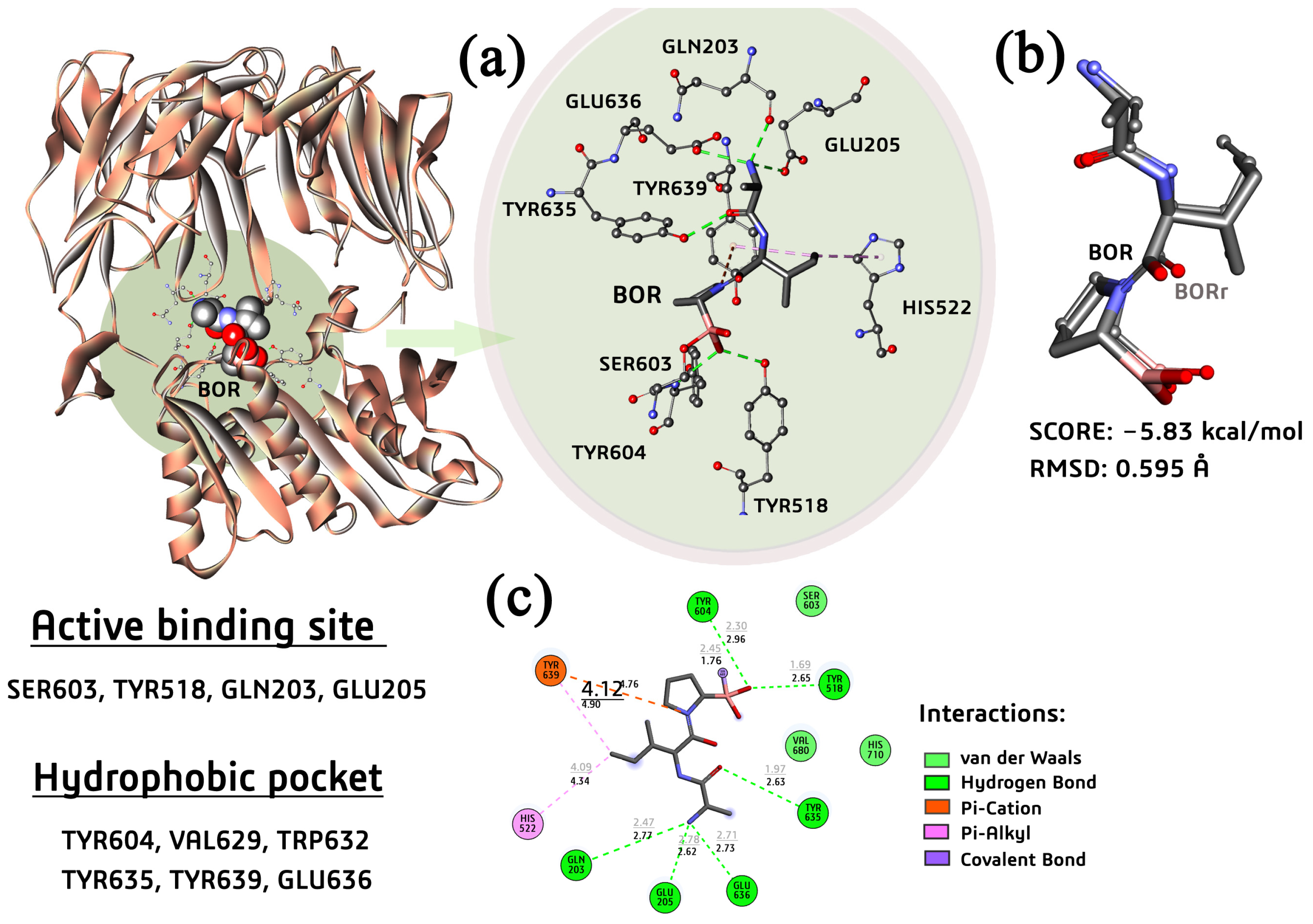
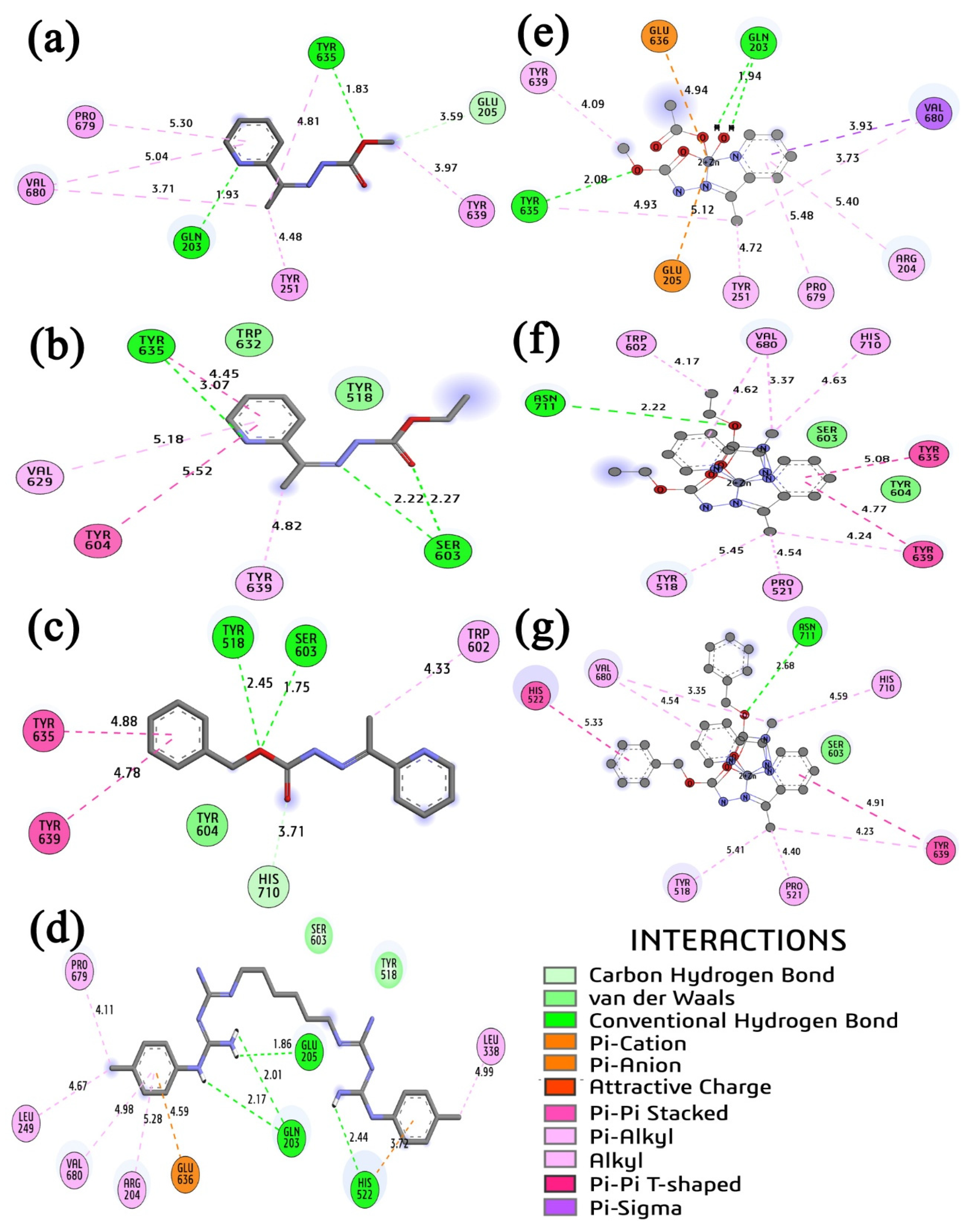
2.7. Molecular Docking Study
2.7.1. Docking of PEP–Ligands Systems
2.7.2. Docking of PTP-39-Ligands Systems
3. Materials and Methods
3.1. Materials, Methods, and Instruments
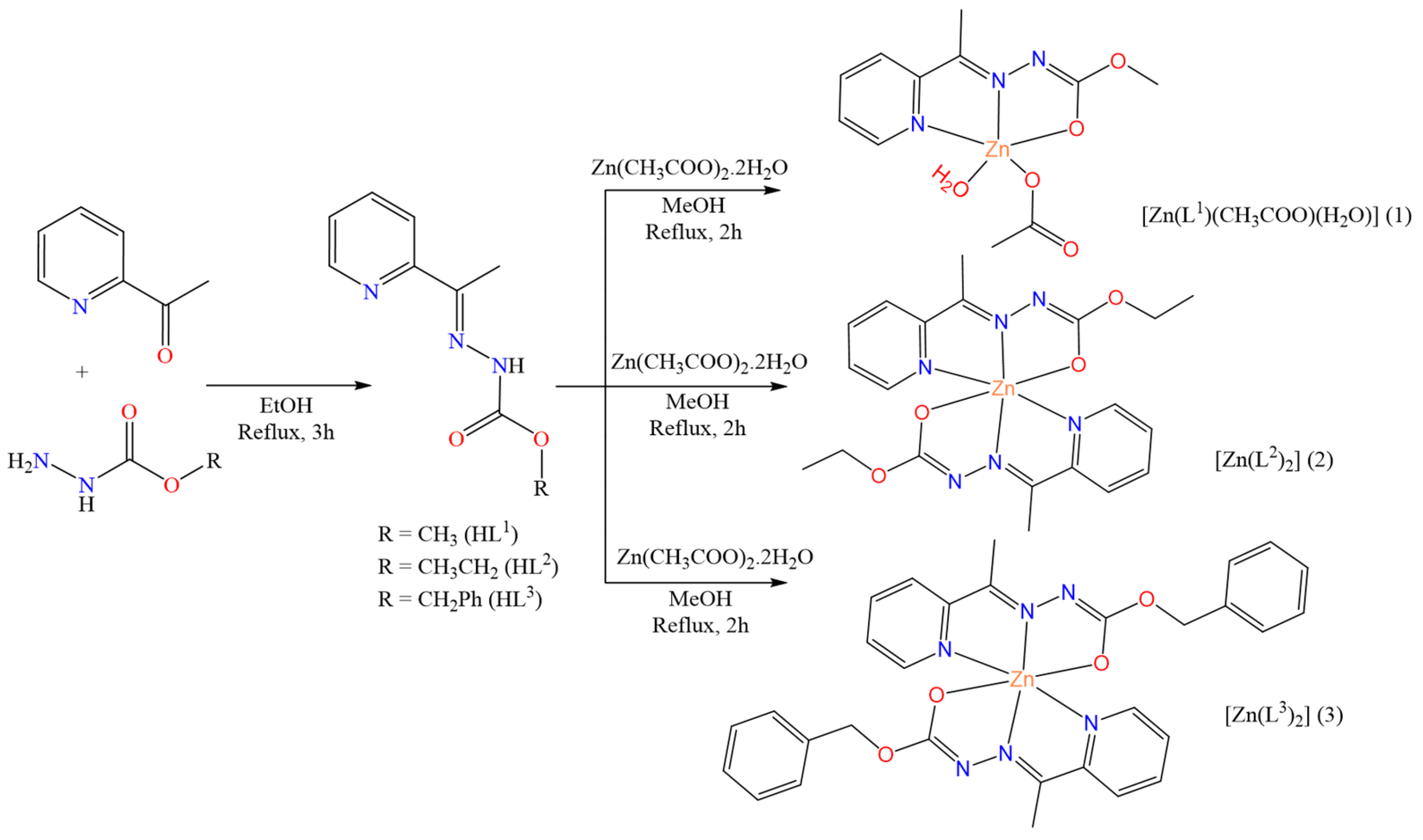
3.2. Synthesis of Carbazate Ligands (HL-HL3)
3.3. Synthesis of the Complexes [Zn(L1)(CH3COO)(H2O)] (1), [Zn(L2)2] (2), and [Zn(L3)2] (3)
3.4. Crystal Structure Determination
3.5. Computational Details
3.6. Biological Activity
3.6.1. Bacteria Used and Cultivation Conditions
3.6.2. Minimal Inhibitory Concentration (MIC)
3.7. Molecular Docking
3.7.1. Setup of the Systems
3.7.2. Protocol of Docking and Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral Microbiota in Human Systematic Diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global Epidemiology of Dental Caries and Severe Periodontitis—A Comprehensive Review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Dhahagani, K.; Mathan Kumar, S.; Chakkaravarthi, G.; Anitha, K.; Rajesh, J.; Ramu, A.; Rajagopal, G. Synthesis and Spectral Characterization of Schiff Base Complexes of Cu(II), Co(II), Zn(II) and VO(IV) Containing 4-(4-Aminophenyl)Morpholine Derivatives: Antimicrobial Evaluation and Anticancer Studies. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Hamid, M.H.S.A.; Said, A.N.A.H.; Mirza, A.H.; Karim, M.R.; Arifuzzaman, M.; Akbar Ali, M.; Bernhardt, P.V. Synthesis, Structures and Spectroscopic Properties of Some Tin(IV) Complexes of the 2-Acetylpyrazine Schiff Bases of S-Methyl- and S-Benzyldithiocarbazates. Inorganica Chim. Acta 2016, 453, 742–750. [Google Scholar] [CrossRef]
- Galván-Hidalgo, J.M.; Chans, G.M.; Ramírez-Apan, T.; Nieto-Camacho, A.; Hernández-Ortega, S.; Gómez, E. Tin(IV) Schiff Base Complexes Derived from Pyridoxal: Synthesis, Spectroscopic Properties and Cytotoxicity. Appl. Organomet. Chem. 2017, 31, e3704. [Google Scholar] [CrossRef]
- Poornima, S.; Premkumar, T.; Butcher, R.J.; Govindarajan, S. Facile One-Pot Template Synthesis of Isotypic Co(II), Ni(II) and Cu(II) Complexes of a Schiff Base Derived from Glyoxylic Acid and Ethyl Carbazate: Spectroscopic, Structural and Thermal Studies. J. Therm. Anal. Calorim. 2019, 138, 3925–3937. [Google Scholar] [CrossRef]
- Sahu, M.; Kumar Manna, A.; Rout, K.; Mondal, J.; Patra, G.K. A Highly Selective Thiosemicarbazone Based Schiff Base Chemosensor for Colorimetric Detection of Cu2+ and Ag+ Ions and Turn-on Fluorometric Detection of Ag+ Ions. Inorganica Chim. Acta 2020, 508, 119633. [Google Scholar] [CrossRef]
- Kolcu, F.; Erdener, D.; Kaya, İ. A Schiff Base Based on Triphenylamine and Thiophene Moieties as a Fluorescent Sensor for Cr(III) Ions: Synthesis, Characterization and Fluorescent Applications. Inorganica Chim. Acta 2020, 509, 119676. [Google Scholar] [CrossRef]
- Poornima, S.; Packiaraj, S.; Pushpaveni, A.; Govindarajan, S.; Butcher, R.J.; Jasinski, J.P.; Zeller, M. Neutral and Ion-Pair Silver(I) Complexes of Schiff Bases Derived from Methyl and Ethyl Carbazates with Glyoxylic Acid: Synthesis, Structure, Thermal Behavior and Cytotoxic Activity. Inorganica Chim. Acta 2019, 497, 119072. [Google Scholar] [CrossRef]
- Fekri, R.; Abdolmaleki, A.; Asadi, A.; Salehi, M.; Karimian, A.; Taghizadehmomen, L.; Raheem, R.K.; Karimian, L. Anticancer Effects of Copper (II) Hydrazone Schiff Base Complex: A Review. Basic Clin. Cancer Res. 2022, 13, 143–155. [Google Scholar] [CrossRef]
- Selvam, P.; Antharjanam, S.; Srinivasan, K.; Premkumar, T. A 1D Silver(I) Coordination Polymer of a New Hydrozone-Hydrazide Ligand: Spectral, Structural, Emission, and Anti-Bacterial Properties and Its Application as a Solid Source Precursor for Silver Oxide Nanoparticles. J. Phys. Chem. Solids 2022, 160, 110368. [Google Scholar] [CrossRef]
- Gungor, E.; Celen, S.; Azaz, D.; Kara, H. Two Tridentate Schiff Base Ligands and Their Mononuclear Cobalt (III) Complexes: Synthesis, Characterization, Antibacterial and Antifungal Activities. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2012, 94, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.J.; Diao, Q.C.; Liang, Y.H.; Zeng, K. Two Novel Co(II) Complexes with Two Different Schiff Bases: Inhibiting Growth of Human Skin Cancer Cells. Braz. J. Med. Biol. Res. 2017, 50, 2–6. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Marzano, I.M.; Ribeiro, G.H.; Colina-Vegas, L.; Pivatto, M.; Fontes, A.P.S.; Ribeiro, C.M.; Pavan, F.R.; De Almeida, K.J.; Batista, A.A.; et al. Platinum(II) Complexes with Carbazates and Hydrazides: Synthesis, Spectral Characterization, Computational Modeling, and Biological Studies. Polyhedron 2015, 98, 146–153. [Google Scholar] [CrossRef]
- Milenković, M.; Bacchi, A.; Cantoni, G.; Radulović, S.; Gligorijević, N.; Arandelović, S.; Sladić, D.; Vujčić, M.; Mitić, D.; Andelković, K. Synthesis, Characterisation and Biological Activity of Co(III) Complex with the Condensation Product of 2-(Diphenylphosphino)Benzaldehyde and Ethyl Carbazate. Inorganica Chim. Acta 2013, 395, 33–43. [Google Scholar] [CrossRef]
- de Siqueira, D.J.; Viana, M.P.; Oliveira, K.M.; Gatto, C.C. Synthesis, Crystal Design and Anticancer Potential of Novel Cu(II), Ni(II), and Pd(II) Complexes with Carbazate Ligand. ACS Omega 2025, 10, 22125–22136. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Kaminskyy, D.; Grellier, P.; Lesyk, R. Trends in Research of Antitrypanosomal Agents among Synthetic Heterocycles. Eur. J. Med. Chem. 2014, 85, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Milenković, M.; Cantoni, G.; Bacchi, A.; Spasojević, V.; Milenković, M.; Sladić, D.; Krstić, N.; Anđelković, K. Synthesis, Characterization and Antimicrobial Activity of Pd(II) and Fe(III) Complexes with Ethyl (2E)-2-[2-(Diphenylphosphino) Benzylidene] Hydrazinecarboxylate. Polyhedron 2014, 80, 47–52. [Google Scholar] [CrossRef]
- Nie, C.; Zhang, Q.; Ding, H.; Huang, B.; Wang, X.; Zhao, X.; Li, S.; Zhou, H.; Wu, J.; Tian, Y. Two Novel Six-Coordinated Cadmium(Ii) and Zinc(Ii) Complexes from Carbazate β-Diketonate: Crystal Structures, Enhanced Two-Photon Absorption and Biological Imaging Application. Dalton Trans. 2014, 43, 599–608. [Google Scholar] [CrossRef]
- de, A. Duarte, E.; Santiago, M.B.; Silva, N.B.S.; Martins, C.H.G.; Gatto, C.C. Crystal Design, Spectroscopic Analyses and Antibacterial Study of New Carbazate Ligands and Their Cu(II) Complexes. Inorganica Chim. Acta 2023, 549, 121421. [Google Scholar] [CrossRef]
- Gatto, C.C.; Duarte, E.D.A.; Liarte, G.S.; Silva, T.S.; Santiago, M.B.; Martins, C.H.G.; Gatto, C.C.; Duarte, E.D.A.; Liarte, G.S.; Thayná, S.; et al. Transition Metal Complexes with 2-Acetylpyridine- Ethylcarbazate: Noncovalent Interactions in Their Structures and Antimicrobial Studies. J. Coord. Chem. 2020, 8972, 1573–1590. [Google Scholar] [CrossRef]
- Dasgupta, S.; Karim, S.; Banerjee, S.; Saha, M.; Das Saha, K.; Das, D. Designing of Novel Zinc(Ii) Schiff Base Complexes Having Acyl Hydrazone Linkage: Study of Phosphatase and Anti-Cancer Activities. Dalton Trans. 2020, 49, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Kaur, G.; Ranjani, A.; Gayathri, L.; Chakraborty, P.; Adhikary, J.; Pasan, J.; Dhanasekaran, D.; Choudhury, A.; Akbarsha, M.; et al. A Trinuclear Zinc-Schiff Base Complex: Biocatalytic Activity and Cytotoxicity. Eur. J. Inorg. Chem. 2014, 2014, 3350–3358. [Google Scholar] [CrossRef]
- Mahato, S.; Meheta, N.; Kotakonda, M.; Joshi, M.; Shit, M.; Choudhury, A.R.; Biswas, B. Synthesis, Structure, Polyphenol Oxidase Mimicking and Bactericidal Activity of a Zinc-Schiff Base Complex. Polyhedron 2021, 194, 114933. [Google Scholar] [CrossRef]
- Majumdar, D.; Biswas, J.K.; Mondal, M.; Surendra Babu, M.S.; Metre, R.K.; Das, S.; Bankura, K.; Mishra, D. Coordination of N,O-Donor Appended Schiff Base Ligand (H2L1) towards Zinc(II) in Presence of Pseudohalides: Syntheses, Crystal Structures, Photoluminescence, Antimicrobial Activities and Hirshfeld Surfaces. J. Mol. Struct. 2018, 1155, 745–757. [Google Scholar] [CrossRef]
- Das, M.; Mukherjee, S.; Koley, B.; Choudhuri, I.; Bhattacharyya, N.; Roy, P.; Samanta, B.C.; Barai, M.; Maity, T. Developing Novel Zinc(II) and Copper(II) Schiff Base Complexes: Combined Experimental and Theoretical Investigation on Their DNA/Protein Binding Efficacy and Anticancer Activity. New J. Chem. 2020, 44, 18347–18361. [Google Scholar] [CrossRef]
- Golbabapour, S.; Gwaram, N.S.; Hassandarvish, P.; Hajrezaie, M.; Kamalidehghan, B.; Abdulla, M.A.; Ali, H.M.; Hadi, A.H.A.; Majid, N.A. Gastroprotection Studies of Schiff Base Zinc (II) Derivative Complex against Acute Superficial Hemorrhagic Mucosal Lesions in Rats. PLoS ONE 2013, 8, e75036. [Google Scholar] [CrossRef]
- Arora, T.; Devi, J.; Boora, A.; Taxak, B.; Rani, S. Synthesis and Characterization of Hydrazones and Their Transition Metal Complexes: Antimicrobial, Antituberculosis and Antioxidant Activity. Res. Chem. Intermed. 2023, 49, 4819–4843. [Google Scholar] [CrossRef]
- Ishii, S.; Fukui, K.; Yokoshima, S.; Kumagai, K.; Beniyama, Y.; Kodama, T.; Fukuyama, T.; Okabe, T.; Nagano, T.; Kojima, H.; et al. High-Throughput Screening of Small Molecule Inhibitors of the Streptococcus Quorum-Sensing Signal Pathway. Sci. Rep. 2017, 7, 4029. [Google Scholar] [CrossRef]
- Xu, Y.; Nakajima, Y.; Ito, K.; Zheng, H.; Oyama, H.; Heiser, U.; Hoffmann, T.; Gärtner, U.-T.; Demuth, H.-U.; Yoshimoto, T. Novel Inhibitor for Prolyl Tripeptidyl Aminopeptidase from Porphyromonas Gingivalis and Details of Substrate-Recognition Mechanism. J. Mol. Biol. 2008, 375, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua [1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane] Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Elkovi, K.A.N.; Jakovljevi, G.; Zlatovi, M. Synthesis and Characterization of Zinc (II), Palladium (II) and Platinum (II) Complex with Structure of Trihydrate. Society 2004, 69, 651–660. [Google Scholar]
- Reena, T.A.; Seena, E.B.; Prathapachandra Kurup, M.R. Zinc(II) Complexes Derived from Di-2-Pyridyl Ketone N4-Phenyl-3-Semicarbazone: Crystal Structures and Spectral Studies. Polyhedron 2008, 27, 3461–3466. [Google Scholar] [CrossRef]
- Gusev, A.; Braga, E.; Karmazina, A.; Karmazin, A.; Konnik, O.; Kiskin, M.; Baryshnikov, G.; Linert, W. Structure-Induced Luminescence and Bioactivities of Zinc(II) Complexes with 2-(2,4-Dichlorophenoxy)-N′-[Pyridin-2-Ylmethylene]Acetohydrazide. Inorganica Chim. Acta 2023, 551, 121481. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Zhao, S.; Yang, Z. Interactions of Novel Pyrazole Ligand and Its Transition Metal Complexes with CT-DNA and BSA: A Combination of Experimental and Computational Studies. Polyhedron 2023, 231, 116273. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel Tools for Visualizing and Exploring Intermolecular Interactions in Molecular Crystals. Acta Crystallogr. Sect. B 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Costamagna, J.; Lillo, L.E.; Matsuhiro, B.; Noseda, M.D.; Villagrán, M. Ni(II) Complexes with Schiff Bases Derived from Amino Sugars. Carbohydr. Res. 2003, 338, 1535–1542. [Google Scholar] [CrossRef]
- Mathews, N.A.; Jose, A.; Kurup, M.R.P. Synthesis and Characterization of a New Aroylhydrazone Ligand and Its Cobalt(III) Complexes: X-Ray Crystallography and in Vitro Evaluation of Antibacterial and Antifungal Activities. J. Mol. Struct. 2019, 1178, 544–553. [Google Scholar] [CrossRef]
- Hong, M.; Yin, H.; Zhang, X.; Li, C.; Yue, C.; Cheng, S. Di- and Tri-Organotin(IV) Complexes with 2-Hydroxy-1-Naphthaldehyde 5-Chloro-2-Hydroxybenzoylhydrazone: Synthesis, Characterization and in Vitro Antitumor Activities. J. Organomet. Chem. 2013, 724, 23–31. [Google Scholar] [CrossRef]
- Singh, P.; Singh, D.P.; Tiwari, K.; Mishra, M.; Singh, A.K.; Singh, V.P. Synthesis, Structural Investigations and Corrosion Inhibition Studies on Mn(Ii), Co(Ii), Ni(Ii), Cu(Ii) and Zn(Ii) Complexes with 2-Amino-Benzoic Acid (Phenyl-Pyridin-2-Yl-Methylene)-Hydrazide. RSC Adv. 2015, 5, 45217–45230. [Google Scholar] [CrossRef]
- Gupta, P.; Das, S.S.; Singh, N.B. Introduction to Spectroscopy, 4th ed.; Lockwood, L., Ed.; Wadsworth Publishing Co., Inc.: Belmont, CA, USA, 2023; Volume 32, ISBN 9781003412588. [Google Scholar]
- Shehnaz; Siddiqui, W.A.; Raza, M.A.; Ashraf, A.; Ashfaq, M.; Tahir, M.N.; Niaz, S. Structure Elucidation {single X-Ray Crystal Diffraction Studies, Hirshfeld Surface Analysis, DFT} and Antibacterial Studies of Sulfonamide Functionalized Schiff Base Copper (II) and Zinc (II) Complexes. J. Mol. Struct. 2024, 1295, 136603. [Google Scholar] [CrossRef]
- Gao, J.-Y.; Zhang, N.; Huang, D.-S.; Liu, X.-R.; Yang, Z.-W.; Zhao, S.-S. Synthesis, Crystal Structures, CT-DNA/BSA Binding Modes and Antibacterial Activities of Zn (II) and Cr (III) with an Acylhydrazone Ligand. Polyhedron 2024, 247, 116736. [Google Scholar] [CrossRef]
- Dinku, D.; Demissie, T.B.; Beas, I.N.; Eswaramoorthy, R.; Abdi, B.; Desalegn, T. Antimicrobial Activities and Docking Studies of New Schiff Base Ligand and Its Cu(II), Zn(II) and Ni (II) Complexes: Synthesis and Characterization. Inorg. Chem. Commun. 2024, 160, 111903. [Google Scholar] [CrossRef]
- Santiago, P.H.O.; Bessa, M.A.S.; Menezes, R.P.; Martins, C.H.G.; Gatto, C.C. Zn(II) Complexes with a New Isoniazid Ligand: Synthesis, Structural Characterization and Antimycobacterial Activity. J. Coord. Chem. 2022, 75, 347–361. [Google Scholar] [CrossRef]
- Gutiérrez Arguelles, D.; Villamizar, C.P.; Brambila-Colombres, E.; Anzaldo, B.; Mendoza, A.; Hernández Téllez, G.; Sharma, P. Synthesis, Crystal Structures, Antimicrobial Activity, and Acute Toxicity Evaluation of Chiral Zn(II) Schiff Base Complexes. Molecules 2024, 29, 5555. [Google Scholar] [CrossRef]
- Caruso, S.; Valenti, C.; Marinucci, L.; Di Pasquale, F.; Truppa, C.; Di Benedetto, G.; Caruso, S.; Pagano, S. Systematic Review of Zinc’s Benefits and Biological Effects on Oral Health. Materials 2024, 17, 800. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT -Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 7th ed.; Document M11-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- Lima, F.C.; Silva, T.S.; Martins, C.H.G.; Gatto, C.C. Synthesis, Crystal Structures and Antimicrobial Activity of Dimeric Copper(II) Complexes with 2-Hydroxyphenyl-Ethylidene-Dithiocarbazates. Inorganica Chim. Acta 2018, 483, 464–472. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Nayak, A.; Sowmya, B.R.; Gandla, H.; Kottrashetti, V.; Ingalagi, P.; Srinivas, V.S.C. Determination and Comparison of Antimicrobial Activity of Aqueous and Ethanolic Extracts of Amorphophallus Paeoniifolius on Periodontal Pathogens: An In Vitro Study. J. Indian Soc. Periodontol. 2023, 27, 40–44. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Biovia Discovery Studio Visualizer. Biovia Discovery Studio Visualizer; Dassault Systèmes, Release: San Diego, CA, USA, 2020. [Google Scholar]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical PKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]

| Bond Lengths (Å) | Bond Angles (°) | ||||||
|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (1) | (2) | (3) | ||
| Zn1–N1 | 2.171 (2) | 2.185 (4) | 2.160 (3) | N1–Zn1–N2 | 75.74 (7) | 75.55 (2) | 76.46 (2) |
| Zn1–N2 | 2.051 (2) | 2.051 (3) | 2.049 (3) | N1–Zn1–O1 | 151.55 (7) | 148.72 (2) | 151.01 (2) |
| Zn1–N4 | - | 2.162 (4) | - | N1–Zn1–N5 | 99.75 (8) | 104.98 (2) | - |
| Zn1–N5 | - | 2.054 (3) | - | N1–Zn1–O3 | 99.60 (7) | 94.37 (2) | - |
| Zn1–O1 | 2.137 (2) | 2.186 (3) | 2.151 (3) | N2–Zn1–O3 | 148.20 (7) | 99.26 (2) | - |
| Zn1–O3 | 1.956 (2) | 2.228 (3) | - | N2–Zn1–N5 | - | 172.79 (4) | - |
| Zn1–O5 | 2.007 (2) | - | - | O1–Zn1–O3 | 104.14 (6) | 96.42 (2) | - |
| C8–O1 | 1.238 (3) | 1.235 (4) | 1.248 (4) | O3–Zn1–N5 | - | 73.54 (2) | - |
| C8–O2 | 1.343 (3) | 1.350 (5) | 1.252 (9) | N2–Zn1–N2i | - | - | 173.29 (3) |
| N2–C6 | 1.286 (3) | 1.283 (5) | 1.297 (5) | C16–N5–N6 | - | 119.30 (4) | - |
| N2–N3 | 1.370 (3) | 1.370 (4) | 1.367 (4) | N3–C8–O2 | 110.51 (2) | 110.10 (4) | 113.30 (4) |
| N3–C8 | 1.348 (3) | 1.334 (5) | 1.336 (5) | C6–N2–N3 | 121.13 (2) | 119.00 (4) | 120.70 (3) |
| Cariogenic Bacterium | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | S. mutans | S. sobrinus | S. oralis | S. sanguinis | S. salivarius | L. paracasei | ||||
| HL1 | >400 | >400 | >400 | >400 | >400 | >400 | ||||
| HL2 | 400 | 400 | >400 | 400 | >400 | >400 | ||||
| HL3 | 12.5 | 25 | 50 | 25 | 100 | 100 | ||||
| (1) | 200 | 200 | 400 | 200 | 400 | 400 | ||||
| (2) | 100 | 100 | 200 | 200 | 200 | 200 | ||||
| (3) | 12.5 | 12.5 | 25 | 25 | 25 | 100 | ||||
| Chlorhexidine | 0.461 | 0.461 | 7.375 | 0.922 | 0.922 | 0.922 | ||||
| Anaerobic Bacterium | ||||||||||
| Compound | A. naeslundii | P. anaerobius | V. parvula | P. gingivalis | F. nucleatum | |||||
| HL1 | >400 | >400 | 400 | 400 | >400 | |||||
| HL2 | >400 | 100 | 400 | 200 | >400 | |||||
| HL3 | 100 | 50 | 100 | 50 | 200 | |||||
| (1) | 200 | 200 | 400 | 100 | 200 | |||||
| (2) | 200 | 100 | 100 | 50 | 400 | |||||
| (3) | 25 | 12.5 | 100 | 100 | 50 | |||||
| Chlorhexidine | 1.844 | 0.230 | 0.922 | 0.922 | 1.844 | |||||
| Carcinogenic Bacteria-S. mutans PEP Enzyme | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Residue | CAB | CABr | CHL | HL1 | HL2 | HL3 | (1) | (2) | (3) |
| ARG66 | 4.45 | 5.47 | 4.99 | 4.83 | 4.97 | 3.66 | 3.59 | 5.48 | 4.84/1.75 |
| ALA70 | 3.85 | 3.70 | 4.06 | -- | -- | -- | >6.00 | 4.43 | -- |
| ASP71 | 3.27 | 1.99 | 2.44 | -- | -- | -- | >6.00 | -- | -- |
| LEU74 | 5.26 | 4.58 | 3.14 | -- | 5.01 | -- | 5.18 | >6.00 | 5.50 |
| TYR77 | 5.45 | 5.44 | -- | 5.18 | 4.06 | 4.96 | >6.00 | 4.96 | 3.15 |
| Kithe | -- | 9.87 | 6.57 | 379.81 | 337.85 | 9.07 | 248.90 | 80.82 | 9.25 |
| Ebind | -- | −6.83 | −7.07 | −4.67 | −4.74 | −6.88 | −4.92 | −5.58 | −6.87 |
| Anaerobic Bacteria–P. gingivalis PTP39 Enzyme | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Residue | BOR | BORr | CHL | HL1 | HL2 | HL3 | (1) | (2) | (3) |
| SER603 | 1.76 | 2.45 | 3.30 | >6.00 | 2.22 | 1.75 | -- | 2.72 | 3.50 |
| TYR518 | 2.65 | 1.69 | 1.79 | >6.00 | 2.08 | 2.45 | -- | 2.90 | 5.41 |
| TYR635 | 2.63 | 1.97 | -- | 1.83 | 3.07 | 4.88 | 2.08 | 5.08 | >6.00 |
| GLU205 | 2.62 | 2.78 | -- | 3.59 | -- | -- | 5.12 | -- | >4.00 |
| GLU636 | 2.73 | 2.71 | 4.59 | -- | -- | -- | 4.94 | -- | -- |
| HIS522 | 4.39 | 4.09 | 2.44 | -- | -- | -- | -- | -- | 5.33 |
| GLN203 | 2.77 | 2.47 | 2.01 | 1.93 | -- | -- | 1.94 | -- | >4.00 |
| TYR604 | 2.96 | 2.30 | -- | -- | 5.52 | 3.31 | -- | -- | -- |
| TYR639 | 4.76 | 4.12 | -- | 3.97 | 4.82 | 4.79 | 4.09 | 4.24 | 4.23 |
| Ebind | -- | −5.83 | −9.43 | −4.21 | −3.99 | −5.33 | −5.00 | −6.39 | −6.62 |
| Kitheo | 53.68 | 0.12 | 815.98 | 1200.0 | 124.53 | 217.33 | 20.86 | 13.97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, C.C.; Siqueira, D.J.d.; Duarte, E.d.A.; Nascimento, É.C.M.; Martins, J.B.L.; Santiago, M.B.; Silva, N.B.S.; Martins, C.H.G. Exploring the Structural Design, Antibacterial Activity, and Molecular Docking of Newly Synthesized Zn(II) Complexes with NNO-Donor Carbazate Ligands. Molecules 2025, 30, 2822. https://doi.org/10.3390/molecules30132822
Gatto CC, Siqueira DJd, Duarte EdA, Nascimento ÉCM, Martins JBL, Santiago MB, Silva NBS, Martins CHG. Exploring the Structural Design, Antibacterial Activity, and Molecular Docking of Newly Synthesized Zn(II) Complexes with NNO-Donor Carbazate Ligands. Molecules. 2025; 30(13):2822. https://doi.org/10.3390/molecules30132822
Chicago/Turabian StyleGatto, Claudia C., Daniel J. de Siqueira, Eduardo de A. Duarte, Érica C. M. Nascimento, João B. L. Martins, Mariana B. Santiago, Nagela B. S. Silva, and Carlos H. G. Martins. 2025. "Exploring the Structural Design, Antibacterial Activity, and Molecular Docking of Newly Synthesized Zn(II) Complexes with NNO-Donor Carbazate Ligands" Molecules 30, no. 13: 2822. https://doi.org/10.3390/molecules30132822
APA StyleGatto, C. C., Siqueira, D. J. d., Duarte, E. d. A., Nascimento, É. C. M., Martins, J. B. L., Santiago, M. B., Silva, N. B. S., & Martins, C. H. G. (2025). Exploring the Structural Design, Antibacterial Activity, and Molecular Docking of Newly Synthesized Zn(II) Complexes with NNO-Donor Carbazate Ligands. Molecules, 30(13), 2822. https://doi.org/10.3390/molecules30132822










