Hydrothermal and Organosolv Treatments for Hydroxycinnamate Release from Corn Stover: Strong versus Mild Alkaline Catalysis
Abstract
1. Introduction
2. Results and Discussion
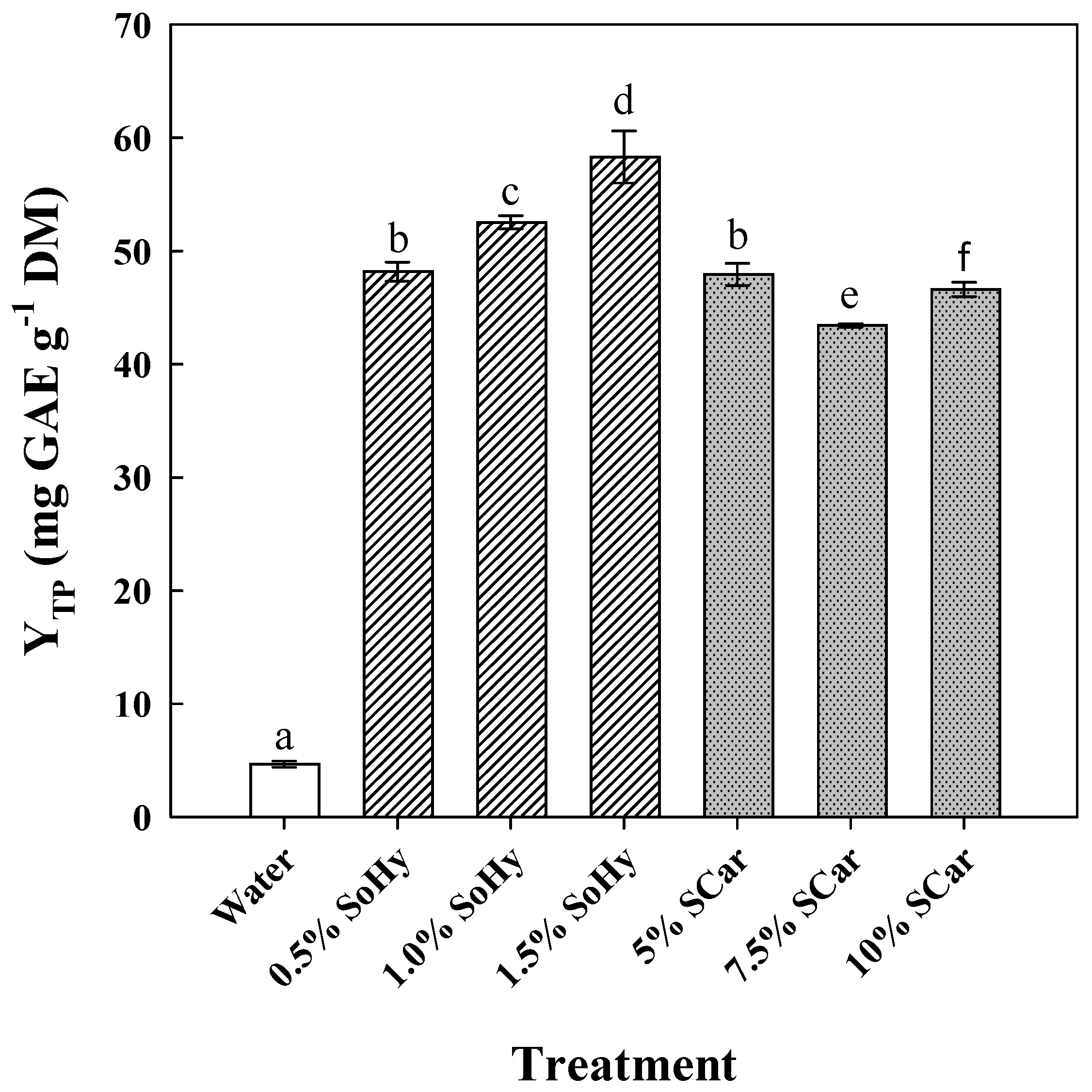
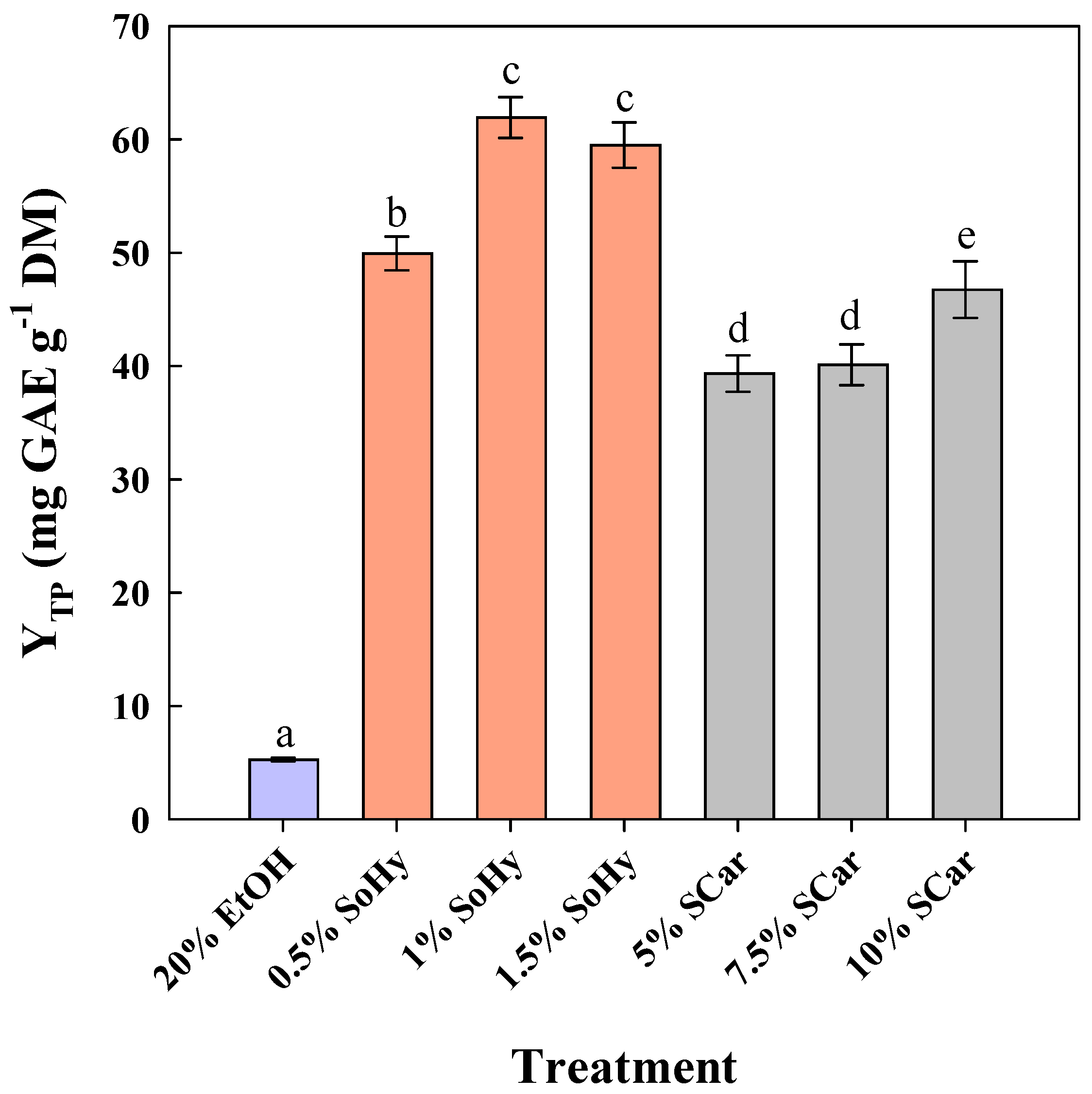
2.1. Effect of Alkali Catalyst Concentration on the Hydrothermal Treatment
2.2. Kinetics of Polyphenol Recovery
2.3. Optimization of Treatments Through Response Surface Methodology
2.4. Hydroxycinnamate Composition
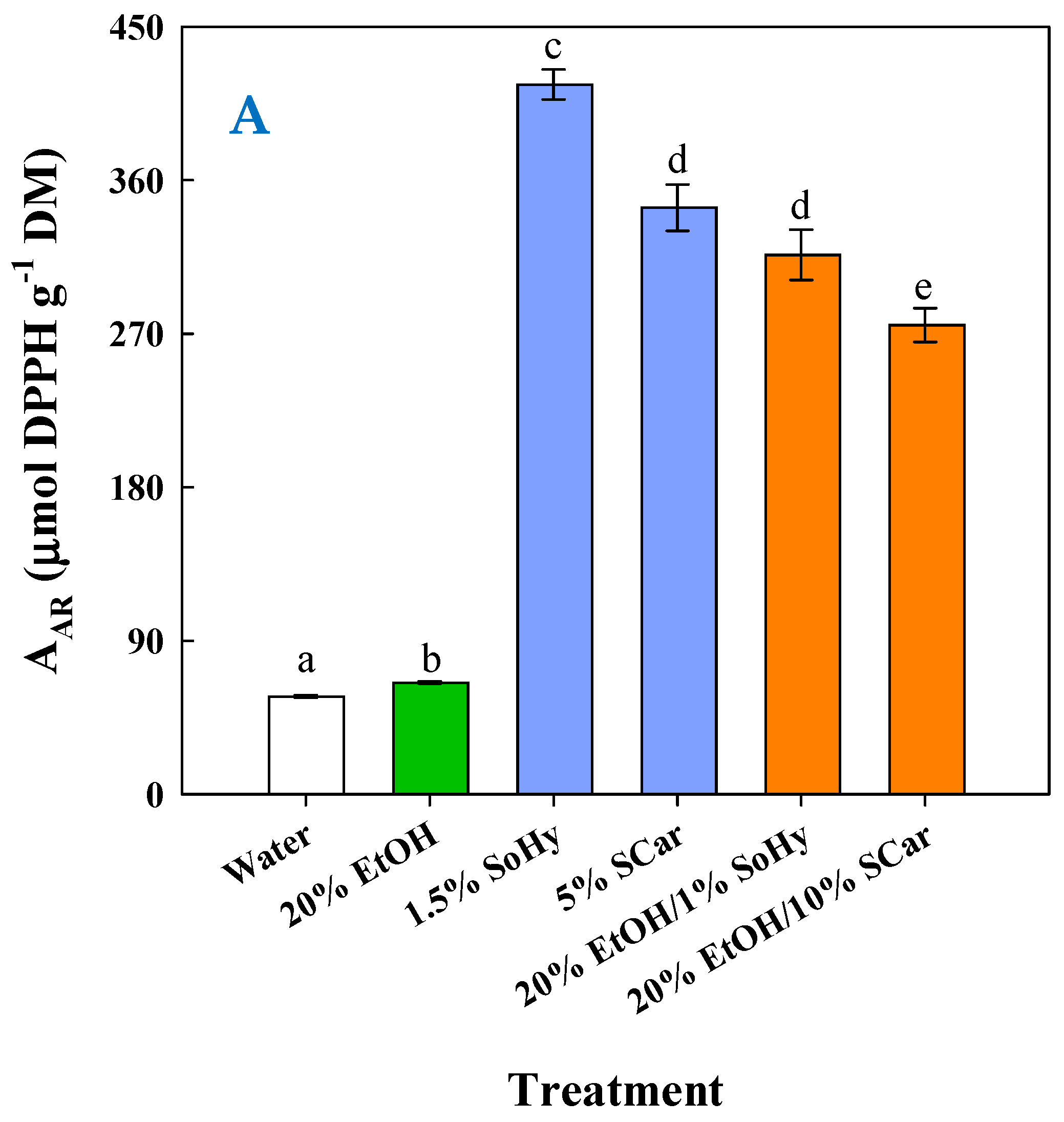
2.5. Antioxidant Effects
3. Materials and Methods
3.1. Chemicals–Reagents
3.2. Corn Stover (CS) Collection and Processing
3.3. Hydrothermal and Organosolv Treatments
3.4. Polyphenol Recovery Kinetics
3.5. Estimation of Severity
3.6. Response Surface-Based Treatment Optimization
3.7. Determination of Treatment Yield and Antioxidant Activity Measurements
3.8. Chromatography
3.9. Statistics and Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, J.; Huang, J.; Yuan, J.; Chen, J.; Pei, Z.; Li, H.; Yang, S. Novel supramolecular deep eutectic solvent-enabled in-situ lignin protection for full valorization of all components of wheat straw. Biores. Technol. 2023, 388, 129722. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Pei, Z.; Huang, J.; Wang, J.; Yang, S.; Li, H. Enhancing lignocellulosic biorefinery sustainability: Mechanisms and optimization of microwave-responsive deep eutectic solvents for rapid delignification. Biofuel Res. J. 2025, 45, 2306–2318. [Google Scholar] [CrossRef]
- Banu, J.R.; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic agricultural waste valorization to obtain valuable products: An overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Srivastava, R.K.; Vivekanand, V.; Goksen, G.; Sahoo, U.K.; Thakur, T.K.; Debeaufort, F.; Uysal-Unalan, I.; Pugazhendhi, A. Recovery of green phenolic compounds from lignin-based source: Role of ferulic acid esterase towards waste valorization and bioeconomic perspectives. Environ. Res. 2024, 256, 119218. [Google Scholar] [CrossRef]
- Ruan, Z.; Wang, X.; Liu, Y.; Liao, W. Corn. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–72. [Google Scholar]
- Postamentel, M.; Coman, C.-N. Analysis of the Main Technical Indicators of Corn, Wheat and Sunflower Crops at the Level of the European Union; University of Agricultural Sciences and Veterinary Medicine: Bucharest, Romania, 2022. [Google Scholar]
- Aghaei, S.; Alavijeh, M.K.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass Bioen. 2022, 161, 106447. [Google Scholar] [CrossRef]
- Sahayaraj, D.V.; Lusi, A.; Mitchell, E.M.; Bai, X.; Tessonnier, J.-P. Comparative study of the solvolytic deconstruction of corn stover lignin in batch and flow-through reactors. Green Chem. 2021, 23, 7731–7742. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; López-Martínez, L.X.; Contreras-Angulo, L.; Heredia, J.B. Antioxidant capacity of lignin and phenolic compounds from corn stover. Waste Biomass Valor. 2019, 10, 95–102. [Google Scholar] [CrossRef]
- Bunzel, M. Chemistry and occurrence of hydroxycinnamate oligomers. Phytochem. Rev. 2010, 9, 47–64. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem. Rev. 2010, 9, 147–170. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Inter. 2013, 2013, 251754. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Goransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic acids and derivatives formulations for skin damages and disorders: A review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Patto, M.C.V.; do Rosário Bronze, M. Relevance, structure and analysis of ferulic acid in maize cell walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, G.; Zarnavalou, V.; Chatzimitakos, T.; Palaiogiannis, D.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Comparison of sodium hydroxide and sodium carbonate as alkali catalysts in ethanol organosolv treatment of cotton stalks for the release of hydroxycinnamates. Recycling 2024, 9, 21. [Google Scholar] [CrossRef]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol release from wheat bran using ethanol-based organosolv treatment and acid/alkaline catalysis: Process modeling based on severity and response surface optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, E.; Grigorakis, S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P. Hydrothermal treatment of wheat bran under mild acidic or alkaline conditions for enhanced polyphenol recovery and antioxidant activity. Molecules 2024, 29, 1193. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.; Liebentritt, S.; Vicente, A.A.; Teixeira, J.A. Lignin from an integrated process consisting of liquid hot water and ethanol organosolv: Physicochemical and antioxidant properties. Inter. J. Biol. Macromol. 2018, 120, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Guenaoui, A.; Casasni, S.; Grigorakis, S.; Makris, D.P. Alkali-catalyzed organosolv treatment of oat bran for enhanced release of hydroxycinnamate antioxidants: Comparison of 1-and 2-propanol. Environments 2023, 10, 118. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, F.; Li, P.; Jiang, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol. Bioeng. 2018, 115, 2683–2702. [Google Scholar] [CrossRef]
- Pazo-Cepeda, M.V.; Aspromonte, S.G.; Alonso, E. Extraction of ferulic acid and feruloylated arabinoxylo-oligosaccharides from wheat bran using pressurized hot water. Food Biosci. 2021, 44, 101374. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Smyrnakis, G.; Stamoulis, G.; Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Recovery of polyphenolic antioxidants from coffee silverskin using acid-catalyzed ethanol organosolv treatment. ChemEngineering 2023, 7, 72. [Google Scholar] [CrossRef]
- Geropoulou, M.; Yiagtzi, E.; Chatzimitakos, T.; Palaiogiannis, D.; Makris, D.P. Organosolv treatment of red grape pomace for effective recovery of antioxidant polyphenols and pigments using a ternary glycerol/ethanol/water system under mild acidic conditions. Molecules 2024, 29, 563. [Google Scholar] [CrossRef]
- Valério, R.; Cadima, M.; Crespo, J.G.; Brazinha, C. Extracting ferulic acid from corn fibre using mild alkaline extraction: A pilot scale study. Waste Biomass Valor. 2022, 13, 287–297. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, S.; Lan, W.; Yue, F. Quantitative extraction of p-coumaric acid and ferulic acid in different gramineous materials and structural changes of residual alkali lignin. J. Renew. Mater. 2023, 11, 555. [Google Scholar] [CrossRef]
- Johnston, P.A.; Zhou, H.; Aui, A.; Wright, M.M.; Wen, Z.; Brown, R.C. A lignin-first strategy to recover hydroxycinnamic acids and improve cellulosic ethanol production from corn stover. Biomass Bioen. 2020, 138, 105579. [Google Scholar] [CrossRef]
- Timokhin, V.I.; Regner, M.; Motagamwala, A.H.; Sener, C.; Karlen, S.D.; Dumesic, J.A.; Ralph, J. Production of p-coumaric acid from corn GVL-lignin. ACS Sust. Chem. Eng. 2020, 8, 17427–17438. [Google Scholar] [CrossRef]
- Valério, R.; Serra, A.T.; Baixinho, J.; Cardeira, M.; Fernández, N.; Bronze, M.R.; Duarte, L.C.; Tavares, M.L.; Crespo, J.G.; Brazinha, C. Combined hydrothermal pre-treatment and enzymatic hydrolysis of corn fibre: Production of ferulic acid extracts and assessment of their antioxidant and antiproliferative properties. Ind. Crops Prod. 2021, 170, 113731. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, S.; Ou, S.; Lin, J.; Wang, Y.; Peng, X.; Li, A.; Yu, B. Preparation of ferulic acid from corn bran: Its improved extraction and purification by membrane separation. Food Bioprod. Proc. 2014, 92, 309–313. [Google Scholar] [CrossRef]
- Li, D.; Long, L.; Ding, S. Alkaline organosolv pretreatment of different sorghum stem parts for enhancing the total reducing sugar yields and p-coumaric acid release. Biotechnol. Biofuels 2020, 13, 106. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D.; Quideau, S.; Helm, R.F.; Grabber, J.H.; Jung, H.-J.G. Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J. Am. Chem. Soc. 1994, 116, 9448–9456. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Bioref. 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Linh, T.N.; Fujita, H.; Sakoda, A. Release kinetics of esterified p-coumaric acid and ferulic acid from rice straw in mild alkaline solution. Biores. Technol. 2017, 232, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sust. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Pazo-Cepeda, V.; Benito-Román, Ó.; Navarrete, A.; Alonso, E. Valorization of wheat bran: Ferulic acid recovery using pressurized aqueous ethanol solutions. Waste Biomass Valor. 2020, 11, 4701–4710. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of insoluble-bound phenolics to the antioxidant potential is dictated by source material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Choo, T.M.; Dhillon, S.; Rabalski, I. Free and bound phenolic acids and total phenolics in black, blue, and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem. 2012, 89, 198–204. [Google Scholar] [CrossRef]
- Gong, L.; Huang, L.; Zhang, Y. Effect of steam explosion treatment on barley bran phenolic compounds and antioxidant capacity. J. Agric. Food Chem. 2012, 60, 7177–7184. [Google Scholar] [CrossRef]
- Boonma, S.; Rangsee, W.; Chaiklangmuang, S. Effect of hydrothermal pre-treatment on ferulic acid content and antioxidant activities of corn hydrolysate. Jpn. J. Food Eng. 2018, 19, 27–34. [Google Scholar] [CrossRef]
- Povilaitis, D.; Šulniūtė, V.; Venskutonis, P.R.; Kraujalienė, V. Antioxidant properties of wheat and rye bran extracts obtained by pressurized liquid extraction with different solvents. J. Cereal Sci. 2015, 62, 117–123. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant properties and efficacies of synthesized alkyl caffeates, ferulates, and coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
- Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C.; Ho, Y.-S. Comparison of kinetic models for the aqueous solid-liquid extraction of Tilia sapwood in a continuous stirred tank reactor. Chem. Eng. Com. 2007, 194, 537–552. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Phil. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Bezerra, M.A.; Ferreira, S.L.C.; Novaes, C.G.; Dos Santos, A.M.P.; Valasques, G.S.; da Mata Cerqueira, U.M.F.; dos Santos Alves, J.P. Simultaneous optimization of multiple responses and its application in Analytical Chemistry—A review. Talanta 2019, 194, 941–959. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D. Polyphenol extraction from Humulus lupulus (hop) using a neoteric glycerol/L-alanine deep eutectic solvent: Optimisation, kinetics and the effect of ultrasound-assisted pretreatment. AgriEngineering 2019, 1, 403–417. [Google Scholar] [CrossRef]
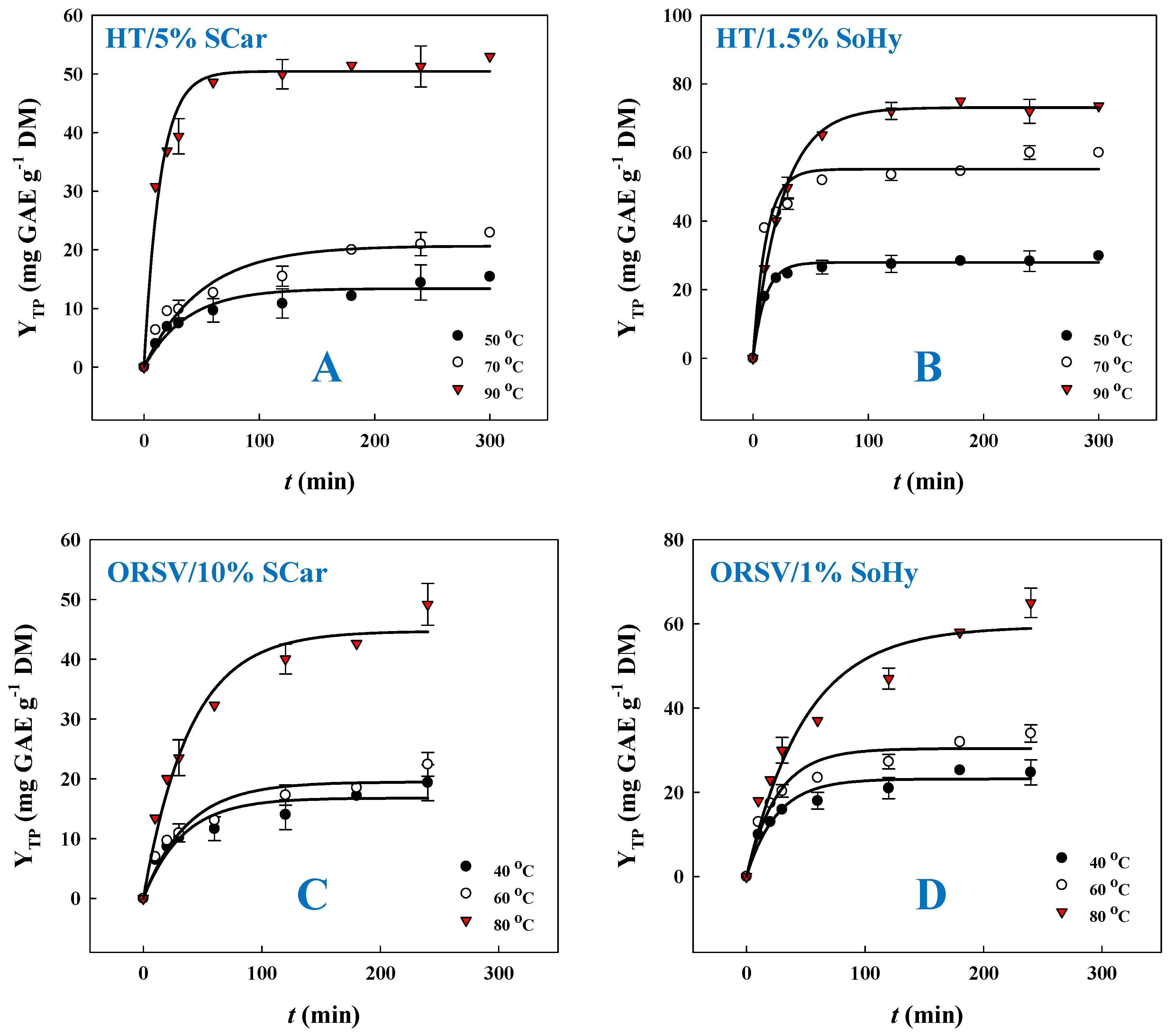



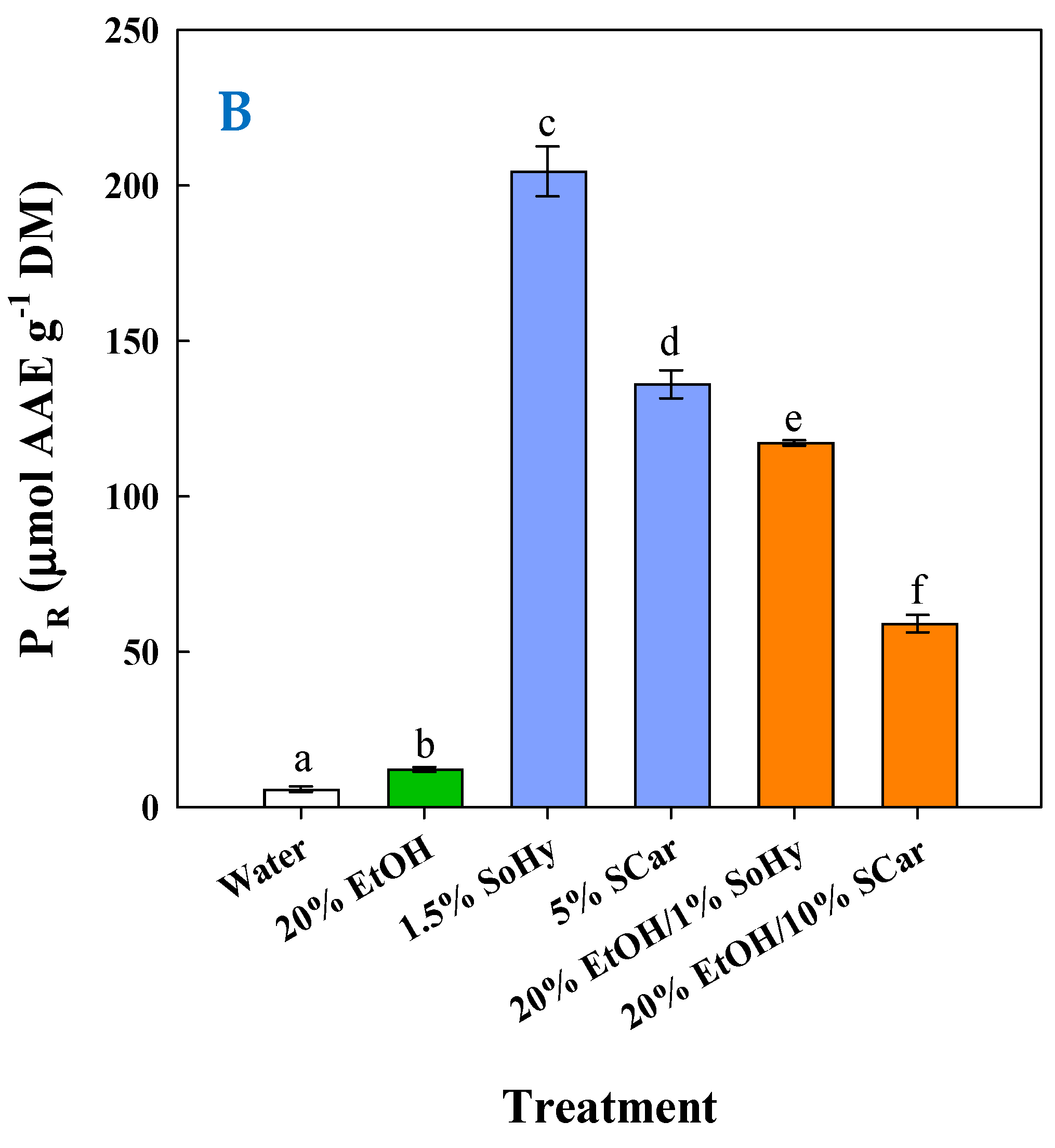
| Treatment | T (°C) | R2 * | k (min−1) × 10−3 | YTP(s) (mg GAE g−1 DM) |
|---|---|---|---|---|
| Hydrothermal | ||||
| 1.5% SoHy | 50 | 0.99 | 93.9 | 27.9 ± 1.9 a |
| 70 | 0.95 | 87.4 | 55.1 ± 3.2 b | |
| 90 | 1.00 | 39.6 | 73.1 ± 5.2 c | |
| 5% SCar | 50 | 0.93 | 26.9 | 13.4 ± 0.8 d |
| 70 | 0.92 | 20.1 | 20.7 ± 1.0 e | |
| 90 | 0.97 | 70.9 | 50.4 ± 3.6 b | |
| Organosolv | ||||
| 1% SoHy | 40 | 0.95 | 39.3 | 23.2 ± 1.7 f |
| 60 | 0.94 | 38.7 | 30.4 ± 2.1 g | |
| 80 | 0.94 | 20.3 | 59.4 ± 3.9 h | |
| 10% SCar | 40 | 0.91 | 30.1 | 16.8 ± 0.9 i |
| 60 | 0.93 | 27.1 | 19.5 ± 1.0 e | |
| 80 | 0.97 | 25.4 | 44.7 ± 2.8 j |
| Treatment | Equation (Model) | R2 | p |
|---|---|---|---|
| Hydrothermal | |||
| 1.5% SoHy | YTP = 61.6 + 22.3X1 − 9.7X12 | 1.00 | 0.0001 |
| 5% SCar | YTP = 22.1 + 20X1 + 1.9X2 + 11.5X12 | 1.00 | 0.0001 |
| Organosolv | |||
| 1% SoHy | YTP = 33.6 + 14.2X1 + 6.8X2 + 5.8X1X2 | 0.97 | 0.0006 |
| 10% SCar | YTP = 18.7 + 13.4X1 + 3.1X2 + 10.9X12 | 1.00 | <0.0001 |
| Treatment | Optimal T (°C) | Optimal t (min) | Maximum Predicted YTP (mg GAE g−1 DM) | Severity (CSF′) |
|---|---|---|---|---|
| Hydrothermal (1.5% SoHy) | 90 | 180 | 74.4 ± 3.6 a | 8.19 |
| Hydrothermal (5% SCar) | 90 | 300 | 57.1 ± 3.7 b | 6.66 |
| Organosolv (20% EtOH/1% SoHy) | 80 | 240 | 64.2 ± 6.8 a | 7.64 |
| Organosolv (20% EtOH/10% SCar) | 80 | 240 | 47.8 ± 1.8 c | 6.55 |
| Treatment | Y (mg g−1 DM) | |||
|---|---|---|---|---|
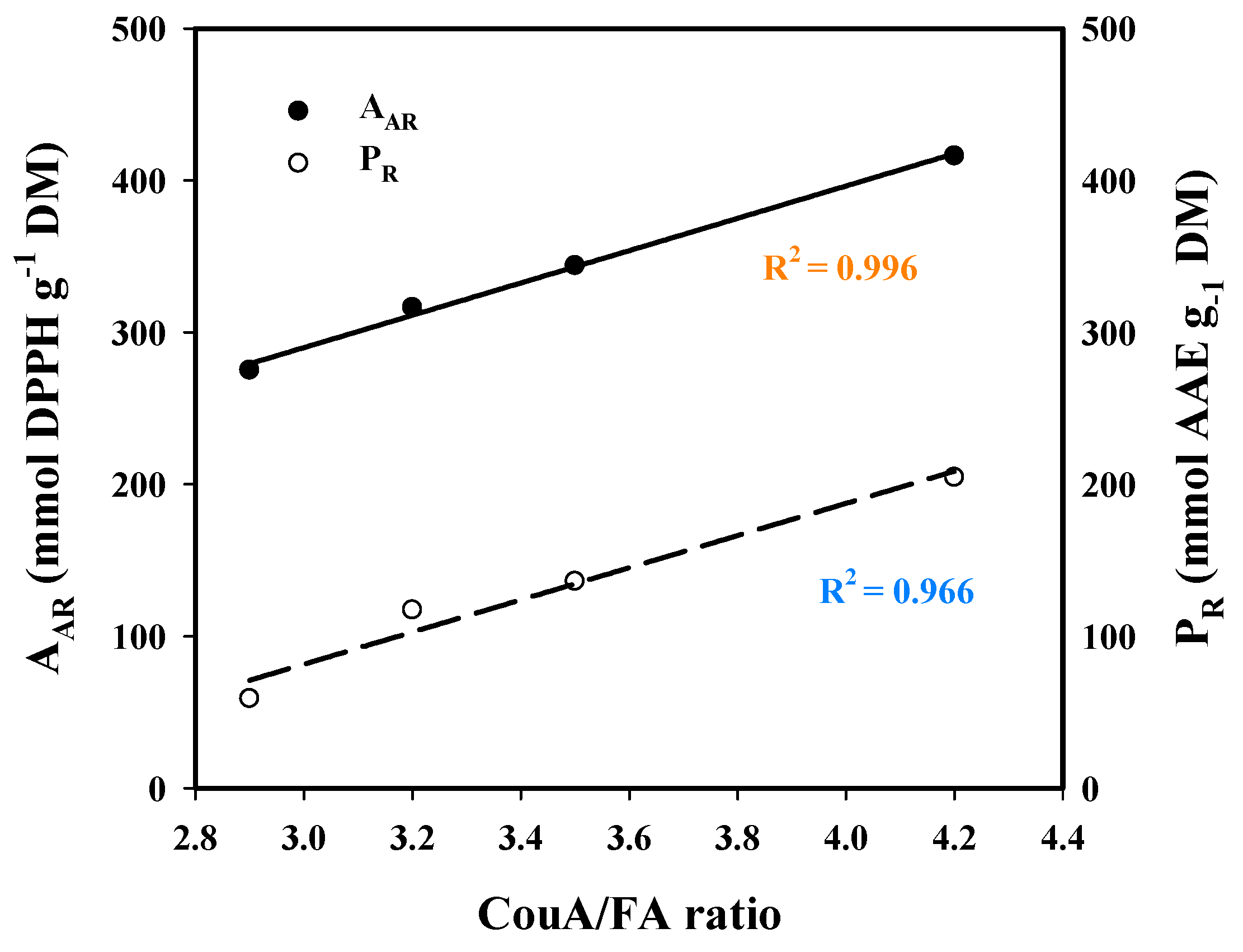
| CouA | FA | Ratio CouA/FA | Total | |
| Water | 0.34 ± 0.01 a | 0.15 ± 0.01 a | 2.3 | 0.49 |
| Hydrothermal (1.5% SoHy) | 25.64 ± 0.5 b | 6.15 ± 0.19 b | 4.2 | 31.79 |
| Hydrothermal (5% SCar) | 20.48 ± 0.94 c | 5.82 ± 0.23 b,e | 3.5 | 26.30 |
| 20% EtOH | 0.31 ± 0.03 a | 0.08 ± 0.01 c | 3.9 | 0.39 |
| Organosolv (20% EtOH/1% SoHy) | 36.06 ± 1.20 d | 11.34 ± 0.82 d | 3.2 | 47.40 |
| Organosolv (20% EtOH/10% SCar) | 16.51 ± 0.20 e | 5.69 ± 0.17 e | 2.9 | 22.20 |
| Process Variables | Codes | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| t (min) | X1 | 60 | 180 | 300 |
| T (°C) (hydrothermal treatment) | X2 | 50 | 70 | 90 |
| T (°C) (organosolv treatment) | 40 | 60 | 80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brimo-Alevra, E.; Koutli, M.; Marielou, E.; Chatzimitakos, T.; Makris, D.P. Hydrothermal and Organosolv Treatments for Hydroxycinnamate Release from Corn Stover: Strong versus Mild Alkaline Catalysis. Molecules 2025, 30, 4297. https://doi.org/10.3390/molecules30214297
Brimo-Alevra E, Koutli M, Marielou E, Chatzimitakos T, Makris DP. Hydrothermal and Organosolv Treatments for Hydroxycinnamate Release from Corn Stover: Strong versus Mild Alkaline Catalysis. Molecules. 2025; 30(21):4297. https://doi.org/10.3390/molecules30214297
Chicago/Turabian StyleBrimo-Alevra, Evangelia, Marina Koutli, Elli Marielou, Theodoros Chatzimitakos, and Dimitris P. Makris. 2025. "Hydrothermal and Organosolv Treatments for Hydroxycinnamate Release from Corn Stover: Strong versus Mild Alkaline Catalysis" Molecules 30, no. 21: 4297. https://doi.org/10.3390/molecules30214297
APA StyleBrimo-Alevra, E., Koutli, M., Marielou, E., Chatzimitakos, T., & Makris, D. P. (2025). Hydrothermal and Organosolv Treatments for Hydroxycinnamate Release from Corn Stover: Strong versus Mild Alkaline Catalysis. Molecules, 30(21), 4297. https://doi.org/10.3390/molecules30214297









