Unveiling Vacancy-Driven Stability: Atomic and Electronic Insights into Ni/Al2O3 Interfaces
Abstract
1. Introduction
2. Results and Discussion
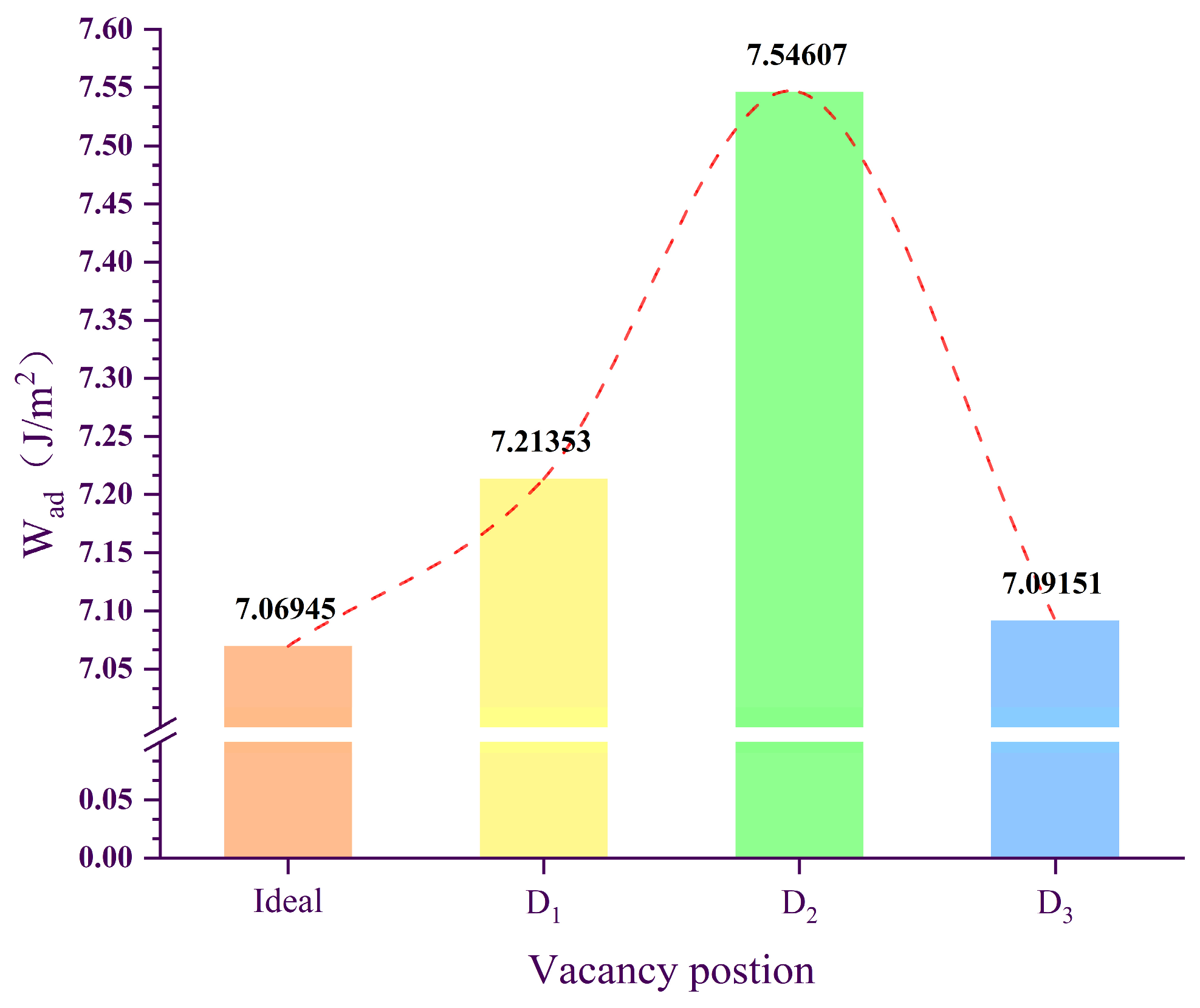
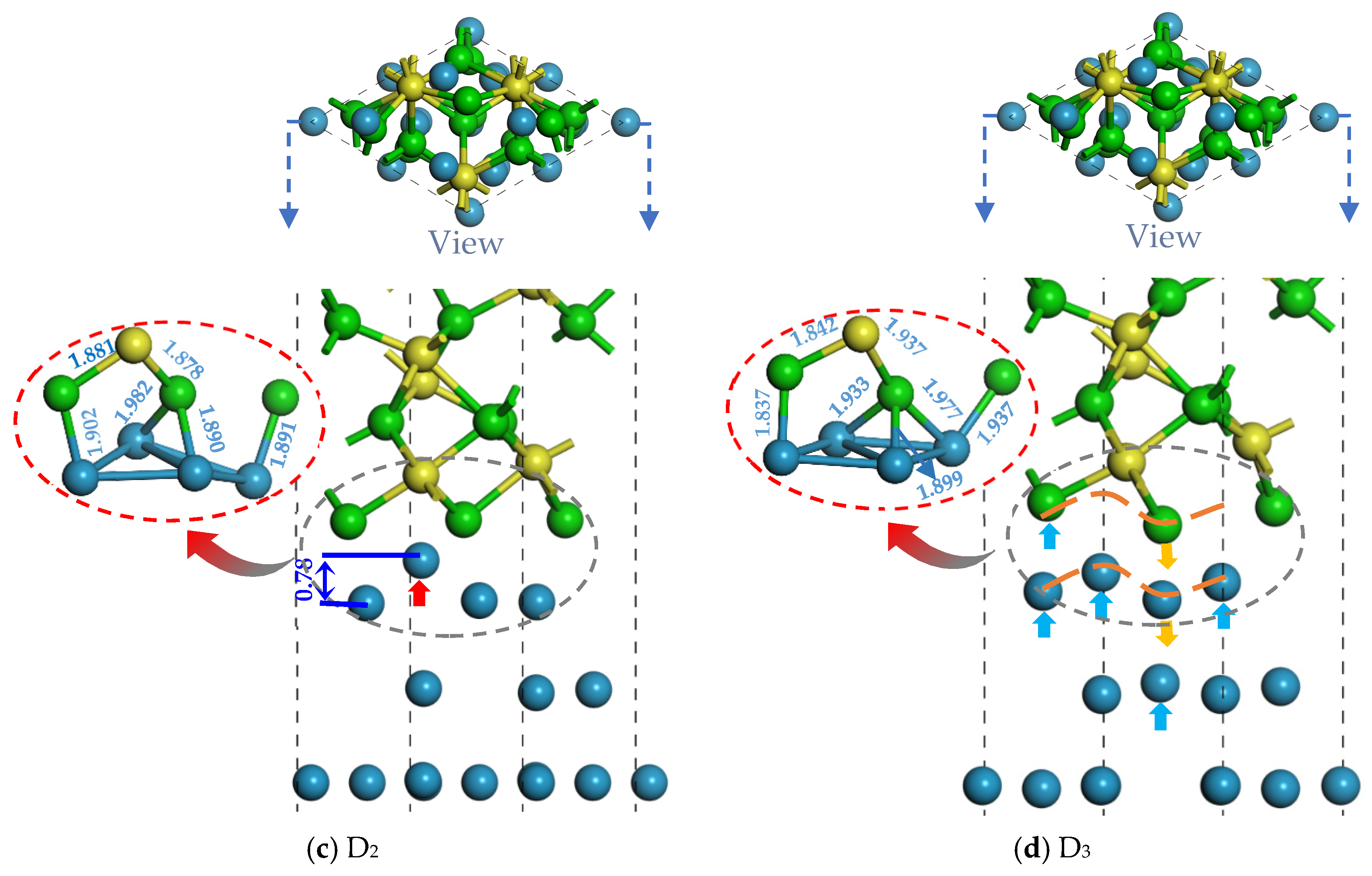
2.1. Bonding Strength of the Interface
2.2. Electronic Properties of Interface
3. Calculation Method and Details
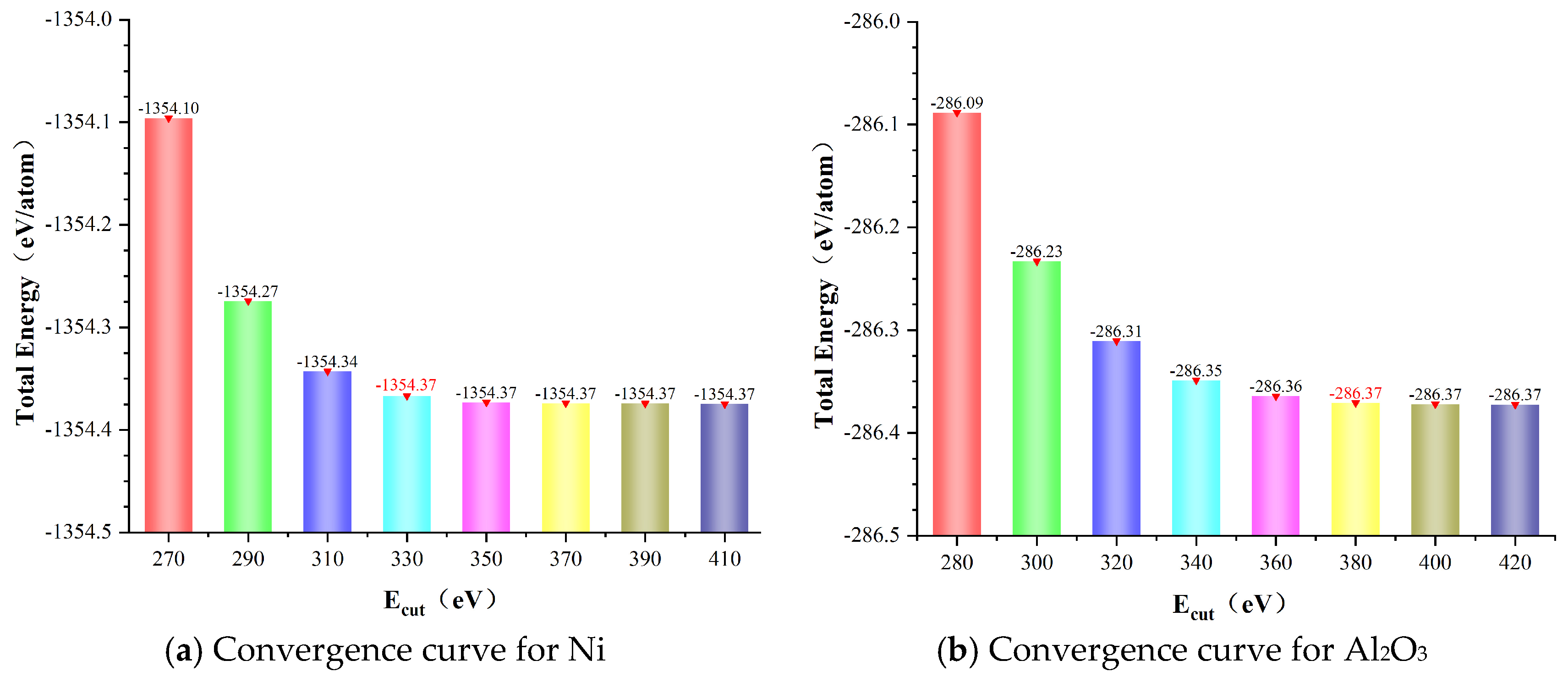
3.1. Calculation Settings
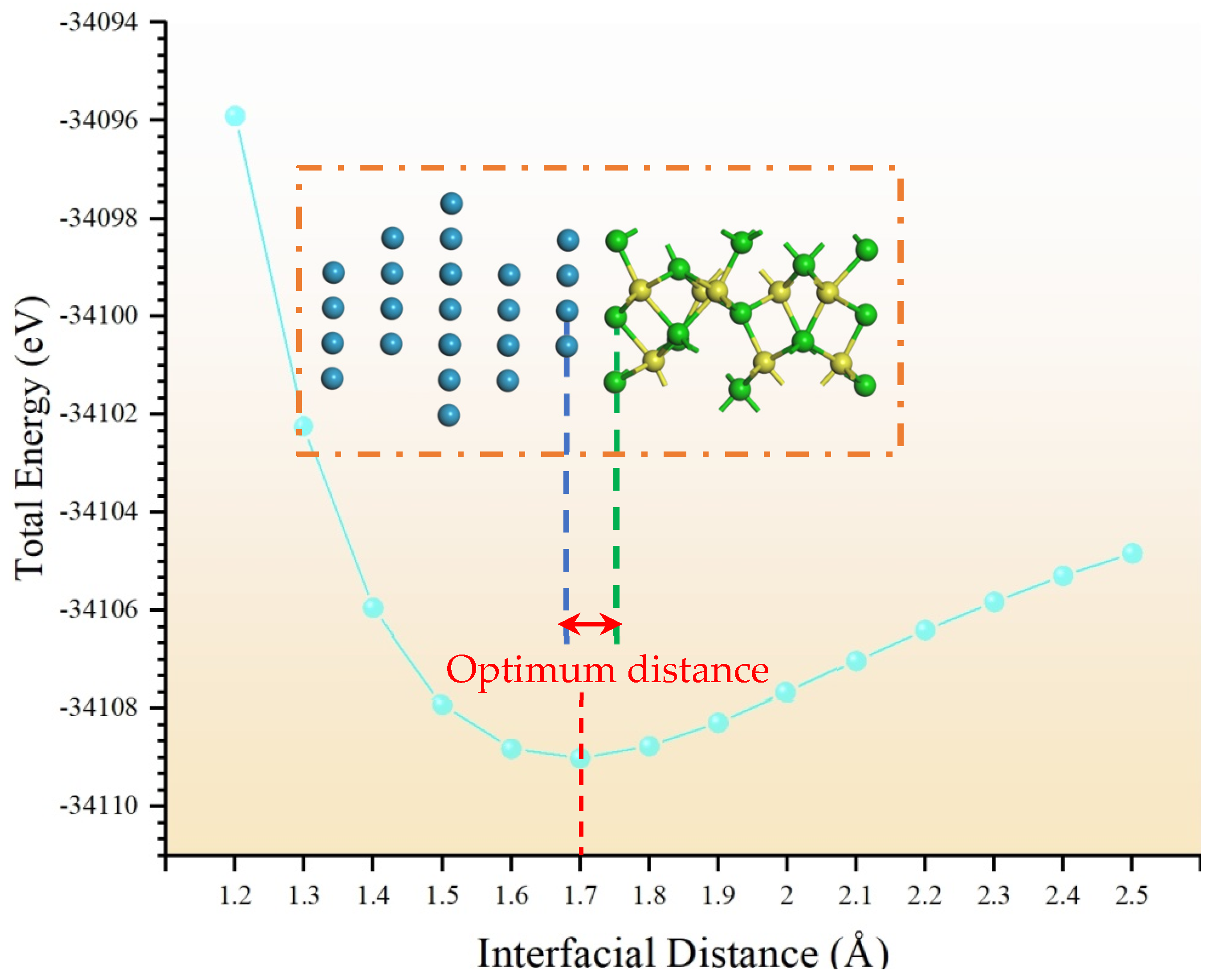
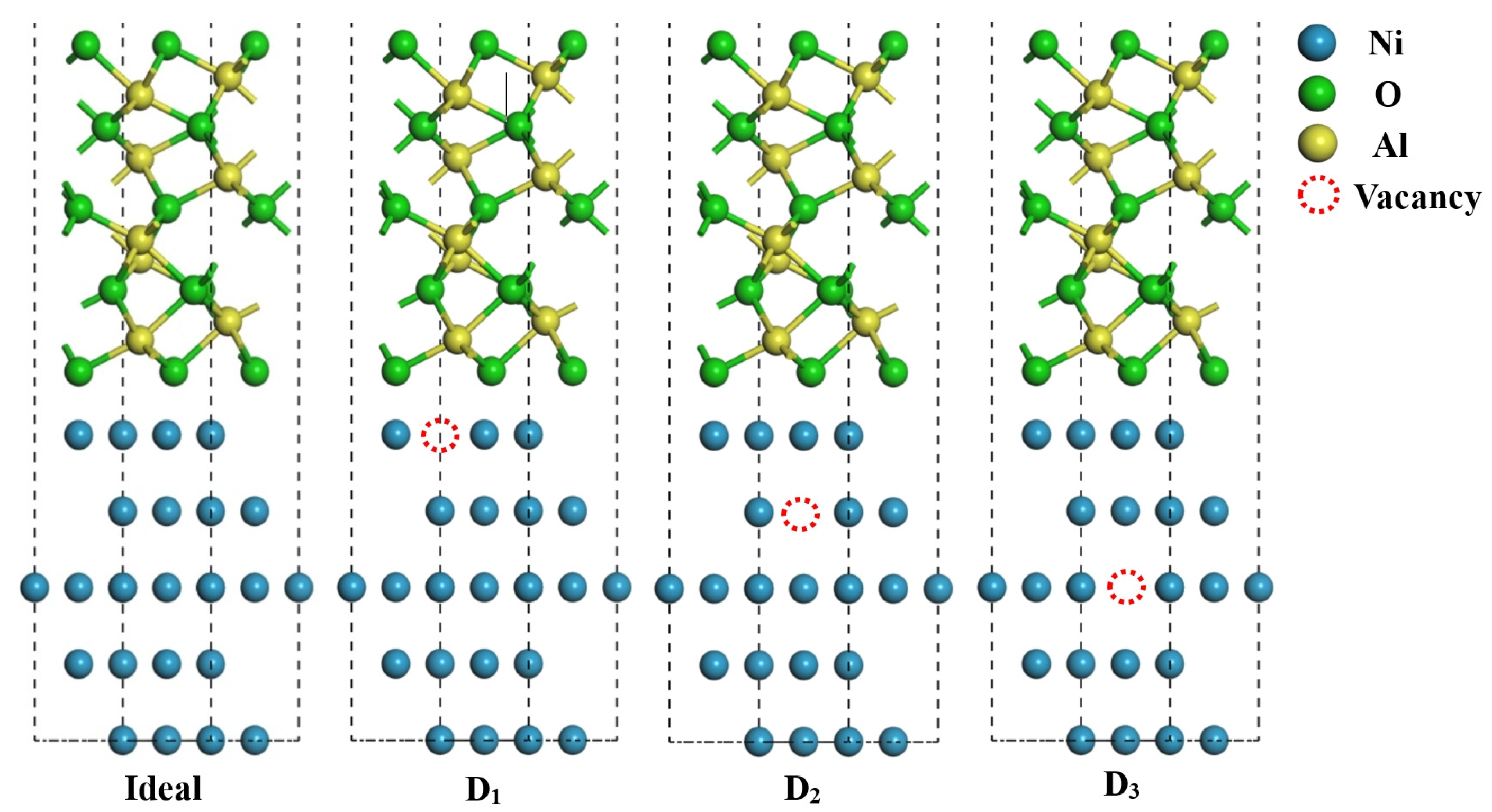
3.2. Model Building
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, X.Q.; Wang, J.J. Application of Surface Engineering Techniques to Corrosion Control over Offshore Engineering Equipment. Key Eng. Mater. 2008, 373, 551–555. [Google Scholar] [CrossRef]
- Abbas, M.K.G.; Ramesh, S.; Tasfy, S.; Lee, K.S. A state-of-the-art review on alumina toughened zirconia ceramic composites. Mater. Today Commun. 2023, 37, 106964. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, L.; Chen, H. Effect of strength and toughness of ceramic particles on impact abrasive wear resistance of iron matrix composites. Mater. Today Commun. 2023, 35, 105689. [Google Scholar] [CrossRef]
- Kota, N.; Charan, M.S.; Laha, T.; Roy, S. Review on development of metal/ceramic interpenetrating phase composites and critical analysis of their properties. Ceram. Int. 2022, 48, 1451–1483. [Google Scholar] [CrossRef]
- Upadhyaya, R.; Tailor, S.; Shrivastava, S.; Gladkova, A.A.; Modi, S.C. Effect of electroless Ni plating on the properties of cold-sprayed Ni-Al2O3 coatings. Surf. Innov. 2017, 5, 97–105. [Google Scholar] [CrossRef]
- Chang, L.M.; Liu, J.H.; Zhang, R.J. Hardness and wear resistance of Ni/Al2O3 composite coatings by electrodeposition. Adv. Mater. Res. 2011, 154, 654–657. [Google Scholar]
- Dhand, D.; Grewal, J.S.; Kumar, P. Study and comparison of wear behaviour of Ni-Al2O3 coatings deposited by hot and cold spray. Surf. Topogr. Metrol. Prop. 2021, 9, 045056. [Google Scholar] [CrossRef]
- Chen, J.S.; Yang, J.M.; Qiao, B. Influence of Laser Remelting on Tensile Properties of Nanocomposite Ni-Al2O3 Coatings. Mater. Trans. 2012, 53, 2205–2207. [Google Scholar]
- Gong, W.B.; Li, R.W.; Li, Y.P.; Sun, D.; Wang, W. Stabilization and corrosion resistance under high-temperature of nanostructured CeO2/ZrO2-Y2O3 thermal barrier coating. Acta Metall. Sin. 2013, 49, 593–598. [Google Scholar] [CrossRef]
- Finnis, W.M.; Kruse, C.; SchÖnberger, U. First principles calculations for metal/ceramic interfaces. MRS Online Proc. Libr. 1994, 357, 427–434. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, X.; Li, W.; Ai, T.; Dong, H.; Yang, Z. Study of Mo3NiB3 and Mo2NiB2 cermets by first-principles calculation and experiment. Next Mater. 2025, 7, 100324. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Bian, Y.; Cen, W.; Yin, D. The energetic, electronic and mechanical properties of metal (Fe/Co/Ni/Cu) || ceramics (TiN) interfaces: A first-principles study. Mater. Today Commun. 2025, 42, 111281. [Google Scholar] [CrossRef]
- Xue, H.; Wei, X.; Guo, W.; Zhang, X. Bonding mechanism study of active Ti element and α-Al2O3 by using first-principle calculation. J. Alloys Compd. 2020, 820, 153070. [Google Scholar] [CrossRef]
- Shi, S.; Tanaka, S.; Kohyama, M. First-principles study of the tensile strength and failure of α-Al2O3 (0001)/Ni (111) interfaces. Phys. Rev. B-Condens. Matter Mater. Phys. 2007, 76, 075431. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Zhang, C.; Dong, N.; Lan, A.; Li, H.; Dong, H.; Han, P. Effects of aluminum diffusion on the adhesive behavior of the Ni (111)/Cr2O3 (0001) interface: First principle study. Comput. Mater. Sci. 2013, 78, 116–122. [Google Scholar]
- Lin, Z.; Peng, X.; Fu, T.; Zhao, Y.; Feng, C.; Huang, C.; Wang, Z. Atomic structures and electronic properties of interfaces between aluminum and carbides/nitrides: A first-principles study. Phys. E Low-Dimens. Syst. Nanostructures 2017, 89, 15–20. [Google Scholar] [CrossRef]
- Li, Y.; Shi, M.; Gao, T.; Chen, C. Adhesion and mechanical properties of Fe/SiC interfaces analyzed at the atomic level: Insight from DFT calculations. Surf. Coat. Technol. 2025, 502, 131983. [Google Scholar] [CrossRef]
- Bao, Z.; Guo, X.; Shang, F. An atomistic investigation into the nature of fracture of Ni/Al2O3 interface with yttrium dopant under tension. Eng. Fract. Mech. 2015, 150, 239–247. [Google Scholar] [CrossRef]
- Li, R.W.; Liu, S.B.; Zhang, X.F.; Chen, Q.; Yang, H. Unveiling the secrets of the nickel-metal/alumina-ceramic interface during stretching: Atomic and electronic insights. Mater. Today Commun. 2024, 39, 109365. [Google Scholar] [CrossRef]
- Sun, F.Q.; Zhang, X.F.; Li, L.; Chen, Q.; Kong, D.; Yang, H.; Li, R. Exploring How Dopants Strengthen Metal-Ni/Ceramic-Al2O3 Interface Structures at the Atomic and Electronic Levels. Molecules 2025, 30, 1990. [Google Scholar] [CrossRef]
- Finnis, M.W. The theory of metal-ceramic interfaces. J. Phys. Condens. Matter 1996, 8, 5811. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, J.R.; Evans, A.G. The connection between ab initio calculations and interface adhesion measurements on metal/oxide systems: Ni/Al2O3 and Cu/Al2O3. Acta Mater. 2002, 50, 3803–3816. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhou, F.; Chen, M.; Qu, N.; Liao, M.; Zhu, J. The interface properties of defective graphene on aluminium: A first-principles calculation. Comput. Mater. Sci. 2021, 188, 110157. [Google Scholar] [CrossRef]
- Liu, Y.; An, L.; Gong, L. First-principles study of Cu adsorption on vacancy-defected/Au-doped graphene. Mod. Phys. Lett. B 2018, 32, 1850139. [Google Scholar] [CrossRef]
- Li, R.W.; Chen, Q.C.; Ouyang, L.; Zhang, Y.; Nie, B.; Ding, Y. Insight into the strengthening mechanism of α-Al2O3/γ-Fe ceramic-metal interface doped with Cr, Ni, Mg, and Ti. Ceram. Int. 2021, 47, 22810–22820. [Google Scholar] [CrossRef]
- Chun, H.; Choi, D.; Kang, J.; Park, J.S.; Han, B. First-Principles computational study of Ni/α-Al2O3 hybrid interface reactions under extreme thermodynamic conditions. Appl. Surf. Sci. 2019, 509, 144861. [Google Scholar] [CrossRef]
- Segall, M.D.; Shah, R.; Pickard, C.J.; Payne, M.C. Population analysis of plane-wave electronic structure calculations of bulk materials. Phys. Rev. B 1996, 54, 16317. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Liu, L.M.; Wang, S.Q. First-principles study of the tensile and fracture of the Al/TiN interface. Comput. Mater. Sci. 2007, 38, 800–806. [Google Scholar] [CrossRef]
- Mattsson, A.E.; Schultz, P.A.; Desjarlais, M.P.; Mattsson, T.R.; Leung, K. Designing meaningful density functional theory calculations in materials science—A primer. Model. Simul. Mater. Sci. Eng. 2004, 13, R1. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Laasonen, K.; Pasquarello, A.; Car, R.; Lee, C.; Vanderbilt, D. Car-Parrinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials. Phys. Rev. B 1993, 47, 10142. [Google Scholar] [CrossRef] [PubMed]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Fischer, T.H.; Almlof, J. General methods for geometry and wave function optimization. J. Phys. Chem. 1992, 96, 9768–9774. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533. [Google Scholar] [CrossRef]
- Fujimura, N.; Nishihara, T.; Goto, S.; Xu, J.; Ito, T. Control of preferred orientation for ZnOx films: Control of self-texture. J. Cryst. Growth 1993, 130, 269–279. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Huang, J.; Ye, Z.; Sun, X.; Chen, S.; Zhao, Y. First-principles calculations on Ni/W interfaces in Steel/Ni/W hot isostatic pressure diffusion bonding layer. Appl. Surf. Sci. 2019, 475, 906–916. [Google Scholar] [CrossRef]
- Wang, X.G.; Chaka, A.; Scheffler, M. Effect of the Environment on α-Al2O3 (0001) Surface Structures. Phys. Rev. Lett. 2000, 84, 3650. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, R.W.; Chen, Q.C.; Kong, D.; Yang, H. Influence of Vacancy on the Bonding Properties of γ-Fe (111)/α-Al2O3 (0001) Interface: A First-Principles Study. Materials 2025, 18, 4666. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, L.; Li, R.; Yang, H.; Kong, D. Unveiling Vacancy-Driven Stability: Atomic and Electronic Insights into Ni/Al2O3 Interfaces. Molecules 2025, 30, 4285. https://doi.org/10.3390/molecules30214285
Duan L, Li R, Yang H, Kong D. Unveiling Vacancy-Driven Stability: Atomic and Electronic Insights into Ni/Al2O3 Interfaces. Molecules. 2025; 30(21):4285. https://doi.org/10.3390/molecules30214285
Chicago/Turabian StyleDuan, Lili, Renwei Li, Haifeng Yang, and Dehao Kong. 2025. "Unveiling Vacancy-Driven Stability: Atomic and Electronic Insights into Ni/Al2O3 Interfaces" Molecules 30, no. 21: 4285. https://doi.org/10.3390/molecules30214285
APA StyleDuan, L., Li, R., Yang, H., & Kong, D. (2025). Unveiling Vacancy-Driven Stability: Atomic and Electronic Insights into Ni/Al2O3 Interfaces. Molecules, 30(21), 4285. https://doi.org/10.3390/molecules30214285




