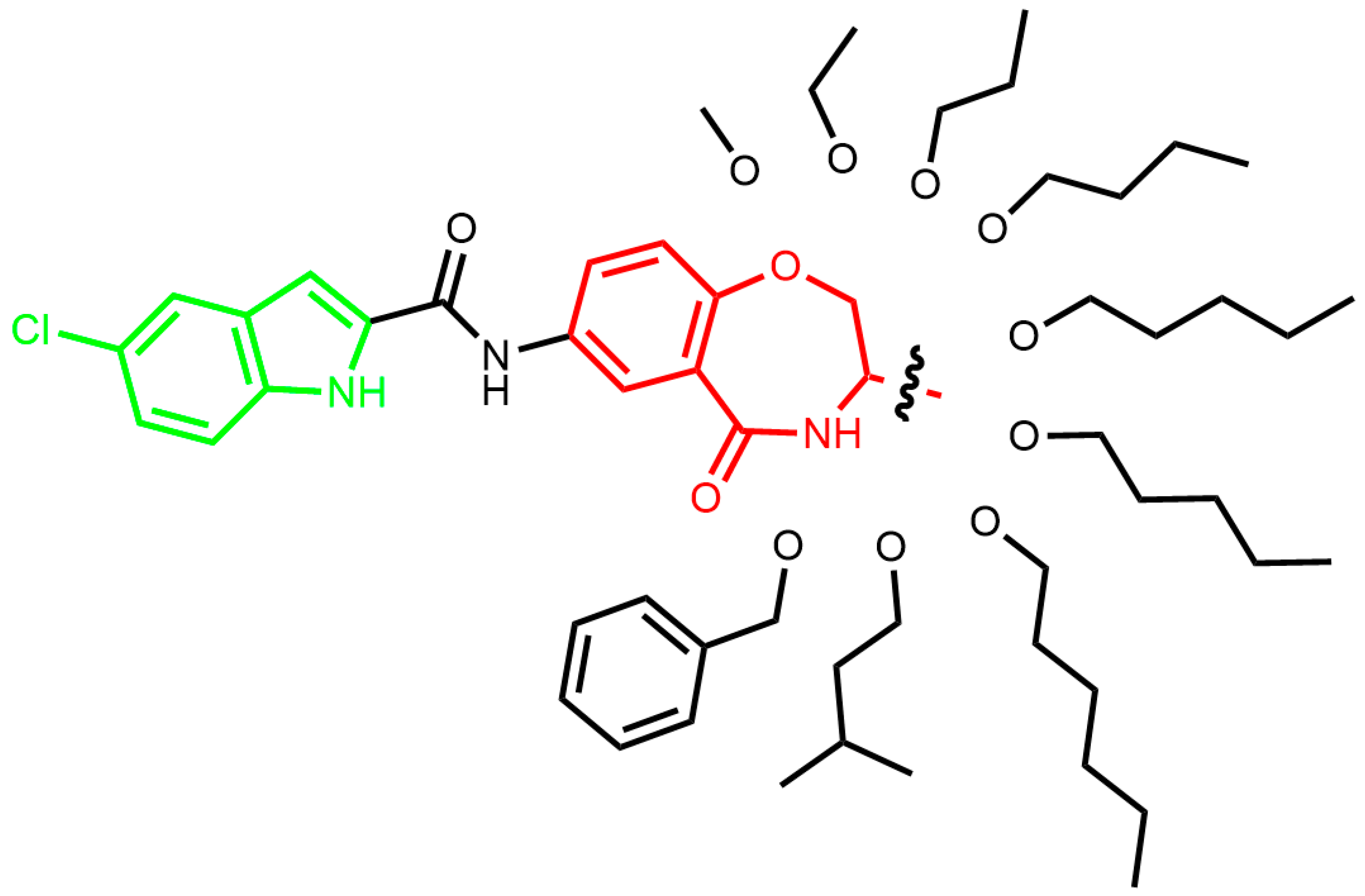
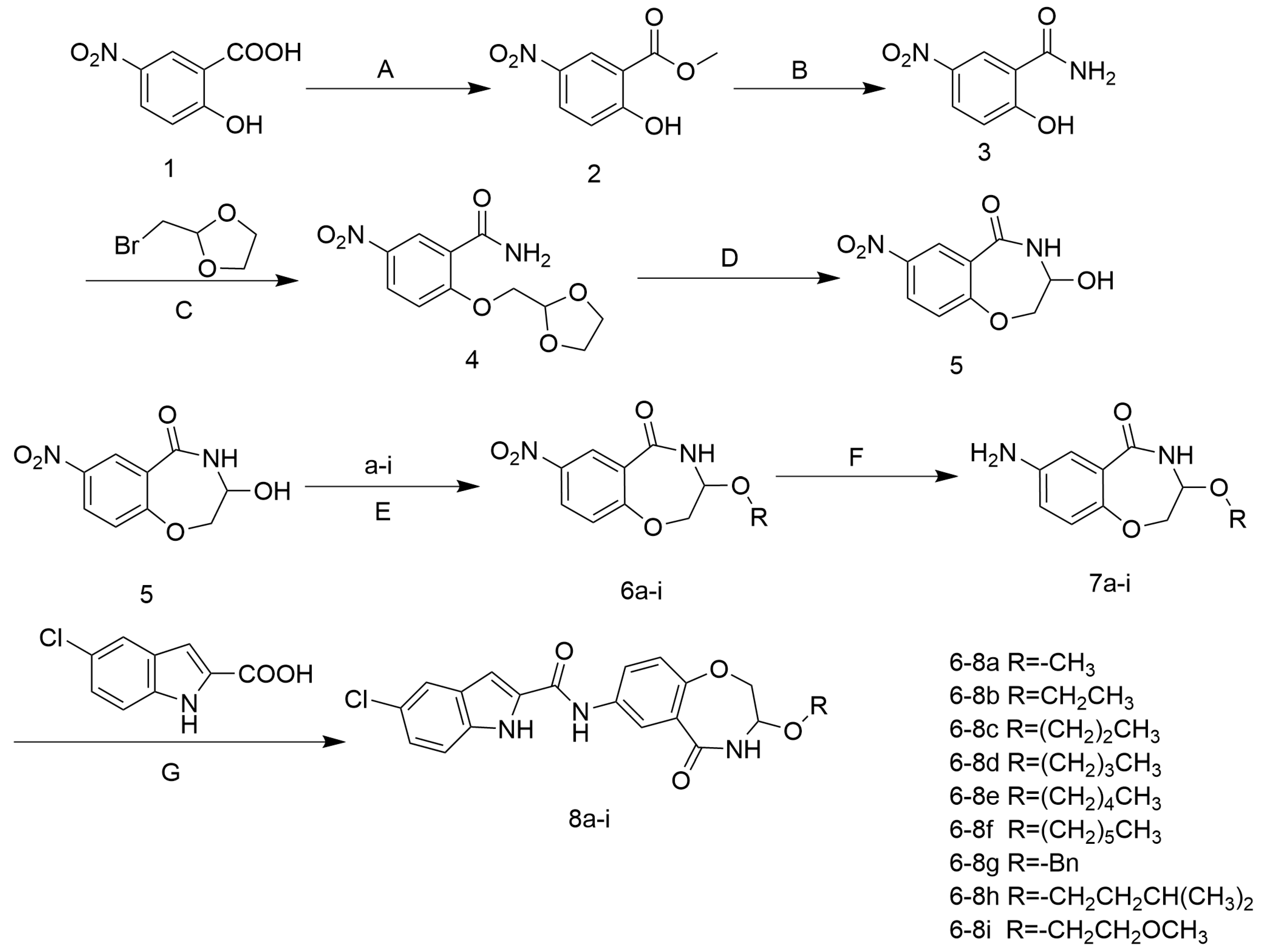
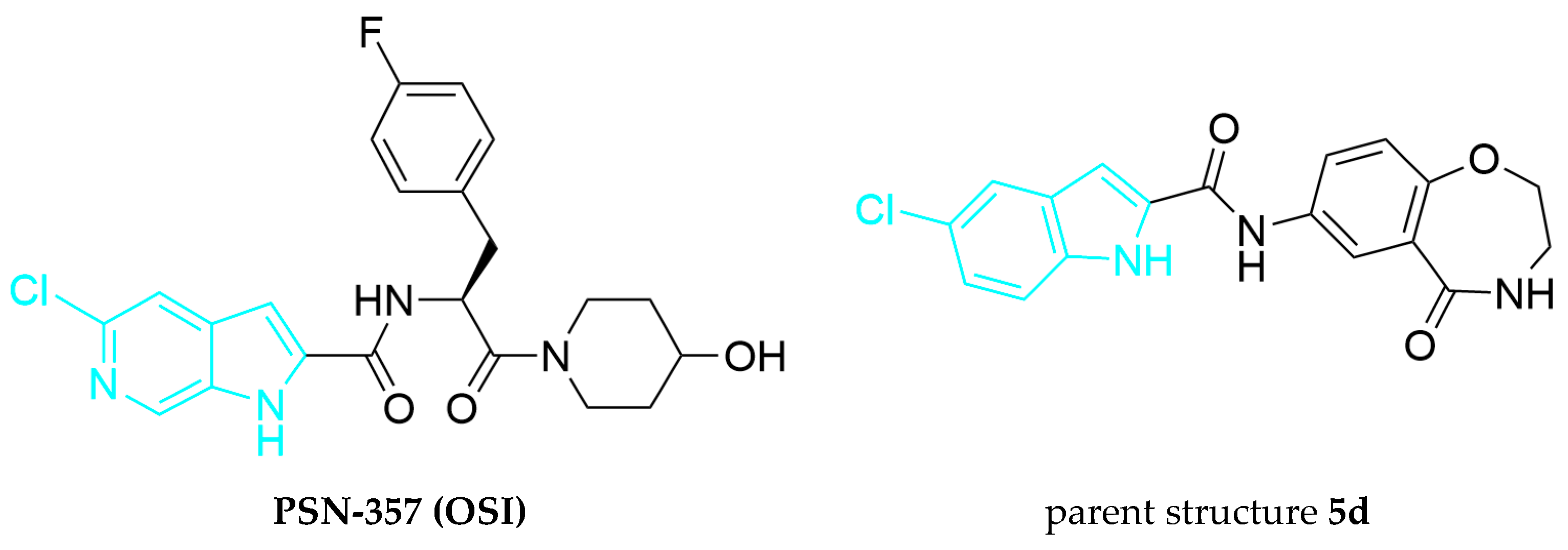
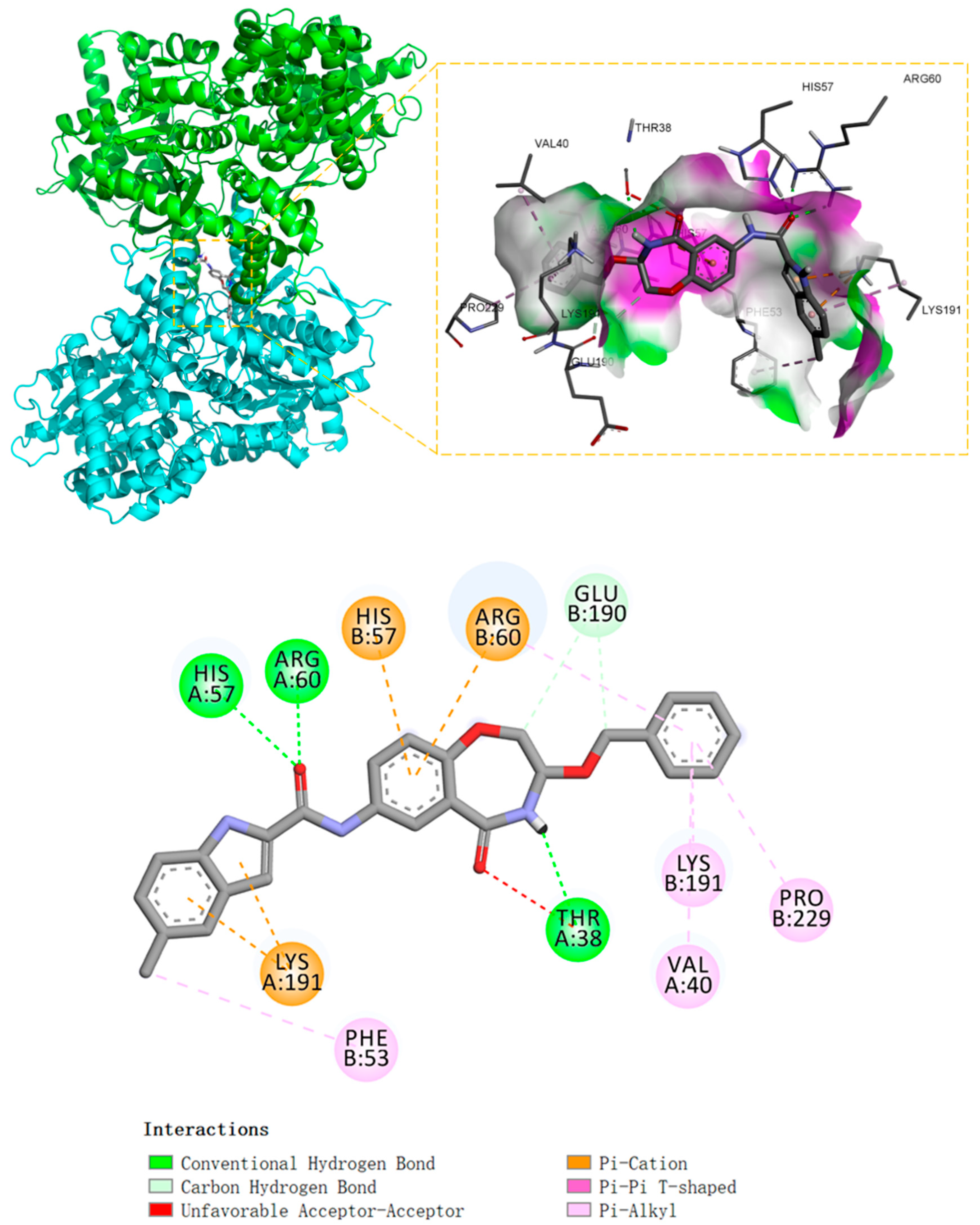
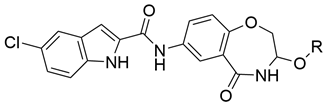
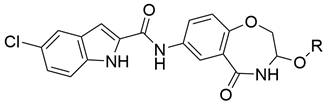
3.2. General Procedure for Compound Synthesis
3.2.1. General Procedure for the Synthesis of Compounds 2–5
Methyl 2-hydroxy-5-nitrobenzoate (2)
A solution of 5-nitrosalicylic acid (4.0 g, 21.84 mmol) in methanol (70 mL) was acidified by dropwise addition of concentrated sulfuric acid (1.5 mL) with stirring. The reaction mixture was placed under a nitrogen atmosphere and refluxed for 48 h. Upon completion, methanol was removed under reduced pressure. Potassium carbonate was added to the residue until gas evolution ceased. Water (20 mL) was added, and the mixture was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were dried over anhydrous sodium sulfate for 2–3 h, filtered, and concentrated under vacuum. Purification by silica gel column chromatography (petroleum ether/ethyl acetate, 15–20%) afforded the product as a white solid (4.0 g, 93%). HPLC analysis: 100%. m.p. 112–114 °C. ESI-MS m/z: 239.2 (M+H)+. 1H NMR (400 MHz, CDCl3) δ 11.43 (s, 1H), 8.79 (d, J = 2.8 Hz, 1H), 8.34 (dd, J = 9.2, 2.8 Hz, 1H), 7.09 (d, J = 9.2 Hz, 1H), 4.04 (s, 3H). 13C-NMR (100 MHz, CDCl3): 169.3, 166.2, 140.0, 130.6, 126.7, 118.7, 112.1, 53.1.
2-Hydroxy-5-nitrobenzamide (3)
Methyl 5-nitrosalicylate (4.0 g, 20.29 mmol) was dissolved in ammonia solution (70 mL) and stirred at 50 °C for 48 h under a nitrogen atmosphere. After completion, the solvent was removed under reduced pressure. The residue was diluted with water (30 mL), carefully acidified to near neutrality with 2 M hydrochloric acid, and extracted with ethyl acetate (3 × 50 mL). The combined organic layers were dried over anhydrous sodium sulfate, filtered, and concentrated. Purification by silica gel column chromatography (petroleum ether/ethyl acetate, 30–50%) yielded the product as a pale yellow solid (2.70 g, 73%). HPLC analysis: 100%. m.p. 130–132 °C. ESI-MS m/z: 180.7 (M-H)−. 1H-NMR (400 MHz, d6-DMSO): 7.10 (d, J = 8.0 Hz, 1H), 8.29 (dd, J = 8.0, 4.0 Hz, 2H), 8.81 (s, 1H), 8.90 (d, J = 2.4 Hz, 1H), 14.16 (s, 1H). 13C-NMR (100 MHz, d6-DMSO): 170.6, 166.8, 139.5, 129.6, 125.5, 119.0, 115.1.
2-[(1,3-Dioxolan-2-yl)methoxy]-5-nitrobenzamide (4)
A solution of 2-hydroxy-5-nitrobenzamide (0.30 g, 1.65 mmol) in DMF (3.0 mL) was treated with potassium carbonate (0.40 g, 2.89 mmol) and stirred at room temperature for 10 min. Subsequently, 2-(bromomethyl)-1,3-dioxolane (0.27 mL, 2.61 mmol) and a catalytic amount of potassium iodide were added. The reaction was carried out under nitrogen at 120 °C for 72 h. The mixture was diluted with brine (20 mL) and extracted with ethyl acetate (3 × 50 mL). The combined organic extracts were washed with saturated brine (3 × 20 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Purification by silica gel column chromatography (petroleum ether/ethyl acetate, 35–60%) provided the product as a pale yellow solid (0.22 g, 50%). HPLC analysis: 98%. m.p. 152–154 °C. ESI-MS m/z: 269.1 (M+H)+. 1H-NMR (400 MHz, CDCl3): 3.99–4.09 (m, 4H), 4.31 (d, J = 3.6 Hz, 2H), 5.35 (t, J = 3.6 Hz, 1H), 6.08 (s, 1H), 7.10 (d, J = 9.2 Hz, 1H), 7.78 (s, 1H), 8.35 (dd, J = 9.2, 2.8 Hz, 1H), 9.10 (d, J = 3.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 164.4, 160.9, 142.3, 128.9, 128.4, 122.4, 113.0, 100.8, 69.3, 65.6.
7-Nitro-3-hydroxy-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (5)
2-[(1,3-Dioxolan-2-yl)methoxy]-5-nitrobenzamide (1.5 g, 5.59 mmol) was dissolved in a mixture of 1,4-dioxane (10 mL) and water (30 mL). Concentrated sulfuric acid (1.5 mL) was added dropwise with stirring. The reaction mixture was heated to 98 °C and maintained for 8 h. After cooling, the mixture was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with saturated brine (3 × 20 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Purification by silica gel column chromatography (dichloromethane/methanol, 1%) yielded the product as a pale yellow solid (0.48 g, 38%). HPLC analysis: 100%. m.p. 163–165 °C. ESI-MS m/z: 222.7 (M-H)−. 1H-NMR (400 MHz, d6-DMSO): 4.34 (d, J = 12.4 Hz, 1H), 4.53 (dd, J = 12.4, 4.4 Hz, 1H), 4.93 (q, J = 8.8 Hz, 1H), 6.33 (d, J = 4.8 Hz, 1H), 7.24 (d, J = 9.2 Hz, 1H), 8.26 (dd, J = 9.2, 3.2 Hz, 1H), 8.96 (d, J = 3.2 Hz, 1H), 9.22 (d, J = 5.6 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 163.8, 162.1, 141.0, 129.6, 127.5, 121.7, 120.7, 73.7, 72.1.
3.2.2. General Procedure for the Synthesis of Compound 6a–i
7-Nitro-3-hydroxy-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (1.0 equiv) was added to a solution containing an excess of esters a–i. p-Toluenesulfonic acid (0.1 equiv) was introduced with continuous stirring. The reaction mixture was maintained at 50 °C for 3 h, cooled to room temperature, and concentrated. The crude product was purified by silica gel column chromatography.
3-Methoxy-7-nitro-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6a)
HPLC analysis: 100%. m.p. 195–197 °C. ESI-MS m/z: 239.2 (M+H)+. 1H-NMR (400 MHz, CDCl3): 3.46 (s, 3H), 4.35 (d, J = 12.3 Hz, 1H), 4.68 (d, J = 4.8 Hz, 1H), 4.74 (dd, J = 12.3, 3.8 Hz, 1H), 7.18 (d, J = 9.0 Hz, 1H), 7.72 (s, 1H), 8.26 (dd, J = 9.4, 1.8 Hz, 1H), 9.19 (s, 1H). 13C-NMR (100 MHz, CDCl3): 165.7, 161.7, 142.4, 130.6, 128.3, 121.8, 119.9, 81.0, 72.0, 55.7.
3-ethoxy-7-nitro-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6b)
HPLC analysis: 96%. m.p. 149–151 °C. ESI-MS m/z: 239.2 (M+H)+. 1H-NMR (400 MHz, CDCl3): 1.21 (t, J = 7.2 Hz, 3H), 3.57–3.64 (m, 1H), 3.76–3.83 (m, 1H), 4.36 (d, J = 12.4 Hz, 1H), 4.72 (dd, J = 12.4, 4.0 Hz, 1H), 4.82 (t, J = 4.0 Hz, 1H), 7.18 (d, J = 9.2 Hz, 1H), 8.26 (dd, J = 8.8, 2.0 Hz, 1H), 8.34 (s, 1H), 9.18 (d, J = 2.0 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 166.0, 162.0, 142.3, 130.5, 128.3, 121.9, 120.1, 79.5, 72.5, 63.9, 14.9.
7-nitro-3-propoxy-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6c)
HPLC analysis: 93%. m.p. 130–132 °C. ESI-MS m/z: 289.2 (M+Na)+. 1H-NMR (400 MHz, CDCl3): 0.82 (t, J = 7.4 Hz, 3H), 1.53–1.62 (m, 2H), 3.51 (dt, J = 9.1, 6.4 Hz, 1H), 3.66 (dt, J = 9.1, 6.8 Hz, 1H), 4.38 (d, J = 12.2 Hz, 1H), 4.72 (dd, J = 12.3, 4.3 Hz, 1H), 4.81 (t, J = 4.8 Hz, 1H), 7.18 (d, J = 9.1 Hz, 1H), 8.26 (dd, J = 9.1, 2.9 Hz, 1H), 8.40 (d, J = 5.2 Hz, 1H), 9.17 (d, J = 2.9 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 166.1, 162.0, 142.2, 130.4, 128.2, 121.8, 120.2, 79.6, 77.4, 77.0, 76.7, 72.6, 69.9, 22.6, 10.4.
3-butoxy-7-nitro-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6d)
HPLC analysis: 96%. m.p. 122–124 °C. ESI-MS m/z: 281.5 (M+H)+. 1H-NMR (500 MHz, CDCl3): 0.84 (t, J = 7.4 Hz, 3H), 1.23–1.28 (m, 2H), 1.51–1.56 (m, 2H), 3.53 (dt, J = 9.2, 6.4 Hz, 1H), 3.70 (dt, J = 9.1, 6.8 Hz, 1H), 4.37 (d, J = 12.2 Hz, 1H), 4.69 (dd, J = 12.3, 4.4 Hz, 1H), 4.79 (t, J = 4.8 Hz, 1H), 7.16 (d, J = 9.1 Hz, 1H), 8.21 (d, J = 4.7 Hz, 1H), 8.25 (dd, J = 9.1, 2.9 Hz, 1H), 9.15 (d, J = 2.9 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 166.0, 161.9, 142.3, 130.3, 128.1, 121.8, 120.3, 79.7, 72.6, 68.0, 31.3, 19.0, 13.6.
7-nitro-3-(pentyloxy)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6e)
HPLC analysis: 99%. m.p. 106–108 °C. ESI-MS m/z: 317.4 (M+Na)+. 1H-NMR (500 MHz, CDCl3): 0.80 (t, J = 7.0 Hz, 3H), 1.15–1.27 (m, 4H), 1.52–1.58 (m, 2H), 3.53 (dt, J = 9.1, 6.4 Hz, 1H), 3.69 (dt, J = 8.8, 6.9 Hz, 1H), 4.38 (d, J = 12.3 Hz, 1H), 4.70 (dd, J = 12.3, 4.3 Hz, 1H), 4.80 (t, J = 4.9 Hz, 1H), 7.17 (d, J = 9.1 Hz, 1H), 8.25 (dd, J = 9.1, 2.9 Hz, 1H), 8.42 (d, J = 4.1 Hz, 1H), 9.14 (d, J = 2.8 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 166.2, 161.9, 142.3, 130.2, 128.1, 121.8, 120.4, 79.6, 72.7, 68.3, 28.9, 28.0, 22.2, 13.8.
3-(hexyloxy)-7-nitro-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6f)
HPLC analysis: 99%. m.p. 93–95 °C. ESI-MS m/z: 331.4 (M+Na)+. 1H-NMR (500 MHz, CDCl3): 0.81 (t, J = 6.7 Hz, 3H), 1.16–1.26 (m, 2H), 1.53 (dd, J = 13.2, 6.5 Hz, 2H), 3.52 (dt, J = 9.1, 6.4 Hz, 1H), 3.69 (dt, J = 9.0, 6.8 Hz, 1H), 4.38 (d, J = 12.3 Hz, 1H), 4.69 (dd, J = 12.3, 4.4 Hz, 1H), 4.79 (t, J = 4.9 Hz, 1H), 7.16 (d, J = 9.1 Hz, 1H), 8.20 (d, J = 3.7 Hz, 1H), 8.25 (dd, J = 9.1, 2.9 Hz, 1H), 9.15 (d, J = 2.9 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 166.0, 161.9, 142.3, 130.2, 128.1, 121.7, 120.4, 79.6, 72.6, 68.3, 31.3, 29.2, 25.5, 22.5, 13.8.
3-(benzyloxy)-7-nitro-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6g)
HPLC analysis: 100%. m.p. 171–173 °C. ESI-MS m/z: 337.2 (M+Na)+. 1H-NMR (500 MHz, CDCl3): 4.35 (d, J = 12.4 Hz, 1H), 4.63–4.70 (m, 2H), 4.80 (dd, J = 14.9, 8.8 Hz, 2H), 7.19 (d, J = 9.0 Hz, 1H), 7.32–7.39 (m, 5H), 8.26 (dd, J = 9.0, 2.7 Hz, 1H), 8.68 (s, 1H), 9.21 (d, J = 2.6 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 166.4, 162.0, 142.3, 136.2, 130.5, 128.8, 128.4, 128.3, 128.2, 121.9, 120.0, 77.6, 72.5, 69.4.
3-(isopentyloxy)-7-nitro-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6h)
HPLC analysis: 100%. m.p. 126–128 °C. ESI-MS m/z: 295.2 (M+H)+. 1H-NMR (500 MHz, CDCl3): 0.82 (dd, J = 9.3, 6.6 Hz, 6H), 1.45 (tt, J = 13.7, 6.7 Hz, 2H), 1.55 (dt, J = 13.4, 6.7 Hz, 1H), 3.56 (dt, J = 9.2, 6.6 Hz, 1H), 3.74 (dt, J = 9.2, 6.9 Hz, 1H), 4.37 (d, J = 12.2 Hz, 1H), 4.70 (dd, J = 12.3, 4.3 Hz, 1H), 4.80 (t, J = 3.6 Hz, 1H), 7.16 (d, J = 9.1 Hz, 1H), 8.25 (dd, J = 9.1, 2.9 Hz, 1H), 8.42 (d, J = 4.9 Hz, 1H), 9.15 (d, J = 2.9 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 166.1, 162.0, 142.3, 130.3, 128.1, 121.8, 120.3, 79.6, 72.6, 66.8, 38.0, 24.9, 22.5, 22.2.
3-(2-methoxyethoxy)-7-nitro-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (6i)
HPLC analysis: 94%. m.p. 115–117 °C. ESI-MS m/z: 305.1 (M+Na)+. 1H-NMR (500 MHz, CDCl3): 3.37 (s, 3H), 3.55–3.56 (m, 2H), 3.78–3.87 (m, 2H), 4.36 (d, J = 12.4 Hz, 1H), 4.70 (dd, J = 12.4, 4.5 Hz, 1H), 4.95 (t, J = 5.0 Hz, 1H), 7.16 (d, J = 9.0 Hz, 1H), 8.12 (d, J = 4.3 Hz, 1H), 8.24 (dd, J = 9.0, 2.9 Hz, 1H), 9.18 (d, J = 2.9 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 165.3, 162.1, 142.4, 130.5, 128.1, 121.7, 120.4, 80.3, 72.41, 72.38, 67.7, 59.0.
3.2.3. General Procedure for the Synthesis of Compounds 7a–i
Compound 6a–i (1.0 eqv) was dissolved in tetrahydrofuran, followed by the addition of HCOONH4 (10.0 eqv) and Pd/C (0.5 eqv). The reaction was carried out with stirring at 50 °C until completion. The reaction mixture was cooled to room temperature and concentrated, and the product was purified by silica gel column chromatography.
7-amino-3-methoxy-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7a)
HPLC analysis: 92.6%. ESI-MS m/z: 209.1 (M+H)+. 1H-NMR (400 MHz, d6-DMSO): 3.17 (s, 3H), 4.15 (d, J = 3.2 Hz, 2H), 4.46 (dt, J = 6.2, 3.2 Hz, 1H), 4.93 (s, 2H), 6.65 (dd, J = 8.5, 2.8 Hz, 1H), 6.72 (d, J = 8.5 Hz, 1H), 7.05 (d, J = 2.8 Hz, 1H), 8.77 (d, J = 5.9 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 167.8, 147.6, 143.9, 124.9, 120.5, 119.0, 115.1, 81.8, 73.8, 54.5.
7-amino-3-ethoxy-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7b)
HPLC analysis: 100%. ESI-MS m/z: 221.6 (M-H)−. 1H-NMR (400 MHz, d6-DMSO): 1.02 (t, J = 6.8 Hz, 3H), 3.36–3.42 (m, 1H), 3.51–3.59 (m, 1H), 4.14 (t, J = 2.8 Hz, 2H), 4.58 (dt, J = 5.6, 3.2 Hz, 1H), 4.92 (s, 2H), 6.66 (dd, J = 8.4, 2.8 Hz, 1H), 6.73 (d, J = 8.4 Hz, 1H), 7.07 (d, J = 2.8 Hz, 1H), 8.72 (d, J = 5.6 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 168.2, 148.3, 144.4, 125.3, 121.0, 119.5, 115.7, 80.7, 74.4, 62.7,15.4.
7-amino-3-propoxy-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7c)
HPLC analysis: 92.6%. ESI-MS m/z: 209.1 (M+H)+. 1H-NMR (400 MHz, d6-DMSO): 0.73 (t, J = 7.4 Hz, 3H), 1.35–1.43 (m, 2H), 3.28 (dt, J = 9.4, 6.4 Hz, 1H), 3.39–3.44 (m, 1H), 4.15 (d, J = 3.1 Hz, 2H), 4.55 (dt, J = 5.9, 3.1 Hz, 1H), 4.94 (s, 2H), 6.64 (dd, J = 8.5, 2.8 Hz, 1H), 6.72 (d, J = 8.5 Hz, 1H), 7.04 (d, J = 2.7 Hz, 1H), 8.73 (d, J = 5.8 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 167.9, 147.7, 143.8, 125.2, 120.5, 118.9, 115.1, 80.3, 74.2, 68.4, 22.2, 10.4.
7-amino-3-butoxy-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7d)
HPLC analysis: 97%. ESI-MS m/z: 251.1 (M+H)+. 1H-NMR (500 MHz, d6-DMSO): 0.80 (t, J = 7.4 Hz, 3H), 1.13–1.21 (m, 2H), 1.34–1.39 (m, 2H), 3.30–3.33 (m, 1H), 3.48 (dt, J = 9.4, 6.7 Hz, 1H), 4.14 (d, J = 3.2 Hz, 2H), 4.55 (dt, J = 6.2, 3.2 Hz, 1H), 4.87 (s, 2H), 6.65 (dd, J = 8.5, 2.8 Hz, 1H), 6.72 (d, J = 8.5 Hz, 1H), 7.05 (d, J = 2.8 Hz, 1H), 8.66 (d, J = 5.8 Hz, 1H). 13C-NMR (125 MHz, d6-DMSO): 167.8, 147.7, 143.8, 125.1, 120.5, 118.9, 115.2, 80.4, 74.2, 66.4, 30.9, 18.6, 13.5.
7-amino-3-(pentyloxy)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7e)
HPLC analysis: 100%. ESI-MS m/z: 265.2 (M+H)+. 1H-NMR (400 MHz, d6-DMSO): 0.81 (t, J = 6.8 Hz, 3H), 1.08–1.16 (m, 2H), 1.17–1.26 (m, 2H), 1.33–1.42 (m, 2H), 3.30 (q, J = 7.2 Hz, 1H), 3.46 (q, J = 8.4 Hz, 1H), 4.14 (s, 2H), 4.54 (d, J = 2.8 Hz, 1H), 4.89 (s, 1H), 6.65 (d, J = 8.4 Hz, 1H), 6.72 (d, J = 8.8 Hz, 1H), 7.04 (s, 1H), 8.69 (d, J = 5.6 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 167.9, 147.7, 143.9, 125.2, 120.5, 118.8, 115.1, 80.4, 74.2, 66.7, 28.6, 27.7, 21.8, 13.9.
7-amino-3-(hexyloxy)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7f)
HPLC analysis: 99%. ESI-MS m/z: 279.1 (M+H)+. 1H-NMR (500 MHz, CDCl3): 0.86 (t, J = 6.9 Hz, 3H), 1.23–1.28 (m, 6H), 1.57–1.48 (m, 2H), 3.44 (dt, J = 9.1, 6.5 Hz, 1H), 3.65 (dt, J = 9.0, 6.8 Hz, 1H), 4.22 (dd, J = 12.0, 2.9 Hz, 1H), 4.31 (dd, J = 12.0, 4.6 Hz, 1H), 4.69 (td, J = 4.8, 3.3 Hz, 1H), 6.77 (dd, J = 8.6, 2.9 Hz, 1H), 6.88 (d, J = 8.6 Hz, 1H), 7.09 (d, J = 3.9 Hz, 1H), 7.36 (d, J = 2.9 Hz, 1H). 13C-NMR (125 MHz, CDCl3): 167.9, 150.4, 141.8, 123.8, 121.5, 121.0, 117.2, 81.8, 73.8, 68.5, 31.5, 29.4, 25.6, 22.5, 13.9.
7-amino-3-(benzyloxy)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7g)
HPLC analysis: 95%. ESI-MS m/z: 285.2 (M+H)+. 1H-NMR (500 MHz, d6-DMSO): 4.17–4.23 (m, 2H), 4.42 (d, J = 12.0 Hz, 1H), 4.56 (d, J = 12.0 Hz, 1H), 4.68–4.69 (m, 1H), 4.91 (s, 2H), 6.66 (dd, J = 8.5, 2.7 Hz, 1H), 6.74 (d, J = 8.5 Hz, 1H), 7.08 (d, J = 2.6 Hz, 1H), 7.20 (d, J = 7.2 Hz, 2H), 7.27 (dq, J = 14.3, 7.2 Hz, 3H), 8.82 (d, J = 5.5 Hz, 1H). 13C-NMR (125 MHz, d6-DMSO): 168.4, 148.3, 144.4, 138.3, 128.6, 128.0, 127.9, 125.5, 121.0, 119.5, 115.7, 80.5, 74.5, 68.8.
7-amino-3-(isopentyloxy)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7h)
HPLC analysis: 92.6%. ESI-MS m/z: 265.2 (M+H)+. 1H-NMR (500 MHz, d6-DMSO): 0.78 (d, J = 6.7 Hz, 6H), 1.20–1.32 (m, 2H), 1.42–1.51 (m, 1H), 3.30–3.35 (m, 1H), 3.52 (dt, J = 9.5, 6.9 Hz, 1H), 4.14 (d, J = 3.2 Hz, 2H), 4.89 (s, 2H), 4.52–4.56 (m, 1H), 6.65 (dd, J = 8.5, 2.8 Hz, 1H), 6.72 (dd, J = 8.5, 2.3 Hz, 1H), 7.05 (d, J = 2.8 Hz, 1H), 8.67 (d, J = 5.9 Hz, 1H). 13C-NMR (125 MHz, d6-DMSO): 168.3, 148.2, 144.3, 125.6, 121.0, 119.4, 115.7, 80.9, 74.6, 65.6, 38.3, 24.8, 23.0, 22.6.
7-amino-3-(2-methoxyethoxy)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one (7i)
HPLC analysis: 92.6%. ESI-MS m/z: 275.1 (M+Na)+. 1H-NMR (500 MHz, d6-DMSO): 3.17 (s, 3H), 3.42–3.34 (m, 2H), 3.53–3.45 (m, 1H), 3.60 (ddd, J = 10.6, 5.5, 4.1 Hz, 1H), 4.15 (qd, J = 12.3, 3.2 Hz, 2H), 4.61 (dt, J = 6.1, 3.2 Hz, 1H), 4.89 (s, 2H), 6.66 (dd, J = 8.5, 2.8 Hz, 1H), 6.73 (d, J = 8.5 Hz, 1H), 7.09 (d, J = 2.7 Hz, 1H), 8.67 (d, J = 5.5 Hz, 1H). 13C-NMR (125 MHz, d6-DMSO): 168.0, 148.4, 144.3, 125.1, 121.0, 119.6, 115.8, 81.3, 74.2, 71.3, 66.7, 58.4.
3.2.4. General Procedure for the Synthesis of Compounds 8a–i
A solution of 5-chloroindole-2-carboxylic acid (1.0 eqv) in DMF was sequentially treated with HATU (1.0 eqv in DMF) and N,N-diisopropylethylamine (DIPEA, 3.0 eqv in DMF). After stirring at room temperature for 10 min, compounds 7a–i (1.0 eqv in DMF) were added. The reaction was heated to 45 °C and stirred for 5 h. Upon completion, the mixture was cooled to room temperature, and 50 mL of purified water was added, followed by suction filtration. The product was purified by silica gel column chromatography.
5-chloro-N-(3-methoxy-5-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-1H-indole-2-carboxamide (8a)
HPLC analysis: 100%. m.p. None. ESI-MS m/z: 420.8 (M+Cl)−. 1H-NMR (400 MHz, d6-DMSO): 3.24 (s, 3H), 4.24 (d, J = 12.2 Hz, 1H), 4.45 (d, J = 12.3 Hz, 1H), 4.55 (s, 1H), 7.05 (d, J = 8.7 Hz, 1H), 7.23 (d, J = 8.2 Hz, 1H), 7.43 (s, 1H), 7.48 (d, J = 8.6 Hz, 1H), 7.78 (s, 1H), 7.98 (d, J = 7.1 Hz, 1H), 8.33 (s, 1H), 9.06 (d, J = 5.2 Hz, 1H), 10.39 (s, 1H), 11.94 (s, 1H). 13C-NMR (100 MHz, d6-DMSO): 166.5, 159.1, 153.1, 135.1, 133.0, 132.9, 128.1, 125.6, 124.3, 123.8, 123.6, 122.2, 120.8, 120.3, 113.9, 103.2, 80.8, 72.4, 54.5.
5-chloro-N-(3-ethoxy-5-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-1H-indole-2-carboxamide (8b)
HPLC analysis: 100%. m.p. 227–229 °C. ESI-MS m/z: 435.8 (M+Cl)−. 1H-NMR (400 MHz, d6-DMSO): 1.05 (t, J = 7.0 Hz, 3H), 3.44 (dq, J = 9.6 Hz, 7.2 Hz, 1H), 3.60 (dq, J = 9.6 Hz, 7.2 Hz, 1H), 4.22 (dd, J = 12.4, 1.6 Hz, 1H), 4.43 (dd, J = 12.4, 3.6 Hz, 1H), 4.65–4.67 (m, 1H), 7.05 (d, J = 8.8 Hz, 1H), 7.23 (dd, J = 8.4, 2.0 Hz, 1H), 7.43 (d, J = 1.2 Hz, 1H), 7.48 (d, J = 8.8 Hz, 1H), 7.78 (d, J = 2.0 Hz, 1H), 7.98 (dd, J = 8.8, 2.4 Hz, 1H), 8.33 (d, J = 2.8 Hz, 1H), 9.04 (d, J = 6.0 Hz, 1H), 10.39 (s, 1H), 11.94 (s, 1H). 13C-NMR (100 MHz, d6-DMSO): 166.4, 159.1, 153.2, 135.1, 132.92, 132.86, 128.1, 125.6, 124.3, 123.8, 123.7, 122.1, 120.8, 120.3, 113.9, 103.2, 79.2, 72.6, 62.2, 14.9.
5-chloro-N-(5-oxo-3-propoxy-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-1H-indole-2-carboxamide (8c)
HPLC analysis: 100%. m.p. 231–233 °C. ESI-MS m/z: 449.8 (M+Na)+. 1H-NMR (400 MHz, d6-DMSO): 0.74 (t, J = 7.4 Hz, 3H), 1.39–1.48 (m, 2H), 3.34–3.39 (m, 1H), 3.45–3.51 (m, 1H), 4.25 (d, J = 12.0 Hz, 1H), 4.43 (dd, J = 12.3, 3.3 Hz, 1H), 4.60–4.69 (m, 1H), 7.06 (d, J = 8.8 Hz, 1H), 7.23 (dd, J = 8.7, 1.8 Hz, 1H), 7.43 (s, 1H), 7.48 (d, J = 8.7 Hz, 1H), 7.78 (d, J = 1.2 Hz, 1H), 7.98 (dd, J = 8.8, 2.6 Hz, 1H), 8.32 (d, J = 2.5 Hz, 1H), 9.03 (d, J = 5.9 Hz, 1H), 10.38 (s, 1H), 11.94 (s, 1H). 13C-NMR (100 MHz, d6-DMSO): 166.6, 159.1, 153.1, 135.1, 133.0, 132.9, 128.1, 125.5, 124.3, 123.8, 123.6, 122.5, 120.8, 120.3, 113.9, 103.2, 79.4, 72.8, 68.3, 22.2, 10.4.
N-(3-butoxy-5-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-5-chloro-1H-indole-2-carboxamide (8d)
HPLC analysis: 100%. m.p. 224–226 °C. ESI-MS m/z: 463.8 (M+Cl)−. 1H-NMR (500 MHz, d6-DMSO): 0.80 (t, J = 7.4 Hz, 3H), 1.16–1.19 (m, 2H), 1.38–1.42 (m, 2H), 3.40 (dt, J = 9.6, 6.3 Hz, 1H), 3.54 (dt, J = 9.4, 6.8 Hz, 1H), 4.24 (dd, J = 12.3, 1.6 Hz, 1H), 4.42 (dd, J = 12.3, 3.4 Hz, 1H), 4.61–4.66 (m, 1H), 7.04 (d, J = 8.8 Hz, 1H), 7.22 (dd, J = 8.7, 1.9 Hz, 1H), 7.42 (d, J = 1.1 Hz, 1H), 7.48 (d, J = 8.7 Hz, 1H), 7.76 (d, J = 1.3 Hz, 1H), 7.96 (dd, J = 8.8, 2.7 Hz, 1H), 8.31 (d, J = 2.6 Hz, 1H), 8.96 (d, J = 6.0 Hz, 1H), 10.34 (s, 1H), 11.88 (s, 1H). 13C-NMR (125 MHz, d6-DMSO): 167.1, 159.6, 153.6, 135.6, 133.5, 133.4, 128.6, 125.9, 124.9, 124.3, 124.1, 123.1, 121.2, 120.8, 114.4, 103.7, 80.0, 73.4, 66.8, 31.4, 19.1, 14.0.
5-chloro-N-(5-oxo-3-(pentyloxy)-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-1H-indole-2-carboxamide (8e)
HPLC analysis: 100%. m.p. 222–224 °C. ESI-MS m/z: 477.9 (M+Cl)−. 1H-NMR (400 MHz, d6-DMSO): 0.78 (t, J = 6.4 Hz, 3H), 1.09–1.14 (m, 2H), 1.16–1.19 (m, 2H), 1.40–1.44 (m, 2H), 3.38 (q, J = 6.0 Hz, 1H), 3.51 (q, J = 8.0 Hz, 1H), 4.25 (d, J = 12.4 Hz, 1H), 4.42 (d, J = 11.6 Hz, 1H), 4.64 (d, J = 2.0 Hz, 1H), 7.05 (d, J = 8.8 Hz, 1H), 7.23 (d, J = 8.8 Hz, 1H), 7.43 (s, 1H), 7.48 (d, J = 8.4 Hz, 1H), 7.77 (s, 1H), 7.98 (d, J = 8.8 Hz, 1H), 8.31 (s, 1H), 8.99 (d, J = 5.6 Hz, 1H), 10.36 (s, 1H), 11.91 (s, 1H). 13C-NMR (100 MHz, d6-DMSO): 166.7, 159.1, 153.1, 135.1, 133.1, 132.9, 128.1, 125.3, 124.4, 123.8, 123.4, 122.9, 120.8, 120.3, 113.9, 103.2, 79.5, 73.1, 66.6, 28.6, 27.7, 21.7, 13.8.
5-chloro-N-(3-(hexyloxy)-5-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-1H-indole-2-carboxamide (8f)
HPLC analysis: 100%. m.p. 187–189 °C. ESI-MS m/z: 490.1 (M+Cl)−. 1H-NMR (500 MHz, d6-DMSO): 0.77 (t, J = 6.2 Hz, 3H), 1.14 (s, 6H), 1.40 (s, 2H), 3.38 (dd, J = 14.6, 5.9 Hz, 1H), 3.51 (dd, J = 15.4, 6.5 Hz, 1H), 4.25 (d, J = 12.0 Hz, 1H), 4.41 (d, J = 10.3 Hz, 1H), 4.63 (s, 1H), 7.04 (d, J = 8.7 Hz, 1H), 7.22 (d, J = 8.5 Hz, 1H), 7.41 (s, 1H), 7.48 (d, J = 8.6 Hz, 1H), 7.76 (s, 1H), 7.97 (d, J = 8.3 Hz, 1H), 8.30 (s, 1H), 8.95 (d, J = 5.5 Hz, 1H), 10.33 (s, 1H), 11.88 (s, 1H). 13C-NMR (125 MHz, d6-DMSO): 167.2, 159.6, 153.5, 135.6, 133.6, 133.4, 128.6, 125.8, 124.8, 124.3, 123.9, 123.4, 121.2, 120.8, 114.4, 103.7, 80.0, 73.6, 67.1, 31.3, 29.3, 25.7, 22.5, 14.3.
N-(3-(benzyloxy)-5-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-5-chloro-1H-indole-2-carboxamide (8g)
HPLC analysis: 100%. m.p. 253–255 °C. ESI-MS m/z: 497.4 (M+Cl)−. 1H-NMR (500 MHz, d6-DMSO): 4.29 (dd, J = 12.3, 1.4 Hz, 1H), 4.47–4.52 (m, 2H), 4.63 (d, J = 12.0 Hz, 1H), 4.77–4.78 (m, 1H), 7.07 (d, J = 8.8 Hz, 1H), 7.24 (dt, J = 6.5, 3.9 Hz, 4H), 7.28–7.31 (m, 2H), 7.43 (s, 1H), 7.49 (d, J = 8.7 Hz, 1H), 7.77 (d, J = 1.3 Hz, 1H), 7.98 (dd, J = 8.8, 2.6 Hz, 1H), 8.36 (d, J = 2.6 Hz, 1H), 9.11 (d, J = 6.0 Hz, 1H), 10.36 (s, 1H), 11.90 (s, 1H). 13C-NMR (125 MHz, d6-DMSO): 167.1, 159.6, 153.7, 138.2, 135.6, 133.6, 133.4, 128.7, 128.6, 128.0, 127.9, 126.1, 124.9, 124.3, 124.1, 123.1, 121.2, 120.8, 114.4, 103.8, 79.6, 73.3, 68.8.
5-chloro-N-(3-(isopentyloxy)-5-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl)-1H-indole-2-carboxamide (8h)
HPLC analysis: 100%. m.p. 95–97 °C. ESI-MS m/z: 477.9 (M+Cl)−. 1H-NMR (500 MHz, d6-DMSO): 0.78 (dd, J = 6.6, 1.7 Hz, 6H), 1.21–1.37 (m, 2H), 1.45–1.53 (m, 1H), 3.41 (dd, J = 15.9, 6.4 Hz, 1H), 3.58 (dd, J = 16.4, 7.0 Hz, 1H), 4.24 (d, J = 12.1 Hz, 1H), 4.42 (dd, J = 12.3, 3.2 Hz, 1H), 4.64 (d, J = 2.2 Hz, 1H), 7.04 (d, J = 8.8 Hz, 1H), 7.22 (dd, J = 8.7, 1.7 Hz, 1H), 7.42 (s, 1H), 7.49 (d, J = 8.7 Hz, 1H), 7.76 (s, 1H), 7.96 (dd, J = 8.8, 2.3 Hz, 1H), 8.29–8.34 (m, 1H), 8.95 (s, 1H), 10.33 (s, 1H), 11.87 (s, 1H). 13C-NMR (125 MHz, d6-DMSO): 167.1, 159.6, 153.6, 135.6, 133.5, 133.4, 128.6, 125.9, 124.9, 124.3, 124.0, 123.1, 121.2, 120.8, 114.4, 103.7, 80.0, 73.4, 65.5, 38.2, 24.9, 22.9, 22.6.
5-chloro-N-[3-(2-methoxyethoxy)-5-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-7-yl]-1H-indole-2-carboxamide (8i)
HPLC analysis: 99%. m.p. 206–208 °C. ESI-MS m/z: 465.9 (M+Cl)−. 1H-NMR (500 MHz, d6-DMSO): 3.18 (s, 3H), 3.39 (tt, J = 10.8, 5.6 Hz, 2H), 3.54–3.58 (m, 1H), 3.64–3.69 (m, 1H), 4.23 (d, J = 12.4 Hz, 1H), 4.44 (dd, J = 12.4, 3.6 Hz, 1H), 4.69–4.70 (m, 1H), 7.05 (d, J = 8.8 Hz, 1H), 7.22 (dd, J = 8.7, 1.9 Hz, 1H), 7.42 (s, 1H), 7.48 (d, J = 8.7 Hz, 1H), 7.76 (s, 1H), 7.98 (dd, J = 8.8, 2.7 Hz, 1H), 8.34 (d, J = 2.7 Hz, 1H), 8.97 (d, J = 5.8 Hz, 1H), 10.35 (s, 1H), 11.89 (s, 1H). 13C-NMR (125 MHz, d6-DMSO): 166.3, 159.1, 153.2, 135.1, 133.0, 132.9, 128.0, 125.5, 124.3, 123.7, 122.1, 120.7, 120.3, 113.9, 103.2, 79.8, 72.4, 70.8, 66.2, 57.9.