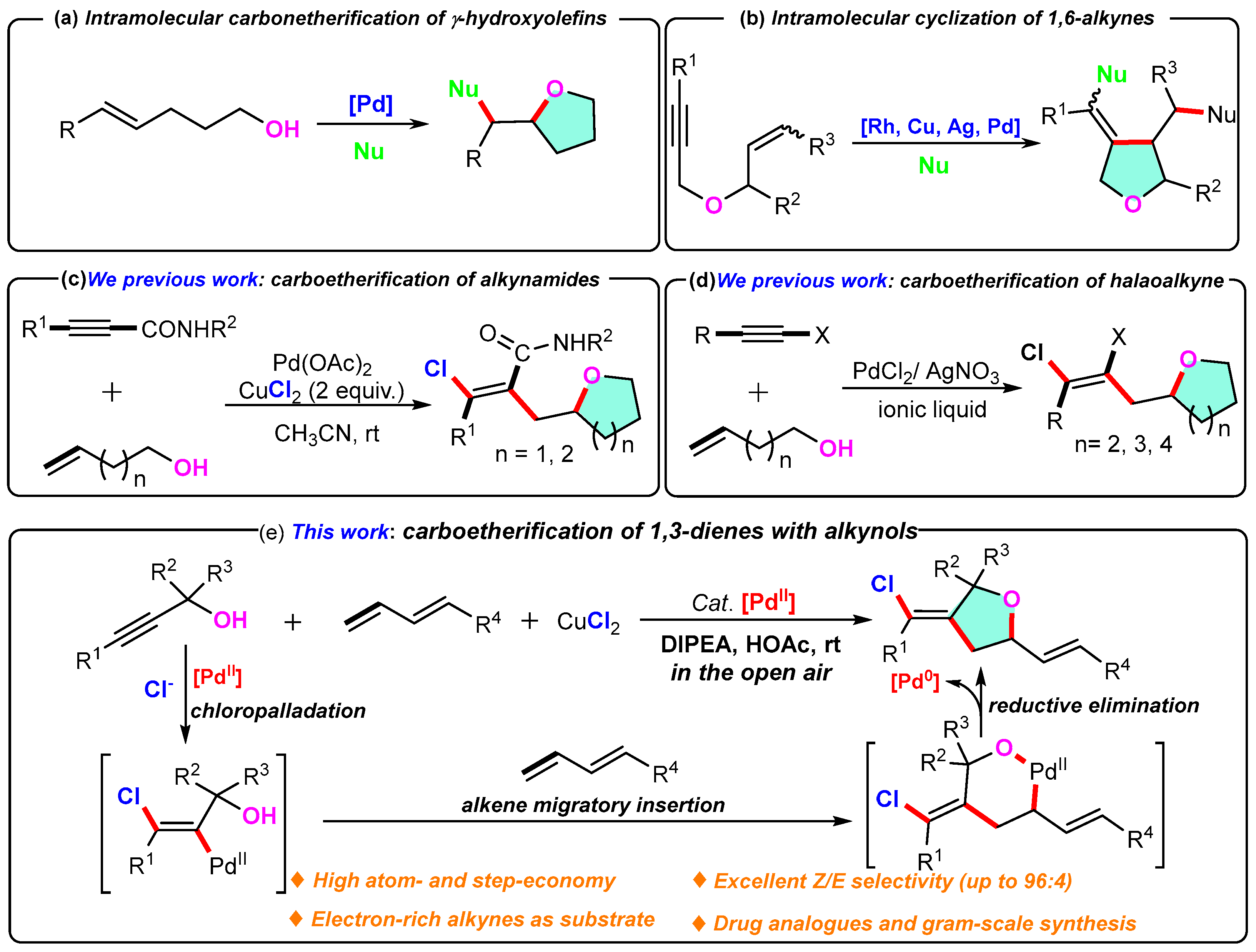
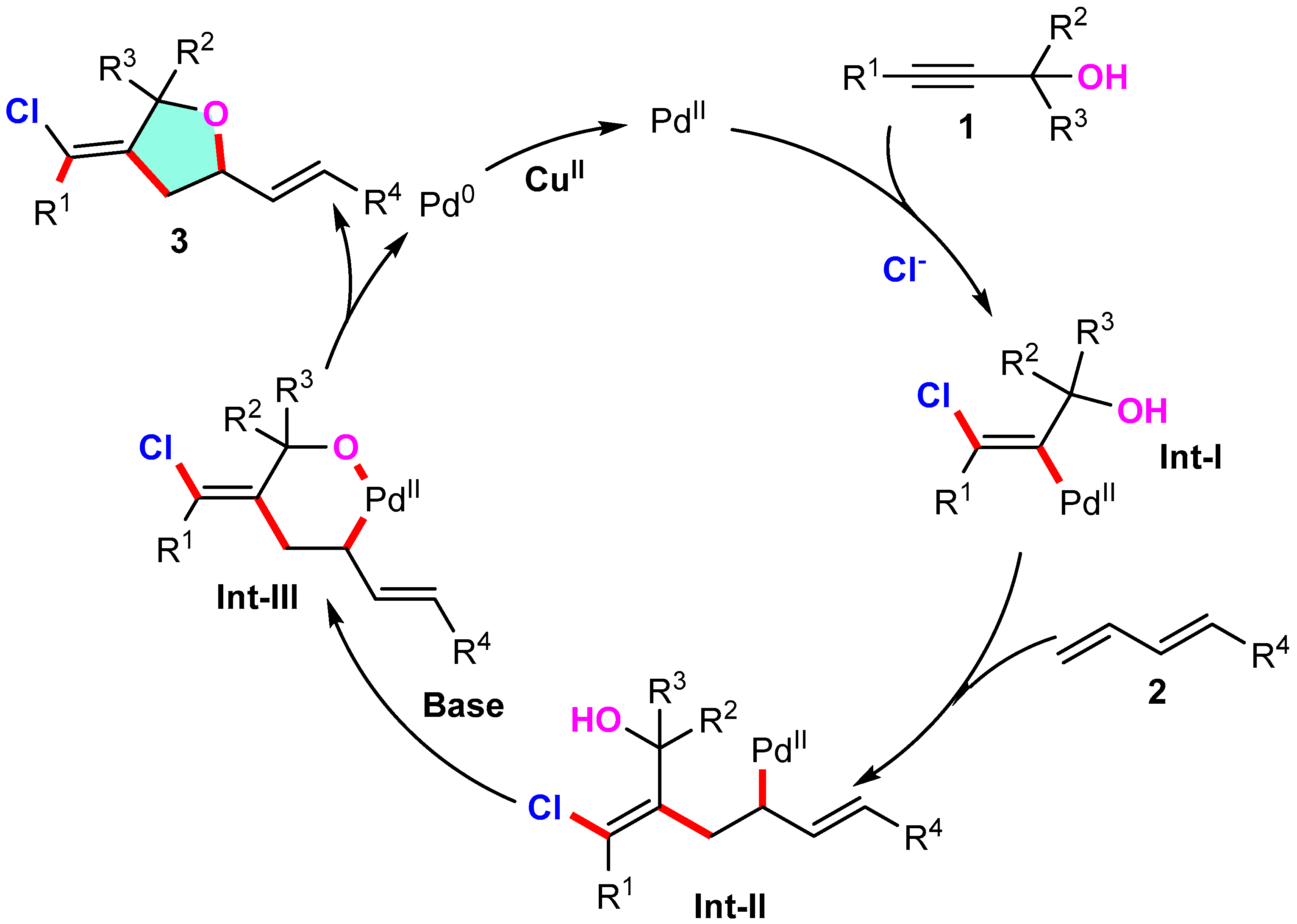
3.2. General Methods for the Preparation of 3-Methylenetetrahydrofuran Derivatives
A mixture of Pd(TFA)2 (10 mol %), CuCl2 (4 equiv.), DIPEA (80 mol%), and HOAc (2 mL) was added to a tube equipped with a stir bar. Then, propargyl alcohols (1, 0.2 mmol) and alkenes (2, 0.3 mmol) were added to the tube under air and stirred at room temperature for 24 h. After the reaction was finished, the reaction was quenched by saturated aqueous NH4Cl and extracted with EtOAc three times. The combined organic layers were dried over anhydrous Na2SO4 and evaporated under a vacuum. The residue was purified by flash column chromatography on silica gel (eluting with petroleum ether/ethyl acetate) to afford the desired products.
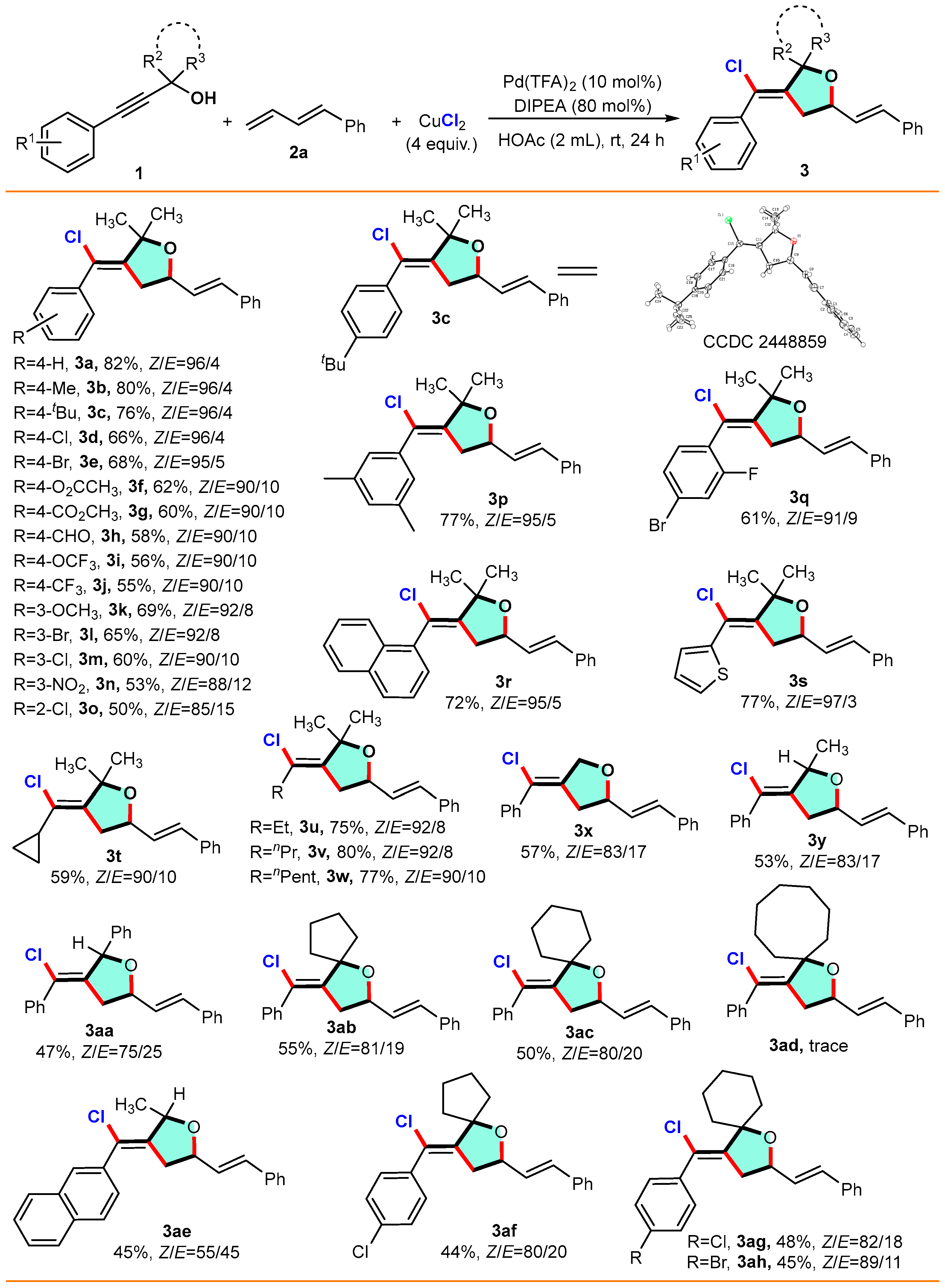
(Z)-3-(chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-styryl)tetrahydrofuran (3a): Alkynol 1a (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3a (82%, 53.2 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.47–7.43 (m, 2H), 7.42–7.38 (m, 4H), 7.37–7.31 (m, 3H), 7.30–7.26 (m, 1H), 6.66 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 7.0 Hz, 1H), 4.59–4.47 (m, 1H), 2.79–2.64 (m, 2H), 1.76 (s, 3H), 1.71 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.6, 140.0, 136.5, 132.4, 128.8, 128.5, 128.3, 128.3, 128.2, 127.8, 126.6, 121.3, 83.6, 42.1, 26.1, 24.0; HRMS (ESI, m/z): calcd for C21H21ClO, [M+H]+: 325.1184, found: 325.1179.
(Z)-3-(chloro(p-tolyl)methylene)-2,2-dimethyl-5-((E)-styryl)tetrahydrofuran (3b): Alkynol 1b (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3b (80%, 54.4 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.42–7.37 (m, 2H), 7.37–7.31 (m, 2H), 7.27 (td, J = 10.7, 8.7, 2.4 Hz, 4H), 7.15 (d, J = 7.5 Hz, 1H), 6.65 (d, J = 15.9 Hz, 1H), 6.24 (dd, J = 15.9, 7.0 Hz, 1H), 4.57–4.49 (m, 1H), 2.71 (d, J = 9.3 Hz, 2H), 2.40 (s, 3H), 1.74 (s, 3H), 1.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.3, 139.9, 138.0, 136.5, 132.4, 129.0, 128.9, 128.8, 128.5, 128.2, 127.8, 126.6, 125.4, 121.4, 83.6, 76.4, 42.1, 26.1, 24.0, 21.4; HRMS (ESI, m/z): calcd for C22H23ClO, [M+H]+: 339.1340, found: 339.1345.
(Z)-3-((4-(tert-butyl)phenyl)chloromethylene)-2,2-dimethyl-5-((E)-styryl)tetrahydrofuran (3c): Alkynol 1c (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3c (76%, 57.5 mg) as a yellow solid, mp = 117.9–118.6 °C; 1H NMR (400 MHz, CDCl3) δ 7.45–7.37 (m, 5H), 6.65 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 7.0 Hz, 1H), 4.58–4.48 (m, 1H), 2.83–2.66 (m, 2H), 1.75 (s, 3H), 1.70 (s, 3H), 1.36 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 151.3, 144.1, 137.0, 136.5, 132.4, 128.9, 128.5, 128.0, 127.8, 126.6, 125.1, 121.4, 83.7, 76.4, 42.2, 34.7, 31.3, 26.2, 24.1; HRMS (ESI, m/z): calcd for C25H29ClO, [M+H]+: 381.1245, found: 381.1246.
(Z)-3-(chloro(4-chlorophenyl)methylene)-2,2-dimethyl-5-((E)-styryl)-tetrahydrofuran (3d): Alkynol 1d (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3d (66%, 47.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.37 (d, J = 6.2 Hz, 7H), 7.32–7.27 (m, 2H), 6.64 (d, J = 15.9 Hz, 1H), 6.22 (dd, J = 15.9, 7.0 Hz, 1H), 4.56–4.49 (m, 1H), 2.68 (d, J = 4.5 Hz, 2H), 1.72 (s, 3H), 1.67 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 145.4, 138.3, 136.4, 134.1, 132.6, 129.7, 128.6, 128.5, 127.9, 126.6, 120.1, 83.7, 76.4, 42.1, 26.0, 23.9; HRMS (ESI, m/z): calcd for C21H20Cl2O, [M+H]+: 359.0794, found: 359.0789.
(Z)-3-((4-bromophenyl)chloromethylene)-2,2-dimethyl-5-((E)-styryl)-tetrahydrofuran (3e): Alkynol 1e (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3e (68%, 55.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.54–7.49 (m, 2H), 7.41–7.37 (m, 2H), 7.36–7.26 (m, 5H), 6.64 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.0 Hz, 1H), 4.56–4.47 (m, 1H), 2.73–2.63 (m, 2H), 1.72 (s, 3H), 1.67 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 145.4, 138.8, 136.4, 132.5, 131.5, 130.0, 128.5, 128.5, 127.9, 126.6, 122.3, 120.2, 83.7, 76.4, 42.1, 26.0, 23.9; HRMS (ESI, m/z): calcd for C21H20BrClO, [M+H]+: 403.0296, found: 403.0293.
4-((Z)-chloro-2,2-dimethyl-5-((E)-styryl)dihydrofuran-3(2H)-ylidene)methyl)-phenyl acetaten (3f): Alkynol 1f (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3f (62%, 47.9 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.46–7.25 (m, 7H), 7.16–7.06 (m, 2H), 6.64 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.0 Hz, 1H), 4.57–4.47 (m, 1H), 2.79–2.62 (m, 2H), 2.33 (s, 3H), 1.72 (s, 3H), 1.67 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.3, 150.3, 145.0, 137.5, 136.4, 132.5, 129.6, 128.6, 128.5, 127.8, 126.6, 121.4, 120.5, 83.7, 76.4, 42.1, 26.1, 23.9, 21.2; HRMS (ESI, m/z): calcd for C23H23ClO3, [M+H]+: 383.1411, found: 383.1408.
methyl 4-((Z)-chloro(2,2-dimethyl-5-((E)-styryl)dihydrofuran-3(2H)-ylidene) methyl)benzoate (3g): Alkynol 1g (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3g (60%, 45.8 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 6.9 Hz, 2H), 7.32 (t, J = 7.7 Hz, 3H), 6.64 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.0 Hz, 1H), 4.57–4.49 (m, 1H), 3.95 (s, 3H), 2.71 (d, J = 7.7 Hz, 2H), 1.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 166.5, 146.1, 144.2, 132.6, 129.8, 129.6, 128.5, 128.4, 127.9, 126.6, 120.2, 83.8, 76.4, 52.3, 42.1, 26.1, 23.9; HRMS (ESI, m/z): calcd for C23H23ClO3, [M+H]+: 383.1410, found: 383.1408.
4-((Z)-Chloro(2,2-dimethyl-5-((E)-styryl)dihydrofuran-3(2H)-ylidene)methyl) benzaldehyde (3h): Alkynol 1h (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3h (58%, 41.1 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 10.04 (s, 1H), 7.95–7.87 (m, 2H), 7.64–7.58 (m, 2H), 7.41–7.36 (m, 2H), 7.34–7.28 (m, 2H), 7.28–7.20 (m, 1H), 6.64 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.0 Hz, 1H), 4.57–4.50 (m, 1H), 2.72 (d, J = 7.6 Hz, 2H), 1.74 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.5, 146.7, 145.6, 136.3, 135.8, 132.7, 129.7, 129.1, 128.6, 128.3, 127.9, 126.6, 120.0, 83.9, 42.1, 26.1, 23.8; HRMS (ESI, m/z): calcd for C22H21ClO2, [M+H]+: 353.1306, found: 353.1303.
(Z)-3-(chloro(4-(trifluoromethoxy)phenyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3i): Alkynol 1i (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3i (56%, 45.5 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 8.5 Hz, 2H), 7.39 (d, J = 7.5 Hz, 2H), 7.33 (d, J = 7.4 Hz, 2H), 7.29–7.22 (m, 3H), 6.65 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.1 Hz, 1H), 4.57–4.48 (m, 1H), 2.74–2.65 (m, 2H), 1.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 148.7, 145.6, 138.5, 136.4, 132.6, 129.9, 128.5, 128.5, 127.9, 126.6, 120.8, 119.9, 83.7, 42.1, 26.0, 23.9; 19F NMR (376 MHz, CDCl3) δ −57.80; HRMS (ESI, m/z): calcd for C22H20ClF3O2, [M+H]+: 409.1000, found: 409.1002.
(Z)-3-(chloro(4-(trifluoromethyl)phenyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3j): Alkynol 1j (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3j (55%, 42.8 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.2 Hz, 2H), 7.55 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 7.7 Hz, 2H), 7.32 (t, J = 7.5 Hz, 2H), 7.29–7.25 (m, 1H), 6.64 (d, J = 15.9 Hz, 1H), 6.22 (dd, J = 15.9, 7.0 Hz, 1H), 4.58–4.49 (m, 1H), 2.70 (d, J = 7.6 Hz, 2H), 1.74 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 146.3, 143.3, 136.3, 132.7, 128.8, 128.5, 128.4, 127.9, 126.6, 125.3 (dd, J = 8.3, 4.0 Hz), 83.8, 42.1, 26.0, 23.8; 19F NMR (376 MHz, CDCl3) δ −62.75; HRMS (ESI, m/z): calcd for C22H20ClF3O, [M+H]+: 393.1053, found: 393.1054.
(Z)-3-(chloro(3-methoxyphenyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3k): Alkynol 1k (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3k (69%, 48.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.39 (dt, J = 6.6, 1.4 Hz, 2H), 7.36–7.25 (m, 4H), 7.06–6.93 (m, 2H), 6.88 (ddd, J = 8.3, 2.7, 1.1 Hz, 1H), 6.64 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.0 Hz, 1H), 4.57–4.48 (m, 1H), 3.85 (s, 3H), 2.80–2.62 (m, 2H), 1.73 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.4, 144.7, 141.2, 136.5, 132.4, 129.3, 128.7, 128.5, 127.8, 126.6, 121.1, 120.7, 114.0, 113.9, 83.6, 76.4, 55.3, 42.1, 26.1, 23.9; HRMS (ESI, m/z): calcd for C22H23ClO2, [M+H]+: 355.1291, found: 355.1284.
(Z)-3-((3-bromophenyl)chloromethylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3l): Alkynol 1l (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3l (65%, 52.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.59 (t, J = 1.9 Hz, 1H), 7.47 (dt, J = 8.1, 1.5 Hz, 1H), 7.41–7.30 (m, 5H), 7.26 (t, J = 7.7 Hz, 2H), 6.65 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.1 Hz, 1H), 4.58–4.48 (m, 1H), 2.76–2.62 (m, 2H), 1.72 (s, 3H), 1.67 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 145.9, 141.8, 136.4, 132.6, 131.4, 131.3, 129.9, 128.6, 128.5, 127.9, 127.0, 126.6, 122.3, 119.7, 83.7, 77.4, 77.1, 76.7, 76.4, 42.1, 26.0, 23.9; HRMS (ESI, m/z): calcd for C21H20BrClO, [M+H]+: 403.0297, found: 403.0293.
(Z)-3-(chloro(3-chlorophenyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3m): Alkynol 1m (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3m (60%, 43.2 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.45–7.37 (m, 3H), 7.36–7.25 (m, 6H), 6.66 (d, J = 15.9 Hz, 1H), 6.24 (dd, J = 15.9, 7.1 Hz, 1H), 4.57–4.48 (m, 1H), 2.77–2.65 (m, 2H), 1.74 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 145.8, 141.5, 136.4, 134.2, 132.6, 129.6, 128.6, 128.5, 128.4, 127.8, 126.6, 126.6, 120.2, 83.7, 76.4, 42.1, 26.1, 23.9; HRMS (ESI, m/z): calcd for C21H20Cl2O, [M+H]+: 359.0794, found: 359.0789.
(Z)-3-(chloro(3-nitrophenyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3n): Alkynol 1n (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3n (53%, 39.2 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.31 (t, J = 2.0 Hz, 1H), 8.22–8.14 (m, 1H), 7.80–7.74 (m, 1H), 7.58 (t, J = 8.0 Hz, 1H), 7.40–7.36 (m, 2H), 7.35–7.26 (m, 3H), 6.65 (d, J = 15.9 Hz, 1H), 6.23 (dd, J = 15.9, 7.1 Hz, 1H), 4.56–4.46 (m, 1H), 2.78–2.67 (m, 2H), 1.74 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 148.1, 147.3, 141.4, 136.3, 134.4, 132.9, 129.4, 128.6, 128.2, 127.9, 126.6, 123.5, 123.1, 118.7, 83.8, 76.4, 42.0, 25.9, 23.8; HRMS (ESI, m/z): calcd for C21H20ClNO3, [M+H]+: 370.1036, found: 370.1029.
(Z)-3-(chloro(2-chlorophenyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3o): Alkynol 1o (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3o (50%, 35.6 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.48–7.43 (m, 1H), 7.36 (d, J = 7.2 Hz, 2H), 7.33–7.28 (m, 5H), 7.25 (d, J = 7.0 Hz, 1H), 6.62 (dd, J = 15.9, 5.6 Hz, 1H), 6.26–6.17 (m, 1H), 4.64–4.52 (m, 1H), 2.54–2.33 (m, 2H), 1.75 (s, 3H), 1.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 138.5, 136.5, 132.4, 130.0, 128.7, 128.5, 127.8, 127.3, 126.5; HRMS (ESI, m/z): calcd for C21H20Cl2O, [M+H]+: 359.0792, found: 359.0789.
(Z)-3-(chloro(3,5-dimethylphenyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3p): Alkynol 1p (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3p (77%, 54.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.3 Hz, 2H), 7.33 (t, J = 7.5 Hz, 2H), 7.29–7.24 (m, 1H), 7.05 (s, 2H), 6.98 (s, 1H), 6.66 (d, J = 15.9 Hz, 1H), 6.26 (dd, J = 15.9, 7.0 Hz, 1H), 4.59–4.49 (m, 1H), 2.80–2.66 (m, 2H), 2.37 (s, 6H), 1.75 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.1, 139.9, 137.9, 136.5, 132.3, 129.9, 128.9, 128.5, 127.8, 126.6, 126.0, 121.6, 83.6, 76.4, 42.1, 26.2, 24.1, 21.3; HRMS (ESI, m/z): calcd for C23H25ClO, [M+H]+: 353.1666, found: 353.1667.
(Z)-3-((4-bromo-2-fluorophenyl)chloromethylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3q): Alkynol 1q (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3q (61%, 50.6 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.39–7.36 (m, 2H), 7.35–7.31 (m, 3H), 7.31–7.24 (m, 3H), 6.64 (d, J = 15.9 Hz, 1H), 6.26–6.13 (m, 1H), 4.60–4.52 (m, 1H), 2.59–2.48 (m, 2H), 1.72 (s, 3H), 1.66 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 158.7 (d, J = 254.2 Hz), 148.6, 136.4, 132.6, 131.7 (d, J = 3.3 Hz), 128.5 (d, J = 3.5 Hz), 127.9, 127.8 (d, J = 3.7 Hz), 126.6, 126.5, 123.0 (d, J = 9.3 Hz), 119.8 (d, J = 25.1 Hz), 113.5, 83.5, 76.3, 41.3, 41.2, 26.1, 23.8; 19F NMR (376 MHz, CDCl3) δ -110.71; HRMS (ESI, m/z): calcd for C21H19BrClFO, [M+H]+: 421.0363, found: 421.0365.
(Z)-3-(chloro(naphthalen-1-yl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3r): Alkynol 1r (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3r (40%, 54.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.0 (d, J = 8.2 Hz, 1H), 7.9 (d, J = 8.2 Hz, 4H), 7.6–7.4 (m, 3H), 7.3–7.2 (m, 4H), 6.6 (d, J = 16.0 Hz, 1H), 6.25–6.12 (m, 1H), 4.61–4.53 (m, 1H), 2.60–2.49 (m, 2H), 1.90 (s, 3H), 1.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 146.9, 137.4, 136.4, 134.0, 132.3, 130.0, 129.0, 128.7, 128.6, 128.5, 127.8, 127.0, 126.8, 126.4, 126.3, 125.6, 124.6, 119.2, 83.4, 76.4, 41.5, 41.1, 26.4, 24.0; HRMS (ESI, m/z): calcd for C25H23ClO, [M+H]+: 375.1340, found: 375.1335.
(Z)-3-(chloro(thiophen-2-yl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3s): Alkynol 1s (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3s (77%, 51.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.46–7.41 (m, 2H), 7.41–7.31 (m, 3H), 7.31–7.21 (m, 2H), 7.06 (dd, J = 5.1, 3.8 Hz, 1H), 6.72 (d, J = 15.9 Hz, 1H), 6.31 (dd, J = 15.9, 7.1 Hz, 1H), 4.67–4.59 (m, 1H), 3.13 (dd, J = 16.2, 5.4 Hz, 1H), 2.82 (dd, J = 16.2, 5.4 Hz, 1H), 1.74 (s, 3H), 1.67 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.6, 142.2, 136.5, 132.6, 128.7, 128.6, 127.9, 127.4, 126.76, 126.6, 126.2, 116.1, 84.5, 76.4, 42.7, 26.2, 23.9; HRMS (ESI, m/z): calcd for C19H19ClOS, [M+H]+: 331.0747, found: 331.0743.
(Z)-3-(chloro(cyclopropyl)methylene)-2,2-dimethyl-5-((E)-styryl) tetrahydrofuran (3t): Alkynol 1t (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3t (59%, 34.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 7.2 Hz, 2H), 7.34 (t, J = 7.4 Hz, 2H), 7.27 (t, J = 7.2 Hz, 1H), 6.70 (d, J = 15.9 Hz, 1H), 6.29 (dd, J = 15.9, 7.0 Hz, 1H), 4.64–4.56 (m, 1H), 3.01 (dd, J = 15.6, 5.4 Hz, 1H), 2.65 (dd, J = 15.5, 5.4 Hz, 1H), 1.75 -1.69 (m, 1H), 1.61 (s, 3H), 1.54 (s, 3H), 0.92–0.81 (m, 2H), 0.78–0.70 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 141.1, 136.6, 132.3, 129.2, 128.5, 127.8, 126.6, 124.4, 83.5, 76.19, 40.2, 26.4, 24.0, 16.6, 5.6, 5.4; HRMS (ESI, m/z): calcd for C18H21ClO, [M+H]+: 289.1355, found: 289.1354.
(Z)-3-(1-chlorobutylidene)-2,2-dimethyl-5-((E)-styryl)tetrahydrofuran (3u): Alkynol 1u (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3u (75%, 43.1 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.46–7.39 (m, 2H), 7.37–7.31 (m, 2H), 7.29–7.24 (m, 1H), 6.69 (d, J = 15.9 Hz, 1H), 6.26 (dd, J = 15.9, 7.1 Hz, 1H), 4.61–4.51 (m, 1H), 2.86 (dd, J = 15.4, 5.5 Hz, 1H), 2.55 (dd, J = 15.5, 10.2 Hz, 1H), 2.34 (t, J = 7.2 Hz, 2H), 1.74–1.64 (m, 2H), 1.63 (s, 3H), 1.52 (s, 3H), 0.96 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 141.6, 136.5, 132.3, 129.1, 128.5, 127.8, 126.6, 124.1, 83.2, 76.0, 40.4, 39.9, 26.4, 23.9, 20.4, 13.0; HRMS (ESI, m/z): calcd for C18H23ClO, [M+H]+: 291.1511, found: 291.1510.
(Z)-3-(1-chlorohexylidene)-2,2-dimethyl-5-((E)-styryl)tetrahydrofuran (3v): Alkynol 1v (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3v (80%, 50.9 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.43–7.39 (m, 2H), 7.36–7.30 (m, 2H), 7.29–7.25 (m, 1H), 6.67 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 7.1 Hz, 1H), 4.62–4.52 (m, 1H), 3.02 (dd, J = 16.6, 5.7 Hz, 1H), 2.74–2.57 (m, 1H), 2.44–2.31 (m, 2H), 1.70–1.57 (m, 3H), 1.53 (s, 3H), 1.45 (s, 3H), 1.42–1.28 (m, 5H), 0.95 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 142.6, 136.6, 132.2, 129.2, 128.5, 127.7, 127.4, 126.6, 81.8, 75.7, 42.0, 35.3, 31.3, 28.2, 27.9, 26.2, 22.5, 14.0, 14.0; HRMS (ESI, m/z): calcd for C20H27ClO, [M+H]+: 319.1826, found: 319.1823.
(Z)-3-(1-chlorononylidene)-2,2-dimethyl-5-((E)-styryl)tetrahydrofuran (3w): Alkynol 1w (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3w (77%, 55.0 mg) as a yellow solid, mp = 69.4–70.2 °C; 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 7.1 Hz, 2H), 7.33 (t, J = 7.5 Hz, 2H), 7.29–7.25 (m, 1H), 6.67 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 7.1 Hz, 1H), 4.63–4.53 (m, 1H), 3.02 (dd, J = 16.6, 5.6 Hz, 1H), 2.69–2.57 (m, 1H), 2.46–2.31 (m, 2H), 1.65–1.58 (m, 2H), 1.45 (s, 3H), 1.39–1.29 (m, 10H), 0.92 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 142.6, 136.6, 132.2, 129.2, 128.5, 127.7, 127.4, 126.6, 81.8, 75.7, 42.0, 35.3, 31.9, 29.5, 29.2, 29.2, 28.3, 28.2, 26.2, 22.7, 14.1; HRMS (ESI, m/z): calcd for C23H33ClO, [M+H]+: 361.2294, found: 361.2293.
(Z)-4-(chloro(phenyl)methylene)-2-((E)-styryl)tetrahydrofuran (3x): Alkynol 1x (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3x (57%, 34.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.55–7.48 (m, 2H), 7.41 (dd, J = 7.9, 2.8 Hz, 4H), 7.34 (t, J = 7.5 Hz, 3H), 7.30–7.26 (m, 1H), 6.66 (d, J = 15.9 Hz, 1H), 6.27 (dd, J = 15.9, 6.7 Hz, 1H), 4.84–4.74 (m, 1H), 4.70–4.54 (m, 2H), 3.00–2.85 (m, 1H), 2.76–2.62 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 138.1, 137.9, 136.4, 132.2, 128.6, 128.4, 128.3, 128.2, 127.9, 127.9, 126.6, 121.9, 81.4, 72.1, 39.5; HRMS (ESI, m/z): calcd for C19H17ClO, [M+H]+: 297.1043, found: 297.1041.
(Z)-3-(chloro(phenyl)methylene)-2-methyl-5-((E)-styryl)tetrahydrofuran (3y): Alkynol 1y (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3y (53%, 33.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.42 (t, J = 7.3 Hz, 4H), 7.34 (dd, J = 8.8, 6.1 Hz, 5H), 7.28 (d, J = 5.5 Hz, 1H), 6.71 (d, J = 15.9 Hz, 1H), 6.32 (dd, J = 15.9, 6.1 Hz, 1H), 4.59–4.52 (m, 1H), 4.50–4.44 (m, 1H), 2.77–2.67 (m, 1H), 2.57–2.49 (m, 1H), 1.59 (d, J = 6.5 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 139.4, 136.5, 132.3, 131.5, 129.8, 128.6, 128.5, 128.3, 128.1, 127.8, 127.6, 126.6, 38.8, 20.3; HRMS (ESI, m/z): calcd for C20H19ClO, [M+H]+: 311.1199, found: 311.1197.
(Z)-3-(chloro(phenyl)methylene)-2-phenyl-5-((E)-styryl)tetrahydrofuran (3aa): Alkynol 1aa (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3aa (47%, 35.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.65–7.53 (m, 3H), 7.53–7.43 (m, 8H), 7.42–7.31 (m, 4H), 7.30 (dd, J = 2.9, 1.5 Hz, 1H), 6.74 (d, J = 16.0 Hz, 1H), 6.38 (dd, J = 16.0, 6.2 Hz, 1H), 5.50 (s, 1H), 4.56–4.49 (m, 1H), 2.99–2.91 (m, 1H), 2.76–2.73 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 139.4, 139.1, 139.0, 138.2, 136.5, 134.3, 133.8, 131.7, 131.5, 129.3, 128.8, 128.7, 128.6, 128.5, 128.4, 128.4, 128.2, 128.1, 128.1, 127.9, 127.9, 127.8, 126.6, 126.5, 125.8, 81.5, 78.7, 68.1, 38.8, 38.2; HRMS (ESI, m/z): calcd for C25H21ClO, [M+H]+: 373.1356, found: 373.1354.
(Z)-4-(chloro(phenyl)methylene)-2-((E)-styryl)-1-oxaspiro[4.4]nonane (3ab): Alkynol 1ab (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3ab (55%, 39.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.48–7.42 (m, 2H), 7.41–7.36 (m, 4H), 7.35–7.29 (m, 3H), 7.29–7.24 (m, 1H), 6.63 (d, J = 15.9 Hz, 1H), 6.26 (dd, J = 15.9, 7.1 Hz, 1H), 4.43–4.35 (m, 1H), 2.72–2.68 (m, 2H), 2.44–2.34 (m, 1H), 2.06–1.80 (m, 7H); 13C NMR (100 MHz, CDCl3) δ 143.7, 140.0, 136.5, 132.4, 128.7, 128.5, 128.3, 128.2, 128.2, 127.8, 126.6, 121.1, 93.9, 76.9, 42.4, 37.6, 36.5, 25.9, 25.5; HRMS (ESI, m/z): calcd for C23H23ClO, [M+H]+: 351.1336, found: 351.1335.
(Z)-4-(chloro(phenyl)methylene)-2-((E)-styryl)-1-oxaspiro[4.5]decane (3ac): Alkynol 1ac (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3ac (50%, 36.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.44–7.35 (m, 6H), 7.34–7.28 (m, 3H), 7.25 (d, J = 7.2 Hz, 1H), 6.61 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 6.8 Hz, 1H), 4.50–4.39 (m, 1H), 2.71–2.59 (m, 2H), 2.57–2.47 (m, 1H), 2.39–2.29 (m, 1H), 1.82–1.62 (m, 7H), 1.36–1.30 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 144.5, 140.5, 136.6, 131.8, 129.3, 128.5, 128.4, 128.3, 128.1, 127.7, 126.5, 121.1, 85.1, 76.1, 42.3, 33.5, 29.8, 25.2, 22.3, 22.1; HRMS (ESI, m/z): calcd for C24H25ClO, [M+H]+: 365.1492, found: 365.1494.
(Z)-3-(chloro(naphthalen-1-yl)methylene)-2-methyl-5-((E)-styryl)tetrahydrofuran (3ae): Alkynol 1ae (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3ae (45%, 34.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 7.8 Hz, 4H), 7.53–7.42 (m, 5H), 7.39–7.33 (m, 2H), 7.32–7.27 (m, 1H), 6.65 (d, J = 15.9 Hz, 1H), 6.28 (dd, J = 15.9, 6.8 Hz, 1H), 4.53–4.42 (m, 1H), 2.82–2.57 (m, 2H), 1.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 140.7, 140.4, 138.3, 138.1, 136.6, 131.9, 131.6, 131.5, 130.9, 130.0, 129.6, 128.8, 128.6, 128.6, 128.5, 128.5, 127.9, 127.4, 127.1, 127.0, 127.0, 126.6, 126.6, 74.3, 72.7, 68.6, 38.7, 38.2, 20.3, 18.4; HRMS (ESI, m/z): calcd for C25H23ClO, [M+H]+: 375.1319, found: 375.1324.
(Z)-4-(chloro(4-chlorophenyl)methylene)-2-((E)-styryl)-1-oxaspiro[4.4]nonane (3af): Alkynol 1af (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3af (44%, 34.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.40 (s, 1H), 7.39–7.35 (m, 5H), 7.34–7.30 (m, 2H), 7.29–7.27 (m, 1H), 6.63 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 7.0 Hz, 1H), 4.46–4.34 (m, 1H), 2.72–2.66 (m, 2H), 2.64–2.59 (m, 1H), 2.41–2.30 (m, 1H), 2.00–1.83 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 144.5, 138.4, 136.4, 134.0, 132.5, 129.7, 128.5, 128.5, 127.8, 126.6, 119.9, 93.9, 76.8, 42.4, 37.6, 36.4, 25.9, 25.5; HRMS (ESI, m/z): calcd for C23H22Cl2O, [M+H]+: 385.0945, found: 385.0944.
(Z)-4-(chloro(4-chlorophenyl)methylene)-2-((E)-styryl)-1-oxaspiro[4.5]decane (3ag): Alkynol 1ag (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3ag (48%, 38.4 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.41–7.38 (m, 2H), 7.36–7.33 (m, 4H), 7.31 (d, J = 8.0 Hz, 2H), 7.28–7.25 (m, 1H), 6.62 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 6.9 Hz, 1H), 4.49–4.41 (m, 1H), 2.68–2.58 (m, 2H), 2.54–2.45 (m, 1H), 2.36–2.26 (m, 1H), 1.81- 1.65 (m, 7H), 1.36–1.30 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 145.3, 138.8, 136.5, 133.9, 131.9, 129.8, 129.1, 128.5, 127.8, 126.6, 119.9, 85.2, 76.1, 42.3, 33.4, 29.7, 25.2, 22.3, 22.1; HRMS (ESI, m/z): calcd for C24H25ClO, [M+H]+: 399.1106, found: 399.1102.
(Z)-4-((4-bromophenyl)chloromethylene)-2-((E)-styryl)-1-oxaspiro[4.5]decane (3ah): Alkynol 1ah (0.20 mmol) with buta-1,3-dien-1-ylbenzene 2a (0.30 mmol, 1.5 equiv.) gave compound 3ah (45%, 40.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.55–7.47 (m, 2H), 7.41–7.37 (m, 2H), 7.34–7.27 (m, 4H), 6.62 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 6.8 Hz, 1H), 4.49–2.41 (m, 1H), 2.69–2.55 (m, 2H), 2.53–2.43 (m, 1H), 2.36–2.26 (m, 1H), 1.83–1.63 (m, 7H), 1.36–1.30 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 145.3, 139.3, 136.5, 131.9, 131.5, 130.1, 129.1, 128.5, 127.8, 126.6, 122.2, 119.9, 85.2, 76.1, 42.3, 33.4, 29.7, 25.2, 22.3, 22.1; HRMS (ESI, m/z): calcd for C24H24BrClO, [M+H]+: 443.0607, found: 443.0606.
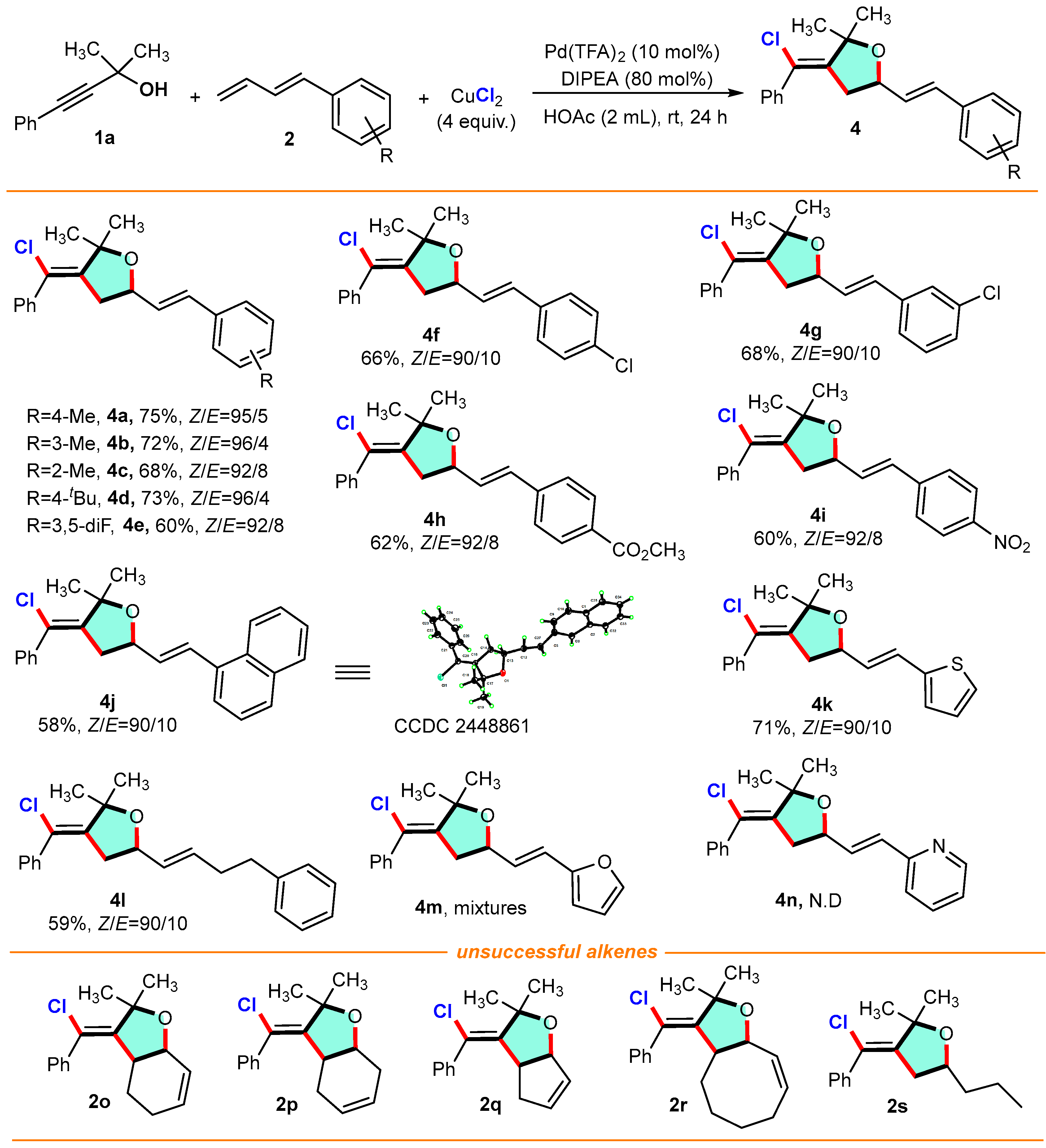
(Z)-3-(chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-4-methylstyryl) tetrahydrofuran (4a): 1-(Buta-1,3-dien-1-yl)-4-methylbenzene 2b (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4a (75%, 51.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.46–7.33 (m, 5H), 7.29 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 8.1 Hz, 2H), 6.61 (d, J = 15.9 Hz, 1H), 6.19 (dd, J = 15.9, 7.1 Hz, 1H), 4.55–4.48 (m, 1H), 2.71 (dd, J = 7.8, 3.2 Hz, 2H), 2.36 (s, 3H), 1.74 (s, 3H), 1.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.7, 139.9, 137.6, 133.7, 132.4, 129.2, 128.3, 128.3, 128.2, 127.7, 126.5, 121.2, 83.6, 76.5, 42.2, 26.1, 24.0, 21.2; HRMS (ESI, m/z): calcd for C22H23ClO, [M+H]+: 339.0754, found: 339.0755.
(Z)-3-(chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-3-methylstyryl) tetrahydrofuran (4b): 1-(Buta-1,3-dien-1-yl)-3-methylbenzene 2c (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4b (72%, 49.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.44–7.29 (m, 5H), 7.24–7.15 (m, 3H), 7.07 (dd, J = 6.9, 2.3 Hz, 1H), 6.60 (d, J = 15.9 Hz, 1H), 6.22 (dd, J = 15.9, 7.1 Hz, 1H), 4.59–4.45 (m, 1H), 2.81–2.66 (m, 2H), 2.35 (s, 3H), 1.73 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.6, 139.9, 138.0, 136.4, 132.5, 128.6, 128.6, 128.4, 128.3, 128.3, 128.2, 127.3, 123.7, 121.3, 83.6, 76.4, 42.1, 26.1, 24.0, 21.4; HRMS (ESI, m/z): calcd for C22H23ClO, [M+H]+: 339.1339, found: 339.1335.
(Z)-3-(chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-2-methylstyryl) tetrahydrofuran (4c): 1-(Buta-1,3-dien-1-yl)-2-methylbenzene 2d (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4c (68%, 45.3 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.49–7.32 (m, 6H), 7.22–7.15 (m, 3H), 6.87 (d, J = 15.7 Hz, 1H), 6.14 (dd, J = 15.7, 7.3 Hz, 1H), 4.60–4.53 (m, 1H), 2.79–2.68 (m, 2H), 2.36 (s, 3H), 1.76 (s, 3H), 1.71 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.7, 140.0, 135.5, 135.5, 130.4, 130.3, 130.1, 128.4, 128.3, 128.3, 127.7, 126.1, 125.8, 121.3, 83.7, 76.8, 42.2, 26.2, 24.1, 19.8; HRMS (ESI, m/z): calcd for C22H23ClO, [M+H]+: 339.1337, found: 339.1335.
(Z)-5-((E)-4-(tert-Butyl)styryl)-3-(chloro(phenyl)methylene)-2,2-dimethyltetrahydro-furan (4d): 1-(Buta-1,3-dien-1-yl)-4-(tert-butyl)benzene 2e (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4d (73%, 55.3 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.44 (dd, J = 8.5, 2.1 Hz, 4H), 7.35 (dd, J = 8.4, 6.6 Hz, 2H), 7.29 (dd, J = 7.8, 5.9 Hz, 3H), 6.71 (d, J = 16.0 Hz, 1H), 6.33 (dd, J = 16.0, 6.0 Hz, 1H), 4.66–4.59 (m, 1H), 2.71 (dd, J = 16.8, 10.6 Hz, 1H), 2.51 (dd, J = 16.8, 3.2 Hz, 1H), 1.62 (s, 6H), 1.38 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 150.3, 136.8, 136.7, 133.0, 131.3, 131.2, 129.1, 128.6, 127.8, 127.8, 126.6, 125.1, 77.0, 69.1, 39.2, 34.6, 31.4, 28.6, 24.3; HRMS (ESI, m/z): calcd for C25H29ClO, [M+H]+: 381.1976, found: 381.1980.
(Z)-3-(chloro(phenyl)methylene)-5-((E)-3,5-difluorostyryl)-2,2-dimethyltetrahydro-furan (4e): 1-(Buta-1,3-dien-1-yl)-3,5-difluorobenzene 2f (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4e (60%, 43.0 mg) as a yellow oil. Yield: 60% (43 mg) as a yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.44–7.30 (m, 6H), 6.88–6.76 (m, 2H), 6.72 (d, J = 16.1 Hz, 1H), 6.25 (dd, J = 16.1, 7.0 Hz, 1H), 4.57–4.47 (m, 1H), 2.78–2.65 (m, 2H), 1.73 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.6 (d, J = 12.1 Hz), 161.3 (dd, J = 45.5, 12.0 Hz), 159.0 (d, J = 12.0 Hz), 144.4, 139.9, 131.0 (dd, J = 4.8, 2.2 Hz), 128.4 (dd, J = 5.8, 3.7 Hz), 123.9, 121.4, 120.6 (dd, J = 12.0, 3.9 Hz), 111.5 (dd, J = 21.4, 3.7 Hz), 104.0 (d, J = 25.8 Hz), 83.8, 42.0, 26.1, 24.0; 19F NMR (376 MHz, CDCl3) δ -110.56, -110.58, -113.59, -113.61; HRMS (ESI, m/z): calcd for C21H19ClF2O, [M+H]+: 361.0997, found: 361.0990.
(Z)-3-(chloro(phenyl)methylene)-5-((E)-4-chlorostyryl)-2,2-dimethyltetrahydro-furan (4f): 1-(Buta-1,3-dien-1-yl)-4-chlorobenzene 2g (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4f (66%, 47.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.45–7.36 (m, 4H), 7.35–7.26 (m, 5H), 6.60 (d, J = 15.9 Hz, 1H), 6.21 (dd, J = 15.9, 7.0 Hz, 1H), 4.56–4.44 (m, 1H), 2.80–2.62 (m, 2H), 1.74 (s, 3H), 1.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.4, 139.9, 134.9, 133.5, 131.1, 129.4, 128.7, 128.3, 127.7, 121.4, 83.7, 76.2, 42.0, 26.1, 24.0; HRMS (ESI, m/z): calcd for C21H20Cl2O, [M+H]+: 359.0795, found: 359.0789.
(Z)-3-(chloro(phenyl)methylene)-5-((E)-3-chlorostyryl)-2,2-dimethyltetrahydrofuran (4g): 1-(Buta-1,3-dien-1-yl)-3-chlorobenzene 2h (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4g (68%, 49.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.44–7.33 (m, 6H), 7.24 (d, J = 3.6 Hz, 3H), 6.58 (d, J = 15.9 Hz, 1H), 6.25 (dd, J = 15.9, 6.8 Hz, 1H), 4.56–4.47 (m, 1H), 2.76–2.63 (m, 2H), 1.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.3, 139.9, 138.4, 134.5, 130.9, 130.4, 129.8, 128.3, 127.8, 126.5, 124.7, 121.4, 83.8, 76.1, 42.0, 26.1, 24.0; HRMS (ESI, m/z): calcd for C21H20Cl2O, [M+H]+: 359.0797, found: 359.0789.
Methyl 4-((E)-2-((Z)-4-(chloro(phenyl)methylene)-5,5-dimethyltetrahydrofuran-2-yl)vinyl)benzoate (4h): Methyl (E)-4-(buta-1,3-dien-1-yl)benzoate 2i (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4h (62%, 47.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 8.2 Hz, 2H), 7.46–7.31 (m, 7H), 6.67 (d, J = 15.9 Hz, 1H), 6.35 (dd, J = 15.9, 6.8 Hz, 1H), 4.58–4.50 (m 1H), 3.92 (s, 3H), 2.78–2.64 (m, 2H), 1.73 (s, 3H), 1.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 166.8, 144.3, 140.9, 139.9, 131.5, 131.2, 129.9, 129.2, 128.3, 126.4, 121.5, 83.8, 52.1, 42.0, 26.1, 24.0; HRMS (ESI, m/z): calcd for C23H23ClO3, [M+H]+: 383.1410, found: 383.1408.
(Z)-3-(chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-4-nitrostyryl)tetrahydrofuran (4i): 1-(Buta-1,3-dien-1-yl)-4-nitrobenzene 2j (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4i (60%, 46.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.24–8.11 (m, 2H), 7.53–7.46 (m, 2H), 7.44–7.31 (m, 5H), 6.71 (d, J = 15.9 Hz, 1H), 6.43 (dd, J = 15.9, 6.5 Hz, 1H), 4.62–4.50 (m, 1H), 2.84–2.64 (m, 2H), 1.74 (s, 3H), 1.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 147.1, 143.9, 142.9, 139.8, 133.8, 129.7, 128.4, 128.3, 128.3, 126.7, 123.9, 121.7, 84.4, 75.8, 41.9, 26.1, 24.0; HRMS (ESI, m/z): calcd for C21H20ClNO3, [M+H]+: 370.1206, found: 370.1204.
(Z)-3-(Chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-2-(naphthalen-1-yl)vinyl)-tetrahydrofuran (4j): 1-(Buta-1,3-dien-1-yl)naphthalene 2k (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4j (58%, 43.0 mg) as a yellow solid, mp = 114.8–115.2 °C. 1H NMR (400 MHz, CDCl3) δ 7.80 (dd, J = 9.0, 6.8 Hz, 3H), 7.74 (d, J = 1.7 Hz, 1H), 7.61 (dd, J = 8.6, 1.7 Hz, 1H), 7.49–7.33 (m, 7H), 6.80 (d, J = 15.9 Hz, 1H), 6.37 (dd, J = 15.9, 7.0 Hz, 1H), 4.62–4.54 (m, 1H), 2.81–2.71 (m, 2H), 1.71 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 144.6, 139.9, 133.9, 133.5, 133.1, 132.5, 129.1, 128.3, 128.3, 128.3, 128.2, 128.0, 127.7, 126.7, 126.3, 125.9, 123.6, 121.3, 83.7, 76.6, 42.2, 26.1, 24.0; HRMS (ESI, m/z): calcd for C25H23ClO, [M+H]+: 375.1338, found: 375.1335.
(Z)-3-(chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-2-(thiophen-2-yl)vinyl)-tetrahydrofuran (4k): 2-(Buta-1,3-dien-1-yl)thiophene 2l (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4k (71%, 48.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.44–7.43 (m, 2H), 7.42–7.32 (m, 3H), 7.30–7.21 (m, 2H), 7.04 (dd, J = 5.1, 3.8 Hz, 1H), 6.70 (d, J = 15.9 Hz, 1H), 6.30 (dd, J = 15.9, 7.1 Hz, 1H), 4.67–4.59 (m, 1H), 3.14 (dd, J = 16.2, 5.3 Hz, 1H), 2.83 (dd, J = 16.2, 10.1 Hz, 1H), 1.72 (s, 3H), 1.65 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.6, 142.2, 136.5, 132.6, 128.7, 128.6, 127.9, 127.4, 126.8, 126.6, 126.2, 116.1, 84.5, 42.7, 26.2, 24.0; HRMS (ESI, m/z): calcd for C19H19ClOS, [M+H]+: 331.0821, found: 331.0819.
(Z)-3-(chloro(phenyl)methylene)-2,2-dimethyl-5-((E)-4-phenylbut-1-en-1-yl)tetrahydrofuran (4l): Hexa-3,5-dien-1-ylbenzene 2m (0.30 mmol, 1.5 equiv.) with alkynol 2-methyl-4-phenylbut-3-yn-2-ol 1a (0.20 mmol) gave compound 4l (59%, 42.0 mg) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.44–7.28 (m, 7H), 7.22–7.15 (m, 3H), 5.85–5.75 (m, 1H), 5.58–5.50 (m, 1H), 4.34–4.26 (m, 1H), 2.74–2.66 (m, 2H), 2.64–2.54 (m, 2H), 2.42–2.32 (m, 2H), 1.69 (s, 3H), 1.63 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144.9, 141.7, 140.0, 133.7, 129.9, 128.4, 128.3, 128.2, 128.2, 125.9, 121.0, 83.4, 76.4, 42.0, 35.4, 34.2, 26.1, 23.9; HRMS (ESI, m/z): calcd for C23H25ClO, [M+H]+: 353.1403, found: 353.1402.