Triaxial Electrospun Nanofiber Membranes for Prolonged Curcumin Release in Dental Applications: Drug Release and Biological Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology
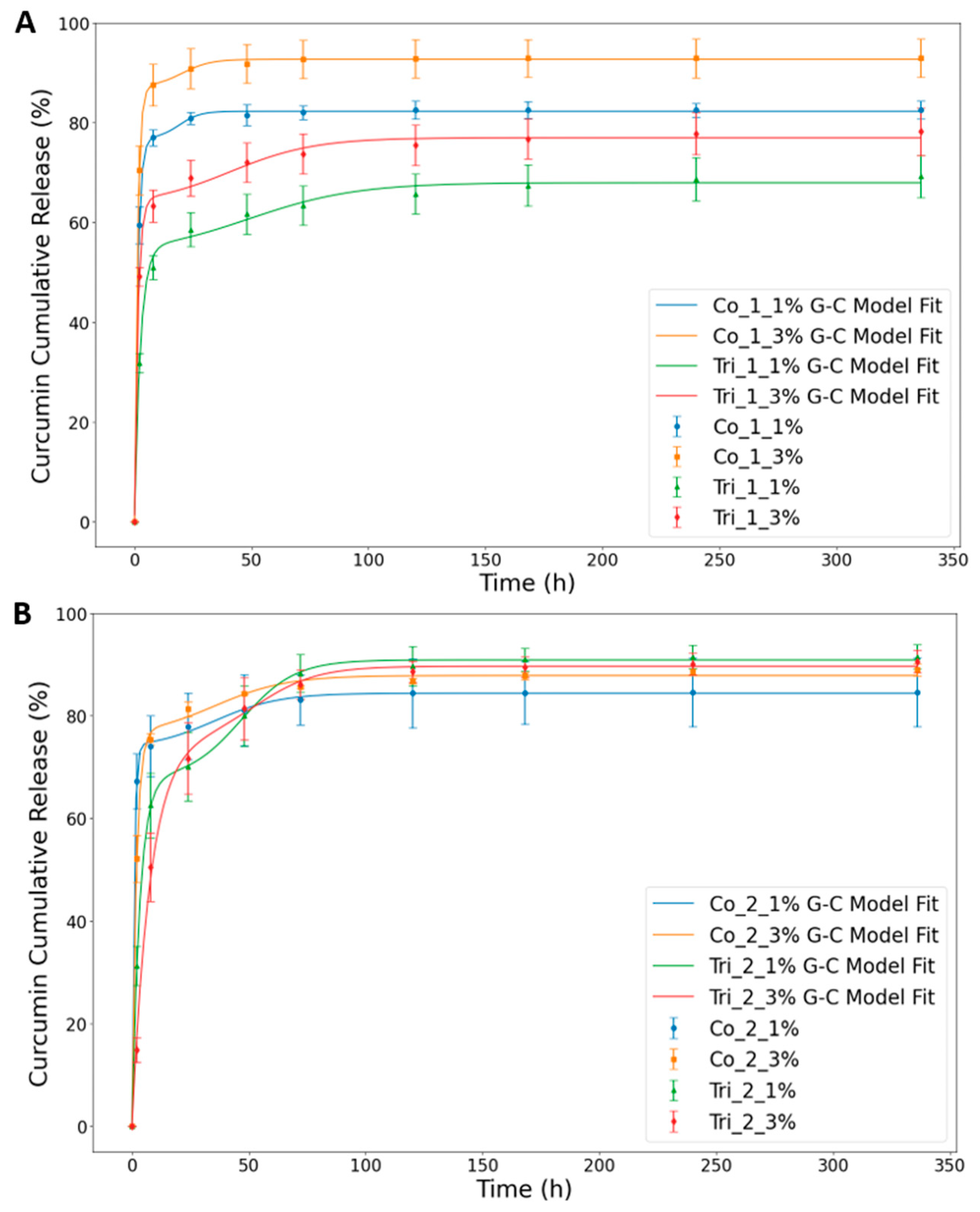
2.2. In Vitro Drug Release Tests and Curcumin Release Kinetics
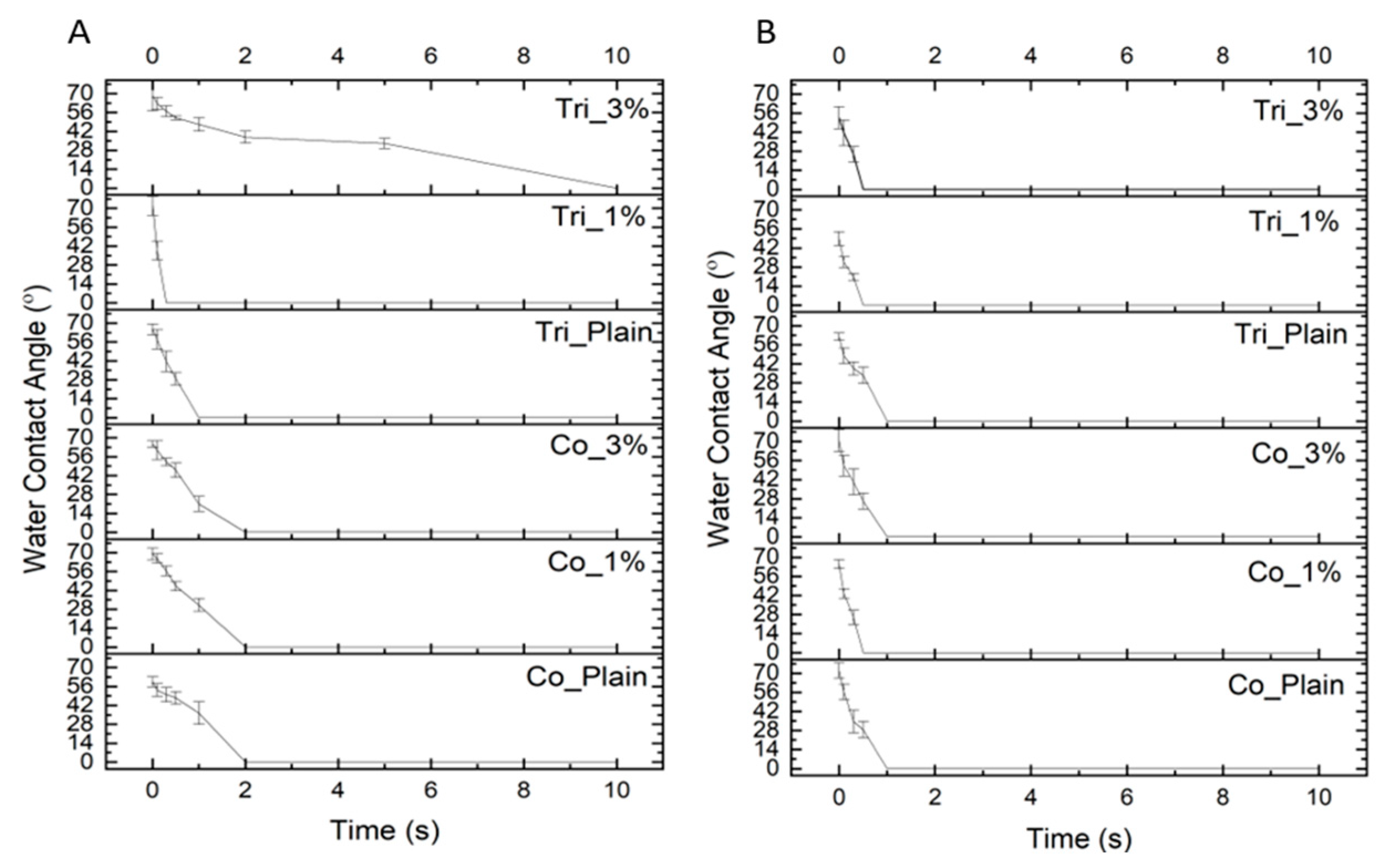
2.3. Surface Wettability
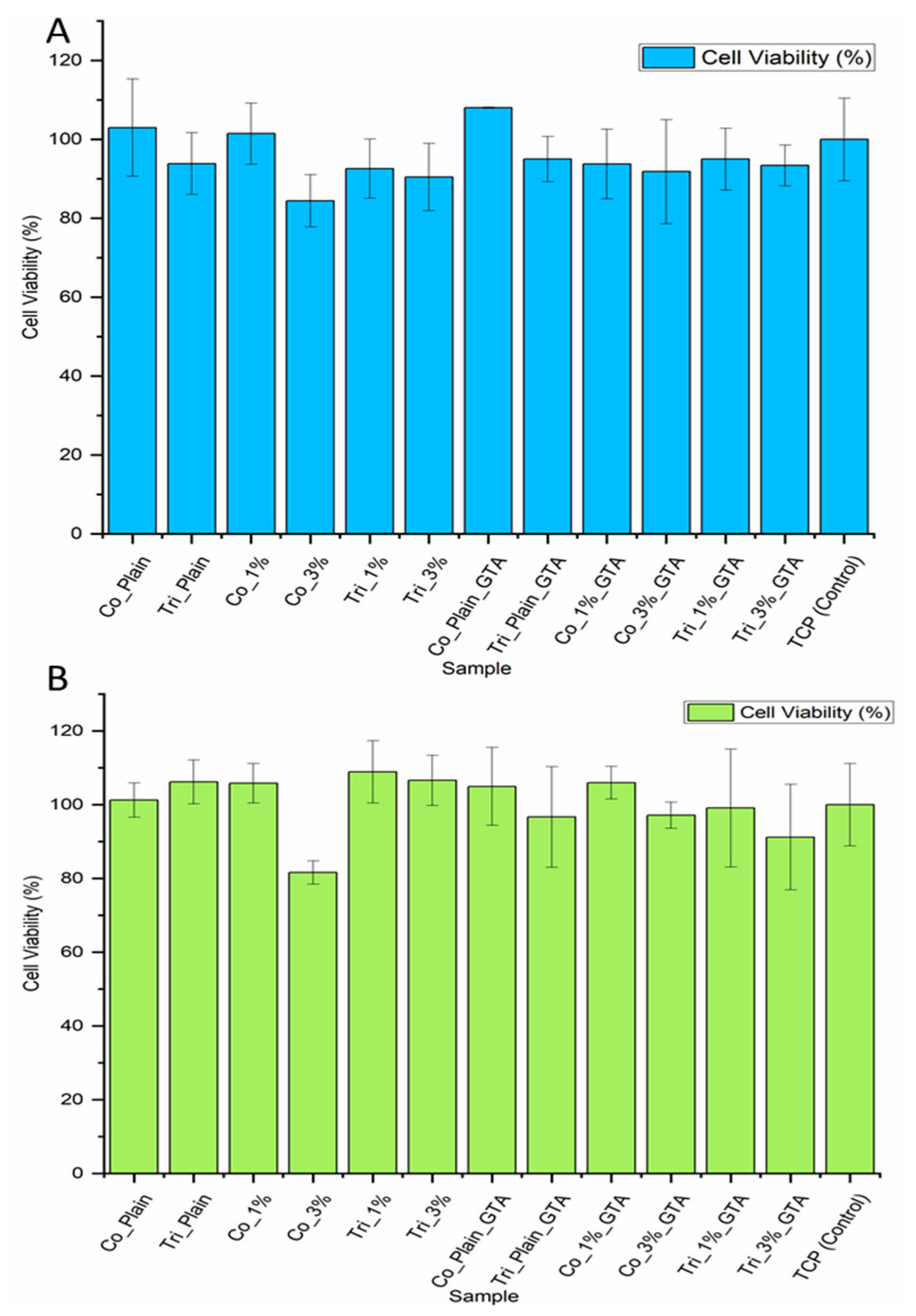
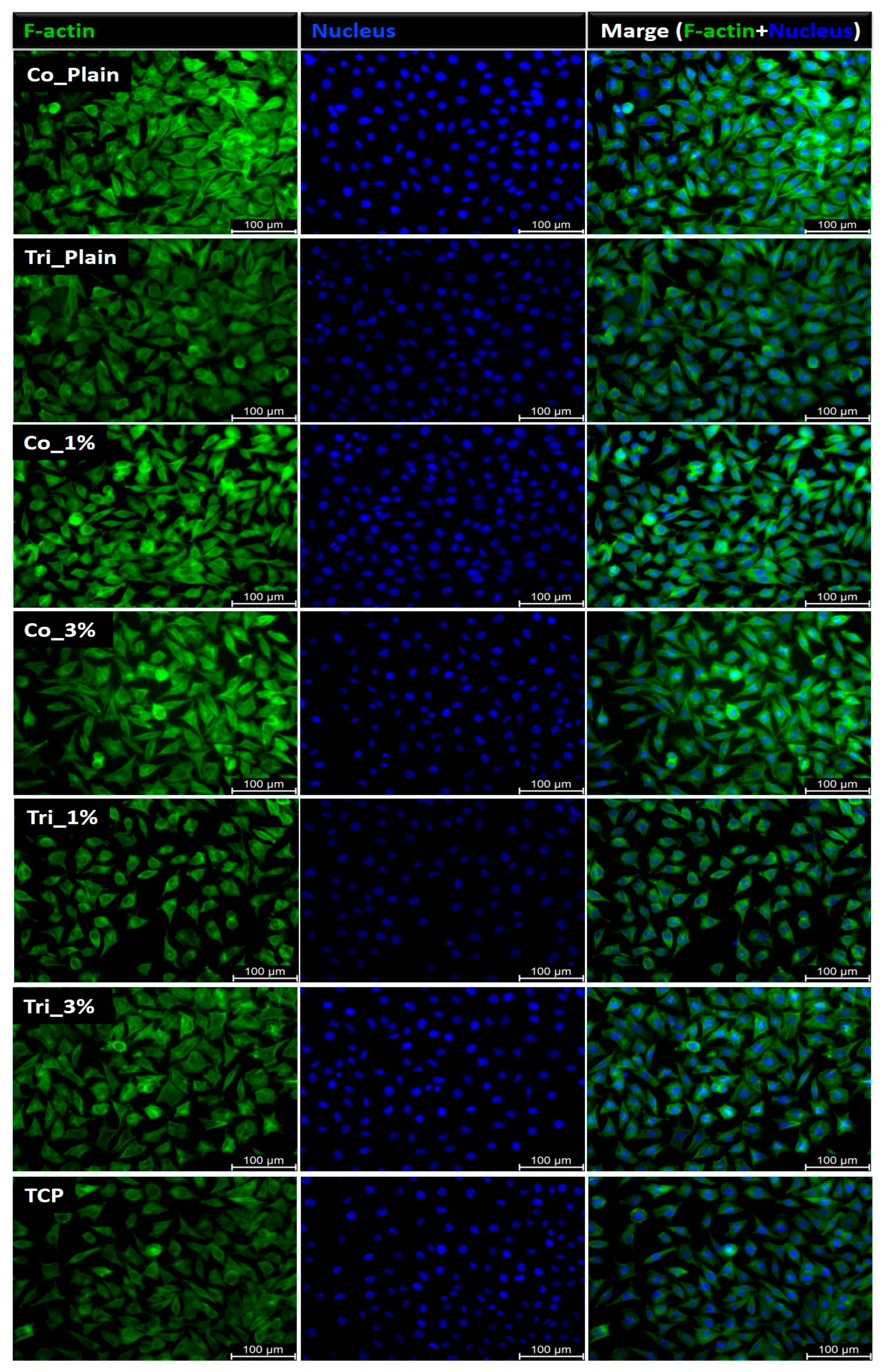
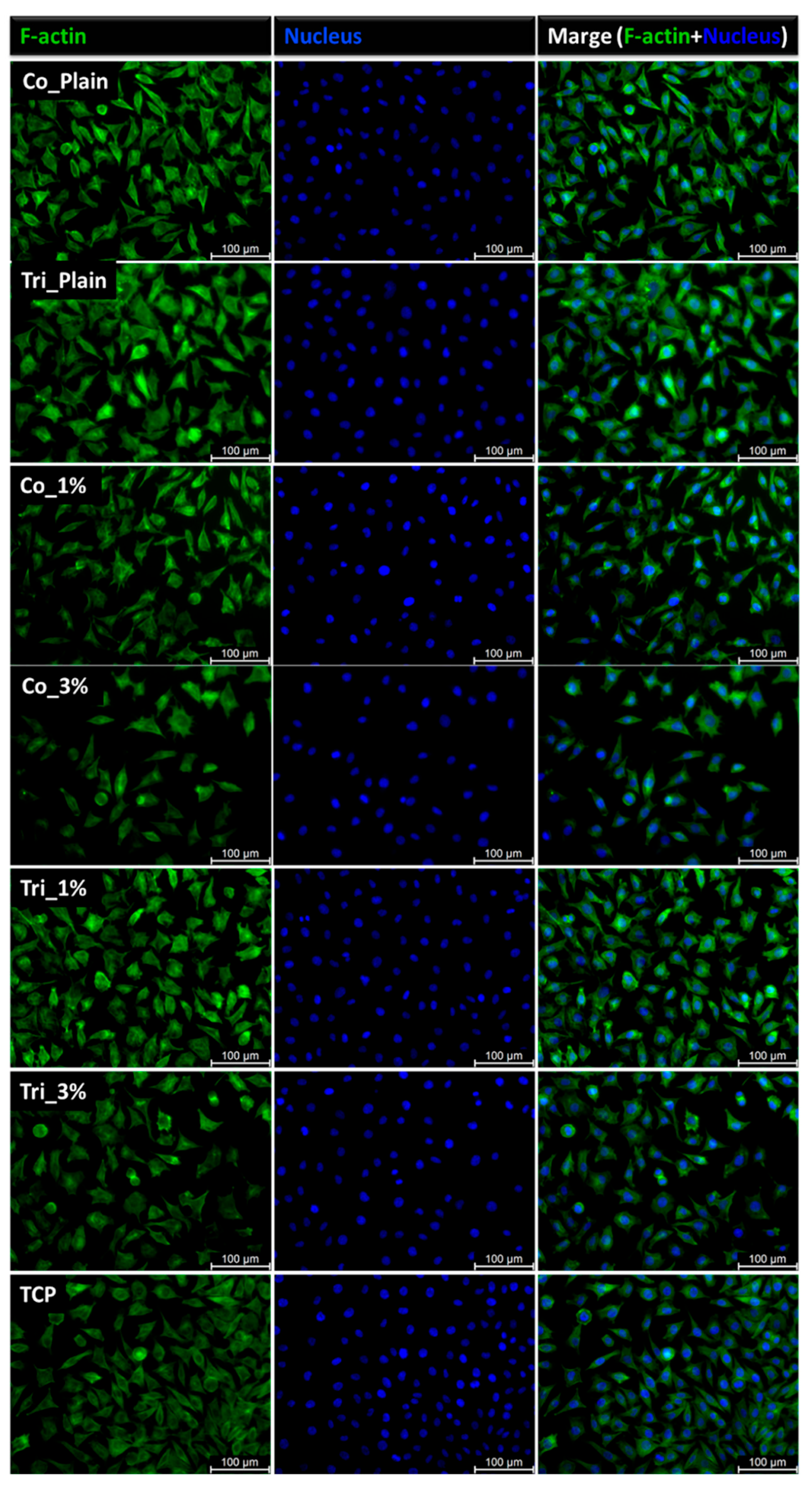
2.4. Biological Evaluation
3. Materials and Methods
3.1. Materials
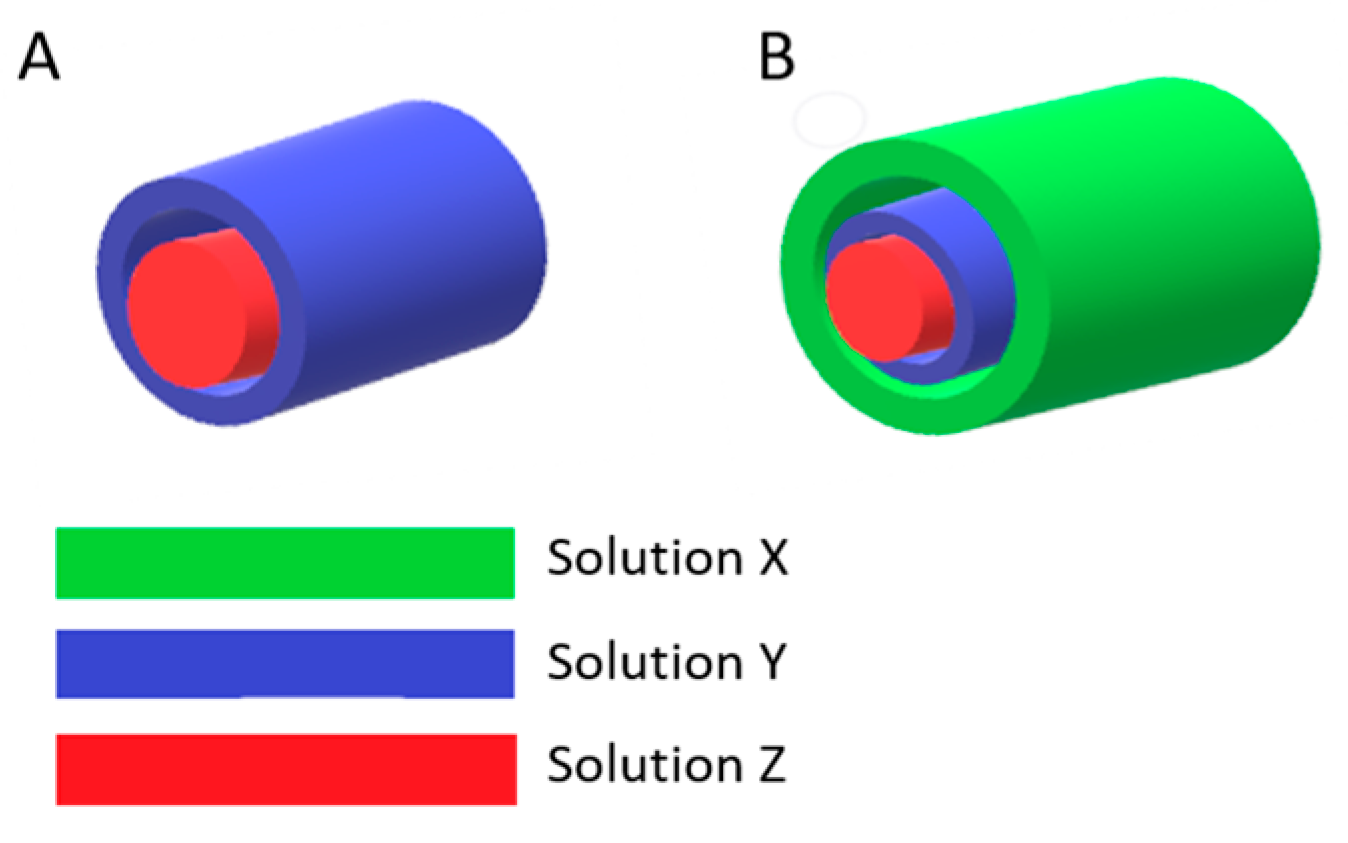
3.2. Electrospinning and Preparation of Samples
3.3. Characterization
3.3.1. Morphology of Fibers
Estimation of Inner Layer Thicknesses
Porosity
3.3.2. In Vitro Drug Release Tests and Release Kinetics
3.3.3. Surface Wettability Test
3.3.4. Cytotoxicity Evaluation
Cytotoxicity Assay
Fibroblast Morphology
3.3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Meinel, A.J.; Germershaus, O.; Luhmann, T.; Merkle, H.P.; Meinel, L. Electrospun Matrices for Localized Drug Delivery: Current Technologies and Selected Biomedical Applications. Eur. J. Pharm. Biopharm. 2012, 81, 1–13. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. CTGF Loaded Electrospun Dual Porous Core-Shell Membrane For Diabetic Wound Healing. Int. J. Nanomed. 2019, 14, 8573–8588. [Google Scholar] [CrossRef]
- Hai, T.; Wan, X.; Yu, D.-G.; Wang, K.; Yang, Y.; Liu, Z.-P. Electrospun Lipid-Coated Medicated Nanocomposites for an Improved Drug Sustained-Release Profile. Mater. Des. 2019, 162, 70–79. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Niu, Y.; Liu, H.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Preparation and in Vivo Efficient Anti-Infection Property of GTR/GBR Implant Made by Metronidazole Loaded Electrospun Polycaprolactone Nanofiber Membrane. Int. J. Pharm. 2014, 475, 566–577. [Google Scholar] [CrossRef]
- Ghosal, K.; Augustine, R.; Zaszczynska, A.; Barman, M.; Jain, A.; Hasan, A.; Kalarikkal, N.; Sajkiewicz, P.; Thomas, S. Novel Drug Delivery Systems Based on Triaxial Electrospinning Based Nanofibers. React. Funct. Polym. 2021, 163, 104895. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Nagiah, N.; Murdock, C.J.; Bhattacharjee, M.; Nair, L.; Laurencin, C.T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Sci. Rep. 2020, 10, 609. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-Drug Delivery Systems: Pros and Cons, Challenges, and Future Directions. Biomater. Sci. 2022, 11, 37–61. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhu, Z.; Lu, Y.; Liu, H.; Yu, D.-G.; Bligh, S.-W.A. A Modified Triaxial Electrospinning for a High Drug Encapsulation Efficiency of Curcumin in Ethylcellulose. Pharmaceutics 2025, 17, 1152. [Google Scholar] [CrossRef] [PubMed]
- Altundag-Erdogan, Ö.; Çetinkaya, H.; Çelebi-Saltik, B.; Altun, B.; Şahin, M.; Mağden, G.K.; Öteyaka, M.Ö. Characterization of a New Triaxial Nanofiber Membrane and Osteogenic Differentiation Capacity of Human Bone Marrow Mesenchymal Stem Cells for Bone Repair. J. Mater. Res. 2025, 40, 1117–1134. [Google Scholar] [CrossRef]
- Miranda, C.S.; Marinho, E.; Rocha, D.; Silva, C.; Silva, M.M.P.; Schlapp-Hackl, I.; Fang, W.; Hummel, M.; Costa, S.P.G.; Homem, N.C.; et al. Pioneering Wound Care Solutions: Triaxial Wet-Spun Fibers with Bioactive Agents for Chronic Wounds—Part I (Production and Characterization of the Triaxial Fibers). Mater. Adv. 2025, 6, 3237–3252. [Google Scholar] [CrossRef]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Annie Bligh, S.W. A Review on Electrospun Poly(amino acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef]
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-Term Antimicrobial Effect of Nisin Released from Electrospun Triaxial Fiber Membranes. Acta Biomater. 2017, 53, 242–249. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.d.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.-T.; Cheng, J.-S.; Zhang, B. Preparation and Characterization of Zein/Carboxymethyl Dextrin Nanoparticles to Encapsulate Curcumin: Physicochemical Stability, Antioxidant Activity and Controlled Release Properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef]
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A.E.; Ganjali, S.; Sahebkar, A. Curcumin Nanofibers for the Purpose of Wound Healing. J. Cell. Physiol. 2019, 234, 5537–5554. [Google Scholar] [CrossRef]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef]
- Liao, H.T.; Lai, Y.-T.; Kuo, C.-Y.; Chen, J.-P. A Bioactive Multi-Functional Heparin-Grafted Aligned Poly(Lactide-Co-Glycolide)/Curcumin Nanofiber Membrane to Accelerate Diabetic Wound Healing. Mater. Sci. Eng. C 2021, 120, 111689. [Google Scholar] [CrossRef]
- Reshmi, C.R.; Suja, P.S.; Manaf, O.; Sanu, P.P.; Sujith, A. Nanochitosan Enriched Poly ε-Caprolactone Electrospun Wound Dressing Membranes: A Fine Tuning of Physicochemical Properties, Hemocompatibility and Curcumin Release Profile. Int. J. Biol. Macromol. 2018, 108, 1261–1272. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yu, D.-G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-Core/PHBV-Shell Fibers to Eliminate Tailing Off for an Improved Sustained Release of Curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef] [PubMed]
- Behtaj, S.; Karamali, F.; Masaeli, E.; Anissimov, Y.G.; Rybachuk, M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and Pure PCL Scaffolds for Retinal Progenitor Cell Cultivation. Biochem. Eng. J. 2021, 166, 107846. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/Gelatin Composite Nanofiber Structures for Effective Guided Bone Regeneration Membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of Gelatin with Tunable Fiber Morphology from Round to Flat/Ribbon. Mater. Sci. Eng. C 2017, 80, 371–378. [Google Scholar] [CrossRef]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D Printed Hybrid Bi-Layer Scaffold for Guided Bone Regeneration. Mater. Des. 2021, 210, 110047. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Vittoria, V.; Gorrasi, G. Coaxial Electrospun Membranes of Poly(ε-Caprolactone)/Poly(Lactic Acid) with Reverse Core-Shell Structures Loaded with Curcumin as Tunable Drug Delivery Systems. Polym. Adv. Technol. 2021, 32, 4005–4013. [Google Scholar] [CrossRef]
- Lian, M.; Sun, B.; Qiao, Z.; Zhao, K.; Zhou, X.; Zhang, Q.; Zou, D.; He, C.; Zhang, X. Bi-Layered Electrospun Nanofibrous Membrane with Osteogenic and Antibacterial Properties for Guided Bone Regeneration. Colloids Surf. B Biointerfaces 2019, 176, 219–229. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films Based on κ-Carrageenan Incorporated with Curcumin for Freshness Monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S.; Jiang, S. Release Kinetics and Antibacterial Activity of Curcumin Loaded Zein Fibers. Food Hydrocoll. 2017, 63, 437–446. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Kokalari, I.; Parnell, S.R.; Smith, G.N.; Zeng, B.-H.; Way, T.-F.; Chuang, F.-S.; Rwei, A.Y. Structure Property Relationship of Micellar Waterborne Poly(Urethane-Urea): Tunable Mechanical Properties and Controlled Release Profiles with Amphiphilic Triblock Copolymers. Langmuir 2023, 39, 10033–10046. [Google Scholar] [CrossRef]
- Jardim, K.V.; Siqueira, J.L.N.; Báo, S.N.; Sousa, M.H.; Parize, A.L. The Role of the Lecithin Addition in the Properties and Cytotoxic Activity of Chitosan and Chondroitin Sulfate Nanoparticles Containing Curcumin. Carbohydr. Polym. 2020, 227, 115351. [Google Scholar] [CrossRef] [PubMed]
- Jardim, K.V.; Palomec-Garfias, A.F.; Araújo, M.V.; Márquez-Beltrán, C.; Bakuzis, A.F.; Moya, S.E.; Parize, A.L.; Sousa, M.H. Remotely Triggered Curcumin Release from Stimuli-Responsive Magneto-Polymeric Layer-by-Layer Engineered Nanoplatforms. J. Appl. Polym. Sci. 2022, 139, 52200. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Chanunpanich, N.; Park, J.S. Porous Core/Sheath Composite Nanofibers Fabricated by Coaxial Electrospinning as a Potential Mat for Drug Release System. Int. J. Pharm. 2012, 439, 296–306. [Google Scholar] [CrossRef]
- Ramos, C.; Lanno, G.-M.; Laidmäe, I.; Meos, A.; Härmas, R.; Kogermann, K. High Humidity Electrospinning of Porous Fibers for Tuning the Release of Drug Delivery Systems. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 880–892. [Google Scholar] [CrossRef]
- McDonald, P.F.; Lyons, J.G.; Geever, L.M.; Higginbotham, C.L. In Vitro Degradation and Drug Release from Polymer Blends Based on Poly(Dl-Lactide), Poly(l-Lactide-Glycolide) and Poly(ε-Caprolactone). J. Mater. Sci. 2010, 45, 1284–1292. [Google Scholar] [CrossRef]
- Liu, G.; McEnnis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, C.; Chen, H.; Wu, J.; Han, C.C.; Xu, S. Electrospun Fibrous Membrane with Confined Chain Configuration: Dynamic Relaxation and Glass Transition. Polymers 2022, 14, 939. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun Curcumin Loaded Poly(ε-Caprolactone)/Gum Tragacanth Nanofibers for Biomedical Application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Fallah, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Fabrication and Characterization of PCL/Gelatin/Curcumin Nanofibers and Their Antibacterial Properties. J. Ind. Text. 2016, 46, 562–577. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, Y.; Li, C.; Zhou, Z.; Liu, Y.; Xu, J.; Wang, L.; Yu, D.-G.; Wang, K. Preparation and Investigation of Cellulose Acetate/Gelatin Janus Nanofiber Wound Dressings Loaded with Zinc Oxide or Curcumin for Enhanced Antimicrobial Activity. Membranes 2024, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Rasouli, S.; Montazeri, M.; Mashayekhi, S.; Sadeghi-Soureh, S.; Dadashpour, M.; Mousazadeh, H.; Nobakht, A.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Synergistic Anticancer Effects of Electrospun Nanofiber-Mediated Codelivery of Curcumin and Chrysin: Possible Application in Prevention of Breast Cancer Local Recurrence. J. Drug Deliv. Sci. Technol. 2020, 55, 101402. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Mutsaers, H.A.M.; Pennings, S.W.C.; Szarek, W.A.; Russel, F.G.M.; Wagener, F.A.D.T.G. Curcumin-Induced Fibroblast Apoptosis and in Vitro Wound Contraction Are Regulated by Antioxidants and Heme Oxygenase: Implications for Scar Formation. J. Cell. Mol. Med. 2009, 13, 712–725. [Google Scholar] [CrossRef]
- Amarjargal, A.; Cegielska, O.; Kolbuk, D.; Kalaska, B.; Sajkiewicz, P. On-Demand Sequential Release of Dual Drug from pH-Responsive Electrospun Janus Nanofiber Membranes toward Wound Healing and Infection Control. ACS Appl. Mater. Interfaces 2024, 16, 153–165. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin Loaded Poly(ε-Caprolactone) Nanofibers: Diabetic Wound Dressing with Antioxidant and Anti-Inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef]
- Duan, G.; Jin, M.; Wang, F.; Greiner, A.; Agarwal, S.; Jiang, S. Core Effect on Mechanical Properties of One Dimensional Electrospun Core-Sheath Composite Fibers. Compos. Commun. 2021, 25, 100773. [Google Scholar] [CrossRef]
- Kolbuk, D.; Guimond-Lischer, S.; Sajkiewicz, P.; Maniura-Weber, K.; Fortunato, G. The Effect of Selected Electrospinning Parameters on Molecular Structure of Polycaprolactone Nanofibers. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 365–377. [Google Scholar] [CrossRef]
- Rose, J.B.; Sidney, L.E.; Patient, J.; White, L.J.; Dua, H.S.; El Haj, A.J.; Hopkinson, A.; Rose, F.R.A.J. In Vitro Evaluation of Electrospun Blends of Gelatin and PCL for Application as a Partial Thickness Corneal Graft. J. Biomed. Mater. Res. A 2019, 107, 828–838. [Google Scholar] [CrossRef]
- Rafiei, M.; Jooybar, E.; Abdekhodaie, M.J.; Alvi, M. Construction of 3D Fibrous PCL Scaffolds by Coaxial Electrospinning for Protein Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110913. [Google Scholar] [CrossRef]
- Jin, G.; Lee, S.; Kim, S.-H.; Kim, M.; Jang, J.-H. Bicomponent Electrospinning to Fabricate Three-Dimensional Hydrogel-Hybrid Nanofibrous Scaffolds with Spatial Fiber Tortuosity. Biomed. Microdevices 2014, 16, 793–804. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Corrigan, O.I. Mechanistic Aspects of the Release of Levamisole Hydrochloride from Biodegradable Polymers. J. Control. Release 2000, 69, 261–272. [Google Scholar] [CrossRef] [PubMed]

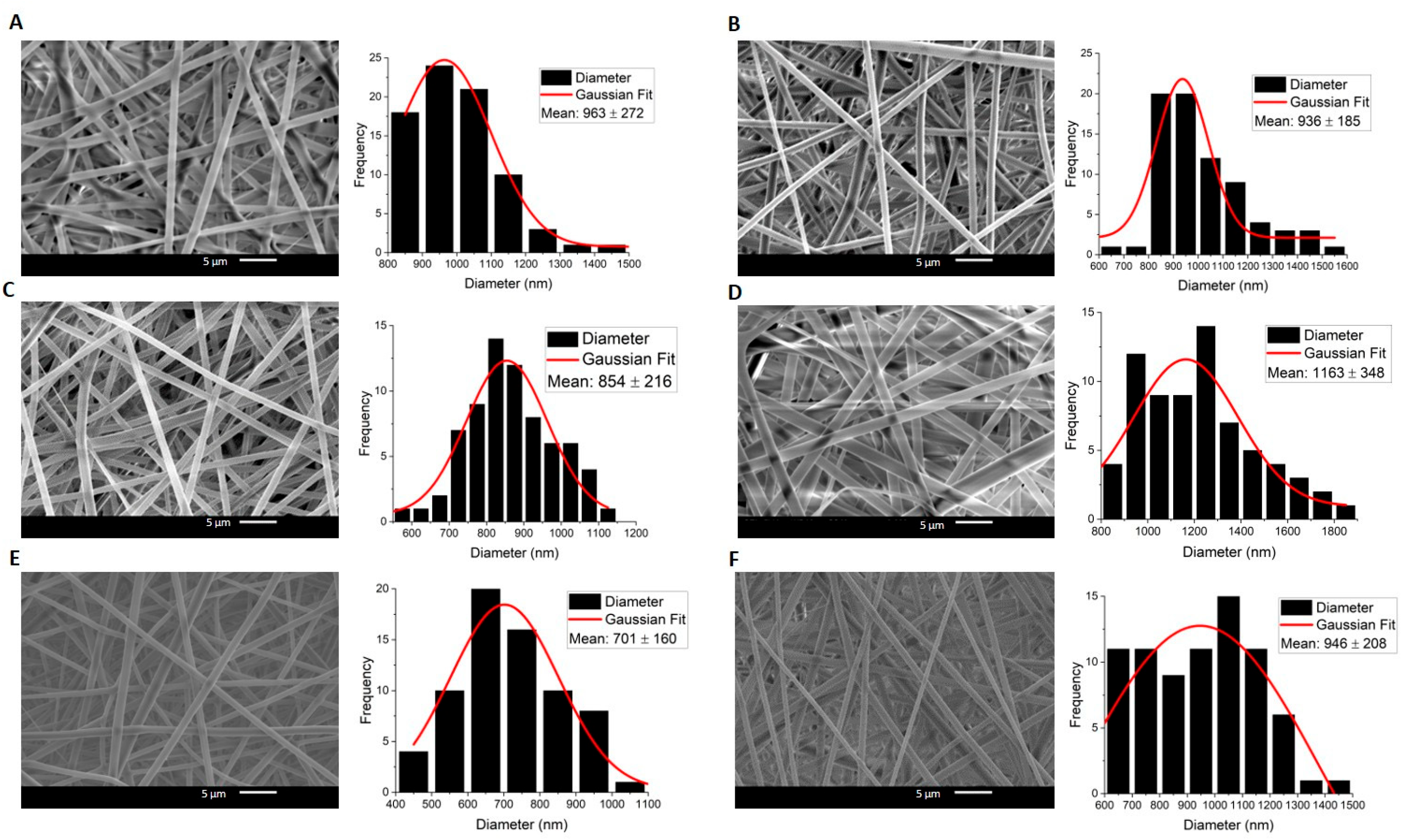
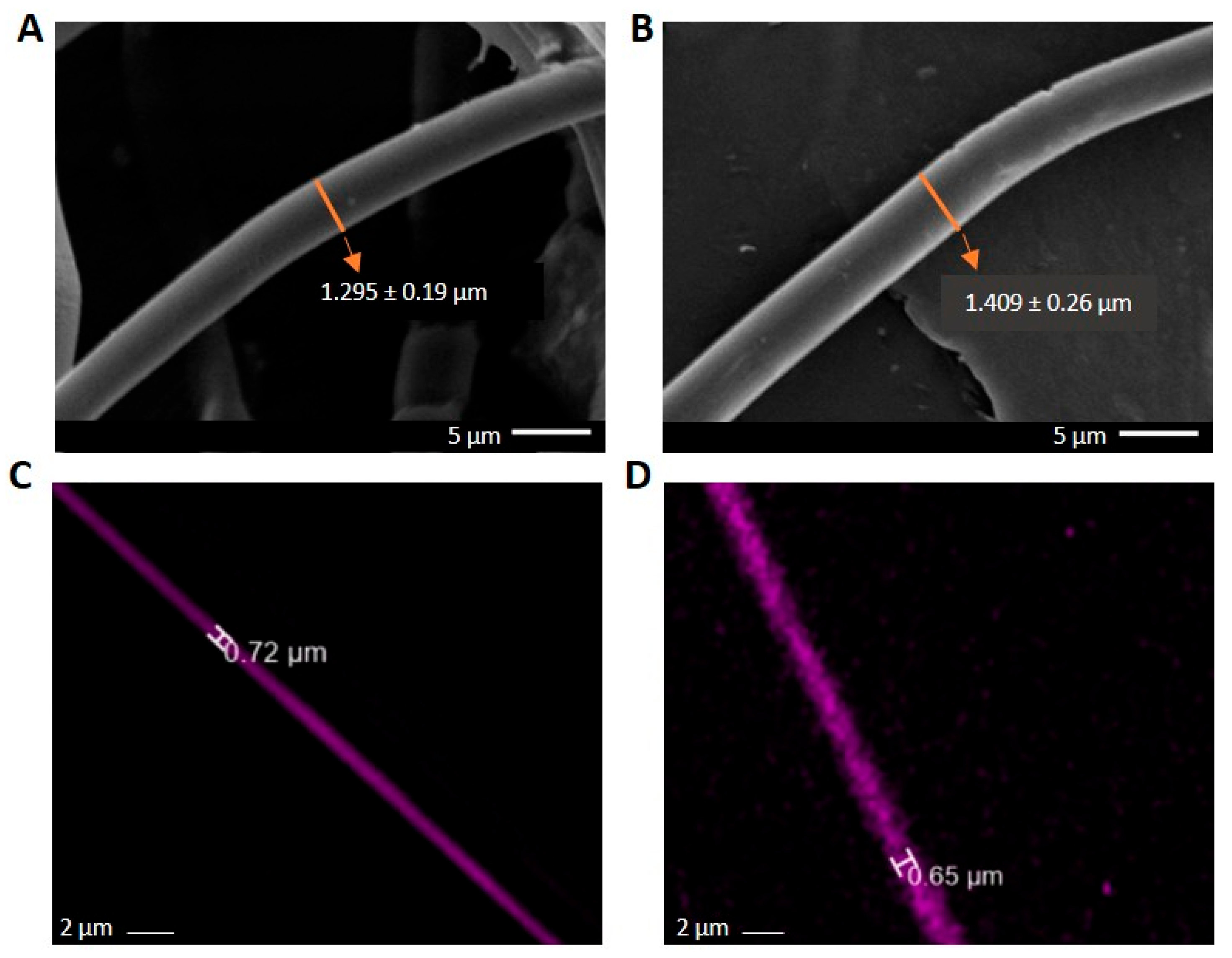
| Sample | C1. Core (nm) | C1. Middle (nm) | C1. Shell (nm) | C2. Core (nm) | C2. Middle (nm) | C2. Shell (nm) |
|---|---|---|---|---|---|---|
| Co_Plain | 238.26 | - | 284.73 | 438.72 | - | 524.27 |
| Tri_Plain | 191.62 | 228.99 | 343.37 | 187.81 | 224.44 | 523.74 |
| Co_1% | 261.50 | - | 312.49 | 389.06 | - | 464.93 |
| Co_3% | 266.96 | - | 319.03 | 529.83 | - | 633.16 |
| Tri_1% | 278.42 | 332.70 | 498.87 | 140.66 | 168.09 | 392.24 |
| Tri_3% | 257.10 | 307.23 | 460.67 | 189.82 | 226.84 | 529.33 |
| Sample | C1. Porosity (%) | C1. Pore Size (µm) | C2. Porosity (%) | C2. Pore Size (µm) |
|---|---|---|---|---|
| Co_Plain | 76 ± 3 | 4.5 ± 0.5 | 82 ± 1 | 11 ± 0.6 |
| Tri_Plain | 79 ± 6 | 7.8 ± 2.4 | 68 ± 8 | 6.1 ± 1.5 |
| Co_1% | 80 ± 1.6 | 5.8 ± 4.5 | 76 ± 8 | 8 ± 3.5 |
| Co_3% | 80 ± 6 | 6.4 ± 2 | 71 ± 3.5 | 8.2 ± 1 |
| Tri_1% | 78 ± 2.5 | 10.3 ± 1.1 | 66 ± 2 | 4.2 ± 2.7 |
| Tri_3% | 81 ± 1 | 11 ± 0.6 | 63 ± 5 | 5.2 ± 0.7 |
| Samples | fB | k1 | k2 | tm | R2 |
|---|---|---|---|---|---|
| Co_1_1% | 77 | 0.739 | 0.225 | 19.11 | 0.9999 |
| Co_1_3% | 87 | 0.818 | 0.164 | 20.27 | 0.9999 |
| Tri_1_1% | 53 | 0.396 | 0.043 | 49.10 | 0.9961 |
| Tri_1_3% | 63 | 0.696 | 0.055 | 40.79 | 0.9975 |
| Co_2_1% | 73 | 1.159 | 0.072 | 35.10 | 0.9996 |
| Co_2_3% | 76 | 0.550 | 0.065 | 35.05 | 0.9987 |
| Tri_2_1% | 68 | 0.299 | 0.083 | 46.98 | 0.9997 |
| Tri_2_3% | 75 | 0.130 | 0.075 | 53.13 | 0.9986 |
| Sample | Core Layer | Middle Layer | Shell Layer |
|---|---|---|---|
| Co_Plain | PCL | GT | - |
| Tri_Plain | PCL | GT | PLGA |
| Co_1% | PCL | GT with 1% Cur (wt.) | - |
| Co_3% | PCL | GT with 3% Cur (wt.) | - |
| Tri_1% | PCL | GT with 1% Cur (wt.) | PLGA |
| Tri_3% | PCL | GT with 3% Cur (wt.) | PLGA |
| Sample | Core Layer | Middle Layer | Shell Layer |
|---|---|---|---|
| Co_Plain | PLGA | GT | - |
| Tri_Plain | PLGA | GT | PCL |
| Co_1% | PLGA | GT with 1% Cur (wt.) | - |
| Co_3% | PLGA | GT with 3% Cur (wt.) | - |
| Tri_1% | PLGA | GT with 1% Cur (wt.) | PCL |
| Tri_3% | PLGA | GT with 3% Cur (wt.) | PCL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Triaxial Electrospun Nanofiber Membranes for Prolonged Curcumin Release in Dental Applications: Drug Release and Biological Properties. Molecules 2025, 30, 4241. https://doi.org/10.3390/molecules30214241
Tabakoglu S, Kołbuk D, Sajkiewicz P. Triaxial Electrospun Nanofiber Membranes for Prolonged Curcumin Release in Dental Applications: Drug Release and Biological Properties. Molecules. 2025; 30(21):4241. https://doi.org/10.3390/molecules30214241
Chicago/Turabian StyleTabakoglu, Sahranur, Dorota Kołbuk, and Paweł Sajkiewicz. 2025. "Triaxial Electrospun Nanofiber Membranes for Prolonged Curcumin Release in Dental Applications: Drug Release and Biological Properties" Molecules 30, no. 21: 4241. https://doi.org/10.3390/molecules30214241
APA StyleTabakoglu, S., Kołbuk, D., & Sajkiewicz, P. (2025). Triaxial Electrospun Nanofiber Membranes for Prolonged Curcumin Release in Dental Applications: Drug Release and Biological Properties. Molecules, 30(21), 4241. https://doi.org/10.3390/molecules30214241









