Tuning Excited-State Properties in Pyrrolo[3,2-b]pyrrole-Based Donor–Acceptor Emitters via Molecular Conformation and Conjugation Control
Abstract
1. Introduction
2. Results and Discussion
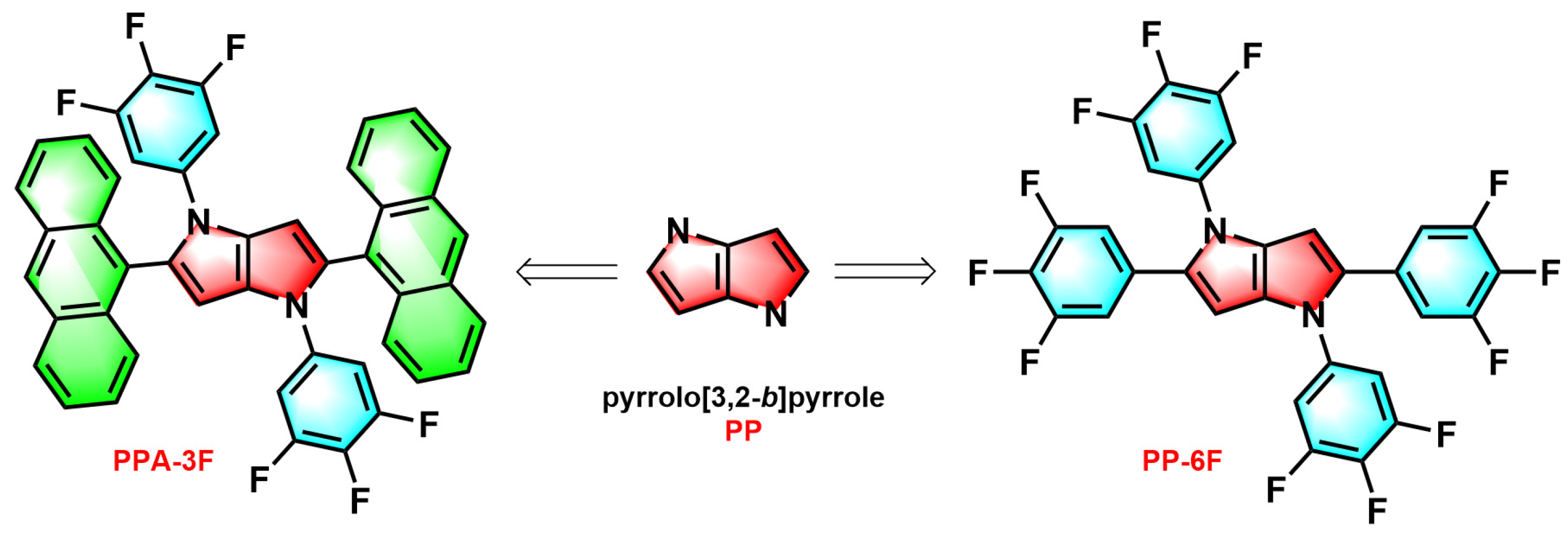
2.1. Synthesis
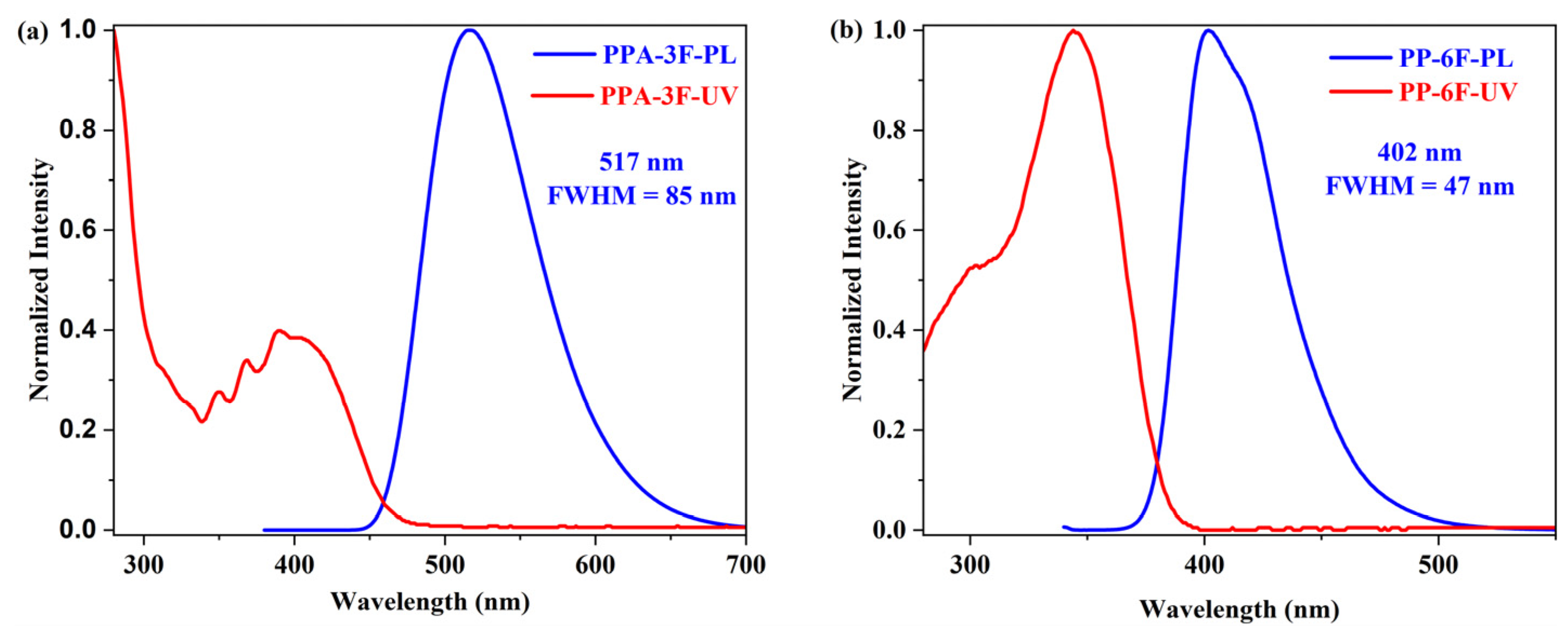
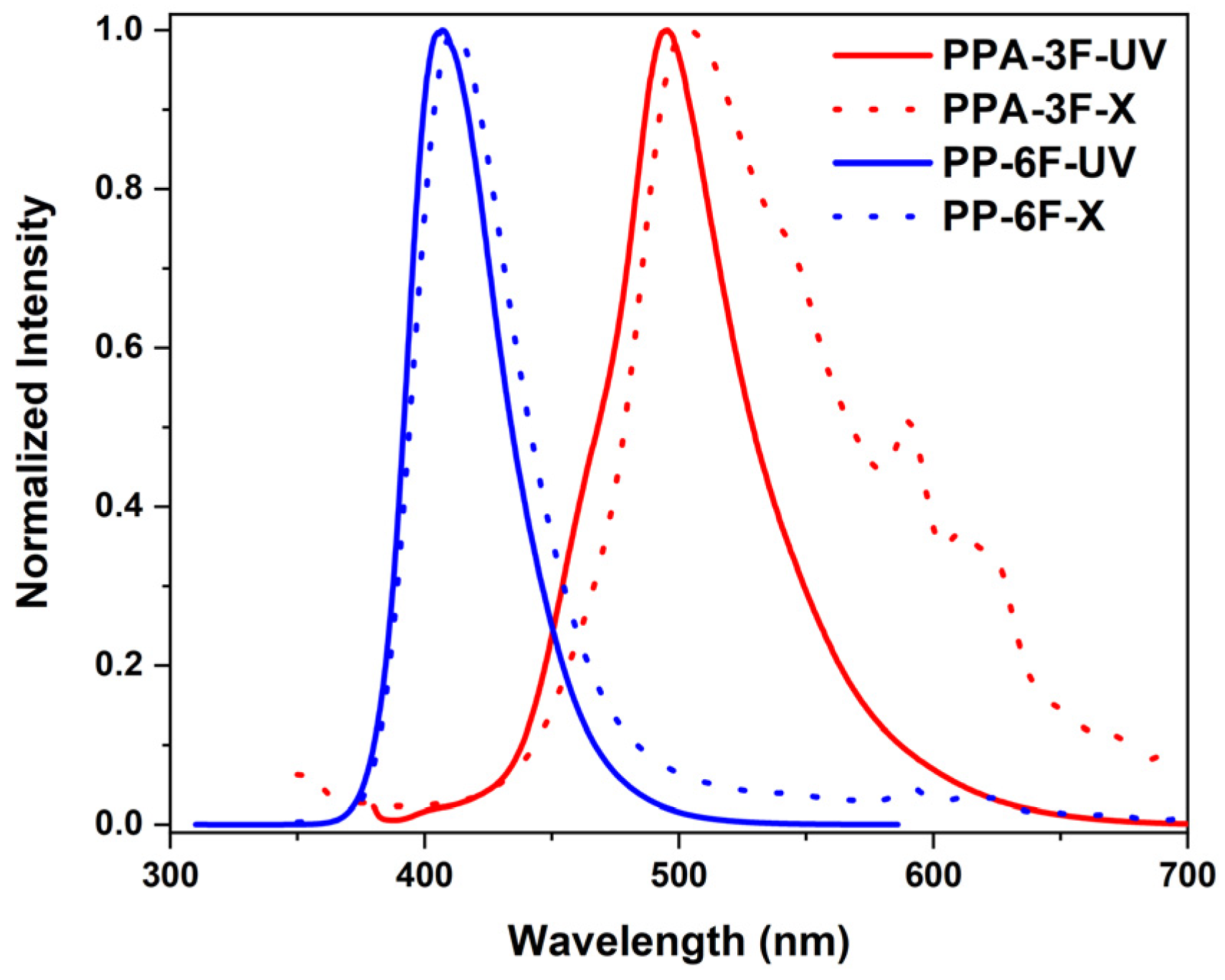
2.2. Photophysical Properties
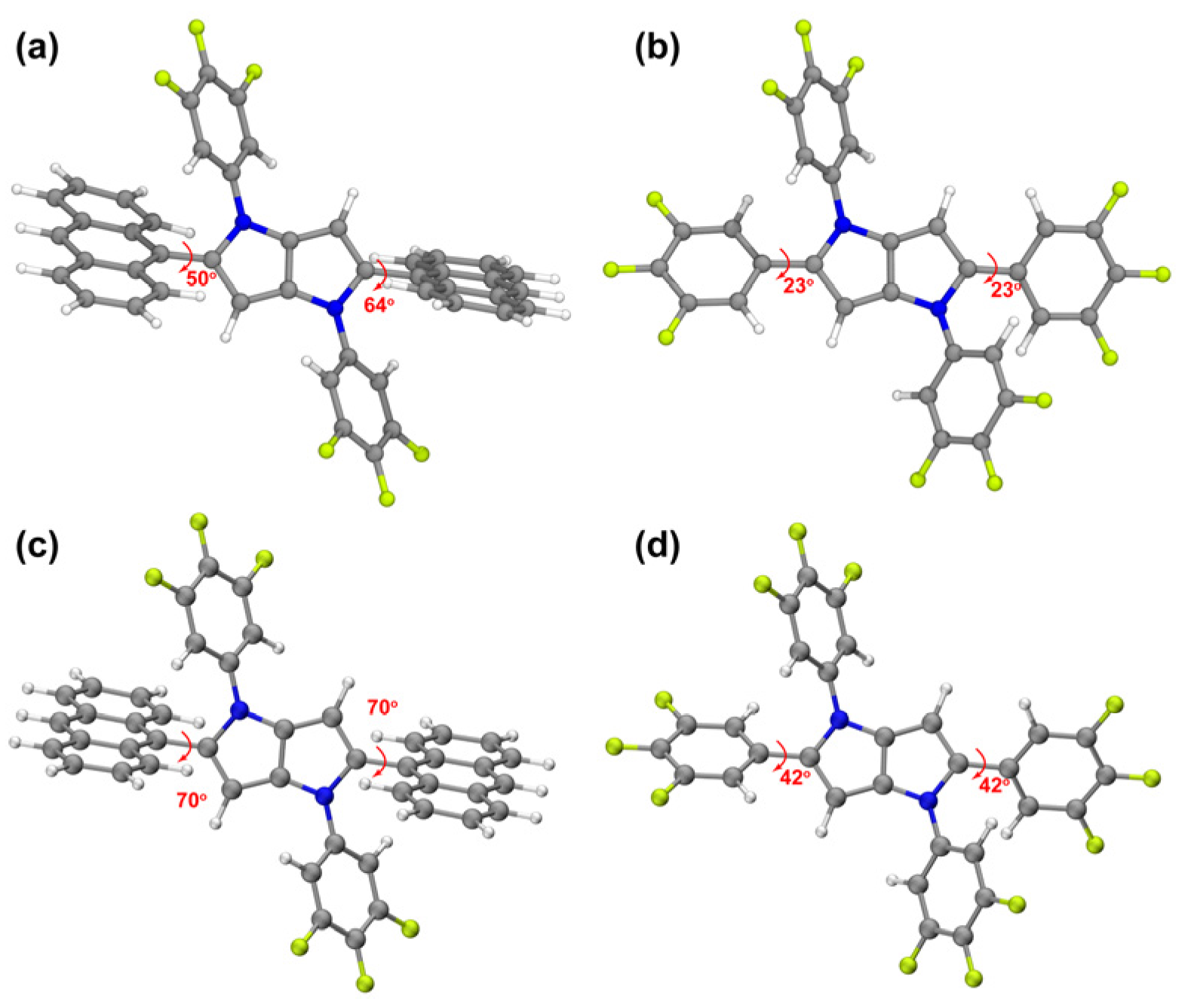
2.3. Single Crystal Structure Analysis
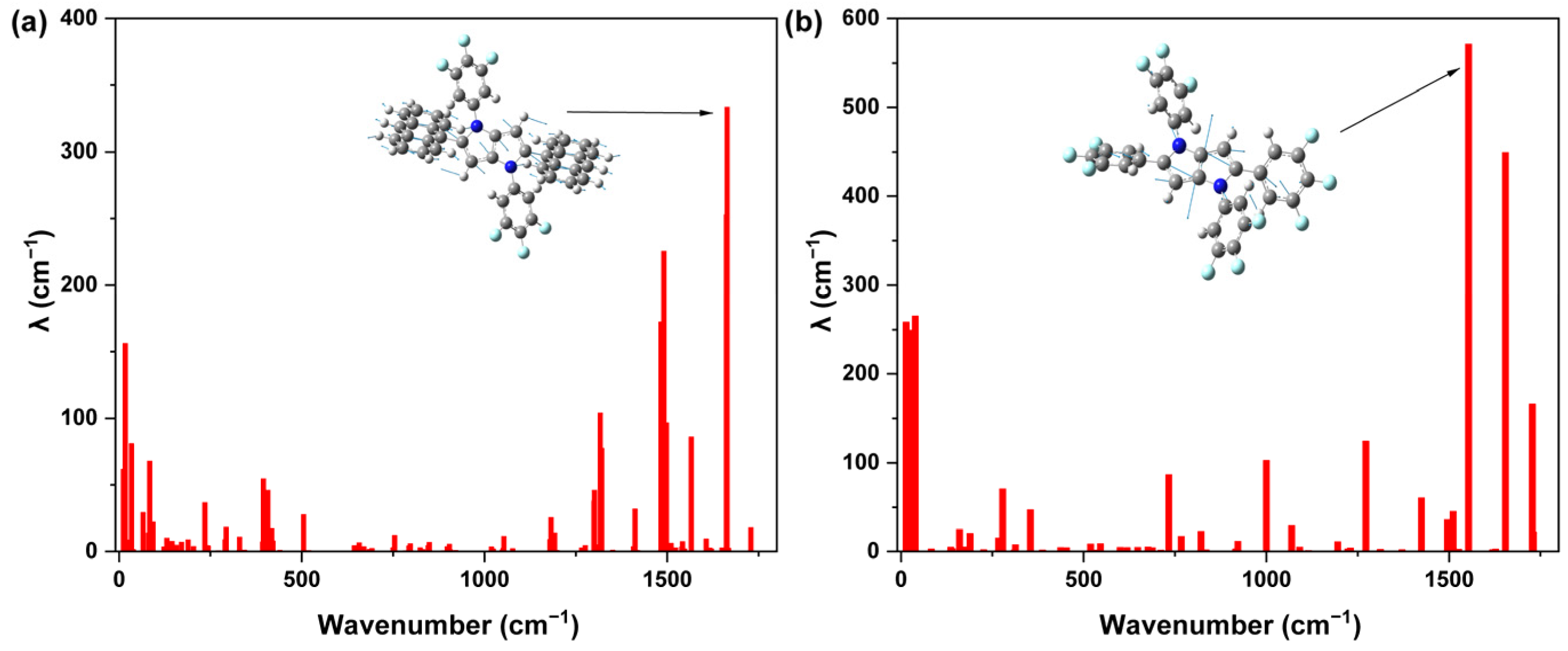
2.4. Theoretical Calculations
3. Conclusions
4. Materials and Methods
4.1. Synthetic Details
4.2. Measurements
4.3. Crystal
4.4. Computational Details
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Ni, S.; Wang, C.-H.; Zhu, W.; Chou, P.-T. Comprehensive Review on the Structural Diversity and Versatility of Multi-Resonance Fluorescence Emitters: Advance, Challenges, and Prospects toward OLEDs. Chem. Rev. 2025, 125, 6685–6752. [Google Scholar] [CrossRef] [PubMed]
- Mamada, M.; Hayakawa, M.; Ochi, J.; Hatakeyama, T. Organoboron-Based Multiple-Resonance Emitters: Synthesis, Structure–Property Correlations, and Prospects. Chem. Soc. Rev. 2024, 53, 1624–1692. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.; Sariciftci, N.S. Organic Solar Cells: An Overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, G.; Yu, H.; Yan, H. Advantages, Challenges and Molecular Design of Different Material Types Used in Organic Solar Cells. Nat. Rev. Mater. 2024, 9, 46–62. [Google Scholar] [CrossRef]
- Liang, W.; Chen, L.; Wang, Z.; Peng, Z.; Zhu, L.; Kwok, C.H.; Yu, H.; Xiong, W.; Li, T.; Zhang, Z.; et al. Oligothiophene Additive-Assisted Morphology Control and Recombination Suppression Enable High-Performance Organic Solar Cells. Adv. Energy Mater. 2024, 14, 2303661. [Google Scholar] [CrossRef]
- Li, J.; Xie, D.; Yuan, X.; Li, Y.; Wei, W.; Zhang, Y.; Feng, H.; Luo, X.; Zhu, J.; Qin, Z.; et al. Nucleation Driving Force-Controlled Fibril Network Formation Using a Non-Halogenated Solvent Enables Polythiophene Solar Cells with over 18% Efficiency. Energy Environ. Sci. 2025, 18, 4384–4395. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, K.; Chen, Z.; Zhang, H. Molecular Design Concept for Enhancement Charge Carrier Mobility in OFETs: A Review. Materials 2023, 16, 6645. [Google Scholar] [CrossRef]
- Fan, X.; Hao, X.; Huang, F.; Yu, J.; Wang, K.; Zhang, X. RGB Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes toward Realizing the BT.2020 Standard. Adv. Sci. 2023, 10, 2303504. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Chen, Z.; Zhang, Q. Organic Cocrystals: Beyond Electrical Conductivities and Field-Effect Transistors (FETs). Angew. Chem. Int. Ed. 2019, 58, 9696–9711. [Google Scholar] [CrossRef]
- Stecko, S.; Gryko, D.T. Multifunctional Heteropentalenes: From Synthesis to Optoelectronic Applications. JACS Au 2022, 2, 1290–1305. [Google Scholar] [CrossRef]
- Górski, K.; Shelton, S.; Lingagouder, J.; Data, P.; Jacquemin, D.; Gryko, D.T. 1,4-Dihydropyrrolo[3,2- b ]Pyrrole Modified with Dibenzoxazepine: A Highly Efficient Core for Charge-Transfer-Based OLED Emitters. Chem. Sci. 2025, 16, 5223–5233. [Google Scholar] [CrossRef]
- Szymański, B.; Sahoo, S.R.; Vakuliuk, O.; Valiev, R.; Ramazanov, R.; Łaski, P.; Jarzembska, K.N.; Kamiński, R.; Teimouri, M.B.; Baryshnikov, G.; et al. Shedding New Light on Quadrupolar 1,4-Dihydropyrrolo[3,2- b ]Pyrroles: Impact of Electron-Deficient Scaffolds over Emission. Chem. Sci. 2025, 16, 2170–2179. [Google Scholar] [CrossRef]
- Govind, C.; Balanikas, E.; Sanil, G.; Gryko, D.T.; Vauthey, E. Structural and Solvent Modulation of Symmetry-Breaking Charge-Transfer Pathways in Molecular Triads. Chem. Sci. 2024, 15, 17362–17371. [Google Scholar] [CrossRef] [PubMed]
- Krzeszewski, M.; Thorsted, B.; Brewer, J.; Gryko, D.T. Tetraaryl-, Pentaaryl-, and Hexaaryl-1,4-Dihydropyrrolo[3,2-b]Pyrroles: Synthesis and Optical Properties. J. Org. Chem. 2014, 79, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Tasior, M.; Vakuliuk, O.; Koga, D.; Koszarna, B.; Gorski, K.; Grzybowski, M.; Kielesinski, L.; Krzeszewski, M.; Gryko, D.T. Method for the Large-Scale Synthesis of Multifunctional 1,4-Dihydro-Pyrrolo[3,2-b]Pyrroles. J. Org. Chem. 2020, 85, 13529–13543. [Google Scholar] [CrossRef] [PubMed]
- Krzeszewski, M.; Gryko, D.T. Chi-Shaped Bis(Areno)-1,4-Dihydropyrrolo[3,2-b]Pyrroles Generated by Oxidative Aromatic Coupling. J. Org. Chem. 2015, 80, 2893–2899. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly Efficient Organic Light-Emitting Diodes from Delayed Fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Su, J.; Wei, J.; Ye, K.; Li, F.; Wang, M.; Li, Q.; Yuan, A.; Zhao, Q.; Shi, C. Tuning Charge Transfer Properties in Symmetric and Asymmetric Pyrrolo[3,2- b ]Pyrrole Derivatives with Hybridized Local and Charge-Transfer Characteristics. Chem. Commun. 2025, 61, 5475–5478. [Google Scholar] [CrossRef]
- Wang, X.; Shi, H.; Ma, H.; Ye, W.; Song, L.; Zan, J.; Yao, X.; Ou, X.; Yang, G.; Zhao, Z.; et al. Organic Phosphors with Bright Triplet Excitons for Efficient X-ray-Excited Luminescence. Nat. Photonics 2021, 15, 187–192. [Google Scholar] [CrossRef]
- Dong, M.; Wang, Z.; Lin, Z.; Zhang, Y.; Chen, Z.; Wu, Y.; Ma, H.; An, Z.; Gu, L.; Huang, W. Temperature-Adaptive Organic Scintillators for X-Ray Radiography. J. Am. Chem. Soc. 2025, 147, 4069–4078. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Lv, A.; Ding, M.; Song, Z.; Ma, H.; An, Z.; Huang, W. Achieving Efficient X-ray Scintillation of Purely Organic Phosphorescent Materials by Chromophore Confinement. Adv. Mater. 2024, 36, 2407916. [Google Scholar] [CrossRef]
- Luo, X.-F.; Xiao, X.; Zheng, Y.-X. Recent Progress in Multi-Resonance Thermally Activated Delayed Fluorescence Emitters with an Efficient Reverse Intersystem Crossing Process. Chem. Commun. 2024, 60, 1089–1099. [Google Scholar] [CrossRef]
- Janiga, A.; Glodkowska-Mrowka, E.; Stoklosa, T.; Gryko, D.T. Synthesis and Optical Properties of Tetraaryl-1,4-dihydropyrrolo[3,2-b ]Pyrroles. Asian J. Org. Chem. 2013, 2, 411–415. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An Sp-Hybridized All-Carboatomic Ring, Cyclo[18]Carbon: Electronic Structure, Electronic Spectrum, and Optical Nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Reimers, J.R. A Practical Method for the Use of Curvilinear Coordinates in Calculations of Normal-Mode-Projected Displacements and Duschinsky Rotation Matrices for Large Molecules. J. Chem. Phys. 2001, 115, 9103–9109. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, T.; Su, J.; Li, F.; Li, Q.; Shi, C. Tuning Excited-State Properties in Pyrrolo[3,2-b]pyrrole-Based Donor–Acceptor Emitters via Molecular Conformation and Conjugation Control. Molecules 2025, 30, 4228. https://doi.org/10.3390/molecules30214228
Gan T, Su J, Li F, Li Q, Shi C. Tuning Excited-State Properties in Pyrrolo[3,2-b]pyrrole-Based Donor–Acceptor Emitters via Molecular Conformation and Conjugation Control. Molecules. 2025; 30(21):4228. https://doi.org/10.3390/molecules30214228
Chicago/Turabian StyleGan, Taotao, Jie Su, Feiyang Li, Qiuxia Li, and Chao Shi. 2025. "Tuning Excited-State Properties in Pyrrolo[3,2-b]pyrrole-Based Donor–Acceptor Emitters via Molecular Conformation and Conjugation Control" Molecules 30, no. 21: 4228. https://doi.org/10.3390/molecules30214228
APA StyleGan, T., Su, J., Li, F., Li, Q., & Shi, C. (2025). Tuning Excited-State Properties in Pyrrolo[3,2-b]pyrrole-Based Donor–Acceptor Emitters via Molecular Conformation and Conjugation Control. Molecules, 30(21), 4228. https://doi.org/10.3390/molecules30214228






