Tri-1,3,4-Oxadiazoles Modified with Nitroimine: Balancing Energy, Sensitivity, and Thermal Stability
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
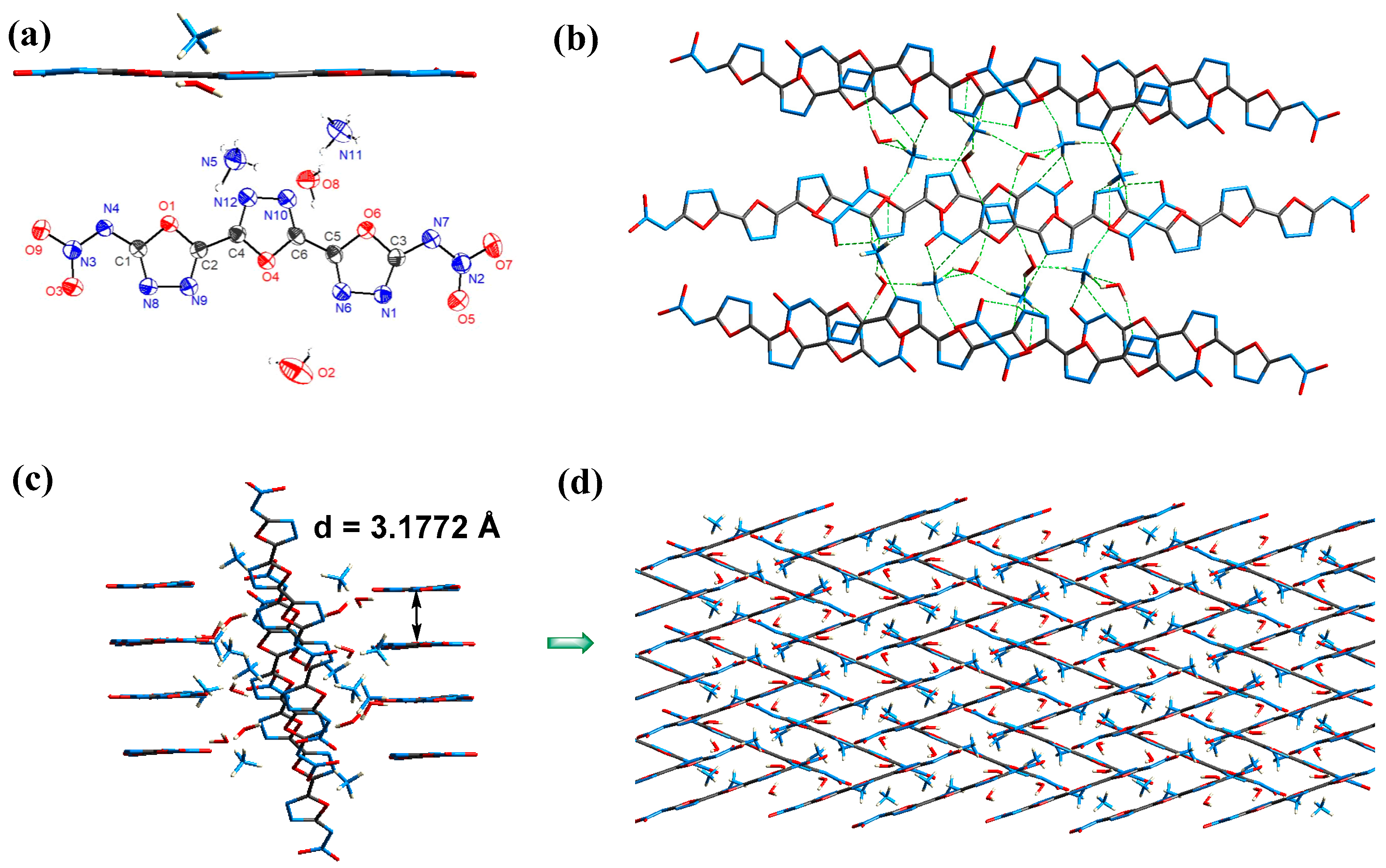
2.2. X-Ray Diffraction
2.3. Weak Interactions
2.4. Physicochemical Properties
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Experimental Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Yu, Q.; Liu, N.; Li, T.; Yi, W. Synthesis of 2-(3,5-dinitrophenyl)-5-(trinitromethyl)-1,3,4-oxadiazole. J. Org. Chem. 2024, 89, 17475–17481. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, Q.; Yi, W. Taming of triazole, tetrazole, and azido groups: Nitrogen-rich energetic materials with high thermal stabilities. ACS Appl. Mater. Interfaces 2024, 16, 63419–63426. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. 3,6-Dinitropyrazolo[4,3-c]pyrazole-based multipurpose energetic materials through versatile N-functionalization strategies. Angew. Chem. Int. Ed. 2016, 55, 12895–12897. [Google Scholar] [CrossRef]
- Yu, Q.; Singh, J.; Staples, R.J.; Shreeve, J.M. Assembling Nitrogen-rich, thermally Stable, and insensitive energetic materials by polycyclization. Chem. Eng. J. 2022, 431, 133235. [Google Scholar] [CrossRef]
- Yu, Q.; Yin, P.; Zhang, J.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Pushing the limits of oxygen balance in 1,3,4-oxadiazoles. J. Am. Chem. Soc. 2017, 139, 8816–8819. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zheng, Z.; Yi, Z.; Yi, W.; Shreeve, J.M. Exploring the maximum potential of initiating ability in metal-free primary explosives. J. Am. Chem. Soc. 2025, 147, 5125–5131. [Google Scholar] [CrossRef]
- Huo, H.; Zhang, J.; Dong, J.; Zhai, L.; Guo, T.; Wang, Z.; Bi, F.; Wang, B. A promising insensitive energetic material based on a fluorodinitromethyl explosophore group and 1,2,3,4-tetrahydro-1,3,5-triazine: Synthesis, crystal structure and performance. RSC Adv. 2020, 10, 11816–11822. [Google Scholar] [CrossRef] [PubMed]
- Kuang, B.; Wang, T.; Li, C.; Sun, M.; Tariq, Q.N.; Zhang, C.; Xie, Z.; Lu, Z.; Zhang, J. Dinitrophenyl-oxadiazole compounds: Design strategy, synthesis, and properties of a series of new melt-cast explosives. Def. Technol. 2024, 35, 100–107. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Petrutik, N.; Wang, K.; Ma, Q.; Shem-Tov, D.; Zhao, F.; Gozin, M. Molecular and crystal features of thermostable energetic materials: Guidelines for architecture of “bridged” compounds. ACS Cen. Sci. 2020, 6, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Song, S.; Yang, Z.; Qi, X.; Wang, K.; Liu, Y.; Zhang, Q.; Tian, Y. Accelerating the discovery of insensitive high-energy-density materials by a materials genome approach. Nat. Commun. 2018, 9, 2444. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; He, C.; Zhang, J.; Shreeve, J.M. Combination of 1,2,4-oxadiazole and 1,2,5-oxadiazole moieties for the generation of high-performance energetic materials. Angew. Chem. Int. Ed. 2015, 54, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J. Synthesis and characterization of diaminobisfuroxane. Eur. J. Inorg. Chem. 2014, 2014, 5808–5811. [Google Scholar] [CrossRef]
- Yu, Q.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Nitromethane bridged bis(1,3,4-oxadiazoles): Trianionic energetic salts with low sensitivities. Chem. Eur. J. 2017, 23, 17682–17686. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Deng, M.; Qi, X.; Nie, F.; Zhang, Q. A promising high-energy-density material. Nat. Commun. 2017, 8, 181. [Google Scholar] [CrossRef]
- Fischer, D.; Klapötke, T.M.; Reymann, M.; Stierstorfer, J. Dense energetic nitraminofurazanes. Chem. Eur. J. 2014, 20, 6401–6411. [Google Scholar] [CrossRef]
- Zhao, G.; He, C.; Yin, P.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Efficient construction of energetic materials via nonmetallic catalytic carbon–carbon cleavage/oxime-release-coupling reactions. J. Am. Chem. Soc. 2018, 140, 3560–3563. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J. 1,5-Di(nitramino)tetrazole: High sensitivity and superior explosive performance. Angew. Chem. Int. Ed. 2015, 54, 10299–10302. [Google Scholar] [CrossRef]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J.; Szimhardt, N. 1,1′-Nitramino-5,5′-bitetrazoles. Chem. Eur. J. 2016, 22, 4966–4970. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, J.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Taming of 3,4-di(nitramino)furazan. J. Am. Chem. Soc. 2015, 137, 15984–15987. [Google Scholar] [CrossRef]
- Fan, H.; Tang, J.; Lei, C.; Hu, W.; Yang, H.; Xiao, C.; Cheng, G. Construction of nitrogen-rich energetic isomers containing multiple hydrogen bond networks. Org. Let. 2024, 26, 8045–8050. [Google Scholar] [CrossRef]
- Yan, T.; Ma, J.; Yang, H.; Cheng, G. Introduction of energetic bis-1,2,4-triazoles bridges: A strategy towards advanced heat resistant explosives. Chem. Eng. J. 2022, 429, 132416. [Google Scholar] [CrossRef]
- Zhao, G.; Kumar, D.; Yin, P.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Construction of polynitro compounds as high-Performance oxidizers via a two-step nitration of various functional groups. Org. Let. 2019, 21, 1073–1077. [Google Scholar] [CrossRef]
- Banik, S.; Kumar, P.; Ghule, V.D.; Khanna, S.; Allimuthu, D.; Dharavath, S. Facile synthesis of nitroamino-1,3,4-oxadiazole with azo linkage: A new family of high-performance and biosafe energetic materials. J. Mater. Chem. A 2022, 10, 22803–22811. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, H.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Syntheses and promising properties of dense energetic 5,5′-dinitramino-3,3′-azo-1,2,4-oxadiazole and its salts. Angew. Chem. Int. Ed. 2016, 55, 3200–3203. [Google Scholar] [CrossRef]
- Zhang, J.; Shreeve, J.M. Nitroaminofurazans with azo and azoxy Linkages: A comparative study of structural, electronic, physicochemical, and energetic properties. J. Phys. Chem. C 2015, 119, 12887–12895. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, X.; Yang, J.; Gong, X.; Fan, G.; Huang, H. Synthesis and performance study of methylene-bridged bis(nitramino-1,2,4-oxadiazole) and its energetic salts. New J. Chem. 2019, 43, 5441–5447. [Google Scholar] [CrossRef]
- Singh, J.; Staples, R.J.; Shreeve, J.M. Balancing Energy and Stability of Nitroamino-1,2,4-oxadiazoles through a planar bridge. Org. Let. 2022, 24, 8832–8836. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chinnam, A.K.; Cheng, G.; Yang, H.; Zhang, J.; Shreeve, J.M. 1,3,4-Oxadiazole Bridges: A Strategy to Improve Energetics at the Molecular Level. Angew. Chem. Int. Ed. 2021, 60, 5497–5504. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, Z.; Ding, N.; Zhao, C.; Tian, B.; Li, S.; Pang, S. Assembly of three oxadiazole isomers toward versatile energetics. J. Mater. Chem. A 2023, 11, 23228–23232. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Tudela, D.; Glasser, L. Lattice potential energy estimation for complex ionic salts from density measurements. Inorg. Chem. 2002, 41, 2364–2367. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Mathieu, D. Sensitivity of energetic materials: Theoretical relationships to detonation performance and molecular structure. Ind. Eng. Chem. Res. 2017, 56, 8191–8201. [Google Scholar] [CrossRef]
- He, X.; Chen, C.; Zhang, Z.; Yu, T.; Wen, L.; Cao, Y.; Liu, Y. Molecule empowerment and crystal desensitization: A multilevel structure-property analysis toward designing high-energy low-sensitivity layered energetic materials. ACS Appl. Mater. Interfaces 2024, 16, 47429–47442. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, H.; Jiang, S.; Yuan, X.; Lu, G.; Lu, M.; Xu, Y. Dense hydrogen-bonded assembly of hydrogen-rich cations and pentazolate anions: A series of highly insensitive ionic salts. Molecules 2025, 30, 2613. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hariharan, P.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 247–257. [Google Scholar] [CrossRef]
- Mathieu, D. Accurate or fast prediction of solid-state formation enthalpies using standard sublimation enthalpies derived from geometrical fragments. Ind. Eng. Chem. Res. 2018, 57, 13856–13865. [Google Scholar] [CrossRef]
- Davis, J.V.; Marrs, F.W.; Cawkwell, M.J.; Manner, V.W. Machine learning models for high explosive crystal density and performance. Chem. Mater. 2024, 36, 11109–11118. [Google Scholar] [CrossRef] [PubMed]
- Sućeska, M. EXPLO5, version 6.05; Brodarski Institute: Zagreb, Croatia, 2020.
- Yadav, A.K.; Ghule, V.D.; Dharavath, S. Dianionic nitrogen-rich triazole and tetrazole-based energetic salts: Synthesis and detonation performance. Mater. Chem. Front. 2021, 5, 8352–8360. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, H.; Zhang, G.; Tang, Y.; Yang, H.; Cheng, G. An advanced primary explosive and secondary explosive based on a zwitterionic pyrazole–triazole derivative. Chem. Commun. 2022, 58, 11847–11850. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, C.; Shreeve, J.M. A furazan-fused pyrazole N-oxide via unusual cyclization. J. Mater. Chem. A 2017, 5, 4314–4319. [Google Scholar] [CrossRef]

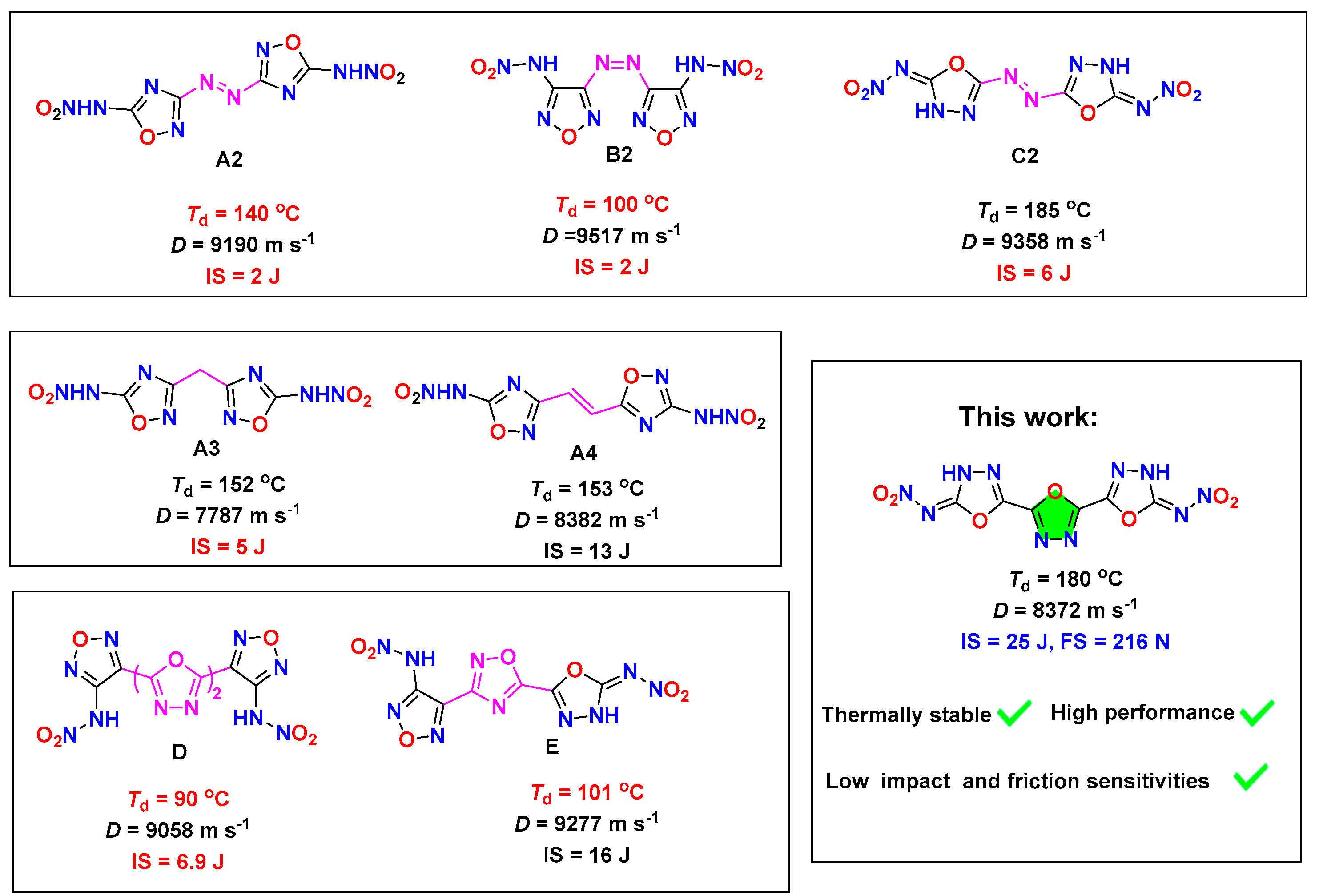



| Comp. | Td a (°C) | ρ b (g cm−3) | ΔfH c (kJ mol−1) | N d (%) | vD e (m s−1) | P f (GPa) | IS g (J) | FS h (N) | ΩCO i (%) |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 276 | 1.67 | 87.95 | 47.45 | 6548 | 18.0 | 40 | 360 | - |
| 5 | 180 | 1.854 | 218.85 | 41.95 | 8372 | 29.5 | 25 | 240 | 0 |
| 6 | 192 | 1.763 | 116.23 | 46.66 | 8076 | 24.03 | 25 | 240 | −13.3 |
| 7 | 171.2 | 1.874 | 397.90 | 50.25 | 8939 | 32.72 | 1.5 | 72 | −17.7 |
| 8 | 204 | 2.12 | −230.81 | 34.82 | 7817 | 25.64 | 25 | 240 | - |
| TNT j | 295 | 1.65 | −67.0 | 18.5 | 6881 | 19.5 | 15 | 360 | −24.7 |
| RDX k | 204 | 1.80 | 70.3 | 37.8 | 8795 | 34.9 | 7.4 | 120 | 0 |
| Pd(N3)2 l | 315 | 4.80 | 450.1 | 28.9 | 5877 | 33.4 | 0.6–4 | 0.3–0.5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Yu, Q.; Li, L.; Peng, K.; Zhu, C.; Yi, W. Tri-1,3,4-Oxadiazoles Modified with Nitroimine: Balancing Energy, Sensitivity, and Thermal Stability. Molecules 2025, 30, 4224. https://doi.org/10.3390/molecules30214224
Chen F, Yu Q, Li L, Peng K, Zhu C, Yi W. Tri-1,3,4-Oxadiazoles Modified with Nitroimine: Balancing Energy, Sensitivity, and Thermal Stability. Molecules. 2025; 30(21):4224. https://doi.org/10.3390/molecules30214224
Chicago/Turabian StyleChen, Fangming, Qiong Yu, Lei Li, Kejia Peng, Chenguang Zhu, and Wenbin Yi. 2025. "Tri-1,3,4-Oxadiazoles Modified with Nitroimine: Balancing Energy, Sensitivity, and Thermal Stability" Molecules 30, no. 21: 4224. https://doi.org/10.3390/molecules30214224
APA StyleChen, F., Yu, Q., Li, L., Peng, K., Zhu, C., & Yi, W. (2025). Tri-1,3,4-Oxadiazoles Modified with Nitroimine: Balancing Energy, Sensitivity, and Thermal Stability. Molecules, 30(21), 4224. https://doi.org/10.3390/molecules30214224






