A Novel DLLME-Based Approach for the Spectrophotometric Determination of Mercury in Environmental Samples Using the Fe(II) Phthalocyanine Sensor
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of DLLME
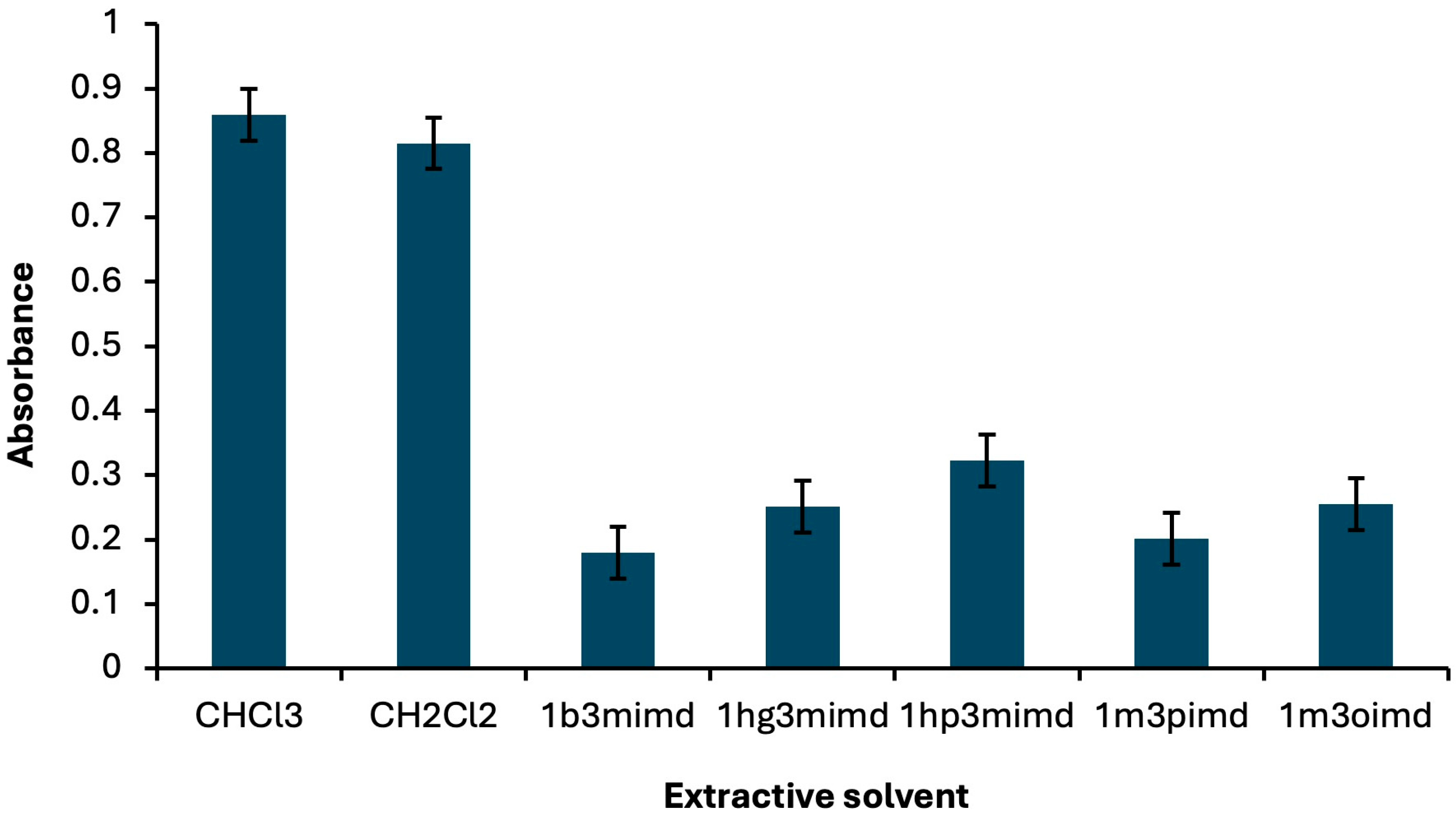
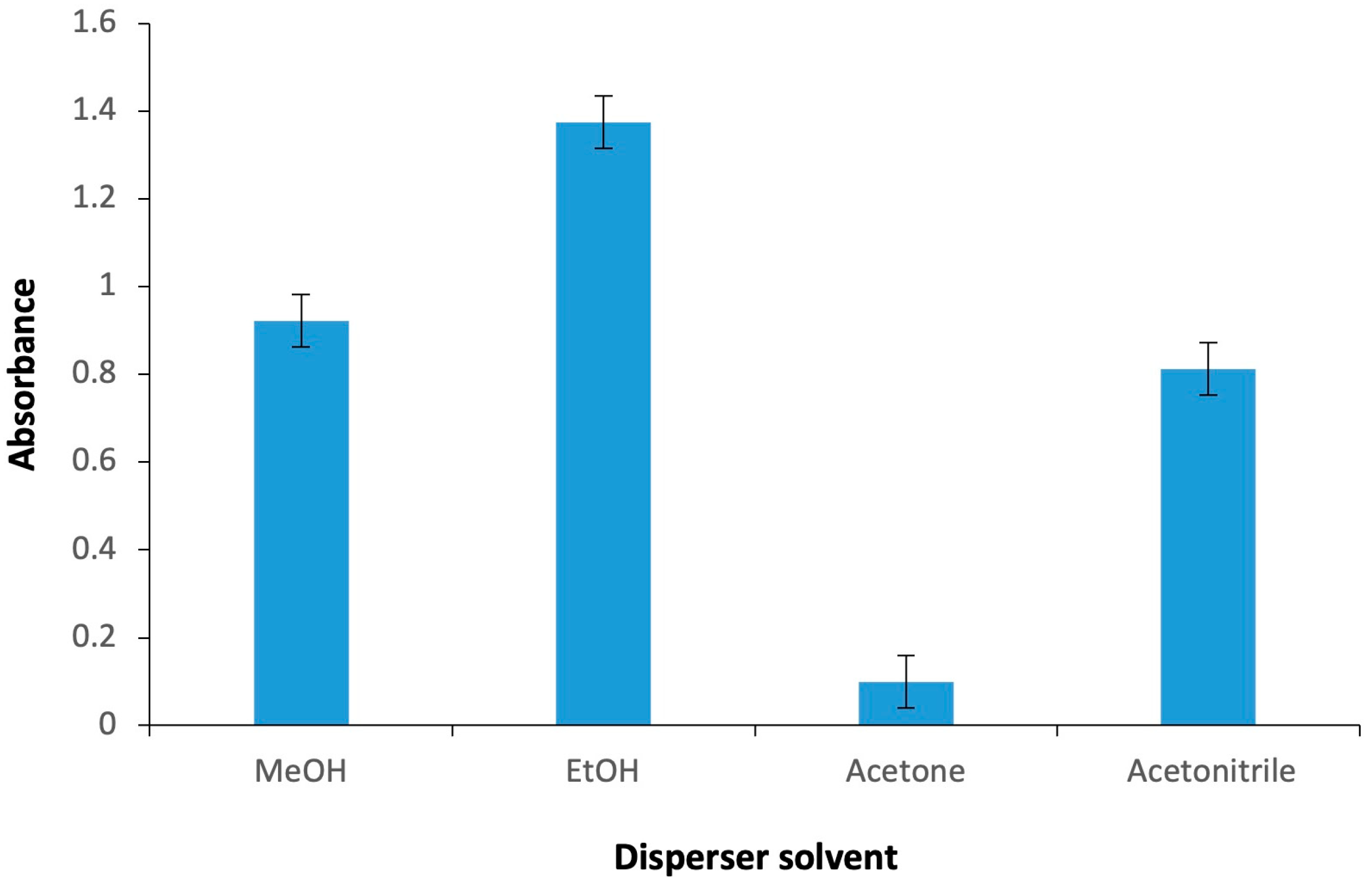
2.1.1. Kind and Volume of Extractive/Disperser Solvents
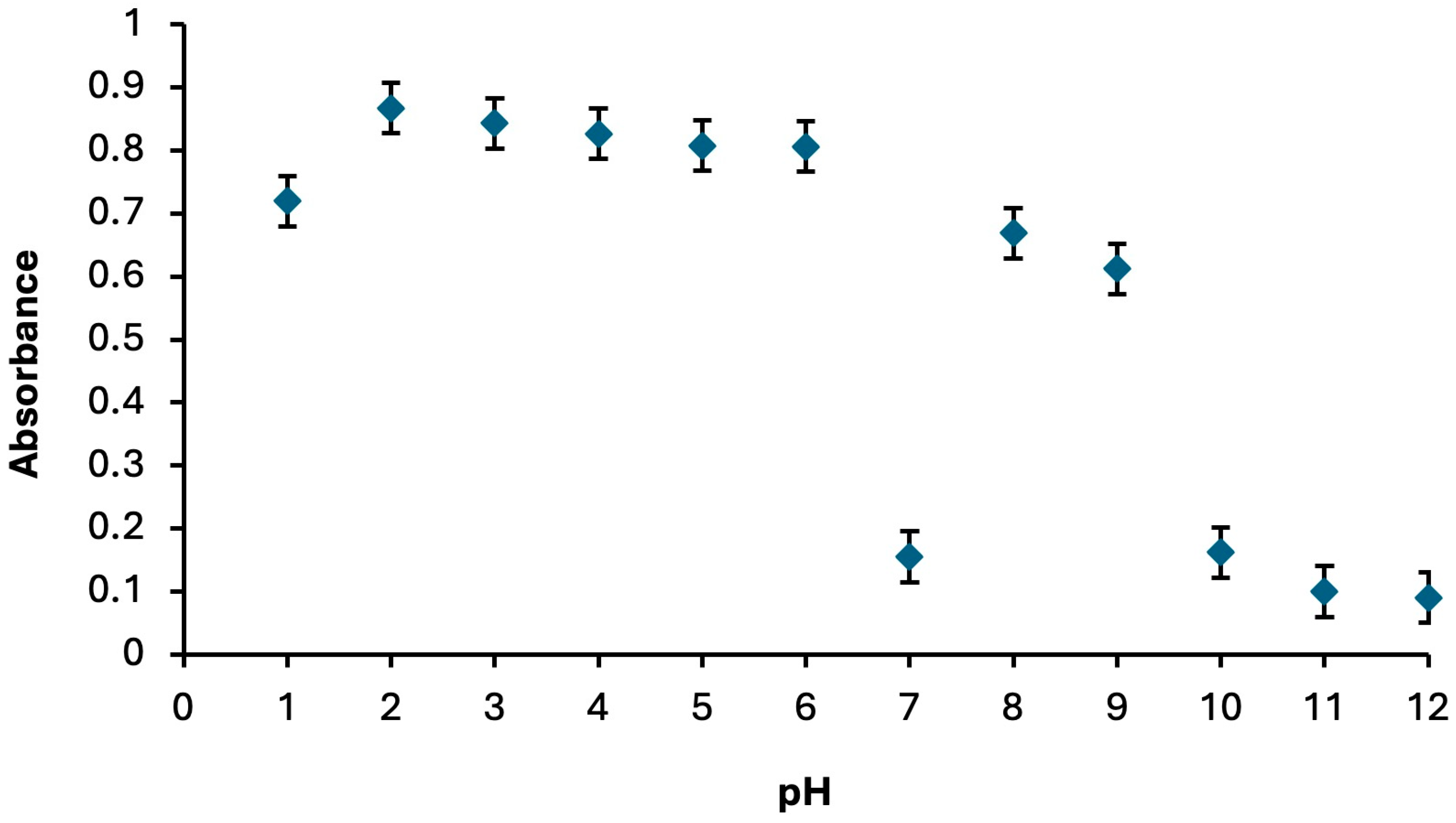
2.1.2. Effect of pH
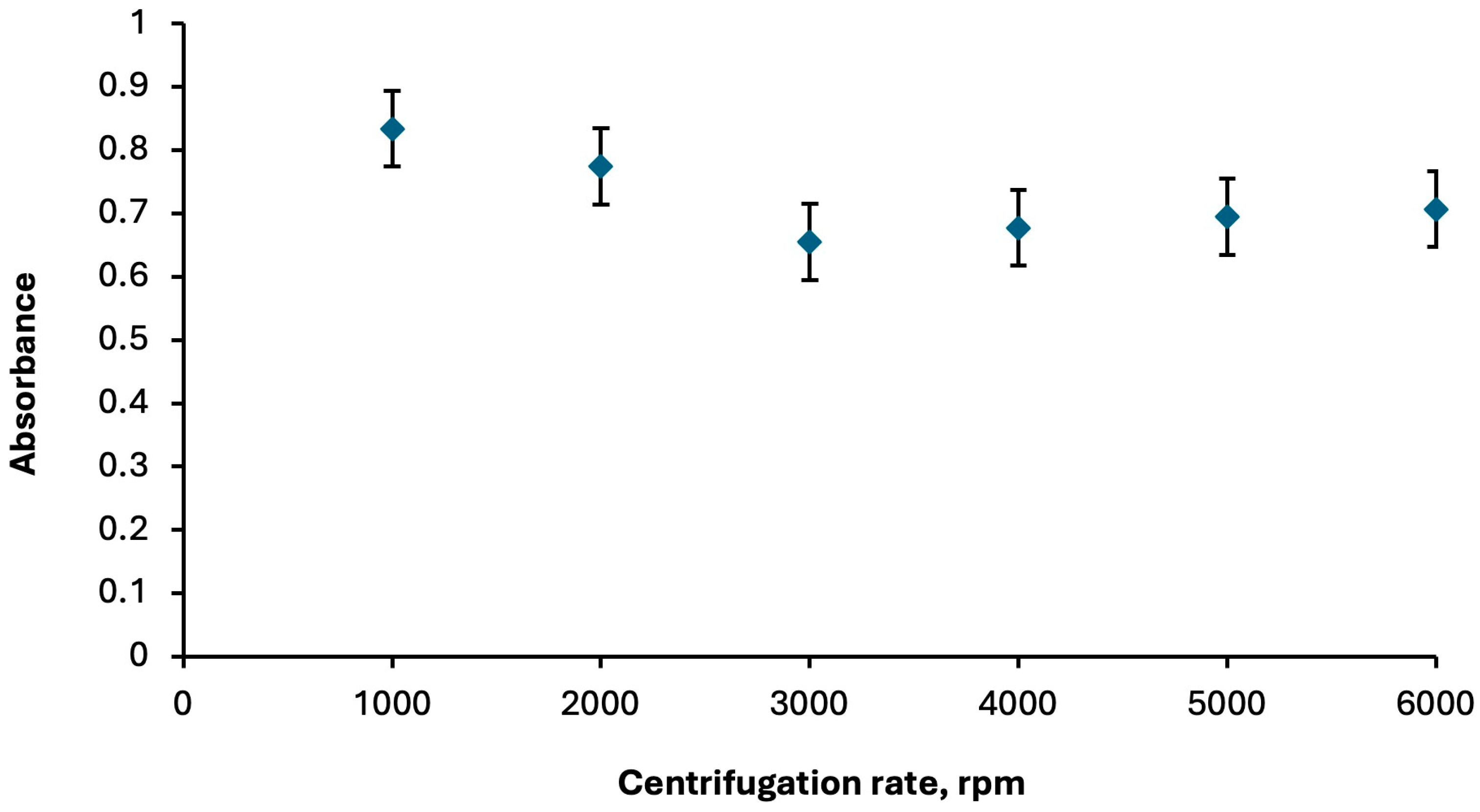
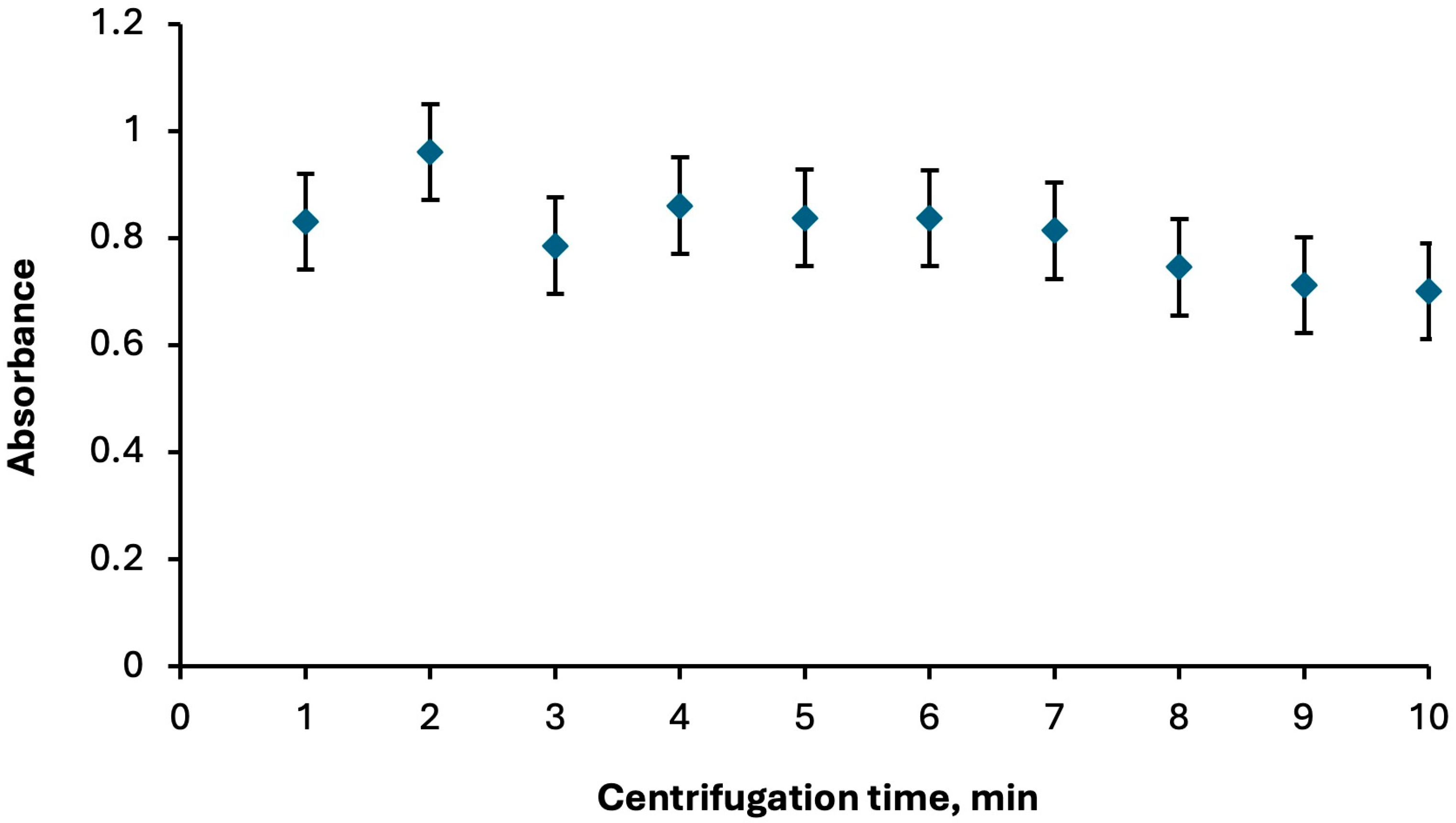
2.1.3. Role of Centrifuge Parameters
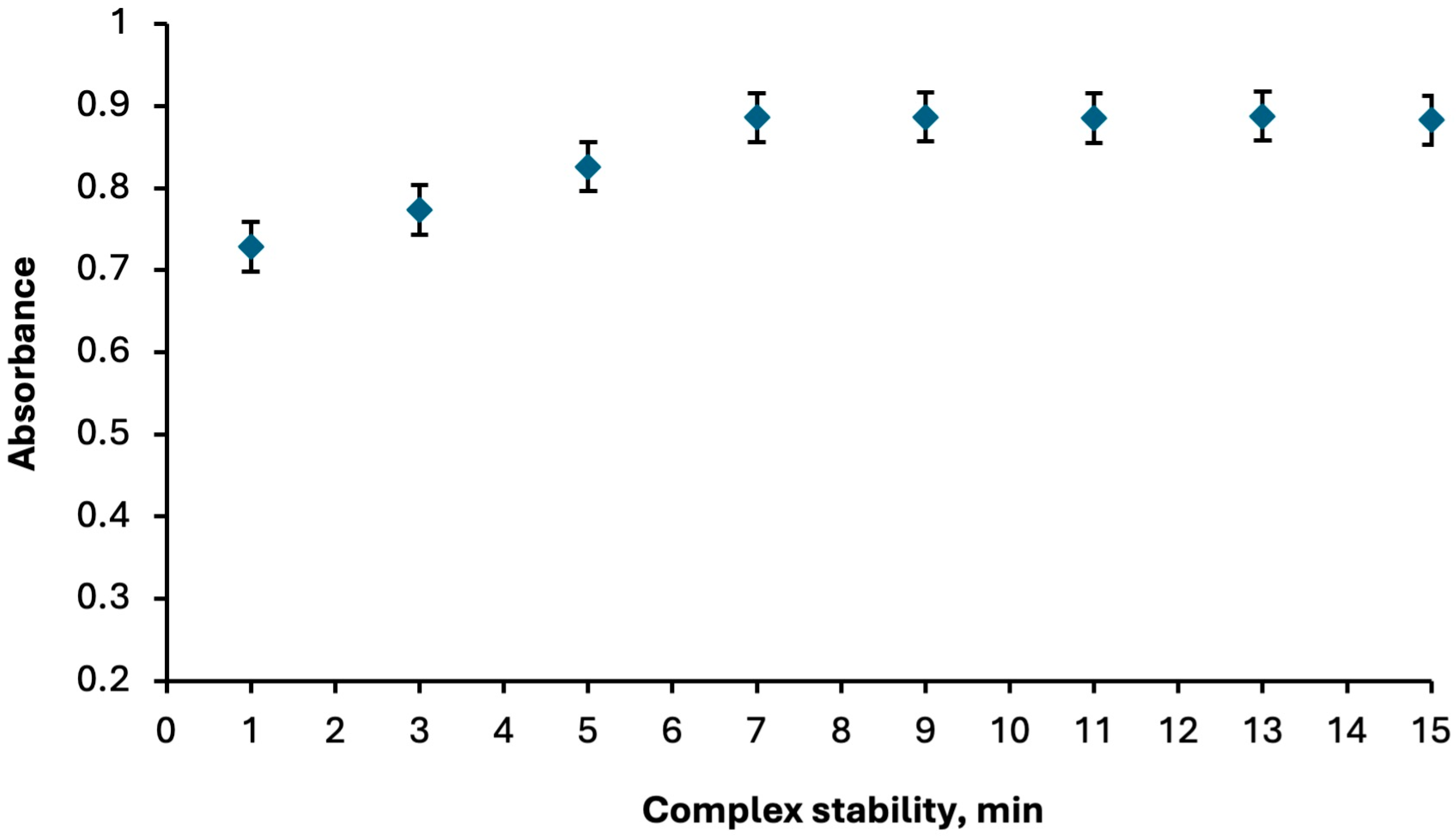
2.1.4. Compex Stability
2.1.5. Effect of Fe(II)Pc Sensor Concentration
2.2. Interferences
2.3. Analytical Figures of Merit
2.4. Comparison with Other Studies
3. Materials and Methods
3.1. Reagents and Instrumentation
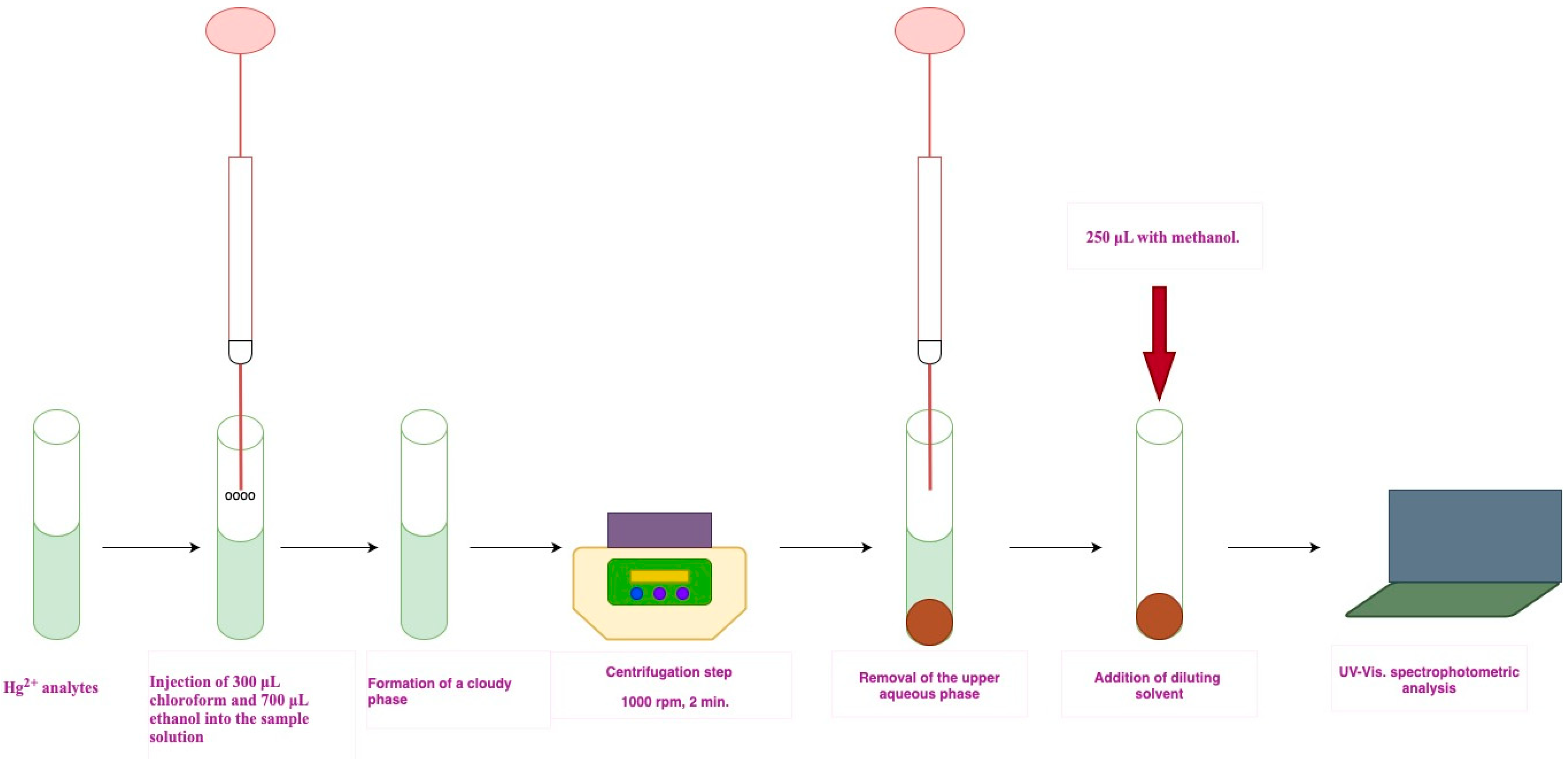
3.2. DLLME Procedure
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibb, H.; O’Leary, K.G. Mercury Exposure and Health Impacts among Individuals in the Artisanal and Small-Scale Gold Mining Community: A Comprehensive Review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, X. Speciation Analysis of Mercury in Water Samples Using Dispersive Liquid-Liquid Microextraction Combined with High-Performance Liquid Chromatography. Anal. Chim. Acta 2011, 702, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Mordukhovich, I.; Wright, R.O.; Hu, H.; Amarasiriwardena, C.; Baccarelli, A.; Litonjua, A.; Sparrow, D.; Vokonas, P.; Schwartz, J. Associations of Toenail Arsenic, Cadmium, Mercury, Manganese, and Lead with Blood Pressure in the Normative Aging Study. Environ. Health Perspect. 2012, 120, 98–104. [Google Scholar] [CrossRef]
- Nemati, I.; Faraji, M.; Jafarinejad, S.; Shirani, M. Development of a Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction Coupled with Spectrophotometer Technique for Determination of Trace Amount of Hg(II) in Water Samples. Chem. Pap. 2023, 77, 909–919. [Google Scholar] [CrossRef]
- Houston, M.C. Role of Mercury Toxicity in Hypertension, Cardiovascular Disease, and Stroke. J. Clin. Hypertens 2011, 13, 621–627. [Google Scholar] [CrossRef]
- Al-Batanony, M.A.; Abdel-Rasul, G.M.; Ma, A.-S.; Al-Dalatony, M.M.; Allam, H.K.; Al-Batanony, M.A. Occupational Exposure to Mercury among Workers in a Fluorescent Lamp Factory, Quisna Industrial Zone, Egypt. Int. J. Occup. Environ. Med. 2013, 4, 149–156. [Google Scholar]
- Farina, M.; Avila, D.S.; Da Rocha, J.B.T.; Aschner, M. Metals, Oxidative Stress and Neurodegeneration: A Focus on Iron, Manganese and Mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef]
- Björkman, L.; Lundekvam, B.F.; Lægreid, T.; Bertelsen, B.I.; Morild, I.; Lilleng, P.; Lind, B.; Palm, B.; Vahter, M. Mercury in Human Brain, Blood, Muscle and Toenails in Relation to Exposure: An Autopsy Study. Environ. Health 2007, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Arantes, F.P.; Savassi, L.A.; Santos, H.B.; Gomes, M.V.T.; Bazzoli, N. Bioaccumulation of Mercury, Cadmium, Zinc, Chromium, and Lead in Muscle, Liver, and Spleen Tissues of a Large Commercially Valuable Catfish Species from Brazil. An. Acad. Bras Cienc. 2016, 88, 137–147. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Yuan, G.; He, X.; Xie, P. Mercury Bioaccumulation in the Food Web of Three Gorges Reservoir (China): Tempo-Spatial Patterns and Effect of Reservoir Management. Sci. Total Environ. 2015, 527–528, 203–210. [Google Scholar] [CrossRef]
- Qu, P.; Pang, M.; Wang, P.; Ma, X.; Zhang, Z.; Wang, Z.; Gong, Y. Bioaccumulation of Mercury along Continuous Fauna Trophic Levels in the Yellow River Estuary and Adjacent Sea Indicated by Nitrogen Stable Isotopes. J. Hazard. Mater. 2022, 432, 128631. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Environmental Exposure to Mercury and Its Toxicopathologic Implications for Public Health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Zulaikhah, S.T.; Wahyuwibowo, J.; Pratama, A.A. Mercury and Its Effect on Human Health: A Review of the Literature. Int. J. Public Health Sci. 2020, 9, 103–114. [Google Scholar] [CrossRef]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low Dose Mercury Toxicity and Human Health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. Human Exposure to Mercury: The Three Modern Dilemmas. J. Trace Elements Exp. Med. 2003, 16, 321–343. [Google Scholar] [CrossRef]
- Burgess, N.M.; Meyer, M.W. Methylmercury Exposure Associated with Reduced Productivity in Common Loons. Ecotoxicology 2007, 17, 83–91. [Google Scholar] [CrossRef]
- Carrasco, L.; Barata, C.; García-Berthou, E.; Tobias, A.; Bayona, J.M.; Díez, S. Patterns of Mercury and Methylmercury Bioaccumulation in Fish Species Downstream of a Long-Term Mercury-Contaminated Site in the Lower Ebro River (NE Spain). Chemosphere 2011, 84, 1642–1649. [Google Scholar] [CrossRef]
- Mei, B.; Fong, W.; Siu, S.; Sai, J.; Tee, K.; Tam, S. Determination of Mercury in Whole Blood and Urine by Inductively Coupled Plasma Mass Spectrometry. J. Anal. Toxicol. 2007, 31, 281–287. [Google Scholar] [CrossRef]
- Teng, H.; Altaf, A.R. Elemental Mercury (Hg0) Emission, Hazards, and Control: A Brief Review. J. Hazard. Mater. Adv. 2022, 5, 100049. [Google Scholar] [CrossRef]
- Natasha; Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Khan Niazi, N.; Dumat, C. A Critical Review of Mercury Speciation, Bioavailability, Toxicity and Detoxification in Soil-Plant Environment: Ecotoxicology and Health Risk Assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. A Review on the Distribution of Hg in the Environment and Its Human Health Impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Bidari, A.; Ganjali, M.R.; Assadi, Y.; Kiani, A.; Norouzi, P. Assay of Total Mercury in Commercial Food Supplements of Marine Origin by Means of DLLME/ICP-AES. Food Anal. Methods 2012, 5, 695–701. [Google Scholar] [CrossRef]
- Pinheiro, F.C.; Aguirre, M.Á.; Nóbrega, J.A.; Canals, A. Dispersive Liquid-Liquid Microextraction of Cd, Hg and Pb from Medicines Prior to ICP OES Determination According to the United States Pharmacopeia. Anal. Methods 2021, 13, 5670–5678. [Google Scholar] [CrossRef]
- Liang, P.; Yu, J.; Yang, E.; Mo, Y. Determination of Mercury in Food and Water Samples by Displacement-Dispersive Liquid-Liquid Microextraction Coupled with Graphite Furnace Atomic Absorption Spectrometry. Food Anal. Methods 2015, 8, 236–242. [Google Scholar] [CrossRef]
- Faraji, M. Novel Hydrophobic Deep Eutectic Solvent for Vortex Assisted Dispersive Liquid-Liquid Micro-Extraction of Two Auxins in Water and Fruit Juice Samples and Determination by High Performance Liquid Chromatography. Microchem. J. 2019, 150, 104130. [Google Scholar] [CrossRef]
- Bagheri, H.; Naderi, M. Immersed Single-Drop Microextraction-Electrothermal Vaporization Atomic Absorption Spectroscopy for the Trace Determination of Mercury in Water Samples. J. Hazard. Mater. 2009, 165, 353–358. [Google Scholar] [CrossRef]
- Martinis, E.M.; Wuilloud, R.G. Cold Vapor Ionic Liquid-Assisted Headspace Single-Drop Microextraction: A Novel Preconcentration Technique for Mercury Species Determination in Complex Matrix Samples. J. Anal. At. Spectrom. 2010, 25, 1432–1439. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Y.; Guo, X.; He, M.; Hu, B. Speciation of Mercury in Various Samples from the Micro-Ecosystem of East Lake by Hollow Fiber-Liquid-Liquid-Liquid Microextraction-HPLC-ICP-MS. J. Anal. Spectrom. 2015, 30, 875–881. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Ioannou, K.I.G. Recent Developments in Homogeneous and Dispersive Liquid-Liquid Extraction for Inorganic Elements Determination. A Review. Talanta 2009, 80, 413–421. [Google Scholar] [CrossRef]
- Dadfarnia, S.; Haji Shabani, A.M. Recent Development in Liquid Phase Microextraction for Determination of Trace Level Concentration of Metals-A Review. Anal. Chim. Acta 2010, 658, 107–119. [Google Scholar] [CrossRef]
- Kamal El-Deen, A.; Elmansi, H.; Belal, F.; Magdy, G. Recent Advances in Dispersion Strategies for Dispersive Liquid–Liquid Microextraction from Green Chemistry Perspectives. Microchem. J. 2023, 191, 108807. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Apyari, V.V.; Tolmacheva, V.V.; Gorbunova, M.V. Dispersive Liquid–Liquid Microextraction of Organic Compounds: An Overview of Reviews. J. Anal. Chem. 2020, 75, 1237–1251. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Deep Eutectic Solvents as Promising Green Solvents in Dispersive Liquid–Liquid Microextraction Based on Solidification of Floating Organic Droplet: Recent Applications, Challenges and Future Perspectives. Molecules 2021, 26, 7406. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of Organic Compounds in Water Using Dispersive Liquid-Liquid Microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Fernández, I.; González-Martín, R.; e Silva, F.A.; Freire, M.G.; Pino, V. Insights into Coacervative and Dispersive Liquid-Phase Microextraction Strategies with Hydrophilic Media—A Review. Anal. Chim. Acta 2021, 1143, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Magqira, B.; Mahlambi, P. Miniaturized Green Liquid-Phase Sample Pre-Concentration Methods for Pharmaceutical Drugs Assessment in Wastewater. Water Environ. J. 2025, 39, 143–167. [Google Scholar] [CrossRef]
- Andruch, V.; Balogh, I.S.; Kocúrová, L.; Šandrejová, J. Five Years of Dispersive Liquid-Liquid Microextraction. Appl. Spectrosc. Rev. 2013, 48, 161–259. [Google Scholar] [CrossRef]
- Leong, M.I.; Fuh, M.R.; Huang, S. Da Beyond Dispersive Liquid-Liquid Microextraction. J. Chromatogr. A 2014, 1335, 2–14. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Emara, A.A.A. The Recent Developments in Dispersive Liquid-Liquid Microextraction for Preconcentration and Determination of Inorganic Analytes. J. Saudi Chem. Soc. 2014, 18, 745–761. [Google Scholar] [CrossRef]
- Rezaee, M.; Yamini, Y.; Faraji, M. Evolution of Dispersive Liquid-Liquid Microextraction Method. J. Chromatogr. A 2010, 1217, 2342–2357. [Google Scholar] [CrossRef]
- Fernández, E.; Vidal, L.; Martín-Yerga, D.; Blanco, M.D.C.; Canals, A.; Costa-García, A. Screen-Printed Electrode Based Electrochemical Detector Coupled with Ionic Liquid Dispersive Liquid-Liquid Microextraction and Microvolume Back-Extraction for Determination of Mercury in Water Samples. Talanta 2015, 135, 34–40. [Google Scholar] [CrossRef]
- Shirkhanloo, H.; Golbabaei, F.; Hassani, H.; Eftekhar, F.; Kian, M.J. Occupational Exposure to Mercury: Air Exposure Assessment and Biological Monitoring Based on Dispersive Ionic Liquid-Liquid Microextraction. Iran. J. Public Health 2014, 43, 793. [Google Scholar]
- Kiwanuka, M.J.; Nomngongo, P.N.; Mketo, N. Rapid and Greener Vortex-Assisted Deep Eutectic Solvent-Based Dispersive Liquid-Liquid Microextraction for Spectroscopic Determination of Hg in Fuels. Green Anal. Chem. 2025, 12, 100236. [Google Scholar] [CrossRef]
- Azhdari, N.; Ayazi, Z.; Habibi, B.; Rahimpour, E. Enhanced Mercury Detection through Ultrasound-Assisted Liquid–Liquid Microextraction and Digital Image Colorimetry Using Hydrophobic Deep Eutectic Solvents. Int. J. Environ. Sci. Technol. 2025, 22, 10067–10082. [Google Scholar] [CrossRef]
- Khan, M.; Soylak, M. Deep Eutectic Solvent Based Liquid-Liquid Microextraction of Mercury in Water, Hair and Fish with Spectrophotometric Determination: A Green Protocol. Anal. Lett. 2023, 56, 1161–1173. [Google Scholar] [CrossRef]
- Conchado-Amado, P.; Sánchez-Piñero, J.; Moreda-Piñeiro, J.; Turnes-Carou, I.; López-Mahía, P.; Muniategui-Lorenzo, S. Ultra-Trace Mercury Determination in Seawater after Vortex-Assisted Liquid-Liquid Micro-Extraction. Spectrochim. Acta Part B At. Spectrosc. 2023, 204, 106683. [Google Scholar] [CrossRef]
- Alzahrani, L.; El-Ghamry, H.A.; Saber, A.L.; Mohammed, G.I. Spectrophotometric Determination of Mercury(II) Ions in Laboratory and Zamzam Water Using Bis Schiff Base Ligand Based on 1,2,4-Triazole-3,5-Diamine and o-Vaniline. Arab. J. Chem. 2023, 16, 104418. [Google Scholar] [CrossRef]
- Liu, Y.M.; Zhang, F.P.; Jiao, B.Y.; Rao, J.Y.; Leng, G. Automated Dispersive Liquid-Liquid Microextraction Coupled to High Performance Liquid Chromatography—Cold Vapour Atomic Fluorescence Spectroscopy for the Determination of Mercury Species in Natural Water Samples. J. Chromatogr. A 2017, 1493, 1–9. [Google Scholar] [CrossRef]
- Leao, D.J.; Silva Junior, M.M.; Silva Junior, J.B.; De Oliveira, D.A.F.; Queiroz, A.F.S.; Ferreira, S.L.C. Ultrasound Assisted Extraction for the Determination of Mercury in Sediment Samples Employing Cold Vapour Atomic Absorption Spectrometry. Anal. Methods 2016, 8, 6554–6559. [Google Scholar] [CrossRef]
- Ma, S.; He, M.; Chen, B.; Deng, W.; Zheng, Q.; Hu, B. Magnetic Solid Phase Extraction Coupled with Inductively Coupled Plasma Mass Spectrometry for the Speciation of Mercury in Environmental Water and Human Hair Samples. Talanta 2016, 146, 93–99. [Google Scholar] [CrossRef]
- Rofouei, M.K.; Rezaei, A.; Masteri-Farahani, M.; Khani, H. Selective Extraction and Preconcentration of Ultra-Trace Level of Mercury Ions in Water and Fish Samples Using Fe 3O 4-Magnetite- Nanoparticles Functionalized by Triazene Compound Prior to Its Determination by Inductively Coupled Plasma-Optical Emission Spectrometry. Anal. Methods 2012, 4, 959–966. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Kiselev, A.N.; Isagulieva, A.K.; Shibaeva, A.V.; Kuzmin, V.A.; Morozov, V.N.; Zevakin, E.A.; Petrova, U.A.; Knyazeva, A.A.; Eroshin, A.V.; et al. Shedding Light on Heavy Metal Contamination: Fluorescein-Based Chemosensor for Selective Detection of Hg2+ in Water. Int. J. Mol. Sci. 2024, 25, 3186. [Google Scholar] [CrossRef]
- Sunitha, P.; Gowda, B.G.; Singh, P.; Vengatesh, G.; Varadaraju, K.; Abass, K.S.; Stupin, V.; Silina, E.; Kollur, S.P. Synthesis of Thiophene Derived Fluorescent Sensor for Hg2+ Ion Detection and Its Applications in Cell Imaging, Latent Fingerprint, and Real Water Analysis. Sci. Rep. 2025, 15, 33207. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Gamov, G.A. Fluorescein Acetone Hydrazone: A Highly Sensitive Probe for Hg2+ Ion Detection and Computational Insights. J. Mol. Struct. 2025, 1348, 1–24. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Sun, M.; Liu, S.; Liu, H.; Pu, X.; Meng, F. Development of a Highly Selective Near-Infrared Fluorescent Probe for Sensitive Detection of Hg2+ in Environmental and Biological Samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2026, 345, 126784. [Google Scholar] [CrossRef]
- Ripoll, L.; Rayos, J.; Aguirre, M.Á.; Vidal, L.; Canals, A. Natural Deep Eutectic Solvent-Based Microextraction for Mercury Speciation in Water Samples. Anal. Bioanal. Chem. 2023, 415, 4435–4444. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Ahmed, H.E.H.; Khan, M. Switchable Hydrophilicity Solvent Based Microextraction of Mercury from Water, Fish and Hair Samples before Its Spectrophotometric Detection. Sustain. Chem. Pharm. 2023, 32, 101006. [Google Scholar] [CrossRef]
- Hayati, M.; Ramezani, M.; Rezanejade Bardajee, G.; Momeni Isfahani, T. Application of Robust Syringe-to-Syringe Dispersive Liquid-Phase Microextraction Method for Preconcentration and Determination of Mercury with the Aid of an Experimental Design. Sep. Sci. Technol. 2022, 57, 274–283. [Google Scholar] [CrossRef]
- El-Feky, H.H.; Mohammed, T.Y.; Amin, A.S.; Kassem, M.A. Rapid Extraction and Separation of Mercury in Water and Food Samples Based on Micelles and Azo-Thiazoles Complexation before Determination by UV-Vis Spectrophotometry. Anal. Methods Environ. Chem. J. 2023, 6, 19–36. [Google Scholar] [CrossRef]
- Liu, X.; Qi, C.; Bing, T.; Cheng, X.; Shangguan, D. Highly Selective Phthalocyanine-Thymine Conjugate Sensor for Hg2+ Based on Target Induced Aggregation. Anal. Chem. 2009, 81, 3699–3704. [Google Scholar] [CrossRef]
- Claessens, C.G.; Hahn, U.; Torres, T. Phthalocyanines: From Outstanding Electronic Properties to Emerging Applications. Chem. Rec. 2008, 8, 75–97. [Google Scholar] [CrossRef]
- Grau, J.; Azorín, C.; Benedé, J.L.; Chisvert, A.; Salvador, A. Use of Green Alternative Solvents in Dispersive Liquid-Liquid Microextraction: A Review. J. Sep. Sci. 2022, 45, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Kokosa, J.M. Selecting an Extraction Solvent for a Greener Liquid Phase Microextraction (LPME) Mode-Based Analytical Method. TrAC Trends Anal. Chem. 2019, 118, 238–247. [Google Scholar] [CrossRef]
- Karimi, M.; Sereshti, H.; Samadi, S.; Parastar, H. Optimization of Dispersive Liquid-Liquid Microextraction and Improvement of Detection Limit of Methyl Tert-Butyl Ether in Water with the Aid of Chemometrics. J. Chromatogr. A 2010, 1217, 7017–7023. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Karimi, M.; Samadi, S. Application of Response Surface Method for Optimization of Dispersive Liquid-Liquid Microextraction of Water-Soluble Components of Rosa Damascena Mill. Essential Oil. J. Chromatogr. A 2009, 1216, 198–204. [Google Scholar] [CrossRef]
- Fattahi, N.; Samadi, S.; Assadi, Y.; Hosseini, M.R.M. Solid-Phase Extraction Combined with Dispersive Liquid-Liquid Microextraction-Ultra Preconcentration of Chlorophenols in Aqueous Samples. J. Chromatogr. A 2007, 1169, 63–69. [Google Scholar] [CrossRef]
- Notardonato, I.; Avino, P. Dispersive Liquid–Liquid Micro Extraction: An Analytical Technique Undergoing Continuous Evolution and Development—A Review of the Last 5 Years. Separations 2024, 11, 203. [Google Scholar] [CrossRef]
- Laosuwan, M.; Mukdasai, S.; Srijaranai, S. A Simple in Syringe Low Density Solvent-Dispersive Liquid Liquid Microextraction for Enrichment of Some Metal Ions Prior to Their Determination by High Performance Liquid Chromatography in Food Samples. Molecules 2020, 25, 552. [Google Scholar] [CrossRef]
- Gharehbaghi, M.; Shemirani, F.; Baghdadi, M. Dispersive Liquid-Liquid Microextraction Based on Ionic Liquid and Spectrophotometric Determination of Mercury in Water Samples. Int. J. Environ. Anal. Chem. 2009, 89, 21–33. [Google Scholar] [CrossRef]
- Hossien-poor-Zaryabi, M.; Chamsaz, M.; Heidari, T.; Zavar, M.H.A.; Behbahani, M.; Salarian, M. Application of Dispersive Liquid-Liquid Micro-Extraction Using Mean Centering of Ratio Spectra Method for Trace Determination of Mercury in Food and Environmental Samples. Food Anal. Methods 2014, 7, 352–359. [Google Scholar] [CrossRef]
- Çağlar, Y.; Biyiklioglu, Z. Spectrophotometric Determination of Hg(II) in Water Samples by Dispersive Liquid Liquid Microextraction with Use Ionic Liquid after Derivatization with a Water Soluble Fe(II) Phthalocyanine. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 331–339. [Google Scholar] [CrossRef]
- Erzunov, D.; Tonkova, S.; Belikova, A.; Vashurin, A. Enhanced Visible Light Absorption and Photophysical Features of Novel Isomeric Magnesium Phthalocyaninates with Cyanophenoxy Substitution. Chemosensors 2022, 10, 503. [Google Scholar] [CrossRef]
- Sun, Q.Z.; Zhao, B.; Chai, L.Y.; Liu, H.; Jin, H.Z.; Liu, H. A 3D Nickel(II) Coordination Polymer Constructed by Mixed- Ligand Strategy: Synthesis, Crystal Structure and Sensing of Hg(II) Ion. Inorg. Nano-Met. Chem. 2022, 52, 711–717. [Google Scholar] [CrossRef]
- Çaǧlar, Y.; Gümrükçüoǧlu, N.; Saka, E.T.; Ocak, M.; Kantekin, H.; Ocak, Ü. Phthalocyanine-Based Fluorescent Chemosensor for the Sensing of Zn (II) in Dimethyl Sulfoxide-Acetonitrile. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 443–447. [Google Scholar] [CrossRef]
| Foreign Ion Added | Tolerance Limit (µg/L) |
|---|---|
| Ag+ | 132 |
| Cu2+ | 119 |
| Cr3+ | 158 |
| Co2+ | 94 |
| Pb2+ | 51 |
| Ni2+ | 51 |
| Cd2+ | 299 |
| Mn2+ | 106 |
| Zn2+ | 427 |
| NO3− | 556 |
| ClO4− | 435 |
| SO42− | 214 |
| PO43− | 278 |
| H2PO4− | 223 |
| Parameters | |
|---|---|
| Linear range, µg/L | 1.00–20.00 |
| Correlation coefficient (R2) | 0.9991 |
| LOD, µg/L | 1.44 |
| LOQ, µg/L | 4.80 |
| Enhancement factor, | 105 |
| RSD, % (n = 6) | 1.27–2.68 a and 1.09–3.01 b |
| Sample | Hg2+ Amount µg/L (n:3) | ||
|---|---|---|---|
| Added | Finded ± SD | Recovery % | |
| Black Sea | 8.00 | 7.49 ± 0.11 | 94.00 |
| Tap water | 8.00 | 7.92 ± 0.04 | 99.00 |
| Sera Lake | 8.00 | 8.17 ± 0.13 | 102.12 |
| Extraction Method | Linear Range (µg/L) | LOD (µg/L) | Enrichment Factor | RSD (%) | Reference |
|---|---|---|---|---|---|
| DLLME | 1.00–100 | 0.30 | 200 | 2.80–7.50 | [5] |
| IL-DLLME | 12.00–140 | 3.90 | 18.80 | 1.70 | [70] |
| DLLME a | 10.00–200 | 3.00 | - | 10.00–12.00 a | [57] |
| DLLME | 0.50–100 | 0.15 | 39 | 2.60 | [71] |
| IL-DLLME | 0.050–1.00 b | 0.010 b | 128 | 0.78 | [72] |
| DLLME | 1.00–20 | 1.44 | 105 | 1.27–2.68 c and 1.09–3.01 d | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çağlar, Y. A Novel DLLME-Based Approach for the Spectrophotometric Determination of Mercury in Environmental Samples Using the Fe(II) Phthalocyanine Sensor. Molecules 2025, 30, 4192. https://doi.org/10.3390/molecules30214192
Çağlar Y. A Novel DLLME-Based Approach for the Spectrophotometric Determination of Mercury in Environmental Samples Using the Fe(II) Phthalocyanine Sensor. Molecules. 2025; 30(21):4192. https://doi.org/10.3390/molecules30214192
Chicago/Turabian StyleÇağlar, Yasemin. 2025. "A Novel DLLME-Based Approach for the Spectrophotometric Determination of Mercury in Environmental Samples Using the Fe(II) Phthalocyanine Sensor" Molecules 30, no. 21: 4192. https://doi.org/10.3390/molecules30214192
APA StyleÇağlar, Y. (2025). A Novel DLLME-Based Approach for the Spectrophotometric Determination of Mercury in Environmental Samples Using the Fe(II) Phthalocyanine Sensor. Molecules, 30(21), 4192. https://doi.org/10.3390/molecules30214192






