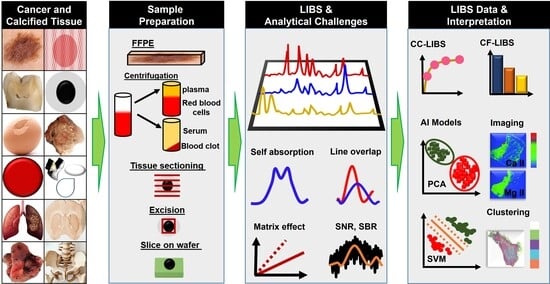
From Fundamentals of Laser-Induced Breakdown Spectroscopy to Recent Advancements in Cancer Detection and Calcified Tissues Analysis: An Overview (2015–2025)
Abstract
1. Introduction
2. Fundamentals of LIBS and Elemental Composition of Human Body
2.1. Matrix Effect
2.2. LIBS Signal Enhancement Strategies
2.3. Artificial Intelligence in LIBS
3. Implementation of LIBS in Oncology
3.1. Skin Cancer
3.2. Breast Cancer
3.3. Colon Cancer
3.4. Stomach Cancer
3.5. Lung Cancer
3.6. Cancer Studies in Animal Samples
3.7. Miscellaneous Cancer Studies
4. LIBS in Calcified Tissues Analysis
4.1. Evolution of LIBS in Dentistry
| Tissues | Ref. | Purpose/Application | Method | Findings | Comments |
|---|---|---|---|---|---|
| Teeth | [221] | Imaging of teeth using long-pulse laser to evaluate LIBS performance/dental anatomy | LIBS elemental imaging | Visualise variation in elemental distribution from enamel to dentine | Limited to major elements, alternative needs to be explored for trace elements |
| [106] | Migration of species from dental restorative materials to tooth matrix/clinical applications (pathological identifications) | Measuring line intensities | One source of trace elemental accumulation in tooth matrix is dental filling materials. | Hg in healthy tissues not detected, LOD higher than concentration (0.10 μg g−1) | |
| [124] | Elemental quantification of teeth using appropriate calibration method/pre-clinical dentistry | CC-LIBS | Quantification of Ca, Mg, C, and Zn, error rate measured for different methods | Considerable error range for LIBS with respect to standard techniques (ICP-MS, EDX); inaccuracies due to matrix effect | |
| [130] | Effect of NPs deposited on teeth on LIBS performance/nano-dentistry | CC-LIBS | Signal enhancement and improved calibration for Ca with ZnO NP | Control of size, shape, and distribution of NPs difficult; reproducibility is of concern | |
| [147] | Sex identification/odontology | LIBS-ANN | Ca, Mg, P, Sr, and H used for classification; accuracy for males 98%, for females 99% | Small sample size (n = 10) and limited number of spectra (N = 500) are insufficient for definitive conclusion | |
| [201] | Classification of teeth according to age and sex for healthy and carious teeth/pre-clinical dentistry | Intensity ratio of spectral lines | High concentration of Mg and Pb in carious teeth; trace elements: intensity decreases with age, female teeth have higher intensity | Small sample size (n = 8); no statistical, cross, and clinical validation performed | |
| [205] | Effect of laser wavelength and irradiance on plasma parameters/pre-clinical dentistry | Boltzmann and Saha–Boltzmann plot methods | High plasma temperature at 1064 nm; plasma frequency and Debye length increase with irradiance | Limited clinical relevance; results published earlier confirmed | |
| [219] | Monitoring the migration of dental filling material into tooth/laser dentistry | Intensity ratio and Stark broadening methods | Plasma temperatures for composite, dentine, and amalgam are different | Element concentration not quantified; limited to ex vivo analysis; other relevant materials are to be analysed | |
| [213] | Effect of antimicrobial agents on remineralisation of dentine/tissue restoration | Changes in intensity vs. wavelength emission profiles | Biomimetic remineralisation of carious dentine by activating an antimicrobial agent using fs laser | Use of different materials will change results (matrix effect); no statistical cross validation and clinical validation | |
| [229] | Variation in elemental composition in ankylotic tissues/orthodontic | Elemental imaging | Ankylotic tissues have higher concentration of Ca and P | Limited spatial resolution of 30 µm (Ca, Mg, P); role of Mg is to be explored | |
| [217] | Variation of Ca and Mg in dental tissues after laser irradiation/laser dentistry | Integrated peak area vs. laser energy density | Threshold laser fluence for Ca in enamel and dentin determined | Threshold laser fluence for Mg not detected (instrumental limitations) | |
| [230] | Age and sex identification/orthodontic treatments | Measured average intensities | Ca, P, and Fe concentrations decrease with age, higher concentrations in females than males | Variation is linked to orthodontic abnormalities; cross validation and clinical validation should be considered | |
| [112] | Diagnosis of dental pathologies/pathological identification | Relative intensity | Ca/P ratio decreases in presence of plaque | Ca/P signal range is close for healthy and pathological regions | |
| [204] | Characterisation of dental tissues/clinical dentistry (dental implants and cavity preparation) | intensity ratio and Stark broadening methods | Plasma temperature higher and electron density lower for Nd:YAG laser wavelength 1064 nm than for 532 nm | Outcomes are to validated by practitioners using other lasers (CO2, diode, Er:YAG) used in laser dentistry | |
| [107] | Assess interaction of laser beam with dental tissues and dental material/dental health and safety | LIBS and photoacoustic sensor | More intense plasma on carious than on healthy tissues. Release of toxic elements (Hg, Ag, Cu, Sn) from amalgam hazardous | Used approximation for acoustic wave propagation may not be appropriate for clinical applications | |
| [202] | Detect early signs of osteoporosis in periodontal patients/clinical dentistry | Mean spectral intensity | Lower (Ca) and higher (K, Mg) content associated with osteoporotic group compared to control group | Control group had periodontal disease, which can have specific effects of osteoporosis (on periodontal tissues) | |
| [231] | Examination of dental tissues, enamel (apical and buccal) and dentine/pre-clinical dentistry | Intensity ratio of spectral lines | Apical enamel is the hardest among buccal enamel and dentine | No statistical, cross, and clinical validation; in vitro analysis | |
| [84] | Thermal effect of fs laser ablation on teeth for caries removal/laser surgery | Simulation method (thermal model) | Minimal thermal damage to surrounding nerve tissues; acceptable removal rates | Optimisation of laser fluence below carbonization threshold of each tissue is challenging | |
| [80] | Evaluation of diffusion of mercury to dental tissues/tooth restoration | Fs-LIBS | Hg penetration depth for deciduous and permanent teeth determined | Spatial resolution limited to 100 µm; penetration depths of other metals in tissues are to be determined | |
| [216] | Ablation threshold fluence for enamel and dentine/laser dentistry | Emission intensity vs. laser energy density | Ablation threshold for enamel and dentine determined | Ablation damages tissues inducing variations in surface topography and structural morphology | |
| [232] | Elemental variations in teeth associated with cariogenic and periodontal pathologies/oral surgery | LIBS intensity of emission lines | Higher concentration of C, O, K, F, Na aggregates in periodontal teeth compared to those with cavities | Statistical cross validation and clinical validations to be done; in vitro analysis | |
| [203] | Detection of toxic elements in smokers, non-smokers, and teeth/periodontal probing | Calibration curve for quantification | Higher concentration of hazardous elements (Pb, Cd, As) in smoker group than in non-smoker group. | No statistical analysis performed; correlation of results to smoking habits or chronic periodontitis difficult | |
| [211] | Examination of deciduous teeth to measure toxic elements/dental health and safety | Comparing spectra with matrix-matched references | High amounts of toxic elements in enamel make it the most affected tissue in the tooth | Small sample size (n = 4); no statistical, cross, and clinical validation; in vitro analysis | |
| [210] | Improve sensitivity of system for caries identification/optical feedback in laser dentistry | Measuring intensity variations | Intensity ratio Zn/Ca increases in presence of caries | Use of two different lasers (Nd:YAG, Er:YAG) makes treatment difficult | |
| Bone | [152] | Performance evaluation of ML models in bone classification/pre-orthopaedic surgery | LIBS-ML (PLS-DA, LDA, LR, SVM, SIMCA, CART, NN) | NN has exceptional performance in terms of sensitivity, robustness, generalisation | Accuracy of NN (100%) may be due to overfitting; other models’ accuracy is 42–66% |
| [83] | Discriminate between normal and pathological bone/optical feedback in orthopaedic surgery | Fs-LIBS-PCA | Higher Mg intensity relative to Ca in pathological bone compared to normal bone | Unable to detect bones suffering from severe pathologies | |
| Kidney stones | [148] | Discrimination of gallbladder stones (mixed vs. pigment GB 2)/diagnosis | LIBS-PCA and PAS | Variation in intensity of Ca, Sr, K, and CN; absence of calcium phosphate in GB 2 | Different sample preparation methods for LIBS and PAS measurements |
| [46,220] | Measure major and trace elements in gallstones/diagnosis | LIBS/WD-XRF/FTIR | Trace element (Zn, Pb, Cr, Cd) concentration exceeded safety limit | LIBS LOD for trace element 10–19 ppm |
4.2. LIBS in Bone and Kidney Stones Analysis
4.3. LIBS in Animal Calcified Tissues
| Tissues | Ref. | Purpose/ Application | Method | Findings | Comments |
|---|---|---|---|---|---|
| Rat dental tissues | [206,207] | Effect of beverages on rat dental tissues/veterinary dental care | Line intensity ratio | Coffee induces loss of Ca and P; decalcification of enamel | Molecular mechanism of enamel protection by green tea needs to be assessed |
| Chameleon oral tissues (tooth and bone) | [212] | Age-related variation in tooth-bone fusion area/physiological condition | Elemental imaging | Intensity of Ca, P, and Mg correlated with age in junction area of tooth and bone | Comparative analysis required to support claim that ankylosis is pathological state in mammals but is physiological for chameleon |
| Pig bones | [215] | Effect of acidic environment on bones/physiological state | Line intensity ratio | Intensity of Ca II/Ca I increases with concentration of sulfuric acid and time; hardness of bone surface is associated to calcium sulphate | Clinical relevance not demonstrated |
| Porcine bones (femora) | [115,146] | Differentiate hard (bone) and soft tissues (muscle and fat)/laser osteotomy | FO-LIBS-ML | Sensitivity of canonical DFA model for differentiating fat (84.6%) is lower than that of bone (100%) | Significant difference in model’s accuracy for soft tissue analysis revealed limitations of LIBS for such tissue examination |
| Boar bones (rib) | [49] | Analysis of light elements/Orthopaedic | CF-LIBS | Ratio of Ca/P measured | Difficult to fulfil prerequisites for CF-LIBS of biological samples; uncertainties of plasma parameters; comparative validation required |
| Bovine bones (femur) | [128] | Discrimination of different bones and different fodders/health and medical | NE-LIBS-PCA | Deposition of Ag NPs on bones and fodder enhances sensitivity of LIBS | Aging of bone and metabolism of cattle affect element concentration; complicates correlation of feeding strategy and bone composition |
| Pig (oral mucosa, peripheral nerve, dental pulp, dentine, enamel, cortical bone, cancellous bone) | [96] | Differentiation of hard and soft tissues/laser surgery | LIBS-PCA-LDA | Ratio of Ca/C identified as classifier (higher in hard tissues than in soft ones) | Sensitivity and specificity of LDA below 70% for enamel-dentine pair classification is serious issue in hard tissues examination (matrix effect lower than in soft tissues) |
| Bovine (bones and muscles) | [222] | Effect of laser repetition rate on ablation of dry bone/laser surgery | Fs-LIBS | Higher repetition rate allows fast cutting of bone (fluence above thresholds) | Temperature rise during ablation of bone should not exceed threshold for irreversible damage of nerves |
| Rat bones (thigh) | [97] | Role of lead in bone/animal health and medical science | Relative line intensity | Ca and Mg signals decrease with increasing Pb concentration | Error (18%) of measured plasma parameters high; quantification of elements hindered |
| Chicken eggshells | [234] | Discrimination of organic eggs from inorganic/food quality control: health and safety | LIBS-NN | Classification accuracy of NN for eggshell across various groups is 100% | Research focused on Ca; quantitative analysis of other elements (Mg, Fe, Al, Sr, Zn, Mn) to be performed |
| Chicken eggshells | [235] | Measurement of plasma parameters/pre-veterinary | Boltzmann plot and Stark broadening | Determination of temperature and electron number density and of inverse Bremsstrahlung absorption coefficient | Large uncertainty (5–50%) of used transition probability data limits precision of determined parameters; practical application is lacking |
| Chicken eggshells | [236] | Determination of heavy and trace elements in organic and regular chicken shell/food quality control: health and safety | CF-LIBS (OLCF and SC) | Ba detected only in organic shell; SC-LIBS is more reliable for quantification of heavy metals and trace elements | LOD values for toxic metals (Pb, Cd, Hg, As) remain uncertain |

5. LIBS Hybrid Technology
6. Limitations, Possible Solutions, and Recommendations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectroscopy |
| AdaBoost | Adaptive Boosting |
| AFM | Atomic force microscopy |
| AI | Artificial intelligence |
| ANN | Artificial neural network |
| AUC | Area under curve |
| BPNN | Back propagation neural network |
| BP_AdaBoost | Backpropagation neural network with adaptive boosting |
| BVF | Bagging voting fusion |
| BT | Boosting tree |
| BCC | Basal cell carcinoma |
| CART | Classification and regression tree |
| CC-LIBS | Calibration curve LIBS |
| CF-LIBS | Calibration-free LIBS |
| CLSM | Confocal laser scanning microscopy |
| CNN | Convolutional neural network |
| CRM | Confocal Raman microspectroscopy |
| CT | Computed tomography |
| DCS | Dual comb spectroscopy |
| DFA | Discriminant function analysis |
| DL | Deep learning |
| DLM | Deep learning model |
| DMF | Dermatofluoroscopy |
| DNN | Deep neural network |
| DP-LIBS | Dual/double pulse LIBS |
| EDS/EDX | Energy dispersive X-ray spectroscopy |
| ED-XRF | Energy dispersive X-ray fluorescence |
| EF-LIBS | Electric field-assisted LIBS |
| EPA | Environmental Protection Agency |
| Er: YAG | Erbium-doped yttrium aluminium garnet |
| ELISA | Enzyme-linked immunosorbent assay |
| FDA | Food and Drug Administration |
| FFF | Front face fluorescence |
| FFPE | Formalin-fixed, paraffin-embedded |
| Fs | Femtosecond pulses |
| Fs-LIBS | Femtosecond LIBS |
| FS-SVM | Feature selection followed by support vector machine |
| Fs-DP-LIBS | Femtosecond double-pulse LIBS |
| FTIR | Fourier transform infrared |
| GIST | Gastrointestinal stromal tumour |
| GRAN | Gradient reversal adversarial network |
| HA | Hydroxyapatite |
| HAZ | Heat-affected zone |
| H&E | Hematoxylin and eosin |
| HSA | Human serum albumin |
| IB | Inverse bremsstrahlung |
| ICP-MS | Inductively coupled plasma mass spectroscopy |
| IL | Intuition learning |
| IR | Infrared |
| KNN | Kernel nearest neighbour |
| KPCA | Kernel principal component analysis |
| KPCA-SVM | Kernel principal component analysis followed by support vector machine |
| KW | Kruskal–Wallis |
| LA-ICP-MS | Laser ablation inductively coupled plasma mass spectroscopy |
| LA-ICP-TOF–MS | Laser ablation mass spectroscopy, inductively coupled time of flight mass spectroscopy |
| LC-OCT | Line-field confocal optical coherence tomography |
| LDA | Linear discriminant analysis |
| LIBS | Laser-induced breakdown spectroscopy |
| LIBS-FTIR | Laser-induced breakdown spectroscopy and Fourier transform infrared |
| LIBS-ML | LIBS-integrated machine learning |
| LIBS-RS | LIBS-integrated Raman spectroscopy |
| LIF | Laser-induced fluorescence |
| LIP | Laser-induced plasma |
| LOD | Limit of detection |
| LR | Logistic regression |
| LTE | Local thermodynamic equilibrium |
| MCC | Merkel cell carcinoma |
| Med | Medical |
| ML | Machine learning |
| MMG | Mammography |
| MLA | Machine learning algorithm |
| MPM | Malignant pleural mesothelioma |
| MRI | Magnetic reasoning imaging |
| Nd:YAG | Neodymium-doped yttrium aluminium garnet laser |
| NE-LIBS | Nanoparticles enhanced LIBS |
| Ns-LIBS | Nanosecond LIBS |
| NN | Neural network |
| OCT | Optical coherence tomography |
| OLCF | One-line calibration free |
| PA | Photoacoustic |
| PAS | Photoacoustic spectroscopy |
| PCA | Principal component analysis |
| PCA-KNN | Principal component analysis followed by kernel nearest neighbour |
| PCA-LDA | Principal component analysis followed by linear discriminant analysis |
| PDMS | Polydimethylsiloxane |
| PET | Positron emission tomography |
| PIXE | Particle-induced X-ray emission technique |
| PLS-DA | Partial least squares discriminant analysis |
| PSCNN | Parallel spectral convolutional neural network |
| Ps-LIBS | Picosecond LIBS |
| QDA | Quadratic discriminant analysis |
| ResNet | Residual network |
| RF | Random forest |
| RF-1D-ResNet | Radio frequency one-dimensional residual network |
| RL | Reinforcement learning |
| ROC | Receiver operating characteristics |
| RS | Raman spectroscopy |
| RS-DLM | Raman spectroscopy-deep learning model |
| RSM | Refined spatial module |
| RSM-LDA | Refined spatial module-linear discriminant analysis |
| SA | Self-absorption |
| SA-LIBS | Spark assisted LIBS |
| SBR | Signal-to-background ratio |
| SC | Self-calibrated |
| SCC | Squamous cell carcinoma |
| SD-LIBS | Spark discharge LIBS |
| SEN-LIBS | Surface-enhanced LIBS |
| SEM | Scanning electron microscopy |
| SIMCA | Soft independent modelling by class analogy |
| SKB | Selectkbest |
| SML | Supervised machine learning |
| SNN | Spiking neural network |
| SNR | Signal-to-noise ratio |
| SOM | Self-organising map |
| SP | Sample preparation |
| SP-LIBS | Single pulse LIBS |
| SVM | Support vector machine |
| TA | Type of analysis |
| ULISA | Upconversion-linked immunosorbent assay |
| Us-LIBS | Ultrashort LIBS |
| UV-Fs-LIBS | Ultraviolet femtosecond LIBS |
| UV-NIR | Ultraviolet to near infrared |
| VUV | Vacuum ultraviolet |
| WHO | World Health Organization |
| XGBoost | Extreme gradient boosting |
| XRF | X-ray fluorescence |
References
- Nicolodelli, G.; Cabral, J.; Menegatti, C.R.; Marangoni, B.; Senesi, G.S. Recent advances and future trends in LIBS applications to agricultural materials and their food derivatives: An overview of developments in the last decade (2010–2019). Part I. Soils and fertilizers. TrAC Trends Anal. Chem. 2019, 115, 70–82. [Google Scholar] [CrossRef]
- Ruan, F.; Zhang, T.; Li, H. Laser-induced breakdown spectroscopy in archeological science: A review of its application and future perspectives. Appl. Spectrosc. Rev. 2019, 54, 573–601. [Google Scholar] [CrossRef]
- Cabral, J.S.; Menegatti, C.R.; Nicolodelli, G. Laser-induced breakdown spectroscopy in cementitious materials: A chronological review of cement and concrete from the last 20 years. TrAC Trends Anal. Chem. 2023, 160, 116948. [Google Scholar] [CrossRef]
- Pedarnig, J.D.; Trautner, S.; Grünberger, S.; Giannakaris, N.; Eschlböck-Fuchs, S.; Hofstadler, J. Review of element analysis of industrial materials by in-line laser—Induced breakdown spectroscopy (LIBS). Appl. Sci. 2021, 11, 9274. [Google Scholar] [CrossRef]
- Ji, H.; Ding, Y.; Zhang, L.; Hu, Y.; Zhong, X. Review of aerosol analysis by laser-induced breakdown spectroscopy. Appl. Spectrosc. Rev. 2021, 56, 193–220. [Google Scholar] [CrossRef]
- Palásti, D.J.; Metzinger, A.; Ajtai, T.; Bozóki, Z.; Hopp, B.; Kovács-Széles, É.; Galbács, G. Qualitative discrimination of coal aerosols by using the statistical evaluation of laser-induced breakdown spectroscopy data. Spectrochim. Acta Part B At. Spectrosc. 2019, 153, 34–41. [Google Scholar] [CrossRef]
- Wang, L.; Tolok, G.; Fu, Y.; Xu, L.; Li, L.; Gao, H.; Zhou, Y. Application and Research Progress of Laser-Induced Breakdown Spectroscopy in Agricultural Product Inspection. ACS Omega 2024, 9, 24203–24218. [Google Scholar] [CrossRef]
- Botto, A.; Campanella, B.; Legnaioli, S.; Lezzerini, M.; Lorenzetti, G.; Pagnotta, S.; Poggialini, F.; Palleschi, V. Applications of laser-induced breakdown spectroscopy in cultural heritage and archaeology: A critical review. J. Anal. At. Spectrom. 2019, 34, 81–103. [Google Scholar] [CrossRef]
- Qiao, S.; Ding, Y.; Tian, D.; Yao, L.; Yang, G. A review of laser-induced breakdown spectroscopy for analysis of geological materials. Appl. Spectrosc. Rev. 2015, 50, 1–26. [Google Scholar] [CrossRef]
- Rifai, K.; Michaud Paradis, M.-C.; Swierczek, Z.; Doucet, F.; Özcan, L.; Fayad, A.; Li, J.; Vidal, F. Emergences of new technology for ultrafast automated mineral phase identification and quantitative analysis using the CORIOSITY Laser-Induced Breakdown Spectroscopy (LIBS) system. Minerals 2020, 10, 918. [Google Scholar] [CrossRef]
- Pedarnig, J. Application of laser-induced breakdown spectroscopy to the analysis of secondary materials in industrial production. In Laser Spectroscopy for Sensing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 496–521. [Google Scholar] [CrossRef]
- Rammelkamp, K.; Schröder, S.; Kubitza, S.; Vogt, D.S.; Frohmann, S.; Hansen, P.B.; Böttger, U.; Hanke, F.; Hübers, H.W. Low-level LIBS and Raman data fusion in the context of in situ Mars exploration. J. Raman Spectrosc. 2020, 51, 1682–1701. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, T.; Li, H. Recent advances in laser-induced breakdown spectroscopy for explosive analysis. TrAC Trends Anal. Chem. 2023, 166, 117197. [Google Scholar] [CrossRef]
- Peng, J.; Song, K.; Zhu, H.; Kong, W.; Liu, F.; Shen, T.; He, Y. Fast detection of tobacco mosaic virus infected tobacco using laser-induced breakdown spectroscopy. Sci. Rep. 2017, 7, 44551. [Google Scholar] [CrossRef]
- Velásquez-Ferrín, A.; Babos, D.V.; Marina-Montes, C.; Anzano, J. Rapidly growing trends in laser-induced breakdown spectroscopy for food analysis. Appl. Spectrosc. Rev. 2021, 56, 492–512. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, V.; Sharma, J. Importance of laser-induced breakdown spectroscopy for hard tissues (bone, teeth) and other calcified tissue materials. Lasers Med. Sci. 2015, 30, 1763–1778. [Google Scholar] [CrossRef] [PubMed]
- Rehse, S.J. A review of the use of laser-induced breakdown spectroscopy for bacterial classification, quantification, and identification. Spectrochim. Acta Part B At. Spectrosc. 2019, 154, 50–69. [Google Scholar] [CrossRef]
- Houghton, S.C.; Hankinson, S.E. Cancer progress and priorities: Breast cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 822–844. [Google Scholar] [CrossRef]
- Wolner, Z.J.; Yélamos, O.; Liopyris, K.; Rogers, T.; Marchetti, M.A.; Marghoob, A.A. Enhancing skin cancer diagnosis with dermoscopy. Dermatol. Clin. 2017, 35, 417–437. [Google Scholar] [CrossRef]
- Tanoue, L.T.; Tanner, N.T.; Gould, M.K.; Silvestri, G.A. Lung cancer screening. Am. J. Respir. Crit. Care Med. 2015, 191, 19–33. [Google Scholar] [CrossRef]
- Meng, X.; Chen, J.; Zhang, Z.; Li, K.; Li, J.; Yu, Z.; Zhang, Y. Non-invasive optical methods for melanoma diagnosis. Photodiagnosis Photodyn. Ther. 2021, 34, 102266. [Google Scholar] [CrossRef]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for breast cancer screening. J. Magn. Reson. Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef]
- Wan, B.; Ganier, C.; Du-Harpur, X.; Harun, N.; Watt, F.; Patalay, R.; Lynch, M. Applications and future directions for optical coherence tomography in dermatology. Br. J. Dermatol. 2021, 184, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E.C. Line-field confocal optical coherence tomography for the in vivo real-time diagnosis of different stages of keratinocyte skin cancer: A preliminary study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Nichols, B.; Migden, M.R.; Rajaram, N.; Reichenberg, J.S.; Markey, M.K.; Ross, M.I.; Tunnell, J.W. Clinical study of noninvasive in vivo melanoma and nonmelanoma skin cancers using multimodal spectral diagnosis. J. Biomed. Opt. 2014, 19, 117003. [Google Scholar] [CrossRef]
- Uddin, A.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M.; Kasmuri, A.; Abbas, S. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 2016, 7, 6. [Google Scholar] [CrossRef]
- Yue, Z.; Sun, C.; Chen, F.; Zhang, Y.; Xu, W.; Shabbir, S.; Zou, L.; Lu, W.; Wang, W.; Xie, Z. Machine learning-based LIBS spectrum analysis of human blood plasma allows ovarian cancer diagnosis. Biomed. Opt. Express 2021, 12, 2559–2574. [Google Scholar] [CrossRef] [PubMed]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman spectroscopy in cancer diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef]
- Bonta, M.; Gonzalez, J.J.; Quarles, C.D.; Russo, R.E.; Hegedus, B.; Limbeck, A. Elemental mapping of biological samples by the combined use of LIBS and LA-ICP-MS. J. Anal. At. Spectrom. 2016, 31, 252–258. [Google Scholar] [CrossRef]
- Li, F.; Ge, L.; Tang, Z.; Chen, Y.; Wang, J. Recent developments on XRF spectra evaluation. Appl. Spectrosc. Rev. 2020, 55, 263–287. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Yang, P.; Chen, Y.; Zhu, Y.; Tong, M.; Hao, Z.; Li, X. Identification of cervical cancer using laser-induced breakdown spectroscopy coupled with principal component analysis and support vector machine. Lasers Med. Sci. 2018, 33, 1381–1386. [Google Scholar] [CrossRef]
- Wang, Q.; Xiangli, W.; Teng, G.; Cui, X.; Wei, K. A brief review of laser-induced breakdown spectroscopy for human and animal soft tissues: Pathological diagnosis and physiological detection. Appl. Spectrosc. Rev. 2021, 56, 221–241. [Google Scholar] [CrossRef]
- Asaduzzaman, K.; Khandaker, M.U.; Baharudin, N.A.B.; Amin, Y.B.M.; Farook, M.S.; Bradley, D.; Mahmoud, O. Heavy metals in human teeth dentine: A bio-indicator of metals exposure and environmental pollution. Chemosphere 2017, 176, 221–230. [Google Scholar] [CrossRef]
- Galbács, G. A critical review of recent progress in analytical laser-induced breakdown spectroscopy. Anal. Bioanal. Chem. 2015, 407, 7537–7562. [Google Scholar] [CrossRef]
- Ghasemi, F.; Parvin, P.; Motlagh, N.S.H.; Amjadi, A.; Abachi, S. Laser induced breakdown spectroscopy and acoustic response techniques to discriminate healthy and cancerous breast tissues. Appl. Opt. 2016, 55, 8227–8235. [Google Scholar] [CrossRef]
- Gazmeh, M.; Bahreini, M.; Tavassoli, S.H. Discrimination of healthy and carious teeth using laser-induced breakdown spectroscopy and partial least square discriminant analysis. Appl. Opt. 2015, 54, 123–131. [Google Scholar] [CrossRef]
- Cáceres, J.O.; de Los Terreros, J.Y.S. A real-world approach to identifying animal bones and Lower Pleistocene fossils by laser induced breakdown spectroscopy. Talanta 2021, 235, 122780. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, Y.; Busser, B.; Trichard, F.; Kulesza, A.; Laurent, J.; Zaun, V.; Lux, F.; Benoit, J.-M.; Panczer, G.; Dugourd, P. 3D imaging of nanoparticle distribution in biological tissue by laser-induced breakdown spectroscopy. Sci. Rep. 2016, 6, 29936. [Google Scholar] [CrossRef]
- Eum, C.; Park, J.; Kumar, S.; Jang, E.; Lee, Y.; Nam, S.-H.; Choi, D.; Chung, H. Feasibility of laser-induced breakdown spectroscopy as a direct raw bile analysis tool for screening of gallbladder cancer. J. Anal. At. Spectrom. 2022, 37, 823–832. [Google Scholar] [CrossRef]
- Khan, M.N.; Wang, Q.; Idrees, B.S.; Xiangli, W.; Teng, G.; Cui, X.; Zhao, Z.; Wei, K.; Abrar, M. A review on laser-induced breakdown spectroscopy in different cancers diagnosis and classification. Front. Phys. 2022, 10, 10. [Google Scholar] [CrossRef]
- Singh, V.K.; Sharma, J.; Pathak, A.K.; Ghany, C.T.; Gondal, M. Laser-induced breakdown spectroscopy (LIBS): A novel technology for identifying microbes causing infectious diseases. Biophys. Rev. 2018, 10, 1221–1239. [Google Scholar] [CrossRef]
- V S, D.; George, S.D.; Kartha, V.; Chidangil, S.; V K, U. Hybrid LIBS-Raman-LIF systems for multi-modal spectroscopic applications: A topical review. Appl. Spectrosc. Rev. 2021, 56, 463–491. [Google Scholar] [CrossRef]
- Gaudiuso, R.; Melikechi, N.; Abdel-Salam, Z.A.; Harith, M.A.; Palleschi, V.; Motto-Ros, V.; Busser, B. Laser-induced breakdown spectroscopy for human and animal health: A review. Spectrochim. Acta Part B At. Spectrosc. 2019, 152, 123–148. [Google Scholar] [CrossRef]
- Khan, Z.; Ullah, M.H.; Rahman, B.; Talukder, A.I.; Wahadoszamen, M.; Abedin, K.; Haider, A. Laser-induced breakdown spectroscopy (LIBS) for trace element detection: A review. J. Spectrosc. 2022, 2022, 3887038. [Google Scholar] [CrossRef]
- Markushin, Y.; Sivakumar, P.; Connolly, D.; Melikechi, N. Tag-femtosecond laser-induced breakdown spectroscopy for the sensitive detection of cancer antigen 125 in blood plasma. Anal. Bioanal. Chem. 2015, 407, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, B.B.S.; Kumar, V.; Sharma, J.; Rai, P.K.; Gondal, M.A.; Gondal, B.; Singh, V.K. Analysis of heterogeneous gallstones using laser-induced breakdown spectroscopy (LIBS) and wavelength dispersive X-ray fluorescence (WD-XRF). Lasers Med. Sci. 2016, 31, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Parvin, P.; Reif, J.; Abachi, S.; Mohebbifar, M.; Razzaghi, M. Laser induced breakdown spectroscopy for the diagnosis of several malignant tissue samples. J. Laser Appl. 2017, 29, 042005. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yu, X.; Chen, D.; Liu, A. Diagnosis of human malignancies using laser-induced breakdown spectroscopy in combination with chemometric methods. Spectrochim. Acta Part B At. Spectrosc. 2018, 139, 63–69. [Google Scholar] [CrossRef]
- Marín-Roldán, A.; Manzoor, S.; Krištof, J.; Veis, P. Enlarged spectral range in Calibration Free-Laser Induced Breakdown Spectroscopy for the qualitative and quantitative analysis of a complex bone matrix. Spectrochim. Acta Part B At. Spectrosc. 2019, 156, 13–19. [Google Scholar] [CrossRef]
- Gondal, M.; Aldakheel, R.; Almessiere, M.; Nasr, M.; Almusairii, J.; Gondal, B. Determination of heavy metals in cancerous and healthy colon tissues using laser induced breakdown spectroscopy and its cross-validation with ICP-AES method. J. Pharm. Biomed. Anal. 2020, 183, 113153. [Google Scholar] [CrossRef]
- Kiss, K.; Šindelářová, A.; Krbal, L.; Stejskal, V.; Mrázová, K.; Vrábel, J.; Kaška, M.; Modlitbová, P.; Pořízka, P.; Kaiser, J. Imaging margins of skin tumors using laser-induced breakdown spectroscopy and machine learning. J. Anal. At. Spectrom. 2021, 36, 909–916. [Google Scholar] [CrossRef]
- Pyun, S.H.; Min, W.; Goo, B.; Seit, S.; Azzi, A.; Wong, D.Y.-S.; Munavalli, G.S.; Huh, C.-H.; Won, C.-H.; Ko, M. Real-time, in vivo skin cancer triage by laser-induced plasma spectroscopy combined with a deep learning–based diagnostic algorithm. J. Am. Acad. Dermatol. 2023, 89, 99–105. [Google Scholar] [CrossRef]
- Livingston, K.M.; Zejdlik, K.; Baudelet, M. Reassociation of Skeletal Remains Using Laser-Induced Breakdown Spectroscopy. Anal. Chem. 2024, 96, 9478–9485. [Google Scholar] [CrossRef]
- Palleschi, V. Laser-induced breakdown spectroscopy: Principles of the technique and future trends. ChemTexts 2020, 6, 18. [Google Scholar] [CrossRef]
- Singh, V.K.; Rai, A.K. Prospects for laser-induced breakdown spectroscopy for biomedical applications: A review. Lasers Med. Sci. 2011, 26, 673–687. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part I: Review of basic diagnostics and plasma–particle interactions: Still-challenging issues within the analytical plasma community. Appl. Spectrosc. 2010, 64, 335A–366A. [Google Scholar] [CrossRef]
- Pasquini, C.; Cortez, J.; Silva, L.; Gonzaga, F.B. Laser induced breakdown spectroscopy. J. Braz. Chem. Soc. 2007, 18, 463–512. [Google Scholar] [CrossRef]
- Song, K.; Lee, Y.-I.; Sneddon, J. Recent developments in instrumentation for laser induced breakdown spectroscopy. Appl. Spectrosc. Rev. 2002, 37, 89–117. [Google Scholar] [CrossRef]
- Fortes, F.J.; Moros, J.; Lucena, P.; Cabalín, L.M.; Laserna, J.J. Laser-induced breakdown spectroscopy. Anal. Chem. 2013, 85, 640–669. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part II: Review of instrumental and methodological approaches to material analysis and applications to different fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Q.; Motto-Ros, V.; Lei, W.; Wang, X.; Bai, X. Generation and expansion of laser-induced plasma as a spectroscopic emission source. Front. Phys. 2012, 7, 649–669. [Google Scholar] [CrossRef]
- Wang, Z.; Afgan, M.S.; Gu, W.; Song, Y.; Wang, Y.; Hou, Z.; Song, W.; Li, Z. Recent advances in laser-induced breakdown spectroscopy quantification: From fundamental understanding to data processing. TrAC Trends Anal. Chem. 2021, 143, 116385. [Google Scholar] [CrossRef]
- Rai, V.; Thakur, S. Physics of plasma in laser-induced breakdown spectroscopy. In Laser-Induced Breakdown Spectroscopy; Singh, J.P., Thakur, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 83–111. [Google Scholar] [CrossRef]
- Pořízka, P.; Klus, J.; Képeš, E.; Prochazka, D.; Hahn, D.W.; Kaiser, J. On the utilization of principal component analysis in laser-induced breakdown spectroscopy data analysis, a review. Spectrochim. Acta Part B At. Spectrosc. 2018, 148, 65–82. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, H.; Li, H. Chemometrics in laser-induced breakdown spectroscopy. J. Chemom. 2018, 32, e2983. [Google Scholar] [CrossRef]
- Limbeck, A.; Brunnbauer, L.; Lohninger, H.; Pořízka, P.; Modlitbová, P.; Kaiser, J.; Janovszky, P.; Kéri, A.; Galbács, G. Methodology and applications of elemental mapping by laser induced breakdown spectroscopy. Anal. Chim. Acta 2021, 1147, 72–98. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, L.; Leprince, M.; Moncayo, S.; Sorbier, L.; Lienemann, C.-P.; Motto-Ros, V. Review of the recent advances and applications of LIBS-based imaging. Spectrochim. Acta Part B At. Spectrosc. 2019, 151, 41–53. [Google Scholar] [CrossRef]
- Messaoud Aberkane, S.; Safi, A.; Botto, A.; Campanella, B.; Legnaioli, S.; Poggialini, F.; Raneri, S.; Rezaei, F.; Palleschi, V. Laser-induced breakdown spectroscopy for determination of spectral fundamental parameters. Appl. Sci. 2020, 10, 4973. [Google Scholar] [CrossRef]
- Zhang, N.; Ou, T.; Wang, M.; Lin, Z.; Lv, C.; Qin, Y.; Li, J.; Yang, H.; Zhao, N.; Zhang, Q. A brief review of calibration-free laser-induced breakdown spectroscopy. Front. Phys. 2022, 10, 887171. [Google Scholar] [CrossRef]
- Poggialini, F.; Campanella, B.; Cocciaro, B.; Lorenzetti, G.; Palleschi, V.; Legnaioli, S. Catching up on calibration-free LIBS. J. Anal. At. Spectrom. 2023, 38, 1751–1771. [Google Scholar] [CrossRef]
- Tognoni, E.; Cristoforetti, G.; Legnaioli, S.; Palleschi, V. Calibration-Free Laser-Induced Breakdown Spectroscopy: State of the art. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 1–14. [Google Scholar] [CrossRef]
- Abubaker Hassan, H. Effects of Different Laser Pulse Regimes (Nanosecond, Picosecond and Femtosecond) on the Ablation of Materials for Production of Nanoparticles in Liquid Solution. In High Energy and Short Pulse Lasers; Richard, V., Ed.; IntechOpen: Rijeka, Croatia, 2016; Volume Chapter 12, pp. 305–325. [Google Scholar] [CrossRef]
- Bäuerle, D. Laser Processing and Chemistry; Springer Science & Business Media: London, UK, 2011; p. 874. [Google Scholar] [CrossRef]
- Méndez-López, C.; González-Gago, C.; Pisonero, J.; Bordel, N. Spatially resolved spectroscopical characterization of one-and two-component structured femtosecond laser induced plasmas. Spectrochim. Acta Part B At. Spectrosc. 2024, 216, 106949. [Google Scholar] [CrossRef]
- Labutin, T.A.; Lednev, V.N.; Ilyin, A.A.; Popov, A.M. Femtosecond laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2016, 31, 90–118. [Google Scholar] [CrossRef]
- Pisonero, J.; Calon, A.; Linares, J.; Mendez-Vicente, A.; Martinez-Nistal, A.; Bordel, N. Evaluation of femtosecond-LA-ICP-TOFMS for multi-elemental mapping at cellular resolution of human-tissue from cancer patients. Opt. Laser Technol. 2025, 180, 111527. [Google Scholar] [CrossRef]
- Al-Bourgol, S.; Machinet, G.; Bakkali, A.; Faucon, M.; Gemini, L. Real-Time Monitoring of Thermal Phenomena during Femtosecond Ablation of Bone Tissue for Process Control. Bioengineering 2024, 11, 309. [Google Scholar] [CrossRef]
- Bello, L.T.; da Ana, P.A.; Santos, D., Jr.; Krug, F.J.; Zezell, D.M.; Vieira, N.D., Jr.; Samad, R.E. Mercury amalgam diffusion in human teeth probed using femtosecond LIBS. Appl. Spectrosc. 2017, 71, 659–669. [Google Scholar] [CrossRef]
- Sarpe, C.; Ciobotea, E.R.; Morscher, C.B.; Zielinski, B.; Braun, H.; Senftleben, A.; Rüschoff, J.; Baumert, T. Identification of tumor tissue in thin pathological samples via femtosecond laser-induced breakdown spectroscopy and machine learning. Sci. Rep. 2023, 13, 9250. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Shin, S.; Moon, Y.; Han, J.H.; Hwang, E.; Jeong, S. High spatial resolution imaging of melanoma tissue by femtosecond laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2021, 179, 106090. [Google Scholar] [CrossRef]
- Gill, R.K.; Smith, Z.J.; Panchal, R.R.; Bishop, J.W.; Gandour-Edwards, R.; Wachsmann-Hogiu, S. Preliminary fsLIBS study on bone tumors. Biomed. Opt. Express 2015, 6, 4850–4858. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, R.F.; Harvey, C.M.; de Martínez Gerbi, M.E.M.; Smith, Z.J.; Smith, D.; Ivaldi, J.C.; Phillips, A.; Chan, J.W.; Wachsmann-Hogiu, S. Fs-laser ablation of teeth is temperature limited and provides information about the ablated components. J. Biophotonics 2017, 10, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Costas-Rodríguez, M.; Delanghe, J.; Vanhaecke, F. High-precision isotopic analysis of essential mineral elements in biomedicine: Natural isotope ratio variations as potential diagnostic and/or prognostic markers. TrAC Trends Anal. Chem. 2016, 76, 182–193. [Google Scholar] [CrossRef]
- Sehrawat, J.S.; Singh, M. Application of Trace Elemental Profile of Known Teeth for Sex and Age Estimation of Ajnala Skeletal Remains: A Forensic Anthropological Cross-Validation Study. Biol. Trace Elem. Res. 2020, 193, 295–310. [Google Scholar] [CrossRef]
- Tamburo, E.; Varrica, D.; Dongarrà, G. Gender as a key factor in trace metal and metalloid content of human scalp hair. A multi-site study. Sci. Total Environ. 2016, 573, 996–1002. [Google Scholar] [CrossRef]
- Schomburg, L. Dietary selenium and human health. Nutrients 2017, 9, 22. [Google Scholar] [CrossRef]
- López-Costas, O.; Lantes-Suarez, O.; Cortizas, A.M. Chemical compositional changes in archaeological human bones due to diagenesis: Type of bone vs soil environment. J. Archaeol. Sci. 2016, 67, 43–51. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Siddiqui, K.; Bawazeer, N.; Scaria Joy, S. Variation in macro and trace elements in progression of type 2 diabetes. Sci. World J. 2014, 2014, 461591. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Korobeinikova, T.V.; Aschner, M.; Baranova, O.V.; Barbounis, E.G.; Tsatsakis, A.; Tinkov, A.A. Medical application of Laser-Induced Breakdown Spectroscopy (LIBS) for assessment of trace element and mineral in biosamples: Laboratory and clinical validity of the method. J. Trace Elem. Med. Biol. 2023, 79, 127241. [Google Scholar] [CrossRef] [PubMed]
- Clases, D.; Gonzalez de Vega, R. Facets of ICP-MS and their potential in the medical sciences—Part 1: Fundamentals, stand-alone and hyphenated techniques. Anal. Bioanal. Chem. 2022, 414, 7337–7361. [Google Scholar] [CrossRef] [PubMed]
- Composition of the Human Body. Available online: https://en.wikipedia.org/wiki/Composition_of_the_human_body (accessed on 6 July 2024).
- Takahashi, T.; Thornton, B. Quantitative methods for compensation of matrix effects and self-absorption in Laser Induced Breakdown Spectroscopy signals of solids. Spectrochim. Acta Part B At. Spectrosc. 2017, 138, 31–42. [Google Scholar] [CrossRef]
- Rohde, M.; Mehari, F.; Klämpfl, F.; Adler, W.; Neukam, F.W.; Schmidt, M.; Stelzle, F. The differentiation of oral soft-and hard tissues using laser induced breakdown spectroscopy–a prospect for tissue specific laser surgery. J. Biophotonics 2017, 10, 1250–1261. [Google Scholar] [CrossRef]
- Shahedi, A.; Eslami, E.; Nourani, M.R. Influence of Lead on the Interpretation of Bone Samples with Laser-Induced Breakdown Spectroscopy. J. Spectrosc. 2016, 2016, 8205479. [Google Scholar] [CrossRef]
- Chan, G.C.Y.; Chan, W.-T. Plasma-related matrix effects in inductively coupled plasma—Atomic emission spectrometry by group I and group II matrix-elements. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 1301–1317. [Google Scholar] [CrossRef]
- Wang, W.; Sun, L.; Zhang, P.; Chen, T.; Zheng, L.; Qi, L. Study of matrix effects in laser-induced breakdown spectroscopy by laser defocus and temporal resolution. J. Anal. At. Spectrom. 2021, 36, 1977–1985. [Google Scholar] [CrossRef]
- Popov, A.M.; Zaytsev, S.M.; Seliverstova, I.V.; Zakuskin, A.S.; Labutin, T.A. Matrix effects on laser-induced plasma parameters for soils and ores. Spectrochim. Acta Part B At. Spectrosc. 2018, 148, 205–210. [Google Scholar] [CrossRef]
- Amais, R.S.; Donati, G.L.; Zezzi Arruda, M.A. ICP-MS and trace element analysis as tools for better understanding medical conditions. TrAC Trends Anal. Chem. 2020, 133, 116094. [Google Scholar] [CrossRef]
- Martinez, M.; Bayne, C.; Aiello, D.; Julian, M.; Gaume, R.; Baudelet, M. Multi-elemental matrix-matched calcium hydroxyapatite reference materials for laser ablation: Evaluation on teeth by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2019, 159, 105650. [Google Scholar] [CrossRef]
- Lin, J.; Li, Y.; Ding, K.; Lin, X.; Che, C. Investigating the mechanistic impact of pork soft tissue preparation techniques on the classification precision of laser-induced breakdown spectroscopy. Anal. Methods 2024, 16, 3654–3662. [Google Scholar] [CrossRef] [PubMed]
- Małajowicz, J.; Khachatryan, K.; Kozłowska, M. Properties of water activated with low-temperature plasma in the context of microbial activity. Beverages 2022, 8, 63. [Google Scholar] [CrossRef]
- Farka, Z.; Vytisková, K.; Makhneva, E.; Zikmundová, E.; Holub, D.; Buday, J.; Prochazka, D.; Novotný, K.; Skládal, P.; Pořízka, P. Comparison of single and double pulse laser-induced breakdown spectroscopy for the detection of biomolecules tagged with photon-upconversion nanoparticles. Anal. Chim. Acta 2024, 1299, 342418. [Google Scholar] [CrossRef]
- Tareq, M.S.; Hamad, T.K. Heavy metal migration from dental filling materials to calcified oral tissues: An in vitro analysis using LIBS and ICP-MS techniques. Odontology 2025, 113, 1613–1623. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Valizadeh, S. Measurement of pulse Nd:YAG laser-induced stress and analysis of dental tissue and amalgam plume using uniaxial polyvinylidene fluoride-based photoacoustic sensor and plasma spectroscopy. Opt. Laser Technol. 2020, 128, 106239. [Google Scholar] [CrossRef]
- Kasem, M.; Gonzalez, J.; Russo, R.; Harith, M. LIBS analysis of artificial calcified tissues matrices. Talanta 2013, 108, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Janovszky, P.; Kéri, A.; Palásti, D.J.; Brunnbauer, L.; Domoki, F.; Limbeck, A.; Galbács, G. Quantitative elemental mapping of biological tissues by laser-induced breakdown spectroscopy using matrix recognition. Sci. Rep. 2023, 13, 10089. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Baudelet, M. Calibration strategies for elemental analysis of biological samples by LA-ICP-MS and LIBS–A review. Anal. Bioanal. Chem. 2020, 412, 27–36. [Google Scholar] [CrossRef]
- Youssef, D.; Motto-Ros, V.; Abdel-Harith, M. Machine learning-based novel approach of image analysis in LIBS elemental mapping for discriminating archeological human bone. Spectrochim. Acta Part B At. Spectrosc. 2025, 224, 107108. [Google Scholar] [CrossRef]
- Nouir, R.; Cherni, I.; Ghalila, H.; Hamzaoui, S. Early diagnosis of dental pathologies by front face fluorescence (FFF) and laser-induced breakdown spectroscopy (LIBS) with principal component analysis (PCA). Instrum. Sci. Technol. 2022, 50, 465–480. [Google Scholar] [CrossRef]
- Mehari, F.; Rohde, M.; Kanawade, R.; Knipfer, C.; Adler, W.; Klämpfl, F.; Stelzle, F.; Schmidt, M. Investigation of the differentiation of ex vivo nerve and fat tissues using laser-induced breakdown spectroscopy (LIBS): Prospects for tissue-specific laser surgery. J. Biophotonics 2016, 9, 1021–1032. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Fan, R.; Yu, X.; Chen, D. Discrimination of soft tissues using laser-induced breakdown spectroscopy in combination with k nearest neighbors (kNN) and support vector machine (SVM) classifiers. Opt. Laser Technol. 2018, 102, 233–239. [Google Scholar] [CrossRef]
- Abbasi, H.; Rauter, G.; Guzman, R.; Cattin, P.C.; Zam, A. Differentiation of femur bone from surrounding soft tissue using laser-induced breakdown spectroscopy as a feedback system for smart laserosteotomy. In Proceedings of the SPIE Photonics Europe 2018, Proceedings Volume 10685. Biophotonics: Photonic Solutions for Better Health Care VI, Strasbourg, France, 23–26 April 2018; pp. 172–179. [Google Scholar] [CrossRef]
- Moncayo, S.; Manzoor, S.; Ugidos, T.; Navarro-Villoslada, F.; Caceres, J. Discrimination of human bodies from bones and teeth remains by laser induced breakdown spectroscopy and neural networks. Spectrochim. Acta Part B At. Spectrosc. 2014, 101, 21–25. [Google Scholar] [CrossRef]
- Kopřivová, H.; Kiss, K.; Krbal, L.; Stejskal, V.; Buday, J.; Pořízka, P.; Kaška, M.; Ryška, A.; Kaiser, J. Imaging the elemental distribution within human malignant melanomas using Laser-Induced Breakdown Spectroscopy. Anal. Chim. Acta 2024, 1310, 342663. [Google Scholar] [CrossRef]
- Khan, M.N.; Wang, Q.; Idrees, B.S.; Teng, G.; Cui, X.; Wei, K. Discrimination of melanoma using laser-induced breakdown spectroscopy conducted on human tissue samples. J. Spectrosc. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yang, S.; Yu, X.; Liu, A. Discrimination of lymphoma using laser-induced breakdown spectroscopy conducted on whole blood samples. Biomed. Opt. Express 2018, 9, 1057–1068. [Google Scholar] [CrossRef]
- Emara, E.M.; Song, H.; Imam, H.; Elwekeel, W.M.; Gao, X.; Mohammed, M.M.; Liu, S. Detection of hypokalemia disorder and its relation with hypercalcemia in blood serum using LIBS technique for patients of colorectal cancer grade I and grade II. Lasers Med. Sci. 2022, 37, 1081–1093. [Google Scholar] [CrossRef]
- Lin, J.; Li, Y.; Lin, X.; Che, C. Decision-level data fusion based on laser-induced breakdown and Raman spectroscopy: A study of bimodal spectroscopy for diagnosis of lung cancer at different stages. Talanta 2024, 275, 126194. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Flores, T.; Ponce, L. Fast Detection of Prostate Malignant Tissue by Multipulsed Laer-Induced Breakdown Spectroscopy (LIBS). Rev. Cuba. De Física 2023, 39, 81. [Google Scholar]
- Seifalinezhad, A.; Bahreini, M.; Matin, M.M.H.; Tavassoli, S.H. Feasibility study on discrimination of neo-plastic and non-neoplastic gastric tissues using spark discharge assisted laser induced breakdown spectroscopy. J. Lasers Med. Sci. 2019, 10, 64. [Google Scholar] [CrossRef]
- Tareq, M.S.; Hamad, T.K. Quantitative analysis of human teeth by using LIBS technology with different calibration methods: In vitro study. J. Opt. 2024, 54, 2064–2074. [Google Scholar] [CrossRef]
- Moon, Y.; Han, J.H.; Choi, J.-h.; Shin, S.; Kim, Y.-C.; Jeong, S. Mapping of cutaneous melanoma by femtosecond laser-induced breakdown spectroscopy. J. Biomed. Opt. 2019, 24, 031011. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Chen, X.; Shi, M.; Ren, T. Evaluation of coffee-ring effect of serum and Ag NPs mixture drop stains using laser-induced breakdown spectroscopy mapping. Spectrochim. Acta Part B At. Spectrosc. 2025, 227, 107158. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Chen, X.; Shi, M.; Ren, T. Nanoparticle-enhanced laser-induced breakdown spectroscopy for serum element analysis using an Ag NP-coated filter paper substrate. J. Anal. At. Spectrom. 2024, 39, 1332–1342. [Google Scholar] [CrossRef]
- Abdel-Salam, Z.; Palleschi, V.; Harith, M. Study of the feeding effect on recent and ancient bovine bones by nanoparticle-enhanced laser-induced breakdown spectroscopy and chemometrics. J. Adv. Res. 2019, 17, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Safi, A.; Landis, J.E.; Adler, H.G.; Khadem, H.; Eseller, K.E.; Markushin, Y.; Honarparvaran, S.; De Giacomo, A.; Melikechi, N. Enhancing biomarker detection sensitivity through tag-laser induced breakdown spectroscopy with NELIBS. Talanta 2024, 271, 125723. [Google Scholar] [CrossRef]
- Tareq, M.S.; Hamad, T.K. Nanoparticle enhanced laser Induced Breakdown Spectroscopy (NELIBS) analysis of human Teeth by using ZnO and Al2O3 nanoparticles. J. Opt. 2024. [Google Scholar] [CrossRef]
- Mohammadimatin, P.; Parvin, P.; Jafargholi, A.; Jahanbakhshi, A.; Ahmadinouri, F.; Tabibkhooei, A.; Heidari, O.; Salarinejad, S. Signal enhancement in spark-assisted laser-induced breakdown spectroscopy for discrimination of glioblastoma and oligodendroglioma lesions. Biomed. Opt. Express 2023, 14, 5795–5816. [Google Scholar] [CrossRef]
- Keerthi, K.; George, S.D.; Ongole, R.; Unnikrishnan, V. Fabrication of a Low Cost Superhydrophobic Substrate for Surface Enhanced Laser-Induced Breakdown Spectroscopy and Its Utility through Identification of Electrolyte Variation for Oral Cancer Detection. ACS Biomater. Sci. Eng. 2024, 10, 1153. [Google Scholar] [CrossRef]
- Ciobotea, E.R.; Sarpe, C.; Zielinski, B.; Braun, H.; Senftleben, A.; Dutta, S.; Mayer, G.; Florian, C.; Baumert, T. Signal enhancement with double-pulse LIBS on biological samples and better discrimination of tissues through machine learning algorithms. Spectrochim. Acta Part B At. Spectrosc. 2024, 222, 107063. [Google Scholar] [CrossRef]
- Grünberger, S.; Ehrentraut, V.; Eschlböck-Fuchs, S.; Hofstadler, J.; Pissenberger, A.; Pedarnig, J.D. Overcoming the matrix effect in the element analysis of steel: Laser ablation-spark discharge-optical emission spectroscopy (LA-SD-OES) and Laser-induced breakdown spectroscopy (LIBS). Anal. Chim. Acta 2023, 1251, 341005. [Google Scholar] [CrossRef]
- Grünberger, S.; Eschlböck-Fuchs, S.; Hofstadler, J.; Pissenberger, A.; Duchaczek, H.; Trautner, S.; Pedarnig, J.D. Analysis of minor elements in steel and chemical imaging of micro-patterned polymer by laser ablation-spark discharge-optical emission spectroscopy and laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2020, 169, 105884. [Google Scholar] [CrossRef]
- Gschwandtner, P.; Rudinger, F.; Trautner, S.; Ramsauer, C.; Hölzl, G.; Röder, T.; Pedarnig, J.D. Quantification of ZnSO4 in aqueous solution by liquid-solid matrix transfer and double-pulse laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2025, 229, 107215. [Google Scholar] [CrossRef]
- Ahamer, C.M.; Pedarnig, J.D. Femtosecond double pulse laser-induced breakdown spectroscopy: Investigation of the intensity enhancement. Spectrochim. Acta Part B At. Spectrosc. 2018, 148, 23–30. [Google Scholar] [CrossRef]
- Giannakaris, N.; Haider, A.; Ahamer, C.M.; Grünberger, S.; Trautner, S.; Pedarnig, J.D. Femtosecond single-pulse and orthogonal double-pulse laser-induced breakdown spectroscopy (LIBS): Femtogram mass detection and chemical imaging with micrometer spatial resolution. Appl. Spectrosc. 2022, 76, 926–936. [Google Scholar] [CrossRef]
- Giannakaris, N.; Siozos, P.; Piñon, V.; Banerjee, S.P.; Sentis, M.; Pedarnig, J.D.; Anglos, D. UV femtosecond single-pulse and collinear double-pulse laser-induced breakdown spectroscopy (LIBS) for depth-resolved characterization of nano-scaled films. Appl. Surf. Sci. 2023, 640, 158354. [Google Scholar] [CrossRef]
- Viljanen, J.; Sun, Z.; Alwahabi, Z.T. Microwave assisted laser-induced breakdown spectroscopy at ambient conditions. Spectrochim. Acta Part B At. Spectrosc. 2016, 118, 29–36. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, T.; Li, H. Applications of laser-induced breakdown spectroscopy (LIBS) combined with machine learning in geochemical and environmental resources exploration. TrAC Trends Anal. Chem. 2020, 133, 116113. [Google Scholar] [CrossRef]
- Debus, B.; Parastar, H.; Harrington, P.; Kirsanov, D. Deep learning in analytical chemistry. TrAC Trends Anal. Chem. 2021, 145, 116459. [Google Scholar] [CrossRef]
- Kościelniak, P. Unified principles of univariate analytical calibration. TrAC Trends Anal. Chem. 2022, 149, 116547. [Google Scholar] [CrossRef]
- Mahmud, M.; Kaiser, M.S.; McGinnity, T.M.; Hussain, A. Deep learning in mining biological data. Cogn. Comput. 2021, 13, 1–33. [Google Scholar] [CrossRef]
- Han, J.H.; Moon, Y.; Lee, J.J.; Choi, S.; Kim, Y.-C.; Jeong, S. Differentiation of cutaneous melanoma from surrounding skin using laser-induced breakdown spectroscopy. Biomed. Opt. Express 2016, 7, 57–66. [Google Scholar] [CrossRef]
- Abbasi, H.; Guzman, R.; Cattin, P.C.; Zam, A. All-fiber-optic LIBS system for tissue differentiation: A prospect for endoscopic smart laser osteotomy. Opt. Lasers Eng. 2022, 148, 106765. [Google Scholar] [CrossRef]
- Tarai, A.K.; Junjuri, R.; Dhobley, A.; Gundawar, M.K. Classification of human tooth using laser-induced breakdown spectroscopy combined with machine learning. J. Opt. 2024, 53, 3810–3820. [Google Scholar] [CrossRef]
- Gazali, Z.; Kumar, R.; Rai, P.K.; Rai, P.K.; Rai, A.K.; Thakur, S.N. Discrimination of gallbladder stone employing laser-induced breakdown spectroscopy (LIBS) and photoacoustic spectroscopy (PAS). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119948. [Google Scholar] [CrossRef]
- Pathak, A.; Singh, A.; Kumar, R.; Rai, A. Laser-induced breakdown spectroscopy coupled with PCA study of human tooth. Natl. Acad. Sci. Lett. 2019, 42, 87–90. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, X.; Bao, M.; Zheng, Y.; Luo, T.; Lu, B.; Teng, G.; Wang, Q.; Khan, M.N.; Yong, J. Early screening and staging of melanoma using blood based on laser-induced breakdown spectroscopy. Microchem. J. 2024, 203, 110955. [Google Scholar] [CrossRef]
- Teng, G.; Wang, Q.; Yang, H.; Qi, X.; Zhang, H.; Cui, X.; Idrees, B.S.; Xiangli, W.; Wei, K.; Khan, M.N. Pathological identification of brain tumors based on the characteristics of molecular fragments generated by laser ablation combined with a spiking neural network. Biomed. Opt. Express 2020, 11, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, S.; Manzoor, S.; Navarro-Villoslada, F.; Caceres, J. Evaluation of supervised chemometric methods for sample classification by Laser Induced Breakdown Spectroscopy. Chemom. Intell. Lab. Syst. 2015, 146, 354–364. [Google Scholar] [CrossRef]
- Winnand, P.; Ooms, M.; Heitzer, M.; Vohl, N.; Lammert, M.; Hölzle, F.; Boernsen, K.O.; Modabber, A. Assessment of the bony resection margin distance in bone-invasive oral cancer using laser-induced breakdown spectroscopy. Clin. Oral Investig. 2024, 28, 474. [Google Scholar] [CrossRef]
- Luo, M.; Li, X.; He, Q.; Liu, J.; Lu, H.; Liu, Q.; Yang, X. Elemental analysis and identification of papillary thyroid cancer tissues using laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2022, 37, 833–840. [Google Scholar] [CrossRef]
- Imam, H.; Mohamed, R.; Eldakrouri, A.A. Primary study of the use of laser-induced plasma spectroscopy for the diagnosis of breast cancer. Opt. Photonics J. 2012, 2, 3. [Google Scholar] [CrossRef]
- Idrees, B.S.; Wang, Q.; Khan, M.N.; Teng, G.; Cui, X.; Xiangli, W.; Wei, K. In-vitro study on the identification of gastrointestinal stromal tumor tissues using laser-induced breakdown spectroscopy with chemometric methods. Biomed. Opt. Express 2022, 13, 26–38. [Google Scholar] [CrossRef]
- Lin, J.; Li, Y.; Lin, X.; Che, C. Precise and rapid diagnosis of lung cancer: Leveraging laser-induced breakdown spectroscopy with optimized kernel methods in machine learning. J. Anal. At. Spectrom. 2024, 39, 2049–2057. [Google Scholar] [CrossRef]
- Li, J.; Pan, X.; Guo, L.; Chen, Y. Cancer diagnosis based on laser-induced breakdown spectroscopy with bagging-voting fusion model. Med. Eng. Phys. 2024, 132, 104207. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, H.; Gao, X.; Xu, Y.; Wang, Z.; Wang, Y. Discrimination of lung tumor and boundary tissues based on laser-induced breakdown spectroscopy and machine learning. Spectrochim. Acta Part B At. Spectrosc. 2021, 180, 106200. [Google Scholar] [CrossRef]
- Li, H.; Sun, H.; Gao, X. Laser-Induced Breakdown Spectroscopy Combined with Machine Learning for the Identification of Lung Cancer Tumors. J. Appl. Spectrosc. 2025, 92, 166–174. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, D.; Shu, K.; Xu, Y.; Duan, Y.; Fan, Q.; Lin, Q.; Tuchin, V.V. Optimization of machine learning classification models for tumor cells based on cell elements heterogeneity with laser-induced breakdown spectroscopy. J. Biophotonics 2023, 16, e202300239. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Pi, L.; Peng, L.; Zhang, D.; Ma, H.; Liu, Y.; Deng, N.; Wang, X.; Guo, L. High-precision identification of breast cancer based on end-to-end parallel spectral convolutional neural network assisted laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2025, 40, 478–486. [Google Scholar] [CrossRef]
- Shi, S.; Pi, L.; Hou, Z.; He, L.; Wang, X.; Guo, L. Batch Effect Correction for LIBS-FTIR Spectral Fusion in Breast Cancer Serum Detection Based on Gradient Reversal Adversarial Network. Talanta 2025, 295, 128324. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, Y.; Lin, X.; Che, C. Fusion of laser-induced breakdown spectroscopy technology and deep learning: A new method to identify malignant and benign lung tumors with high accuracy. Anal. Bioanal. Chem. 2024, 416, 993–1000. [Google Scholar] [CrossRef]
- Teng, G.; Wang, Q.; Zhang, H.; Xiangli, W.; Yang, H.; Qi, X.; Cui, X.; Idrees, B.S.; Wei, K.; Khan, M.N. Discrimination of infiltrative glioma boundary based on laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2020, 165, 105787. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, F.; Sheng, Z.; Zhang, D.; Zhang, S.; Wang, W.; Jin, H.; Qi, J.; Guo, L. Blood cancer diagnosis using ensemble learning based on a random subspace method in laser-induced breakdown spectroscopy. Biomed. Opt. Express 2020, 11, 4191–4202. [Google Scholar] [CrossRef]
- Kumar, A.; Yueh, F.-Y.; Singh, J.P.; Burgess, S. Characterization of malignant tissue cells by laser-induced breakdown spectroscopy. Appl. Opt. 2004, 43, 5399–5403. [Google Scholar] [CrossRef]
- Gaudiuso, R.; Ewusi-Annan, E.; Melikechi, N.; Sun, X.; Liu, B.; Campesato, L.F.; Merghoub, T. Using LIBS to diagnose melanoma in biomedical fluids deposited on solid substrates: Limits of direct spectral analysis and capability of machine learning. Spectrochim. Acta Part B At. Spectrosc. 2018, 146, 106–114. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, Z.; Lin, Q.; Duan, Y. Study on the molecular mechanisms against human breast cancer from insight of elemental distribution in tissue based on laser-induced breakdown spectroscopy (LIBS). Biol. Trace Elem. Res. 2021, 199, 1686–1692. [Google Scholar] [CrossRef]
- Sancey, L.; Motto-Ros, V.; Busser, B.; Kotb, S.; Benoit, J.-M.; Piednoir, A.; Lux, F.; Tillement, O.; Panczer, G.; Yu, J. Laser spectrometry for multi-elemental imaging of biological tissues. Sci. Rep. 2014, 4, 6065. [Google Scholar] [CrossRef]
- Kiss, K.; Kopřivová, H.; Stejskal, V.; Krbal, L.; Buday, J.; Brunnbauer, L.; Képeš, E.; Pořízka, P.; Ryška, A.; Kaška, M. Assessing spatial distribution of bioindicator elements in various cutaneous tumors using correlative imaging with laser-ablation-based analytical methods. Talanta 2024, 279, 126651. [Google Scholar] [CrossRef]
- Melikechi, N.; Markushin, Y.; Connolly, D.C.; Lasue, J.; Ewusi-Annan, E.; Makrogiannis, S. Age-specific discrimination of blood plasma samples of healthy and ovarian cancer prone mice using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 123, 33–41. [Google Scholar] [CrossRef]
- Zikmundová, E.; Sklenárová, D.; Kočí, E.; Zatloukalová, T.; Bačová, T.; Makhneva, E.; Holub, D.; Macháčová, E.; Kopřivová, H.; Vytisková, K. Magnetic microbead-based upconversion immunoassay with laser-induced breakdown spectroscopy readout for the detection of prostate-specific antigen. Microchim. Acta 2024, 191, 656. [Google Scholar] [CrossRef]
- Khan, M.N.; Wang, Q.; Idrees, B.S.; Teng, G.; Xiangli, W.; Cui, X.; Wei, K. Evaluation of human melanoma and normal formalin paraffin-fixed samples using Raman and LIBS fused data. Lasers Med. Sci. 2022, 37, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Idrees, B.S.; Teng, G.; Israr, A.; Zaib, H.; Jamil, Y.; Bilal, M.; Bashir, S.; Khan, M.N.; Wang, Q. Comparison of whole blood and serum samples of breast cancer based on laser-induced breakdown spectroscopy with machine learning. Biomed. Opt. Express 2023, 14, 2492–2509. [Google Scholar] [CrossRef] [PubMed]
- Orthaber, K.; Pristovnik, M.; Skok, K.; Perić, B.; Maver, U. Skin cancer and its treatment: Novel treatment approaches with emphasis on nanotechnology. J. Nanomater. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, J.N.; Baugh, E.G.; Fast, A.; Lentsch, G.; Balu, M.; Lee, B.A.; Kelly, K.M. A clinical perspective on the automated analysis of reflectance confocal microscopy in dermatology. Lasers Surg. Med. 2021, 53, 1011–1019. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Diagnostic criteria of common benign and malignant neoplasms, dermoscopic and histopathologic correlates of key confocal criteria, and diagnostic algorithms. J. Am. Acad. Dermatol. 2021, 84, 17–31. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Keim, U.; Jagoda, A.; Forschner, A.; Fink, C.; Spaenkuch, I.; Tampouri, I.; Eigentler, T.; Weide, B.; Haenssle, H.A. Dermatofluoroscopy diagnostics in different pigmented skin lesions: Strengths and weaknesses. JDDG J. Der Dtsch. Dermatol. Ges. 2020, 18, 682–690. [Google Scholar] [CrossRef]
- Barbierato, E.; Gatti, A. The challenges of machine learning: A critical review. Electronics 2024, 13, 416. [Google Scholar] [CrossRef]
- Zhao, J.; Lui, H.; Kalia, S.; Lee, T.K.; Zeng, H. Improving skin cancer detection by Raman spectroscopy using convolutional neural networks and data augmentation. Front. Oncol. 2024, 14, 1320220. [Google Scholar] [CrossRef]
- Ayadh, M.; Waszczuk, L.; Ogien, J.; Dauce, G.; Augis, L.; Tfaili, S.; Tfayli, A.; Perrot, J.-L.; Dubois, A. AI-assisted identification of nonmelanoma skin cancer structures based on combined line-field confocal optical coherence tomography and confocal Raman microspectroscopy. J. Biomed. Opt. 2025, 30, 076008. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wu, X.; Fang, X.; Fu, Q.; Wang, P.; Wang, X.; Li, S.; Li, Y. Raman spectroscopy combined with deep learning for rapid detection of melanoma at the single cell level. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 122029. [Google Scholar] [CrossRef] [PubMed]
- Weihmann, K.; Schleusener, J.; Eigentler, T.K.; Ghoreschi, F.C.; Moritz, R.K.; Szyc, L.; Dronnik, E.; Meinke, M.C. In vivo study to evaluate an intelligent algorithm for time efficient detection of malignant melanoma using dermatofluoroscopy. Ski. Pharmacol. Physiol. 2024, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Peñaranda, F.; Naranjo, V.; Lloyd, G.R.; Kastl, L.; Kemper, B.; Schnekenburger, J.; Nallala, J.; Stone, N. Discrimination of skin cancer cells using Fourier transform infrared spectroscopy. Comput. Biol. Med. 2018, 100, 50–61. [Google Scholar] [CrossRef]
- Huttunen, M.J.; Hristu, R.; Dumitru, A.; Floroiu, I.; Costache, M.; Stanciu, S.G. Multiphoton microscopy of the dermoepidermal junction and automated identification of dysplastic tissues with deep learning. Biomed. Opt. Express 2019, 11, 186–199. [Google Scholar] [CrossRef]
- Ruini, C.; Schlingmann, S.; Jonke, Ž.; Avci, P.; Padrón-Laso, V.; Neumeier, F.; Koveshazi, I.; Ikeliani, I.U.; Patzer, K.; Kunrad, E. Machine learning based prediction of squamous cell carcinoma in ex vivo confocal laser scanning microscopy. Cancers 2021, 13, 5522. [Google Scholar] [CrossRef] [PubMed]
- Cancer Today. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900 (accessed on 7 March 2024).
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Benbrahim-Tallaa, L.; Bouvard, V.; Bianchini, F.; Straif, K. Breast-cancer screening—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2015, 372, 2353–2358. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387. [Google Scholar] [CrossRef]
- Escudero-Cernuda, S.; Clases, D.; Eiro, N.; González, L.O.; Fraile, M.; Vizoso, F.J.; Fernández-Sánchez, M.L.; Gonzalez de Vega, R. Quantitative distribution of essential elements and non-essential metals in breast cancer tissues by LA-ICP-TOF–MS. Anal. Bioanal. Chem. 2025, 417, 361–371. [Google Scholar] [CrossRef]
- Naidu, B.G.; Srikanth, S.; Raju, G.N.; Sarita, P. PIXE analysis of blood serum of breast cancer patients undergoing successive chemotherapy. J. Radioanal. Nucl. Chem. 2020, 323, 1307–1316. [Google Scholar] [CrossRef]
- Huthwelker, T.; Borca, C.N.; Altamura, D.; De Caro, L.; Vanna, R.; Corsi, F.; Morasso, C.; Banfi, G.; Arpa, G.; Bunk, O. Microcalcifications in breast cancer tissue studied by X-ray absorption, emission, scattering and diffraction. Appl. Crystallogr. 2025, 58, 233–250. [Google Scholar] [CrossRef]
- Eyecioğlu, Ö. Determination of Se, Cr, Mn, Zn, Co, Na, and K in blood samples of breast cancer patients to investigate their variation using ICP-MS and ICP-OES. At. Spectrosc. 2019, 40, 11–16. [Google Scholar] [CrossRef]
- Balakrishnan, M.; George, R.; Sharma, A.; Graham, D.Y. Changing trends in stomach cancer throughout the world. Curr. Gastroenterol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Visser, M.; Van Grimbergen, I.; Hölters, J.; Barendregt, W.; Vermeer, L.; Vreuls, W.; Janssen, J. Performance insights of endobronchial ultrasonography (EBUS) and mediastinoscopy for mediastinal lymph node staging in lung cancer. Lung Cancer 2021, 156, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Winnand, P.; Boernsen, K.O.; Ooms, M.; Heitzer, M.; Vohl, N.; Lammert, M.; Hölzle, F.; Modabber, A. Assessment of the electrolyte heterogeneity of tissues in mandibular bone-infiltrating head and neck cancer using laser-induced breakdown spectroscopy. Int. J. Mol. Sci. 2024, 25, 2607. [Google Scholar] [CrossRef]
- Samek, O.; Beddows, D.; Telle, H.; Kaiser, J.; Liška, M.; Caceres, J.; Urena, A.G. Quantitative laser-induced breakdown spectroscopy analysis of calcified tissue samples. Spectrochim. Acta Part B At. Spectrosc. 2001, 56, 865–875. [Google Scholar] [CrossRef]
- Abdullah, K.F.; Kadhem, S.J. Analysis of laser induced breakdown spectra for distinguish between healthy and carious teeth. J. Opt. 2024, 54, 1100–1107. [Google Scholar] [CrossRef]
- Ayed, M.S.; Shafiq, S.S.; Diab, H.M.; Alahmari, A.D.; Divakar, D.D. Assessing periapical dental radiographs as a screening parameter for early indications of osteoporosis in postmenopausal periodontal patients and root surface evaluation using spectrochemical analysis. Saudi Med. J. 2018, 39, 719. [Google Scholar] [CrossRef] [PubMed]
- Alhasmi, A.M.; Gondal, M.A.; Nasr, M.M.; Shafik, S.; Habibullah, Y.B. Detection of toxic elements using laser-induced breakdown spectroscopy in smokers’ and nonsmokers’ teeth and investigation of periodontal parameters. Appl. Opt. 2015, 54, 7342–7349. [Google Scholar] [CrossRef]
- Batool, J.; Amin, N.; Jamil, Y.; Shaikh, N.; Al Islam, S. Rapid elemental analysis of human teeth using laser induced breakdown spectroscopy. Phys. B Condens. Matter 2021, 602, 412495. [Google Scholar] [CrossRef]
- Dakhil, S.S.; Hamad, T.K. Spectrometric measurement of human teeth plasma produced by nanosecond Nd:YAG pulsed laser. J. Opt. 2024, 53, 4107–4112. [Google Scholar] [CrossRef]
- Manno, S.H.; Manno, F.A.; Tian, L.; Khan, M.S.; Ahmed, I.; Liu, Y.; Li, V.W.; Xu, S.; Xie, F.; Hung, T.F. Spectroscopic and microscopic examination of teeth exposed to green tea at different temperatures. PLoS ONE 2020, 15, e0244542. [Google Scholar] [CrossRef] [PubMed]
- Manno, S.H.; Manno, F.A.; Ahmed, I.; Ahmed, R.; Shu, L.; Li, L.; Xu, S.; Xie, F.; Li, V.W.; Ho, J. Spectroscopic examination of enamel staining by coffee indicates dentin erosion by sequestration of elements. Talanta 2018, 189, 550–559. [Google Scholar] [CrossRef]
- Ahmed, A.N.; Alwazzan, M.J.; Ismael, M.A. Study effects of pulse laser energy on human primary teeth and extraction caries area by using image processing techniques. NeuroQuantology 2020, 18, 36. [Google Scholar] [CrossRef]
- Hamzaoui, S.; Nouir, R.; Jaidene, N. The study of carious teeth by laser-induced breakdown spectroscopy. J. Appl. Spectrosc. 2017, 84, 82–86. [Google Scholar] [CrossRef]
- Sasazawa, S.; Kakino, S.; Matsuura, Y. Optical-fiber-based laser-induced breakdown spectroscopy for detection of early caries. J. Biomed. Opt. 2015, 20, 065002. [Google Scholar] [CrossRef]
- Khalid, A.; Bashir, S.; Akram, M.; Hayat, A. Laser-induced breakdown spectroscopy analysis of human deciduous teeth samples. Lasers Med. Sci. 2015, 30, 2233–2238. [Google Scholar] [CrossRef]
- Dosedělová, H.; Štěpánková, K.; Zikmund, T.; Lesot, H.; Kaiser, J.; Novotný, K.; Štembírek, J.; Knotek, Z.; Zahradníček, O.; Buchtová, M. Age-related changes in the tooth–bone interface area of acrodont dentition in the chameleon. J. Anat. 2016, 229, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Kandil, H.; Ahmed, E.; Fouad, N.; Ali Dabbous, O.; Niazy, M.; Mohamed, T. Using Femtosecond Laser Light-Activated Materials: The Biomimetic Dentin Remineralization Was Monitored by Laser-Induced Breakdown Spectroscopy. Medicina 2023, 59, 591. [Google Scholar] [CrossRef]
- Tofanelli, M.; Pardini, L.; Borrini, M.; Bartoli, F.; Bacci, A.; D’Ulivo, A.; Pitzalis, E.; Mascherpa, M.C.; Legnaioli, S.; Lorenzetti, G. Spectroscopic analysis of bones for forensic studies. Spectrochim. Acta Part B At. Spectrosc. 2014, 99, 70–75. [Google Scholar] [CrossRef]
- Marković, M.; Kuzmanović, M.; Ranković, D.; Bajuk-Bogdanović, D.; Šajić, A.; Dimić, D. From Structure to Strength: Analyzing the Impact of Sulfuric Acid on Pig Bone Demineralization Through FTIR, LIBS, and AAS. Int. J. Mol. Sci. 2024, 25, 12250. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Al-Jedani, S.; Razvi, M.; Saeed, A.; Abdel-Daiem, A.; Ansari, M.S.; Babkair, S.S.; Salah, N.A.; Al-Mujtaba, A. Data fitting to study ablated hard dental tissues by nanosecond laser irradiation. PLoS ONE 2016, 11, e0156093. [Google Scholar] [CrossRef]
- Mustafa, M.; Latif, A.; Jehangir, M.; Siraj, K. Nd:YAG laser irradiation consequences on calcium and magnesium in human dental tissues. Lasers Dent. Sci. 2022, 6, 107–115. [Google Scholar] [CrossRef]
- Mustafa, M.; Jehangir, M.; Latif, A. Laser-Induced Breakdown Spectroscopy and Microscopy Study of Human Dental Tissues. In Electron Microscopy; Mhadhbi, M., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- PetroviĆ, J.; MarinkoviĆ, J.; ŽivkoviĆ, S.; RadenkoviĆ, M.; CiganoviĆ, J.; MarkoviĆ, D.; MomČiloviĆ, M. The TEA CO2 Laser as a Promising tool for Elemental Analysis of Restored Teeth and Evaluation of Restoration Constituent Penetration into Dental Tissues. Plasma Chem. Plasma Process. 2023, 43, 1237–1253. [Google Scholar] [CrossRef]
- Khalil, A.A.I.; Gondal, M.A.; Shemis, M.; Khan, I.S. Detection of carcinogenic metals in kidney stones using ultraviolet laser-induced breakdown spectroscopy. Appl. Opt. 2015, 54, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Teng, F.; Ren, L.; Yan, Q.; Ye, W.; Tian, Y.; Li, Y.; Guo, J.; Deguchi, Y.; Zheng, R. Long-pulsed laser-induced breakdown spectroscopy for elemental imaging: An evaluation with human teeth. Spectrochim. Acta Part B At. Spectrosc. 2025, 229, 107201. [Google Scholar] [CrossRef]
- Gill, R.K.; Smith, Z.J.; Lee, C.; Wachsmann-Hogiu, S. The effects of laser repetition rate on femtosecond laser ablation of dry bone: A thermal and LIBS study. J. Biophotonics 2016, 9, 171–180. [Google Scholar] [CrossRef]
- Goldman, L.; Hornby, P.; Meyer, R.; Goldman, B. Impact of the laser on dental caries. Nature 1964, 203, 417. [Google Scholar] [CrossRef]
- Kareem, R.O.; Bulut, N.; Kaygili, O. Hydroxyapatite biomaterials: A comprehensive review of their properties, structures, medical applications, and fabrication methods. J. Chem. Rev. 2024, 6, 1–26. [Google Scholar] [CrossRef]
- Sarna-Boś, K.; Boguta, P.; Skic, K.; Wiącek, D.; Maksymiuk, P.; Sobieszczański, J.; Chałas, R. Physicochemical properties and surface characteristics of ground human teeth. Molecules 2022, 27, 5852. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escudero, A.C.; Legaz, I.; Prieto-Bonete, G.; López-Nicolás, M.; Maurandi-López, A.; Pérez-Cárceles, M.D. Aging and trace elements in human coronal tooth dentine. Sci. Rep. 2020, 10, 9964. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Vakhnovetsky, J.; Vakhnovetsky, A.; Ghobrial, M.; Nath, D.; Morgano, S.M. Functional role of inorganic trace elements in dentin apatite tissue—Part 1: Mg, Sr, Zn, and Fe. J. Trace Elem. Med. Biol. 2022, 71, 126932. [Google Scholar] [CrossRef]
- Kunin, A.A.; Evdokimova, A.Y.; Moiseeva, N.S. Age-related differences of tooth enamel morphochemistry in health and dental caries. EPMA J. 2015, 6, 3. [Google Scholar] [CrossRef]
- Pořízka, P.; Konečná, A.; Šindelářová, A.; Šulcová, M.; Modlitbová, P.; Prochazka, D.; Nevoránková, P.; Navrátil, M.; Vrlíková, L.; Buchtová, M. Feasibility of laser-induced breakdown spectroscopy to elucidate elemental changes in human tooth ankylosis. Spectrochim. Acta Part B At. Spectrosc. 2023, 206, 106727. [Google Scholar] [CrossRef]
- Unnikrishnan, V.; Mitra, P.; Srikant, N.; Baptist, J.; Keerthi, K.; Ongole, R. Validity of laser induced breakdown spectroscopy (LIBS) in determining age and sex from tooth specimens. Heliyon 2022, 8, e10946. [Google Scholar] [CrossRef]
- Suyanto, H.; Trisnawati, N.; Putra, I.; Suprihatin, I. Characterization of human teeth by laser-induced breakdown spectroscopy. J. Phys. Conf. Ser. 2018, 1120, 012018. [Google Scholar] [CrossRef]
- Gassama, B.C.; Gueye, M.; Traore, A.; Tamba, B.; Babamou, K.; Tine, S.D.; Wague, A.; Rey, G.; Faye, B. Laser-induced breakdown spectroscopy of pathological mineralized dental tissues in a population of Senegalese individuals: A study of 75 cases. J. Dent. Oro. Surg. 2016, 1, 115. [Google Scholar] [CrossRef]
- Tariq, U.; Haider, Z.; Hussain, R.; Tufail, K.; Ali, J. LIBS analysis of hydroxyapatite extracted from bovine bone for Ca/P ratio measurements. AIP Conf. Proc. 2017, 1824, 030027. [Google Scholar] [CrossRef]
- Subki, K.; Putri, A.; Qusthalani, A.; Sari, N.; Subianto, M.; Sadami, K.; Ramli, M.; Lahna, K.; Idris, N. Analysis of organic egg authenticity determination using laser-induced breakdown spectroscopy (LIBS) assisted by artificial neural networks (ANN). IOP Conf. Ser. Earth Environ. Sci. 2025, 1510, 012088. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, X.; Lv, S.; Zhu, H. Measurements of egg shell plasma parameters using laser-induced breakdown spectroscopy. Pramana 2015, 85, 105–114. [Google Scholar] [CrossRef]
- Fahad, M.; Shah, K.H.; Ali, S.; Abrar, M. Spectroscopic diagnostics of eggshells plasma using LIBS with self-absorption correction in conjunction with WDXRF, EDS and FTIR techniques. Eur. Phys. J. D 2024, 78, 153. [Google Scholar] [CrossRef]
- Manard, B.T.; Hintz, C.J.; Quarles, C.D., Jr.; Burns, W.; Zirakparvar, N.A.; Dunlap, D.R.; Beiswenger, T.; Cruz-Uribe, A.M.; Petrus, J.A.; Hexel, C.R. Determination of fluorine distribution in shark teeth by laser-induced breakdown spectroscopy. Metallomics 2022, 14, mfac050. [Google Scholar] [CrossRef]
- Abbasi, H.; Rauter, G.; Guzman, R.; Cattin, P.C.; Zam, A. Laser-induced breakdown spectroscopy as a potential tool for autocarbonization detection in laserosteotomy. J. Biomed. Opt. 2018, 23, 071206. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Yanhong, G.; Ying, Z.; Yuandong, L.; Yuan, L. Analytical study of seashell using laser-induced breakdown spectroscopy. Plasma Sci. Technol. 2017, 19, 52–55. [Google Scholar] [CrossRef]
- Chirinos, J.R.; Oropeza, D.D.; Gonzalez, J.J.; Hou, H.; Morey, M.; Zorba, V.; Russo, R.E. Simultaneous 3-dimensional elemental imaging with LIBS and LA-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 1292–1298. [Google Scholar] [CrossRef]
- Jantzi, S.C.; Motto-Ros, V.; Trichard, F.; Markushin, Y.; Melikechi, N.; De Giacomo, A. Sample treatment and preparation for laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 115, 52–63. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectrochim. Acta Part B At. Spectrosc. 2021, 175, 106013. [Google Scholar] [CrossRef]
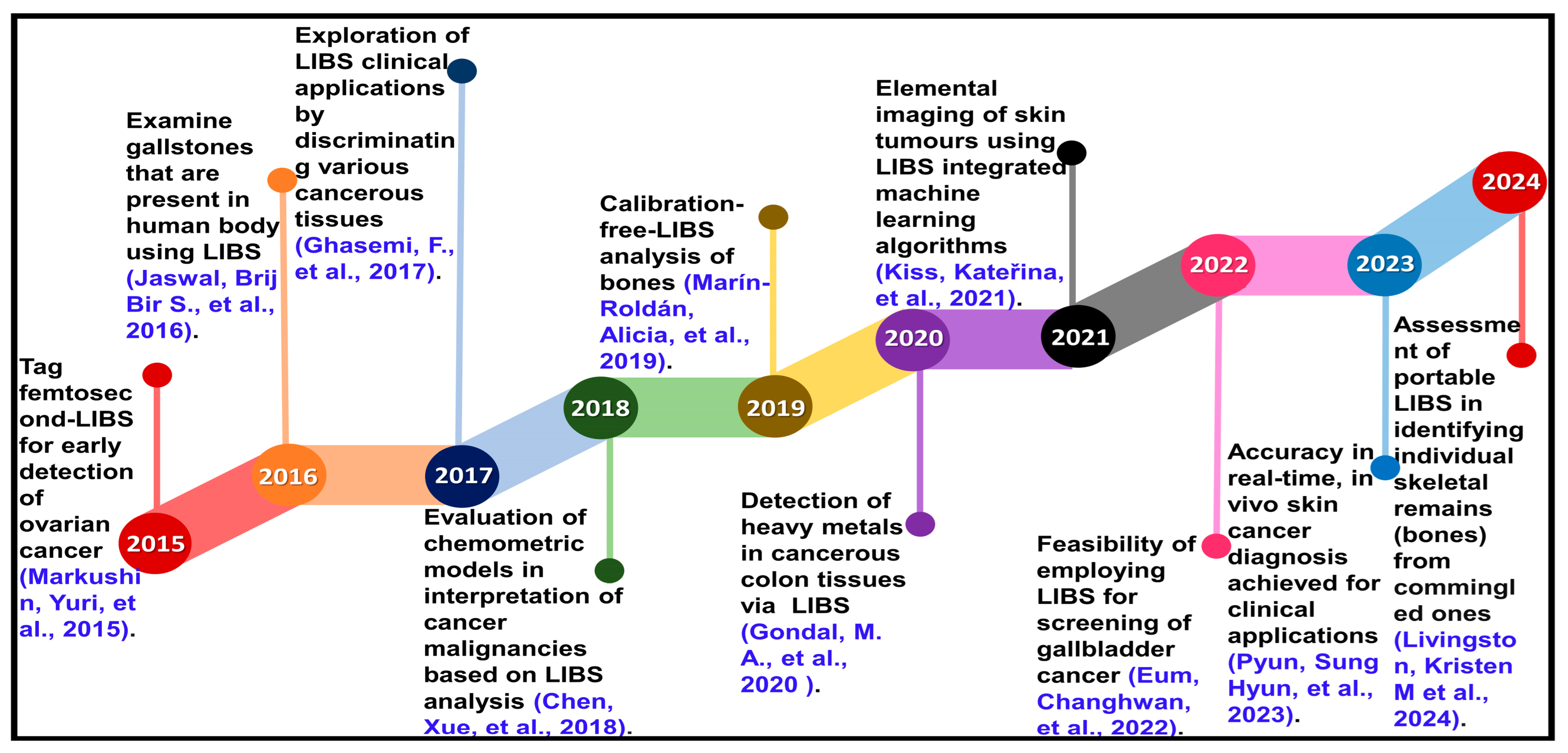

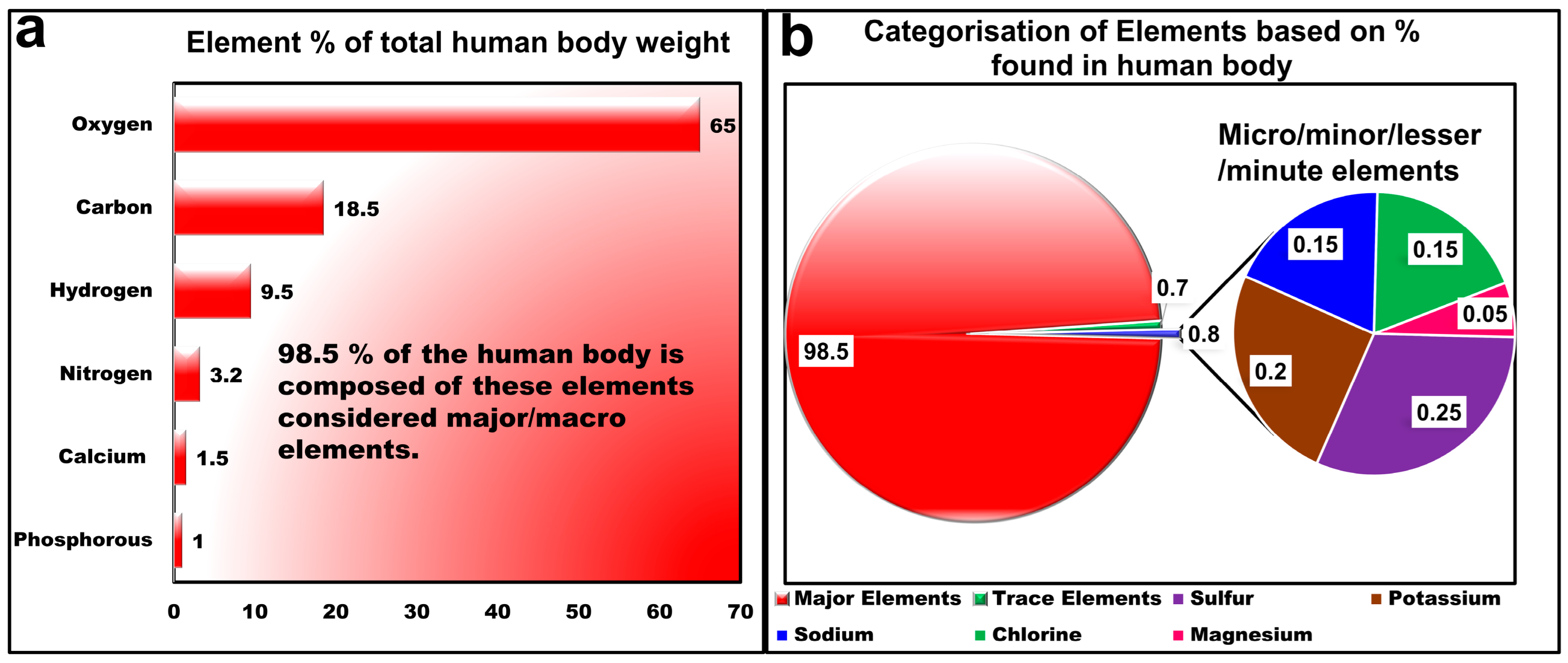
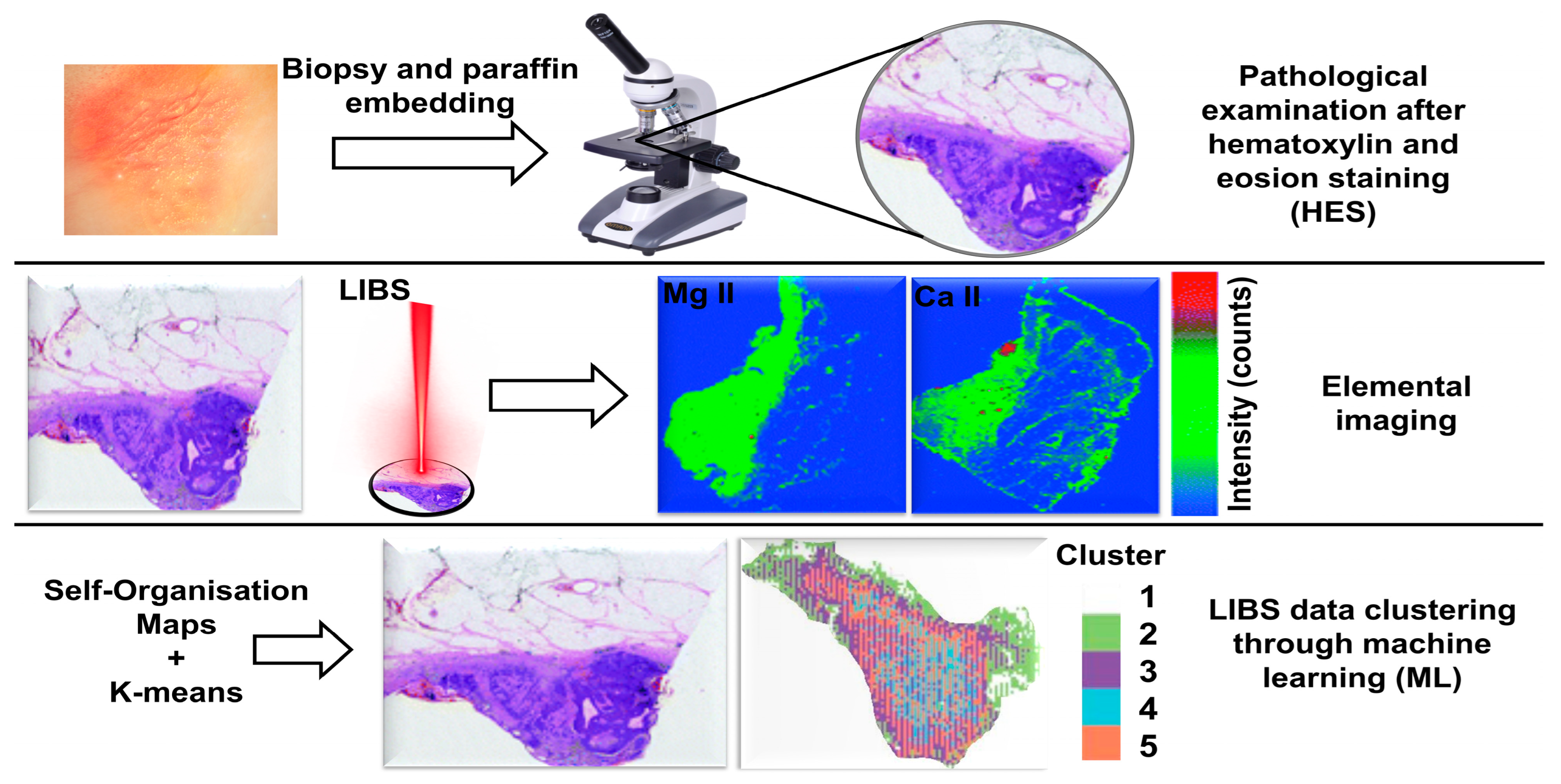
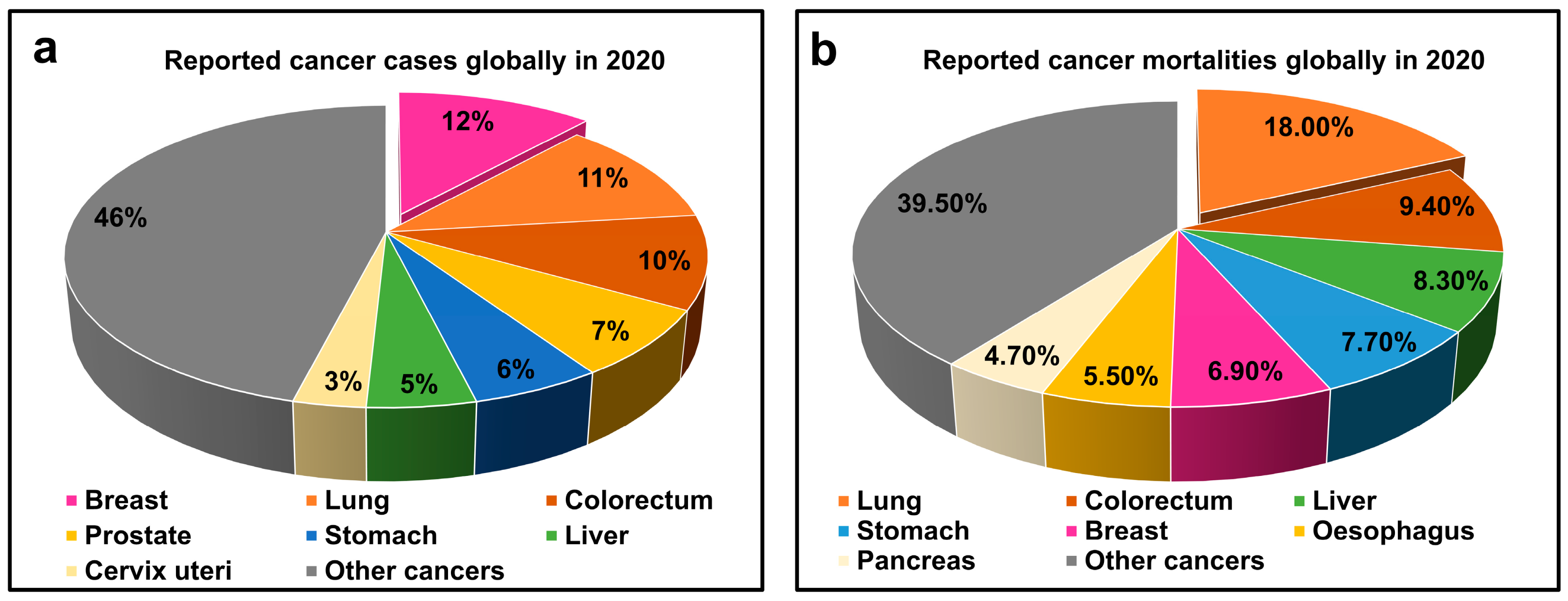
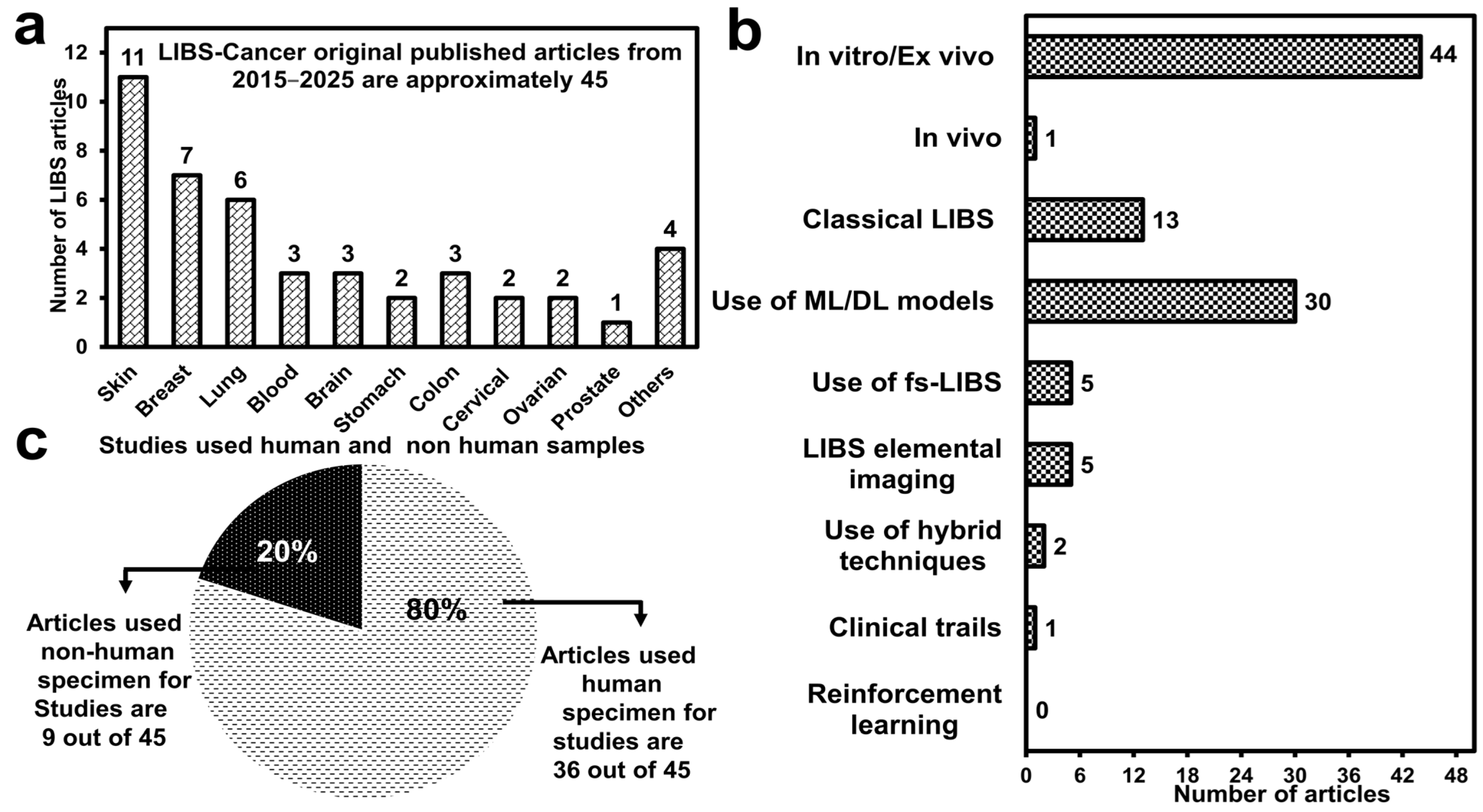
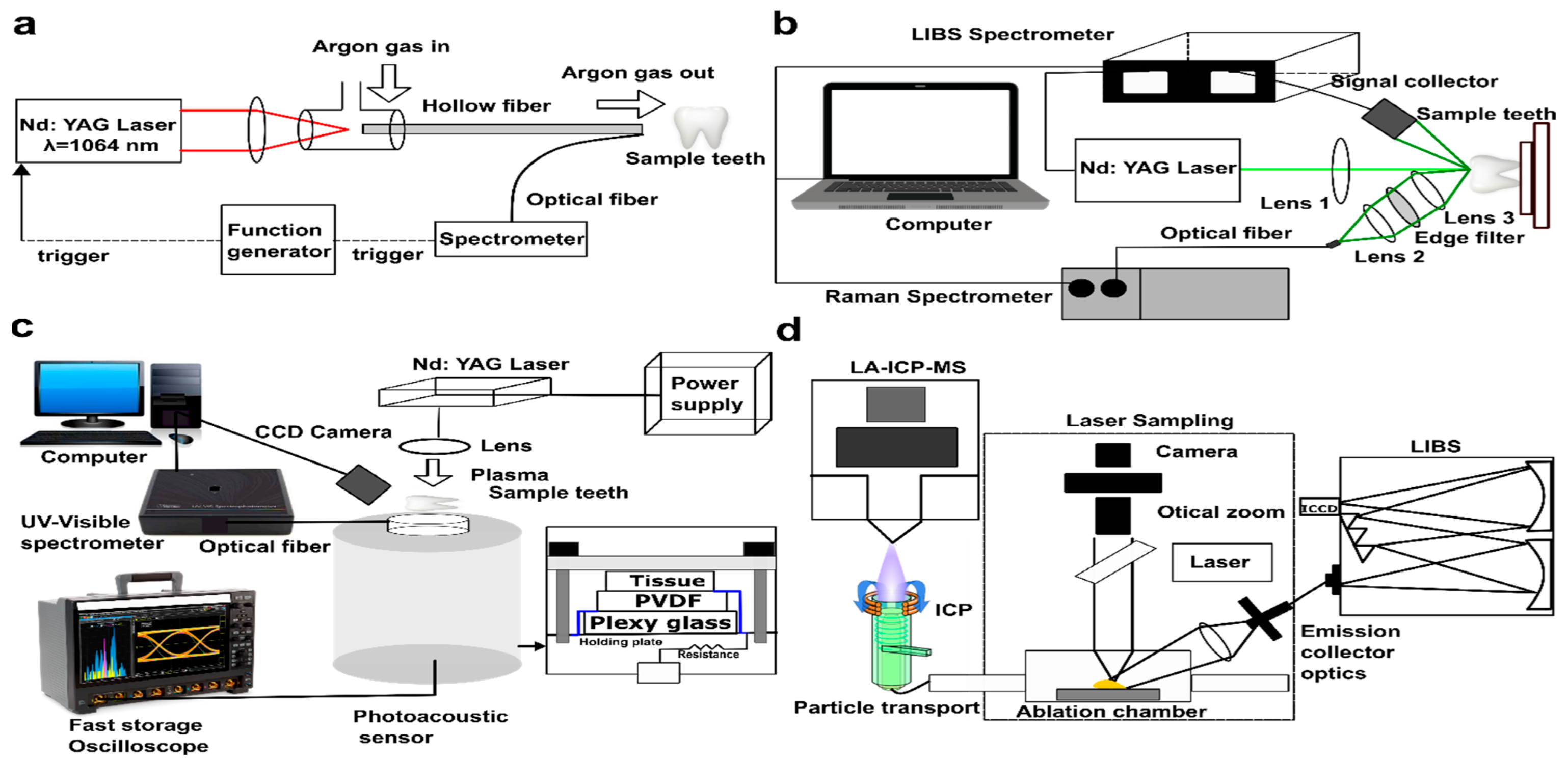
| Material/Tissues | Matrix Elements/Non-Matrix Elements | Challenges | Comments | Ref. | |
|---|---|---|---|---|---|
| Human samples | Skin tissues (cancerous and healthy) | C, N, H, O/Ca, Mg, Na, K | Limited lateral resolution, unable to map trace elements with precision | Ratio of intensities used for standardisation to eliminate ME; spatial resolution in LIBS imaging lower than for LA-ICP-MS | [51,117] |
| Skin tissues | C, N, H, O/Ca, Mg, K, P, Fe, Na | Spectral fluctuation | Extensive pre-processing methods (standard normal variate, autoscaling, auto centring, normalisation by area) used to enhance model efficiencies | [118] | |
| Blood | C, N, O, H, Ca, P/Fe, K, Na, Mg | Spectral fluctuation | ME of filter paper substrate and blood add up; signals of filter paper not subtracted, normalisation of intensities insufficient | [119] | |
| Serum | K, Na, Ca, Mg/Zn, Cu | Poor signal-to-noise ratio (SNR) | Self-absorption factor (0.6) to be reduced for reliable analysis | [120] | |
| Lung tumour | C, N, H, O, P, S/Ca, Mg, Cu, Fe | Detection of anomalous spectra | Pre-processing and z-score method used to reduce fluctuation; uncertainty about variation in intensity (ME or abnormalities) | [121] | |
| Prostate malignant tissue | C, N, H, O/Na, Fe | Low intensities, high background noise | Trace elemental detection and quantification are lacking | [122] | |
| Gastric tissues | C, N, H, O, Ca, Na/Mg | Poor SNR | Biopsies and extensive sample preparation required | [123] | |
| Teeth | Ca, P/- | Poor quantification precision | Certified reference materials for dental tissues not available; plasma properties of tooth samples and reference materials considerably different | [124] | |
| Teeth | Ca, P/Al, Ba, Hg, Pb, Sr | High limit of detection for trace elements | Non-matrix elements from filling materials migrate to matrix, unreliable quantification | [106] | |
| Teeth, bones | Ca, P/Mg, Sr | Poor repeatability, LIBS-ML model inefficient for classification | LIBS-NN model cannot be generalised; different models used for classification, even for specimens having similar matrices | [116] | |
| Animal samples | Soft pork tissues (fat, skin, and muscle) | C, N, H, O, K, Ca, Na/- | Spectral fluctuation | LIBS-SVM classification sensitivity (74%) for similar muscle tissues is limited | [114] |
| Porcine organic tissues (liver, brain, kidney, heart, lung, and skeletal muscle) | C, N, H, O/Ca, Mg, Na, K, Fe | Poor quantitative analysis | Matrix-matched reference materials required; larger data set of animal tissues required for validation | [109] | |
| Porcine tissues (bone, muscle, and fat) | C, N, H, O/Ca, K, Na, Zn, Fe, Cl | Background noise | LIBS-DFA model sensitivity and specificity for discrimination of soft tissues (muscle and fat) 10% less than that for bones | [115] | |
| Pig’s soft tissues (fats and nerves) | C, N, H, O, P, S/Ca, Na, K | Poor classification accuracy | Model performances to be improved; ex vivo analysis may not be applicable for in vivo studies | [113] | |
| Rib bones of boar | Ca, P/Mg, Sr | Uncertainty of measured plasma parameters restricts accurate elemental quantification | Optimisation of experimental parameters (gate delay, gas pressure, spectral range); enhanced CF-LIBS performance for all matrix elements of all tissues uncertain | [49] | |
| Mice skin tissues (melanoma lesion and normal) | C, N, H, O, P/Mg | Low average spatial resolution due to instrumental limitations | Unable to discriminate between highly similar tissues; conventional histological imaging used for cross-validation | [125] |
| Type of Cancer/Methods/Refs. | Pitfalls | Remarks |
|---|---|---|
| Gastric/LIBS-KNN/[156] | KNN: poor scalability, curse of dimensionality, lack of interpretability | Generalised additive models (GAM) and XGBoost could be better alternatives |
| Lungs/LIBS-PCA/[159] | PCA: compromises important features having low variations, ineffective for non-linear relationships | For non-linear issues, kernel PCA can be employed |
| Lungs/LIBS-1D-ResNet/[164] | 1D ResNet: domain-sensitive means model trained on one type of spectrometer; for data from another spectrometer transfer learning methods required | Fine-tuning can be effective in resolving domain sensitivity; regularising techniques and weight loss functions can reduce over-fitting |
| Lungs/LIBS-KPCA/[157] | Kernel has its own critical hyperparameters for tuning bandwidth and their values are not reported; KPCA is sensitive to outliers | It can be useful for a non-linear data set when the goal is to get predictive outcomes without understanding the features |
| Lungs/LIBS-Bagged tree model/[160] | Bagged tree model suffers from overfitting when a smaller number of spectra (280) is used | Overfitting can be reduced by eliminating noise from the LIBS spectrum |
| Cervical/LIBS-SVM/[31] | SVM is kernel-dependent, wrong selection leads to poor accuracy; effective for binary classification, unable to deal with multiple stages of cancer | Use of cross-validation is recommended for kernel selection because they have different regularisation parameters |
| Cervical/LIBS-CNN/[161] | CNN decision interpretation is not convincing; vulnerable to adversarial attacks; difficult to handle and has poor robustness | Adversarial training and multiple CNNs can provide generalisation |
| Brain/LIBS-SNN/[151] | Heuristic algorithms (cuckoo search algorithm used) computationally challenging; new method, not yet applied in other LIBS-medical studies | Usage of neuromorphic hardware can increase computational speed; hybrid architectures (ANN-SNN or CNN-SNN) can be more effective |
| Brain/LIBS-SVM/[165] | Identification accuracy of SVM is low (~60%); least reliable in intraoperative tumour classifications | Bayesian optimisation method can be applied for tuning penalty and kernel function parameters instead of using particle swarm optimisation (PSO) |
| Ovarian/LIBS-BPNN/[27] | BPNN model detail activation functions, regularisation and training strategy (epochs, batch size, and learning rate) is missing | Model description is required for reproducing the results in other labs |
| Breast/LIBS-PSCC/[162] | Significant drop in performance of CNN when shared pre-processing module is removed (accuracy decreased from 90% to 82%); PSCNN outcomes are instrument-dependent | In limited scenarios, shared pre-processing can be effective |
| Blood/LIBS-RSM-LDA/[166] | Randomness excludes critical features; can repeatedly pick correlated features, which reduces diversity among learners and weakens ensemble effect | Mechanistic correlation improvement in subtype discrimination |
| Teeth/LIBS-ANN/[147] | ANN requires large data set, 500 spectra are insufficient for analysis; it is an extension of SVM based on sigmoid functions | Tools (LIME and SHAP) can interpret ANN predictions for medical use |
| Bones/LIBS-SIMCA/[152] | Non-discriminative model which requires pre-processing and PCA before applying SIMCA, limited accuracy of 58% is attained | Kernel PCA or non-linear relationship kernel SIMCA can be used for biomedical data |
| Ref. | Laser (Parameters) | Spectrometer | Spectral Resolution | Spectral Range | Gate Delay | Integration Time | Environment | Cancer Type |
|---|---|---|---|---|---|---|---|---|
| [150] | Nd:YAG (1064 nm, 1 Hz, 10 ns, 30 mJ) | Avantes AvaSpec 2048 | 0.20–0.30 nm | 190–1100 nm | 1.28 µs | 1.05 ms | Air | Skin |
| [145] | Nd:YAG (532 nm, 5 Hz, 5 ns, 7.49 mJ) | Multichannel Instruments | 0.1 nm | 197–1045 nm | 0.2 µs | 1.05 ms | Argon | Skin |
| [48] | Nd:YAG (1064 nm, 5 Hz, 8 ns, 73 mJ) | Avantes AvaSpec ULS2048-4 | 0.09–0.22 nm | 200–850 nm | 5 µs | - | Air | Skin, Blood |
| [118] | Nd:YAG (1064 nm, 1 Hz, 5 ns, 64 mJ) | Avantes AvaSpec 2048-2-USB2 | 0.2–0.3 nm | 190–1100 nm | 1.28 µs | 2 ms | Air | Skin |
| [51] | Nd:YAG (532 nm, 20 Hz, 10 ns, 7.49 mJ) | Czerny Turner (SR-500i-B2-R) | - | 275–775 nm | 0.5 µs | - | Ar | Skin |
| [117] | Nd:YAG (266 nm, 50 Hz, 8 ns, 8 mJ) | Czerny Turner | - | 240–407 nm | 0.3 µs | - | Ar | Skin |
| [52] | Nd:YAG (1064 nm, 4 ns) | Single channel | 0.7 nm | 270–800 nm | - | 1 ms | Air | Skin |
| [168] | Ti:Sapphire (775 nm, 150 fs, 1.20 mJ & 1.44 mJ) | Echelle | - | - | 50 ns | 700 µs | Helium | Skin * |
| [125] | Ytterbium (1030 nm, 550 fs, 250 µJ) | - | 0.1 nm | 200–900 nm | 0.1 µs | - | Ar | Skin * |
| [82] | Ytterbium (1030 nm, 343 nm, 550 fs, 30–80 µJ, 7.42 J/cm2) | Single spectrometer | 0.4 nm | 240–800 nm | 20 ns | - | Air | Skin * |
| [175] | Nd:YAG (532 nm, 10 Hz, 103 mJ) | Avantes Ava Spec 2048 | 0.08 nm | 190–770 nm | 1 µs | 2 ms | Air | Breast |
| [162,163] | Nd:YAG (532 nm, 1 Hz, 98.6 mJ) | Avantes AvaSpec ULS4096CL-Evo | - | 200–900 nm | 2 µs | - | Air | Breast |
| [35,47] | Nd:YAG (1064 nm, 1 Hz, 10 ns, 150 mJ) | Avantes Ava Spec 2048 | 0.4 nm | 200–1100 nm | 1.28 µs | - | Air | Breast, Colon, Larynx, Tongue |
| [169] | Nd:YAG (1064 nm, 10 Hz, 6 mJ) | Avantes | - | 182–600 nm | 2 µs | - | Ar | Breast * |
| [81] | Ti:Sapph. (785 nm, 1 KHz, 30 fs, 7 µJ) | L.O.T. Oriel Multispec MS125 | 1 nm | 23 ns | - | Air | Breast, Liver | |
| [158] | Nd:YAG (532 nm, 10 Hz, 8 ns, 175 mJ) | Avantes AvaSpec ULS4096CL-EVO | - | 200–950 nm | 2 µs | - | Air | Lungs, Esophageal |
| [160] | Nd:YAG (1064 nm, 10 Hz, 10 ns, 65 mJ) | Mechelle Me5000 | 200–850 nm | 1 µs | 1 µs | Air | Lungs | |
| [159] | Nd:YAG (1064 nm, 10 Hz, 10 ns, 65 mJ) | Mechelle Me5000 | - | 200–850 nm | 1 µs | 1 ms | Air | Lungs |
| [164] | Nd:YAG (1064 nm, 10 Hz, 40 mJ) | - | - | 240–850 nm | 6 µs | - | Air | Lungs |
| [121] | Nd:YAG (1064 nm, 10 Hz, 5 ns, 50 mJ) | Mechelle Me5000 | - | 200–900 nm | 3 µs | - | Air | Lungs |
| [157] | Nd:YAG (10 Hz, 10 ns, 40 mJ) | - | - | 200–900 nm | 3 µs | - | Air | Lungs |
| [166] | Nd:YAG (532 nm, 10 Hz, 8 ns, 30 mJ) | Echelle | - | 200–950 nm | 1 µs | - | Air | Blood |
| [119] | Nd:YAG (1064 nm, 5 Hz, 8 ns, 73 mJ) | Avantes AvaSpec ULS2048-4 | 0.09–0.22 nm | 200–850 nm | 5 µs | - | Air | Blood |
| [50] | Nd:YAG (266 nm, 20 Hz, 8 ns, 50 mJ) | SR 500i-A | - | 280–900 nm | 500 ns | - | Air | Colon |
| [120] | Nd:YAG (1064 nm, 10 Hz, 10 ns, 20–30 mJ) | Mechelle Me5000 | - | 200–975 nm | 300 ns | - | Air | Colon |
| [123] | Nd:YAG (1064 nm, 1 Hz, 6 ns, 30 mJ) | Echelle (Kestrel, SE200) | - | 200–800 nm | 1 µs | - | Air | Stomach |
| [31] | Nd:YAG (532 nm, 5 Hz, 8 ns, 30 mJ) | Mechelle Me5000 | - | 200–900 nm | 0.9 µs | 1 s | Air | Cervical |
| [161] | Nd:YAG (1064 nm, 10 Hz, 6 ns, 50 mJ) | Echelle (Aryelle 200) | - | 193–840 nm | - | - | Air | Cervical |
| [27] | Nd:YAG (1064 nm, 7 ns, 30 mJ) | Mechelle Me5000 | 230–900 nm | 0.8 µs | - | Air | Ovarian | |
| [45] | Ti:Sapphire (775 nm, 150 fs, 1.6 mJ) | Mechelle Me5000 | - | - | 50 ns | 700 µs | Air | Ovarian |
| [172] | Ti:Sapphire (775 nm, 150 fs, 1.54 mJ) | Mechelle Me5000 | 0.013–0.056 nm | 220–850 nm | 50 ns | 700 µs | Helium | Ovarian* |
| [151,165] | Nd:YAG (1064 nm, 1 Hz, 5 ns, 40 mJ) | Avantes AvaSpec 2048-2-USB2 | 0.2–0.3 nm | 190–1100 nm | 1.29 µs | 2 ms | Air | Brain |
| [131] | Nd:YAG (1064 nm, 1 Hz, 10 ns, 50 mJ) | Avantes AvaSpec 2048 | 0.4 nm | 200–1100 nm | 1.2 µs | 2 ms | Air | Brain |
| [39] | Nd:YAG (1064 nm, 1 kHz, 7 ns, 270 µJ) | Five channels | - | 187–887 nm | 0.5 µs | - | Air | Gallbladder |
| [122] | Nd:YAG (1064 nm, 10 Hz, 8 ns, 40 mJ) | Czerny-Turner | 0.3 nm | 250–800 nm | 2 µs, 10 1 µs | - | Air | Prostrate |
| [153] | Nd:YAG (1064 nm, 10 Hz, 8 ns, 270 µJ) | - | - | 127–868 nm | - | - | Air | Oral |
| [132] | Nd:YAG (1064 nm, 10 Hz, 6 ns, 8 mJ) | Mechelle (Me5000), Czerny-Turner | 0.05 nm, 0.1 nm | 250–900 nm | 900 ns | - | Air | Oral |
| Laser-Induced Breakdown Spectroscopy (LIBS) | Other Optical/Spectroscopical Modalities | ||||||
|---|---|---|---|---|---|---|---|
| Objectives | SP/TA | Biomarkers | Remarks | Objectives | SP/TA | Biomarkers | Remarks |
| Diagnostic accuracy of LIBS-DNN for skin cancer | No/in vivo | Higher intensities of Ca, Na, and Fe for cancerous tissues | Use of limited dataset, further clinical studies required | Improvement in skin cancer detection by RS-DLM | No/in vivo | Higher line intensities at certain wavenumbers for cancer tissues | Binary classification restricted to exploring staging of disease |
| Evaluation of LIBS-Raman data fusion method in melanoma diagnosis | FFPE/in vitro | Higher intensities (Ca, Mg), alterations in Raman bands for cancer tissues | Assumption-based study performed only on two subjects | Morpho-chemical characterisation of skin cancer using LC-OCT and CRM | No/ex vivo | Higher intensities of SCC at 821, 1012, 1220, 1446, 1580, 2931 cm−1 | Fluctuation of intensity for the same tissue, same band shift for different pathologies |
| * Identification of melanoma lesions from surrounding dermis | Embedded and slicing/in vitro | Higher intensities (Ca, Mg) in affected tissues | Injection of melanoma into mice, cannot apply to human studies | Melanoma cell identification from melanocyte cells by RS | Incubation and centrifugation/in vitro | Higher intensities of Melanoma cells at 645, 947, 1030, 1453, 1582 cm−1 | Sample preparation involved; limited number of samples being used |
| LIBS imaging of cutaneous tumours correlated with LA-ICP-MS imaging | FFPE/invitro | Higher Ca and Mg content in tumour region | Poor spatial resolution due to ablation; low sensitivity due to matrix effect and self-absorption | LA-ICP-MS elemental imaging of cutaneous tissue as a complementary method | Tissues on glass slide/in vitro | High intensities of Ca, Mg, P, and Zn in tumour tissues | Spatial and in-depth resolution limited, ablation heterogeneous and destructive, matrix effects |
| Spectral analysis using ML algorithms for classification of melanoma stages | FFPE/in vitro | P, Ca, Mg, K | Semi-destructive nature of LIBS restricts in vivo analysis | DMF images of skin lesions processed by AI models to achieve diagnostic accuracy | No/in vivo | Cancer-induced alteration in signals of molecules (melanin and keratin) | Accuracy of diagnostic model not high, time-consuming procedure |
| Classification of melanoma, lymphoma, and healthy subjects based on LIBS-ML algorithms | Serum drops on dry filter paper/in vitro | Ca, Na, K, H, O, N | Reproducibility issues due to shot-to-shot fluctuations, use of filter paper causes uncertainties | Multiphoton microscopy with DL model for diagnostic information on non-melanoma cancer | FFPE/in vitro | MPM images exhibit distinct features for both healthy and abnormal surfaces | Results not very reliable, cross-validation is required |
| Diagnosis of subtype of melanoma malignancies using LIBS elemental imaging | FFPE/in vitro | Ca, Mg | Small sample size (17) used to classify six subtypes of melanoma | CLSM image classification for diagnostic prediction of skin cancer | Staining procedure/in vitro | Alteration in image processing for two groups of normal and SCC cells | Training of technicians required due to complex and extensive diagnostic procedure |
| * Examine the melanoma malignancy using fs-LIBS elemental imaging | Frozen sectioning/in vitro | Ca, Mg | Variation of line intensities (plasma fluctuation); low spatial resolution | Processing of FTIR hyperspectral images to classify skin tumour cells | Cells grown on crystal surface/Ex vivo | Malignant cell lines grow disordered, skin cells are flattened | Misclassification by environmental water vapour interference and artefacts |
| Methods | Objectives | SP/TA | Findings | Challenges | Ref. |
|---|---|---|---|---|---|
| Laser-induced breakdown spectroscopy (LIBS) | Identify cancerous tissues (breast, colon, larynx, and tongue) from normal ones. | Tissues kept in formalin, cut in slices of ~5 × 5 × 2 mm3 /in vitro | Higher plasma temperature for cancerous tissues due to presence of trace elements | Errors in measuring plasma parameters cause uncertainty in element quantification | [35,47] |
| Laser ablation inductively coupled plasma time of flight mass spectroscopy (LA-ICP-TOF–MS) | Comparative analysis of LA-ICP-TOF-MS and histological staining for breast cancer investigation | Paraffin-embedded breast tissues/in vitro | Both methods revealed elevated levels of Cu, Zn, Sr, and Ba in abnormal tissues | Calibration range 0–33 µg g−1, limit of quantification for various metals 11–83 ng g−1. | [193] |
| LIBS and Fourier transform infrared spectroscopy (LIBS-FTIR) | LIBS and FTIR data fusion correction of batch effects in serum spectra for accurate classification of breast cancer using GRAN framework | Centrifugation and storage of the serum sample/in vitro | Superior feature extraction from combined elemental and molecular spectra by CNN model improved diagnosis by mitigating batch effects | Different sample compatibility, computational complexity, concern about reproducibility of results, lack of interpretation of an observed effect | [163] |
| Particle-induced X-ray emission (PIXE) | Measuring alteration of trace elements in breast cancer patients undergoing chemotherapy and restoration to normal levels after chemotherapy | Centrifugation and freezing of serum samples/in vitro | Elevation of Ca, Cr, Fe, and Cu and alleviation of Ti, Zn, and Se in breast cancer tissues | Requires solid sample, light elements not detected, high uncertainty in quantification heterogeneous samples | [194] |
| X-ray fluorescence (XRF), X-ray absorption spectroscopy, wide-angle X-ray scattering | Determining the degree of microcalcification (MC) and trace elements association with breast cancer malignancies | FFPE/in vitro | Irregular hydroxyapatite crystal arrangement and distribution of trace elements (Na, S, Cl, Sr and Y) of cancer-affected tissues | Difficult to detect light elements, overlapping of spectral peaks, requirement of matrix match standards | [195] |
| Inductively coupled plasma mass spectroscopy (ICP-MS), Inductively coupled plasma optical emission spectroscopy (ICP-OES) | Quantification of variation in elemental composition in blood samples of patients | Digestion in microwave oven/in vitro | Reduction in Se and Cr and elevation in Na content in blood of breast cancer patients | Digestion of sample in acid, unable to detect light elements and to quantify halogens, spectral drift; spectral interferences | [196] |
| Cancer Type and Ref. | Sample Preparation | Sample Size | Number of Spectra | Data Preprocessing | Plasma Parameters | Dimensionality Reduction | Model | Model Accuracy |
|---|---|---|---|---|---|---|---|---|
| Blood and skin [48] | ✓ | Moderate | Moderate | ✓ | ✕ | ✓ | KNN | High |
| Skin [118] | ✓ | - | Small | ✓ | ✕ | ✕ | ANN/PLS-DA | High |
| Skin * [150] | ✓ | Moderate | Small | ✓ | ✕ | ✓ | BP_AdaBoost | High |
| Skin [52] | ✓ | Large | Large | ✓ | ✕ | ✓ | DNN | - |
| Skin * [168] | ✓ | - | - | ✓ | ✓ | ✓ | Gradient boosting | High |
| Breast [163] | ✓ | Large | Small | ✓ | ✕ | ✕ | CNN | Low |
| Breast [175] | ✓ | Large | Moderate | ✓ | ✕ | ✓ | SVM | Low |
| Breast [162] | ✓ | Large | Moderate | ✓ | ✕ | ✓ | PSCNN | High |
| Breast/liver [81] | ✓ | Small | Large | ✓ | ✕ | ✓ | RF/ANN | High |
| Breast [35] | ✓ | Large | Large | ✓ | ✓ | ✕ | ✕ | ✕ |
| Breast/colon/larynx/tongue [47] | ✓ | Large | Large | ✓ | ✓ | ✕ | ✕ | ✕ |
| Lungs/liver/oesophageal [158] | ✓ | Large | Moderate | ✓ | ✕ | ✓ | SVM | Moderate |
| Lungs [121] | ✓ | Large | Small | ✓ | ✕ | ✕ | XGBoost | Low |
| Lungs [159] | ✓ | Large | Small | ✓ | ✕ | ✓ | PCA-Boosting tree | High |
| Lungs [164] | ✓ | Large | Small | ✓ | ✕ | ✓ | RF-1D ResNet | High |
| Lungs [160] | ✓ | Moderate | Small | ✓ | ✕ | ✓ | KNN | High |
| Lungs [157] | ✓ | Moderate | Small | ✓ | ✕ | ✓ | KPCA-SVM | High |
| Blood [166] | ✓ | Moderate | Small | - | ✕ | - | RSM-LDA | High |
| Blood [119] | ✓ | Moderate | Moderate | ✓ | ✕ | ✓ | LDA/KNN | High |
| Brain [131] | ✓ | - | - | - | ✓ | ✕ | ✕ | ✕ |
| Brain [165] | ✓ | Small | Small | ✓ | ✕ | ✓ | SVM | High |
| Brain [151] | ✓ | Small | Small | ✓ | ✕ | ✓ | SNN | Moderate |
| Colon [120] | ✓ | Small | Small | ✓ | ✓ | ✕ | ✕ | ✕ |
| Colon [50] | ✓ | Small | Small | ✓ | ✓ | ✕ | ✕ | ✕ |
| Stomach [123] | ✓ | Small | - | ✓ | ✕ | ✕ | ✕ | ✕ |
| Stomach [156] | ✓ | Small | Small | ✓ | ✕ | ✓ | SVM/KNN/PLS-DA | High |
| * Ovarian [172] | ✓ | Large | Large | ✓ | ✕ | ✕ | RF | Low |
| Ovarian [27] | ✓ | Large | Moderate | ✓ | ✕ | ✓ | BPNN | - |
| Cervical [31] | ✓ | Small | Small | ✓ | ✕ | ✓ | PCA-SVM | High |
| Oral [153] | ✓ | Small | Moderate | ✓ | ✕ | ✕ | LR | - |
| AI Model/Sample | Accuracy (%) | Sensitivity (%) | Specificity (%) | ROC Curve (AUC) | Cross-Validation | Cancer Type, Ref. |
|---|---|---|---|---|---|---|
| PCA-KNN/Serum | 96 | 97 | 95.6 | 0.99 | 10-folds | Blood [48] |
| PCA-KNN/Serum | 96 | 89.2 | 99.4 | 0.986 | 10-folds | Skin [48] |
| DNN/skin tissues | - | 94.6 | 88.9 | 10-folds | Skin [52] | |
| PCA-LDA/pellets for melanoma | - | 99.4 | 100 | - | 10-folds | Skin * [145] |
| PCA-LDA/excised tissues of melanoma | - | 96.7 | 99.7 | - | 10-folds | Skin * [145] |
| BP_AdaBoost/serum for early screening | 86.1 | - | - | - | 10-folds | Skin * [150] |
| BP_AdaBoost/serum for staging | 96.1 | - | - | - | 10-folds | Skin * [150] |
| ANN/melanoma FFPE | 100 | 100 | 100 | 1 | - | Skin [118] |
| PLS-DA/melanoma FFPE | 100 | 100 | 100 | 1 | - | Skin [118] |
| Gradient boosting/Serum on Cu substrate | 96.3 | - | - | - | 5-folds | Skin * [168] |
| CNN/serum (batch 2) | 59.9 | 0.48 | 0.71 | 0.64 | - | Breast [163] |
| GRAN/serum (batch 2) | 89.7 | 0.99 | 0.80 | 0.950 | - | Breast [163] |
| Narrow NN/whole blood | 91.7 | 97.2 | 87.5 | 0.93 | 10-fold | Breast [175] |
| Decision fine Tree/serum | 89.7 | 95.2 | 83.3 | 0.87 | 10-fold | Breast [175] |
| PSCNN/blood plasma | 90 | 86 | 94 | 0.95 | 5-folds | Breast [162] |
| RF, ANN, KNN/breast and liver tissue on quartz glass substrate | >94 | - | - | - | 10-folds | Breast Liver [81] |
| BVF/serum samples on silicon substrate | 92.53 | 92.92 | - | - | - | Lungs Liver Esophageal [158] |
| CNN/lung tissues | 99.17 | 99.17 | 99.88 | 1 | - | Lungs [121] |
| RF boosting tree/lung tissues | 98.9 | 99.3 | 98.6 | 0.982 | 10-folds | Lungs [159] |
| RF-1D ResNet/lung tissues | 91.1 | 91.3 | 91.3 | 0.99 | - | Lungs [164] |
| Bagged tree/tumour and normal tissues | 98.9 | 98.6 | 99.3 | 0.982 | 10-folds | Lungs [160] |
| KPCA-SVM/tumour and normal tissues | 99.03 | 99.72 | 98.89 | 0.970 | - | Lungs [157] |
| RSM-LDA/serum | 91 | - | - | - | - | Blood [166] |
| PCA-LDA/blood | 99.78 | 99.6 | 99.8 | 1 | 10-folds | Blood [119] |
| PCA-KNN/blood | 99.72 | 99.7 | 99.7 | 1 | 10-folds | Blood [119] |
| FS-SVM/glioma and infiltrative tissue samples | 95 | - | - | - | - | Brain [165] |
| SNN/tumour tissues | 88.62 | - | - | - | - | Brain [151] |
| BPNN/blood | - | 71.4 | 86.5 | - | Ovarian [27] | |
| CNN/cervical cancer cells on silicon wafer | 97.92 | - | - | - | - | Cervical [161] |
| PCA-SVM/cervical tissues embedded in paraffin wax | 94.4 | - | - | - | - | Cervical [31] |
| PCA-NN/prostate tissue microarrays | 97 | - | - | - | 5-folds | Prostrate [122] |
| PCA/blood and biological fluids on superhydrophobic (PDMS) substrate | - | 88 | 96 | - | - | Oral [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dastageer, M.M.; Siraj, K.; Pedarnig, J.D.; Zhang, D.; Qasim, M.; Rahim, M.S.A.; Mushtaq, S.; Younas, Q.; Hussain, B. From Fundamentals of Laser-Induced Breakdown Spectroscopy to Recent Advancements in Cancer Detection and Calcified Tissues Analysis: An Overview (2015–2025). Molecules 2025, 30, 4176. https://doi.org/10.3390/molecules30214176
Dastageer MM, Siraj K, Pedarnig JD, Zhang D, Qasim M, Rahim MSA, Mushtaq S, Younas Q, Hussain B. From Fundamentals of Laser-Induced Breakdown Spectroscopy to Recent Advancements in Cancer Detection and Calcified Tissues Analysis: An Overview (2015–2025). Molecules. 2025; 30(21):4176. https://doi.org/10.3390/molecules30214176
Chicago/Turabian StyleDastageer, Muhammad Mustafa, Khurram Siraj, Johannes David Pedarnig, Dacheng Zhang, Muhammad Qasim, Muhammad Shahzad Abdul Rahim, Saba Mushtaq, Qaneeta Younas, and Bareera Hussain. 2025. "From Fundamentals of Laser-Induced Breakdown Spectroscopy to Recent Advancements in Cancer Detection and Calcified Tissues Analysis: An Overview (2015–2025)" Molecules 30, no. 21: 4176. https://doi.org/10.3390/molecules30214176
APA StyleDastageer, M. M., Siraj, K., Pedarnig, J. D., Zhang, D., Qasim, M., Rahim, M. S. A., Mushtaq, S., Younas, Q., & Hussain, B. (2025). From Fundamentals of Laser-Induced Breakdown Spectroscopy to Recent Advancements in Cancer Detection and Calcified Tissues Analysis: An Overview (2015–2025). Molecules, 30(21), 4176. https://doi.org/10.3390/molecules30214176