Coumarin Derivatives as Anticancer Agents: Mechanistic Landscape with an Emphasis on Breast Cancer
Abstract
1. Introduction
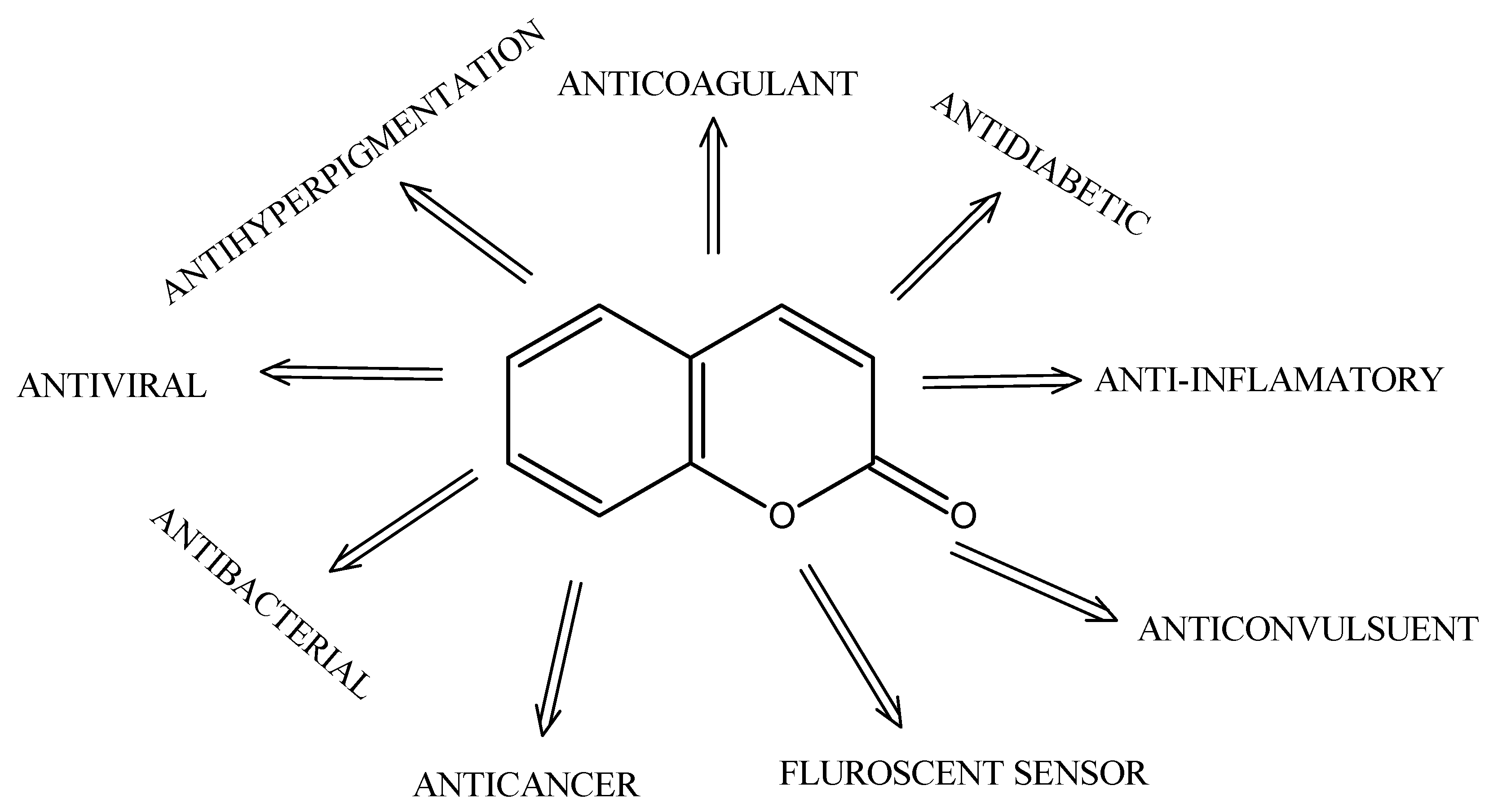
2. Coumarin Characteristics
3. Mechanistic Exploration of Coumarin Derivatives as Promising Anticancer Agents
4. Coumarin-Based Derivatives for Targeted Prostate Cancer Therapy
4.1. Esculetin (6,7-Dihydroxycoumarin): Induction of Apoptosis and G1 Arrest in Human Prostate Cancer Cells
4.2. Benzylidene Coumarin Hybrids as Targeted Anti-Prostate Cancer Agents: Dual Inhibition of EGFR and PI3Kβ
4.3. Coumarin–Benzimidazole Compounds as Emerging Anticancer Agents in Prostate Cancer Therapy
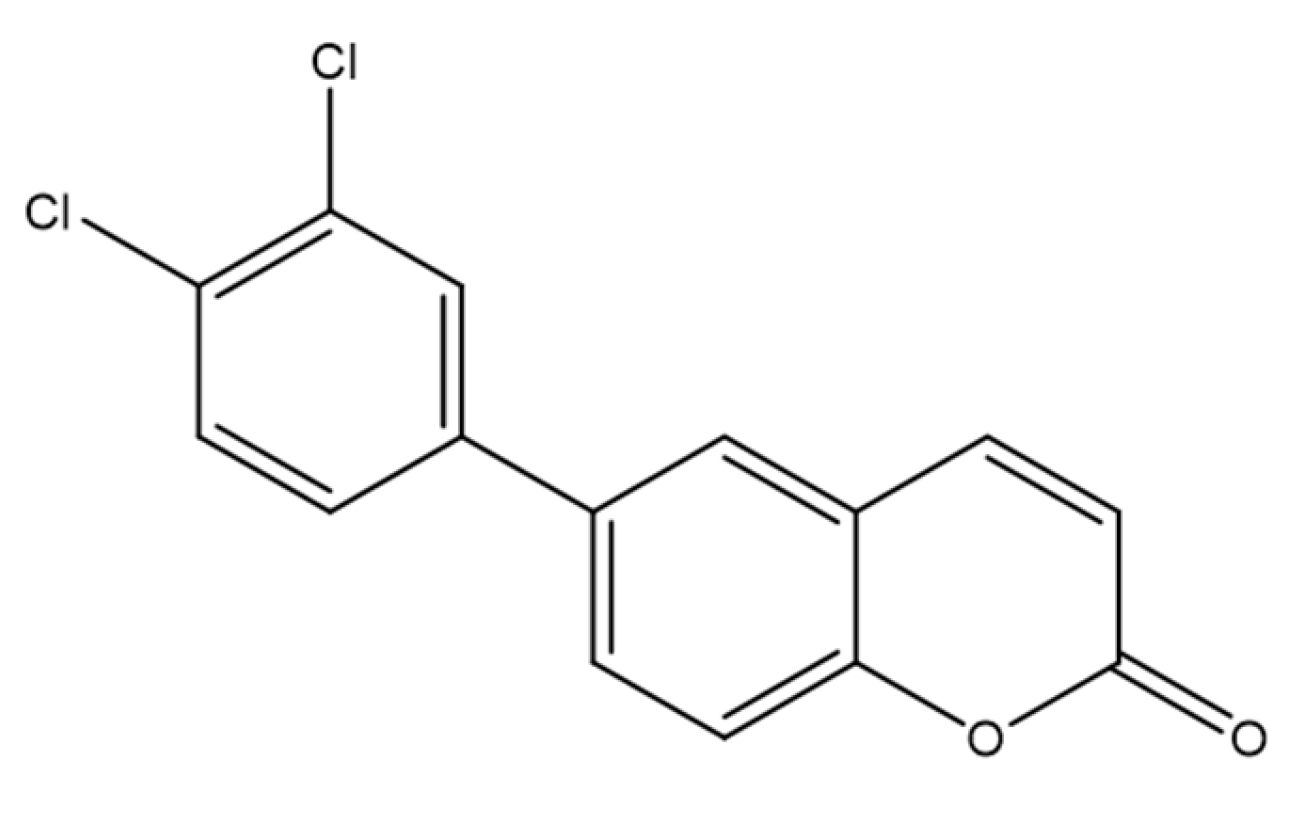
4.4. 3-(4-Nitrophenyl)coumarin Derivatives as Emerging Anticancer Agents in Prostate Cancer Therapy
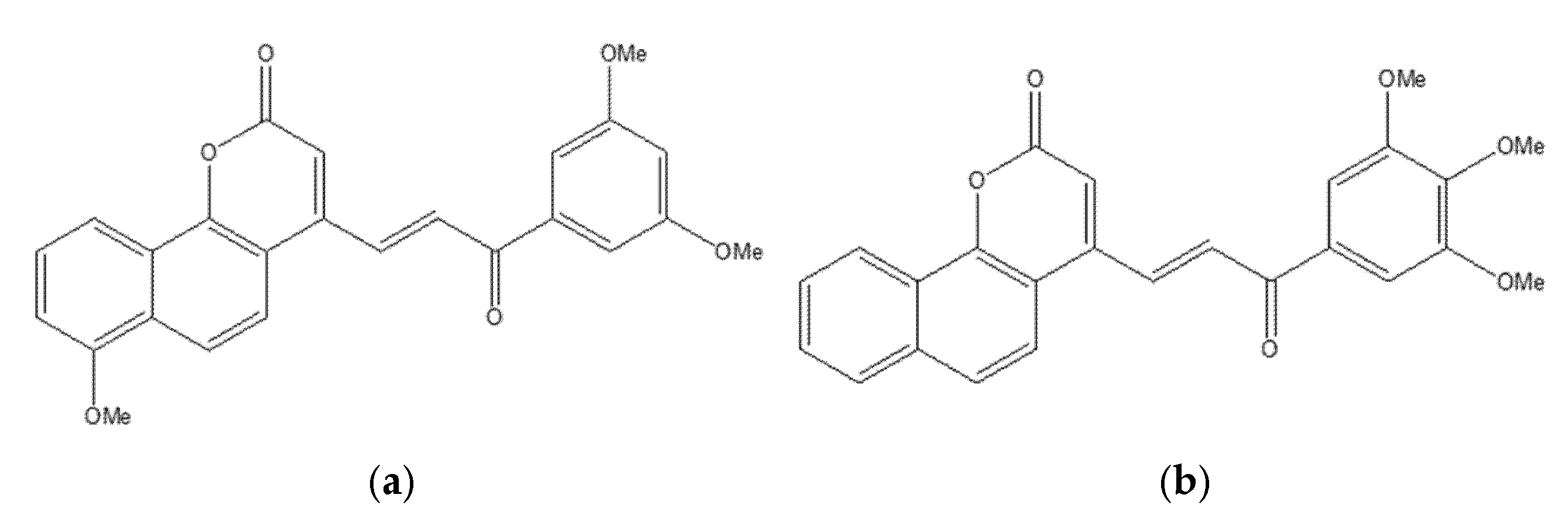
4.5. Linear Furanocoumarin Hybrids as Emerging Anticancer Agents in Breast and Prostate Cancer Therapy
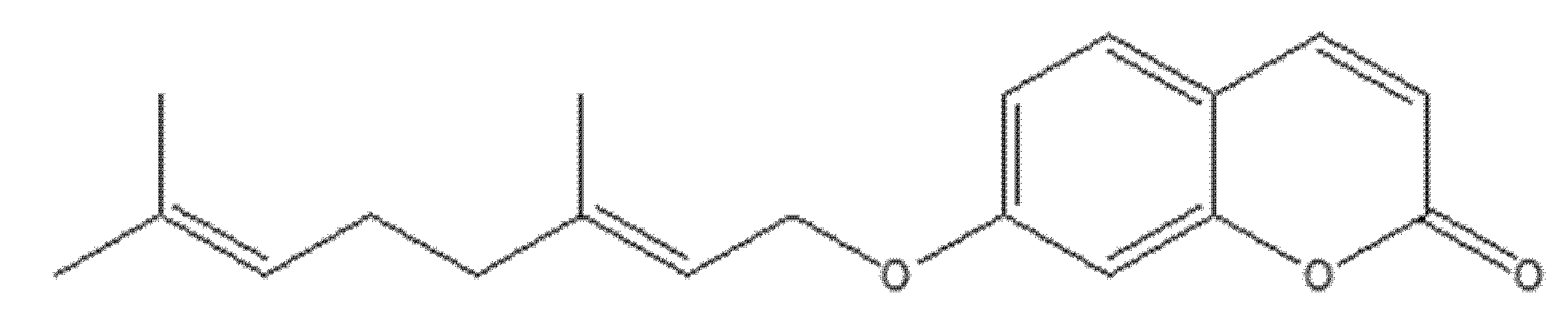
4.6. Antiproliferative Activity of 8-Isopentenyloxy Coumarin in Prostate Cancer Cells
4.7. Evaluation of Coumarin-Based Compounds in PC-3 Prostate Cancer Cells
5. Coumarin-Based Derivatives for Targeted Lung Cancer Therapy
5.1. Coumarin Hybrids as Modulators of Epithelial–Mesenchymal Transition and Cell Migration in Lung Cancer Models
5.2. Coumarin Hybrids as Dual-Acting Topoisomerase Inhibitors with Antiproliferative Potential in A549 Lung Cancer Cells
5.3. Novel Coumarin–Piperazine-2(5H)-Furanone Hybrids as Potential Anti-Lung Cancer Agents: Synthesis, Biological Evaluation, and Molecular Docking Studies
5.4. Benzyl Coumarin–Furoxan Derivatives as Inducers of Apoptosis and Autophagy in Non-Small-Cell Lung Cancer
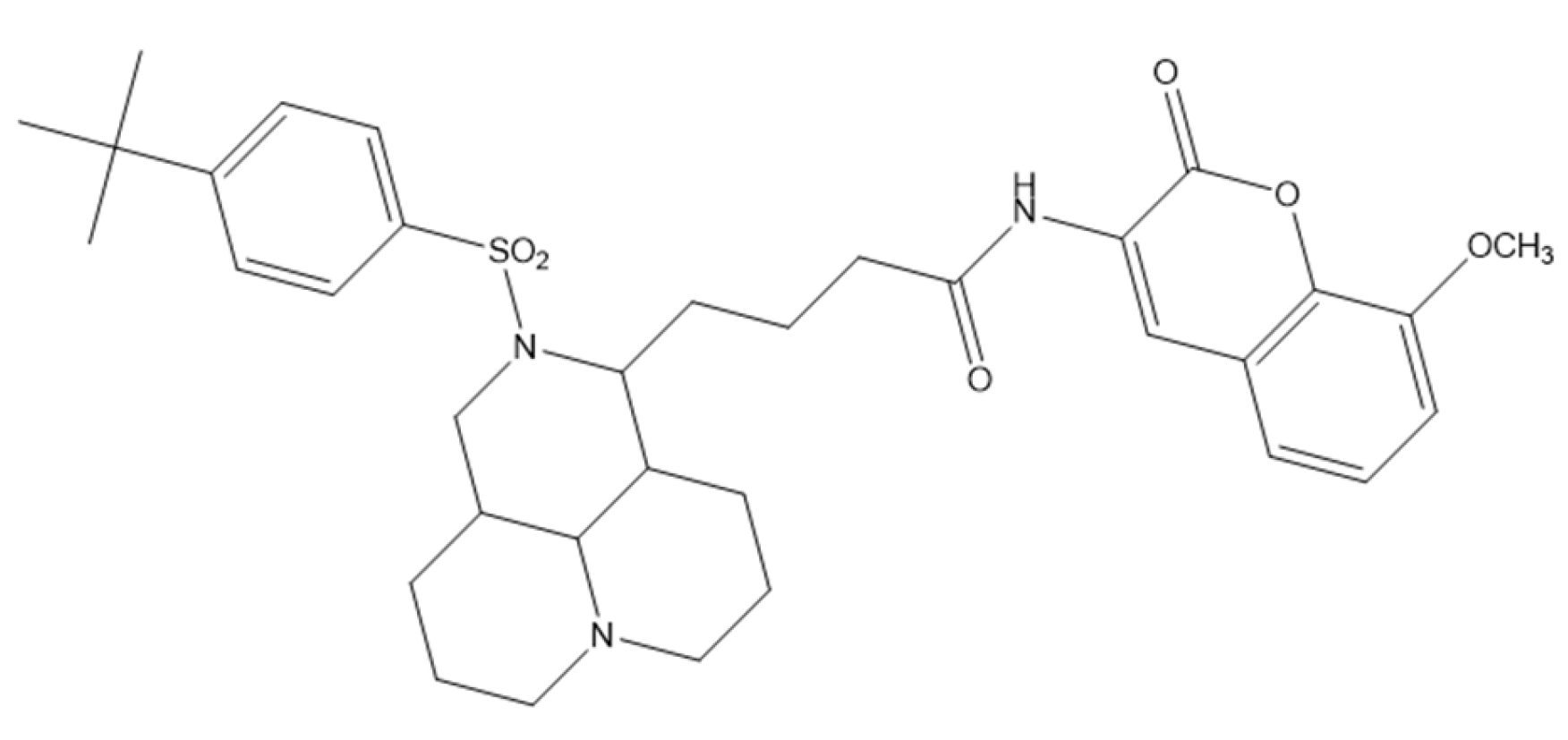
5.5. Coumarin–Matrine Hybrids as Selective Hsp90 (NTD and CTD) Isoform Inhibitors for Lung Carcinoma Therapy
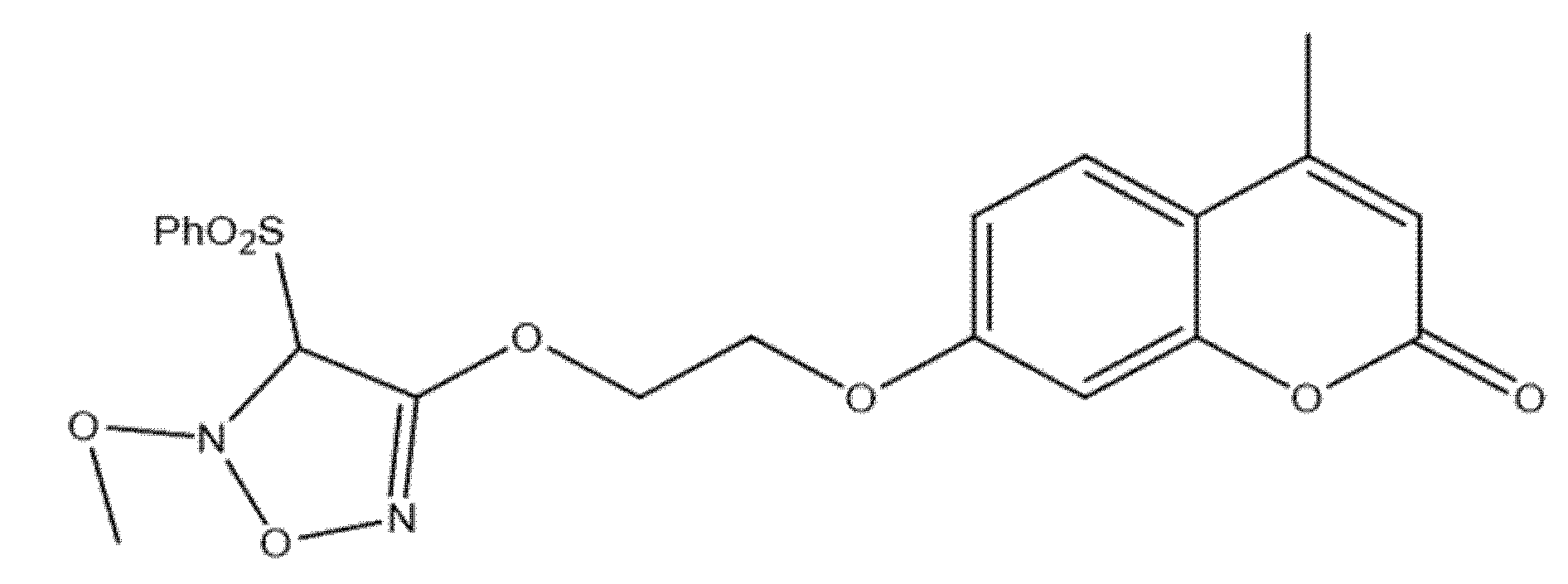
5.6. Coumarin–Phenylsulfonyl Furoxan Hybrids as Modulators of Programmed Cell Death Through Apoptosis and Autophagy in Lung Adenocarcinoma (A549)
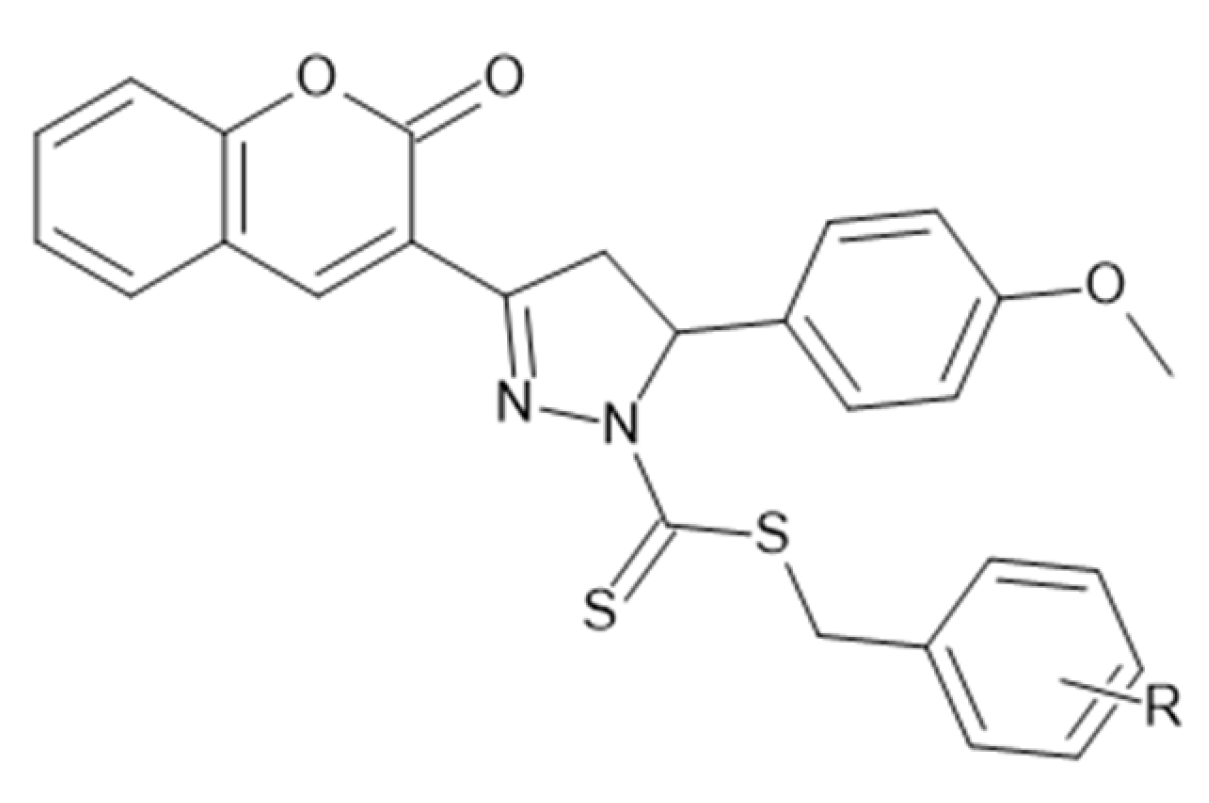
5.7. Coumarin–Pyrazole Carbodithioate Hybrids as Potent CDK2 Inhibitors for Lung Carcinoma Therapy (A549; PDB: 1DI8)
5.8. Azaheterocyclic Coumarin Derivatives as Multitargeted Kinase Inhibitors Induce Apoptosis in Non-Small-Cell Lung Carcinoma (A549; PDB: 2OH4)
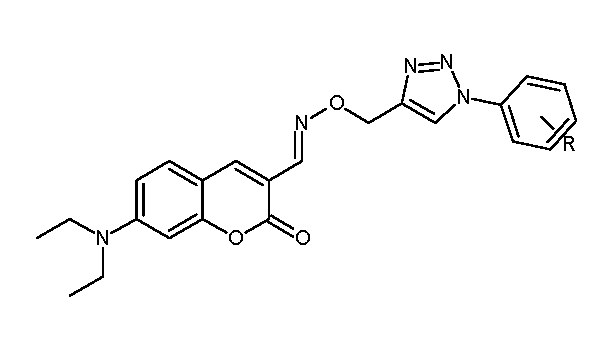
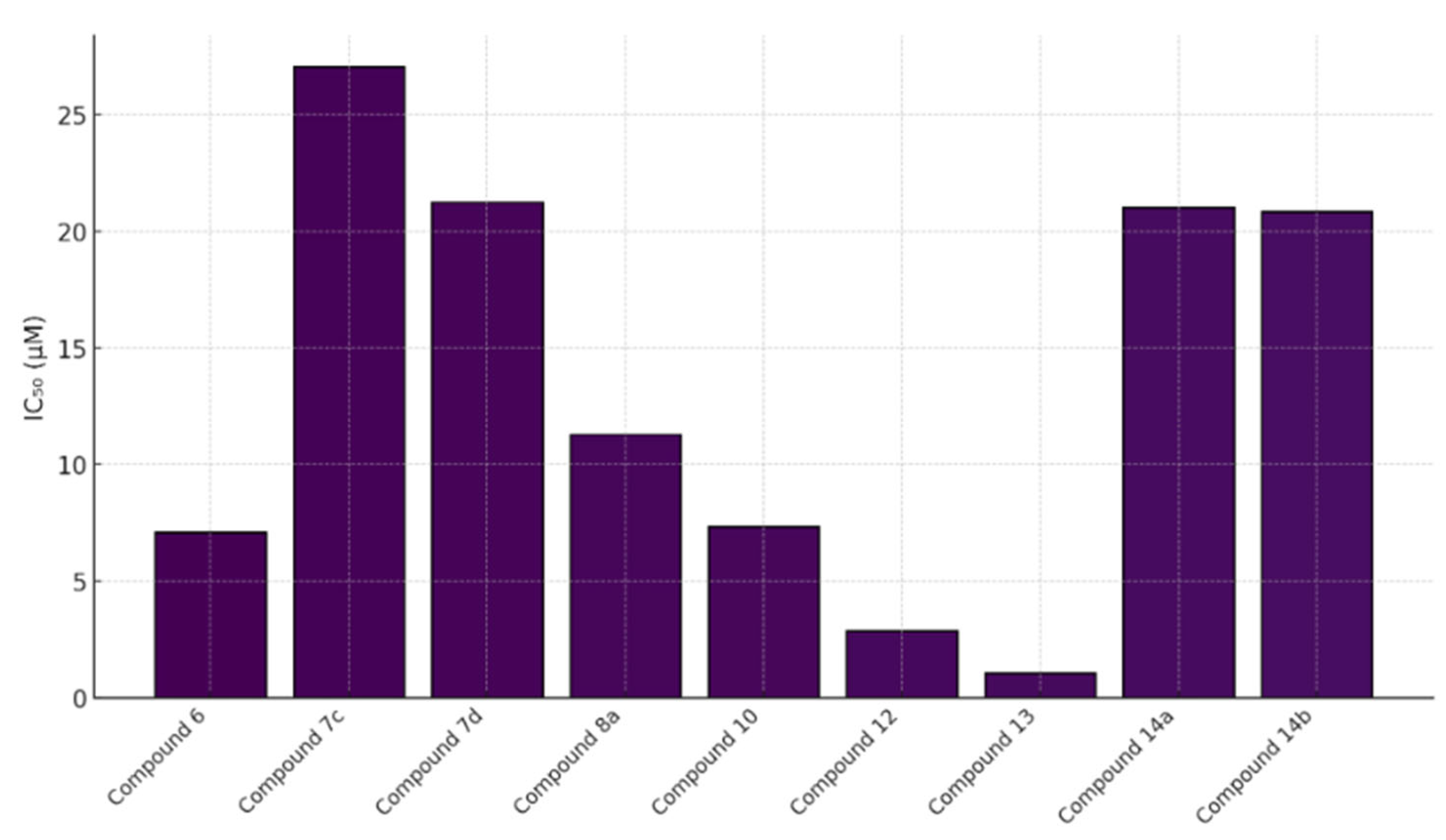
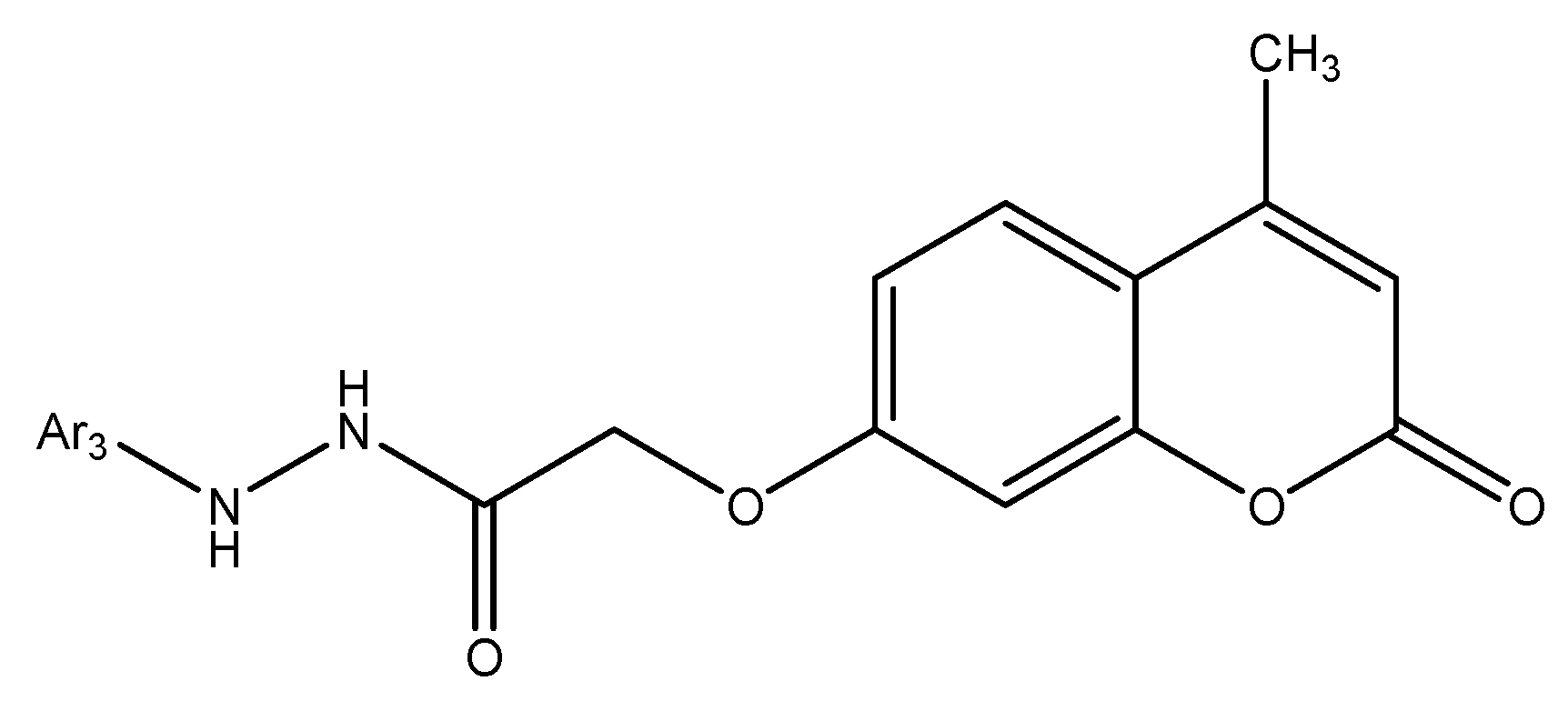
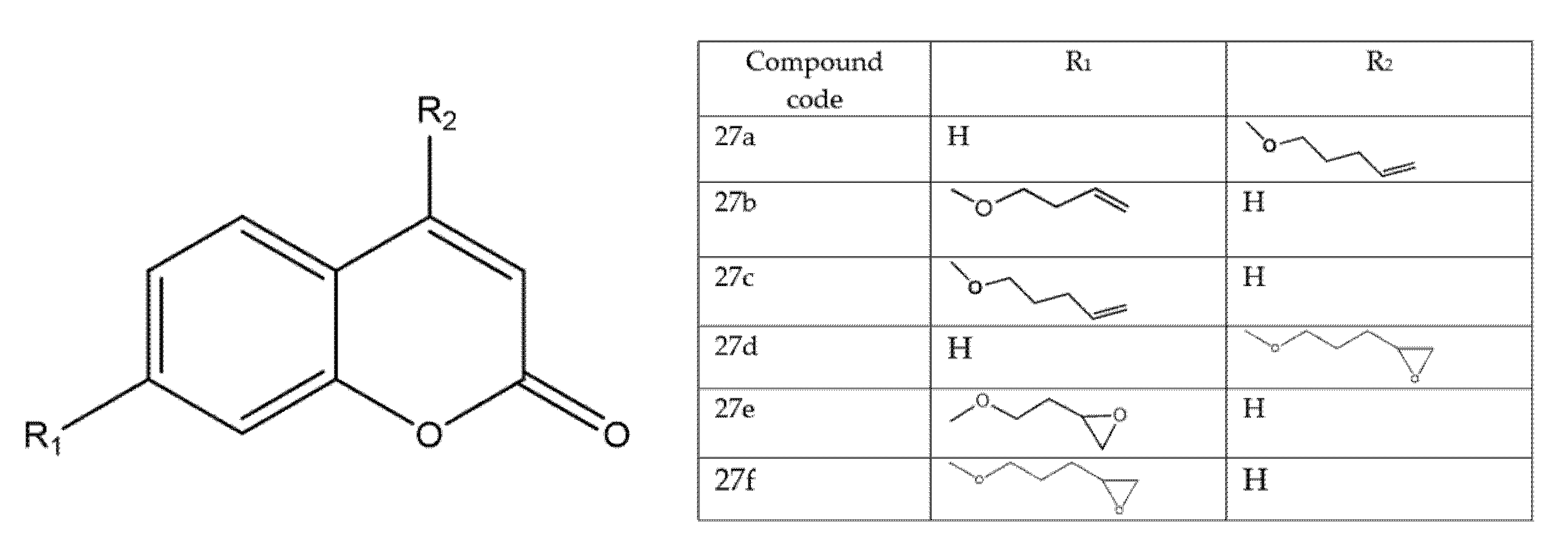
5.9. Coumarin-Based 1,2,3-Triazole-Linked Hybrids as Promising Anticancer Agents Against Lung Cancer: Design, Synthesis, and Cytotoxicity Evaluation
6. Coumarin-Based Derivatives for Targeted Breast Cancer Therapy
6.1. Coumarin Hybrids as Promising VEGFR-2 Inhibitors: Molecular Modeling and Anticancer Potential Against Breast Cancer
6.2. Coumarin Hybrids as Promising Dual-Target Agents Against Breast and Cervical Cancer Through Modulation of VEGFR-2 and p38α MAPK Pathways
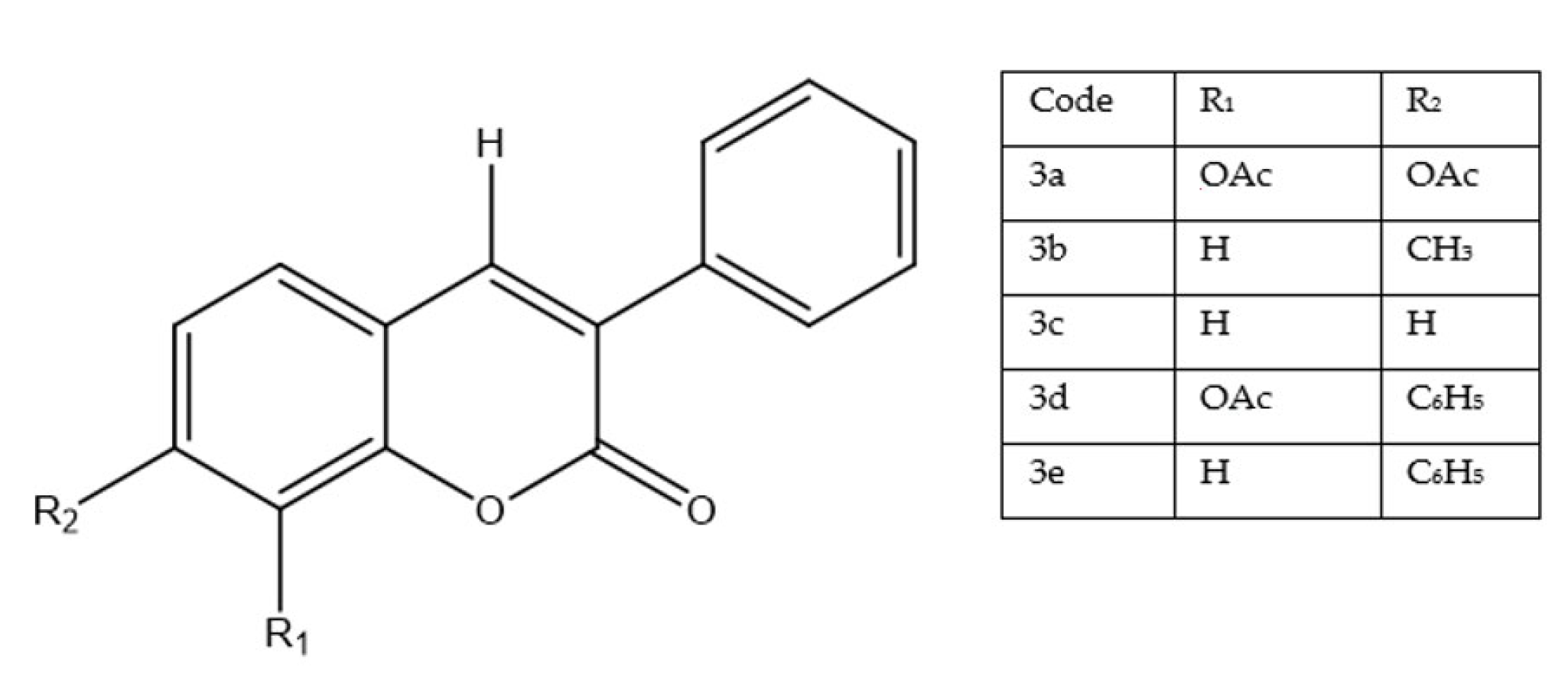
6.3. 4,7-Disubstituted Coumarin Derivatives as Dual Aromatase and EGFR Inhibitors in Breast Carcinoma (MCF-7, MDA-MB-231; PDB: 3EQM, 1M17)
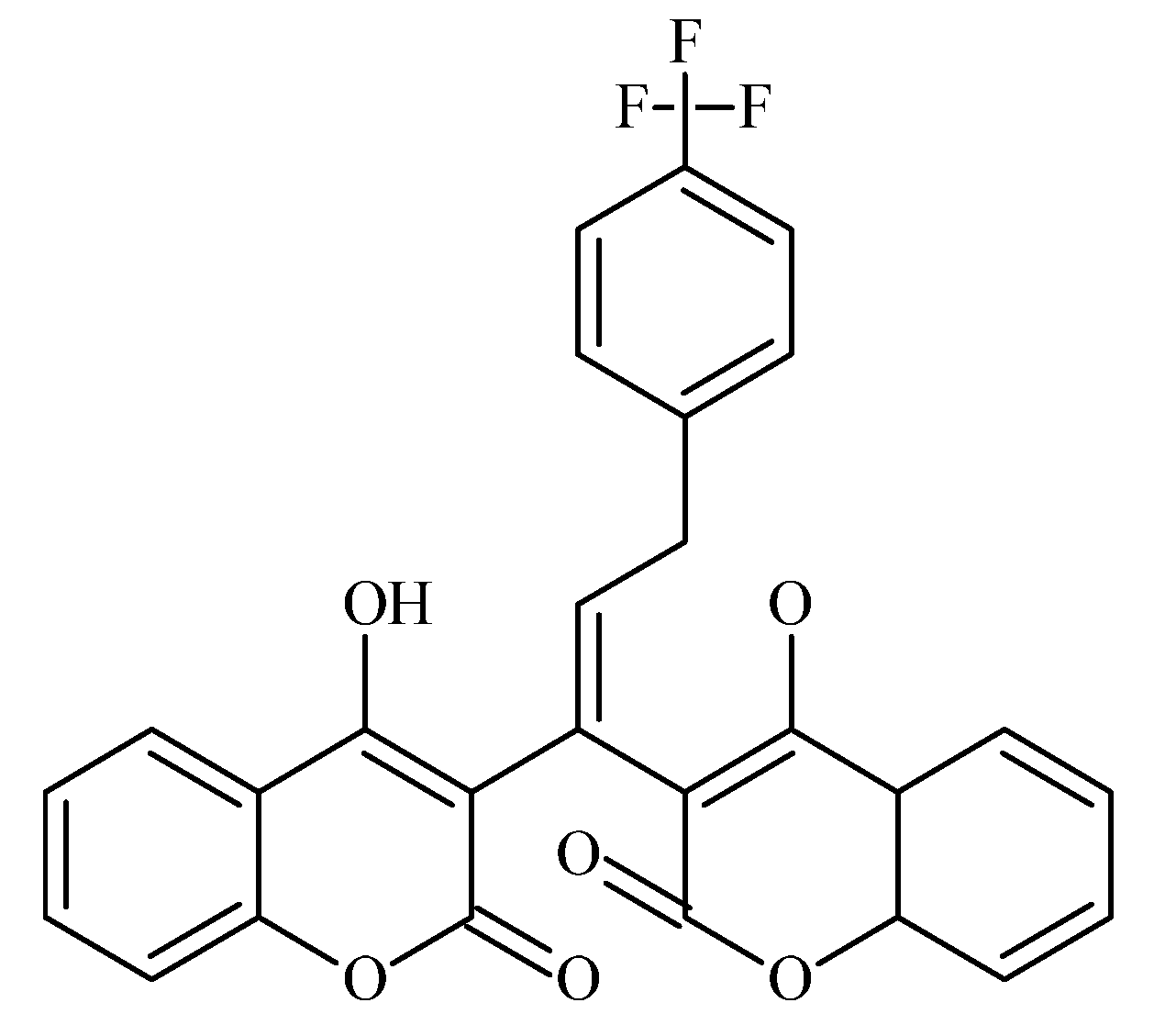
6.4. Neo-Tanshinlactone–Chalcone Coumarin Hybrids as TNF-α–Targeted Anticancer Agents in Breast Carcinoma (MCF-7, MDA-MB-231; PDB: 2AZ5)
6.5. Fluorinated Coumarin Derivatives as Dual VEGFR-2 and p38α MAPK Inhibitors in Breast and Cervical Carcinoma (MCF-7, HeLa; PDB: 3U6J, 3FMK)
6.6. Coumarin–Pyrimidine–Triazole Hybrids as Multitargeted Computational Leads for Breast Carcinoma (MCF-7; PDB: 3SRQ, 4KD7; Tubulin Colchicine/Vinblastine Sites)
6.7. Coumarin-Based Derivatives as Caspase-9 and BCL-2 Modulators Inducing Apoptosis in Breast Carcinoma (MCF-7; PDB: 1NW9, 4MAN)
6.8. Coumarin–1,3,4-Oxadiazole Hybrids as Estrogen-Receptor Modulators in Breast Carcinoma (MCF-7, MDA-MB-231; PDB: 3ERT)
7. Coumarin-Based Derivatives for Targeted Blood Cancer Therapy
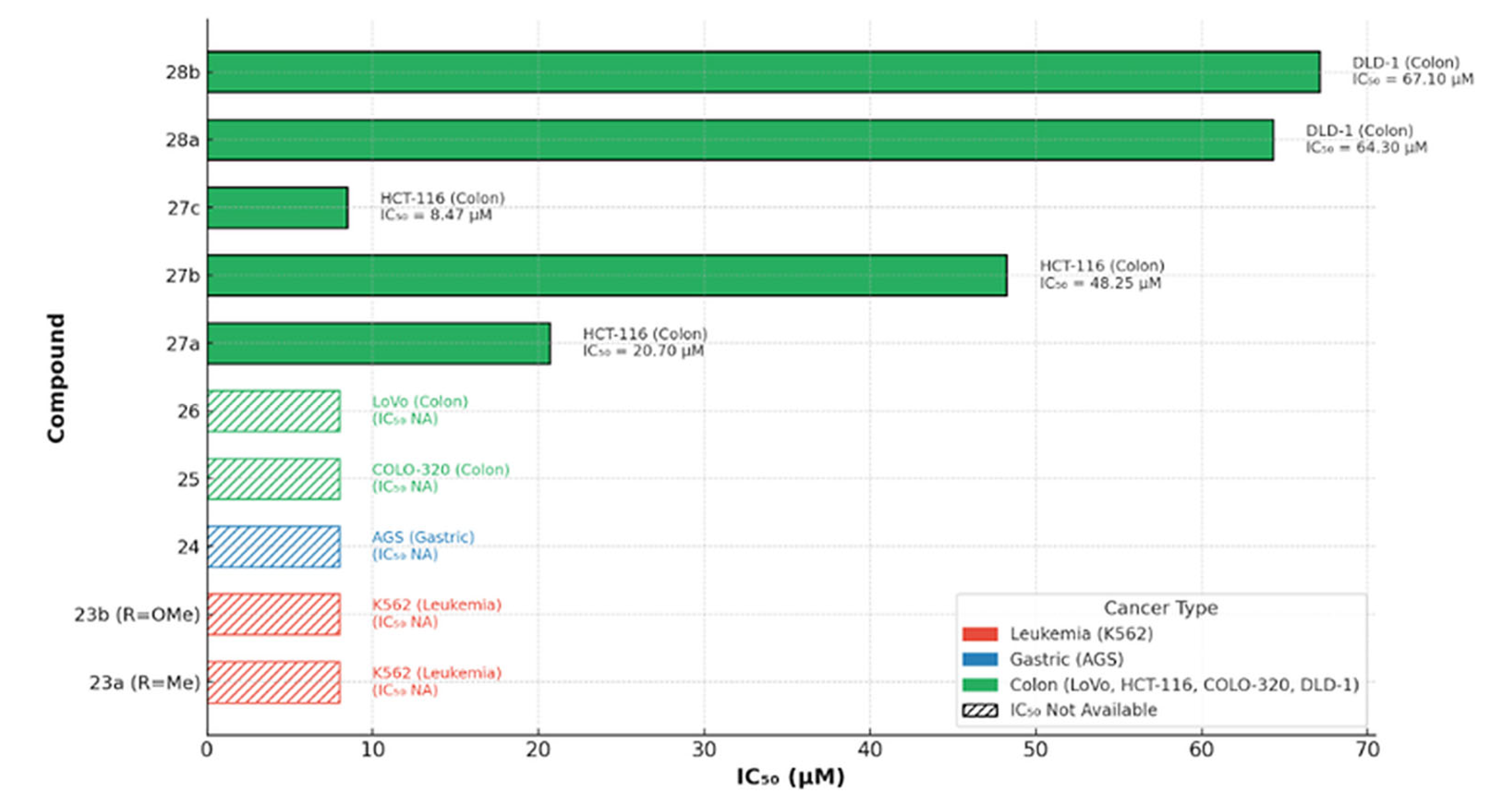
Cytotoxic Potential of Benzofuran–Chromone–Coumarin Hybrids Against Leukemia Cells
8. Coumarin-Based Derivatives for Targeted Stomach Cancer Therapy
8.1. Coumarin-Based Hybrids (SSBC, Compound 24) as Pro-Apoptotic Agents in Gastric Carcinoma (AGS; BH3 Domain)
8.2. Coumarin and Furocoumarin Hybrids from Citrus trifoliata as Dual Antiproliferative and P-Glycoprotein Inhibitors in Colorectal Carcinoma (COLO 320; P-gp)
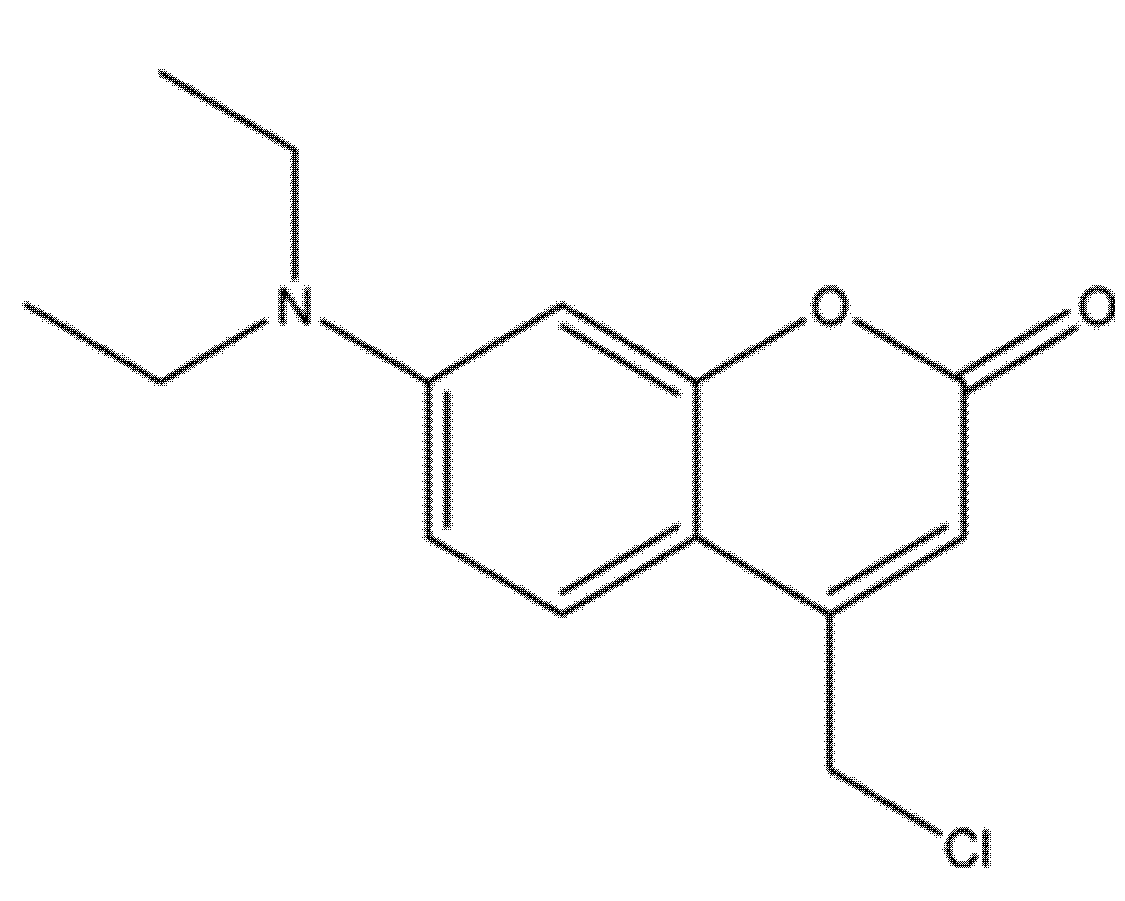
8.3. 7-Diethylamino-4-Chloromethylcoumarin (7D4C) as a Selective Inducer of Mitochondria- and p53-Mediated Apoptosis in Colorectal Carcinoma (LoVo)
8.4. Coumarin-Based Hybrids as Dual DNA-Polymerase Inhibitors and Antiproliferative Agents in Colorectal Cancer (HCT-116)
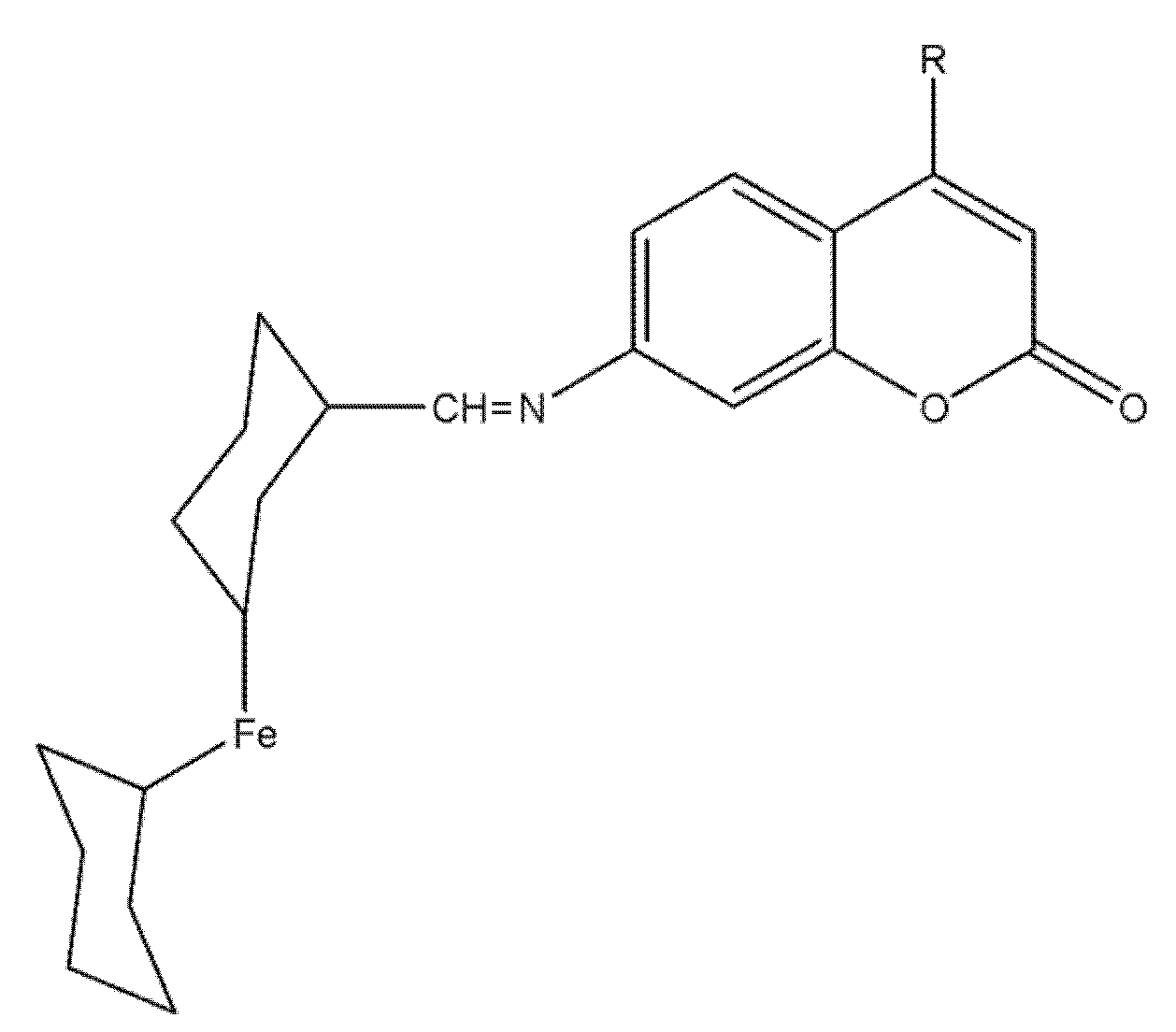
8.5. Ferrocene–Coumarin Hybrids as Selective Antiproliferative Agents in Colorectal Carcinoma (DLD-1)
9. Structure–Activity Relationships and Recent Design Trends in Coumarin-Based Anticancer Agents
| Chemotype | Primary Target(s) | Model | Potency (IC50/Ki) | Dominant Phenotype | Citation |
|---|---|---|---|---|---|
| VEGFR-2-active coumarin (compound 4a) | VEGFR-2 kinase | MCF-7 | Cell IC50 1.24 µM; VEGFR-2 IC50 0.36 µM | G2/M arrest; caspase-9; pre-G1 apoptosis | [36] |
| 7-substituted multitarget coumarins | EGFR; aromatase (CYP19A1) | MDA-MB-231 > MCF-7 | MDA-MB-231 IC50 ≈ 1.9–3.5 µM; MCF-7 IC50 ≈ 3.9–6.0 µM | Apoptosis; G0/G1 or S arrest | [64] |
| Fluorinated coumarins (series) | VEGFR-2; p38α MAPK | MCF-7 | VEGFR-2 inhibition up to 94%; cell IC50 7.9–8.3 µg/mL | Antiproliferative; kinase inhibition | [61] |
| Benzimidazole–coumarin hybrids | PI3K/Akt/mTOR axis | Breast-cancer models | Pathway suppression (biochemical) | Caspase-dependent apoptosis | [15] |
| Coumarin PARP-1 inhibitors (8-carbamyl-3-arylcoumarin) | PARP-1/2 | BRCA-mutant lines (e.g., SUM149PT, HCC1937) | PARP-1 IC50 2.53 nM; PARP-2 IC50 6.45 nM; antiproliferative IC50 0.62–4.26 µM | G2/M arrest; ROS↑; DSB↑; apoptosis | [85] |
| P-gp (ABCB1) modulator LL-348 (coumarin) | MDR/P-gp chemosensitization | PK/transport models; combo with paclitaxel | Enhanced oral absorption and tumor uptake of paclitaxel | MDR reversal; chemosensitization | [86] |
| Aromatase-active coumarin (4-benzyl-3-(4′-chlorophenyl)-7-methoxy) | CYP19A1 (aromatase) | MCF-7aro | Ki ≈ 84 nM (competitive) | Suppresses androgen-driven proliferation (3D) | [87] |
| Hsp90-engaging coumarins (NTD/CTD concepts) | Hsp90 (NTD/CTD) | A549 ± in vivo | Cell IC50 (series-dependent) | Chaperone disruption; tumor-growth inhibition signals | [88] |
| Coumarin hybrids (Topo I/CDK2-oriented) | Topoisomerase I; CDK2 | A549 | Cell IC50 (series-dependent) | Cell-cycle arrest; apoptosis | [89,90] |
9.1. VEGFR-2–Oriented Coumarins: 4-Aryl/Benzyl Patterns and G2/M-Linked Apoptosis
9.2. EGFR/Aromatase-Biased Designs vs. Breast-Cancer Subtype (ER+ vs. TNBC)
9.3. PI3K/Akt/mTOR ± Autophagy: Protective vs. Non-Protective Settings
9.4. Hsp90 Engagement (NTD/CTD): Dual-Domain Strategies and In Vivo Signals
9.5. Topoisomerase I/CDK2 and Linker/Halogen SAR in A549
9.6. MDR Chemosensitization (P-gp) and Delivery-Aware Optimization
9.7. Translational Design Rules: Potency ↔ Exposure ↔ Selectivity Funnel
9.8. Cross-Indication Synthesis and Outlook
- (1)
- (2)
- Resistance-aware combinations—pre-plan co-therapies to neutralize protective autophagy or efflux-mediated resistance, reserving systemic P-gp inhibition for delivery strategies that bias tumor exposure; consensus reviews highlight autophagy’s dual role and the translational challenges of ABC-transporter blockade [103,107,121].
- (3)
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-MA | 3-Methyladenine (autophagy inhibitor) |
| ADME | Absorption, Distribution, Metabolism, Excretion |
| ADMET | Absorption, Distribution, Metabolism, Excretion, Toxicity |
| AEs | Adverse Events |
| AGS | Human gastric adenocarcinoma cell line |
| Akt | Protein kinase B |
| ALB/Alamar Blue | Resazurin-based cell viability assay |
| A549 | Human lung adenocarcinoma cell line |
| ATG5 | Autophagy-related 5 |
| ATP | Adenosine triphosphate |
| BAX | Bcl-2–associated X protein |
| BCL-2 | B-cell lymphoma 2 |
| BEAS-2B | Human normal bronchial epithelial cell line |
| BBB | Blood–brain barrier |
| BH3 | BCL-2 homology 3 |
| CAR | Cytarabine |
| Casp-3/-9 | Caspase-3/Caspase-9 |
| CDK2 | C-C motif chemokine 2 |
| CC50 | 50% cytotoxic concentration |
| CDK CDK2 | Cyclin-dependent kinase/Cyclin-dependent kinase 2 |
| CTD | C-terminal domain (e.g., Hsp90-CTD) |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DDI | Drug–drug interaction |
| DHFR | Dihydrofolate reductase |
| DLD-1 | Human colon adenocarcinoma cell line |
| DMSO | Dimethyl sulfoxide |
| DOX | Doxorubicin |
| DU145 | Human prostate carcinoma cell line |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| ER/ERα | Estrogen receptor/Estrogen receptor-alpha |
| ERK1/2 | Extracellular signal-regulated kinases 1/2 |
| FACS | Fluorescence-activated cell sorting |
| FITC | Fluorescein isothiocyanate |
| FGFR | Fibroblast growth factor receptor |
| FT-IR | Fourier-transform infrared spectroscopy |
| GSH | Reduced glutathione |
| H1299/H460/Calu-1 | Human NSCLC cell lines |
| HCT-116 | Human colorectal carcinoma cell line |
| HEK-293 | Human embryonic kidney cell line |
| HeLa | Human cervical carcinoma cell line |
| Hsp90 | Heat shock protein 90 |
| IC50 | Half-maximal inhibitory concentration |
| iCa2+ | Intracellular calcium |
| iROS | Intracellular reactive oxygen species |
| K562 | Human chronic myelogenous leukemia cell line |
| LC3-II | Microtubule-associated proteins 1A/1B light chain 3B (lipidated form) |
| LoVo | Human colorectal adenocarcinoma cell line |
| LNCaP | Human androgen-responsive prostate cancer cell line |
| LY294002 | PI3K inhibitor |
| MALDI-TOF-MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MAPK (p38α) | Mitogen-activated protein kinase (p38 alpha) |
| MCF-7 | Human ER-positive breast cancer cell line |
| MCF-10A | Human normal breast epithelial cell line |
| MDA-MB-231 | Human triple-negative breast cancer cell line |
| MDR | Multidrug resistance |
| MMP (ΔΨm) | Mitochondrial membrane potential |
| MST3 | Mammalian Ste20-like protein kinase 3 |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay |
| mTOR | Mechanistic target of rapamycin |
| NIH-3T3 | Mouse embryonic fibroblast cell line |
| NMR | Nuclear magnetic resonance |
| NSCLC | Non-small-cell lung cancer |
| NTD | N-terminal domain (e.g., Hsp90-NTD) |
| PAINS | Pan-assay interference compounds |
| PARP | Poly(ADP-ribose) polymerase |
| PC-3 | Human prostate carcinoma cell line |
| PD | Pharmacodynamics |
| PDB | Protein Data Bank |
| PDGFR | Platelet-derived growth factor receptor |
| PI (stain) | Propidium iodide |
| PI3K | Phosphoinositide 3-kinase |
| PK/PD | Pharmacokinetics/Pharmacodynamics |
| P-gp | P-glycoprotein (ABCB1) |
| PTEN | Phosphatase and tensin homolog |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| QSAR | Quantitative structure–activity relationship |
| RMSD | Root-mean-square deviation |
| ROS | Reactive oxygen species |
| SAR | Structure–activity relationship |
| SI | Selectivity index |
| SKOV-3 | Human ovarian carcinoma cell line |
| TGI | Tumor growth inhibition |
| TNBC | Triple-negative breast cancer |
| TNF-α | Tumor necrosis factor-alpha |
| Topo I | Topoisomerase I |
| UV–Vis | Ultraviolet–visible spectroscopy |
| VEGFR-2 | Vascular endothelial growth factor receptor-2 |
| WI-38 | Human lung fibroblast cell line |
| ΔΨm | Mitochondrial membrane potential |
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 Cancer Estimates: Data Sources, Methods, and a Snapshot of the Cancer Burden Worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef]
- Elshemy, S.; Abbas, S. Latest Updates in Coumarin Scaffolds as Potent Antiproliferative Agents (2018–2023). Sphinx J. Pharm. Med. Sci. 2025, 9, 77–90. [Google Scholar] [CrossRef]
- Emami, S.; Dadashpour, S. Current Developments of Coumarin-Based Anti-Cancer Agents in Medicinal Chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent Advancements of Coumarin-Based Anticancer Agents: An up-to-Date Review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Bhattarai, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer Potential of Coumarin and Its Derivatives. Mini-Reviews Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Makuch-Kocka, A.; Aebisher, D. Coumarins in Anticancer Therapy: Mechanisms of Action, Potential Applications and Research Perspectives. Pharmaceutics 2025, 17, 595. [Google Scholar] [CrossRef] [PubMed]
- Aqib, M.; Khatoon, S.; Ali, M.; Sajid, S.; Assiri, M.A.; Ahamad, S.; Saquib, M.; Hussain, M.K. Exploring the Anticancer Potential and Mechanisms of Action of Natural Coumarins and Isocoumarins. Eur. J. Med. Chem. 2025, 282, 117088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singla, R.; Dandriyal, J.; Jaitak, V. Coumarin Derivatives as Anticancer Agents for Lung Cancer Therapy: A Review. Anticancer Agents Med. Chem. 2018, 18, 964–984. [Google Scholar] [CrossRef]
- Song, X.; Fan, J.; Liu, L.; Liu, X.; Gao, F. Coumarin Derivatives with Anticancer Activities: An Update. Arch. Pharm. 2020, 353, 2000025. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef]
- Jigar, L.P. The Introduction of Coumarin; Blue Rose Publishers: Noida, India, 2021. [Google Scholar]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as Anticancer Agents: A Review on Synthetic Strategies, Mechanism of Action and SAR Studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Koley, M.; Han, J.; Soloshonok, V.A.; Mojumder, S.; Javahershenas, R.; Makarem, A. Latest Developments in Coumarin-Based Anticancer Agents: Mechanism of Action and Structure–Activity Relationship Studies. RSC Med. Chem. 2024, 15, 10–54. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Sharma, A.; Mao, R.; Jiang, N.; Dun, B.; She, J.-X. Derivatives Containing Both Coumarin and Benzimidazole Potently Induce Caspase-Dependent Apoptosis of Cancer Cells through Inhibition of PI3K-AKT-MTOR Signaling. Anticancer Drugs 2015, 26, 667–677. [Google Scholar] [CrossRef]
- Kerekes, D.; Horváth, A.; Kúsz, N.; Borcsa, B.L.; Szemerédi, N.; Spengler, G.; Csupor, D. Coumarins, Furocoumarins and Limonoids of Citrus Trifoliata and Their Effects on Human Colon Adenocarcinoma Cell Lines. Heliyon 2022, 8, e10453. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Jiang, S.; Dong, M.; Kuerban, K.; Li, J.; Feng, M.; Chen, Y.; Ye, L. A Hybrid of Coumarin and Phenylsulfonylfuroxan Induces Caspase-Dependent Apoptosis and Cytoprotective Autophagy in Lung Adenocarcinoma Cells. Phytomedicine 2018, 39, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Al Hasan, M.S.; Emon, Y.; Alshahrani, M.Y.; Mizan, M.; Uddin, M.B.; Hasan, A.M.W.; Sayeed, M.A.; Mia, E.; Yana, N.T.; Hossan, R.; et al. Coumarin Derivatives as Anticancer Agents Targeting PI3K-AKT-MTOR Pathway: A Comprehensive Literature Review. Med. Oncol. 2025, 42, 301. [Google Scholar] [CrossRef]
- Turkekul, K.; Colpan, R.D.; Baykul, T.; Ozdemir, M.D.; Erdogan, S. Esculetin Inhibits the Survival of Human Prostate Cancer Cells by Inducing Apoptosis and Arresting the Cell Cycle. J. Cancer Prev. 2018, 23, 10–17. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Jang, J.-Y.; Shim, J.-H.; Myung, P.K.; Chae, J.-I. Esculetin, a Coumarin Derivative, Exhibits Anti-Proliferative and Pro-Apoptotic Activity in G361 Human Malignant Melanoma. J. Cancer Prev. 2015, 20, 106–112. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol Induces Cell Cycle Arrest and Apoptosis with Docetaxel in Prostate Cancer Cells via a P53/P21WAF1/CIP1 and P27KIP1 Pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Serttas, R.; Turkekul, K.; Dibirdik, I.; Bilir, A. The Flavonoid Apigenin Reduces Prostate Cancer CD44 + Stem Cell Survival and Migration through PI3K/Akt/NF-ΚB Signaling. Life Sci. 2016, 162, 77–86. [Google Scholar] [CrossRef]
- Abdel Ghany, L.M.A.; Ibrahim, T.S.; Alharbi, A.S.; Abdel-Aziz, M.S.; El-labbad, E.M.; Elagawany, M.; Ryad, N. Development of Certain Benzylidene Coumarin Derivatives as Anti-Prostate Cancer Agents Targeting EGFR and PI3Kβ Kinases. J. Enzyme Inhib. Med. Chem. 2024, 39, 2311157. [Google Scholar] [CrossRef]
- Buckup, M.; Rice, M.A.; Hsu, E.-C.; Garcia-Marques, F.; Liu, S.; Aslan, M.; Bermudez, A.; Huang, J.; Pitteri, S.J.; Stoyanova, T. Plectin Is a Regulator of Prostate Cancer Growth and Metastasis. Oncogene 2021, 40, 663–676. [Google Scholar] [CrossRef]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Lacy, A. Studies on Coumarins and Coumarin-Related Compounds to Determine Their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.S.; Payá, M. Pharmacological and Biochemical Actions of Simple Coumarins: Natural Products with Therapeutic Potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Latinwo, L.M.; Virgile, C.; Badisa, V.L.D.; Gbadebo, A.J. Synthesis and in Vitro Evaluation of 3-(4-Nitrophenyl)Coumarin Derivatives in Tumor Cell Lines. Bioorg. Chem. 2015, 58, 96–103. [Google Scholar] [CrossRef]
- Mohler, J.L.; Gomella, L.G.; Crawford, E.D.; Glode, L.M.; Zippe, C.D.; Fair, W.R.; Marshall, M.E. Phase II Evaluation of Coumarin (1,2-benzopyrone) in Metastatic Prostatic Carcinoma. Prostate 1992, 20, 123–131. [Google Scholar] [CrossRef]
- Simons, J.W.; Mikhak, B.; Chang, J.F.; DeMarzo, A.M.; Carducci, M.A.; Lim, M.; Weber, C.E.; Baccala, A.A.; Goemann, M.A.; Clift, S.M.; et al. Induction of Immunity to Prostate Cancer Antigens: Results of a Clinical Trial of Vaccination with Irradiated Autologous Prostate Tumor Cells Engineered to Secrete Granulocyte-Macrophage Colony-Stimulating Factor Using Ex Vivo Gene Transfer. Cancer Res. 1999, 59, 5160–5168. [Google Scholar]
- Chauthe, S.K.; Mahajan, S.; Rachamalla, M.; Tikoo, K.; Singh, I.P. Synthesis and Evaluation of Linear Furanocoumarins as Potential Anti-Breast and Anti-Prostate Cancer Agents. Med. Chem. Res. 2015, 24, 2476–2484. [Google Scholar] [CrossRef]
- Pae, H.; Oh, H.; Yun, Y.; Oh, G.; Jang, S.I.; Hwang, K.; Kwon, T.; Lee, H.; Chung, H. Imperatorin, a Furanocoumarin from Angelica dahurica (Umbelliferae), Induces Cytochrome C-Dependent Apoptosis in Human Promyelocytic Leukaemia, HL-60 Cells. Pharmacol. Toxicol. 2002, 91, 40–48. [Google Scholar] [CrossRef]
- Hosseinymehr, M.; Matin, M.M.; Sadeghian, H.; Bahrami, A.R.; Kaseb-Mojaver, N. 8-Farnesyloxycoumarin Induces Apoptosis in PC-3 Prostate Cancer Cells by Inhibition of 15-Lipoxygenase-1 Enzymatic Activity. Anticancer Drugs 2016, 27, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.Y.; Abdel Latif, N.A.; El-Mansy, M.F.; Elserwy, W.S.; Abdelhafez, O.M. VEGFR-2 Inhibiting Effect and Molecular Modeling of Newly Synthesized Coumarin Derivatives as Anti-Breast Cancer Agents. Bioorg. Med. Chem. 2020, 28, 115328. [Google Scholar] [CrossRef]
- Rawat, A.; Vijaya Bhaskar Reddy, A. Recent Advances on Anticancer Activity of Coumarin Derivatives. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- de Araújo, R.S.A.; Carmo, J.D.O.D.S.; de Omena Silva, S.L.; Costa da Silva, C.R.A.; Souza, T.P.M.; Mélo, N.B.D.; Bourguignon, J.-J.; Schmitt, M.; Aquino, T.M.D.; Rodarte, R.S.; et al. Coumarin Derivatives Exert Anti-Lung Cancer Activity by Inhibition of Epithelial–Mesenchymal Transition and Migration in A549 Cells. Pharmaceuticals 2022, 15, 104. [Google Scholar] [CrossRef]
- Marcucci, F.; Stassi, G.; De Maria, R. Epithelial–Mesenchymal Transition: A New Target in Anticancer Drug Discovery. Nat. Rev. Drug Discov. 2016, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Kang, H.E.; Kim, N.H.; Yook, J.I. Therapeutic Implications of Cancer Epithelial-Mesenchymal Transition (EMT). Arch. Pharm. Res. 2019, 42, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Konkoľová, E.; Hudáčová, M.; Hamuľaková, S.; Jendželovský, R.; Vargová, J.; Ševc, J.; Fedoročko, P.; Kožurková, M. Tacrine-Coumarin Derivatives as Topoisomerase Inhibitors with Antitumor Effects on A549 Human Lung Carcinoma Cancer Cell Lines. Molecules 2021, 26, 1133. [Google Scholar] [CrossRef]
- Liang, X.; Wu, Q.; Luan, S.; Yin, Z.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. A Comprehensive Review of Topoisomerase Inhibitors as Anticancer Agents in the Past Decade. Eur. J. Med. Chem. 2019, 171, 129–168. [Google Scholar] [CrossRef]
- Zhang, S.-S.; He, Y.; Wei, M.-X. Novel Coumarin-Piperazine-2(5H)-Furanone Hybrids as Potential Anti-Lung Cancer Agents: Synthesis, Biological Evaluation and Molecular Docking Studies. Fitoterapia 2024, 177, 106105. [Google Scholar] [CrossRef]
- Ma, X.; Sui, X.; Liu, C.; Li, H.; Han, C.; Xu, T.; Zhang, H.; Zhang, Y.; Cai, D.; Li, Y.; et al. Co-Delivery of Berberine and Magnolol Targeted Liposomes for Synergistic Anti-Lung Cancer. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131773. [Google Scholar] [CrossRef]
- Bisi, A.; Cappadone, C.; Rampa, A.; Farruggia, G.; Sargenti, A.; Belluti, F.; Di Martino, R.M.C.; Malucelli, E.; Meluzzi, A.; Iotti, S.; et al. Coumarin Derivatives as Potential Antitumor Agents: Growth Inhibition, Apoptosis Induction and Multidrug Resistance Reverting Activity. Eur. J. Med. Chem. 2017, 127, 577–585. [Google Scholar] [CrossRef]
- Dong, M.; Ye, T.; Bi, Y.; Wang, Q.; Kuerban, K.; Li, J.; Feng, M.; Wang, K.; Chen, Y.; Ye, L. A Novel Hybrid of 3-Benzyl Coumarin Seco-B-Ring Derivative and Phenylsulfonylfuroxan Induces Apoptosis and Autophagy in Non-Small-Cell Lung Cancer. Phytomedicine 2019, 52, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, J.; Wang, G.; Jiang, Y.; Shang, L.; Zhao, Y.; Huang, J.; Yang, S.; Wang, J.; Yu, Y. Design, Synthesis and Biological Evaluation of Coumarin Derivatives as Novel Acetylcholinesterase Inhibitors That Attenuate H2O2-Induced Apoptosis in SH-SY5Y Cells. Bioorg. Chem. 2016, 68, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, M.; Wang, K.; Gu, Y.; Guo, S.; Wang, W.; Ma, Y.; Liu, H.; Chen, Y. Novel Hybrids of 3-Substituted Coumarin and Phenylsulfonylfuroxan as Potent Antitumor Agents with Collateral Sensitivity against MCF-7/ADR. J. Med. Chem. 2022, 65, 9328–9349. [Google Scholar] [CrossRef]
- Kowah, J.A.H.; Liu, X.; Han, K.; Guan, C.; Qiu, G.; Jiang, M.; Wang, L.; Gao, R.; Li, Y.; Yan, S. Rational Design and Synthesis of Matrine Containing Coumarin Derivatives as Hsp90 (NTD&CTD) Isoform Selective Inhibitors for the Treatment of Lung Carcinoma. 2024. Available online: https://discovery.researcher.life/article/rational-design-and-synthesis-of-matrine-containing-coumarin-derivatives-as-hsp90-ntd-amp-amp-ctd-isoform-selective-inhibitors-for-the-treatment-of-lung-carcinoma/17053f5a82ad3244ac785f773699dfe7 (accessed on 21 October 2025).
- Xu, Y.; Zeng, P.; Wang, H.; Han, K.; Qiu, G.; Wei, Y.; Chen, R.; Wang, L.; Liu, X. Novel Matrinic Acid Derivatives Bearing 2-anilinothiazole Structure for Non-small Cell Lung Cancer Treatment with Improved Hsp90 Targeting Effect. Drug Dev. Res. 2022, 83, 1434–1454. [Google Scholar] [CrossRef]
- Garg, G.; Khandelwal, A.; Blagg, B.S.J. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. Adv. Cancer Res. 2016, 129, 51–88. [Google Scholar]
- Goel, A.; Prasad, A.K.; Parmar, V.S.; Ghosh, B.; Saini, N. 7,8-Dihydroxy-4-methylcoumarin Induces Apoptosis of Human Lung Adenocarcinoma Cells by ROS-independent Mitochondrial Pathway through Partial Inhibition of ERK/MAPK Signaling. FEBS Lett. 2007, 581, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Akki, M.; Reddy, D.S.; Katagi, K.S.; Kumar, A.; Babagond, V.; Munnolli, R.S.; Joshi, S.D. Coumarin-Pyrazole Linked Carbodithioates as Potential Anti-Cancer Agents: Design, Synthesis, Biological, and Molecular Docking Investigation. Russ. J. Gen. Chem. 2022, 92, 2092–2107. [Google Scholar] [CrossRef]
- Maciejewska, N.; Olszewski, M.; Jurasz, J.; Serocki, M.; Dzierzynska, M.; Cekala, K.; Wieczerzak, E.; Baginski, M. Novel Chalcone-Derived Pyrazoles as Potential Therapeutic Agents for the Treatment of Non-Small Cell Lung Cancer. Sci. Rep. 2022, 12, 3703. [Google Scholar] [CrossRef]
- Sun, B.; Gao, L.; Ahsan, A.; Chu, P.; Song, Y.; Li, H.; Zhang, Z.; Lin, Y.; Peng, J.; Song, Z.; et al. Anticancer Effect of SZC015 on Lung Cancer Cells through ROS-Dependent Apoptosis and Autophagy Induction Mechanisms in Vitro. Int. Immunopharmacol. 2016, 40, 20230069. [Google Scholar] [CrossRef]
- Premsagar Miriyala, V.; Raj Thommandru, P.; Kashanna, J.; Govinda, V.; Ravi, G.; Kishore, R. Design, Synthesis and Cytotoxicity of New Coumarin-1,2,3-triazole Derivatives: Evaluation of Anticancer Activity and Molecular Docking Studies. Chem. Biodivers. 2023, 20, e202300269. [Google Scholar] [CrossRef]
- Toan, V.N.; Thanh, N.D. Synthesis and Antiproliferative Activity of Hybrid Thiosemicarbazone Derivatives Bearing Coumarin and D-Galactose Moieties with EGFR Inhibitory Activity and Molecular Docking Study. Med. Chem. Res. 2021, 30, 1868–1885. [Google Scholar] [CrossRef]
- Batran, R.Z.; Kassem, A.F.; Abbas, E.M.H.; Elseginy, S.A.; Mounier, M.M. Design, Synthesis and Molecular Modeling of New 4-Phenylcoumarin Derivatives as Tubulin Polymerization Inhibitors Targeting MCF-7 Breast Cancer Cells. Bioorg. Med. Chem. 2018, 26, 3474–3490. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Cooperwood, J.; Khan, M.O. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary Isoflavone Intake and All-cause Mortality in Breast Cancer Survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079. [Google Scholar] [CrossRef]
- Batran, R.Z.; Dawood, D.H.; El-Seginy, S.A.; Ali, M.M.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. New Coumarin Derivatives as Anti-Breast and Anti-Cervical Cancer Agents Targeting VEGFR-2 and P38α MAPK. Arch. Pharm. 2017, 350, 1700064. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.C.; Wong, F.L.; Jamison, P.M.; Jones, S.F.; Galaska, L.; Brady, K.T.; Wethers, B.; Stokes-Townsend, G. Implementation of the National Breast and Cervical Cancer Early Detection Program: The Beginning. Cancer 2014, 120, 2540–2548. [Google Scholar] [CrossRef]
- Fox, S.B.; Generali, D.G.; Harris, A.L. Breast Tumour Angiogenesis. Breast Cancer Res. 2007, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Takla, F.N.; Bayoumi, W.A.; El-Messery, S.M.; Nasr, M.N.A. Developing Multitarget Coumarin Based Anti-Breast Cancer Agents: Synthesis and Molecular Modeling Study. Sci. Rep. 2023, 13, 1178223421995854. [Google Scholar] [CrossRef]
- Schick, J.; Ritchie, R.P.; Restini, C. Breast Cancer Therapeutics and Biomarkers: Past, Present, and Future Approaches. Breast Cancer Basic Clin. Res. 2021, 15, 1231450. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Saquib, M.; Sanobar; Khan, M.F.; Ansari, W.A.; Arif, D.O.; Irfan, M.; Khan, M.I.; Hussain, M.K. Natural Product-Inspired Synthesis of Coumarin–Chalcone Hybrids as Potential Anti-Breast Cancer Agents. Front. Pharmacol. 2023, 14, 1231450. [Google Scholar] [CrossRef]
- Saquib, M.; Baig, M.H.; Khan, M.F.; Azmi, S.; Khatoon, S.; Rawat, A.K.; Dong, J.J.; Asad, M.; Arshad, M.; Hussain, M.K. Design and Synthesis of Bioinspired Benzocoumarin-Chalcones Chimeras as Potential Anti-Breast Cancer Agents. ChemistrySelect 2021, 6, 8754–8765. [Google Scholar] [CrossRef]
- Subramani, A.K.; Sivaperuman, A.; Natarajan, R.; Bhandare, R.R.; Shaik, A.B. QSAR and Molecular Docking Studies of Pyrimidine-Coumarin-Triazole Conjugates as Prospective Anti-Breast Cancer Agents. Molecules 2022, 27, 1845. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bria, E.; Giannarelli, D.; Felici, A.; Papaldo, P.; Fabi, A.; Di Cosimo, S.; Ruggeri, E.M.; Milella, M.; Ciccarese, M.; et al. Second- and Third-Generation Aromatase Inhibitors as First-Line Endocrine Therapy in Postmenopausal Metastatic Breast Cancer Patients: A Pooled Analysis of the Randomised Trials. Br. J. Cancer 2006, 94, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. The Cytoskeleton and Cancer. Cancer Metastasis Rev. 2009, 28, 5–14. [Google Scholar] [CrossRef]
- Mehran, M.A.; Monireh, K.; Dariush, S.; Farinaz, J.A.; Reza, T.M.; Shabnam, T.; Mohammad, S.A.; Fateme, E.V.; Yousef, P. Apoptosis Induction by New Coumarin Derivatives in a Mice Model of Breast Cancer. Arch. Razi Inst. 2023, 78, 1430–1440. [Google Scholar] [CrossRef]
- Molnár, T.; Pallagi, P.; Tél, B.; Király, R.; Csoma, E.; Jenei, V.; Varga, Z.; Gogolák, P.; Odile Hueber, A.; Máté, Z.; et al. Caspase-9 Acts as a Regulator of Necroptotic Cell Death. FEBS J. 2021, 288, 6476–6491. [Google Scholar] [CrossRef]
- Dhawan, S.; Kerru, N.; Awolade, P.; Singh-Pillay, A.; Saha, S.T.; Kaur, M.; Jonnalagadda, S.B.; Singh, P. Synthesis, Computational Studies and Antiproliferative Activities of Coumarin-Tagged 1,3,4-Oxadiazole Conjugates against MDA-MB-231 and MCF-7 Human Breast Cancer Cells. Bioorg. Med. Chem. 2018, 26, 5612–5623. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Ramazani, A.; Razzaghi-Asl, N. Anti-Cancer Nitrogen-Containing Heterocyclic Compounds. Curr. Org. Chem. 2018, 22, 2256–2279. [Google Scholar] [CrossRef]
- Zwergel, C.; Valente, S.; Salvato, A.; Xu, Z.; Talhi, O.; Mai, A.; Silva, A.; Altucci, L.; Kirsch, G. Novel Benzofuran–Chromone and –Coumarin Derivatives: Synthesis and Biological Activity in K562 Human Leukemia Cells. Medchemcomm 2013, 4, 1571. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-Containing Hybrids and Their Anticancer Activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Sankarapandian, K.; Veerappan, K.; Natarajan, S.; Kandaswamy, N.; Thangavelu, L.; Balusamy, S.R. In Silico and in Vitro Analysis of Coumarin Derivative Induced Anticancer Effects by Undergoing Intrinsic Pathway Mediated Apoptosis in Human Stomach Cancer. Phytomedicine 2018, 46, 119–130. [Google Scholar] [CrossRef]
- Lin, M.-H.; Cheng, C.-H.; Chen, K.-C.; Lee, W.-T.; Wang, Y.-F.; Xiao, C.-Q.; Lin, C.-W. Induction of ROS-Independent JNK-Activation-Mediated Apoptosis by a Novel Coumarin-Derivative, DMAC, in Human Colon Cancer Cells. Chem. Biol. Interact. 2014, 218, 42–49. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Yi, H.-K.; Yun, B.-S.; Lee, D.-Y.; Hwang, P.H.; Park, H.-R.; Kim, M.S. The Extract of the Immature Fruit of Poncirus Trifoliata Induces Apoptosis in Colorectal Cancer Cells via Mitochondrial Autophagy. Food Sci. Hum. Wellness 2020, 9, 237–244. [Google Scholar] [CrossRef]
- Beyaztas, H.; Bozali, K.; Koc, S.; Ozdemir, M.; Yalcin, B.; Guler, E.M. Synthesis and Characterization of 7-Diethylamino-4-Chloromethyl Coumarin: Spectroscopic Analysis, Molecular Docking, and Anticancer Activity on Large Intestine Carcinoma Cells. Chem. Biol. Interact. 2024, 404, 111287. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Bruna-Haupt, E.F.; Perretti, M.D.; Garro, H.A.; Carrillo, R.; Machín, F.; Lorenzo-Castrillejo, I.; Gutiérrez, L.; Vega-Hissi, E.G.; Mamberto, M.; Menacho-Marquez, M.; et al. Synthesis of Structurally Related Coumarin Derivatives as Antiproliferative Agents. ACS Omega 2023, 8, 26479–26496. [Google Scholar] [CrossRef]
- Taner, B.; Mahmood Mahmood, A.H.; Karakurt, S.; Altuner, E.E.; Dar, U.A. Synthesis, Characterization and Biological Evaluation of New Coumarin-Ferrocene Conjugates as Antitumor Agents. Inorganica Chim. Acta 2025, 578, 122534. [Google Scholar] [CrossRef]
- El-Sherief, H.A.M.; Youssif, B.G.M.; Abbas Bukhari, S.N.; Abdelazeem, A.H.; Abdel-Aziz, M.; Abdel-Rahman, H.M. Synthesis, Anticancer Activity and Molecular Modeling Studies of 1,2,4-Triazole Derivatives as EGFR Inhibitors. Eur. J. Med. Chem. 2018, 156, 774–789. [Google Scholar] [CrossRef]
- Lu, G.; Zou, Z.; Xin, M.; Meng, Y.; Cheng, Z.; Du, Z.; Gu, J.; Zhang, X.; Zou, Y. Carbamoylation at C-8 Position of Natural 3-Arylcoumarin Scaffold for the Discovery of Novel PARP-1 Inhibitors with Potent Anticancer Activity. Eur. J. Med. Chem. 2024, 277, 116726. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Chae, S.W.; Xia, Y.; Kim, N.H.; Kim, H.J.; Rhie, S.; Lee, H.J. Effect of Coumarin Derivative-Mediated Inhibition of P-Glycoprotein on Oral Bioavailability and Therapeutic Efficacy of Paclitaxel. Eur. J. Pharmacol. 2014, 723, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R.; Shemon, A.N.; Barden, J.A.; Wiley, J.S. Extracellular ATP Increases Cation Fluxes in Human Erythrocytes by Activation of the P2X7 Receptor. J. Biol. Chem. 2004, 279, 44749–44755. [Google Scholar] [CrossRef]
- Roe, S.M.; Prodromou, C.; Pearl, L.H. Structural Basis for Inhibition of the Hsp90 Molecular Chaperone by the Antitumor Antibiotics Radicicol and Geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef]
- Schulze-Gahmen, U.; De Bondt, H.L.; Kim, S.-H. High-Resolution Crystal Structures of Human Cyclin-Dependent Kinase 2 with and without ATP: Bound Waters and Natural Ligand as Guides for Inhibitor Design. J. Med. Chem. 1996, 39, 4540–4546. [Google Scholar] [CrossRef]
- Staker, B.L.; Hjerrild, K.; Feese, M.D.; Behnke, C.A.; Burgin, A.B., Jr.; Stewart, L.J. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc. Natl. Acad. Sci. USA 2002, 99, 15387–15392. [Google Scholar] [CrossRef]
- Herencia-Ropero, A.; Llop-Guevara, A.; Staniszewska, A.D.; Domènech-Vivó, J.; García-Galea, E.; Moles-Fernández, A.; Pedretti, F.; Domènech, H.; Rodríguez, O.; Guzmán, M.; et al. The PARP1 Selective Inhibitor Saruparib (AZD5305) Elicits Potent and Durable Antitumor Activity in Patient-Derived BRCA1/2-Associated Cancer Models. Genome Med. 2024, 16, 107. [Google Scholar] [CrossRef]
- Abdelnaby, R.M.; Rateb, H.S.; Ali, O.; Saad, A.S.; Nadeem, R.I.; Abou-Seri, S.M.; Amin, K.M.; Younis, N.S.; Abdelhady, R. Dual PI3K/Akt Inhibitors Bearing Coumarin-Thiazolidine Pharmacophores as Potential Apoptosis Inducers in MCF-7 Cells. Pharmaceuticals 2022, 15, 428. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Y.; Liang, Q.; Yang, X.; Huang, J.; Wang, J.; Zhang, H.; Shi, J. Recent Advances in Dual PI3K/MTOR Inhibitors for Tumour Treatment. Front. Pharmacol. 2022, 13, 875372. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of Anti-Angiogenic Therapy and Immune Checkpoint Blockade Normalizes Vascular-Immune Crosstalk to Potentiate Cancer Immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Abolibda, T.Z.; Fathalla, M.; Farag, B.; Zaki, M.E.A.; Gomha, S.M. Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase. Molecules 2023, 28, 689. [Google Scholar] [CrossRef]
- Halder, J.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Nanotherapeutics Approaches to Overcome P-Glycoprotein-Mediated Multi-Drug Resistance in Cancer. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102494. [Google Scholar] [CrossRef] [PubMed]
- Haibe-Kains, B.; El-Hachem, N.; Birkbak, N.J.; Jin, A.C.; Beck, A.H.; Aerts, H.J.W.L.; Quackenbush, J. Inconsistency in Large Pharmacogenomic Studies. Nature 2013, 504, 389–393. [Google Scholar] [CrossRef]
- Iorio, F.; Knijnenburg, T.A.; Vis, D.J.; Bignell, G.R.; Menden, M.P.; Schubert, M.; Aben, N.; Gonçalves, E.; Barthorpe, S.; Lightfoot, H.; et al. A Landscape of Pharmacogenomic Interactions in Cancer. Cell 2016, 166, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Siranosian, B.; Ha, G.; Tang, H.; Oren, Y.; Hinohara, K.; Strathdee, C.A.; Dempster, J.; Lyons, N.J.; Burns, R.; et al. Genetic and Transcriptional Evolution Alters Cancer Cell Line Drug Response. Nature 2018, 560, 325–330. [Google Scholar] [CrossRef]
- Ferreira, L.; Dos Santos, R.; Oliva, G.; Andricopulo, A. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Ferreira, R.S.; Simeonov, A.; Jadhav, A.; Eidam, O.; Mott, B.T.; Keiser, M.J.; McKerrow, J.H.; Maloney, D.J.; Irwin, J.J.; Shoichet, B.K. Complementarity Between a Docking and a High-Throughput Screen in Discovering New Cruzain Inhibitors. J. Med. Chem. 2010, 53, 4891–4905. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting Autophagy in Cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Mohsen, S.; Sobash, P.T.; Algwaiz, G.F.; Nasef, N.; Al-Zeidaneen, S.A.; Karim, N.A. Autophagy Agents in Clinical Trials for Cancer Therapy: A Brief Review. Curr. Oncol. 2022, 29, 1695–1708. [Google Scholar] [CrossRef]
- Tian, Y.; Lei, Y.; Wang, Y.; Lai, J.; Wang, J.; Xia, F. Mechanism of Multidrug Resistance to Chemotherapy Mediated by P-glycoprotein (Review). Int. J. Oncol. 2023, 63, 561936. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-I.; Tseng, Y.-J.; Chen, M.-H.; Huang, C.-Y.F.; Chang, P.M.-H. Clinical Perspective of FDA Approved Drugs With P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Syvänen, S.; Eriksson, J. Advances in PET Imaging of P-Glycoprotein Function at the Blood-Brain Barrier. ACS Chem. Neurosci. 2013, 4, 225–237. [Google Scholar] [CrossRef]
- van Assema, D.M.; Lubberink, M.; Rizzu, P.; van Swieten, J.C.; Schuit, R.C.; Eriksson, J.; Scheltens, P.; Koepp, M.; Lammertsma, A.A.; van Berckel, B.N. Blood–Brain Barrier P-Glycoprotein Function in Healthy Subjects and Alzheimer’s Disease Patients: Effect of Polymorphisms in the ABCB1 Gene. EJNMMI Res. 2012, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Matos, J.; Morales, A.I.; Prieto, M.; Remião, F.; Silva, R. Study Models of Drug–Drug Interactions Involving P-Glycoprotein: The Potential Benefit of P-Glycoprotein Modulation at the Kidney and Intestinal Levels. Molecules 2023, 28, 7532. [Google Scholar] [CrossRef]
- Li, Z.-N.; Luo, Y. HSP90 Inhibitors and Cancer: Prospects for Use in Targeted Therapies (Review). Oncol. Rep. 2022, 49, 6. [Google Scholar] [CrossRef]
- Amatya, E.; Blagg, B.S.J. Recent Advances toward the Development of Hsp90 C-Terminal Inhibitors. Bioorg. Med. Chem. Lett. 2023, 80, 129111. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Spanier, L.; Bickel, D.; Dienstbier, N.; Woloschin, V.; Vogt, M.; Pols, H.; Lungerich, B.; Reiners, J.; Aghaallaei, N.; et al. Development of a First-in-Class Small-Molecule Inhibitor of the C-Terminal Hsp90 Dimerization. ACS Cent. Sci. 2022, 8, 636–655. [Google Scholar] [CrossRef] [PubMed]
- Zajec, Ž.; Dernovšek, J.; Cingl, J.; Ogris, I.; Gedgaudas, M.; Zubrienė, A.; Mitrović, A.; Golič Grdadolnik, S.; Gobec, M.; Tomašič, T. New Class of Hsp90 C-Terminal Domain Inhibitors with Anti-Tumor Properties against Triple-Negative Breast Cancer. J. Med. Chem. 2024, 67, 12984–13018. [Google Scholar] [CrossRef]
- Kamal, A.; Thao, L.; Sensintaffar, J.; Zhang, L.; Boehm, M.F.; Fritz, L.C.; Burrows, F.J. A High-Affinity Conformation of Hsp90 Confers Tumour Selectivity on Hsp90 Inhibitors. Nature 2003, 425, 407–410. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings 1PII of Original Article: S0169-409X(96)00423-1. The Article Was Originally Published in Advanced Drug Delivery Reviews 23 (1997). Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Jørgensen, J.T. A Paradigm Shift in Biomarker Guided Oncology Drug Development. Ann. Transl. Med. 2019, 7, 148. [Google Scholar] [CrossRef]
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, T.M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-Derived Tumour Xenografts as Models for Oncology Drug Development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014, 4, 648407. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef] [PubMed]
- Park, K. A Review of Modeling Approaches to Predict Drug Response in Clinical Oncology. Yonsei Med. J. 2017, 58, 1. [Google Scholar] [CrossRef]
- Ireson, C.R.; Alavijeh, M.S.; Palmer, A.M.; Fowler, E.R.; Jones, H.J. The Role of Mouse Tumour Models in the Discovery and Development of Anticancer Drugs. Br. J. Cancer 2019, 121, 101–108. [Google Scholar] [CrossRef] [PubMed]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacholli, V.B.; R., S.M.; H., P.B.; M., L.; S., P.; Kumar, A.; Szeleszczuk, Ł.; Gackowski, M. Coumarin Derivatives as Anticancer Agents: Mechanistic Landscape with an Emphasis on Breast Cancer. Molecules 2025, 30, 4167. https://doi.org/10.3390/molecules30214167
Hacholli VB, R. SM, H. PB, M. L, S. P, Kumar A, Szeleszczuk Ł, Gackowski M. Coumarin Derivatives as Anticancer Agents: Mechanistic Landscape with an Emphasis on Breast Cancer. Molecules. 2025; 30(21):4167. https://doi.org/10.3390/molecules30214167
Chicago/Turabian StyleHacholli, Veda B., Shubha M. R., Prabhanajan B. H., Lavanya M., Pramod S., Abhishek Kumar, Łukasz Szeleszczuk, and Marcin Gackowski. 2025. "Coumarin Derivatives as Anticancer Agents: Mechanistic Landscape with an Emphasis on Breast Cancer" Molecules 30, no. 21: 4167. https://doi.org/10.3390/molecules30214167
APA StyleHacholli, V. B., R., S. M., H., P. B., M., L., S., P., Kumar, A., Szeleszczuk, Ł., & Gackowski, M. (2025). Coumarin Derivatives as Anticancer Agents: Mechanistic Landscape with an Emphasis on Breast Cancer. Molecules, 30(21), 4167. https://doi.org/10.3390/molecules30214167









