New Dihalogenated Derivatives of Condensed Benzimidazole Diones Promotes Cancer Cell Death Through Regulating STAT3/HK2 Axis/Pathway
Abstract
1. Introduction
2. Results and Discussion
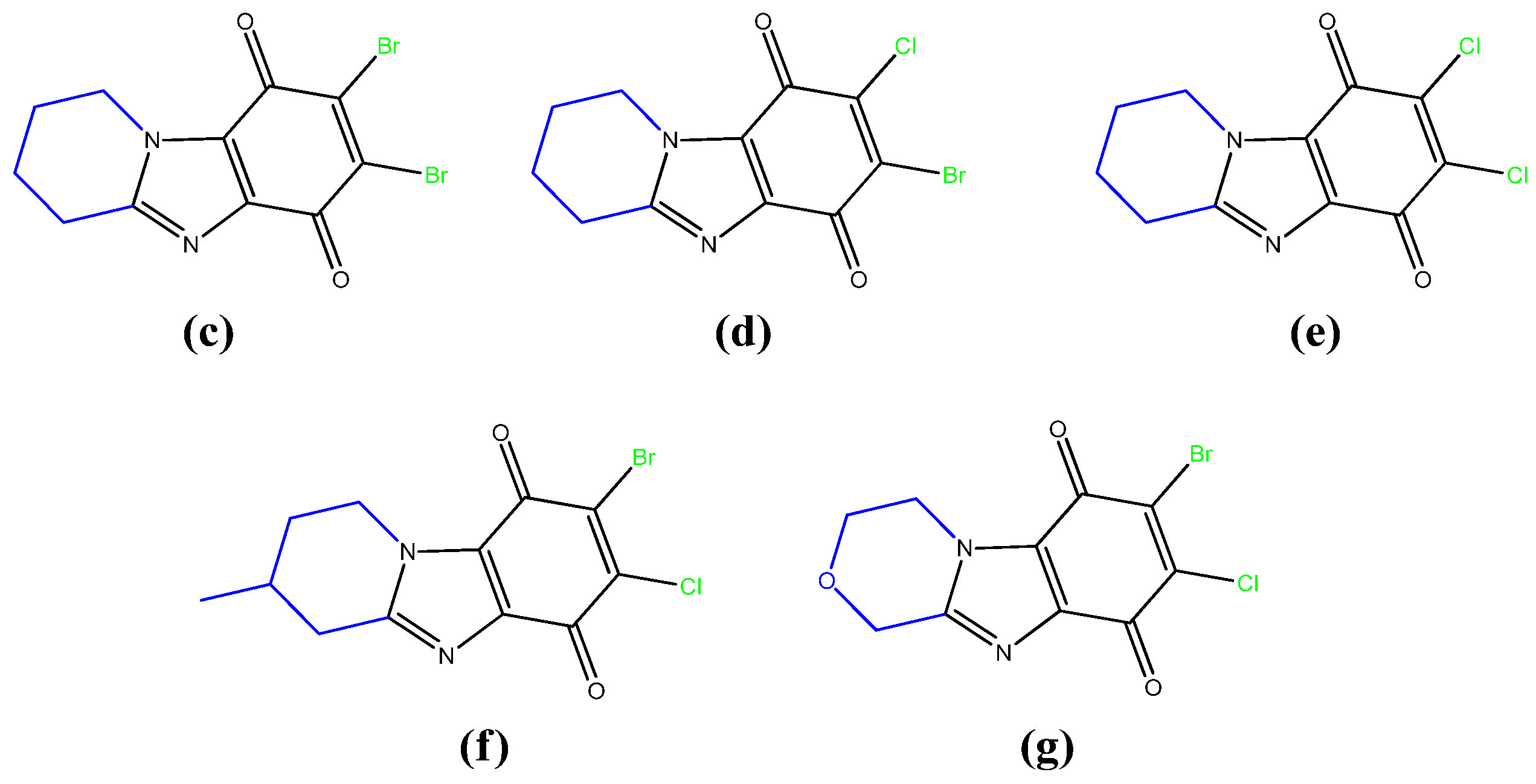
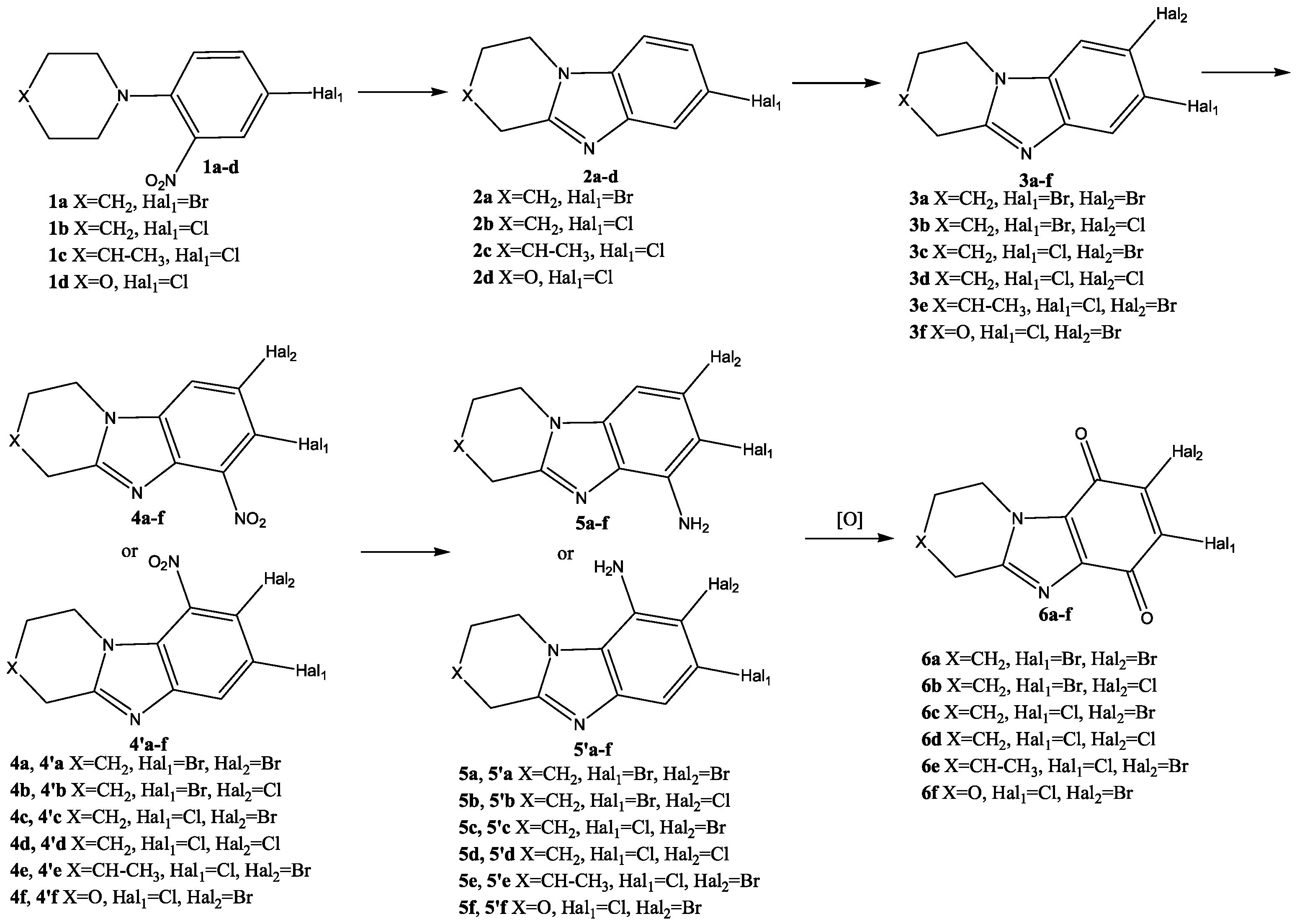
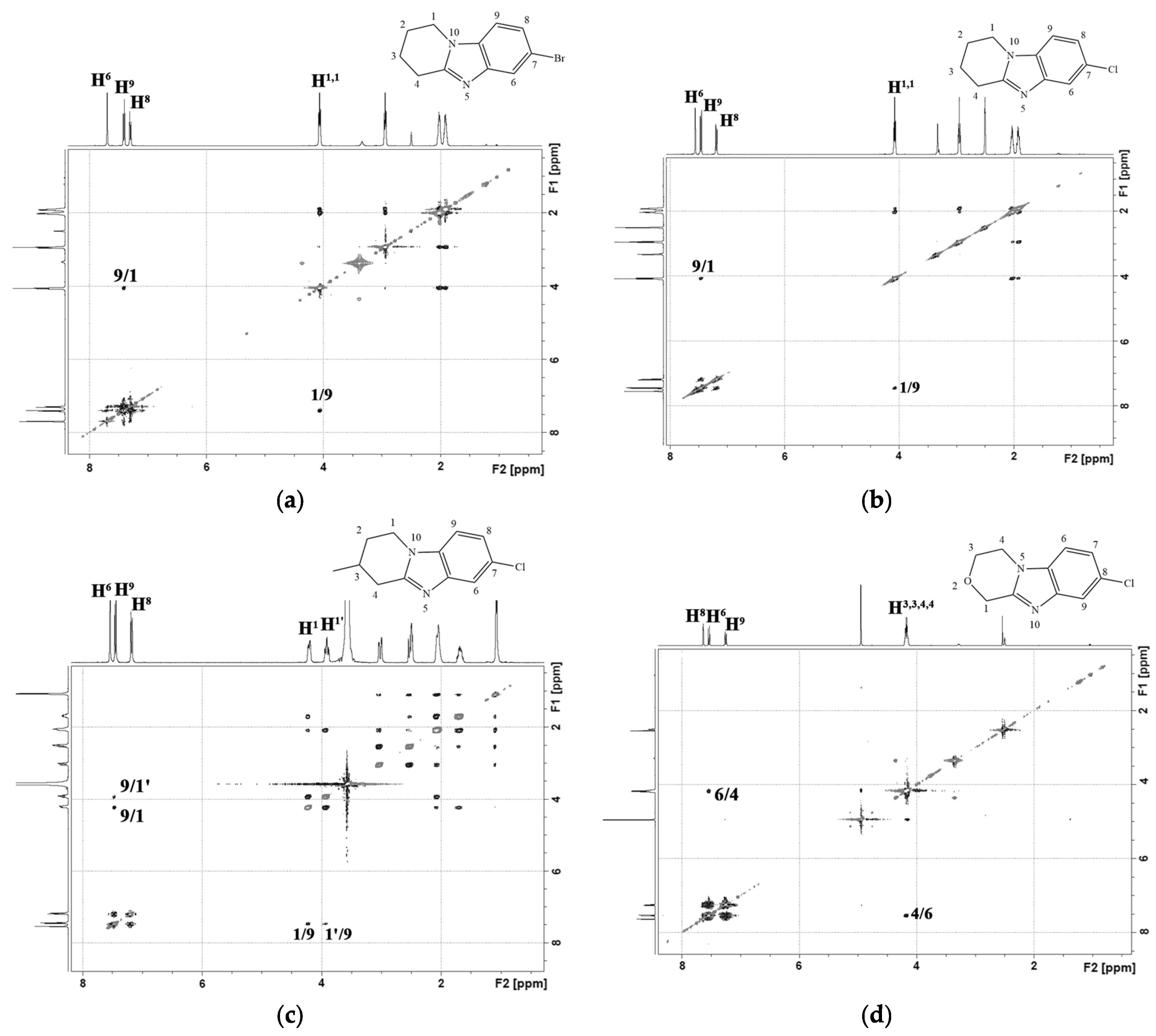
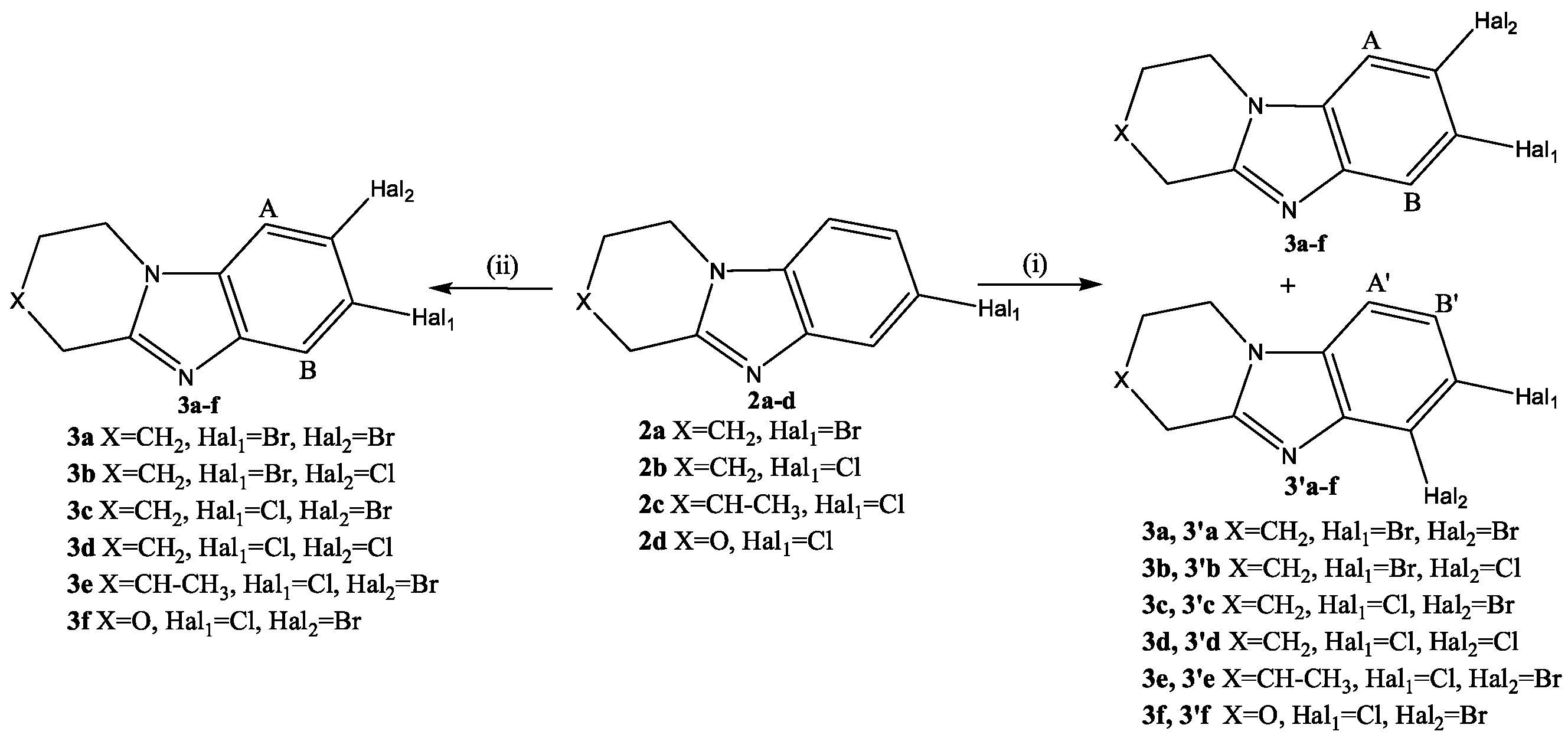
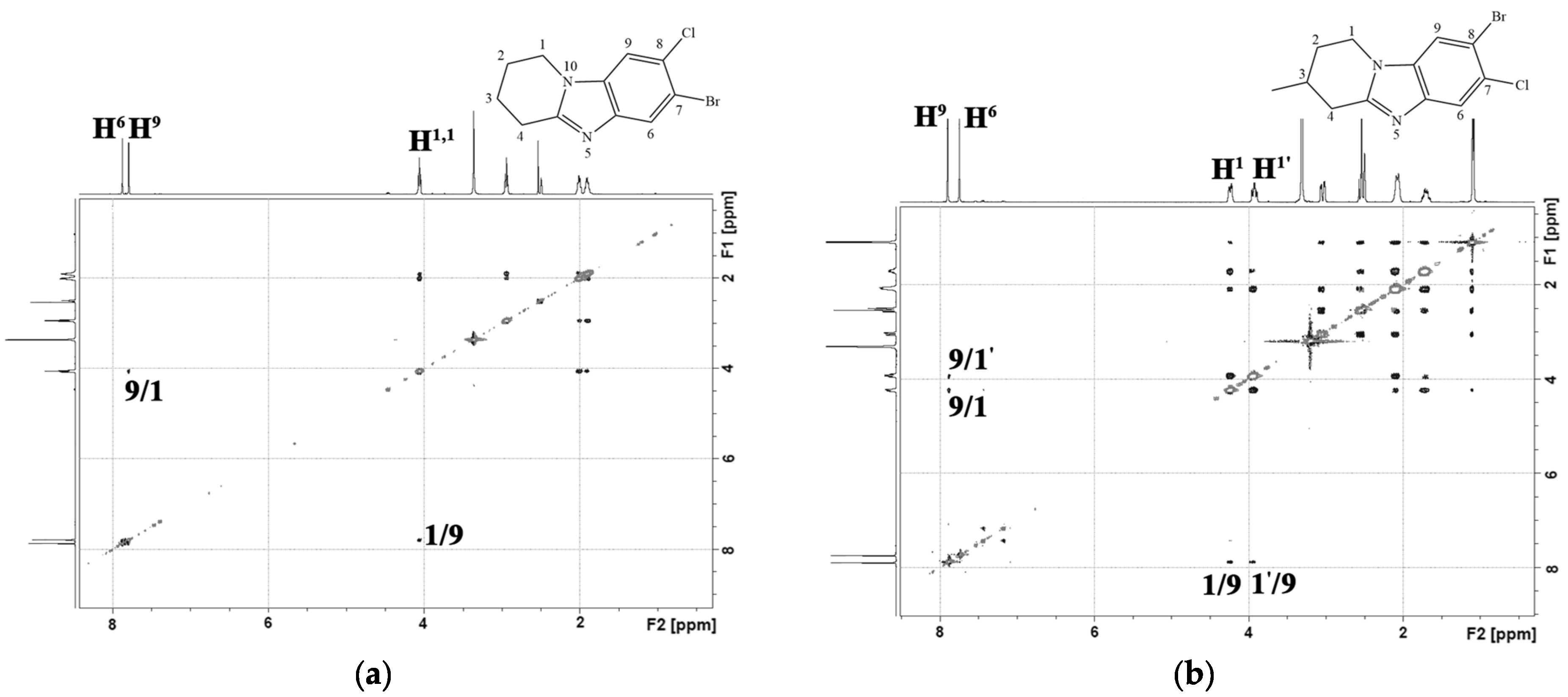
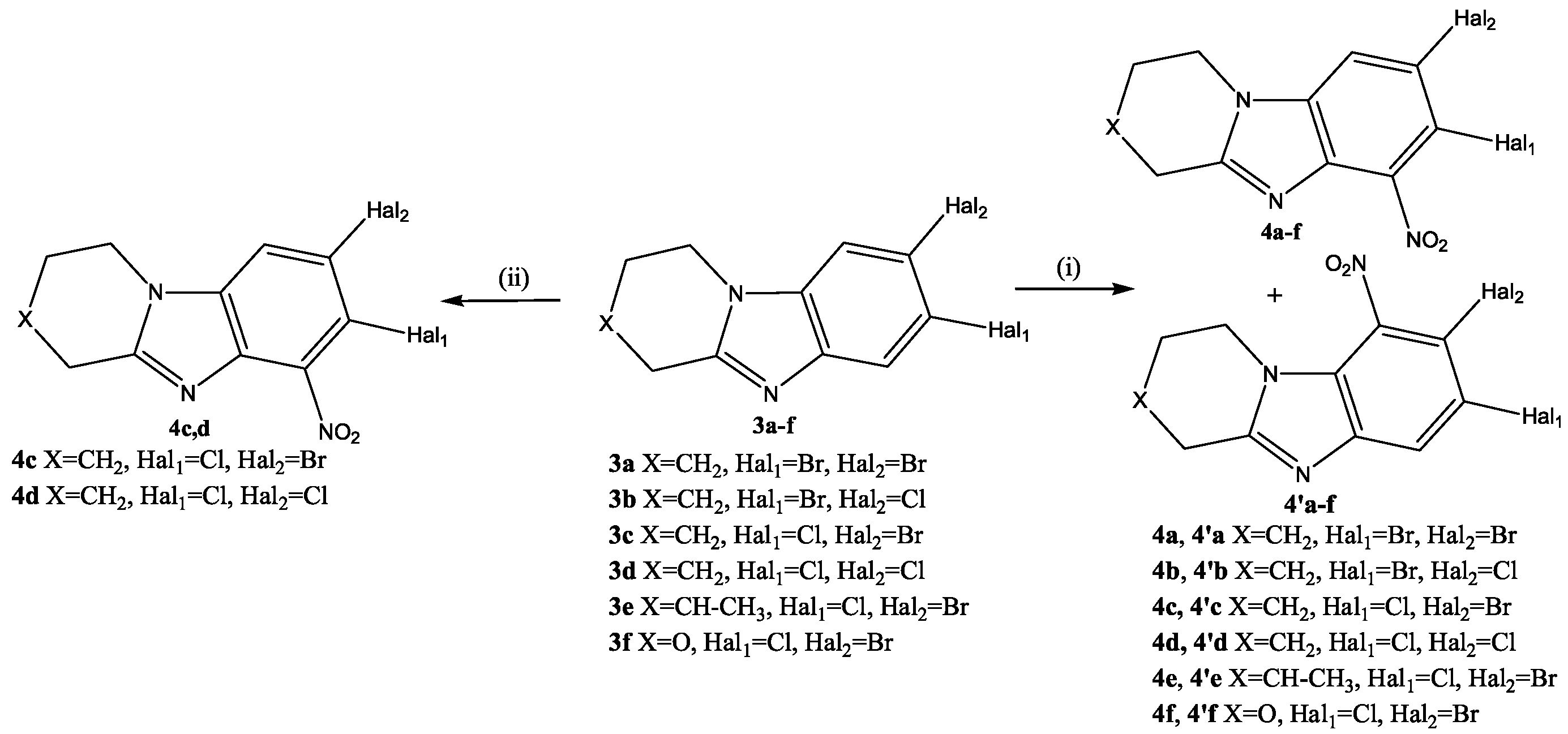
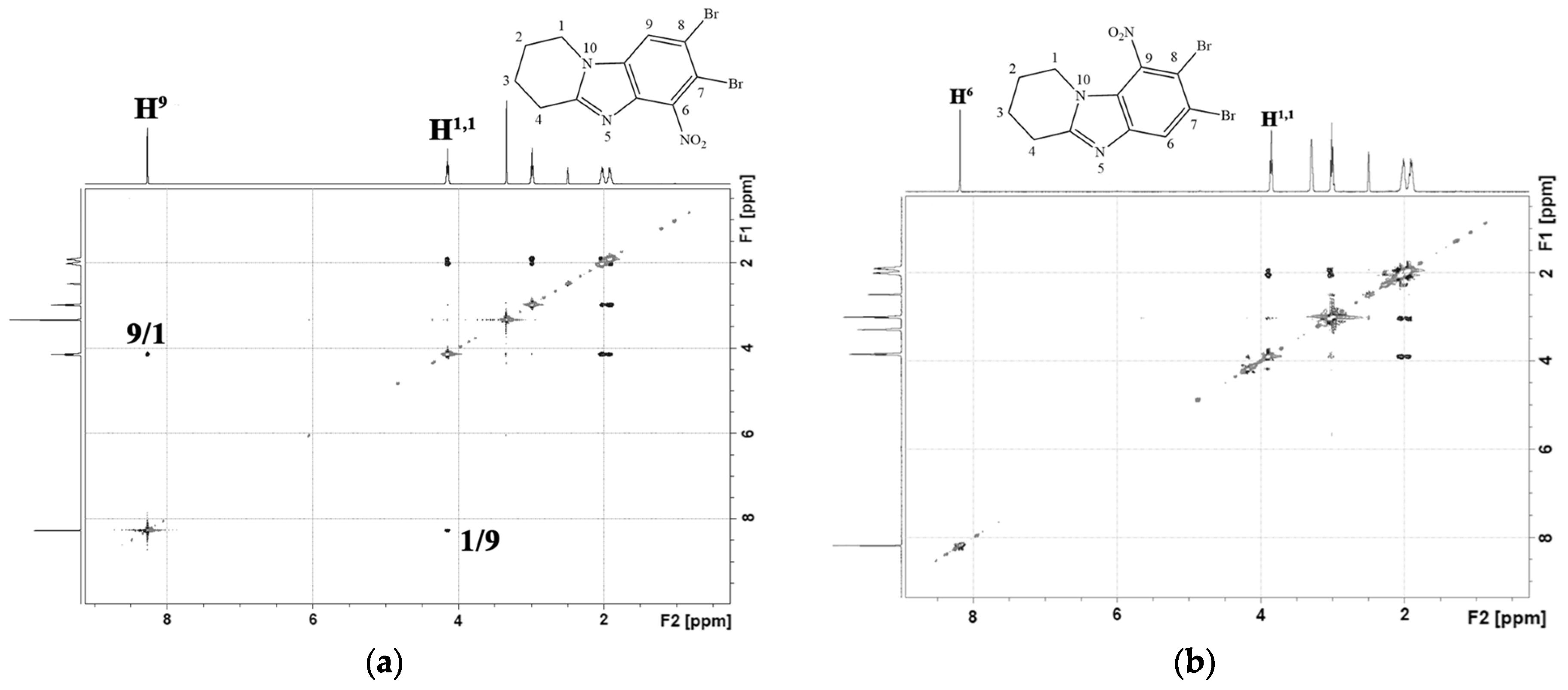
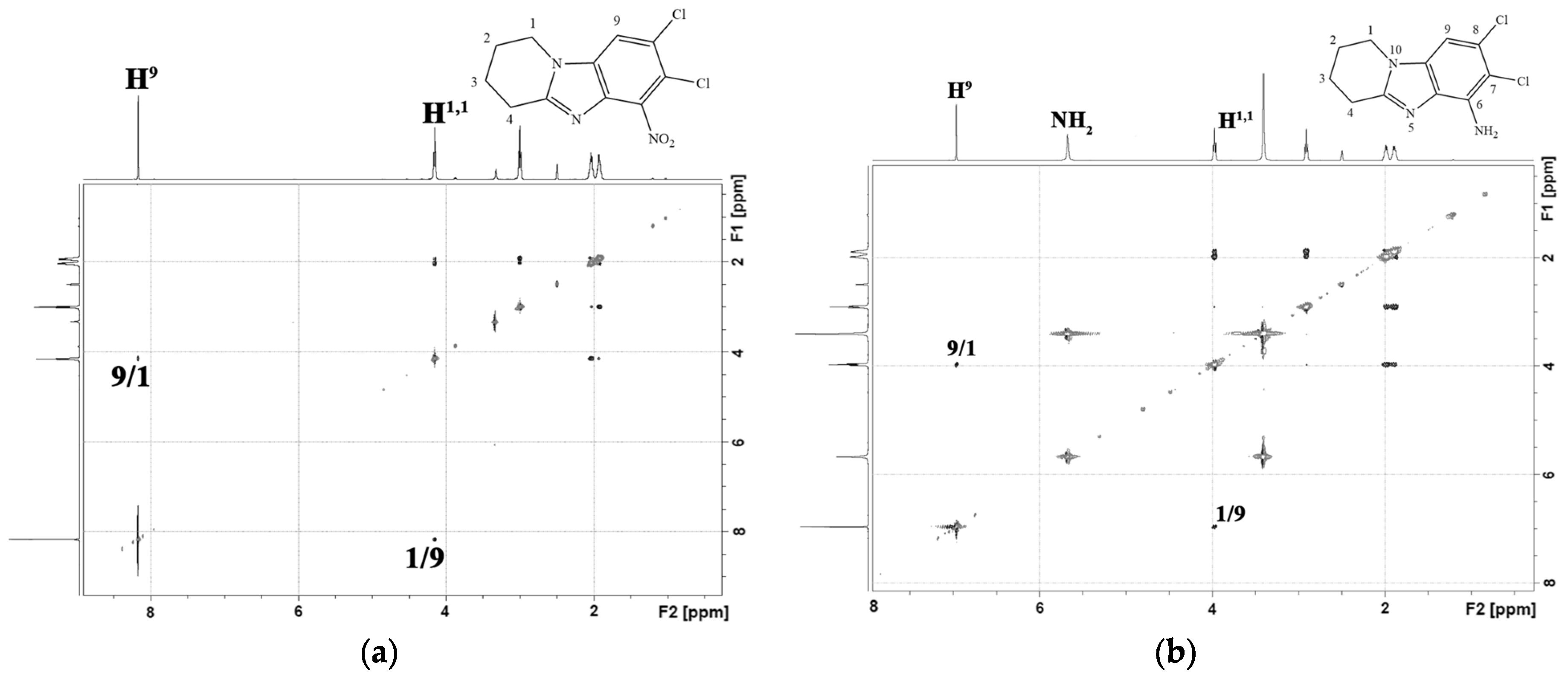
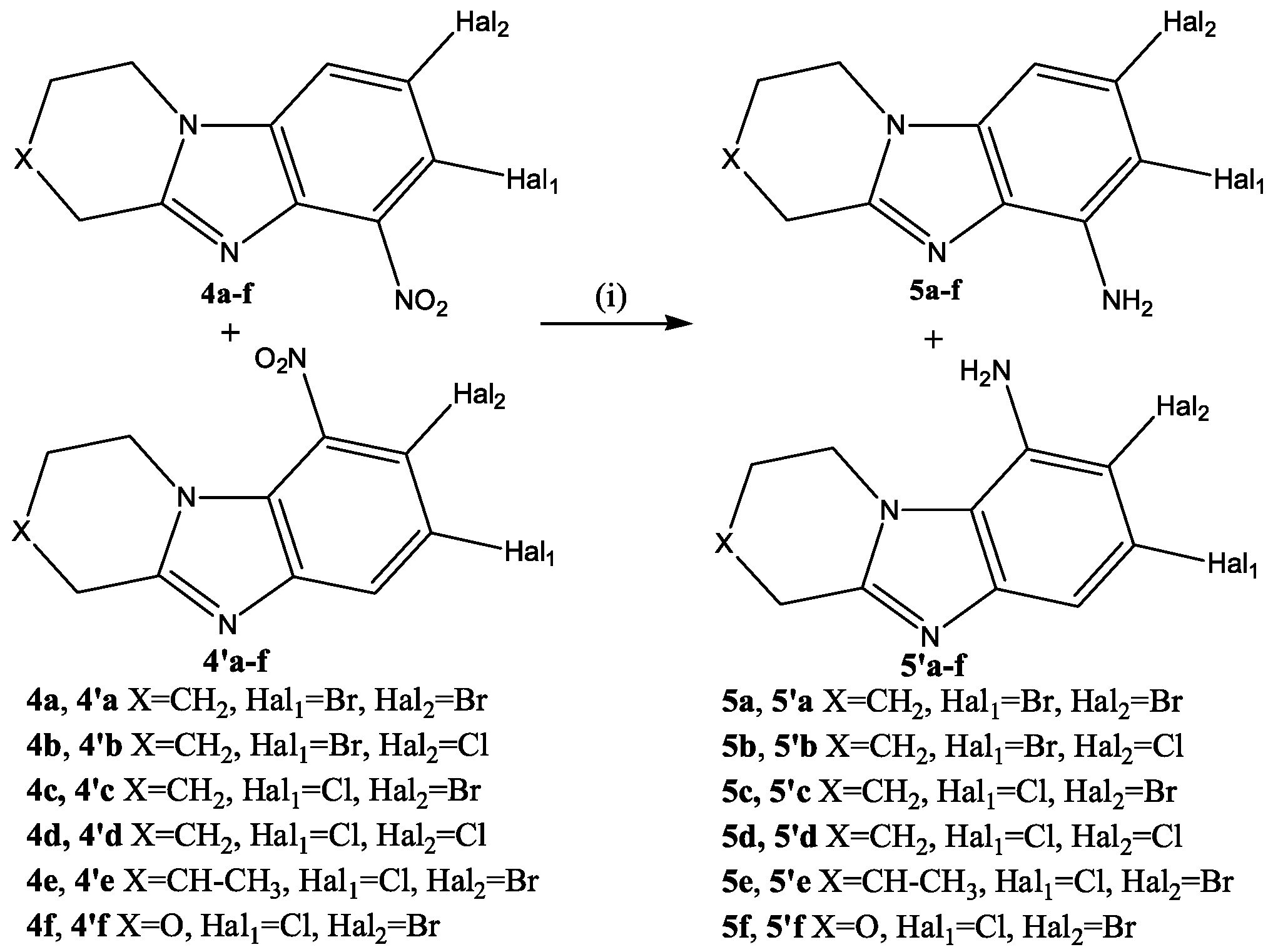
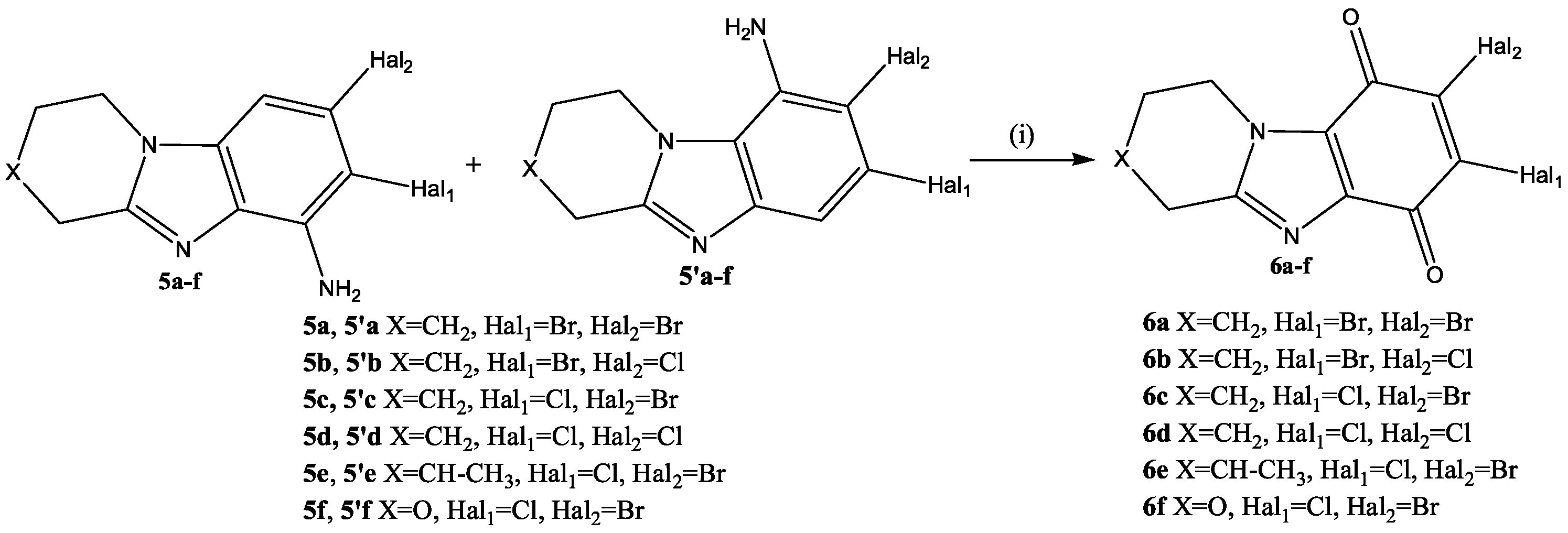
2.1. Chemistry
2.2. In Vitro Study and In Silico Prediction of the Cytotoxic Profile of New Heterocyclic Quinones 6a–6f
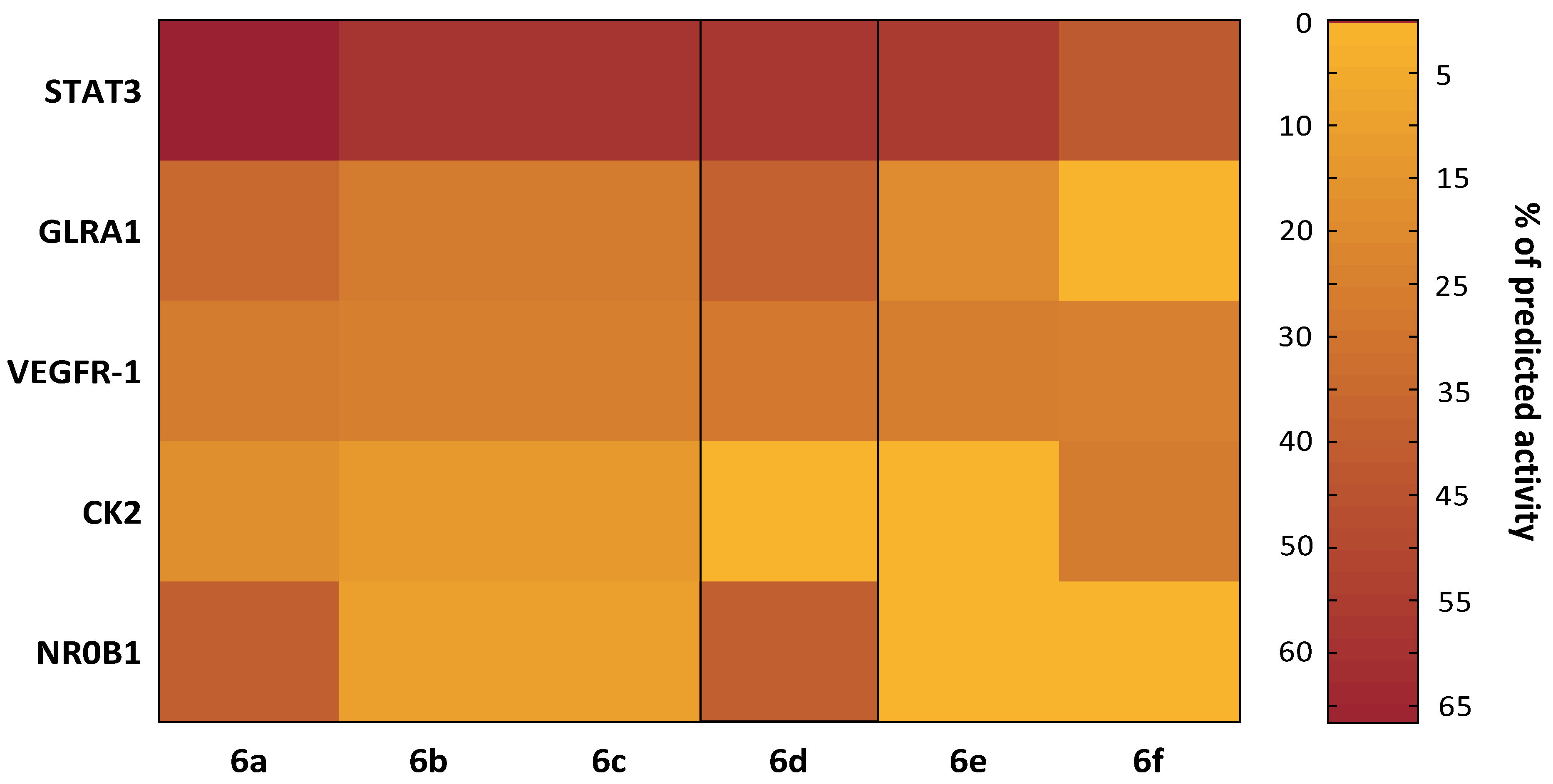
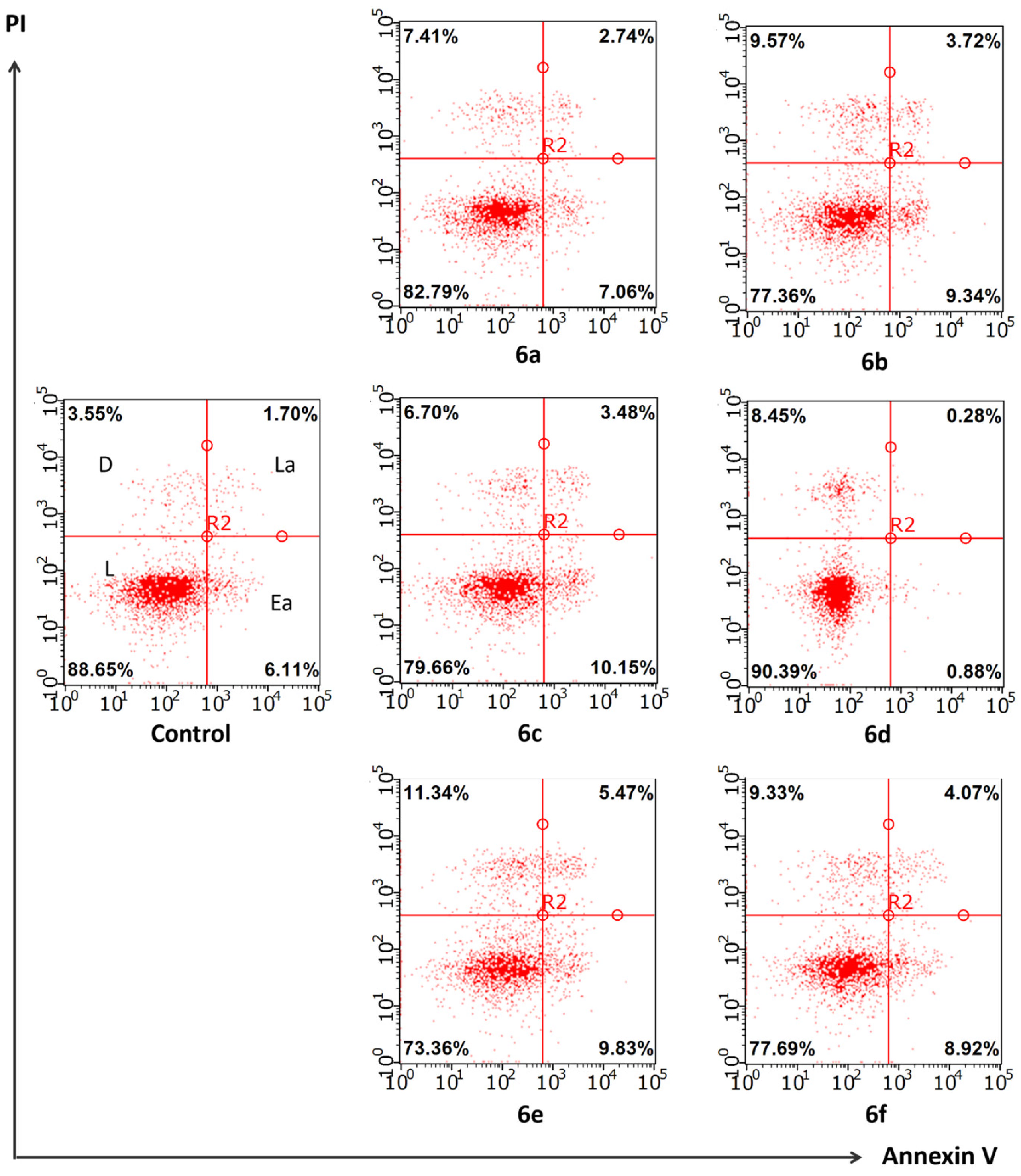
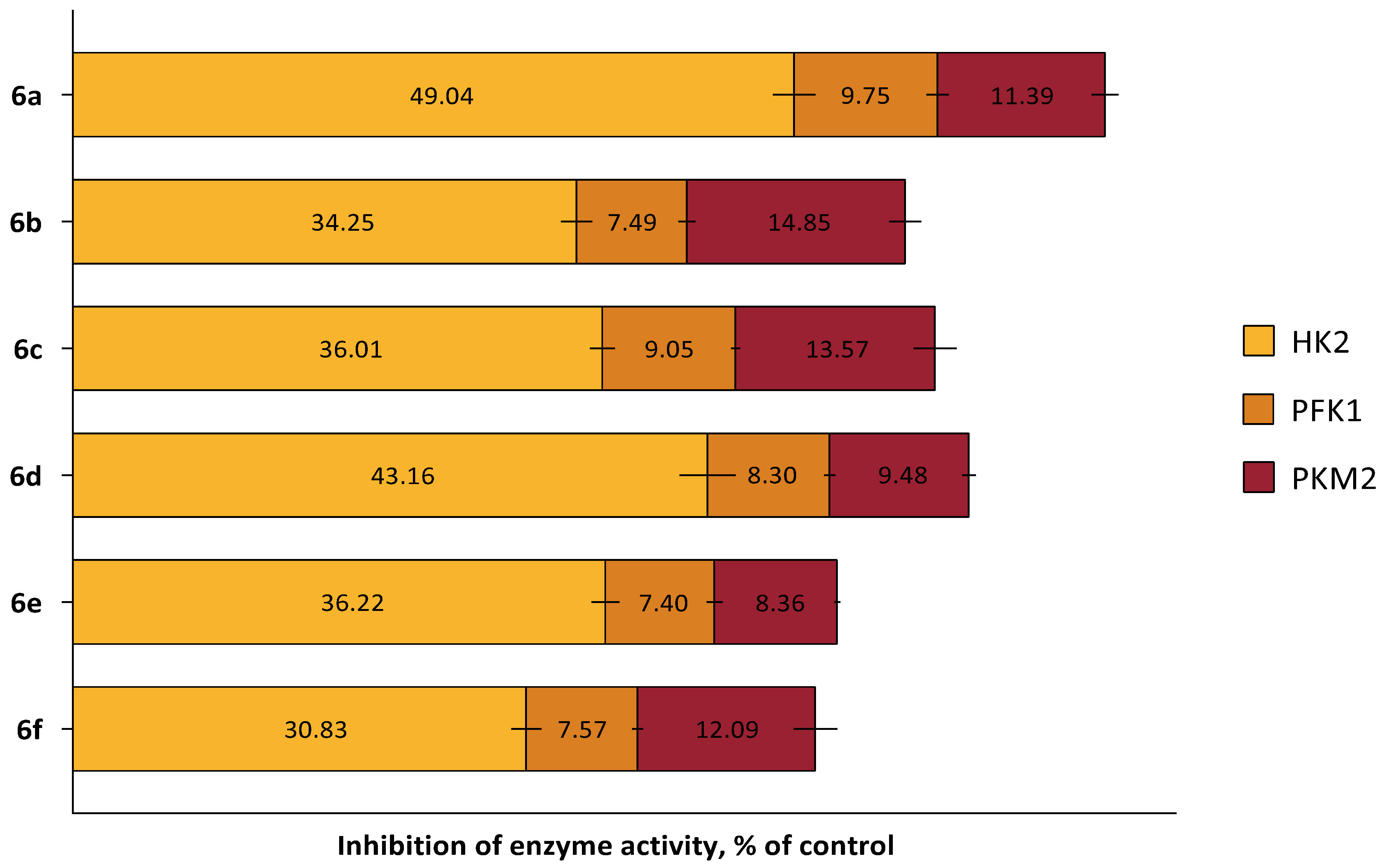
2.3. In Silico and In Vitro Study of the Cytotoxic Mechanism of Heterocyclic Quinones 6a–6f
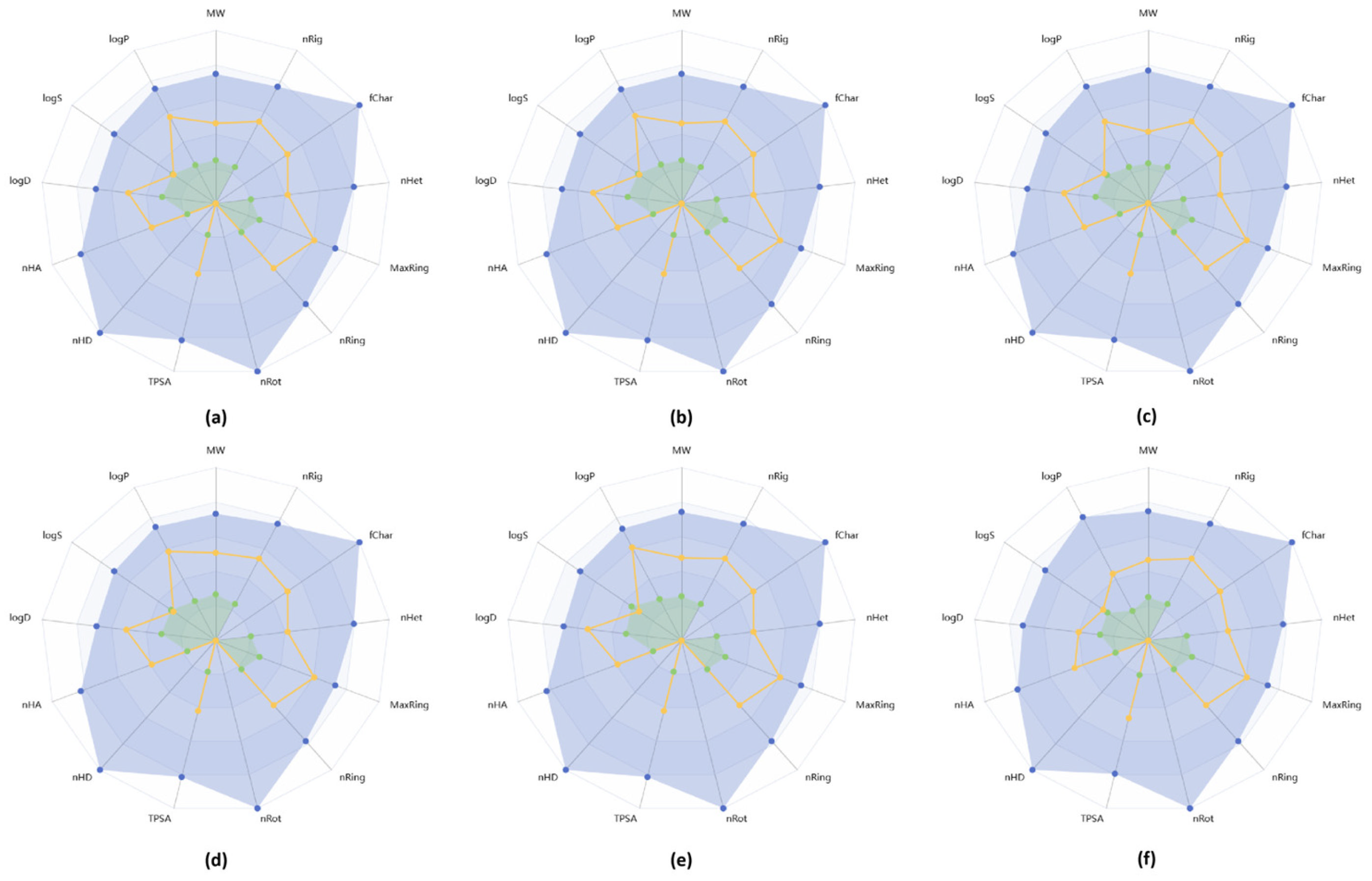
2.4. In Silico ADME Profile of New Heterocyclic Quinones 6a–6f
3. Materials and Methods
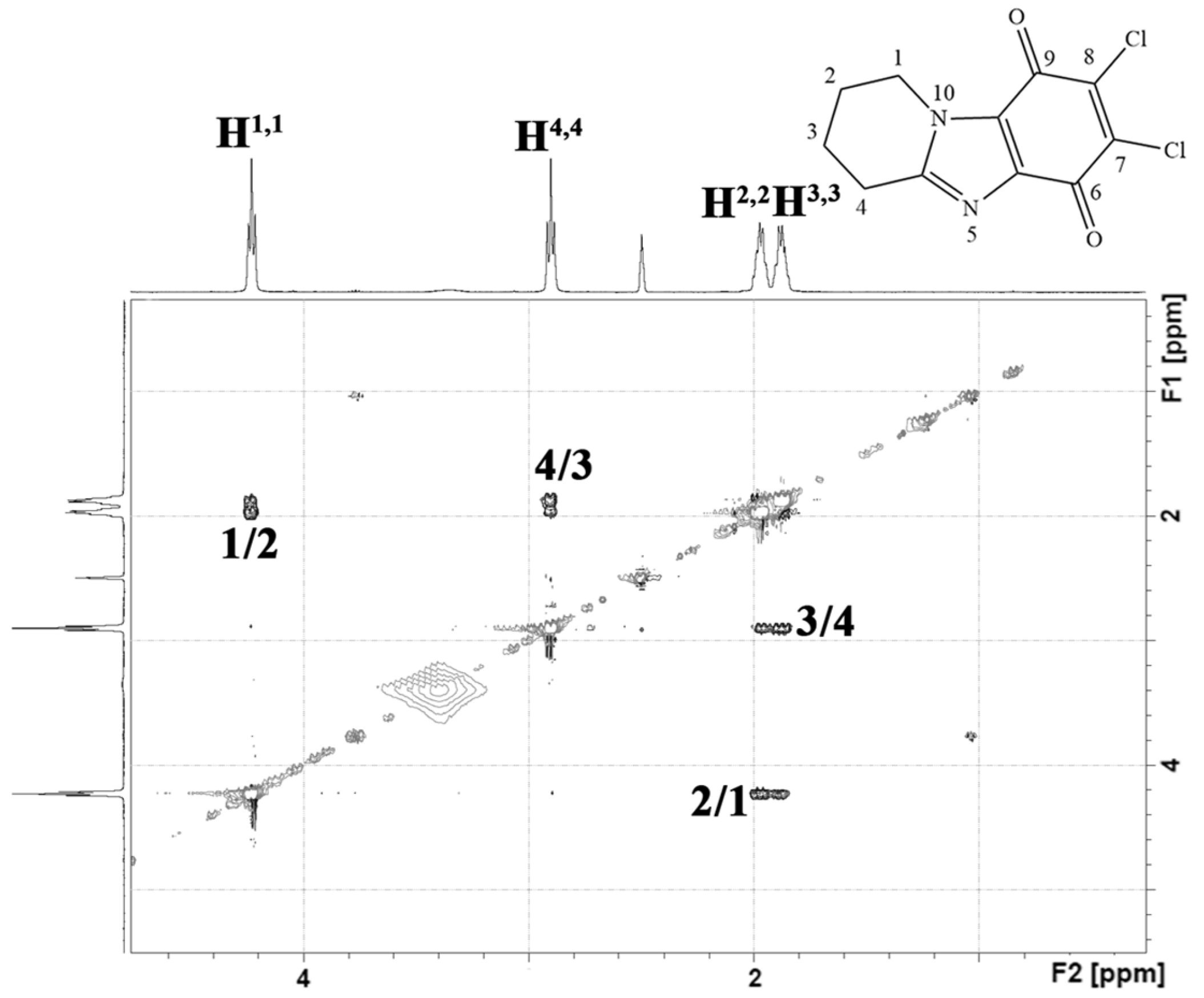
3.1. Chemistry
3.1.1. Reagents and Materials
3.1.2. Synthesis and General Procedures
3.2. Biological Evaluation
3.2.1. Cell Lines and Their Cultivation
3.2.2. Cytotoxicity Profile Study
3.2.3. Study of the Compounds’ Effect on Apoptosis
3.2.4. Study of the Compounds’ Effect on the Activity of Allosteric Glycolytic Enzymes
3.2.5. In Silico Toxicological Characteristics and Molecular Targets of the Compounds
3.2.6. In Silico ADME Profile Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Fu, G.; Zhong, W. Natural quinazolinones: From a treasure house to promising anticancer leads. Eur. J. Med. Chem. 2023, 245 Pt 1, 114915. [Google Scholar] [CrossRef]
- Lopez-Fernandez, T.; Marco, I.; Aznar, M.C.; Barac, A.; Bergler-Klein, J.; Meattini, I.; Scott, J.M.; Cardinale, D.; Dent, S. Breast cancer and cardiovascular health. Eur. Heart J. 2024, 45, 4366–4382. [Google Scholar] [CrossRef]
- Michel, L.; Schadendorf, D.; Rassaf, T. Oncocardiology: New challenges, new opportunities. Herz 2020, 45, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Saman, S.; Srivastava, N.; Yasir, M.; Chauhan, I. A Comprehensive Review on Current Treatments and Challenges Involved in the Treatment of Ovarian Cancer. Curr. Cancer Drug Targets 2024, 24, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, B.; Dabrowska, A.; Kulbacka, J.; Baczynska, D. Chemoresistance and the tumor microenvironment: The critical role of cell-cell communication. Cell Commun. Signal. 2024, 22, 486. [Google Scholar] [CrossRef] [PubMed]
- Babaker, M.A.; Aljoud, F.A.; Alkhilaiwi, F.; Algarni, A.; Ahmed, A.; Khan, M.I.; Saadeldin, I.M.; Alzahrani, F.A. The Therapeutic Potential of Milk Extracellular Vesicles on Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 6812. [Google Scholar] [CrossRef]
- Karati, D.; Kumar, D. A Comprehensive Review on Targeted Cancer Therapy: New Face of Treatment Approach. Curr. Pharm. Des. 2023, 29, 3282–3294. [Google Scholar] [CrossRef]
- Tian, X.H.; Hong, L.L.; Jiao, W.H.; Lin, H.W. Natural sesquiterpene quinone/quinols: Chemistry, biological activity, and synthesis. Nat. Prod. Rep. 2023, 40, 718–749. [Google Scholar] [CrossRef]
- Andrades-Lagos, J.; Campanini-Salinas, J.; Pedreros-Riquelme, A.; Mella, J.; Choquesillo-Lazarte, D.; Zamora, P.P.; Pessoa-Mahana, H.; Burbulis, I.; Vasquez-Velasquez, D. Design, Synthesis, and Structure-Activity Relationship Studies of New Quinone Derivatives as Antibacterial Agents. Antibiotics 2023, 12, 1065. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, M.; Li, K.; Liu, F.; Zhang, F.; Zhang, Y.; Wang, K.; Fang, W. 1,4,6-trihydroxy-8-alkylated-9,10-anthraquinones with antibacterial activities from soil-derived Streptomyces sp. WS-13394. J. Antibiot. 2022, 75, 375–379. [Google Scholar] [CrossRef]
- Jiao, S.; Huang, H.; Wang, L.; Wuken, S.; Liu, C.; Kang, L.; Liu, J.; Hu, Z.; Tu, P.; Huang, L.; et al. Three Quinone-Terpenoid Alkaloids from Syringa pinnatifolia with Cytotoxic Potential by Activation of ERK. J. Org. Chem. 2023, 88, 7096–7103. [Google Scholar] [CrossRef]
- Sagurna, L.; Heinrich, S.; Kaufmann, L.S.; Ruckert-Reed, C.; Busche, T.; Wolf, A.; Eickhoff, J.; Klebl, B.; Kalinowski, J.; Bandow, J.E. Characterization of the Antibacterial Activity of Quinone-Based Compounds Originating from the Alnumycin Biosynthetic Gene Cluster of a Streptomyces Isolate. Antibiotics 2023, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Dahlem Junior, M.A.; Nguema Edzang, R.W.; Catto, A.L.; Raimundo, J.M. Quinones as an Efficient Molecular Scaffold in the Antibacterial/Antifungal or Antitumoral Arsenal. Int. J. Mol. Sci. 2022, 23, 14108. [Google Scholar] [CrossRef]
- Dong, F.R.; Gao, L.; Wang, L.; Jiang, Y.Y.; Jin, Y.S. Natural Products as Antifungal Agents against Invasive Fungi. Curr. Top. Med. Chem. 2023, 23, 1859–1917. [Google Scholar] [CrossRef]
- Shoaib, A.; Javed, S.; Wahab, S.; Azmi, L.; Tabish, M.; Sultan, M.H.; Abdelsalam, K.; Alqahtani, S.S.; Ahmad, M.F. Cellular, Molecular, Pharmacological, and Nano-Formulation Aspects of Thymoquinone—A Potent Natural Antiviral Agent. Molecules 2023, 28, 5435. [Google Scholar] [CrossRef]
- Mohamad Ishak, N.S.; Numaguchi, T.; Ikemoto, K. Antiviral Effects of Pyrroloquinoline Quinone through Redox Catalysis to Prevent Coronavirus Infection. ACS Omega 2023, 8, 44839–44849. [Google Scholar] [CrossRef]
- Paulin, J.V.; Bayram, S.; Graeff, C.F.O.; Bufon, C.C.B. Exploring the Charge Transport of a Natural Eumelanin for Sustainable Technologies. ACS Appl. Bio. Mater. 2023, 6, 3633–3637. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ni, Y.; Lu, Y.; Zhang, Q.; Hou, J.; Yang, G.; Liu, X.; Xie, W.; Yan, Z.; Zhao, Q.; et al. Designing Quinone-Based Anodes with Rapid Kinetics for Rechargeable Proton Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209642. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, J.; Song, E.; Song, Y. Polychlorinated biphenyl quinone induces immunotoxicity via lymphocytes apoptosis and Th1-Th2 cell imbalance in C57BL/6 mice. Sci. Total Environ. 2022, 824, 153870. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.; Song, E.; Song, Y. Polybrominated diphenyl ethers quinone exhibits neurotoxicity by inducing DNA damage, cell cycle arrest, apoptosis and p53-driven adaptive response in microglia BV2 cells. Toxicology 2021, 457, 152807. [Google Scholar] [CrossRef]
- Yu, J.; Li, S.; Zeng, X.; Song, J.; Hu, S.; Cheng, S.; Chen, C.; Luo, H.; Pan, W. Design, synthesis, and evaluation of proliferation inhibitory activity of novel L-shaped ortho-quinone analogs as anticancer agents. Bioorganic Chem. 2021, 117, 105383. [Google Scholar] [CrossRef]
- Verweij, J.; Pinedo, H.M. Mitomycin C: Mechanism of action, usefulness and limitations. Anticancer Drugs 1990, 1, 5–13. [Google Scholar] [CrossRef]
- Avendano, C.; Menendez, J.C. Medicinal Chemistry of Anticancer Drugs, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 243–271. [Google Scholar]
- Navarrete, A.; Armitage, E.G.; Musteanu, M.; Garcia, A.; Mastrangelo, A.; Bujak, R.; Lopez-Casas, P.P.; Hidalgo, M.; Barbas, C. Metabolomic evaluation of Mitomycin C and rapamycin in a personalized treatment of pancreatic cancer. Pharmacol. Res. Perspect. 2014, 2, e00067. [Google Scholar] [CrossRef]
- Varzandeh, M.; Varshosaz, J.; Labbaf, S.; Esmaeil, N. Sodium-borohydride exfoliated bismuthene loaded with Mitomycin C for chemo-photo-radiotherapy of triple negative breast cancer. Int. J. Pharm. 2023, 636, 122825. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Chen, S.; Ye, Q.; Basappa, B.; Zhu, T.; Lobie, P.E.; Pandey, V. Combining Mitomycin C with inhibition of BAD phosphorylation enhances apoptotic cell death in advanced cervical cancer. Transl. Oncol. 2024, 49, 102103. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Hussain, S.A.; Porta, N.; Lewis, R.; Crundwell, M.; Jenkins, P.; Rawlings, C.; Tremlett, J.; Sreenivasan, T.; Wallace, J.; et al. Chemoradiotherapy in Muscle-invasive Bladder Cancer: 10-yr Follow-up of the Phase 3 Randomised Controlled BC2001 Trial. Eur. Urol. 2022, 82, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Scilipoti, P.; Slusarczyk, A.; de Angelis, M.; Soria, F.; Pradere, B.; Krajewski, W.; D’Andrea, D.; Mari, A.; Giudice, F.D.; Pichler, R.; et al. The Role of Mitomycin C in Intermediate-risk Non-muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2024, 7, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, F.; Zhang, H.; To, K.K.W.; Wu, S.; Chen, Z.; Liang, S.; Fu, L. Mitomycin C enhanced the efficacy of PD-L1 blockade in non-small cell lung cancer. Signal Transduct. Target. Ther. 2020, 5, 141. [Google Scholar] [CrossRef]
- Ko, J.C.; Chen, J.C.; Wang, T.J.; Zheng, H.Y.; Chen, W.C.; Chang, P.Y.; Lin, Y.W. Astaxanthin down-regulates Rad51 expression via inactivation of AKT kinase to enhance mitomycin C-induced cytotoxicity in human non-small cell lung cancer cells. Biochem. Pharmacol. 2016, 105, 91–100. [Google Scholar] [CrossRef]
- Wang, L.W.; Liu, Y.S.; Jiang, J.K. The effect of Mitomycin-C in neoadjuvant concurrent chemoradiotherapy for rectal cancer. J. Chin. Med. Assoc. 2022, 85, 1120–1125. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z.; Deng, W.; Lan, J.; Zhu, Y.; Wang, H.; Xu, X.; Zhang, Y.; Wu, X.; Yang, K.; et al. An Organoid Model for the Therapeutic Effect of Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer. Ann. Surg. Oncol. 2025, 32, 1925–1940. [Google Scholar] [CrossRef]
- Carlos de Oliveira, R.; Wilson, S.E. Biological effects of mitomycin C on late corneal haze stromal fibrosis following PRK. Exp. Eye Res. 2020, 200, 108218. [Google Scholar] [CrossRef]
- Shen, C.Y.; Chen, L.H.; Lin, Y.F.; Lai, L.C.; Chuang, E.Y.; Tsai, M.H. Mitomycin C treatment induces resistance and enhanced migration via phosphorylated Akt in aggressive lung cancer cells. Oncotarget 2016, 7, 79995–80007. [Google Scholar] [CrossRef]
- Sweeney, M.; Conboy, D.; Mirallai, S.I.; Aldabbagh, F. Advances in the Synthesis of Ring-Fused Benzimidazoles and Imidazobenzimidazoles. Molecules 2021, 26, 2684. [Google Scholar] [CrossRef]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive Review in Current Developments of Benzimidazole-Based Medicinal Chemistry. Chem. Biol. Drug Des. 2015, 86, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorganic Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef] [PubMed]
- Begunov, R.S.; Aleksandrova, Y.R.; Yandulova, E.Y.; Nikolaeva, N.S.; Neganova, M.E. Synthesis and cytotoxicity of 7,8-dihalopyrido[1,2-a]benzimidazole-6,9-dione and its 1,2,3,4-tetrahydro analogue. Mendeleev Commun. 2023, 33, 237–239. [Google Scholar] [CrossRef]
- Begunov, R.S.; Sakulina, V.O.; Syroeshkin, M.A.; Saverina, E.A.; Sokolov, A.A.; Minyaev, M.E. Electroreductive heterocyclization of ortho-piperidino substituted nitro(het)arenas. Mendeleev Commun. 2020, 30, 633–635. [Google Scholar] [CrossRef]
- Hubbard, J.W.; Piegols, A.M.; Söderberg, B.C.G. Palladium-catalyzed N-heteroannulation of N-allyl- or N-benzyl-2-nitrobenzenamines: Synthesis of 2-substituted benzimidazoles. Tetrahedron 2007, 63, 7077–7085. [Google Scholar] [CrossRef]
- Meth-Cohn, O.; Suschitzky, H. Heterocycles by ring closure of ortho-substituted t-anilines (The t-amino effect). Adv. Heterocycl. Chem. 1972, 14, 211–278. [Google Scholar]
- Joardar, S.; Bhattacharyya, A. A palladium on carbon catalyzed one-pot synthesis of substituted benzimidazoles. Synthesis 2014, 46, 3121–3132. [Google Scholar] [CrossRef]
- Duchek, J.; Vasella, A. Synthesis of Benzimidazoles by Phosphine-Mediated Reductive Cyclisation of ortho-Nitro-anilides. Helv. Chim. Acta 2011, 94, 977–986. [Google Scholar] [CrossRef]
- Alonso, J.; Halland, N.; Nazaré, M.; R’kyek, O.; Urmann, M.; Lindenschmidt, A.A. Direct, Regioselective Palladium-Catalyzed Synthesis of N-Substituted Benzimidazoles and Imidazopyridines. Eur. J. Org. Chem. 2011, 2011, 234–237. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Al-Mourabit, A. Formic acid as a sustainable and complementary reductant: An approach to fused benzimidazoles by molecular iodine-catalyzed reductive redox cyclization of o-nitro-t-anilines. Green Chem. 2016, 18, 2966–2970. [Google Scholar] [CrossRef]
- Zou, D.; Wang, W.; Hu, Y.; Jia, T. Nitroarenes and nitroalkenes as potential amino sources for the synthesis of N-heterocycles. Org. Biomol. Chem. 2023, 21, 2254–2271. [Google Scholar] [CrossRef]
- Zhu, H.; Driver, T.G. Recent Advances to Mediate Reductive Processes of Nitroarenes Using Single-Electron Transfer, Organomagnesium, or Organozinc Reagents. Synthesis 2022, 54, 3142–3161. [Google Scholar] [CrossRef] [PubMed]
- Haines, A. Methods for Oxidation of Organic Compounds, Alcohols, Alcohol Derivatives, Alky Halides, Nitroalkanes, Alkyl Azides, Carbonyl Compounds Hydroxyarenes and Aminoarenes; Academic Press: London, UK, 1988; Volume 2, p. 388. [Google Scholar]
- Meyer, A.; Fischer, K. Oxidative transformation processes and products of para-phenylenediamine (PPD) and para-toluenediamine (PTD)—A review. Environ. Sci. Eur. 2015, 2015, 11. [Google Scholar] [CrossRef]
- Begunov, R.S.; Sokolov, A.A.; Fedyanin, I.V.; Sakulina, V.O.; Syroeshkin, M.A. A simple and convenient method for synthesizing dipyrido[1,2-a:1′,2′-a′]benzo[1,2-d:5,4-d]diimidazole-6,13-dione. Mendeleev Commun. 2019, 29, 184–186. [Google Scholar] [CrossRef]
- Ismail, F.M.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef]
- Xiao, S.; Lin, Z.; Wang, X.; Lu, J.; Zhao, Y. Synthesis and Cytotoxicity Evaluation of Panaxadiol Derivatives. Chem. Biodivers. 2020, 17, e1900516. [Google Scholar] [CrossRef]
- Laffon, B.; Fernandez-Bertolez, N.; Costa, C.; Pasaro, E.; Valdiglesias, V. Comparative study of human neuronal and glial cell sensitivity for in vitro neurogenotoxicity testing. Food Chem. Toxicol. 2017, 102, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Fohlen, A.; Bordji, K.; Assenat, E.; Gongora, C.; Bazille, C.; Boulonnais, J.; Naveau, M.; Breuil, C.; Peres, E.A.; Bernaudin, M.; et al. Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time. Pharmaceuticals 2021, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- Strese, S.; Fryknas, M.; Larsson, R.; Gullbo, J. Effects of hypoxia on human cancer cell line chemosensitivity. BMC Cancer 2013, 13, 331. [Google Scholar] [CrossRef]
- Colomer Gallardo, A.; Martínez Rodríguez, R.; Castillo Pacheco, C.; González Satue, C.; Ibarz Servio, L. Dermatological side effects of intravesical Mitomycin C: Delayed hypersensitivity. Efectos secundarios de la administración de Mitomicina C intravesical: Dermatitis por hipersensibilidad retardada. Arch. Esp. Urol. 2016, 69, 89–91. [Google Scholar]
- Cicek, D.; Cobanoglu, B.; Inci, R.; Dertlioglu, S.B.; Kokcam, I.; Elkiran, T. A very rare side effect of mitomycin-C: Psoriasiform drug eruption. Int. J. Dermatol. 2013, 52, 1572–1574. [Google Scholar] [CrossRef]
- Fang, Y.P.; Hu, P.Y.; Huang, Y.B. Diminishing the side effect of mitomycin C by using pH-sensitive liposomes: In vitro characterization and in vivo pharmacokinetics. Drug Des. Devel. Ther. 2018, 12, 159–169. [Google Scholar] [CrossRef]
- Mathes, J.; Todenhofer, T. Managing Toxicity of Intravesical Therapy. Eur. Urol. Focus 2018, 4, 464–467. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Rudik, A.V.; Pogodin, P.V.; Savosina, P.I.; Tarasova, O.A.; Dmitriev, A.V.; Ivanov, S.M.; Biziukova, N.Y.; Druzhilovskiy, D.S.; Filimonov, D.A.; et al. CLC-Pred 2.0: A Freely Available Web Application for In Silico Prediction of Human Cell Line Cytotoxicity and Molecular Mechanisms of Action for Druglike Compounds. Int. J. Mol. Sci. 2023, 24, 1689. [Google Scholar] [CrossRef]
- Marminon, C.; Werner, C.; Gast, A.; Herfindal, L.; Charles, J.; Lindenblatt, D.; Aichele, D.; Mularoni, A.; Doskeland, S.O.; Jose, J.; et al. Exploring the biological potential of the brominated indenoindole MC11 and its interaction with protein kinase CK2. Biol. Chem. 2025, 406, 125–138. [Google Scholar] [CrossRef]
- Bayrak, N.; Yildirim, H.; Yildiz, M.; Radwan, M.O.; Otsuka, M.; Fujita, M.; Ciftci, H.I.; Tuyun, A.F. A novel series of chlorinated plastoquinone analogs: Design, synthesis, and evaluation of anticancer activity. Chem. Biol. Drug Des. 2020, 95, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, S.; Corona, P.; Sanna, L.; Borghi, F.; Bordoni, V.; Asproni, B.; Pinna, G.A.; Bagella, L.; Murineddu, G. Novel 1,3,4-oxadiazole chalcogen analogues: Synthesis and cytotoxic activity. Eur. J. Med. Chem. 2022, 238, 114440. [Google Scholar] [CrossRef]
- Way2Drug. Available online: https://way2drug.com/index.php (accessed on 20 May 2025).
- Oda, T.; Tian, T.; Inoue, M.; Ikeda, J.; Qiu, Y.; Okumura, M.; Aozasa, K.; Morii, E. Tumorigenic role of orphan nuclear receptor NR0B1 in lung adenocarcinoma. Am. J. Pathol. 2009, 175, 1235–1245. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, H.; Hu, S.J.; Liao, S.Y.; Tao, D.C.; Tan, X.L.; Yi, M.; Leng, X.Y.; Wang, Z.K.; Shi, J.Y.; et al. NR0B1 suppresses ferroptosis through upregulation of NRF2/c-JUN-CBS signaling pathway in lung cancer cells. Am. J. Cancer Res. 2023, 13, 5174–5196. [Google Scholar]
- Hanif, I.M.; Hanif, I.M.; Shazib, M.A.; Ahmad, K.A.; Pervaiz, S. Casein Kinase II: An attractive target for anti-cancer drug design. Int. J. Biochem. Cell Biol. 2010, 42, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, K.H.; Kim, H.M.; Kim, T.S.; Zhang, X.; Park, S.M.; Beom, S.H.; Kim, H.S.; Cheong, J.H.; Chung, H.C.; et al. Inhibiting casein kinase 2 overcomes paclitaxel resistance in gastric cancer. Gastric Cancer 2019, 22, 1153–1163. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, J.H.; Lee, S.H. Casein Kinase 2alpha Augments Oxaliplatin Resistance in Colorectal Cancer Cells by Increasing ABCE1 Expression. Anticancer Res. 2023, 43, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Bulanova, D.; Akimov, Y.; Senkowski, W.; Oikkonen, J.; Gall-Mas, L.; Timonen, S.; Elmadani, M.; Hynninen, J.; Hautaniemi, S.; Aittokallio, T.; et al. A synthetic lethal dependency on casein kinase 2 in response to replication-perturbing therapeutics in RB1-deficient cancer cells. Sci. Adv. 2024, 10, e1564. [Google Scholar] [CrossRef] [PubMed]
- Gowda, C.; Sachdev, M.; Muthusami, S.; Kapadia, M.; Petrovic-Dovat, L.; Hartman, M.; Ding, Y.; Song, C.; Payne, J.L.; Tan, B.H.; et al. Casein Kinase II (CK2) as a Therapeutic Target for Hematological Malignancies. Curr. Pharm. Des. 2017, 23, 95–107. [Google Scholar] [CrossRef]
- Kopparapu, P.K.; Boorjian, S.A.; Robinson, B.D.; Downes, M.; Gudas, L.J.; Mongan, N.P.; Persson, J.L. Expression of VEGF and its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res. 2013, 33, 2381–2390. [Google Scholar]
- Pal, S.K.; Vuong, W.; Zhang, W.; Deng, J.; Liu, X.; Carmichael, C.; Ruel, N.; Pinnamaneni, M.; Twardowski, P.; Lau, C.; et al. Clinical and Translational Assessment of VEGFR1 as a Mediator of the Premetastatic Niche in High-Risk Localized Prostate Cancer. Mol. Cancer Ther. 2015, 14, 2896–2900. [Google Scholar] [CrossRef]
- Abbasi, O.; Mashayekhi, F.; Mirzajani, E.; Fakhriyeh Asl, S.; Mahmoudi, T.; Saeedi Saedi, H. Soluble VEGFR1 concentration in the serum of patients with colorectal cancer. Surg. Today 2015, 45, 215–220. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Liu, G.; Li, J.; Chen, J.; Wang, X.; Lui, W.O.; Lu, G. Molecular mechanism of long chain non coding RNA LINC00511 influencing breast cancer stem cells: Mechanism of VEGFR1 protein. Int. J. Biol. Macromol. 2025, 302, 140437. [Google Scholar] [CrossRef]
- Gurrola-Diaz, C.; Lacroix, J.; Dihlmann, S.; Becker, C.M.; von Knebel Doeberitz, M. Reduced expression of the neuron restrictive silencer factor permits transcription of glycine receptor alpha1 subunit in small-cell lung cancer cells. Oncogene 2003, 22, 5636–5645. [Google Scholar]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Nakano-Tateno, T.; Satou, M.; Chik, C.; Tateno, T. Emerging role of signal transducer and activator of transcription 3 (STAT3) in pituitary adenomas. Endocr. J. 2021, 68, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Li, H.; Lou, L.; Huang, Q.; Zhang, Z.; Mo, J.; Li, M.; Lu, J.; Zhu, K.; Chu, Y.; et al. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022, 52, 102317. [Google Scholar] [CrossRef]
- Sabarwal, A.; van Rooyen, J.C.; Caburet, J.; Avgenikos, M.; Dheeraj, A.; Ali, M.; Mishra, D.; de Meester, J.S.B.; Stander, S.; van Otterlo, W.A.L.; et al. A novel 4’-brominated derivative of fisetin induces cell cycle arrest and apoptosis and inhibits EGFR/ERK1/2/STAT3 pathways in non-small-cell lung cancer without any adverse effects in mice. FASEB J. 2022, 36, e22654. [Google Scholar] [CrossRef]
- Kumar, R.M.; Kumar, H.; Bhatt, T.; Jain, R.; Panchal, K.; Chaurasiya, A.; Jain, V. Fisetin in Cancer: Attributes, Developmental Aspects, and Nanotherapeutics. Pharmaceuticals 2023, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, C.M.; Gonzalez-Berdullas, P.; Pereira, M.; Duarte, D.; Vale, N.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Investigation of the Anticancer and Drug Combination Potential of Brominated Coelenteramines toward Breast and Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 13981. [Google Scholar] [CrossRef]
- Schmitt, F.; Subramaniam, D.; Anant, S.; Padhye, S.; Begemann, G.; Schobert, R.; Biersack, B. Halogenated Bis(methoxybenzylidene)-4-piperidone Curcuminoids with Improved Anticancer Activity. ChemMedChem 2018, 13, 1115–1123. [Google Scholar] [CrossRef]
- Galoczova, M.; Coates, P.; Vojtesek, B. STAT3, stem cells, cancer stem cells and p63. Cell Mol. Biol. Lett. 2018, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Cheung, S.T. STAT3: An Emerging Therapeutic Target for Hepatocellular Carcinoma. Cancer 2019, 11, 1646. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Ma, Y.; Wright, G.M.; Huang, M.; Irby, R.; Briggs, J.; Karras, J.; Cress, W.D.; Pardoll, D.; et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell Biol. 2005, 25, 7432–7440. [Google Scholar] [CrossRef]
- Xiong, A.; Yang, Z.; Shen, Y.; Zhou, J.; Shen, Q. Transcription Factor STAT3 as a Novel Molecular Target for Cancer Prevention. Cancers 2014, 6, 926–957. [Google Scholar] [CrossRef]
- Afshari, H.; Noori, S.; Nourbakhsh, M.; Daraei, A.; Azami Movahed, M.; Zarghi, A. A novel imidazo[1,2-a]pyridine derivative and its co-administration with curcumin exert anti-inflammatory effects by modulating the STAT3/NF-kappaB/iNOS/COX-2 signaling pathway in breast and ovarian cancer cell lines. Bioimpacts 2024, 14, 27618. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Budi, H.S.; Izadi, S.; Timoshin, A.; Asl, S.H.; Beyzai, B.; Ghaderpour, A.; Alian, F.; Eshaghi, F.S.; Mousavi, S.M.; Rafiee, B.; et al. Blockade of HIF-1alpha and STAT3 by hyaluronate-conjugated TAT-chitosan-SPION nanoparticles loaded with siRNA molecules prevents tumor growth. Nanomedicine 2021, 34, 102373. [Google Scholar] [CrossRef]
- Qu, H.; Yu, Q.; Jia, B.; Zhou, W.; Zhang, Y.; Mu, L. HIF-3alpha affects preeclampsia development by regulating EVT growth via activation of the Flt-1/JAK/STAT signaling pathway in hypoxia. Mol. Med. Rep. 2021, 23, 68. [Google Scholar] [CrossRef]
- Huang, J.; Sun, W.; Wang, Z.; Lv, C.; Zhang, T.; Zhang, D.; Dong, W.; Shao, L.; He, L.; Ji, X.; et al. FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J. Exp. Clin. Cancer Res. 2022, 41, 42. [Google Scholar] [CrossRef]
- Wang, T.X.; Zhang, Z.Q.; Cong, Y.; Shi, X.Y.; Liu, Y.H.; Zhao, F.L. Prosapogenin A induces apoptosis in human cancer cells in vitro via inhibition of the STAT3 signaling pathway and glycolysis. Oncol. Lett. 2013, 6, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D.; et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef]
- Li, J.; Liu, T.; Zhao, L.; Chen, W.; Hou, H.; Ye, Z.; Li, X. Ginsenoside 20(S)-Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int. J. Oncol. 2015, 46, 775–781. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, Z.; Guo, X.; Miao, Z.; Ma, S. Atractylenolide I Induces Apoptosis and Suppresses Glycolysis by Blocking the JAK2/STAT3 Signaling Pathway in Colorectal Cancer Cells. Front. Pharmacol. 2020, 11, 273. [Google Scholar] [CrossRef]
- Douros, J.D.; Baltzegar, D.A.; Reading, B.J.; Seale, A.P.; Lerner, D.T.; Grau, E.G.; Borski, R.J. Leptin Stimulates Cellular Glycolysis Through a STAT3 Dependent Mechanism in Tilapia. Front. Endocrinol. 2018, 9, 465. [Google Scholar] [CrossRef]
- Bai, Z.; Ye, Y.; Ye, X.; Yuan, B.; Tang, Y.; Wei, J.; Jin, M.; Wang, G.; Li, X. Leptin promotes glycolytic metabolism to induce dendritic cells activation via STAT3-HK2 pathway. Immunol. Lett. 2021, 239, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Xu, M.; Yuan, X.; Xie, H.; Zhao, J. Circular RNA circCUL3 Accelerates the Warburg Effect Progression of Gastric Cancer through Regulating the STAT3/HK2 Axis. Mol. Ther. Nucleic Acids 2020, 22, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liu, Q.; Zheng, S.; Liu, T.; Tan, D.; Lu, X. PKM2-regulated STAT3 promotes esophageal squamous cell carcinoma progression via TGF-beta1-induced EMT. J. Cell Biochem. 2019, 120, 11539–11550. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, H.; Shen, K.; Feng, J.; Yang, K.; Shi, T.; Xi, Q. SPARC Promotes Aerobic Glycolysis and 5-Fluorouracil Resistance in Colorectal Cancer Through the STAT3/HK2 Axis. Cancer Med. 2025, 14, e70972. [Google Scholar] [CrossRef]
- Ou, B.; Sun, H.; Zhao, J.; Xu, Z.; Liu, Y.; Feng, H.; Peng, Z. Polo-like kinase 3 inhibits glucose metabolism in colorectal cancer by targeting HSP90/STAT3/HK2 signaling. J. Exp. Clin. Cancer Res. 2019, 38, 426. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.X.; Zhou, Y.Z.; Qin, X.M. Baicalein Attenuates Neuroinflammation in LPS-Treated BV-2 Cells by Inhibiting Glycolysis via STAT3/c-Myc Pathway. Neurochem. Res. 2023, 48, 3363–3377. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic reprogramming and therapeutic resistance in primary and metastatic breast cancer. Mol. Cancer 2024, 23, 261. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, Z.; Xia, W.; Jiang, P. Metabolic reprogramming, sensing, and cancer therapy. Cell Rep. 2024, 43, 115064. [Google Scholar] [CrossRef]
- Hyrossova, P.; Milosevic, M.; Skoda, J.; Vachtenheim, J., Jr.; Rohlena, J.; Rohlenova, K. Effects of metabolic cancer therapy on tumor microenvironment. Front. Oncol. 2022, 12, 1046630. [Google Scholar] [CrossRef]
- Arundhathi, J.R.D.; Mathur, S.R.; Gogia, A.; Deo, S.V.S.; Mohapatra, P.; Prasad, C.P. Metabolic changes in triple negative breast cancer-focus on aerobic glycolysis. Mol. Biol. Rep. 2021, 48, 4733–4745. [Google Scholar] [CrossRef]
- Chen, X.; Su, W.; Chen, J.; Ouyang, P.; Gong, J. ST3GAL4 promotes tumorigenesis in breast cancer by enhancing aerobic glycolysis. Hum. Cell 2024, 38, 1. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.F.; Miao, Y.; Xi, Y.; Li, X.; Liu, M.F.; Zhang, M.; Li, B. Verteporfin Suppresses YAP-Induced Glycolysis in Breast Cancer Cells. J. Investig. Surg. 2023, 36, 2266732. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Huang, Q.; Jin, M.; Huang, G. Ginsenoside Rh2 shifts tumor metabolism from aerobic glycolysis to oxidative phosphorylation through regulating the HIF1-alpha/PDK4 axis in non-small cell lung cancer. Mol. Med. 2024, 30, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, L.; Zhang, M.; Du, Y.; Li, C.; Ren, H.; Zheng, L. H3K18 Lactylation Potentiates Immune Escape of Non-Small Cell Lung Cancer. Cancer Res. 2024, 84, 3589–3601. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Guo, S.; Wang, S.; Zhang, Y.; Chen, H.; Wang, Y.; Liu, R.; Niu, Y.; Xu, Y. EIF4A3-Induced circARHGAP29 Promotes Aerobic Glycolysis in Docetaxel-Resistant Prostate Cancer through IGF2BP2/c-Myc/LDHA Signaling. Cancer Res. 2022, 82, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Uo, T.; Ojo, K.K.; Sprenger, C.C.T.; Epilepsia, K.S.; Perera, B.G.K.; Damodarasamy, M.; Sun, S.; Kim, S.; Hogan, H.H.; Hulverson, M.A.; et al. A Compound That Inhibits Glycolysis in Prostate Cancer Controls Growth of Advanced Prostate Cancer. Mol. Cancer Ther. 2024, 23, 973–994. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Huang, Y.; Yu, J.; Wang, T.; Li, C.; Yang, L.; Zhang, P.; Shi, L.; Yin, Y.; et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed. Pharmacother. 2023, 159, 114301. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Shi, H.; Wang, M.; Yu, D.; Fu, M.; Qian, Y.; Zhang, X.; Ji, R.; Wang, S.; et al. M2 Tumor-Associated Macrophages-Derived Exosomal MALAT1 Promotes Glycolysis and Gastric Cancer Progression. Adv. Sci. 2024, 11, e2309298. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, F.Y.; Shen, Y.; Tian, Y.; Han, F.J. Research progress on the mechanism of glycolysis in ovarian cancer. Front. Immunol. 2023, 14, 1284853. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhu, W.; Han, J.; Yang, X.; Zhou, R.; Lu, H.C.; Yu, H.; Yuan, W.B.; Li, P.C.; Tao, J.; et al. The role of the HIF-1alpha/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 2021, 41, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Rao, D.; Zhang, M.; Gao, Q. Metabolic reprogramming in the tumor microenvironment of liver cancer. J. Hematol. Oncol. 2024, 17, 6. [Google Scholar] [CrossRef]
- Behl, T.; Singh, S.; Sharma, N.; Zahoor, I.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules 2022, 27, 3705. [Google Scholar] [CrossRef]
- Madden, J.C.; Thompson, C.V. Pharmacokinetic Tools and Applications. Methods Mol. Biol. 2022, 2425, 57–83. [Google Scholar]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef]
- Panse, N.; Gerk, P.M. The Caco-2 Model: Modifications and enhancements to improve efficiency and predictive performance. Int. J. Pharm. 2022, 624, 122004. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhang, X.; Pan, X.; Wang, B.; Ji, C.; Qi, Y.; Zhang, J.Z.H. HobPre: Accurate prediction of human oral bioavailability for small molecules. J. Cheminform. 2022, 14, 1. [Google Scholar] [CrossRef]
- Rocha, B.; de Morais, L.A.; Viana, M.C.; Carneiro, G. Promising strategies for improving oral bioavailability of poor water-soluble drugs. Expert. Opin. Drug Discov. 2023, 18, 615–627. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]



| IC50 of Cytotoxic Effect, µM | |||||
|---|---|---|---|---|---|
| Compound | A549 1 | MCF-7 2 | SW-480 3 | SH-SY5Y 4 | Hek-293 5 |
| 6a | 9.53 ± 0.59 | 8.81 ± 1.65 | 9.11 ± 0.13 | 9.66 ± 0.57 | 24.77 ± 0.42 |
| 6b | 8.86 ± 3.19 | 7.79 ± 0.03 | 10.54 ± 0.68 | 8.60 ± 0.06 | 16.52 ± 0.15 |
| 6c | 7.81 ± 0.64 | 8.35 ± 0.32 | 10.22 ± 0.04 | 9.49 ± 0.80 | 16.01 ± 0.18 |
| 6d | 7.76 ± 0.17 | 8.52 ± 0.23 | 8.68 ± 0.20 | 8.52 ± 0.49 | 15.09 ± 0.38 |
| 6e | 8.23 ± 0.39 | 8.97 ± 0.05 | 9.53 ± 0.51 | 9.28 ± 0.15 | 20.17 ± 0.30 |
| 6f | 11.28 ± 2.49 | 8.90 ± 0.19 | 8.44 ± 0.07 | 10.75 ± 0.93 | 25.38 ± 0.02 |
| Mytomycin C | 18.09 ± 1.80 | 20.37 ± 1.74 | 11.28 ± 0.46 | 7.04 ± 0.02 | 6.59 ± 1.46 |
| Compound | Physicochemical Properties | ||||
|---|---|---|---|---|---|
| MW 1 | nHA 2 | nHD 3 | logS 4 | LogP 5 | |
| 6a | 357.90 | 4 | 0 | −4.18 | 4.02 |
| 6b | 313.95 | 4 | 0 | −4.08 | 4.27 |
| 6c | 313.95 | 4 | 0 | −4.05 | 3.91 |
| 6d | 270.00 | 4 | 0 | −3.82 | 4.74 |
| 6e | 327.96 | 4 | 0 | −4.66 | 5.66 |
| 6f | 315.93 | 5 | 0 | −3.69 | 3.25 |
| Compound | Properties of Drug-Likeness | ||||||
|---|---|---|---|---|---|---|---|
| Caco-2 Permeability | MDCK Permeability | F20% 1 | F30% 2 | F50% 3 | CLplasma 4 | T1/2 5 | |
| 6a | −4.55 | −4.42 | excellent | excellent | excellent | 2.96 | 1.65 |
| 6b | −4.46 | −4.51 | excellent | excellent | excellent | 4.33 | 1.11 |
| 6c | −4.46 | −4.51 | excellent | excellent | excellent | 4.72 | 1.08 |
| 6d | −4.34 | −4.71 | excellent | excellent | excellent | 6.01 | 0.81 |
| 6e | −4.57 | −4.45 | excellent | excellent | excellent | 6.05 | 1.04 |
| 6f | −4.48 | −4.56 | excellent | excellent | excellent | 4.47 | 1.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, Y.; Savina, L.; Shagina, I.; Lyubina, A.; Zubishina, A.; Makarova, S.; Bagylly, A.; Khokhlov, A.; Begunov, R.; Neganova, M. New Dihalogenated Derivatives of Condensed Benzimidazole Diones Promotes Cancer Cell Death Through Regulating STAT3/HK2 Axis/Pathway. Molecules 2025, 30, 4150. https://doi.org/10.3390/molecules30214150
Aleksandrova Y, Savina L, Shagina I, Lyubina A, Zubishina A, Makarova S, Bagylly A, Khokhlov A, Begunov R, Neganova M. New Dihalogenated Derivatives of Condensed Benzimidazole Diones Promotes Cancer Cell Death Through Regulating STAT3/HK2 Axis/Pathway. Molecules. 2025; 30(21):4150. https://doi.org/10.3390/molecules30214150
Chicago/Turabian StyleAleksandrova, Yulia, Luiza Savina, Inna Shagina, Anna Lyubina, Alla Zubishina, Svetlana Makarova, Anna Bagylly, Alexander Khokhlov, Roman Begunov, and Margarita Neganova. 2025. "New Dihalogenated Derivatives of Condensed Benzimidazole Diones Promotes Cancer Cell Death Through Regulating STAT3/HK2 Axis/Pathway" Molecules 30, no. 21: 4150. https://doi.org/10.3390/molecules30214150
APA StyleAleksandrova, Y., Savina, L., Shagina, I., Lyubina, A., Zubishina, A., Makarova, S., Bagylly, A., Khokhlov, A., Begunov, R., & Neganova, M. (2025). New Dihalogenated Derivatives of Condensed Benzimidazole Diones Promotes Cancer Cell Death Through Regulating STAT3/HK2 Axis/Pathway. Molecules, 30(21), 4150. https://doi.org/10.3390/molecules30214150







