Bioactivity and Compound Identification in Extracts from Three Australian Populations of Portulaca oleracea: Full NMR Structural Characterisation of Oleracein Australis 1
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Australian Populations of Purslane
2.2. In Vitro Bioactivity Assays
2.2.1. Total Phenolic Content (TPC)
2.2.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.2.3. Ferric-Reducing Antioxidant Potential (FRAP)
2.2.4. Antioxidant Activity in a Linoleic Acid Emulsion
2.3. Profiling of Purslane Extracts by UHPLC-DAD
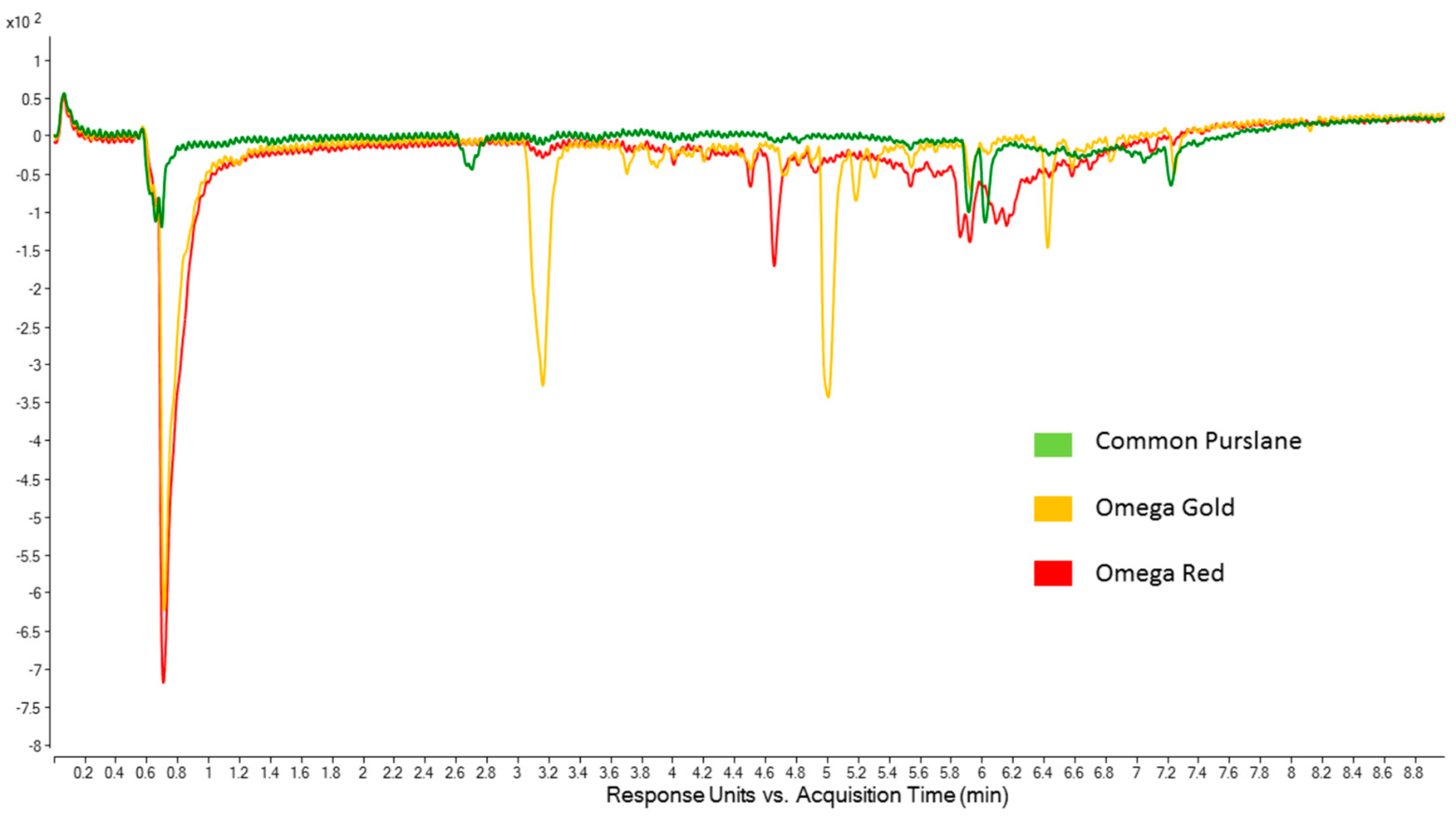
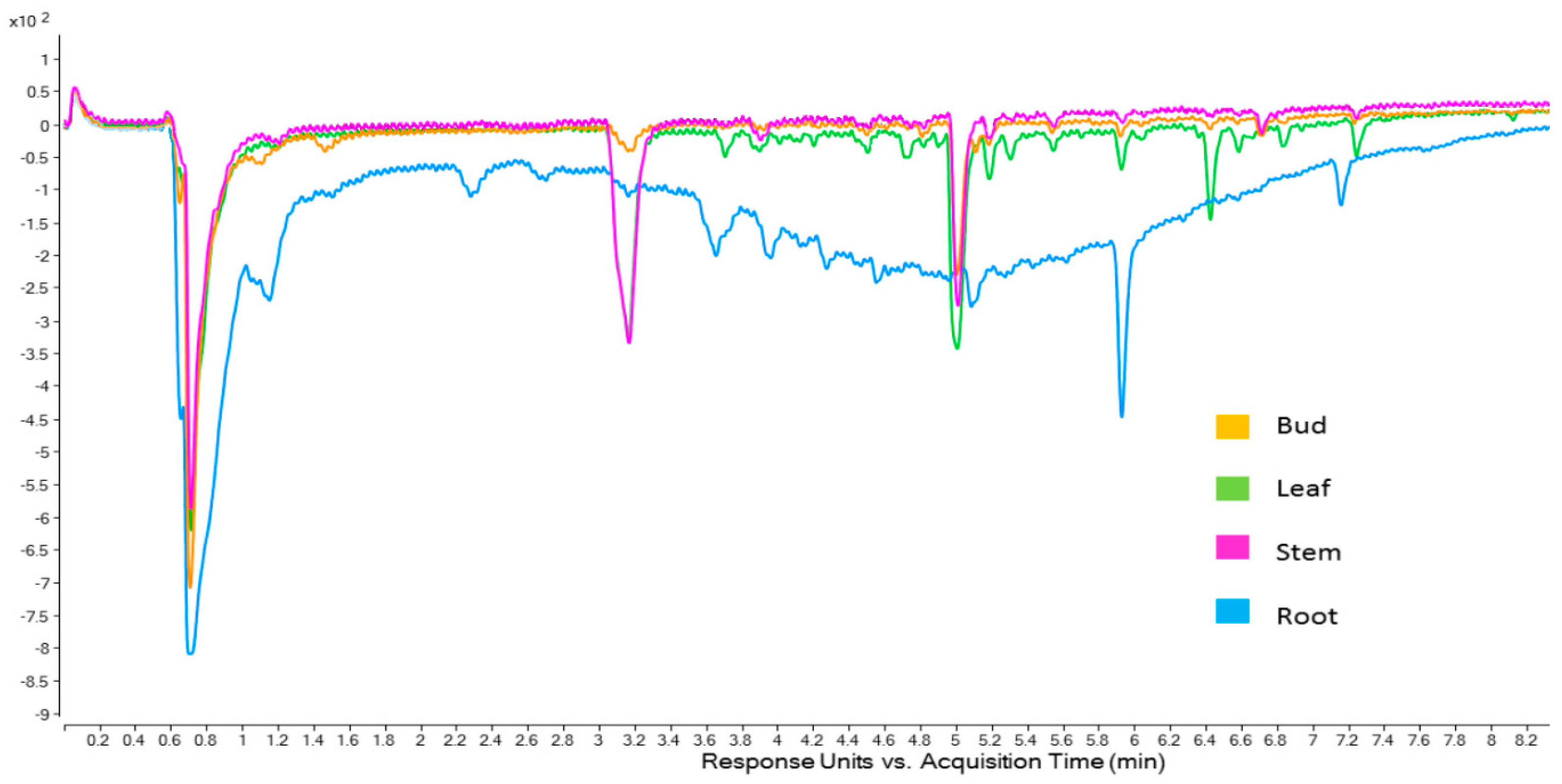
2.4. Online ABTS Chromatograms
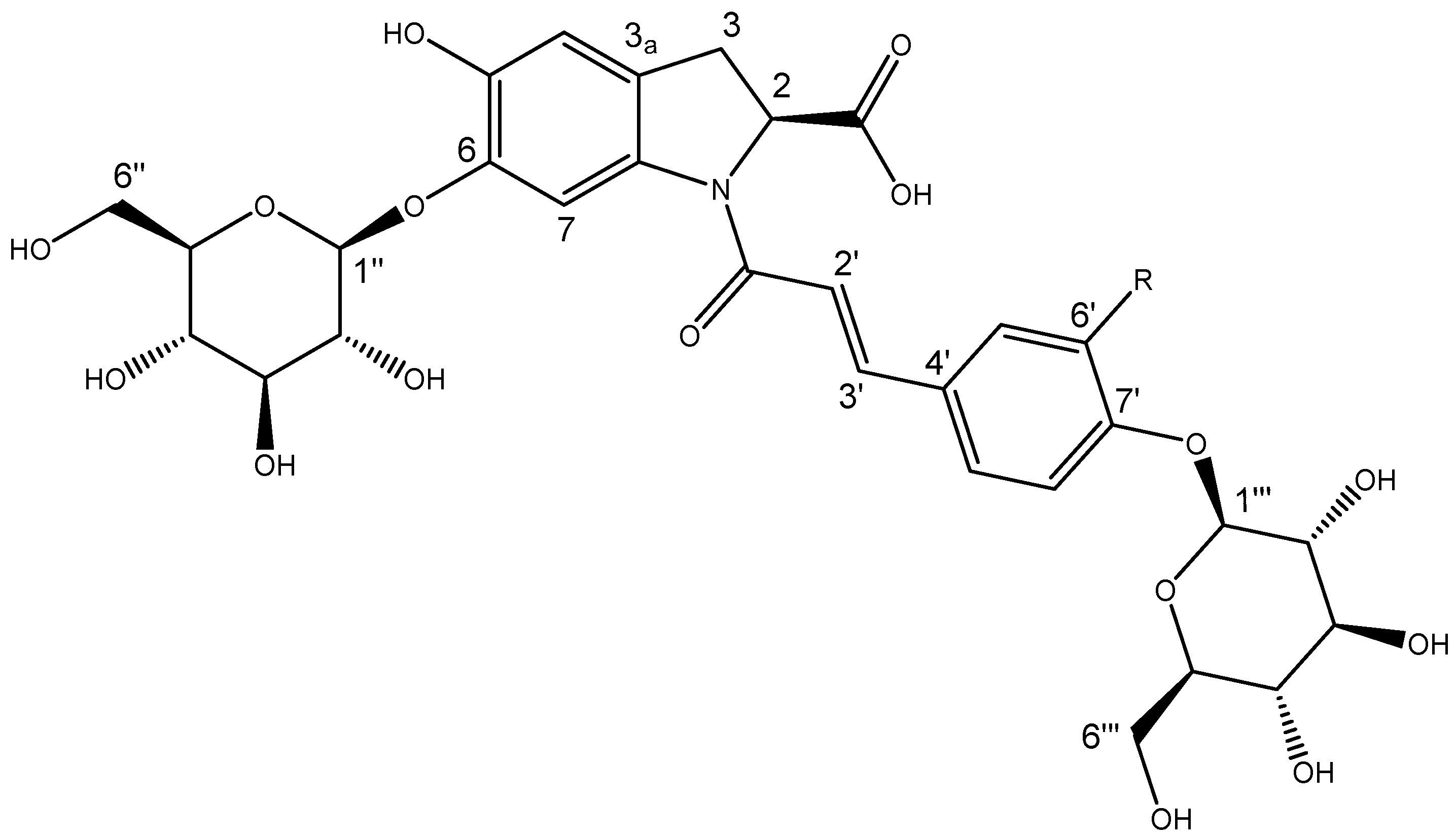
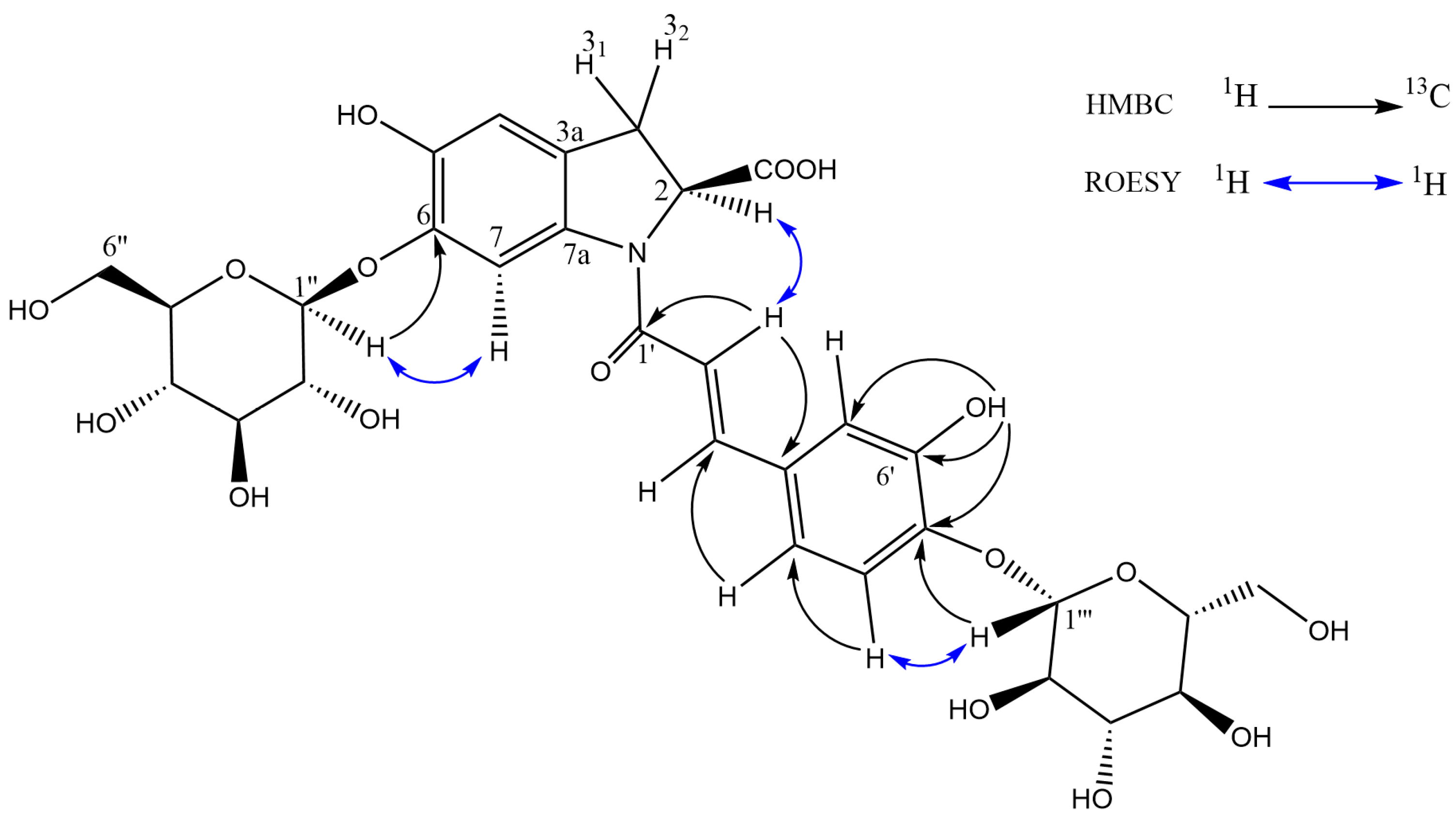
2.5. Isolation and NMR Structural Characterisation of Oleracein Australis 1 and Oleracein C
2.6. Qualitative Profiling of Purslane Extracts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation of Purslane Extracts
3.4. In Vitro Bioactivity Assays
3.5. Determination of Total Phenolic Content (TPC) Using Folin–Ciocalteu (FC) Reagent
3.6. Trolox Equivalence Antioxidant Capacity (TEAC) Assay
3.7. Ferric-Reducing Antioxidant Potential (FRAP) Capacity
3.8. DPPH Antioxidant Assay
3.9. Antioxidant Activity in a Linoleic Acid Emulsion
3.10. Isolation of Oleracein Australis 1
3.11. Chromatographic Analyses of Plant Portions
3.12. Ultra-High Performance Liquid Chromatography-Diode Array (UHPLC-DAD) with Online ABTS Detection
3.13. LC–QTOF-MS Analysis
3.14. LC-QQQ-MS/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A Review of Traditional Uses, Phytochemistry and Pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Cane, S. Australian Aboriginal Subsistence in the Western Desert. Hum. Ecol. 1987, 15, 391–434. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.U.; Ali, M.E.; Rahman, M.M. Purslane Weed (Portulaca oleracea): A Prospective Plant Source of Nutrition, Omega-3 Fatty Acid, and Antioxidant Attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. Biomed. Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Xu, X.; Yu, L.; Chen, G. Determination of Flavonoids in Portulaca oleracea L. By Capillary Electrophoresis with Electrochemical Detection. J. Pharm. Biomed. Anal. 2006, 41, 493–499. [Google Scholar] [CrossRef]
- Yan, J.; Sun, L.-R.; Zhou, Z.-Y.; Chen, Y.-C.; Zhang, W.-M.; Dai, H.-F.; Tan, J.-W. Homoisoflavonoids from the Medicinal Plant Portulaca oleracea. Phytochemistry 2012, 80, 37–41. [Google Scholar] [CrossRef]
- Li, Y.X.; Xiao, L.G.; Yan, H.; Wu, M.Y.; Hao, X.J.; Liu, H.Y. Nutritional Values, Bioactive Compounds and Health Benefits of Purslane (Portulaca oleracea L.): A Comprehensive Review. Food Sci. Hum. Wellness 2024, 13, 2480–2501. [Google Scholar] [CrossRef]
- Li, K.; Xia, T.S.; Jiang, Y.P.; Wang, N.N.; Lai, L.Y.; Xu, S.Y.; Yue, X.Q.; Xin, H.L. A Review on Ethnopharmacology, Phytochemistry, Pharmacology and Potential Uses of Portulaca oleracea L. J. Ethnopharmacol. 2024, 319, 117211C. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Borji, A.; Farkhondeh, T. Attenuation of Oxidative Stress and Inflammation by Portulaca oleracea in Streptozotocin-Induced Diabetic Rats. J. Evid. Based Complement. Altern. Med. 2017, 22, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Mashatan, N.; Farmani, E.; Khodashenas, M.; Musazadeh, V.; Ahrabi, S.S.; Moridpour, A.H.; Faghfouri, A.H. The Effects of Purslane (Portulaca oleracea) on Glycemic Indices: A Grade-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2023, 37, 5529–5540. [Google Scholar] [CrossRef]
- Nkhumeleni, Z.; Phoswa, W.N.; Mokgalaboni, K. Purslane Ameliorates Inflammation and Oxidative Stress in Diabetes Mellitus: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12276. [Google Scholar] [CrossRef]
- Barroso, T.; Alexandre, J.D.; da Cruz, E.P.; Dias, A.R.G.; Forster-Carneiro, T.; Bastos, C.P. An Updated on Applications and Future Perspectives for the Valorization of Purslane (Portulaca oleracea): A Comprehensive Review and Bibliometric Analysis. Eur. Food Res. Technol. 2024, 250, 1285–1306. [Google Scholar] [CrossRef]
- Sun, H.X.; He, X.Q.; Lin, C.J.; Li, L.Y.; Zhou, R.Y.; Jin, T.Y.; Yue, S.; Feng, D.; Gong, J.; Sun, J.W.; et al. Effect of Oleracein E, a Neuroprotective Tetrahydroisoquinoline, on Rotenone-Induced Parkinson’s Disease Cell and Animal Models. ACS Chem. Neurosci. 2017, 8, 155–164. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, A.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I. Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar]
- Kapitany, A. Australian Pigface and Pigweed; Kapitany Enterprises: Melbourne, Australia, 2013. [Google Scholar]
- Mulry, K.R.; Hanson, B.A.; Dudle, D.A. Alternative Strategies in Response to Saline Stress in Two Varieties of Portulaca oleracea (Purslane). PLoS ONE 2015, 10, e0138723. [Google Scholar] [CrossRef]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I. Phytochemical Composition and Bioactive Compounds of Common Purslane (Portulaca oleracea L.) as Affected by Crop Management Practices. Trends Food Sci. Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Mattera, M.; Pilla, N.; Aguzzi, A.; Gabrielli, P.; Di Lena, G.; Durazzo, A.; Lucarini, M. Portulaca oleracea L.: Literature Quantitative Research Analysis. Nat. Prod. Res. 2024, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; Monroy-Torres, R.; Cariño-Cortés, R.; Jiménez-Alvarado, R. Physicochemical, Nutritional and Antioxidant Characterization of Three Vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as Potential Sources of Phytochemicals and Bioactive Compounds. J. Food Meas. Charact. 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Melilli, M.G.; Pagliaro, A.; Bognanni, R.; Scandurra, S.; Di Stefano, V. Antioxidant Activity and Fatty Acids Quantification in Sicilian Purslane Germplasm. Nat. Prod. Res. 2020, 34, 26–33. [Google Scholar] [CrossRef]
- Martelin, A.; Picaud, J.C.; Faton, S.; Pradat, P.; Pastor-Diez, B.; Haÿs, S.; Armoiry, X. Optimizing Polyphenol Content and Extraction Methods for Antioxidant Constituents from Portulaca oleracea: Comparing Reflux and Maceration Methods with Various Solvents. J. Hosp. Infect. 2024, 152, 114–121. [Google Scholar] [CrossRef]
- Rinaldi, R.; Amodio, M.L.; Colelli, G. Effect of Temperature and Exogenous Ethylene on the Physiological and Quality Traits of Purslane (Portulaca oleracea L.) Leaves during Storage. Postharvest Biol. Technol. 2010, 58, 147–156. [Google Scholar] [CrossRef]
- Nagarani, G.; Abirami, A.; Nikitha, P.; Siddhuraju, P. Effect of Hydrothermal Processing on Total Polyphenolics and Antioxidant Potential of Underutilized Leafy Vegetables, Boerhaavia diffusa and Portulaca oleracea. Asian Pac. J. Trop. Biomed. 2014, 4, S468–S477. [Google Scholar] [CrossRef]
- Erkan, N. Antioxidant Activity and Phenolic Compounds of Fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Quah, E.P.L. Antioxidant Properties of Different Cultivars of Portulaca oleracea. Food Chem. 2007, 103, 734–740. [Google Scholar] [CrossRef]
- Amirul Alam, M.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Alam, M.Z. Effects of Salinity and Salinity-Induced Augmented Bioactive Compounds in Purslane (Portulaca oleracea L.) for Possible Economical Use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Meyer, A.S. The Problems of Using One-Dimensional Methods to Evaluate Multifunctional Food and Biological Antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of Antioxidant Activity with the Thiobarbituric Acid Reactive Substances Assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Development of a Method Suitable for High-Throughput Screening to Measure Antioxidant Activity in a Linoleic Acid Emulsion. Antioxidants 2019, 8, 366. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Qiao, J.Y.; Li, H.W.; Liu, F.G.; Li, Y.C.; Tian, S.; Cao, L.H.; Hu, K.; Wu, X.X.; Miao, M.S. Effects of Portulaca oleracea Extract on Acute Alcoholic Liver Injury of Rats. Molecules 2019, 24, 2887. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Shin, J.S.; Park, S.K.; Kang, S.; Jeong, S.C.; Moon, J.K.; Choi, Y. Differences in the Metabolic Profiles and Antioxidant Activities of Wild and Cultivated Black Soybeans Evaluated by Correlation Analysis. Food Res. Int. 2017, 100, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Effect of Processing Conditions, Prestorage Treatment, and Storage Conditions on the Phenol Content and Antioxidant Activity of Olive Mill Waste. J. Agric. Food Chem. 2008, 56, 3925–3932. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; García, P.; Garrido, A. Hydroxytyrosol 4-Β-D-Glucoside, an Important Phenolic Compound in Olive Fruits and Derived Products. J. Agric. Food Chem. 2002, 50, 3835–3839. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.; Llorent-Martínez, E.J.; Ruiz-Medina, A. Phytochemical Composition and Antioxidant Activity of Portulaca oleracea: Influence of the Steaming Cooking Process. Foods 2021, 10, 94. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.M.; Wang, W.; Wang, R.F.; Ding, Y.; Du, L.J. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.-Z.; Yue, S.; Sun, H.-X.; Jin, T.-Y.; Wang, H.-N.; Zhu, R.-X.; Xiang, L. Indoline Amide Glucosides from Portulaca oleracea: Isolation, Structure, and Dpph Radical Scavenging Activity. J. Nat. Prod. 2015, 78, 2588–2597. [Google Scholar] [CrossRef]
- Voynikov, Y.; Nedialkov, P.; Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Dimitrov, I. UHPLC-Orbitrap-MS Tentative Identification of 51 Oleraceins (Cyclo-Dopa Amides) in Portulaca oleracea L. Cluster Analysis and MS2 Filtering by Mass Difference. Plants 2021, 10, 1921. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Napolitano, A.; Dirsch, V.M.; Heiss, E.H.; Masullo, M.; Piacente, S. Oleraceins from Portulaca oleracea Leaves: Quali-Quantitative Determination and Antioxidant Potential. Curr. Res. Food Sci. 2025, 10, 100992. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (Cawg) Metabolomics Standards Initiative (Msi). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Xiong, L.; Peng, C.; Xie, X.F.; Guo, L.; He, C.J.; Geng, Z.; Wan, F.; Dai, O.; Zhou, Q.M. Alkaloids Isolated from the Lateral Root of Aconitum carmichaelii. Molecules 2012, 17, 9939–9946. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Jiao, Y.N.; Jin, T.Y.; Sun, H.X.; Li, S.Q.; Jin, C.R.; Hu, S.Y.; Ji, J.B.; Xiang, L. Phenolic Alkaloid Oleracein E Attenuates Oxidative Stress and Neurotoxicity in AlCl3-Treated Mice. Life Sci. 2017, 191, 211–218. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Investigation of Australian Olive Mill Waste for Recovery of Biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Schwarz, L.J.; Santhakumar, A.B.; Blanchard, C.L. Stabilization Treatment of Rice Bran Alters Phenolic Content and Antioxidant Activity. Cereal Chem. 2020, 97, 281–292. [Google Scholar] [CrossRef]
- Rao, S.; Callcott, E.T.; Santhakumar, A.B.; Chinkwo, K.A.; Vanniasinkam, T.; Luo, J.X.; Blanchard, C.L. Profiling Polyphenol Composition and Antioxidant Activity in Australian-Grown Rice Using UHPLC Online-ABTS System. J. Cereal Sci. 2018, 80, 174–179. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Ksouri, W.M.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. Assessment of Antioxidant Activity and Neuroprotective Capacity on Pc12 Cell Line of Frankenia Thymifolia and Related Phenolic LC-MS/MS Identification. Evid.-Based Complement. Altern. Med. 2016, 2016, 2843463. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Burrows, G.E.; Ryan, D.; Prenzler, P.D. Multi-Dimensional Antioxidant Screening of Selected Australian Native Plants and Putative Annotation of Active Compounds. Molecules 2023, 28, 3106. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Gao, P.; Li, Q.; Li, H.; Huang, R.; Wu, M. Inhibition of Lipid Oxidation in Foods and Feeds and Hydroxyl Radical-Treated Fish Erythrocytes: A Comparative Study of Ginkgo Biloba Leaves Extracts and Synthetic Antioxidants. Anim. Nutr. 2016, 2, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Li, C.; Gao, M.; Ying, X.; Yang, G. Pharmacokinetics and Metabolism of Olerciamide A from Portulaca oleracea L. in rats by UHPLC-UV and UHPLC-ESI-QTOF/MS. Biomed. Chromatogr. 2018, 32, e4061. [Google Scholar] [CrossRef] [PubMed]
- Brenton, A.G.; Godfrey, A.R. Accurate mass measurement: Terminology and treatment of data. J. Am. Soc. Mass Spectrom. 2010, 21, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Santiago, E.; Yahia, E.M. Identification and Quantification of Betalains from the Fruits of 10 Mexican Prickly Pear Cultivars by High-Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef]


| Plant Part | Common Purslane (mg GAE/g DW) 1 | Omega Gold (mg GAE/g DW) | Omega Red (mg GAE/g DW) |
|---|---|---|---|
| Bud | 8.97 ± 0.28 a,A | 5.47 ± 0.82 b,C | 3.89 ± 0.77 b,C |
| Leaf | 8.24 ± 0.22 a,A | 8.67 ± 0.41 a,B | 8.97 ± 0.59 a,B |
| Stem | 2.4 ± 0.34 b,B | 4.19 ± 0.32 a,C | 4.65 ± 0.47 a,C |
| Root | 3.09 ± 0.69 c,B | 31.13 ± 0.77 b,A | 36.50 ± 9.13 a,A |
| Plant Part | Common Purslane 1 | Omega Gold | Omega Red |
|---|---|---|---|
| Bud | 33.88 ± 0.97 b,C | 47.68 ± 0.86 a,B | 53.69 ± 8.58 a,B |
| Leaf | 57.80 ± 2.40 b,A,B | 58.92 ± 1.63 b,A,B | 67.58 ± 2.40 a,A |
| Stem | 51.03 ± 2.32 b,B | 61.84 ± 6.43 a,A | 58.83 ± 1.72 a,b,A,B |
| Root | 61.41 ± 7.03 a,A | 68.01 ± 7.98 a,A | 68.87 ± 4.89 a,A |
| Oleracein Australis 1 | Oleracein C | |||
|---|---|---|---|---|
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 2 | 59.9 | 5.53 d C (10.8) | 60.2 | 5.52 dd (10.8, 1.9) |
| 2-COOH B | 173.4 | 13.20 bs | 173.4 | 13.18 |
| 31 | 32.1 | 3.15 m | 32.5 | 3.16 m |
| 32 A | 3.51 m | 3.52 m | ||
| 3a | 124.6 | na | 124.6 | na |
| 4 | 111.5 | 6.73 s | 111.8 | 6.73 s |
| 5 | 143.6 | na | 143.6 | na |
| 5-OH B | na | 8.45 bs | na | 8.51 s |
| 6 | 144.0 | na | 144.0 | na |
| 7 | 107.5 | 8.11 s | 107.9 | 8.12 s |
| 7a | 135.3 | na | 135.3 | na |
| 1′ | 163.6 | na | 163.5 | na |
| 2′ | 117.6 | 6.77 d (15.2) | 117.7 | 6.83 d (15.2) |
| 3′ | 141.2 | 7.47 d (15.2) | 141.2 | 7.56 d (15.2) |
| 4′ | 129.6 | na | 128.6 | na |
| 5′ | 114.1 | 7.19 d (1.6) | 129.5 | 7.64 d (8.7) |
| 6′ | 146.8 | na | 116.5 | 7.07 d (8.7) |
| 6′-OH B | na | 8.75 bs | na | na |
| 7′ | 147.0 | na | 158.7 | na |
| 8′ | 115.9 | 7.14 d (8.5) | 116.5 | 7.07 d (8.7) |
| 9′ | 120.5 | 7.06 d C (8.5) | 129.5 | 7.64 d (8.7) |
| 1″ | 103.2 | 4.57 d (6.2) | 103.6 | 4.61 d (7.4) |
| 2″ A | 73.1 | 3.27 m | 73.4 | 3.26 m |
| 3″ A | 75.8 | 3.2–3.55 m | 77.1 | 3.2–3.55 m |
| 4″ A | 69.3 | 3.2–3.55 m | 69.7 | 3.2–3.55 m |
| 5″ A | 76.8 | 3.2–3.55 m | 75.0 | 3.2–3.55 m |
| 61″ | 60.4 | 3.72 m | 60.6 | 3.71 m |
| 62″ A | 3.48 m | 3.47 m | ||
| 6″-OH B | na | 4.47 t (5.9) | na | 4.47 bs |
| 1‴ | 101.4 | 4.75 d (7.2) | 100.1 | 4.92 d (7.4) |
| 2‴ A | 72.9 | 3.30 m | 73.2 | 3.29 m |
| 3‴ A | 75.6 | 3.2–3.55 m | 77.0 | 3.2–3.55 m |
| 4‴ A | 69.1 | 3.2–3.55 m | 69.2 | 3.2–3.55 m |
| 5‴ A | 77.1 | 3.2–3.55 m | 76.6 | 3.2–3.55 m |
| 61‴ | 60.1 | 3.72 m | 60.3 | 3.71 m |
| 62‴ | 3.61 m | 3.61 m | ||
| 6‴-OH B | na | 4.61 B t (5.9) | na | 4.56 bs |
| Antioxidant Assay | Oleracein Australis 1 1 | Oleracein C |
|---|---|---|
| ABTS | 1.98 ± 0.07 | 1.42 ± 0.05 |
| DPPH | 1.62 ± 0.07 | 1.17 ± 0.04 |
| TPC | 2.08 ± 0.01 | 1.64 ± 0.01 |
| FRAP | 1.51 ± 0.09 | 1.36 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geier, C.; Micklewright, R.; Barrow, R.; Jamie, J.F.; Prenzler, P.D.; Ryan, D.; Schwarz, L. Bioactivity and Compound Identification in Extracts from Three Australian Populations of Portulaca oleracea: Full NMR Structural Characterisation of Oleracein Australis 1. Molecules 2025, 30, 4147. https://doi.org/10.3390/molecules30204147
Geier C, Micklewright R, Barrow R, Jamie JF, Prenzler PD, Ryan D, Schwarz L. Bioactivity and Compound Identification in Extracts from Three Australian Populations of Portulaca oleracea: Full NMR Structural Characterisation of Oleracein Australis 1. Molecules. 2025; 30(20):4147. https://doi.org/10.3390/molecules30204147
Chicago/Turabian StyleGeier, Colette, Rachael Micklewright, Russell Barrow, Joanne F. Jamie, Paul D. Prenzler, Danielle Ryan, and Lachlan Schwarz. 2025. "Bioactivity and Compound Identification in Extracts from Three Australian Populations of Portulaca oleracea: Full NMR Structural Characterisation of Oleracein Australis 1" Molecules 30, no. 20: 4147. https://doi.org/10.3390/molecules30204147
APA StyleGeier, C., Micklewright, R., Barrow, R., Jamie, J. F., Prenzler, P. D., Ryan, D., & Schwarz, L. (2025). Bioactivity and Compound Identification in Extracts from Three Australian Populations of Portulaca oleracea: Full NMR Structural Characterisation of Oleracein Australis 1. Molecules, 30(20), 4147. https://doi.org/10.3390/molecules30204147










