Under ONIOM Layers: Analysis of BCR-ABL Enzyme Inhibitors Through Bond-Critical Points and Natural Orbitals
Abstract
1. Introduction
2. Results and Discussion
3. Methods
3.1. Docking Protocol
3.2. ONIOM Calculations
- 1.
- The T315I isoform of the BCR-ABL enzyme in the absence of any ligands;
- 2.
- BCR-ABL(T315I) complexed with rebastinib;
- 3.
- BCR-ABL(T315I) complexed with ponatinib.
3.3. NCI, BCP, and NBO Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sampaio, M.M.; Santos, M.L.C.; Marques, H.S.; Gonçalves, V.L.d.S.; Araújo, G.R.L.; Lopes, L.W.; Apolonio, J.S.; Silva, C.S.; Santos, L.K.d.S.; Cuzzuol, B.R.; et al. Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review. World J. Clin. Oncol. 2021, 12, 69–94. [Google Scholar] [CrossRef]
- Pricl, S.; Fermeglia, M.; Ferrone, M.; Tamborini, E. T315I-mutated Bcr-Abl in chronic myeloid leukemia and imatinib: Insights from a computational study. Mol. Cancer Ther. 2005, 4, 1167–1174. [Google Scholar] [CrossRef]
- Cilloni, D.; Saglio, G. Molecular pathways: BCR-ABL. Clin. Cancer Res. 2012, 18, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.E.; Deininger, M.W. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. 2021, 49, 100825. [Google Scholar] [CrossRef] [PubMed]
- Rohrbacher, M.; Hasford, J. Epidemiology of chronic myeloid leukaemia (CML). Best Pract. Res. Clin. Haematol. 2009, 22, 295–302. [Google Scholar] [CrossRef]
- Zámečníkova, A. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia as a model of rational drug design in cancer. Expert Rev. Hematol. 2010, 3, 45–56. [Google Scholar] [CrossRef]
- Tosta Perez, M.; Herrera Belen, L.; Letelier, P.; Calle, Y.; Pessoa, A.; Farias, J.G. l-Asparaginase as the gold standard in the treatment of acute lymphoblastic leukemia: A comprehensive review. Med. Oncol. 2023, 40, 150. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.S.; Lemos, L.G.T.; Moraes, G.N.d.; Maia, R.C. Unraveling survivin expression in chronic myeloid leukemia: Molecular interactions and clinical implications. Blood Rev. 2020, 43, 100671. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Rana, A.; Datoguia, T.S.; Hamerschlak, N.; Brumatti, G. BCR-ABL1 Tyrosine Kinase Complex Signaling Transduction: Challenges to Overcome Resistance in Chronic Myeloid Leukemia. Pharmaceutics 2022, 14, 215. [Google Scholar] [CrossRef]
- Kang, Z.J.; Liu, Y.F.; Xu, L.Z.; Long, Z.J.; Huang, D.; Yang, Y.; Liu, B.; Feng, J.X.; Pan, Y.J.; Yan, J.S.; et al. The Philadelphia chromosome in leukemogenesis. Chin. J. Cancer 2016, 35, 48. [Google Scholar] [CrossRef]
- Watt, J.L.; Page, B.M. Reciprocal translocation and the Philadelphia chromosome. Hum. Genet. 1978, 42, 163–170. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Kumar, R.; Krause, D.S. Chronic Myeloid Leukemia: A Model Disease of the Past, Present and Future. Cells 2021, 10, 117. [Google Scholar] [CrossRef]
- Dobrovic, A.; Peters, G.B.; Ford, J.H. Review: Molecular analysis of the Philadelphia chromosome. Chromosoma 1991, 100, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Davulcu, E.A.; Pekerbas, M.; Karaca, E.; Durmaz, B.; Özsan, N.; Akın, H.; Saydam, G. Complex karyotype with double Philadelphia chromosome and T315I mutation results in blastic phase and extensive extramedullary infiltration in a chronic myeloid leukemia patient. Cancer Genet. 2022, 266–267, 74–80. [Google Scholar] [CrossRef]
- Reinhold, U.; Hennig, E.; Leiblein, S.; Niederwieser, D.; Deininger, M.W.N. FISH for BCR-ABL on interphases of peripheral blood neutrophils but not of unselected white cells correlates with bone marrow cytogenetics in CML patients treated with imatinib. Leukemia 2003, 17, 1925–1929. [Google Scholar] [CrossRef][Green Version]
- Dewald, G.W.; Wyatt, W.A.; Juneau, A.L.; Carlson, R.O.; Zinsmeister, A.R.; Jalal, S.M.; Spurbeck, J.L.; Silver, R.T. Highly sensitive fluorescence in situ hybridization method to detect double BCR/ABL fusion and monitor response to therapy in chronic myeloid leukemia. Blood 1998, 91, 3357–3365. [Google Scholar] [CrossRef][Green Version]
- Chopra, R.; Pu, Q.Q.; Elefanty, A.G. Biology of BCR-ABL. Blood Rev. 1999, 13, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Malagrinò, F.; Puglisi, E.; Pagano, L.; Travaglini-Allocatelli, C.; Toto, A. GRB2: A dynamic adaptor protein orchestrating cellular signaling in health and disease. Biochem. Biophys. Rep. 2024, 39, 101803. [Google Scholar] [CrossRef]
- Bar-Sagi, D. The Sos (Son of sevenless) protein. Trends Endocrinol. Metab. TEM 1994, 5, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Bedi, A.; Zehnbauer, B.A.; Barber, J.P.; Sharkis, S.J.; Jones, R.J. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood 1994, 83, 2038–2044. [Google Scholar] [CrossRef]
- Stein, S.J.; Baldwin, A.S. NF-B suppresses ROS levels in BCR-ABL+ cells to prevent activation of JNK and cell death. Oncogene 2011, 30, 4557–4566. [Google Scholar] [CrossRef]
- Wertheim, J.A.; Forsythe, K.; Druker, B.J.; Hammer, D.; Boettiger, D.; Pear, W.S. BCR-ABL-induced adhesion defects are tyrosine kinase-independent. Blood 2002, 99, 4122–4130. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Sausville, E.A. Imatinib for chronic myelogenous leukaemia: A 9 or 24 carat gold standard? Lancet 2003, 361, 1400–1401. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A Breakthrough of Targeted Therapy in Cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef]
- Peng, B.; Lloyd, P.; Schran, H. Clinical pharmacokinetics of imatinib. Clin. Pharmacokinet. 2005, 44, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Rosti, G.; Iacobucci, I.; Baccarani, M.; Martinelli, G. Choosing the Best Second-Line Tyrosine Kinase Inhibitor in Imatinib-Resistant Chronic Myeloid Leukemia Patients Harboring Bcr-Abl Kinase Domain Mutations: How Reliable Is the IC50? Oncologist 2011, 16, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Volpe, G.; Panuzzo, C.; Ulisciani, S.; Cilloni, D. Imatinib resistance in CML. Cancer Lett. 2009, 274, 1–9. [Google Scholar] [CrossRef]
- Cang, S.; Liu, D. P-loop mutations and novel therapeutic approaches for imatinib failures in chronic myeloid leukemia. J. Hematol. Oncol. 2008, 1, 15. [Google Scholar] [CrossRef]
- Rosari, F.; Minutolo, F.; Orciuolo, E. Past, present, and future of Bcr-Abl inhibitors: From chemical development to clinical efficacy. J. Hematol. Oncol. 2018, 11, 106156. [Google Scholar] [CrossRef] [PubMed]
- Pandrala, M.; Bruyneel, A.A.N.; Hnatiuk, A.P.; Mercola, M.; Malhotra, S.V. Designing Novel BCR-ABL Inhibitors for Chronic Myeloid Leukemia with Improved Cardiac Safety. J. Med. Chem. 2022, 65, 10898–10919. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.S.; Xu, Q.; et al. AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance. Cancer Cell 2009, 16, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Tanneeru, K.; Guruprasad, L. Ponatinib Is a Pan-BCR-ABL Kinase Inhibitor: MD Simulations and SIE Study. PLoS ONE 2013, 8, e78556. [Google Scholar] [CrossRef]
- Marto, J.P.; Strambo, D.; Livio, F.; Michel, P. Drugs Associated With Ischemic Stroke: A Review for Clinicians. Stroke 2021, 52, E646–E659. [Google Scholar] [CrossRef]
- Tousif, S.; Singh, A.P.; Umbarkar, P.; Galindo, C.; Wheeler, N.; Toro Cora, A.; Zhang, Q.; Prabhu, S.D.; Lal, H. Ponatinib Drives Cardiotoxicity by S100A8/A9-NLRP3-IL-1β Mediated Inflammation. Circ. Res. 2023, 132, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, G.; Qian, B.; Okhowat, A.; Sabha, N.; Kontos, C.D.; Guha, A. Targeting the Tie2/Tek Receptor in Astrocytomas. Am. J. Pathol. 2004, 164, 467–476. [Google Scholar] [CrossRef]
- Huang, H.; Bhat, A.; Woodnutt, G.; Lappe, R. Targeting the ANGPT–TIE2 pathway in malignancy. Nat. Rev. Cancer 2010, 10, 575–585. [Google Scholar] [CrossRef]
- Hasenstein, J.R.; Kasmerchak, K.; Buehler, D.; Hafez, G.R.; Cleary, K.; Moody, J.S.; Kozak, K.R. Efficacy of Tie2 receptor antagonism in Angiosarcoma. Neoplasia 2012, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Liu, D.; Fueyo, J.; Gomez-Manzano, C. Tie2: A journey from normal angiogenesis to cancer and beyond. Histol. Histopathol. 2008, 23, 773–780. [Google Scholar] [CrossRef]
- Ashaq, M.S.; Zhou, Q.; Li, Z.; Zhao, B. Novel targeted therapies in chronic myeloid leukemia. Pharm. Sci. Adv. 2024, 2, 100052. [Google Scholar] [CrossRef]
- Cortes, J.; Talpaz, M.; Smith, H.P.; Snyder, D.S.; Khoury, J.; Bhalla, K.N.; Pinilla-Ibarz, J.; Larson, R.; Mitchell, D.; Wise, S.C.; et al. Phase 1 dose-finding study of rebastinib (DCC-2036) in patients with relapsed chronic myeloid leukemia and acute myeloid leukemia. Haematologica 2017, 102, 519–528. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Yazdanpanah, N.; Rezaei, N. Chronic myeloid leukemia stem cells: Targeting therapeutic implications. Stem Cell Res. Ther. 2021, 12, 603. [Google Scholar] [CrossRef]
- Chan, W.W.; Wise, S.C.; Kaufman, M.D.; Ahn, Y.M.; Ensinger, C.L.; Haack, T.; Hood, M.M.; Jones, J.; Lord, J.W.; Lu, W.P.; et al. Conformational Control Inhibition of the BCR-ABL1 Tyrosine Kinase, Including the Gatekeeper T315I Mutant, by the Switch-Control Inhibitor DCC-2036. Cancer Cell 2011, 19, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Antolíková, E.; Žáková, L.; Turkenburg, J.P.; Watson, C.J.; Hančlová, I.; Šanda, M.; Cooper, A.; Kraus, T.; Brzozowski, A.M.; Jiráček, J. Non-equivalent role of inter- and intramolecular hydrogen bonds in the insulin dimer interface. J. Biol. Chem. 2011, 286, 36968–36977. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Reynolds, C.A.; Koole, C.; Smith, K.J.; Mobarec, J.C.; Simms, J.; Quon, T.; Coudrat, T.; Furness, S.G.; Miller, L.J.; et al. A hydrogen-bonded polar network in the core of the glucagon-like peptide-1 receptor is a fulcrum for biased agonism: Lessons from class B crystal structuress. Mol. Pharmacol. 2016, 89, 335–347. [Google Scholar] [CrossRef]
- Venugopal, P.P.; Das, B.K.; Soorya, E.; Chakraborty, D. Effect of hydrophobic and hydrogen bonding interactions on the potency of ß-alanine analogs of G-protein coupled glucagon receptor inhibitors. Proteins Struct. Funct. Bioinform. 2020, 88, 327–344. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Kamran Haider, M. Hydrogen Bonds in Proteins: Role and Strength. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- Xing, L.; Klug-Mcleod, J.; Rai, B.; Lunney, E.A. Kinase hinge binding scaffolds and their hydrogen bond patterns. Bioorganic Med. Chem. 2015, 23, 6520–6527. [Google Scholar] [CrossRef] [PubMed]
- Li, G.C.; Srivastava, A.K.; Kim, J.; Taylor, S.S.; Veglia, G. Mapping the Hydrogen Bond Networks in the Catalytic Subunit of Protein Kinase A Using H/D Fractionation Factors. Biochemistry 2015, 54, 4042–4049. [Google Scholar] [CrossRef]
- Ding, Y.; Fang, Y.; Moreno, J.; Ramanujam, J.; Jarrell, M.; Brylinski, M. Assessing the similarity of ligand binding conformations with the Contact Mode Score. Comput. Biol. Chem. 2016, 64, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alvarez, A.; Costa, A.M.; Vilarrasa, J. The Performance of several docking programs at reproducing protein-macrolide-like crystal structures. Molecules 2017, 22, 136. [Google Scholar] [CrossRef]
- Reddy, E.P.; Aggarwal, A.K. The Ins and Outs of Bcr-Abl Inhibition. Genes Cancer 2012, 3, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.M.; Nascimento, É.C.; Martins, J.B. Investigation on the interaction behavior of afatinib, dasatinib, and imatinib docked to the BCR-ABL protein. J. Mol. Model. 2021, 27, 309. [Google Scholar] [CrossRef]
- Rocha, K.M.L.; Nascimento, E.C.M.; de Jesus, R.C.C.; Martins, J.B.L. In Silico Molecular Modeling of Four New Afatinib Derived Molecules Targeting the Inhibition of the Mutated Form of BCR-ABL T315I. Molecules 2024, 29, 4254. [Google Scholar] [CrossRef]
- Pereira, W.A.; Nascimento, É.C.M.; Martins, J.B.L. Electronic and structural study of T315I mutated form in DFG-out conformation of BCR-ABL inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 9774–9788. [Google Scholar] [CrossRef]
- Miar, M.; Shiroudi, A.; Pourshamsian, K.; Oliaey, A.R.; Hatamjafari, F. Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo[d]thiazole-2(3H)-imine and its para-substituted derivatives: Solvent and substituent effects. J. Chem. Res. 2021, 45, 147–158. [Google Scholar] [CrossRef]
- Yu, J.; Su, N.Q.; Yang, W. Describing Chemical Reactivity with Frontier Molecular Orbitalets. JACS Au 2022, 2, 1383–1394. [Google Scholar] [CrossRef]
- Bryenton, K.R.; Adeleke, A.A.; Dale, S.G.; Johnson, E.R. Delocalization error: The greatest outstanding challenge in density-functional theory. WIREs Comput. Mol. Sci. 2022, 13, e1631. [Google Scholar] [CrossRef]
- Jursic, B. A B3LYP hybrid density functional theory study of structural properties, energies, and heats of formation for silicon–hydrogen compounds. J. Mol. Struct. THEOCHEM 2000, 497, 65–73. [Google Scholar] [CrossRef]
- Costa, R.J.; Castro, E.A.S.; Politi, J.R.S.; Gargano, R.; Martins, J.B.L. Methanol, ethanol, propanol, and butanol adsorption on H-ZSM-5 zeolite: An ONIOM study. J. Mol. Model. 2019, 25, 34. [Google Scholar] [CrossRef]
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.C.M.; Martins, J.B.L. Electronic structure and PCA analysis of covalent and non-covalent acetylcholinesterase inhibitors. J. Mol. Model. 2010, 17, 1371–1379. [Google Scholar] [CrossRef]
- Nascimento, L.A.; Nascimento, É.C.; Martins, J.B. In silico study of tacrine and acetylcholine binding profile with human acetylcholinesterase: Docking and electronic structure. J. Mol. Model. 2022, 28, 252. [Google Scholar] [CrossRef] [PubMed]
- Herschlag, D.; Pinney, M.M. Hydrogen Bonds: Simple after All? Biochemistry 2018, 57, 3338–3352. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Kruse, H.; Goerigk, L.; Grimme, S. Why the standard B3LYP/6-31G* model chemistry should not be used in DFT calculations of molecular thermochemistry: Understanding and correcting the problem. J. Org. Chem. 2012, 77, 10824–10834. [Google Scholar] [CrossRef]
- van der Lubbe, S.C.; Guerra, C.F. The Nature of Hydrogen Bonds: A Delineation of the Role of Different Energy Components on Hydrogen Bond Strengths and Lengths. Chem. Asian J. 2019, 14, 2760–2769. [Google Scholar] [CrossRef]
- van der Lubbe, S.C.; Guerra, C.F. Hydrogen-Bond Strength of CC and GG Pairs Determined by Steric Repulsion: Electrostatics and Charge Transfer Overruled. Chem. A Eur. J. 2017, 23, 10249–10253. [Google Scholar] [CrossRef]
- Mao, Y.; Horn, P.R.; Head-Gordon, M. Energy decomposition analysis in an adiabatic picture. Phys. Chem. Chem. Phys. 2017, 19, 5944–5958. [Google Scholar] [CrossRef]
- Gilli, G.; Bellucci, F.; Ferretti, V.; Bertolasi, V. Evidence for resonance-assisted hydrogen bonding from crystal-structure correlations on the enol form of the .beta.-diketone fragment. J. Am. Chem. Soc. 1989, 111, 1023–1028. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. Evidence for resonance-assisted hydrogen bonding. 2. Intercorrelation between crystal structure and spectroscopic parameters in eight intramolecularly hydrogen bonded 1,3-diaryl-1,3-propanedione enols. J. Am. Chem. Soc. 1991, 113, 4917–4925. [Google Scholar] [CrossRef]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef]
- Kar, T.; Scheiner, S. Comparison of cooperativity in CH...O and OH...O hydrogen bonds. J. Phys. Chem. A 2004, 108, 9161–9168. [Google Scholar] [CrossRef]
- Chen, Y.F.; Dannenberg, J.J. Cooperative 4-pyridone H-bonds with extraordinary stability. A DFT molecular orbital study. J. Am. Chem. Soc. 2006, 128, 8100–8101. [Google Scholar] [CrossRef]
- Kobko, N.; Paraskevas, L.; Del Rio, E.; Dannenberg, J.J. Cooperativity in amide hydrogen bonding chains: Implications for protein-folding models. J. Am. Chem. Soc. 2001, 123, 4348–4349. [Google Scholar] [CrossRef]
- Mahmoudi Gomari, M.; Rostami, N.; Ghodrati, A.; Hernandez, Y.; Fadaie, M.; Sadegh Eslami, S.; Tarighi, P. Implementation of docking, molecular dynamics and free energy to investigate drug potency of novel BCR-ABLT315I inhibitors as an alternative to ponatinib. Comput. Toxicol. 2021, 20, 100180. [Google Scholar] [CrossRef]
- Contreras-García, J.; Yang, W.; Johnson, E.R. Analysis of Hydrogen-Bond Interaction Potentials from the Electron Density: Integration of Noncovalent Interaction Regions. J. Phys. Chem. A 2011, 115, 12983–12990. [Google Scholar] [CrossRef]
- Castro, T.S.; Martins, G.F.; de Alcântara Morais, S.F.; Ferreira, D.A.C. Aromaticity of Cope and Claisen rearrangements. Theor. Chem. Accounts 2023, 142, 40. [Google Scholar] [CrossRef]
- Martins, G.F.; Castro, T.S.; Ferreira, D.A.C. Theoretical investigation of anion perfluorocubane. J. Mol. Model. 2023, 29, 319. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Sandak, A.; Sandak, J. Multiscale modelling investigation of wood modification with acetic anhydride †. Phys. Chem. Chem. Phys. 2020, 22, 28448–28458. [Google Scholar] [CrossRef]
- Zhou, T.; Commodore, L.; Huang, W.S.; Wang, Y.; Thomas, M.; Keats, J.; Xu, Q.; Rivera, V.M.; Shakespeare, W.C.; Clackson, T.; et al. Structural Mechanism of the Pan-BCR-ABL Inhibitor Ponatinib (AP24534): Lessons for Overcoming Kinase Inhibitor Resistance. Chem. Biol. Drug Des. 2011, 77, 1–11. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Shivanika, C.; Deepak Kumar, S.; Ragunathan, V.; Tiwari, P.; Sumitha, A.; Brindha Devi, P. Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J. Biomol. Struct. Dyn. 2022, 40, 585–611. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef]
- Silva Andrade, B.; Ghosh, P.; Barh, D.; Tiwari, S.; José Santana Silva, R.; Rodrigues de Assis Soares, W.; Silva Melo, T.; Santos Freitas, A.; González-Grande, P.; Sousa Palmeira, L.; et al. Computational screening for potential drug candidates against the SARS-CoV-2 main protease. F1000Research 2020, 9, 514. [Google Scholar] [CrossRef]
- Biovia, R.D.S. Discovery Studio Modeling Environment; Science and Education: Newark, DE, USA, 2017. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 27–28. [Google Scholar] [CrossRef]
- Chung, L.W.; Sameera, W.M.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.; Liu, F.; et al. The ONIOM Method and Its Applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 720–723. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Spicher, S.; Caldeweyher, E.; Hansen, A.; Grimme, S. Benchmarking London dispersion corrected density functional theory for noncovalent ion–π interactions. Phys. Chem. Chem. Phys. 2021, 23, 11635–11648. [Google Scholar] [CrossRef]
- Liao, M.S.; Huang, M.J.; Watts, J.D. Assessment of dispersion corrections in DFT calculations on large biological systems. Mol. Phys. 2012, 110, 3061–3076. [Google Scholar] [CrossRef]
- Körzdörfer, T.; Sears, J.S.; Sutton, C.; Brédas, J.L. Long-range corrected hybrid functionals for π-conjugated systems: Dependence of the range-separation parameter on conjugation length. J. Chem. Phys. 2011, 135, 204107. [Google Scholar] [CrossRef]
- Szczepanik, D.W.; Solà, M.; Andrzejak, M.; Pawełek, B.; Dominikowska, J.; Kukułka, M.; Dyduch, K.; Krygowski, T.M.; Szatylowicz, H. The role of the long-range exchange corrections in the description of electron delocalization in aromatic species. J. Comput. Chem. 2017, 38, 1640–1654. [Google Scholar] [CrossRef]
- Guan, H.; Sun, H.; Zhao, X. Application of Density Functional Theory to Molecular Engineering of Pharmaceutical Formulations. Int. J. Mol. Sci. 2025, 26, 3262. [Google Scholar] [CrossRef]
- Ouma, R.B.O.; Ngari, S.M.; Kibet, J.K. A review of the current trends in computational approaches in drug design and metabolism. Discov. Public Health 2024, 21, 108. [Google Scholar] [CrossRef]
- Lu, L.; Hu, H.; Hou, H.; Wang, B. An improved B3LYP method in the calculation of organic thermochemistry and reactivity. Comput. Theor. Chem. 2013, 1015, 64–71. [Google Scholar] [CrossRef]
- Ye, N.; Yang, Z.; Liu, Y. Applications of density functional theory in COVID-19 drug modeling. Drug Discov. Today 2022, 27, 1411–1419. [Google Scholar] [CrossRef]
- Lozynski, M.; Rusinska-Roszak, D.; Mack, H.G. Hydrogen Bonding and Density Functional Calculations: The B3LYP Approach as the Shortest Way to MP2 Results. J. Phys. Chem. A 1998, 102, 2899–2903. [Google Scholar] [CrossRef]
- Schmidt, T.C.; Welker, A.; Rieger, M.; Sahu, P.K.; Sotriffer, C.A.; Schirmeister, T.; Engels, B. Protocol for Rational Design of Covalently Interacting Inhibitors. ChemPhysChem 2014, 15, 3226–3235. [Google Scholar] [CrossRef]
- Mihalovits, L.M.; Ferenczy, G.G.; Keserű, G.M. The role of quantum chemistry in covalent inhibitor design. Int. J. Quantum Chem. 2022, 122, e26768. [Google Scholar] [CrossRef]
- Tóth, L.; Muszbek, L.; Komáromi, I. Mechanism of the irreversible inhibition of human cyclooxygenase-1 by aspirin as predicted by QM/MM calculations. J. Mol. Graph. Model. 2013, 40, 99–109. [Google Scholar] [CrossRef]
- Cuadrado, C.; Daranas, A.H.; Sarotti, A.M. May the Force (Field) Be with You: On the Importance of Conformational Searches in the Prediction of NMR Chemical Shifts. Mar. Drugs 2022, 20, 699. [Google Scholar] [CrossRef]
- Yildiz, I.; Yildiz, B.S. Computational Analysis of Histone Deacetylase 10 Mechanism by the ONIOM Method: A Complementary Approach to X-ray and Kinetics Studies. ACS Omega 2022, 7, 6393–6402. [Google Scholar] [CrossRef]
- Kar, R.K. Benefits of hybrid QM/MM over traditional classical mechanics in pharmaceutical systems. Drug Discov. Today 2023, 28, 103374. [Google Scholar] [CrossRef]
- Potier, N.; Barth, P.; Tritsch, D.; Biellmann, J.F.; Van Dorsselaer, A. Study of non-covalent enzyme-inhibitor complexes of aldose reductase by electrospray mass spectrometry. Eur. J. Biochem. 1997, 243, 274–282. [Google Scholar] [CrossRef]
- Ponder, J.W.; Case, D.A. Force Fields for Protein Simulations. Adv. Protein Chem. 2003, 66, 27–85. [Google Scholar]
- Momany, F.; Willett, J. Computational studies on carbohydrates: In vacuo studies using a revised AMBER force field, AMB99C, designed for α-(1→4) linkages. Carbohydr. Res. 2000, 326, 194–209. [Google Scholar] [CrossRef]
- Freindorf, M.; Shao, Y.; Furlani, T.R.; Kong, J. Lennard–Jones parameters for the combined QM/MM method using the B3LYP/6-31G*/AMBER potential. J. Comput. Chem. 2005, 26, 1270–1278. [Google Scholar] [CrossRef]
- Kellie, J.L.; Wetmore, S.D. Selecting DFT methods for use in optimizations of enzyme active sites: Applications to ONIOM treatments of DNA glycosylases. Can. J. Chem. 2013, 91, 559–572. [Google Scholar] [CrossRef]
- Lundberg, M.; Sasakura, Y.; Zheng, G.; Morokuma, K. Case studies of ONIOM(DFT:DFTB) and ONIOM(DFT:DFTB:MM) for enzymes and enzyme mimics. J. Chem. Theory Comput. 2010, 6, 1413–1427. [Google Scholar] [CrossRef]
- Sharma, H.; Raju, B.; Narendra, G.; Motiwale, M.; Sharma, B.; Verma, H.; Silakari, O. QM/MM studies on enzyme catalysis and insight into designing of new inhibitors by ONIOM approach: Recent update. ChemistrySelect 2023, 8, e202203319. [Google Scholar] [CrossRef]
- Nadia, L.; Djameleddine, K.; Rayenne, D. Theoretical study of the inclusion processes of octopamine with β-cyclodextrin: PM6, ONIOM, and NBO analysis. Comptes Rendus. Chim. 2014, 17, 1169–1175. [Google Scholar] [CrossRef]
- Tantirungrotechai, Y.; Roddecha, S.; Punyain, K.; Toochinda, P. Assessment of mixed basis set and ONIOM methods on the activation energy of ring opening reactions of substituted cyclobutenes. J. Mol. Struct. THEOCHEM 2009, 893, 98–105. [Google Scholar] [CrossRef]
- Piyaauksornsak, S.; Tangthongkul, T.; Wanbayor, R.; Wanno, B.; Ruangpornvisuti, V. Molecular structures of 8,8’-dithioureido-2,2’-binaphthalene derivatives and their anions recognition: An ONIOM investigation. Struct. Chem. 2009, 20, 767–780. [Google Scholar] [CrossRef]
- Heerdt, G.; Morgon, N.H. Theoretical study of thermochemical properties using composite methods adapted to ONIOM. J. Braz. Chem. Soc. 2012, 23, 1741–1746. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian˜16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Keith, T. AIMAll, Version 19.10.12; TK Gristmill Software: Overland Park, KS, USA, 2019.
- Matta, C.F.; Lombardi, O.; Jaimes Arriaga, J. Two-step emergence: The quantum theory of atoms in molecules as a bridge between quantum mechanics and molecular chemistry. Found. Chem. 2020, 22, 107–129. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Araújo, R.C.; Ramos, M.N. The QTAIM Molecular Topology and the Quantum-Mechanical Description of Hydrogen Bonds and Dihydrogen Bonds. Quim. Nova 2010, 33, 1155–1162. [Google Scholar] [CrossRef]
- Oliveira, V.; Kraka, E. Systematic Coupled Cluster Study of Noncovalent Interactions Involving Halogens, Chalcogens, and Pnicogens. J. Phys. Chem. A 2017, 121, 9544–9556. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density — Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Cox, D.F.; Crawford, T.D.; Rosso, K.M.; Ross, N.L.; Downs, R.T. Classification of metal-oxide bonded interactions based on local potential- and kinetic-energy densities. J. Chem. Phys. 2006, 124, 084704. [Google Scholar] [CrossRef]
- Martins, J.B.; Quintino, R.P.; Politi, J.R.d.S.; Sethio, D.; Gargano, R.; Kraka, E. Computational analysis of vibrational frequencies and rovibrational spectroscopic constants of hydrogen sulfide dimer using MP2 and CCSD(T). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118540. [Google Scholar] [CrossRef]
- Freindorf, M.; Kraka, E.; Cremer, D. A comprehensive analysis of hydrogen bond interactions based on local vibrational modes. Int. J. Quantum Chem. 2012, 112, 3174–3187. [Google Scholar] [CrossRef]
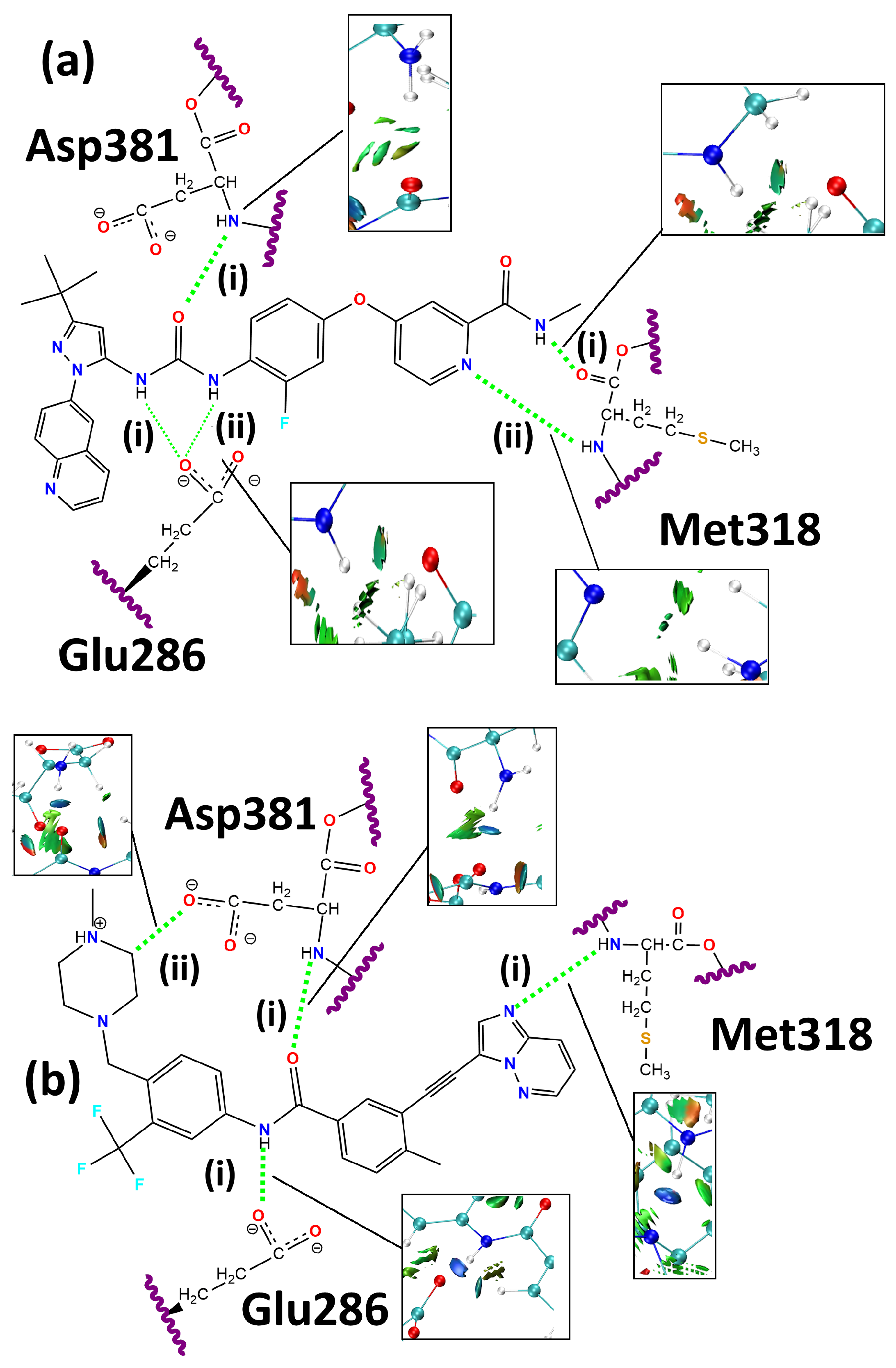
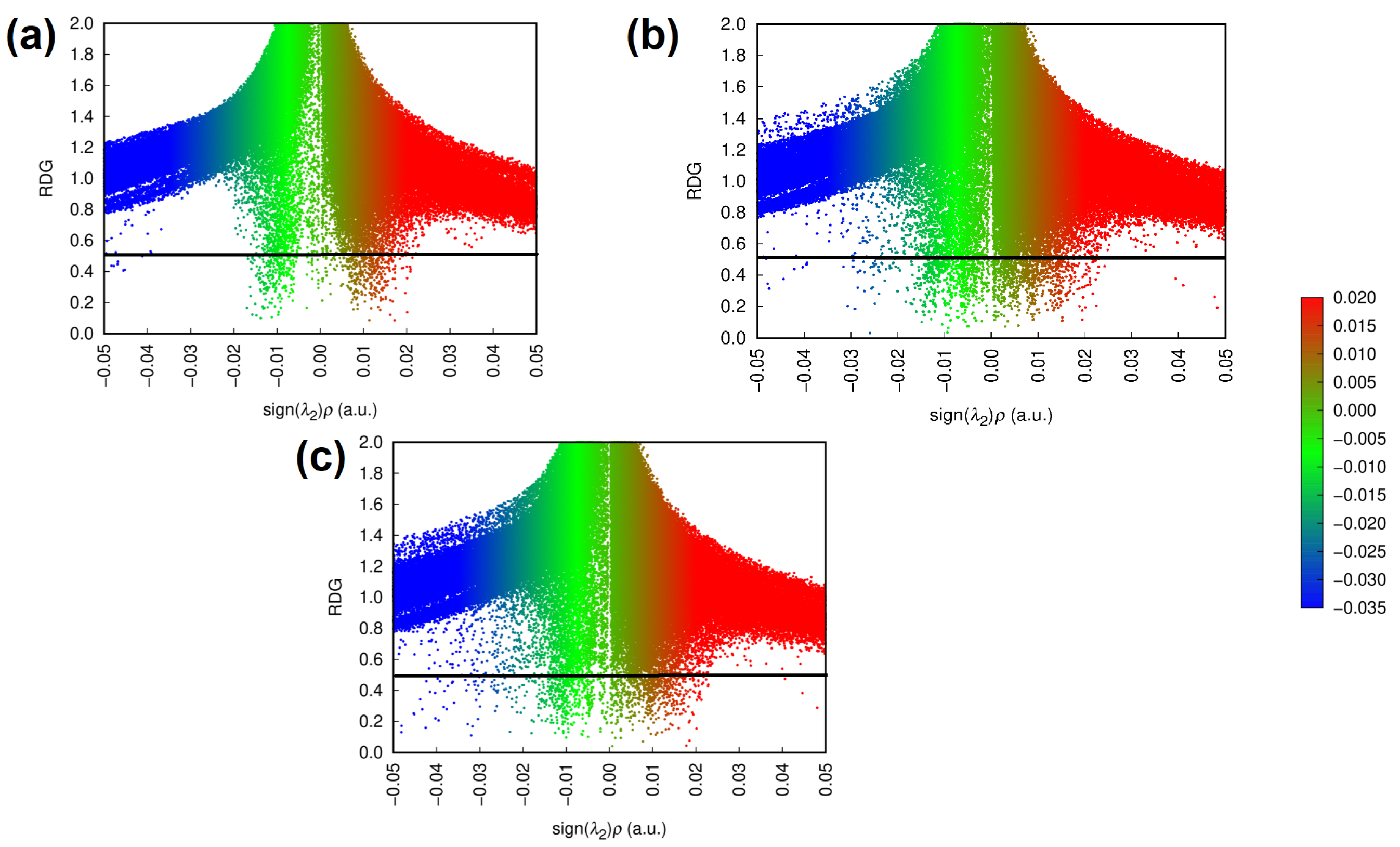
| Parameter | BCR-ABL | BCR-ABL + Rebastinib | BCR-ABL + Ponatinib |
|---|---|---|---|
| HOMO | |||
| LUMO | |||
| Gap |
| Interaction | Interatomic Distance (Å) | Bond Angle (Degrees) | ||
|---|---|---|---|---|
| Docking | ONIOM | Docking | ONIOM | |
| Rebastinib:Glu286 (i) | 2.029 | 1.910 | 141.6 | 152.1 |
| Rebastinib:Glu286 (ii) | 2.040 | 1.923 | 132.5 | 151.3 |
| Rebastinib:Met318 (i) | 2.218 | 2.144 | 127.4 | 130.2 |
| Rebastinib:Met318 (ii) | 2.218 | 2.371 | 163.3 | 170.2 |
| Rebastinib:Asp381 (i) | 1.979 | 2.175 | 163.6 | 171.3 |
| Ponatinib:Glu286 (i) | 1.823 | 1.864 | 165.2 | 167.9 |
| Ponatinib:Met318 (i) | 1.922 | 1.924 | 171.2 | 171.5 |
| Ponatinib:Asp381 (i) | 1.888 | 1.905 | 170.2 | 168.9 |
| Ponatinib:Asp381 (ii) | 1.808 | 1.806 | 165.5 | 160.5 |
| Interaction | Energy Density (a.u.) | Electron Density (e·Å−3) | Laplacian (e·Å−5) | E(2) (kcal·mol−1) |
|---|---|---|---|---|
| Rebastinib:Glu286 (i) | 0.206 | 2.087 | 8.38 (a) | |
| Rebastinib:Glu286 (ii) | 0.206 | 2.087 | 14.09 (a) | |
| Rebastinib:Met318 (i) | 0.115 | 1.465 | 1.84 (b) | |
| Rebastinib:Met318 (ii) | 0.086 | 0.919 | 4.35 (c) | |
| Rebastinib:Asp381(i) | 0.101 | 1.199 | 2.47 (d) | |
| Ponatinib:Glu286 (i) | 0.219 | 2.304 | 10.68 (a) | |
| Ponatinib:Met318 (i) | 0.222 | 2.216 | 19.96 (c) | |
| Ponatinib:Asp381 (i) | 0.182 | 2.229 | 7.11 (d) | |
| Ponatinib:Asp381 (ii) | 0.285 | 2.914 | 3.95 (e) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, K.M.L.; Nascimento, É.C.M.; Martins, J.B.L. Under ONIOM Layers: Analysis of BCR-ABL Enzyme Inhibitors Through Bond-Critical Points and Natural Orbitals. Molecules 2025, 30, 4145. https://doi.org/10.3390/molecules30204145
Rocha KML, Nascimento ÉCM, Martins JBL. Under ONIOM Layers: Analysis of BCR-ABL Enzyme Inhibitors Through Bond-Critical Points and Natural Orbitals. Molecules. 2025; 30(20):4145. https://doi.org/10.3390/molecules30204145
Chicago/Turabian StyleRocha, Kelvyn M. L., Érica C. M. Nascimento, and João B. L. Martins. 2025. "Under ONIOM Layers: Analysis of BCR-ABL Enzyme Inhibitors Through Bond-Critical Points and Natural Orbitals" Molecules 30, no. 20: 4145. https://doi.org/10.3390/molecules30204145
APA StyleRocha, K. M. L., Nascimento, É. C. M., & Martins, J. B. L. (2025). Under ONIOM Layers: Analysis of BCR-ABL Enzyme Inhibitors Through Bond-Critical Points and Natural Orbitals. Molecules, 30(20), 4145. https://doi.org/10.3390/molecules30204145







