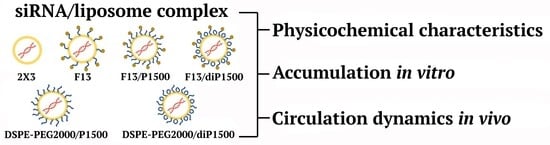
Loop-Structured PEG-Lipoconjugate Enhances siRNA Delivery Mediated by Liner-PEG Containing Liposomes
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of the Liposomes and Their Complexes with siRNA
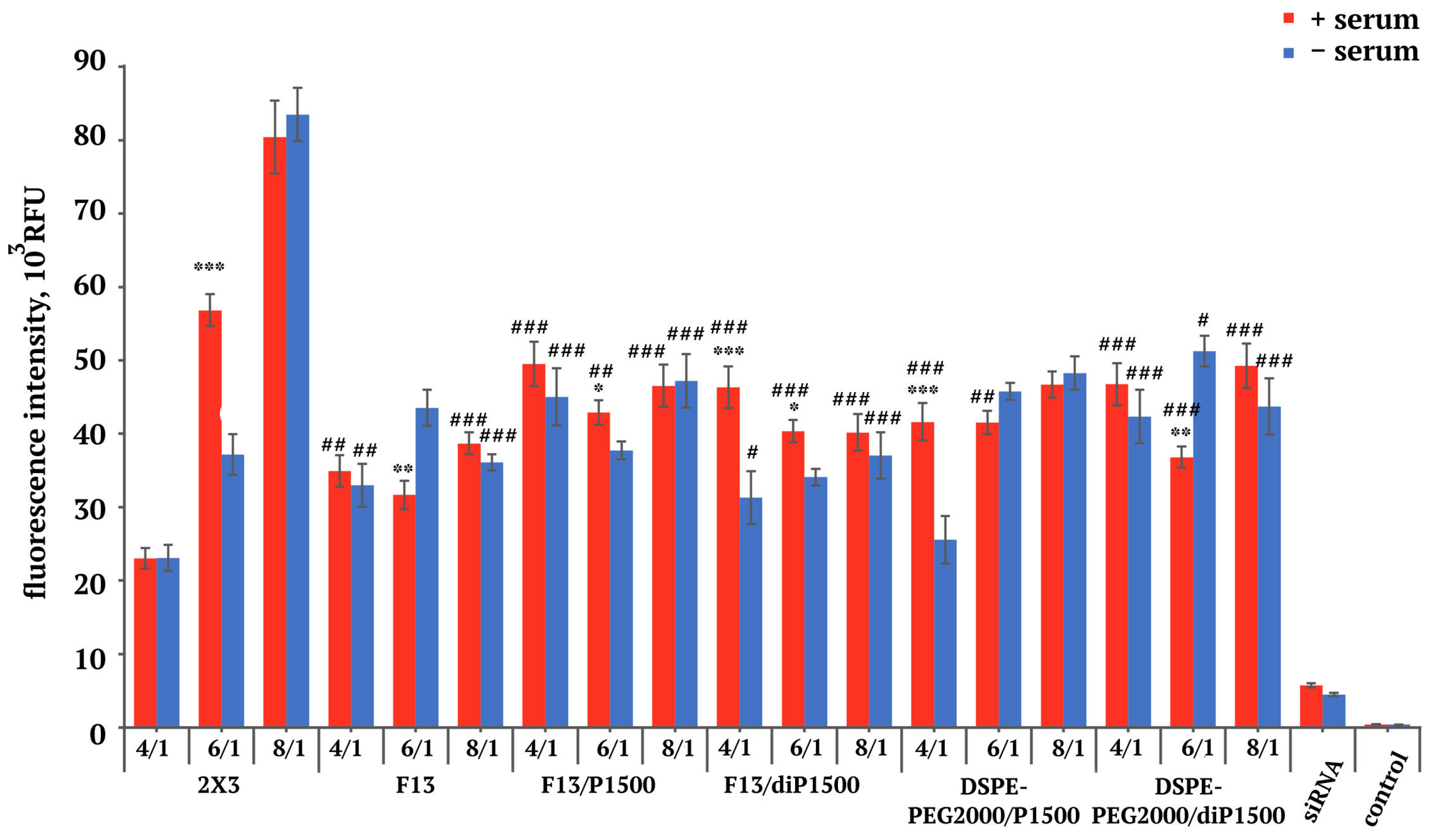
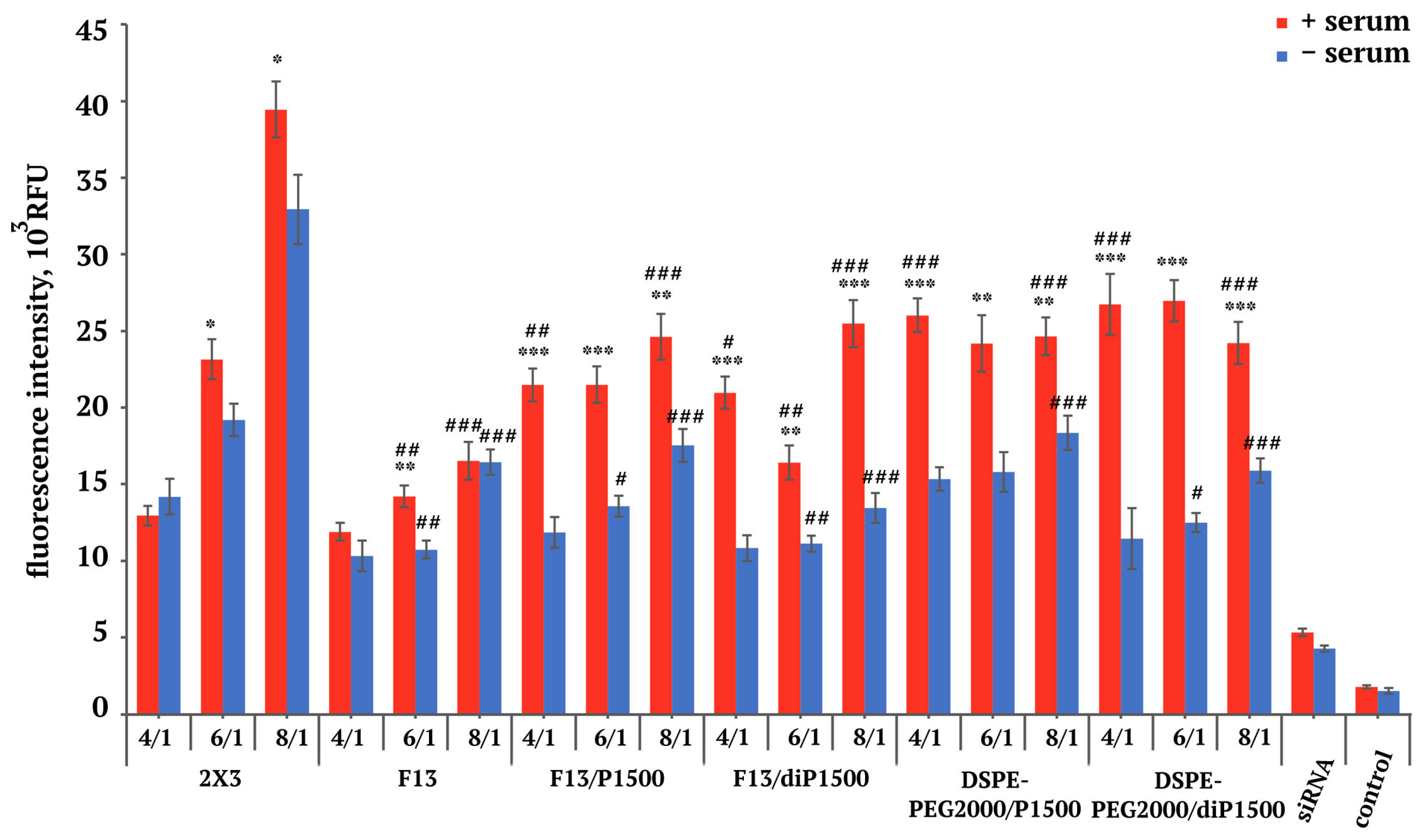
2.2. Accumulation of siRNA/Liposome Complexes in KB-3-1 Cells
2.3. siRNA Concentration Dynamics in Mouse Blood Plasma
3. Materials and Methods
3.1. Lipoconjugate Synthesis and Liposome Preparation
3.2. Synthesis of siRNAs and siRNA/Liposome Complex Preparation
3.3. Liposome Sizes and Zeta Potentials
3.4. Animals
3.5. Tumor Cell Line
3.6. Cellular Accumulation Assay
3.7. Quantitative Stem-Loop Real-Time PCR Analysis of the Dynamics of siRNA Concentration in Blood Plasma
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traber, G.M.; Yu, A.-M. RNAi-Based Therapeutics and Novel RNA Bioengineering Technologies. J. Pharmacol. Exp. Ther. 2023, 384, 133–154. [Google Scholar] [CrossRef]
- Padda, I.S.; Mahtani, A.U.; Patel, P.; Parmar, M. Small Interfering RNA (siRNA) Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Smith, E.S.; Whitty, E.; Yoo, B.; Moore, A.; Sempere, L.F.; Medarova, Z. Clinical Applications of Short Non-Coding RNA-Based Therapies in the Era of Precision Medicine. Cancers 2022, 14, 1588. [Google Scholar] [CrossRef]
- Syed, Y.Y. Nedosiran: First Approval. Drugs 2023, 83, 1729–1733. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid Nanoparticles for Nucleic Acid Delivery: Current Perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Singha, K.; Namgung, R.; Kim, W.J. Polymers in Small-Interfering RNA Delivery. Nucleic Acid. Ther. 2011, 21, 133–147. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.-Y.; Lu, A.; Wang, X.-Y.; Jiang, L.-X.; Wang, J.-C. Non-Viral Vectors for RNA Delivery. J. Control. Release 2022, 342, 241–279. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Polymerized Selenium Nanoparticles for Folate-Receptor-Targeted Delivery of Anti-Luc-siRNA: Potential for Gene Silencing. Biomedicines 2020, 8, 76. [Google Scholar] [CrossRef]
- Ultav, G.; Tonbul, H.; Salva, E. An Effective VEGF-siRNA Delivery via Folic Acid Decorated and Pegylated Silica Nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 76, 103828. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.-C.; Cho, C.-F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- Dennahy, I.S.; Han, Z.; MacCuaig, W.M.; Chalfant, H.M.; Condacse, A.; Hagood, J.M.; Claros-Sorto, J.C.; Razaq, W.; Holter-Chakrabarty, J.; Squires, R.; et al. Nanotheranostics for Image-Guided Cancer Treatment. Pharmaceutics 2022, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dkhar, D.S.; Kumari, R.; Divya Mahapatra, S.; Srivastava, A.; Dubey, V.K.; Chandra, P. Ligand Conjugated Lipid-Based Nanocarriers for Cancer Theranostics. Biotechnol. Bioeng. 2022, 119, 3022–3043. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Kabilova, T.; Shmendel, E.; Gladkikh, D.; Morozova, N.; Maslov, M.; Chernolovskaya, E.; Vlassov, V.; Zenkova, M. Novel PEGylated Liposomes Enhance Immunostimulating Activity of isRNA. Molecules 2018, 23, 3101. [Google Scholar] [CrossRef] [PubMed]
- Vysochinskaya, V.; Shishlyannikov, S.; Zabrodskaya, Y.; Shmendel, E.; Klotchenko, S.; Dobrovolskaya, O.; Gavrilova, N.; Makarova, D.; Plotnikova, M.; Elpaeva, E.; et al. Influence of Lipid Composition of Cationic Liposomes 2X3-DOPE on mRNA Delivery into Eukaryotic Cells. Pharmaceutics 2022, 15, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Huang, L. Influence of Polyethylene Glycol Density and Surface Lipid on Pharmacokinetics and Biodistribution of Lipid-Calcium-Phosphate Nanoparticles. Biomaterials 2014, 35, 3027–3034. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Chan, C.-L.; Majzoub, R.N.; Shirazi, R.S.; Ewert, K.K.; Chen, Y.-J.; Liang, K.S.; Safinya, C.R. Endosomal Escape and Transfection Efficiency of PEGylated Cationic Liposome-DNA Complexes Prepared with an Acid-Labile PEG-Lipid. Biomaterials 2012, 33, 4928–4935. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, Z.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Paradox of PEGylation in Fabricating Hybrid Nanoparticle-Based Nicotine Vaccines. Biomaterials 2018, 182, 72–81. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A Strategy for Overcoming the “PEG Dilemma” in Efficient Drug Delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef]
- Shmendel, E.V.; Puchkov, P.A.; Maslov, M.A. Design of Folate-Containing Liposomal Nucleic Acid Delivery Systems for Antitumor Therapy. Pharmaceutics 2023, 15, 1400. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The Folate Receptor as a Rational Therapeutic Target for Personalized Cancer Treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate Receptor Expression in Carcinomas and Normal Tissues Determined by a Quantitative Radioligand Binding Assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Nikam, R.R.; Gore, K.R. Folate Receptor-Mediated siRNA Delivery: Recent Developments and Future Directions for RNAi Therapeutics. Nucleic Acid. Ther. 2021, 31, 245–270. [Google Scholar] [CrossRef]
- Kabilova, T.O.; Shmendel, E.V.; Gladkikh, D.V.; Chernolovskaya, E.L.; Markov, O.V.; Morozova, N.G.; Maslov, M.A.; Zenkova, M.A. Targeted Delivery of Nucleic Acids into Xenograft Tumors Mediated by Novel Folate-Equipped Liposomes. Eur. J. Pharm. Biopharm. 2018, 123, 59–70. [Google Scholar] [CrossRef]
- Shmendel, E.V.; Kabilova, T.O.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Targeted Delivery of Nucleic Acids by Folate-Containing Liposomes into KB-3-1 and HEK 293 Cells. Russ. J. Bioorg. Chem. 2019, 45, 719–725. [Google Scholar] [CrossRef]
- Gladkikh, D.V.; Sen’kova, A.V.; Chernikov, I.V.; Kabilova, T.O.; Popova, N.A.; Nikolin, V.P.; Shmendel, E.V.; Maslov, M.A.; Vlassov, V.V.; Zenkova, M.A.; et al. Folate-Equipped Cationic Liposomes Deliver Anti-MDR1-siRNA to the Tumor and Increase the Efficiency of Chemotherapy. Pharmaceutics 2021, 13, 1252. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tian, J.; Chen, X. Effect of Surface Properties on Liposomal siRNA Delivery. Biomaterials 2016, 79, 56–68. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational Design of Cationic Lipids for siRNA Delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. “Stealth” Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Composition on Phagocytic Uptake and Plasma Protein Adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Maslov, M.A.; Kabilova, T.O.; Petukhov, I.A.; Morozova, N.G.; Serebrennikova, G.A.; Vlassov, V.V.; Zenkova, M.A. Novel Cholesterol Spermine Conjugates Provide Efficient Cellular Delivery of Plasmid DNA and Small Interfering RNA. J. Control. Release 2012, 160, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L.-S. Delivery of siRNA Therapeutics: Barriers and Carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Hattori, Y.; Shimizu, S.; Ozaki, K.; Onishi, H. Effect of Cationic Lipid Type in Folate-PEG-Modified Cationic Liposomes on Folate Receptor-Mediated siRNA Transfection in Tumor Cells. Pharmaceutics 2019, 11, 181. [Google Scholar] [CrossRef]
- Lee, R.J.; Low, P.S. Delivery of Liposomes into Cultured KB Cells via Folate Receptor-Mediated Endocytosis. J. Biol. Chem. 1994, 269, 3198–3204. [Google Scholar] [CrossRef]
- Dahlman, J.E.; Barnes, C.; Khan, O.F.; Thiriot, A.; Jhunjunwala, S.; Shaw, T.E.; Xing, Y.; Sager, H.B.; Sahay, G.; Speciner, L.; et al. In Vivo Endothelial siRNA Delivery Using Polymeric Nanoparticles with Low Molecular Weight. Nat. Nanotechol. 2014, 9, 648–655. [Google Scholar] [CrossRef]
- Palchetti, S.; Colapicchioni, V.; Digiacomo, L.; Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; La Barbera, G.; Laganà, A. The Protein Corona of Circulating PEGylated Liposomes. Biochim. Biophys. Acta 2016, 1858, 189–196. [Google Scholar] [CrossRef]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef]
- Reddy, J.A.; Abburi, C.; Hofland, H.; Howard, S.J.; Vlahov, I.; Wils, P.; Leamon, C.P. Folate-Targeted, Cationic Liposome-Mediated Gene Transfer into Disseminated Peritoneal Tumors. Gene Ther. 2002, 9, 1542–1550. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based Analysis of Lipid Nanoparticle-Mediated siRNA Delivery, Intracellular Trafficking and Endosomal Escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Leamon, C.P.; Cooper, S.R.; Hardee, G.E. Folate-Liposome-Mediated Antisense Oligodeoxynucleotide Targeting to Cancer Cells: Evaluation in Vitro and in Vivo. Bioconjugate Chem. 2003, 14, 738–747. [Google Scholar] [CrossRef]
- Antony, A.C. The Biological Chemistry of Folate Receptors. Blood 1992, 79, 2807–2820. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.K.; Sarkar, A.; Feldmann, D.P.; Hoffmann, P.; Merkel, O.M. Revisiting the Value of Competition Assays in Folate Receptor-Mediated Drug Delivery. Biomaterials 2017, 138, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Suzuki, T.; Matsunaga, M.; Nakamura, K.; Moriyoshi, N.; Ishida, T.; Kiwada, H. Anti-Angiogenic Therapy via Cationic Liposome-Mediated Systemic siRNA Delivery. Int. J. Pharm. 2012, 422, 280–289. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The Accelerated Blood Clearance (ABC) Phenomenon: Clinical Challenge and Approaches to Manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.C.; Matthay, K.K.; Newman, M.S.; Hidayat, J.E.; Collins, L.R.; Redemann, C.; Martin, F.J.; Papahadjopoulos, D. Versatility in Lipid Compositions Showing Prolonged Circulation with Sterically Stabilized Liposomes. Biochim. Biophys. Acta 1992, 1105, 193–200. [Google Scholar] [CrossRef]
- Ezzat, A.A.; Tammam, S.N.; Hanafi, R.S.; Rashad, O.; Osama, A.; Abdelnaby, E.; Magdeldin, S.; Mansour, S. Different Serum, Different Protein Corona! The Impact of the Serum Source on Cellular Targeting of Folic Acid-Modified Chitosan-Based Nanoparticles. Mol. Pharm. 2022, 19, 1635–1646. [Google Scholar] [CrossRef]
- Lin, Y.; Wilk, U.; Pöhmerer, J.; Hörterer, E.; Höhn, M.; Luo, X.; Mai, H.; Wagner, E.; Lächelt, U. Folate Receptor-Mediated Delivery of Cas9 RNP for Enhanced Immune Checkpoint Disruption in Cancer Cells. Small 2023, 19, 2205318. [Google Scholar] [CrossRef]
- Fanciullino, R.; Ciccolini, J. Liposome-Encapsulated Anticancer Drugs: Still Waiting for the Magic Bullet? Curr. Med. Chem. 2009, 16, 4361–4371. [Google Scholar] [CrossRef]
- Mishra, S.; Webster, P.; Davis, M.E. PEGylation Significantly Affects Cellular Uptake and Intracellular Trafficking of Non-Viral Gene Delivery Particles. Eur. J. Cell Biol. 2004, 83, 97–111. [Google Scholar] [CrossRef]
- Shmendel, E.V.; Bakhareva, S.A.; Makarova, D.M.; Chernikov, I.V.; Morozova, N.G.; Chernolovskaya, E.L.; Zenkova, M.A.; Maslov, M.A. Uncharged Gemini-Amphiphiles as Components of Cationic Liposomes for Delivery of Nucleic Acids. Russ. J. Bioorg Chem. 2020, 46, 1250–1260. [Google Scholar] [CrossRef]
- Luneva, A.; Puchkov, P.; Shmendel, E.; Zenkova, M.; Kuzevanova, A.; Alimov, A.; Karpukhin, A.; Maslov, M. Optimization of the Technology for the Preparation of Cationic Liposomes for the Delivery of Nucleic Acids. Russ. J. Bioorganic Chem. 2018, 44, 724–731. [Google Scholar] [CrossRef]
- Petukhov, I.; Maslov, M.; Morozova, N.; Serebrennikova, G. Synthesis of Polycationic Lipids Based on Cholesterol and Spermine. Russ. Chem. Bull. 2010, 59, 260–268. [Google Scholar] [CrossRef]
- Bishani, A.; Makarova, D.M.; Shmendel, E.V.; Maslov, M.A.; Sen’kova, A.V.; Savin, I.A.; Gladkikh, D.V.; Zenkova, M.A.; Chernolovskaya, E.L. Influence of the Composition of Cationic Liposomes on the Performance of Cargo Immunostimulatory RNA. Pharmaceutics 2023, 15, 2184. [Google Scholar] [CrossRef]
- Landesman, Y.; Svrzikapa, N.; Cognetta, A.; Zhang, X.; Bettencourt, B.R.; Kuchimanchi, S.; Dufault, K.; Shaikh, S.; Gioia, M.; Akinc, A.; et al. In Vivo Quantification of Formulated and Chemically Modified Small Interfering RNA by Heating-in-Triton Quantitative Reverse Transcription Polymerase Chain Reaction (HIT qRT-PCR). Silence 2010, 1, 16. [Google Scholar] [CrossRef]
- Czimmerer, Z.; Hulvely, J.; Simandi, Z.; Varallyay, E.; Havelda, Z.; Szabo, E.; Varga, A.; Dezso, B.; Balogh, M.; Horvath, A.; et al. A Versatile Method to Design Stem-Loop Primer-Based Quantitative PCR Assays for Detecting Small Regulatory RNA Molecules. PLoS ONE 2013, 8, e55168. [Google Scholar] [CrossRef]
- Xu, L.; Anchordoquy, T.J. Cholesterol Domains in Cationic Lipid/DNA Complexes Improve Transfection. Biochim. Biophys. Acta 2008, 1778, 2177–2181. [Google Scholar] [CrossRef]


| Liposome | Target Component | PEG Component | Z-Ave, d.nm | PdI | ZP, mV | |
|---|---|---|---|---|---|---|
| F13 | DSPE-PEG2000-folate 2% | - | 128.6 ± 5.9 | 0.7 | 32.4 ± 9.0 | |
| 4/1 | 156.7 ± 12.3 | 0.5 | 23.1 ± 3.8 | |||
| 6/1 | 144.6 ± 22.4 | 0.6 | 30.5 ± 5.1 | |||
| 8/1 | 158.2 ± 10.2 | 0.4 | 35.4 ± 2.4 | |||
| F13/P1500 | DSPE-PEG2000-folate 2% | P1500 2% | 241.8 ± 65.7 | 0.4 | 39.2 ± 8.1 | |
| 4/1 | 201.5 ± 39.4 | 0.4 | 3.9 ± 3.3 | |||
| 6/1 | 191.1 ± 5.5 | 0.5 | 7.4 ± 1.2 | |||
| 8/1 | 171.7 ± 8.6 | 0.5 | 9.1 ± 0.3 | |||
| F13/diP1500 | DSPE-PEG2000-folate 2% | diP1500 2% | 126.0 ± 23.0 | 0.4 | 28.8 ± 12.8 | |
| 4/1 | 214.7 ± 14.0 | 0.5 | 25.4 ± 3.5 | |||
| 6/1 | 138.9 ± 8.3 | 0.7 | 30.5 ± 7.3 | |||
| 8/1 | 172.5 ± 113.6 | 0.8 | 21.6 ± 7.6 | |||
| DSPE-PEG2000/P1500 | - | DSPE-PEG2000 2% P1500 2% | 116.1 ± 17.9 | 0.7 | 26.8 ± 4.7 | |
| 4/1 | 353.3 ± 31.3 | 0.5 | 36.6 ± 13.5 | |||
| 6/1 | 215.7 ± 13.7 | 0.4 | 25.0 ± 7.5 | |||
| 8/1 | 145.2 ± 44.9 | 0.7 | 35.6 ± 10.1 | |||
| DSPE-PEG2000/diP1500 | - | DSPE-PEG2000 2% diP1500 2% | 198.0 ± 99.2 | 0.5 | 26.8 ± 2.0 | |
| 4/1 | 165.8 ± 21.6 | 0.3 | 14.0 ± 3.6 | |||
| 6/1 | 202.3 ± 22.7 | 0.5 | 6.9 ± 0.5 | |||
| 8/1 | 214.1 ± 38.4 | 0.5 | 35.5 ± 2.6 | |||
| 2X3 | - | - | 88.0 ± 7.1 | 0.2 | 39.5 ± 2.2 | |
| 4/1 | 318.0 ± 14.5 | 0.4 | 21.6 ± 1.6 | |||
| 6/1 | 155.5 ± 7.3 | 0.3 | 18.0 ± 1.4 | |||
| 8/1 | 238.5 ± 24.1 | 0.4 | 23.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladkikh, D.V.; Shmendel, E.V.; Makarova, D.M.; Maslov, M.A.; Zenkova, M.A.; Chernolovskaya, E.L. Loop-Structured PEG-Lipoconjugate Enhances siRNA Delivery Mediated by Liner-PEG Containing Liposomes. Molecules 2025, 30, 4127. https://doi.org/10.3390/molecules30204127
Gladkikh DV, Shmendel EV, Makarova DM, Maslov MA, Zenkova MA, Chernolovskaya EL. Loop-Structured PEG-Lipoconjugate Enhances siRNA Delivery Mediated by Liner-PEG Containing Liposomes. Molecules. 2025; 30(20):4127. https://doi.org/10.3390/molecules30204127
Chicago/Turabian StyleGladkikh, Daniil V., Elena V. Shmendel, Darya M. Makarova, Mikhail A. Maslov, Marina A. Zenkova, and Elena L. Chernolovskaya. 2025. "Loop-Structured PEG-Lipoconjugate Enhances siRNA Delivery Mediated by Liner-PEG Containing Liposomes" Molecules 30, no. 20: 4127. https://doi.org/10.3390/molecules30204127
APA StyleGladkikh, D. V., Shmendel, E. V., Makarova, D. M., Maslov, M. A., Zenkova, M. A., & Chernolovskaya, E. L. (2025). Loop-Structured PEG-Lipoconjugate Enhances siRNA Delivery Mediated by Liner-PEG Containing Liposomes. Molecules, 30(20), 4127. https://doi.org/10.3390/molecules30204127