Influence of Arabic Gum/Gelatin/Ascorbyl Palmitate Coating on Quality Parameters of Hazelnut Kernels Stored in Plastic Boxes
Abstract
1. Introduction
2. Results
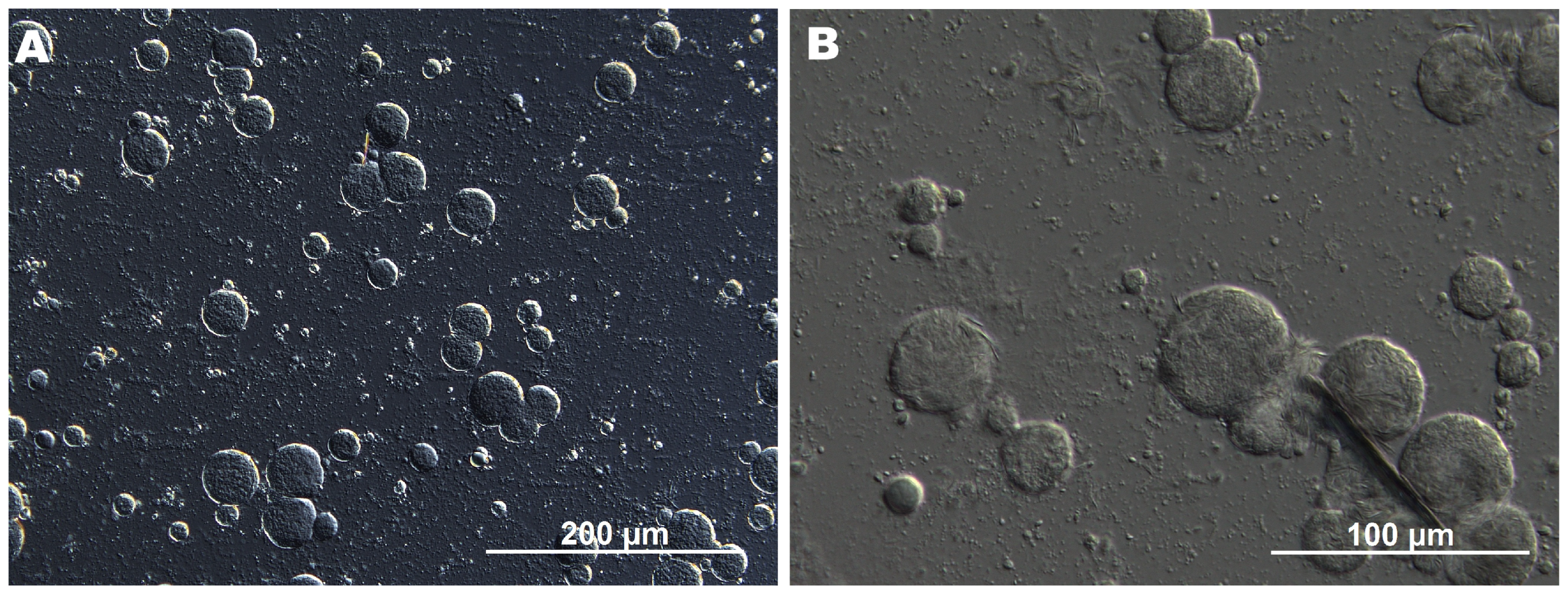
2.1. Characterization of Coating-Forming Emulsion (CFE)
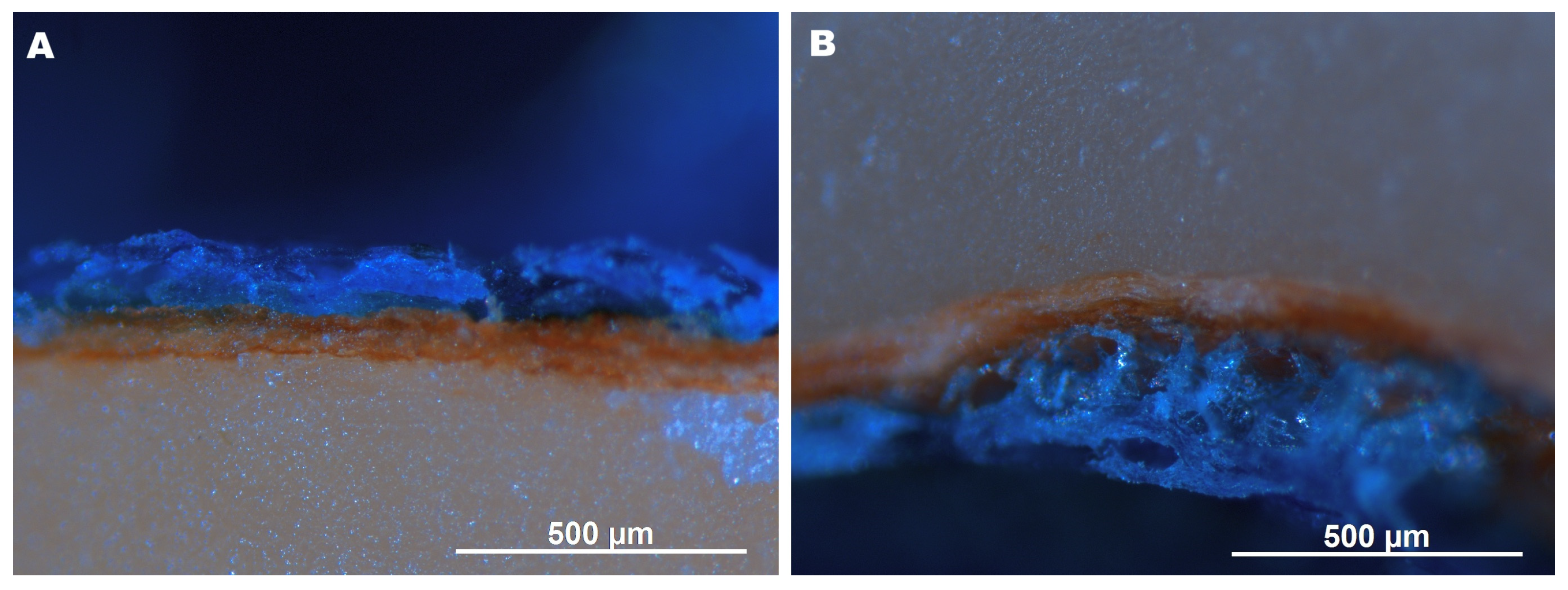
2.2. Microstructure of Hazelnuts
2.3. pH, Weight Loss (WL), Moisture Content (MC), Hardness, and Color Parameters
2.4. Antiradical Activity, Acid Value (AV), Peroxide Value (PV), and Thiobarbituric Acid Reactive Substances (TBARS) Concentration
3. Materials and Methods
3.1. Materials
3.2. Coating Formulation and Procedure
3.3. Analysis of CFE
3.4. Microstructure
3.5. pH
3.6. Determination of Weight Loss (WL), Color Parameters, Hardness, and Moisture Content (MC)
3.7. Determination of Antiradical Properties
3.8. Determination of Acid Value (AV), Peroxide Value (PV), and Thiobarbituric Acid Reactive Substances (TBARS) Concentration in Hazelnut Oil
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| a* | Red/Green coordinate |
| AA | Ascorbic Acid |
| AP | Ascorbyl Palmitate |
| AV | Acid Value |
| b* | Yellow/Blue coordinate |
| CFE | Coating-Forming Emulsion |
| DPPH* | 2,2-Diphenyl-1-picrylhydrazyl radical |
| GAR | Gum Arabic |
| GEL | Gelatin |
| L* | Lightness |
| MC | Moisture Content |
| PV | Peroxide Value |
| Rq | Root-Mean-Square Roughness |
| TBARS | Thiobarbituric Acid Reactive Substances |
| WL | Weight Loss |
| η | Viscosity |
References
- European Commission. EU Actions Against Food Waste. Food Safety—Food Waste. European Commission. Available online: https://food.ec.europa.eu/food-safety/food-waste/eu-actions-against-food-waste_en (accessed on 21 August 2025).
- European Commission. Research and Innovation for Food Waste Prevention and Reduction at Household Level Through Measurement, Monitoring and New Technologies (HORIZON-CL6-2025-02-FARM2FORK-04-Two-Stage). CORDIS–EU Research Results. European Union. Available online: https://cordis.europa.eu/programme/id/HORIZON_HORIZON-CL6-2025-02-FARM2FORK-04-two-stage (accessed on 21 August 2025).
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What Makes Good Antioxidants in Lipid-Based Systems? The Next Theories Beyond the Polar Paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef]
- Mukwevho, P.L.; Kaseke, T.; Fawole, O.A. Innovations in Biodegradable Packaging and Edible Coating of Shelled Temperate Nuts. Food Bioprocess. Technol. 2025, 18, 7763–7794. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of nuts and their potential health benefits—An overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 18 August 2025).
- Kowalczyk, D.; Zięba, E.; Skrzypek, T.; Baraniak, B. Effect of carboxymethyl cellulose/candelilla wax coating containing ascorbic acid on quality of walnut (Juglans regia L.) kernels. Int. J. Food Sci. Technol. 2017, 52, 1425–1431. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Drozłowska, E. Polysaccharide/gelatin blend films as carriers of ascorbyl palmitate—A comparative study. Food Chem. 2020, 333, 127465. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.M.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Microfibrillated cellulose addition improved the physicochemical and bioactive properties of biodegradable films based on soy protein and clove essential oil. Food Hydrocoll. 2018, 79, 416–427. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Han, J.H.; Hwang, H.M.; Min, S.; Krochta, J.M. Coating of peanuts with edible whey protein film containing α-tocopherol and ascorbyl palmitate. J. Food Sci. 2008, 73, 349–355. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, A.; Rocha-Uribe, A. Antioxidant hydrophobicity and emulsifier type influences the partitioning of antioxidants in the interface improving oxidative stability in O/W emulsions rich in n-3 fatty acids. Eur. J. Lipid Sci. Technol. 2017, 121, 1700277. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Baraniak, B. Effect of candelilla wax on functional properties of biopolymer emulsion films—A comparative study. Food Hydrocoll. 2014, 41, 195–209. [Google Scholar] [CrossRef]
- Kowalski, Z.; Banach, M.; Makara, A. Otrzymywanie białka niskotemperaturowego mocno żelującego (żelatyny) metodami chemicznymi. Chemik 2011, 65, 1085–1092. [Google Scholar]
- Benedini, L.; Messina, P.V.; Palma, S.D.; Allemandi, D.A.; Schulz, P.C. The ascorbyl palmitate-polyethyleneglycol 400-water system phase behavior. Colloids Surf. B Biointerfaces 2012, 89, 265–270. [Google Scholar] [CrossRef]
- Kowalczyk, D. Biopolymer/candelilla wax emulsion films as carriers of ascorbic acid—A comparative study. Food Hydrocoll. 2016, 52, 543–553. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kazimierczak, W.; Zięba, E.; Lis, M.; Wawrzkiewicz, M. Structural and physicochemical properties of glycerol-plasticized edible films made from pea protein-based emulsions containing increasing concentrations of candelilla wax or oleic acid. Molecules 2024, 29, 5998. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. Mycotoxin Contamination in Hazelnut: Current Status, Analytical Strategies, and Future Prospects. Toxins 2023, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Brar, P.K.; Danyluk, M.D. Nuts and grains: Microbiology and preharvest contamination risks. Microbiol. Spectr. 2018, 6, 1128. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1284/2002 of 15 July 2002 Laying Down the Marketing Standard for Hazelnuts in Shell. Official Journal of the European Communities L 182. 25 July 2002, pp. 14–18. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002R1284 (accessed on 21 August 2025).
- Turan, A. Effect of drying on the chemical composition of Çakıldak (cv) hazelnuts during storage. Grasas y Aceites 2019, 70, 296. [Google Scholar] [CrossRef]
- DKS 2992:2023; Kenya Bureau of Standards. Hazelnut Kernels—Specification. Kenya Bureau of Standards: Nairobi, Kenya, 2023. Available online: https://www.kebs.org/wp-content/uploads/2024/01/Hazelnut-Kernels-Specification-1.pdf (accessed on 21 August 2025).
- Ghirardello, D.; Contessa, C.; Valentini, N.; Zeppa, G.; Rolle, L.; Gerbi, V.; Botta, R. Effect of storage conditions on chemical and physical characteristics of hazelnut (Corylus avellana L.). Postharvest Biol. Technol. 2013, 81, 37–43. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Almeida, C.F.F.; Correia, P.M.R. Influence of packaging and storage on some properties of hazelnuts. J. Food Meas. Charact. 2015, 9, 11–19. [Google Scholar] [CrossRef]
- Razavi, R.; Maghsoudlou, Y.; Aalami, M.; Ghorbani, M. Impact of carboxymethyl cellulose coating enriched with Thymus vulgaris L. extract on physicochemical, microbial, and sensorial properties of fresh hazelnut (Corylus avellana L.) during storage. J. Food Process. Preserv. 2021, 45, 15313. [Google Scholar] [CrossRef]
- Correia, P.; Filipe, A.; Ferrão, A.C.; Ramalhosa, E.; Guiné, R.P.F. Effect of moisture on the characteristics of hazelnut kernel during storage. In Proceedings of the Livro de Resumos do XVI Encontro de Química dos Alimentos: Bio-Sustentabilidade e Bio-Segurança Alimentar, Castelo Branco, Portugal, 10–13 October 2022; p. 104. [Google Scholar]
- Pfeil, J.A.; Zhao, Y.; McGorrin, R.J. Chemical composition, phytochemical content, and antioxidant activity of hazelnut (Corylus avellana L.) skins from Oregon. LWT 2024, 201, 116204. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G. Changes in Antioxidant Activity, Profile, and Content of Polyphenols and Tocopherols in Common Hazel Seed (Corylus avellana L.) Depending on Variety and Harvest Date. Molecules 2020, 25, 43. [Google Scholar] [CrossRef]
- Alasalvar, C.; Karamać, M.; Amarowicz, R.; Shahidi, F. Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover. J. Agric. Food Chem. 2006, 54, 4826–4832. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A.; Hallmann, E. Effect of climate and roasting on polyphenols and tocopherols in the kernels and skin of six hazelnut cultivars (Corylus avellana L.). Agriculture 2020, 10, 36. [Google Scholar] [CrossRef]
- Ingram, L.O.; Buttke, T.M. Effects of alcohols on micro-organisms. In Advances in Microbial Physiology; Rose, A.H., Tempest, D.W., Eds.; Academic Press: Cambridge, MA, USA, 1985; Volume 25, pp. 253–300. [Google Scholar]
- Dobarganes, C.; Márquez-Ruiz, G. Rancidity in Nuts: Mechanisms and Control. Food Rev. Int. 1999, 15, 309–333. [Google Scholar] [CrossRef]
- Gull, A.; Masoodi, F.A.; Masoodi, L.; Gani, A.; Muzaffar, S. Effect of sodium alginate coatings enriched with α-tocopherol on quality of fresh walnut kernels. Food Chem. Adv. 2023, 2, 100169. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Shakerardekani, A.; Mirdehghan, S.H. Effect of alginate coating enriched with Shirazi thyme essential oil on quality of the fresh pistachio (Pistacia vera L.). J. Food Sci. Technol. 2021, 58, 34–43. [Google Scholar] [CrossRef]
- Habashi, R.; Zomorodi, S.; Talaie, A.; Jari, S. Effects of chitosan coating enriched with thyme essential oil and packaging methods on a postharvest quality of Persian walnut under cold storage. Foods Raw Mater. 2019, 7, 18–25. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Seyhan, F.; Tijskens, L.M.M.; Evranuz, O. Modelling temperature and pH dependence of lipase and peroxidase activity in Turkish hazelnuts. J. Food Eng. 2002, 52, 387–395. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Karaś, M.; Kazimierczak, W.; Skrzypek, T.; Wiater, A.; Bartkowiak, A.; Basiura-Cembala, M. A Comparative study on the structural, physicochemical, release, and antioxidant properties of sodium casein and gelatin films containing sea buckthorn oil. Polymers 2025, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Karaś, M.; Kordowska-Wiater, M.; Skrzypek, T.; Kazimierczak, W. Inherently acidic films based on chitosan lactate-doped starches and pullulan as carries of nisin: A comparative study of controlled-release and antimicrobial properties. Food Chem. 2023, 404, 134760. [Google Scholar] [CrossRef] [PubMed]
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Pegg, R.B. Spectrophotometric Measurement of Secondary Lipid Oxidation Products. Curr. Protoc. Food Anal. Chem. 2001, 1, D2.4.1–D2.4.18. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| pH of CFE | 5.10 ± 0.03 |
| η of CFE (mPa·s) | 48.50 ± 5.87 |
| Coating thickness at the top (μm) | 93.11 ± 23.23 |
| Coating thickness at the bottom (μm) | 112.71 ± 76.27 |
| Parameter | Control | Coated |
|---|---|---|
| Rq | 57.57± 10.37 b | 40.22 ± 6.13 a |
| pH of surface | 5.60 ± 0.08 b | 5.14 ± 0.04 a |
| pH of interior surface | 6.09 ± 0.14 a | 6.10 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, D.; Niedźwiadek, K.; Skrzypek, T.; Zięba, E.; Jarecki, J. Influence of Arabic Gum/Gelatin/Ascorbyl Palmitate Coating on Quality Parameters of Hazelnut Kernels Stored in Plastic Boxes. Molecules 2025, 30, 4126. https://doi.org/10.3390/molecules30204126
Kowalczyk D, Niedźwiadek K, Skrzypek T, Zięba E, Jarecki J. Influence of Arabic Gum/Gelatin/Ascorbyl Palmitate Coating on Quality Parameters of Hazelnut Kernels Stored in Plastic Boxes. Molecules. 2025; 30(20):4126. https://doi.org/10.3390/molecules30204126
Chicago/Turabian StyleKowalczyk, Dariusz, Katarzyna Niedźwiadek, Tomasz Skrzypek, Emil Zięba, and Jaromir Jarecki. 2025. "Influence of Arabic Gum/Gelatin/Ascorbyl Palmitate Coating on Quality Parameters of Hazelnut Kernels Stored in Plastic Boxes" Molecules 30, no. 20: 4126. https://doi.org/10.3390/molecules30204126
APA StyleKowalczyk, D., Niedźwiadek, K., Skrzypek, T., Zięba, E., & Jarecki, J. (2025). Influence of Arabic Gum/Gelatin/Ascorbyl Palmitate Coating on Quality Parameters of Hazelnut Kernels Stored in Plastic Boxes. Molecules, 30(20), 4126. https://doi.org/10.3390/molecules30204126







