Sodium-Glucose Cotransporter-2 Inhibitors in Diabetes and Beyond: Mechanisms, Pleiotropic Benefits, and Clinical Use—Reviewing Protective Effects Exceeding Glycemic Control
Abstract
1. Introduction
2. Historical Background and Development of SGLT2 Inhibitors
3. Mechanism of Action and Pharmacodynamics of SGLT2 Inhibitors
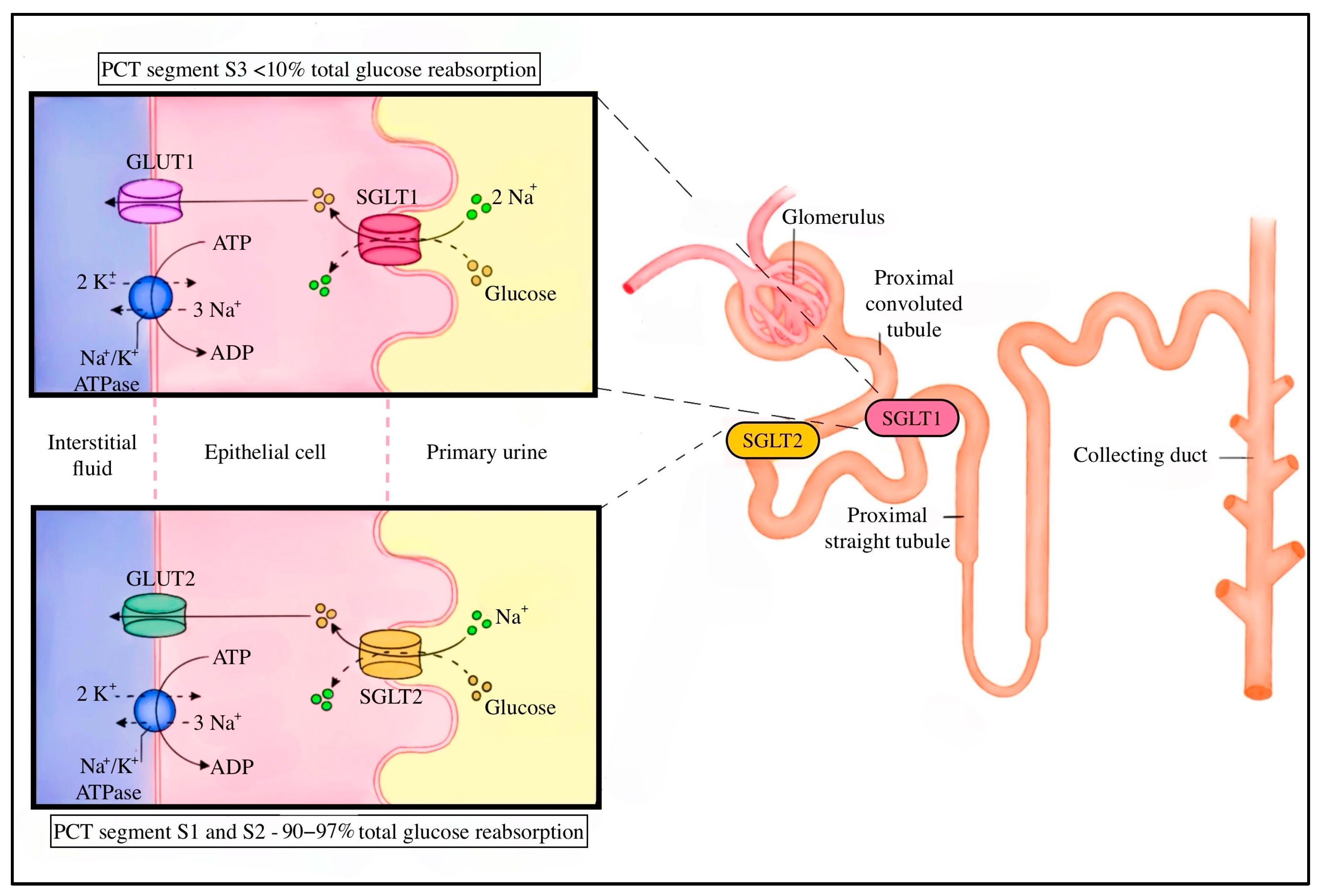
3.1. SGLT’s Function in Renal Glucose Homeostasis
3.2. Renal Effects of SGLT2 Blockade
3.3. Pharmacodynamic Outcomes and Glycemic Efficacy
4. Genomic Localization and Structure of SGLT Family Members
5. Effects of SGLT2 Inhibitors—Evidence and Clinical Recommendations
5.1. Improved Glycemic Control
5.2. Weight Loss and Lipid Metabolism
5.3. Renoprotective Effects
5.4. Reduction in Hospitalization and Mortality in Heart Failure
5.5. Inhibition of Hyperuricemia
5.6. Anti-Inflammatory Properties
5.7. Combating Anemia
5.8. Hepatic Effects
5.9. Potential Impact on Cognitive Function
6. Adverse Effects of SGLT2 Inhibitors: A Comprehensive Clinical Overview
6.1. Diabetic Ketoacidosis
6.2. Infections
6.2.1. Genital Infections
6.2.2. Urinary Tract Infections
6.2.3. Fournier’s Gangrene
6.3. Increased Risk of Fractures
6.4. Risk of Lower Limb Amputation
6.5. Potential Association with Malignancies
6.6. Acute Kidney Injury
7. Recommendations and Guidelines from Around the World
7.1. Heart Failure
7.2. Chronic Kidney Disease (CKD)
7.3. Diabetes Mellitus Type 2
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| SGLT2 | Sodium-Glucose Cotransporter-2 |
| T2DM | Type 2 diabetes mellitus |
| SGLT1 | Sodium-Glucose Cotransporter-1 |
| HbA1c | Glycated Hemoglobin |
| EMA | European Medicines Agency |
| FDA | United States Food and Drug Administration |
| SGLT | Sodium-glucose transporter |
| PCT | Proximal Convoluted Tubule |
| TmG | Glucose Reabsorptive Capacity |
| GLUT2 | Glucose Transporter 2 |
| eGFR | Estimated Glomerular Filtration Rate |
| FISH | Fluorescence In Situ Hybridization |
| FPG | Fasting Plasma Glucose |
| DPP-4 | Dipeptidyl Peptidase 4 |
| ADA | American Diabetes Association |
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| NHE3 | Sodium/Hydrogen Exchanger Isoform 3 |
| MI | Myocardial Infarction |
| MACE | Major Adverse Cardiovascular Events |
| PWV | Pulse Wave Velocity |
| βOHB | Beta-Hydroxybutyrate |
| ROS | Reactive Oxygen Species |
| HDACs | Histone Deacetylases |
| AMPK | AMP-Activated Protein Kinase |
| mTOR | Mammalian Target of Rapamycin |
| Bcl-2 | B cell Lymphoma 2 |
| Nrf2 | Nuclear Factor Erythroid 2-related Factor 2 |
| SOD | Superoxide Dismutase |
| PRDX | Peroxiredoxins |
| GPX | Glutathione Peroxidase |
| HO-1 | Heme Oxygenase-1 |
| NO | Nitric Oxide |
| HF | Heart Failure |
| LDL | Low-Density Lipoprotein |
| HDL | High-Density Lipoprotein |
| LPL | Lipoprotein Lipase |
| VLDL | Very Low-Density Lipoprotein |
| AE/AEs | Adverse Event(s); |
| AKI | Acute Kidney Injury |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| CKD | Chronic Kidney Disease |
| CV | Cardiovascular |
| DKA | Diabetic Ketoacidosis |
| ESKD | End-stage Kidney Disease |
| HFmrEF | Heart Failure with Mildly Reduced Ejection Fraction |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HHF | Hospitalization for Heart Failure |
| HR | Hazard Ratio |
| LVEF | Left Ventricular Ejection Fraction |
| UTIs | Urinary Tract Infections |
| UA | Uric Acid |
| RAAS | Renin–Angiotensin–Aldosterone System |
| URAT1 | Urate Transporter 1 |
| GLUT9 | Glucose Transporter 9 |
| OAT4/OAT10 | Organic Anion Transporter 4/10 |
| FE-UA | Fractional Excretion of Uric Acid |
| PUA | Plasma Uric Acid |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-1β | Interleukin-1 Beta |
| NF-κB | Nuclear Factor Kappa B |
| NLRP3 | NOD-like Receptor Protein 3 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| IL-10 | Interleukin-10 |
| EPO | Erythropoietin |
| HIF | Hypoxia-Inducible Factor |
| ESA | Erythropoiesis-Stimulating Agent |
| NAFLD | Nonalcoholic Fatty Liver Disease |
| BMI | Body Mass Index |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| GGTP | Gamma-Glutamyl Transferase |
| LSM | Liver Stiffness Measurement |
| CAP | CAP—Controlled Attenuation Parameter |
| MRI-PDFF | Magnetic Resonance Imaging Proton Density Fat Fraction |
| ZAG | Zinc-α2-Glycoprotein |
| AD | Alzheimer’s Disease |
| AChE | Acetylcholinesterase |
| BDNF | Brain-Derived Neurotrophic Factor |
| RCT | Randomized Controlled Trial |
| FAERS | FDA Adverse Event Reporting System |
| ESC | European Society of Cardiology |
| MRAs | Mineralocorticoid Receptor Antagonists |
| AHA | American Heart Association |
| ACC | American College of Cardiology |
| HFSA | Heart Failure Society of America |
| AACE | American Association of Clinical Endocrinology |
References
- Chao, E.C.; Henry, R.R. SGLT2 Inhibition—A Novel Strategy for Diabetes Treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef]
- Fonseca-Correa, J.I.; Correa-Rotter, R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front. Med. 2021, 8, 777861. [Google Scholar] [CrossRef]
- Madaan, T.; Akhtar, M.; Najmi, A.K. Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors: Current Status and Future Perspective. Eur. J. Pharm. Sci. 2016, 93, 244–252. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Hompesch, M.; Kasichayanula, S.; Liu, X.; Hong, Y.; Pfister, M.; Morrow, L.A.; Leslie, B.R.; Boulton, D.W.; Ching, A.; et al. Characterization of Renal Glucose Reabsorption in Response to Dapagliflozin in Healthy Subjects and Subjects with Type 2 Diabetes. Diabetes Care 2013, 36, 3169–3176. [Google Scholar] [CrossRef]
- Ferrannini, E. Sodium-Glucose Co-Transporters and Their Inhibition: Clinical Physiology. Cell Metab. 2017, 26, 27–38. [Google Scholar] [CrossRef]
- Yokono, M.; Takasu, T.; Hayashizaki, Y.; Mitsuoka, K.; Kihara, R.; Muramatsu, Y.; Miyoshi, S.; Tahara, A.; Kurosaki, E.; Li, Q.; et al. SGLT2-Selective Inhibitor Ipragliflozin Reduces Body Fat Mass by Increasing Fatty Acid Oxidation in High-Fat Diet-Induced Obese Rats. Eur. J. Pharmacol. 2014, 727, 66–74. [Google Scholar] [CrossRef]
- Pratama, K. Weight Loss Effect of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors in Patients with Obesity without Diabetes: A Systematic Review. Acta Endocrinol. 2022, 18, 216–224. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Neuen, B.L.; Young, T.; Heerspink, H.J.L.; Neal, B.; Perkovic, V.; Billot, L.; Mahaffey, K.W.; Charytan, D.M.; Wheeler, D.C.; Arnott, C.; et al. SGLT2 Inhibitors for the Prevention of Kidney Failure in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Lancet Diabetes Endocrinol. 2019, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Rashed, A.; Wasef, M.; Kalra, P.R. The 2023 ESC Heart Failure Guideline Update and Its Implications for Clinical Practice. Br. J. Cardiol. 2024, 31, 23. [Google Scholar] [CrossRef] [PubMed]
- Care, D. American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S219–S230. [Google Scholar] [CrossRef]
- Vasquez-Rios, G.; Nadkarni, G.N. SGLT2 Inhibitors: Emerging Roles in the Protection Against Cardiovascular and Kidney Disease Among Diabetic Patients. Int. J. Nephrol. Renovasc. Dis. 2020, 13, 281–296. [Google Scholar] [CrossRef]
- Xing, B.; Zhao, Y.; Dong, B.; Zhou, Y.; Lv, W.; Zhao, W. Effects of Sodium–Glucose Cotransporter 2 Inhibitors on Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. J. Diabetes Investig. 2020, 11, 1238–1247. [Google Scholar] [CrossRef]
- Zhou, P.; Tan, Y.; Hao, Z.; Xu, W.; Zhou, X.; Yu, J. Effects of SGLT2 Inhibitors on Hepatic Fibrosis and Steatosis: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1144838. [Google Scholar] [CrossRef]
- Dwinata, M.; Putera, D.; Hasan, I.; Raharjo, M. SGLT2 Inhibitors for Improving Hepatic Fibrosis and Steatosis in Non-Alcoholic Fatty Liver Disease Complicated with Type 2 Diabetes Mellitus: A Systematic Review. Clin. Exp. Hepatol. 2020, 6, 339–346. [Google Scholar] [CrossRef]
- Alami, M.; Zerif, E.; Khalil, A.; Hajji, N.; Ramassamy, C.; Lacombe, G.; Laurent, B.; Cohen, A.A.; Wikowski, J.M.; Gris, D.; et al. Neuroprotective Effects of SGLT2 Inhibitors Empagliflozin and Dapagliflozin on Aβ1-42-Induced Neurotoxicity and Neuroinflammation in Cellular Models of Alzheimer’s Disease. J. Alzheimers Dis. 2025, 105, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yang, H.; Lu, J.; Chen, L.; Wen, T.; Zhao, S.; Shi, L. The Impact of SGLT2 Inhibitors on Dementia Onset in Patients with Type 2 Diabetes: A Meta-Analysis of Cohort Studies. Neuroendocrinology 2025, 115, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. SGLT2 Inhibitors: The Statins of the 21st Century. Eur. Heart J. 2022, 43, 1029–1030. [Google Scholar] [CrossRef]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, Metabolic and Cardiovascular Considerations of SGLT2 Inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Ronald Kahn, C.; Roth, J. Phlorizin: A Review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef]
- Jörgens, V. Josef von Mering: The Baron Who Discovered SGLT Inhibition. In Frontiers in Diabetes; Jörgens, V., Porta, M., Eds.; S. Karger AG: Basel, Switzerland, 2020; Volume 29, pp. 134–141. ISBN 978-3-318-06733-0. [Google Scholar]
- Rossetti, L.; Smith, D.; Shulman, G.I.; Papachristou, D.; DeFronzo, R.A. Correction of Hyperglycemia with Phlorizin Normalizes Tissue Sensitivity to Insulin in Diabetic Rats. J. Clin. Investig. 1987, 79, 1510–1515. [Google Scholar] [CrossRef]
- Arakawa, K.; Ishihara, T.; Oku, A.; Nawano, M.; Ueta, K.; Kitamura, K.; Matsumoto, M.; Saito, A. Improved Diabetic Syndrome in C57BL/KsJ-db/db Mice by Oral Administration of the Na+-Glucose Cotransporter Inhibitor T-1095. Br. J. Pharmacol. 2001, 132, 578–586. [Google Scholar] [CrossRef]
- Haas, B.; Eckstein, N.; Pfeifer, V.; Mayer, P.; Hass, M.D.S. Efficacy, Safety and Regulatory Status of SGLT2 Inhibitors: Focus on Canagliflozin. Nutr. Diabetes 2014, 4, e143. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 Inhibitors on UTIs and Genital Infections in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef]
- Scheen, A.J. Beneficial Effects of SGLT2 Inhibitors on Fatty Liver in Type 2 Diabetes: A Common Comorbidity Associated with Severe Complications. Diabetes Metab. 2019, 45, 213–223. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Kanai, Y.; Lee, W.S.; You, G.; Brown, D.; Hediger, M.A. The Human Kidney Low Affinity Na+/Glucose Cotransporter SGLT2: Delineation of the Major Renal Reabsorptive Mechanism for D-Glucose. J. Clin. Investig. 1994, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Role of the Kidney in Normal Glucose Homeostasis and in the Hyperglycaemia of Diabetes Mellitus: Therapeutic Implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of Renal Glucose Handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef]
- Farber, S.J.; Berger, E.Y.; Earle, D.P. Effect of Diabetes and Insulin on the Maximum Capacity of the Renal Tubules to Reabsorb Glucose. J. Clin. Investig. 1951, 30, 125–129. [Google Scholar] [CrossRef]
- Mogensen, C.E. Maximum Tubular Reabsorption Capacity for Glucose and Renal Hemodynamics during Rapid Hypertonic Glucose Infusion in Normal and Diabetic Subjects. Scand. J. Clin. Lab. Investig. 1971, 28, 101–109. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. The Tubular Hypothesis of Nephron Filtration and Diabetic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef]
- Faham, S.; Watanabe, A.; Besserer, G.M.; Cascio, D.; Specht, A.; Hirayama, B.A.; Wright, E.M.; Abramson, J. The Crystal Structure of a Sodium Galactose Transporter Reveals Mechanistic Insights into Na+/Sugar Symport. Science 2008, 321, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Silverthorn, D.U. Fizjologia Człowieka; Ponikowska, B., Ed.; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2018; 940p, ISBN 978-83-200-5536-8. [Google Scholar]
- Hansen, H.H.; Jelsing, J.; Hansen, C.F.; Hansen, G.; Vrang, N.; Mark, M.; Klein, T.; Mayoux, E. The Sodium Glucose Cotransporter Type 2 Inhibitor Empagliflozin Preserves β-Cell Mass and Restores Glucose Homeostasis in the Male Zucker Diabetic Fatty Rat. J. Pharmacol. Exp. Ther. 2014, 350, 657–664. [Google Scholar] [CrossRef]
- Garg, S.K.; Henry, R.R.; Banks, P.; Buse, J.B.; Davies, M.J.; Fulcher, G.R.; Pozzilli, P.; Gesty-Palmer, D.; Lapuerta, P.; Simó, R.; et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N. Engl. J. Med. 2017, 377, 2337–2348. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel Hypothesis to Explain Why SGLT2 Inhibitors Inhibit Only 30–50% of Filtered Glucose Load in Humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Nardini, C.; Mannucci, E. Efficacy and Safety of Sodium Glucose Co-Transport-2 Inhibitors in Type 2 Diabetes: A Meta-Analysis of Randomized Clinical Trials. Diabetes Obes. Metab. 2014, 16, 457–466. [Google Scholar] [CrossRef]
- Hediger, M.A.; Budarf, M.L.; Emanuel, B.S.; Mohandas, T.K.; Wright, E.M. Assignment of the Human Intestinal Na+/Glucose Cotransporter Gene (SGLT1) to the q11.2→qter Region of Chromosome 22. Genomics 1989, 4, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; Klisak, I.; Bacallao, R.; Sparkes, R.S.; Wright, E.M. Assignment of the Human Na+/Glucose Cotransporter Gene SGLT1 to Chromosome 22q13.1. Genomics 1993, 17, 752–754. [Google Scholar] [CrossRef]
- Turk, E.; Martín, M.G.; Wright, E.M. Structure of the Human Na+/Glucose Cotransporter Gene SGLT1. J. Biol. Chem. 1994, 269, 15204–15209. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018: A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and Safety of Sodium–Glucose Co-Transporter-2 Inhibitors in Type 2 Diabetes Mellitus: Systematic Review and Network Meta-Analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef]
- Matsumura, M.; Nakatani, Y.; Tanka, S.; Aoki, C.; Sagara, M.; Yanagi, K.; Suzuki, K.; Aso, Y. Efficacy of Additional Canagliflozin Administration to Type 2 Diabetes Patients Receiving Insulin Therapy: Examination of Diurnal Glycemic Patterns Using Continuous Glucose Monitoring (CGM). Diabetes Ther. 2017, 8, 821–827. [Google Scholar] [CrossRef]
- Nishimura, R.; Osonoi, T.; Kanada, S.; Jinnouchi, H.; Sugio, K.; Omiya, H.; Ubukata, M.; Sakai, S.; Samukawa, Y. Effects of Luseogliflozin, a Sodium–Glucose Co-Transporter 2 Inhibitor, on 24-h Glucose Variability Assessed by Continuous Glucose Monitoring in Japanese Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Diabetes Obes. Metab. 2015, 17, 800–804. [Google Scholar] [CrossRef]
- Merovci, A.; Mari, A.; Solis, C.; Xiong, J.; Daniele, G.; Chavez-Velazquez, A.; Tripathy, D.; Urban McCarthy, S.; Abdul-Ghani, M.; DeFronzo, R.A. Dapagliflozin Lowers Plasma Glucose Concentration and Improves β-Cell Function. J. Clin. Endocrinol. Metab. 2015, 100, 1927–1932. [Google Scholar] [CrossRef]
- Merovci, A.; Solis-Herrera, C.; Daniele, G.; Eldor, R.; Fiorentino, T.V.; Tripathy, D.; Xiong, J.; Perez, Z.; Norton, L.; Abdul-Ghani, M.A.; et al. Dapagliflozin Improves Muscle Insulin Sensitivity but Enhances Endogenous Glucose Production. J. Clin. Investig. 2014, 124, 509–514. [Google Scholar] [CrossRef]
- Merovci, A.; Abdul-Ghani, M.; Mari, A.; Solis-Herrera, C.; Xiong, J.; Daniele, G.; Tripathy, D.; DeFronzo, R.A. Effect of Dapagliflozin with and without Acipimox on Insulin Sensitivity and Insulin Secretion in T2DM Males. J. Clin. Endocrinol. Metab. 2016, 101, 1249–1256. [Google Scholar] [CrossRef]
- Bonora, B.M.; Avogaro, A.; Fadini, G.P. Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence. Diabetes Metab. Syndr. Obes. 2020, 13, 161–174. [Google Scholar] [CrossRef]
- American Diabetes Association. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S122–S137. [Google Scholar] [CrossRef]
- Del Prato, S.; Nauck, M.; Durán-Garcia, S.; Maffei, L.; Rohwedder, K.; Theuerkauf, A.; Parikh, S. Long-Term Glycaemic Response and Tolerability of Dapagliflozin versus a Sulphonylurea as Add-On Therapy to Metformin in Patients with Type 2 Diabetes: 4-Year Data. Diabetes Obes. Metab. 2015, 17, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Sasako, T.; Kubota, N.; Sakurai, Y.; Takamoto, I.; Kubota, T.; Inagi, R.; Seki, G.; Goto, M.; Ueki, K.; et al. Dual Regulation of Gluconeogenesis by Insulin and Glucose in the Proximal Tubules of the Kidney. Diabetes 2017, 66, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Queniat, G.; Moerman, E.; Thévenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; Malaisse, W.J.; et al. Inhibition of the Glucose Transporter SGLT2 with Dapagliflozin in Pancreatic Alpha Cells Triggers Glucagon Secretion. Nat. Med. 2015, 21, 512–517. [Google Scholar] [CrossRef]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.-J. Metabolic Response to Sodium–Glucose Cotransporter 2 Inhibition in Type 2 Diabetic Patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef]
- Tentolouris, A.; Vlachakis, P.; Tzeravini, E.; Eleftheriadou, I.; Tentolouris, N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int. J. Environ. Res. Public Health 2019, 16, 2965. [Google Scholar] [CrossRef]
- Suzuki, M.; Takeda, M.; Kito, A.; Fukazawa, M.; Yata, T.; Yamamoto, M.; Nagata, T.; Fukuzawa, T.; Yamane, M.; Honda, K.; et al. Tofogliflozin, a Sodium/Glucose Cotransporter 2 Inhibitor, Attenuates Body Weight Gain and Fat Accumulation in Diabetic and Obese Animal Models. Nutr. Diabetes 2014, 4, e125. [Google Scholar] [CrossRef]
- Wu, P.; Wen, W.; Li, J.; Xu, J.; Zhao, M.; Chen, H.; Sun, J. Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effect of SGLT2 Inhibitor on Blood Leptin and Adiponectin Level in Patients with Type 2 Diabetes. Horm. Metab. Res. 2019, 51, 487–494. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diab. Rep. 2022, 22, 39. [Google Scholar] [CrossRef]
- Cassis, P.; Locatelli, M.; Cerullo, D.; Corna, D.; Buelli, S.; Zanchi, C.; Villa, S.; Morigi, M.; Remuzzi, G.; Benigni, A.; et al. SGLT2 Inhibitor Dapagliflozin Limits Podocyte Damage in Proteinuric Nondiabetic Nephropathy. JCI Insight 2018, 3, e98720. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ma, Y.; Liu, Y.; Liang, W.; Chen, X.; Ren, Z.; Wang, H.; Singhal, P.C.; Ding, G. Angiotensin II Down-Regulates Nephrin–Akt Signaling and Induces Podocyte Injury: Role of c-Abl. Mol. Biol. Cell 2016, 27, 197–208. [Google Scholar] [CrossRef]
- Thomson, S.C.; Vallon, V. Renal Effects of Sodium–Glucose Co-Transporter Inhibitors. In Proceedings of the American Society of Nephrology Kidney Week, Virtual, 22–25 October 2020. [Google Scholar]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of Diabetic Kidney Disease: Impact of SGLT2 Inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
- Palm, F.; Cederberg, J.; Hansell, P.; Liss, P.; Carlsson, P.-O. Reactive Oxygen Species Cause Diabetes-Induced Decrease in Renal Oxygen Tension. Diabetologia 2003, 46, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Hansell, P.; Ronquist, G.; Waldenström, A.; Liss, P.; Carlsson, P.-O. Polyol-Pathway-Dependent Disturbances in Renal Medullary Metabolism in Experimental Insulin-Deficient Diabetes Mellitus in Rats. Diabetologia 2004, 47, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 Inhibitor Empagliflozin Reduces Renal Growth and Albuminuria in Proportion to Hyperglycemia and Prevents Glomerular Hyperfiltration in Diabetic Akita Mice. Am. J. Physiol. Renal Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Rose, M.; Gerasimova, M.; Satriano, J.; Platt, K.A.; Koepsell, H.; Cunard, R.; Sharma, K.; Thomson, S.C.; Rieg, T. Knockout of Na-Glucose Transporter SGLT2 Attenuates Hyperglycemia and Glomerular Hyperfiltration but Not Kidney Growth or Injury in Diabetes Mellitus. Am. J. Physiol. Renal Physiol. 2013, 304, F156–F167. [Google Scholar] [CrossRef]
- Nagata, T.; Fukuzawa, T.; Takeda, M.; Fukazawa, M.; Mori, T.; Nihei, T.; Honda, K.; Suzuki, Y.; Kawabe, Y. Tofogliflozin, a Novel Sodium–Glucose Co-Transporter 2 Inhibitor, Improves Renal and Pancreatic Function in db/db Mice. Br. J. Pharmacol. 2013, 170, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-Term Treatment with the Sodium Glucose Cotransporter 2 Inhibitor, Dapagliflozin, Ameliorates Glucose Homeostasis and Diabetic Nephropathy in db/db Mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Williams, J.M.; Takahashi, T.; Miyata, N.; Roman, R.J. Effects of a New SGLT2 Inhibitor, Luseogliflozin, on Diabetic Nephropathy in T2DN Rats. J. Pharmacol. Exp. Ther. 2013, 345, 464–472. [Google Scholar] [CrossRef]
- Gangadharan Komala, M.; Gross, S.; Mudaliar, H.; Huang, C.; Pegg, K.; Mather, A.; Shen, S.; Pollock, C.A.; Panchapakesan, U. Inhibition of Kidney Proximal Tubular Glucose Reabsorption Does Not Prevent against Diabetic Nephropathy in Type 1 Diabetic eNOS Knockout Mice. PLoS ONE 2014, 9, e108994. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; De Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin: A Glucose-Regulating Drug with Diuretic Properties in Subjects with Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, Y.; Li, Y.; Zhang, J.; Zhang, H.; Fu, X.; Guo, Z.; Yang, Y.; Kang, K.; et al. Effects of First-Line Antidiabetic Drugs on the Improvement of Arterial Stiffness: A Bayesian Network Meta-Analysis. J. Diabetes 2023, 15, 685–698. [Google Scholar] [CrossRef]
- Tassone, F.; Ferreri, C.; Rossi, A.; Borretta, G.; Pastorini, G.; Anastasio, F.; Feola, M. Empagliflozin and Arterial Stiffness in Patients with Type 2 Diabetes: A Real-World Case-Control Study. Endocr. Metab. Immune Disord. Drug Targets, 2025; Epub ahead of printing. [Google Scholar] [CrossRef]
- Bosch, A.; Ott, C.; Jung, S.; Striepe, K.; Karg, M.V.; Kannenkeril, D.; Dienemann, T.; Schmieder, R.E. How Does Empagliflozin Improve Arterial Stiffness in Patients with Type 2 Diabetes Mellitus? Subanalysis of a Clinical Trial. Cardiovasc. Diabetol. 2019, 18, 44. [Google Scholar] [CrossRef]
- Rizos, E.C.; Tagkas, C.F.; Asimakopoulos, A.-G.I.; Tsimihodimos, V.; Anastasiou, G.; Rizzo, M.; Agouridis, A.P.; Ntzani, E.E. The Effect of SGLT2 Inhibitors and GLP-1 Receptor Agonists on Arterial Stiffness: A Meta-Analysis of Randomized Controlled Trials. J. Diabetes Complicat. 2024, 38, 108781. [Google Scholar] [CrossRef]
- Mudaliar, S.; Alloju, S.; Henry, R.R. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care 2016, 39, 1115–1122. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Hu, T.; Bazzano, L.A. The Low-Carbohydrate Diet and Cardiovascular Risk Factors: Evidence from Epidemiologic Studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 337–343. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium–Glucose Cotransporter Inhibitors and Oxidative Stress: An Update. J. Cell. Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z.I. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Curtain, J.P.; Docherty, K.F.; Jhund, P.S.; Petrie, M.C.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; et al. Effect of Dapagliflozin on Ventricular Arrhythmias, Resuscitated Cardiac Arrest, or Sudden Death in DAPA-HF. Eur. Heart J. 2021, 42, 3727–3738. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Bellocchio, G.; Telesca, M.; Cianflone, E.; Torella, D.; Rossi, F.; Urbanek, K.; Berrino, L. Sodium-Glucose Cotransporter 2 Inhibitors and Heart Failure: A Bedside-to-Bench Journey. Front. Cardiovasc. Med. 2021, 8, 810791. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 3651. [Google Scholar] [CrossRef] [PubMed]
- Corral, P.; Nardelli, N.; Elbert, A.; Aranguren, F.; Schreier, L. Impact of SGLT2 Inhibitors on Lipoproteins in Type 2 Diabetes. Curr. Diabetes Rep. 2025, 25, 16. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Simental-Mendía, M.; Millán-Alanís, J.M.; Simental-Mendía, L.E. Effect of Sodium-Glucose Co-Transporter 2 Inhibitors on Lipid Profile: A Systematic Review and Meta-Analysis of 48 Randomized Controlled Trials. Pharmacol. Res. 2020, 160, 105068. [Google Scholar] [CrossRef]
- Bechmann, L.E.; Emanuelsson, F.; Nordestgaard, B.G.; Benn, M. SGLT2-Inhibition Increases Total, LDL, and HDL Cholesterol and Lowers Triglycerides: Meta-Analyses of 60 Randomized Trials, Overall and by Dose, Ethnicity, and Drug Type. Atherosclerosis 2024, 394, 117236. [Google Scholar] [CrossRef]
- Calapkulu, M.; Cander, S.; Gul, O.O.; Ersoy, C. Lipid Profile in Type 2 Diabetic Patients with New Dapagliflozin Treatment; Actual Clinical Experience Data of Six Months Retrospective Lipid Profile from Single Center. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1031–1034. [Google Scholar] [CrossRef]
- Taheri, H.; Chiti, H.; Reshadmanesh, T.; Gohari, S.; Jalilvand, A.; Arsang-Jang, S.; Ismail-Beigi, F.; Ghanbari, S.; Dadashi, M.; Asgari, A.; et al. Empagliflozin Improves High-Sensitive Cardiac Troponin-I and High-Density Lipoprotein Cholesterol in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease: A Post-Hoc Analysis of EMPA-CARD Trial. J. Diabetes Metab. Disord. 2023, 22, 1723–1730. [Google Scholar] [CrossRef]
- Kwak, S.H.; Han, K.A.; Kim, K.; Yu, J.M.; Kim, E.; Won, J.C.; Kang, J.G.; Chung, C.H.; Oh, S.; Choi, S.H.; et al. Efficacy and Safety of Enavogliflozin, a Novel SGLT2 Inhibitor, in Korean People with Type 2 Diabetes: A 24-Week, Multicentre, Randomized, Double-Blind, Placebo-Controlled, Phase III Trial. Diabetes Obes. Metab. 2023, 25, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Osto, E.; Bonacina, F.; Pirillo, A.; Norata, G.D. Neutral Effect of SGLT2 Inhibitors on Lipoprotein Metabolism: From Clinical Evidence to Molecular Mechanisms. Pharmacol. Res. 2023, 188, 106667. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Huggins, L.-A.; Scerbo, D.; Obunike, J.; Mullick, A.E.; Rothenberg, P.L.; Prospero, N.A.D.; Eckel, R.H.; Goldberg, I.J. Mechanism of Increased LDL and Decreased Triglycerides with SGLT2 Inhibition. Arter. Thromb. Vasc. Biol. 2019, 38, 2207–2216. [Google Scholar] [CrossRef]
- Albitar, O.; D’Souza, C.M.; Adeghate, E.A. Effects of Lipoproteins on Metabolic Health. Nutrients 2024, 16, 2156. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Fukui, T.; Nakanishi, N.; Yamamoto, S.; Tomoyasu, M.; Osamura, A.; Ohara, M.; Yamamoto, T.; Ito, Y.; Hirano, T. Dapagliflozin Decreases Small Dense Low-Density Lipoprotein-Cholesterol and Increases High-Density Lipoprotein 2-Cholesterol in Patients with Type 2 Diabetes: Comparison with Sitagliptin. Cardiovasc. Diabetol. 2017, 16, 8. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 Inhibitor Empagliflozin in Patients Hospitalized for Acute Heart Failure: A Multinational Randomized Trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Jones, W.S.; Udell, J.A.; Anker, S.D.; Petrie, M.C.; Harrington, J.; Mattheus, M.; Zwiener, I.; Amir, O.; Bahit, M.C.; et al. Empagliflozin after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1455–1466. [Google Scholar] [CrossRef]
- James, S.; Erlinge, D.; Storey, R.F.; McGuire, D.K.; De Belder, M.; Eriksson, N.; Andersen, K.; Austin, D.; Arefalk, G.; Carrick, D.; et al. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evid. 2024, 3, EVIDoa2300286. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Perkins, B.A.; Cherney, D.Z.I. Uric Acid as a Biomarker and a Therapeutic Target in Diabetes. Can. J. Diabetes 2015, 39, 239–246. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Škrtić, M.; Yang, G.K.; Yip, P.M.; Perkins, B.A.; Cherney, D.Z.I. Glycosuria-Mediated Urinary Uric Acid Excretion in Patients with Uncomplicated Type 1 Diabetes Mellitus. Am. J. Physiol.-Ren. Physiol. 2015, 308, F77–F83. [Google Scholar] [CrossRef]
- Lewis, D.G.; Mrcp, M. Risk Factor Control Is Key in Diabetic Nephropathy. Practitioner 2014, 258, 13–17. [Google Scholar]
- Kanbay, M.; Jensen, T.; Solak, Y.; Le, M.; Roncal-Jimenez, C.; Rivard, C.; Lanaspa, M.A.; Nakagawa, T.; Johnson, R.J. Uric Acid in Metabolic Syndrome: From an Innocent Bystander to a Central Player. Eur. J. Intern. Med. 2016, 29, 3–8. [Google Scholar] [CrossRef]
- Cheeseman, C. Solute Carrier Family 2, Member 9 and Uric Acid Homeostasis. Curr. Opin. Nephrol. Hypertens. 2009, 18, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Yip, A.S.Y.; Leong, S.; Teo, Y.H.; Teo, Y.N.; Syn, N.L.X.; See, R.M.; Wee, C.F.; Chong, E.Y.; Lee, C.-H.; Chan, M.Y.; et al. Effect of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors on Serum Urate Levels in Patients with and without Diabetes: A Systematic Review and Meta-Regression of 43 Randomized Controlled Trials. Ther. Adv. Chronic Dis. 2022, 13, 20406223221083509. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Hyperuricemia and Gout Reduction by SGLT2 Inhibitors in Diabetes and Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Das, N.A.; Carpenter, A.J.; Belenchia, A.; Aroor, A.R.; Noda, M.; Siebenlist, U.; Chandrasekar, B.; DeMarco, V.G. Empagliflozin Reduces High Glucose-Induced Oxidative Stress and miR-21-Dependent TRAF3IP2 Induction and RECK Suppression, and Inhibits Human Renal Proximal Tubular Epithelial Cell Migration and Epithelial-to-Mesenchymal Transition. Cell. Signal. 2020, 68, 109506. [Google Scholar] [CrossRef]
- Pirklbauer, M.; Sallaberger, S.; Staudinger, P.; Corazza, U.; Leierer, J.; Mayer, G.; Schramek, H. Empagliflozin Inhibits IL-1β-Mediated Inflammatory Response in Human Proximal Tubular Cells. Int. J. Mol. Sci. 2021, 22, 5089. [Google Scholar] [CrossRef]
- Tan, S.A.; Tan, L. Empagliflozin and Canagliflozin Attenuate Inflammatory Cytokines Interferon-λ, Tumor Necrosis Factor-α, Interleukin-6: Possible Mechanism of Decreasing Cardiovascular Risk in Diabetes Mellitus. J. Am. Coll. Cardiol. 2018, 71, A1830. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Butler, A.E.; Atkin, S.L.; Katsiki, N.; Sahebkar, A. Sodium–Glucose Cotransporter 2 Inhibitors and Inflammation in Chronic Kidney Disease: Possible Molecular Pathways. J. Cell. Physiol. 2019, 234, 223–230. [Google Scholar] [CrossRef]
- Lee, T.-M.; Chang, N.-C.; Lin, S.-Z. Dapagliflozin, a Selective SGLT2 Inhibitor, Attenuated Cardiac Fibrosis by Regulating the Macrophage Polarization via STAT3 Signaling in Infarcted Rat Hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Van Gaal, L.; Leiter, L.A.; Vijapurkar, U.; List, J.; Cuddihy, R.; Ren, J.; Davies, M.J. Effects of Canagliflozin versus Glimepiride on Adipokines and Inflammatory Biomarkers in Type 2 Diabetes. Metabolism 2018, 85, 32–37. [Google Scholar] [CrossRef]
- Shaheer, A.; Kumar, A.; Menon, P.; Jallo, M.; Basha, S. Effect of Add-On Therapy of Sodium-Glucose Cotransporter 2 Inhibitors and Dipeptidyl Peptidase 4 Inhibitors on Adipokines in Type 2 Diabetes Mellitus. J. Clin. Med. Res. 2021, 13, 355–362. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin Resistance. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Scheen, A.J. Effects of SGLT2 Inhibitors on Systemic and Tissue Low-Grade Inflammation: The Potential Contribution to Diabetes Complications and Cardiovascular Disease. Diabetes Metab. 2018, 44, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Hejna, J.; Green, K.; Batra, M.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Dapagliflozin Suppresses Hepcidin and Increases Erythropoiesis. J. Clin. Endocrinol. Metab. 2020, 105, e1056–e1063. [Google Scholar] [CrossRef]
- Packer, M. Mechanisms of Enhanced Renal and Hepatic Erythropoietin Synthesis by Sodium–Glucose Cotransporter 2 Inhibitors. Eur. Heart J. 2023, 44, 5027–5035. [Google Scholar] [CrossRef]
- Tziastoudi, M.; Pissas, G.; Golfinopoulos, S.; Filippidis, G.; Dousdampanis, P.; Eleftheriadis, T.; Stefanidis, I. Sodium–Glucose Transporter 2 (SGLT2) Inhibitors and Iron Deficiency in Heart Failure and Chronic Kidney Disease: A Literature Review. Life 2023, 13, 2338. [Google Scholar] [CrossRef]
- Sato, K.; Babazono, T. Successful Withdrawal of Erythropoiesis-Stimulating Agent after Administration of an SGLT2 Inhibitor, Tofogliflozin, in People with Diabetes. Diabet. Med. 2022, 39, e14632. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Shimoda, M.; Sanada, J.; Fushimi, Y.; Hirata, Y.; Irie, S.; Obata, A.; Kimura, T.; Hirukawa, H.; Kohara, K.; et al. There Is a Close Association between the Recovery of Liver Injury and Glycemic Control after SGLT2 Inhibitor Treatment in Japanese Subjects with Type 2 Diabetes: A Retrospective Clinical Study. Diabetes Ther. 2018, 9, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; De Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Latva-Rasku, A.; Honka, M.-J.; Kullberg, J.; Mononen, N.; Lehtimäki, T.; Saltevo, J.; Kirjavainen, A.K.; Saunavaara, V.; Iozzo, P.; Johansson, L.; et al. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study with 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care 2019, 42, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Kabil, S.L.; Mahmoud, N.M. Canagliflozin Protects against Non-Alcoholic Steatohepatitis in Type-2 Diabetic Rats through Zinc Alpha-2 Glycoprotein Up-Regulation. Eur. J. Pharmacol. 2018, 828, 135–145. [Google Scholar] [CrossRef]
- Liao, X.; Wang, X.; Li, H.; Li, L.; Zhang, G.; Yang, M.; Yuan, L.; Liu, H.; Yang, G.; Gao, L. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Increases Circulating Zinc-α2-Glycoprotein Levels in Patients with Type 2 Diabetes. Sci. Rep. 2016, 6, 32887. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.-U. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes Mellitus and Risk of Dementia: A Meta-Analysis of Prospective Observational Studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef]
- Cao, F.; Yang, F.; Li, J.; Guo, W.; Zhang, C.; Gao, F.; Sun, X.; Zhou, Y.; Zhang, W. The Relationship between Diabetes and the Dementia Risk: A Meta-Analysis. Diabetol. Metab. Syndr. 2024, 16, 101. [Google Scholar] [CrossRef]
- Parra Bravo, C.; Naguib, S.A.; Gan, L. Cellular and Pathological Functions of Tau. Nat. Rev. Mol. Cell Biol. 2024, 25, 845–864. [Google Scholar] [CrossRef]
- Iannantuoni, F.; De Marañon, A.M.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef]
- Gunawan, P.Y.; Gunawan, P.A.; Hariyanto, T.I. Risk of Dementia in Patients with Diabetes Using Sodium-Glucose Transporter 2 Inhibitors (SGLT2i): A Systematic Review, Meta-Analysis, and Meta-Regression. Diabetes Ther. 2024, 15, 663–675. [Google Scholar] [CrossRef]
- Youn, Y.J.; Kim, S.; Jeong, H.-J.; Ah, Y.-M.; Yu, Y.M. Sodium-Glucose Cotransporter-2 Inhibitors and Their Potential Role in Dementia Onset and Cognitive Function in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Neuroendocrinol. 2024, 73, 101131. [Google Scholar] [CrossRef]
- Arafa, N.M.S.; Ali, E.H.A.; Hassan, M.K. Canagliflozin Prevents Scopolamine-Induced Memory Impairment in Rats: Comparison with Galantamine Hydrobromide Action. Chem. Biol. Interact. 2017, 277, 195–203. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic Control with Empagliflozin, a Novel Selective SGLT2 Inhibitor, Ameliorates Cardiovascular Injury and Cognitive Dysfunction in Obese and Type 2 Diabetic Mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Li, Y.; Niu, L.; Liang, R.; Tang, M.; Cai, Q.; Xu, J.; Zhang, D.; Yin, X.; Liu, X.; et al. SGLT2 Inhibitors: A Novel Therapy for Cognitive Impairment via Multifaceted Effects on the Nervous System. Transl. Neurodegener. 2024, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.A.; Yusuf, D.; Christy, J.; Solmaz, V.; Erdogan, A.; Taskiran, E.; Erbas, O. Highly Selective SGLT2 Inhibitor Dapagliflozin Reduces Seizure Activity in Pentylenetetrazol-Induced Murine Model of Epilepsy. BMC Neurol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Thompson, M.J.; Sharp, S.J.; Walter, F.M. Factors Associated with the Presence of Diabetic Ketoacidosis at Diagnosis of Diabetes in Children and Young Adults: A Systematic Review. BMJ 2011, 343, d4092. [Google Scholar] [CrossRef]
- Goldenberg, R.M.; Berard, L.D.; Cheng, A.Y.Y.; Gilbert, J.D.; Verma, S.; Woo, V.C.; Yale, J.-F. SGLT2 Inhibitor–Associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin. Ther. 2016, 38, 2654–2664.e1. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.L.; Buschur, E.O.; Buse, J.B.; Cohan, P.; Diner, J.C.; Hirsch, I.B. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment with Sodium–Glucose Cotransporter 2 Inhibition. Diabetes Care 2015, 38, 1687–1693. [Google Scholar] [CrossRef]
- Wang, Y.; Desai, M.; Ryan, P.B.; DeFalco, F.J.; Schuemie, M.J.; Stang, P.E.; Berlin, J.A.; Yuan, Z. Incidence of Diabetic Ketoacidosis among Patients with Type 2 Diabetes Mellitus Treated with SGLT2 Inhibitors and Other Antihyperglycemic Agents. Diabetes Res. Clin. Pract. 2017, 128, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mohammed, A.S.; Raut, A.; Moore, S.; Houlden, R.; Awad, S. Prevalence and Clinical Characteristics of Adults Presenting with Sodium-Glucose Cotransporter-2 Inhibitor-Associated Diabetic Ketoacidosis at a Canadian Academic Tertiary Care Hospital. Can. J. Diabetes 2021, 45, 214–219. [Google Scholar] [CrossRef]
- Erondu, N.; Desai, M.; Ways, K.; Meininger, G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care 2015, 38, 1680–1686. [Google Scholar] [CrossRef]
- Burke, K.R.; Schumacher, C.A.; Harpe, S.E. SGLT2 Inhibitors: A Systematic Review of Diabetic Ketoacidosis and Related Risk Factors in the Primary Literature. Pharmacotherapy 2017, 37, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Nair, A. A Literature Review of the Therapeutic Perspectives of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitor-Induced Euglycemic Diabetic Ketoacidosis. Cureus 2022, 14, e29652. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.-H.; Lee, J.-K.; Chen, W.-J. Clinical Adverse Events Associated with Sodium–Glucose Cotransporter 2 Inhibitors: A Meta-Analysis Involving 10 Randomized Clinical Trials and 71 553 Individuals. J. Clin. Endocrinol. Metab. 2021, 106, 2133–2145. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings About Too Much Acid in the Blood and Serious Urinary Tract Infections. 2016. Available online: https://www.fda.gov/drugs (accessed on 24 September 2025).
- Thompson, A.; Fleischmann, K.E.; Smilowitz, N.R.; De Las Fuentes, L.; Mukherjee, D.; Aggarwal, N.R.; Ahmad, F.S.; Allen, R.B.; Altin, S.E.; Auerbach, A.; et al. 2024 AHA/ACC/ACS/ASNC/HRS/SCA/SCCT/SCMR/SVM Guideline for Perioperative Cardiovascular Management for Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 150, 1869–1969. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. 1), S321–S334. [Google Scholar] [CrossRef]
- Packer, M.; Butler, J.; Zeller, C.; Pocock, S.J.; Brueckmann, M.; Ferreira, J.P.; Filippatos, G.; Usman, M.S.; Zannad, F.; Anker, S.D. Blinded Withdrawal of Long-Term Randomized Treatment with Empagliflozin or Placebo in Patients with Heart Failure. Circulation 2023, 148, 1011–1022. [Google Scholar] [CrossRef]
- Geerlings, S.; Fonseca, V.; Castro-Diaz, D.; List, J.; Parikh, S. Genital and Urinary Tract Infections in Diabetes: Impact of Pharmacologically-Induced Glucosuria. Diabetes Res. Clin. Pract. 2014, 103, 373–381. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Pan, H.; Zou, Y.; Wang, B.; Wang, G.; Zhu, H. Safety and Efficiency of SGLT2 Inhibitor Combining with Insulin in Subjects with Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2017, 96, e6944. [Google Scholar] [CrossRef]
- Shi, Q.; Nong, K.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Rydén, L.; Marx, N.; Brosius, F.C.; Mustafa, R.A.; Agarwal, A.; et al. Benefits and Harms of Drug Treatment for Type 2 Diabetes: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. BMJ 2023, 381, e074068. [Google Scholar] [CrossRef]
- Patel, S.; Hickman, A.; Frederich, R.; Johnson, S.; Huyck, S.; Mancuso, J.P.; Gantz, I.; Terra, S.G. Safety of Ertugliflozin in Patients with Type 2 Diabetes Mellitus: Pooled Analysis of Seven Phase 3 Randomized Controlled Trials. Diabetes Ther. 2020, 11, 1347–1367. [Google Scholar] [CrossRef]
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium–Glucose Cotransporter 2 Inhibitors for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2013, 159, 262–274. [Google Scholar] [CrossRef]
- Bersoff-Matcha, S.J.; Chamberlain, C.; Cao, C.; Kortepeter, C.; Chong, W.H. Fournier Gangrene Associated with Sodium–Glucose Cotransporter-2 Inhibitors: A Review of Spontaneous Postmarketing Cases. Ann. Intern. Med. 2019, 170, 764–769. [Google Scholar] [CrossRef]
- Fisher, A.; Fralick, M.; Filion, K.B.; Dell’Aniello, S.; Douros, A.; Tremblay, É.; Shah, B.R.; Ronksley, P.E.; Alessi-Severini, S.; Hu, N.; et al. Sodium-Glucose Co-Transporter-2 Inhibitors and the Risk of Urosepsis: A Multi-Site, Prevalent New-User Cohort Study. Diabetes Obes. Metab. 2020, 22, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Ruanpeng, D.; Ungprasert, P.; Sangtian, J.; Harindhanavudhi, T. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors and Fracture Risk in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis. Diabetes Metab. Res. Rev. 2017, 33, e2903. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Li, D.D.; Zhang, J.J.; Hsu, Y.H.; Wang, T.S.; Zhai, S.D.; Song, Y.Q. Lack of Evidence for a Harmful Effect of Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors on Fracture Risk among Type 2 Diabetes Patients: A Network and Cumulative Meta-Analysis of Randomized Controlled Trials. Diabetes Obes. Metab. 2016, 18, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Van Hulten, V.; Driessen, J.H.M.; Starup-Linde, J.K.; Al-Mashhadi, Z.K.; Viggers, R.; Klungel, O.H.; Souverein, P.C.; Vestergaard, P.; Stehouwer, C.D.A.; Van Den Bergh, J.P. The Associations of Sodium-Glucose Cotransporter-2 Inhibitors versus Dipeptidyl Peptidase-4 Inhibitors as Add-On to Metformin with Fracture Risk in Patients with Type 2 Diabetes Mellitus. Diabetes Obes. Metab. 2023, 25, 3235–3247. [Google Scholar] [CrossRef]
- Mostafa, M.E.A.; Alrasheed, T. Risk of Bone Fracture by Using Dipeptidyl Peptidase-4 Inhibitors, Glucagon-Like Peptide-1 Receptor Agonists, or Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus: A Network Meta-Analysis of Population-Based Cohort Studies. Front. Endocrinol. 2024, 15, 1410883. [Google Scholar] [CrossRef]
- Ueda, P.; Svanström, H.; Melbye, M.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Pasternak, B. Sodium Glucose Cotransporter 2 Inhibitors and Risk of Serious Adverse Events: Nationwide Register-Based Cohort Study. BMJ 2018, 363, k4365. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.Y.; Wang, T.; Shen, S.; Tang, H. Risks of Diabetic Foot Syndrome and Amputation Associated with Sodium Glucose Co-Transporter 2 Inhibitors: A Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. 2018, 44, 410–414. [Google Scholar] [CrossRef]
- Kohler, S.; Salsali, A.; Hantel, S.; Kaspers, S.; Woerle, H.J.; Kim, G.; Broedl, U.C. Safety and Tolerability of Empagliflozin in Patients with Type 2 Diabetes. Clin. Ther. 2016, 38, 1299–1313. [Google Scholar] [CrossRef]
- Jabbour, S.; Seufert, J.; Scheen, A.; Bailey, C.J.; Karup, C.; Langkilde, A.M. Dapagliflozin in Patients with Type 2 Diabetes Mellitus: A Pooled Analysis of Safety Data from Phase IIb/III Clinical Trials. Diabetes Obes. Metab. 2018, 20, 620–628. [Google Scholar] [CrossRef]
- American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care 2017, 40, S64–S74. [Google Scholar] [CrossRef]
- Tang, H.; Dai, Q.; Shi, W.; Zhai, S.; Song, Y.; Han, J. SGLT2 Inhibitors and Risk of Cancer in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Diabetologia 2017, 60, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Nreu, B.; Mannucci, E.; Monami, M. Sodium-Glucose Co-Transporter-2 (SGLT-2) Inhibitors and Cancer: A Meta-Analysis of Randomized Controlled Trials. Diabetes Obes. Metab. 2019, 21, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Emberson, J.R.; Haynes, R.; Herrington, W.G.; Judge, P.; Landray, M.J.; Mayne, K.J.; Ng, S.Y.A.; Preiss, D.; Roddick, A.J.; et al. Impact of Diabetes on the Effects of Sodium Glucose Co-Transporter-2 Inhibitors on Kidney Outcomes: Collaborative Meta-Analysis of Large Placebo-Controlled Trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Deng, W.; Liu, C.; Yang, J.; Li, Y.; Cai, G.; Chen, X.; Dong, Z. Influence of Sodium/Glucose Cotransporter-2 Inhibitors on the Incidence of Acute Kidney Injury: A Meta-Analysis. Front. Pharmacol. 2024, 15, 1372421. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e263–e421. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Vellanki, P.; Blonde, L.; Christofides, E.A.; Galindo, R.J.; Hirsch, I.B.; Isaacs, S.D.; Izuora, K.E.; Low Wang, C.C.; Twining, C.L.; et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm–2023 Update. Endocr. Pract. 2023, 29, 305–340. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. 1), S207–S238. [Google Scholar] [CrossRef]

| Study | Year | Drug | Condition/ Population | Median Follow-Up | Primary Endpoint | Overall Result | Key Adverse Events/ Complications (Reported) |
|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME [9] | 2015 | Empagliflozin 10 mg/25 mg | T2DM with ASCVD (7020 patients) | 3.1 years | 3-point MACE | ↓ MACE (HR ≈ 0.86); large ↓ CV death (~38%); ↓ HHF | ↑ genital mycotic infections |
| CANVAS Program [14] | 2017 | Canagliflozin 100 mg/300 mg | T2DM with ASCVD or high CV risk (10,142 participants) | 3.6 years (mean 188 weeks) | 3-point MACE | ↓ MACE (HR ≈ 0.86); renal-benefit signals | ↑ lower-limb amputations (HR~1.97) and fracture signal; ↑ genital mycotic infections |
| DECLARE–TIMI 58 [10] | 2019 | Dapagliflozin 10 mg | T2DM with multiple risk factors or ASCVD (17,160 patients) | 4.2 years | Dual: MACE; CV death or HHF | Neutral MACE; ↓ CV death/HHF (HR~0.83) | ↑ DKA (0.3% vs. 0.1%); ↑ serious genital infections |
| VERTIS CV [105] | 2020 | Ertugliflozin 5 mg/15 mg | T2DM with established ASCVD (8246 patients) | 3.5 years (mean) | 3-point MACE (non-inferiority) | Non-inferior for MACE; trend ↓ HHF | ↑ genital mycotic infections/UTIs vs. placebo; overall safety otherwise balanced |
| DAPA-HF [106] | 2019 | Dapagliflozin 10 mg | HFrEF (with/without diabetes) (4744 patients) | 18.2 months | CV death or worsening HF | ↓ primary composite (HR~0.74) | ↑ genital infections; DKA rare |
| EMPEROR-Reduced [8] | 2020 | Empagliflozin 10 mg | HFrEF (with/without diabetes) (3730 patients) | 16 months | CV death or HHF | ↓ primary composite (HR~0.75) | ↑ hypotension/volume depletion and genital infections |
| EMPEROR-Preserved [107] | 2021 | Empagliflozin 10 mg | HFpEF/HFmrEF (LVEF > 40%) 5988 patients | 26.2 months | CV death or HHF | ↓ primary composite (HR~0.79) | ↑ genital/urinary infections and hypotension vs. placebo |
| DELIVER [108] | 2022 | Dapagliflozin 10 mg | HFpEF/HFmrEF (LVEF > 40%) 6263 patients | 2.3 years | Worsening HF or CV death | ↓ primary composite (HR~0.82) | ↑ genital infections; hypotension |
| DAPA-CKD [109] | 2020 | Dapagliflozin 10 mg | CKD with/without T2DM 4304 participants | 2.4 years | ≥50% eGFR decline, ESKD or kidney/CV death | ↓ renal composite and ↓ all-cause mortality | ↑ genital mycotic infections; DKA rare |
| EMPA-KIDNEY [110] | 2023 | Empagliflozin 10 mg | CKD with/without diabetes (broad eGFR/albuminuria) 6609 patients | 2.0 years | Kidney disease progression or CV death | ↓ primary composite (HR~0.72) | AKI events not increased; ↑ genital infections (class-typical) |
| CREDENCE [12] | 2019 | Canagliflozin 100 mg | T2DM with CKD (albuminuric) 4401 patients | 2.62 years | ESKD, doubling of creatinine, or renal/CV death | ↓ primary renal composite (HR~0.70) | No significant ↑ amputations or fractures vs. placebo; ↑ male genital infections; rare DKA |
| EMPULSE [111] | 2022 | Empagliflozin 10 mg | Acute HF (initiated in-hospital, stabilized 566 patients | 90 days (hierarchical endpoint) | Win ratio composite (death, HF events, time to first HF event) | Significant clinical benefit at 90 days (win ratio ~1.36) | Well tolerated |
| EMPACT-MI [112] | 2024 | Empagliflozin 10 mg | Recent MI with high HF risk (without established HF) 3260 patients | 17.9 months | First HHF or all-cause death | Neutral for primary endpoint; HHF component reduced | AEs similar between groups; class-consistent safety |
| DAPA-MI [113] | 2024 | Dapagliflozin 10 mg | Recent MI without diabetes or chronic HF 4017 patients | ≈1 year | Hierarchical cardiometabolic win composite | Win for cardiometabolic outcomes; no difference in CV death/HHF | AEs comparable to placebo; genital infections uncommon in non-diabetics |
| SOLOIST-WHF (SGLT1/2) [114] | 2021 | Sotagliflozin 200 mg/400 mg | T2DM with recent worsening HF 1222 patients | ≈9 months | Total CV death + HF hospitalizations/urgent visits | ↓ total CV death/HF events | Class-typical genital infections |
| SCORED (SGLT1/2) [115] | 2021 | Sotagliflozin 200 mg/400 mg | T2DM with CKD and CV risk 10,584 patients | ≈16 months | CV death + HF hospitalization/urgent visits | ↓ primary composite; cardiorenal benefits in analyses | ↑ diarrhea, genital mycotic infections, volume depletion, and DKA vs. placebo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanke, J.; Romejko, K.; Niemczyk, S. Sodium-Glucose Cotransporter-2 Inhibitors in Diabetes and Beyond: Mechanisms, Pleiotropic Benefits, and Clinical Use—Reviewing Protective Effects Exceeding Glycemic Control. Molecules 2025, 30, 4125. https://doi.org/10.3390/molecules30204125
Hanke J, Romejko K, Niemczyk S. Sodium-Glucose Cotransporter-2 Inhibitors in Diabetes and Beyond: Mechanisms, Pleiotropic Benefits, and Clinical Use—Reviewing Protective Effects Exceeding Glycemic Control. Molecules. 2025; 30(20):4125. https://doi.org/10.3390/molecules30204125
Chicago/Turabian StyleHanke, Julia, Katarzyna Romejko, and Stanisław Niemczyk. 2025. "Sodium-Glucose Cotransporter-2 Inhibitors in Diabetes and Beyond: Mechanisms, Pleiotropic Benefits, and Clinical Use—Reviewing Protective Effects Exceeding Glycemic Control" Molecules 30, no. 20: 4125. https://doi.org/10.3390/molecules30204125
APA StyleHanke, J., Romejko, K., & Niemczyk, S. (2025). Sodium-Glucose Cotransporter-2 Inhibitors in Diabetes and Beyond: Mechanisms, Pleiotropic Benefits, and Clinical Use—Reviewing Protective Effects Exceeding Glycemic Control. Molecules, 30(20), 4125. https://doi.org/10.3390/molecules30204125







