Nanofiber Networks from Self-Assembling Cardanol Amphiphiles: Toward Renewable Multifunctional Surfactants
Abstract
1. Introduction
2. Results and Discussion
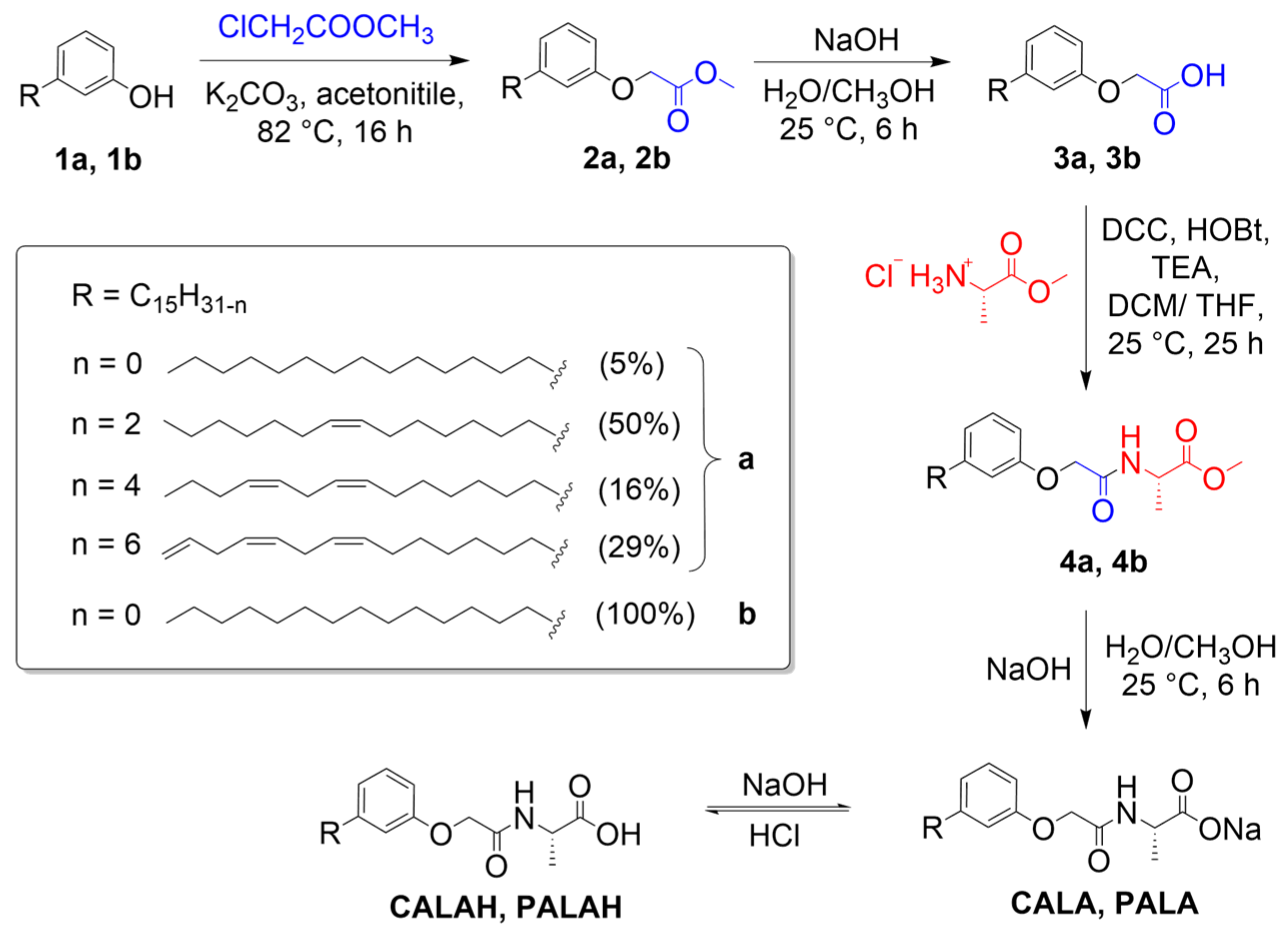
2.1. Synthesis of Amphiphilic Molecules CALAH and PALAH
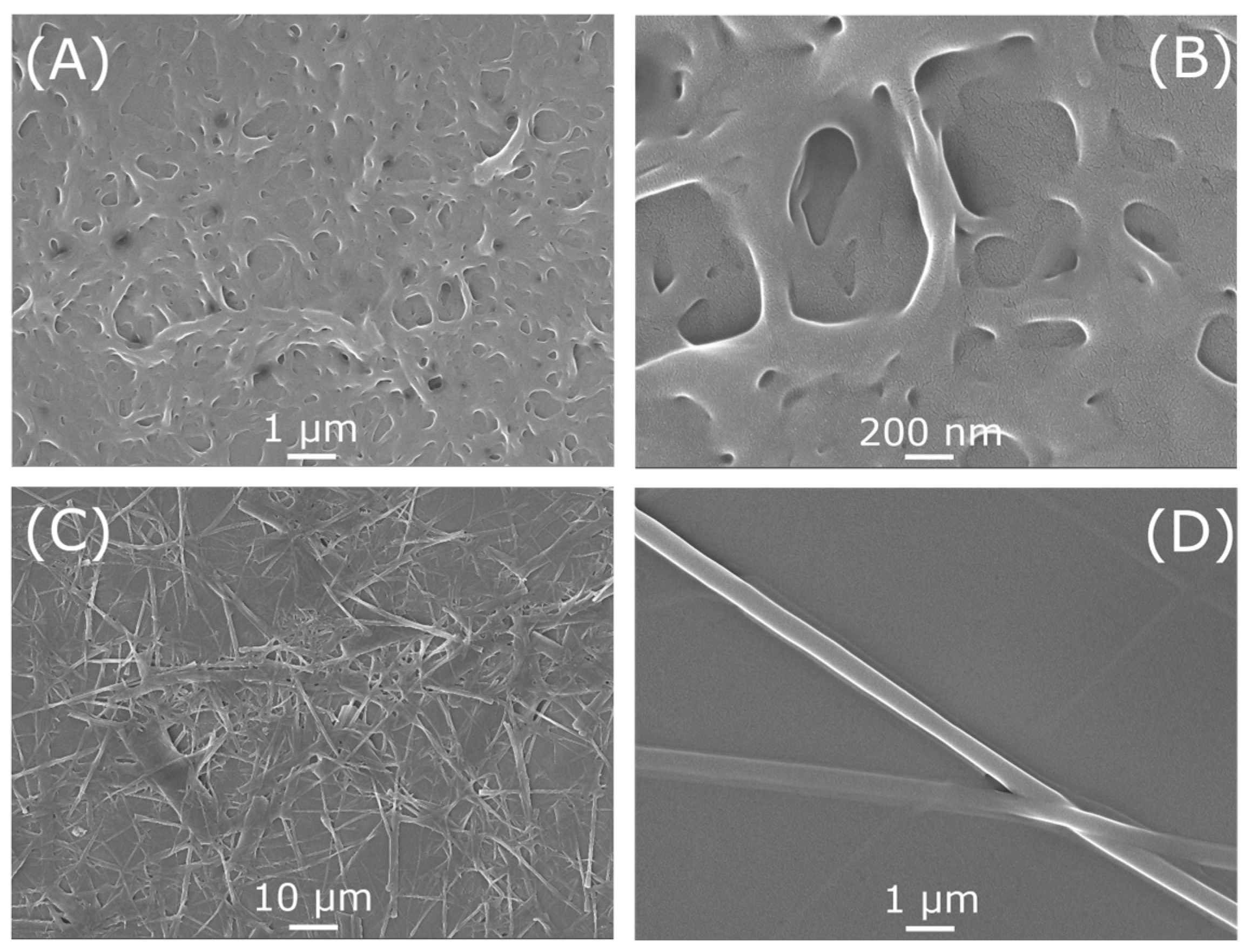
2.2. Morphological Structures
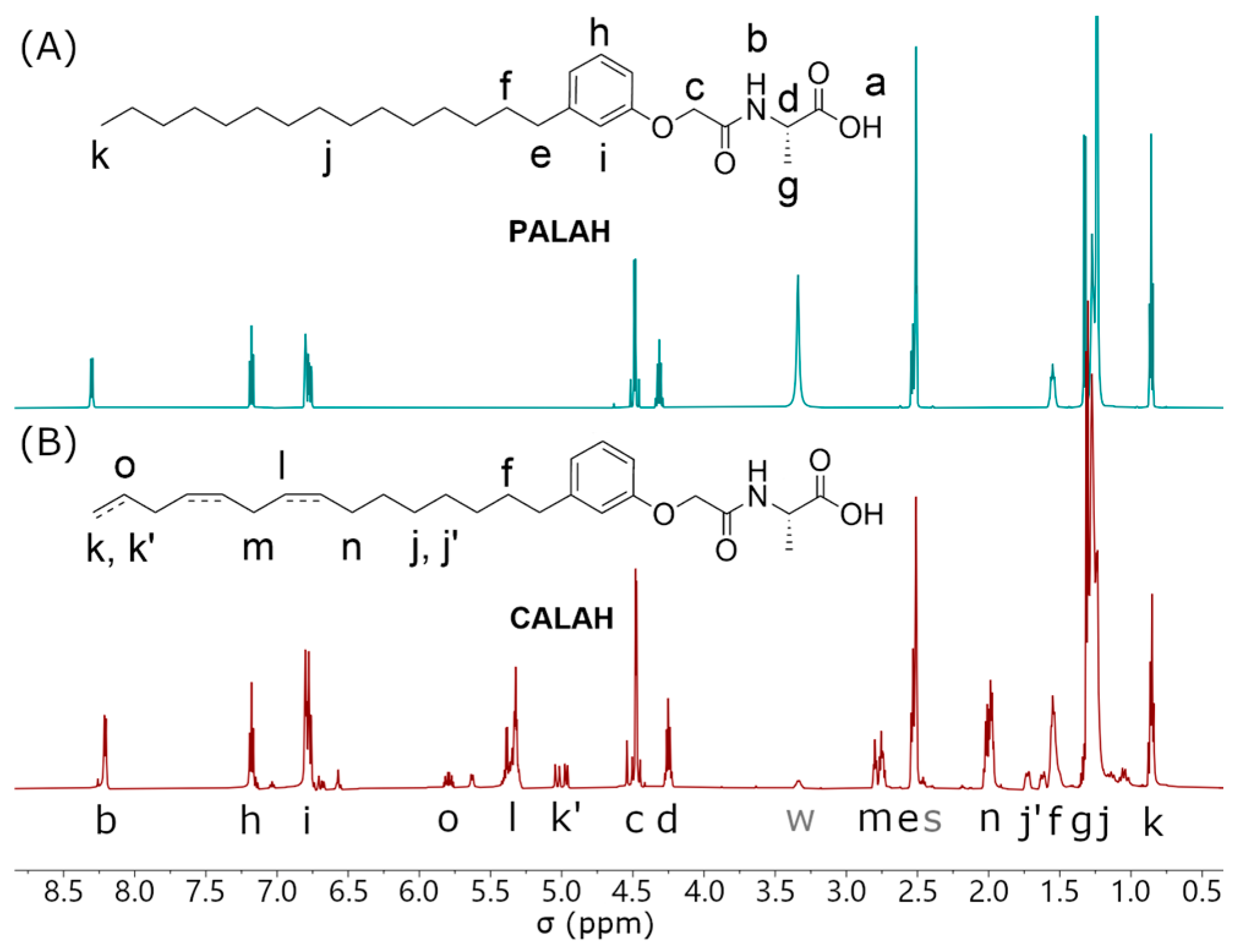
2.3. 1H NMR Self-Assembled Structures
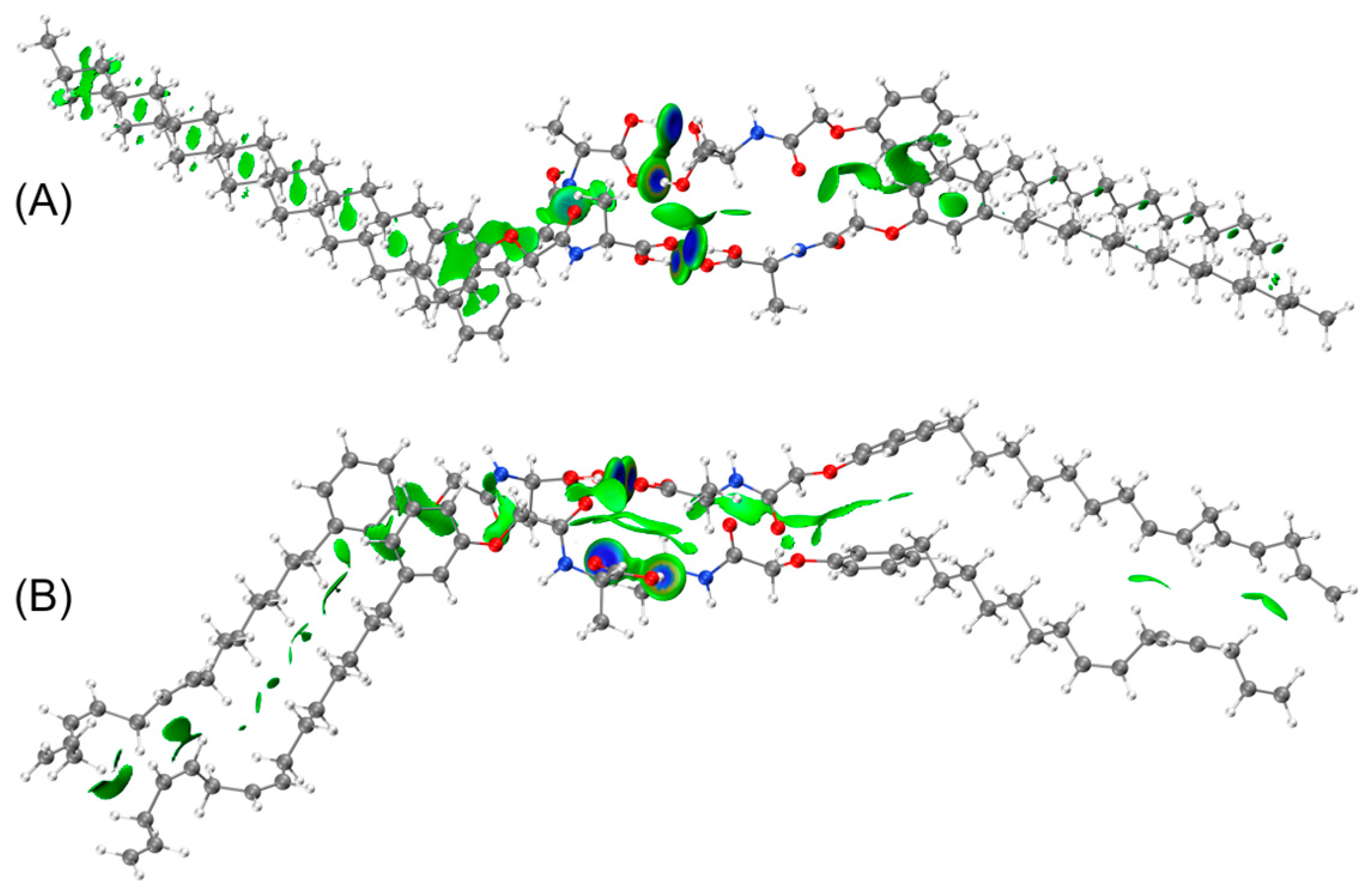
2.4. DFT Calculations
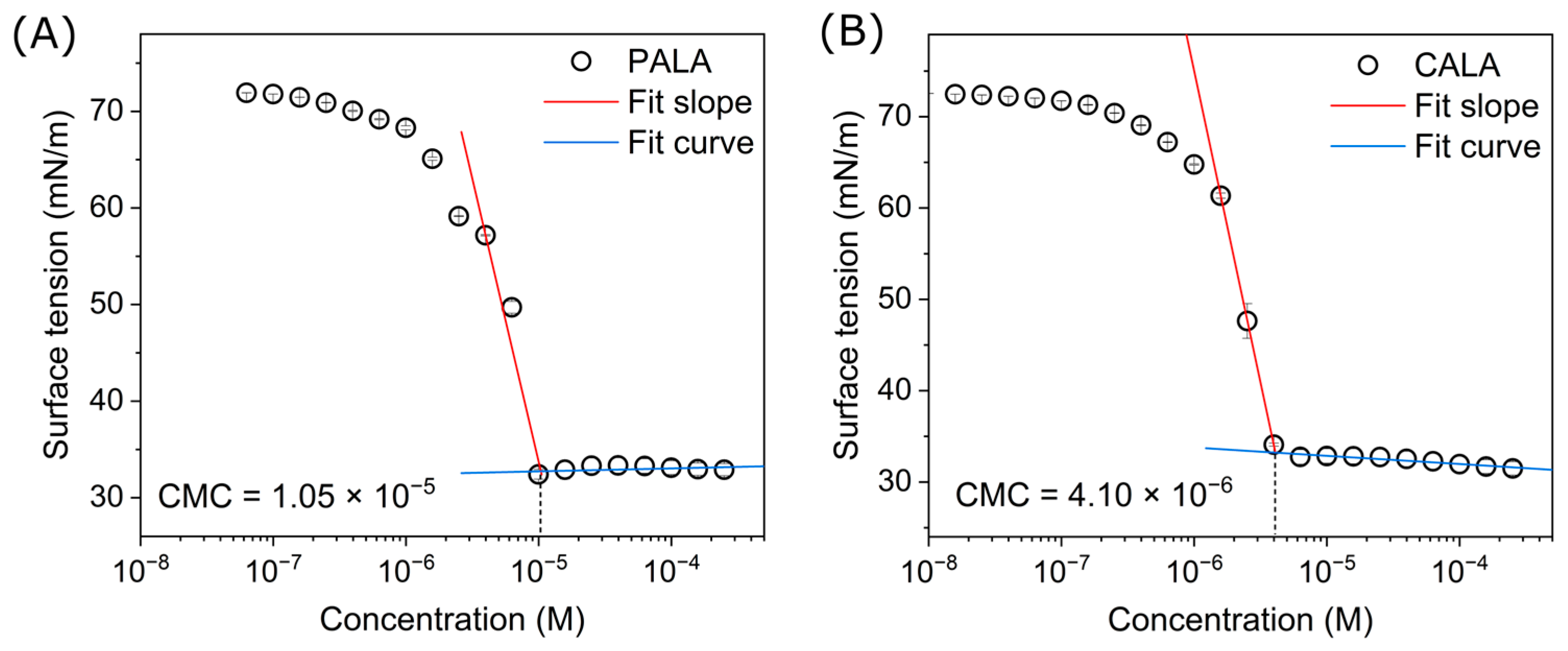
2.5. Application of Characteristics as a Surfactant
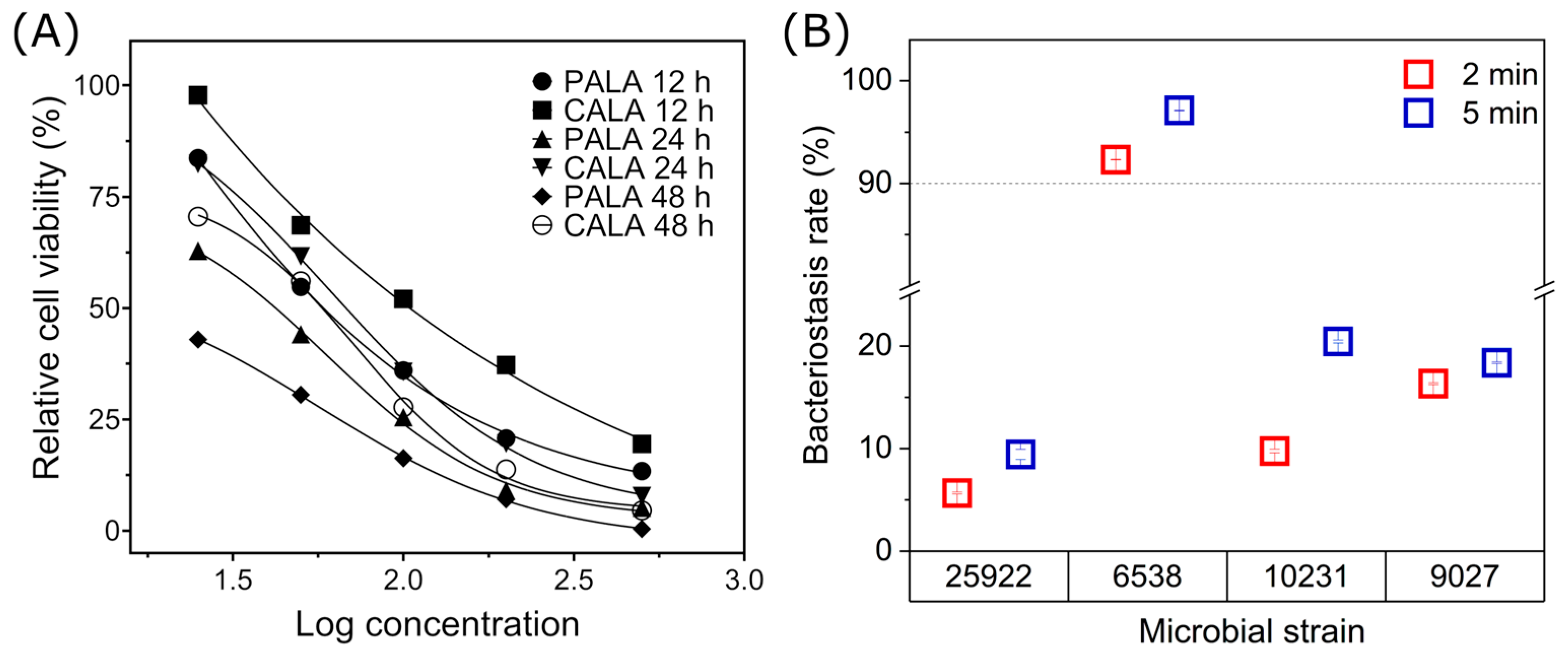
2.6. Cell and Microbial Inhibitory Properties
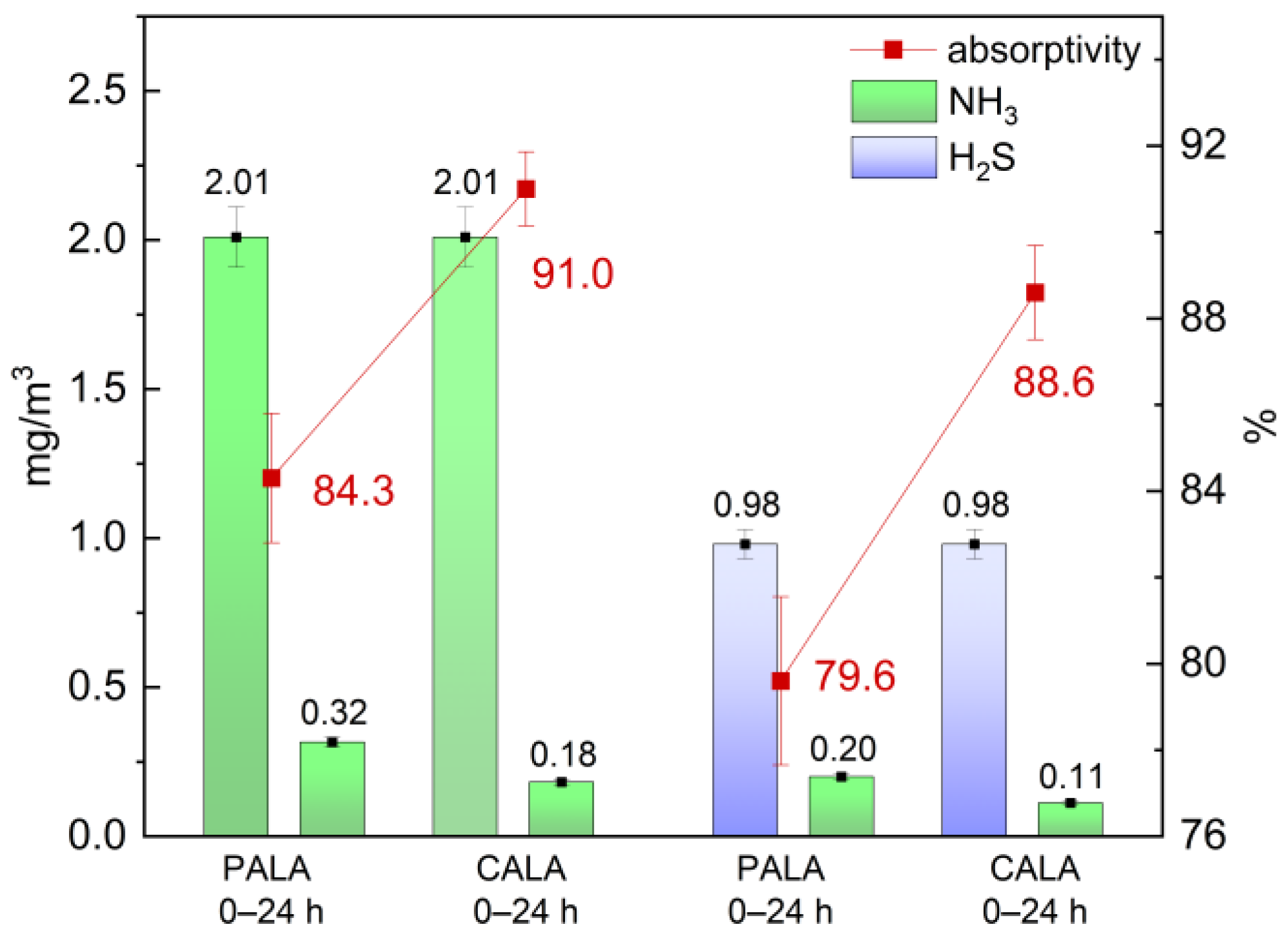
2.7. Gas Adsorption Properties
3. Materials and Methods
3.1. Synthesis Materials and Characterization
3.2. Synthesis of Amphiphilic Molecules CALAH and PALAH
3.2.1. Methyl Cardenolphenoxyacetate (2a)
3.2.2. Cardenolphenoxyacetic Acid (3a)
3.2.3. Methyl (Cardenolphenoxy)acetyl-L-alaninate (4a)
3.2.4. Sodium (Cardenolphenoxy)acetyl-L-alanine (CALA) and (Cardenolphenoxy)acetyl-L-alanine (CALAH)
3.2.5. Methyl 3-Pentadecylphenoxyacetate (2b)
3.2.6. 3-Pentadecylphenoxyacetic Acid (3b)
3.2.7. Methyl (3-Pentadecylphenoxy)acetyl-L-alaninate (4b)
3.2.8. Sodium (3-Pentadecylphenoxy)acetyl-L-alanine (PALA) and (3-Pentadecylphenoxy)acetyl-L-alanine (PALAH)
3.3. Morphological Structures
3.4. 1H NMR Self-Assembled Structures
3.5. DFT Calculations
3.6. Application Characteristics as Surfactant
3.7. Cell and Microbial Inhibitory Properties
3.8. Gas Adsorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNSL | Cashew nut shell liquid |
| NMR | Nuclear magnetic resonance |
| HRMS | High-resolution mass spectrometry |
| FT-IR | Fourier-transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
| DFT | Density functional theory |
| HLB | Hydrophilic lipophilic balance |
| CMC | Critical micelle concentration |
| APGs | Alkyl polyglycolides |
| DCC | N,N′-dicyclohexylcarbodiimide |
| HOBt | 1-Hydroxybenzotriazole |
| THF | Tetrahydrofuran |
| Co., Ltd. | Company limited by shares |
| DCM | Dichloromethane |
| Rf | Retention factor value |
| r.t. | Room temperature |
| mol | Mole |
| M | Mole per liter |
| CDCl3 | Chloroform |
| Ph | Phenyl group |
| J | Coupling constant |
| t | Triplet |
| s | Singlet |
| q | Quartet |
| m | Multiplet |
| dd | Double doublet |
| dq | Double quartet |
| ddt | Double double triplet |
| pH | Potential of hydrogen |
| h | Hour |
| g | Gram |
| mL | Milliliter |
| mM | Millimole per liter |
| mg | Milligram |
| mmol | Millimole |
| equiv. | Equivalents |
| DMSO | Dimethyl sulfoxide |
| MHz | Mega Hertz |
| cm−1 | Reciprocal centimeter |
| °C | Degree Celsius |
| ppm | Parts per million |
| μm | Micrometer |
| calc. | Calculated |
| Å | Angstrom |
| kJ | Kilojoule |
| TEA | Triethylamine |
| ESI | Electrospray ionization |
| v/v | Volume to Volume Ratio |
| δ | Chemical shift |
| ATR | Attenuated Total Reflectance |
| [α] | Specific optical rotation |
| EtOH | Ethanol |
| c | Concentration |
| SMD | Solvation model density |
| IGMH | Independent gradient model based on Hirshfeld partition |
| E | Energy |
| RDG | Reduced density gradient |
| CO2 | Carbon dioxide |
| CCK8 | Cell Counting Kit-8 |
| PBS | Fetal bovine serum |
| EDTA | Ethylenediaminetetraacetic Acid |
| L929 | NCTC clone 929 |
| MEM | Minimum essential medium |
| rpm | Revolutions per minute |
| PBS | Phosphate-buffered saline |
| eV | Electron volt |
| SDS | Sodium dodecyl sulfate |
| IC50 | Half maximal inhibitory concentration |
| NH3 | Ammonia |
| H2S | Hydrogen sulfide |
References
- Saleem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, e08905. [Google Scholar] [CrossRef]
- Malik, K.; Capareda, S.C.; Kamboj, B.R.; Malik, S.; Singh, K.; Arya, S.; Bishnoi, D.K. Biofuels production: A review on sustainable alternatives to traditional fuels and energy sources. Fuels 2024, 5, 157–175. [Google Scholar] [CrossRef]
- Deshmukh, M.K.G.; Sameeroddin, M.; Abdul, D.; Sattar, M.A. Renewable energy in the 21st century: A review. Mater. Today Proc. 2023, 80, 1756–1759. [Google Scholar] [CrossRef]
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable fossil fuels in a 1.5 C world. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef]
- Stubbs, S.; Yousaf, S.; Khan, I. A review on the synthesis of bio-based surfactants using green chemistry principles. DARU J. Pharm. Sci. 2022, 30, 407–426. [Google Scholar] [CrossRef]
- Romero Vega, G.; Gallo Stampino, P. Bio-Based surfactants and biosurfactants: An overview and main characteristics. Molecules 2025, 30, 863. [Google Scholar] [CrossRef]
- Igwebuike, C.M.; Awad, S.; Andrès, Y. Renewable energy potential: Second-generation biomass as feedstock for bioethanol production. Molecules 2024, 29, 1619. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.Y.; Siti-Dina, R.P.; Leng, S.T.K.; Er, A.; Show, P.L. Circular bioeconomy in palm oil industry: Current practices and future perspectives. Environ. Technol. Innov. 2023, 30, 103050. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. From renewable biomass to nanomaterials: Does biomass origin matter? Prog. Mater. Sci. 2022, 130, 100999. [Google Scholar] [CrossRef]
- Luengo, G.S.; Leonforte, F.; Greaves, A.; Rubio, R.G.; Guzman, E. Physico-chemical challenges on the self-assembly of natural and bio-based ingredients on hair surfaces: Towards sustainable haircare formulations. Green Chem. 2023, 25, 7863–7882. [Google Scholar] [CrossRef]
- Kalak, T. Potential use of industrial biomass waste as a sustainable energy source in the future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Agger, J.W.; Zeuner, B. Bio-based surfactants: Enzymatic functionalization and production from renewable resources. Curr. Opin. Biotechnol. 2022, 78, 102842. [Google Scholar] [CrossRef]
- Vo, T.-V.; Chou, Y.-Y.; Chen, B.-H. Preparation of microemulsion from an alkyl polyglycoside surfactant and tea tree oil. Molecules 2021, 26, 1971. [Google Scholar] [CrossRef] [PubMed]
- Veronico, L.; Colafemmina, G.; Gentile, L. Enhancing oil-uptake efficiency with an alkyl polyglycoside–dodecanol formulation. Colloids Interfaces 2024, 8, 6. [Google Scholar] [CrossRef]
- Liu, H.; Qian, Z.; Wang, Q.; Wu, D.; Wang, X. Development of renewable biomass-derived carbonaceous aerogel/mannitol phase-change composites for high thermal-energy-release efficiency and shape stabilization. ACS Appl. Energy Mater. 2021, 4, 1714–1730. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Khoo, K.S.; Gupta, V.K.; Sharma, M.; Show, P.L.; Yap, P.-S. Exploitation of lignocellulosic-based biomass biorefinery: A critical review of renewable bioresource, sustainability and economic views. Biotechnol. Adv. 2023, 69, 108265. [Google Scholar] [CrossRef]
- Sun, S.; De Angelis, G.; Bertella, S.; Jones, M.J.; Dick, G.R.; Amstad, E.; Luterbacher, J.S. Integrated Conversion of Lignocellulosic Biomass to Bio-Based Amphiphiles Using a Functionalization-Defunctionalization Approach. Angew. Chem. Int. Ed. 2024, 63, e202312823. [Google Scholar] [CrossRef]
- Sharma, K.; Negi, S.; Thakur, N.; Kishore, K. Partial glycerides-an important nonionic surfactant for industrial applications: An overview. J. Biol. Chem. Chron 2017, 3, 10–19. [Google Scholar]
- Kobayashi, Y.; Li, Q.; Ushimaru, K.; Hirota, M.; Morita, T.; Fukuoka, T. Isolation and characterization of novel naturally occurring sophorolipid glycerides. Bioresour. Technol. Rep. 2023, 22, 101399. [Google Scholar] [CrossRef]
- Callender, S.P.; Wettig, S.D. Phase Behavior of Non-Ionic Surfactant-Medium Chain Triglyceride-Water Microemulsion Systems. J. Surfactants Deterg. 2021, 24, 603–629. [Google Scholar] [CrossRef]
- Roy, A.; Fajardie, P.; Lepoittevin, B.; Baudoux, J.; Lapinte, V.; Caillol, S.; Briou, B. CNSL, a promising building blocks for sustainable molecular design of surfactants: A critical review. Molecules 2022, 27, 1443. [Google Scholar] [CrossRef]
- Veeramanoharan, A.; Kim, S.-C. A comprehensive review on sustainable surfactants from CNSL: Chemistry, key applications and research perspectives. RSC Adv. 2024, 14, 25429–25471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, S.; Song, K.; Gong, X.; Zhang, S.; Gao, S.; Lu, Z. A bio-based benzoxazine surfactant from amino acids. Green Chem. 2020, 22, 3481–3488. [Google Scholar] [CrossRef]
- Vichitra, M.; Sunil Kumar, M.; Beemkumar, N.; Subbiah, G.; Kamakshi Priya, K. Advances in extraction and sustainable utilization of cashew nut shell liquid (CNSL) for industrial applications. Int. J. Chem. React. Eng. 2025, 3, 529–539. [Google Scholar] [CrossRef]
- Uliassi, E.; de Oliveira, A.S.; de Camargo Nascente, L.; Romeiro, L.A.S.; Bolognesi, M.L. Cashew nut shell liquid (CNSL) as a source of drugs for Alzheimer’s disease. Molecules 2021, 26, 5441. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, M.; Hu, Y.; Wang, Y.; Li, P.; Cao, J.; Shi, M.; Tan, J.; Zhang, M.; Xiao, X. Efficacy and safety of CD19-specific CAR T cell–based therapy in B-cell acute lymphoblastic leukemia patients with CNSL. Blood J. Am. Soc. Hematol. 2022, 139, 3376–3386. [Google Scholar] [CrossRef]
- León, J.; Ortiz, G.; Barrios, A.F.G.; Álvarez, O.; Maranon, A.; Hernandez, C.; Ayala-Garcia, C.; Porras, A. Understanding the effects of pre-treatment and extraction methods on lipid fingerprint of cashew nut shell liquid (CNSL) by non-targeted lipidomics analysis. Sci. Rep. 2025, 15, 18103. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.J.; Marsh, R. The crystal structure of L-alanine. Acta Crystallogr. 1966, 20, 550–555. [Google Scholar] [CrossRef]
- Laerdahl, J.K.; Wesendrup, R.; Schwerdtfeger, P. d- or l-Alanine: That Is the Question. ChemPhysChem 2000, 1, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Sunde, M.; Harper, A. Effect of D-alanine and D-aspartic acid on the chick. J. Nutr. 1972, 102, 1441–1451. [Google Scholar] [CrossRef]
- Prasad, Y.S.; Miryala, S.; Lalitha, K.; Ranjitha, K.; Barbhaiwala, S.; Sridharan, V.; Maheswari, C.U.; Srinandan, C.; Nagarajan, S. Disassembly of bacterial biofilms by the self-assembled glycolipids derived from renewable resources. ACS Appl. Mater. Interfaces 2017, 9, 40047–40058. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 6.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2025, 15, e70019. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial activity, in vitro cytotoxicity, and cell cycle arrest of gemini quaternary ammonium surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Ahire, M.B.; Bhagwat, S.S. Novel ester-linked anionic gemini surfactant: Synthesis, surface-active properties and antimicrobial Study. J. Surfactants Deterg. 2017, 20, 789–797. [Google Scholar] [CrossRef]
- Freud, B.; Freud, H. A theory of the ring method for the determination of surface tension. J. Am. Chem. Soc. 1930, 52, 1772–1782. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Surface tension and its measurement. In Handbook of Adhesives and Surface Preparation; Ebnesajjad, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 21–30. [Google Scholar]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–325. [Google Scholar]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Rosen, M.; Solash, J. Factors affecting initial foam height in the Ross-Miles foam test. J. Am. Oil Chem. Soc. 1969, 46, 399–402. [Google Scholar] [CrossRef]
- Sanchez, L.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Potential irritation of lysine derivative surfactants by hemolysis and HaCaT cell viability. Toxicol. Lett. 2006, 161, 53–60. [Google Scholar] [CrossRef]
- Long, X.; Zeng, Y.-F.; Liu, Y.; Liu, Y.; Li, T.; Liao, L.; Guo, Y. Synthesis of novel genistein amino acid derivatives and investigation on their interactions with bovine serum albumin by spectroscopy and molecular docking. RSC Adv. 2018, 8, 31201–31212. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Nusca, T.D.; Hochbaum, A.I. Rhamnolipids mediate an interspecies biofilm dispersal signaling pathway. ACS Chem. Biol. 2016, 11, 3068–3076. [Google Scholar] [CrossRef]
- Banat, I.M.; De Rienzo, M.A.D.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef] [PubMed]
- QB/T 2761-2024; Method for Measuring Purification Effect of Indoor Air Purification Products. Ministry of Industry and Information Technology: Beijing, China, 2024.
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]

| Molecular | kJ/mol | eV |
|---|---|---|
| PALAH dimer | −37.30 | −0.39 |
| PALAH tetramer | −206.91 | −2.14 |
| CALAH dimer | −31.88 | −0.33 |
| CALAH tetramer | −216.99 | −2.25 |
| Surfactants | CMC (mol/L) | γCMC (mN/m) | ΠCMC 1 (mN/m) |
|---|---|---|---|
| PALA | 1.05 × 10−5 | 32.5 | 39.5 |
| CALA | 4.10 × 10−6 | 33.2 | 38.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, L.; Liu, B.; Deng, L.; Wu, Z. Nanofiber Networks from Self-Assembling Cardanol Amphiphiles: Toward Renewable Multifunctional Surfactants. Molecules 2025, 30, 4119. https://doi.org/10.3390/molecules30204119
Wang Y, Zhao L, Liu B, Deng L, Wu Z. Nanofiber Networks from Self-Assembling Cardanol Amphiphiles: Toward Renewable Multifunctional Surfactants. Molecules. 2025; 30(20):4119. https://doi.org/10.3390/molecules30204119
Chicago/Turabian StyleWang, Yichuan, Leilei Zhao, Bao Liu, Longhui Deng, and Zhenqiang Wu. 2025. "Nanofiber Networks from Self-Assembling Cardanol Amphiphiles: Toward Renewable Multifunctional Surfactants" Molecules 30, no. 20: 4119. https://doi.org/10.3390/molecules30204119
APA StyleWang, Y., Zhao, L., Liu, B., Deng, L., & Wu, Z. (2025). Nanofiber Networks from Self-Assembling Cardanol Amphiphiles: Toward Renewable Multifunctional Surfactants. Molecules, 30(20), 4119. https://doi.org/10.3390/molecules30204119







