Sodium Benzoate and Potassium Sorbate Inhibit Proteolysis and Promote Lipid Oxidation in Atlantic Herring Marinades Produced on an Industrial Scale
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties and Proximate Composition
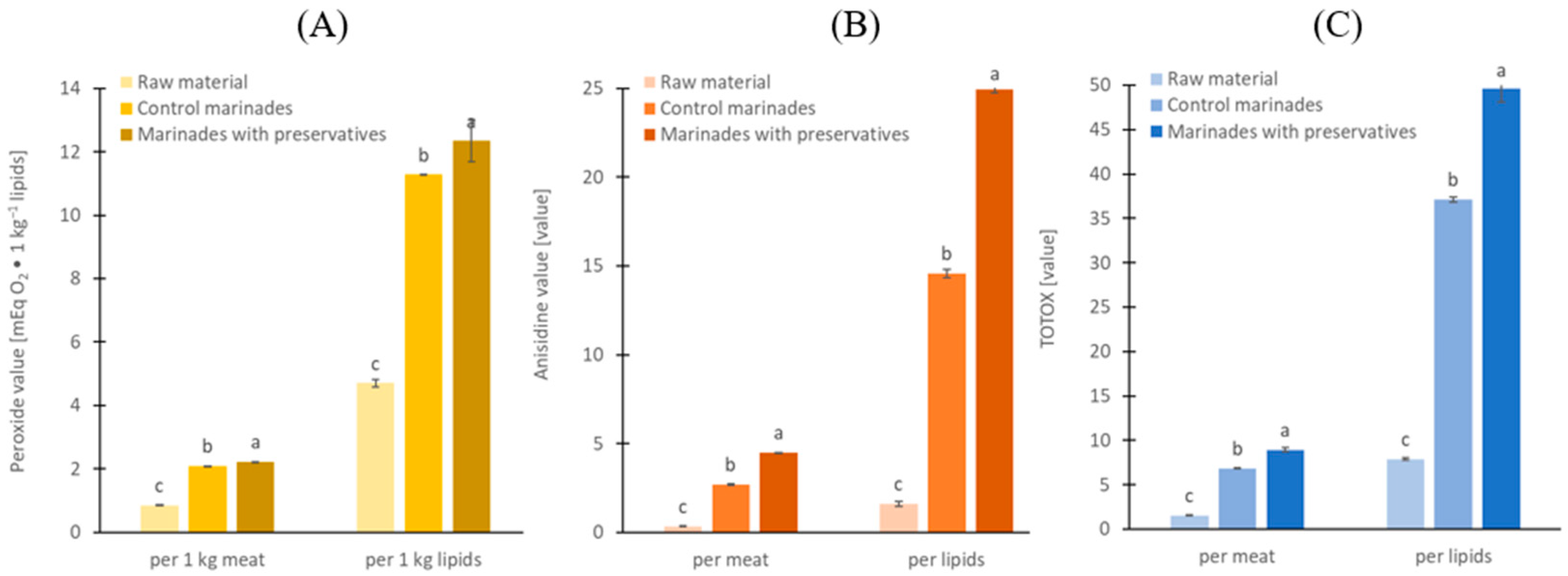
2.2. Lipid Oxidation
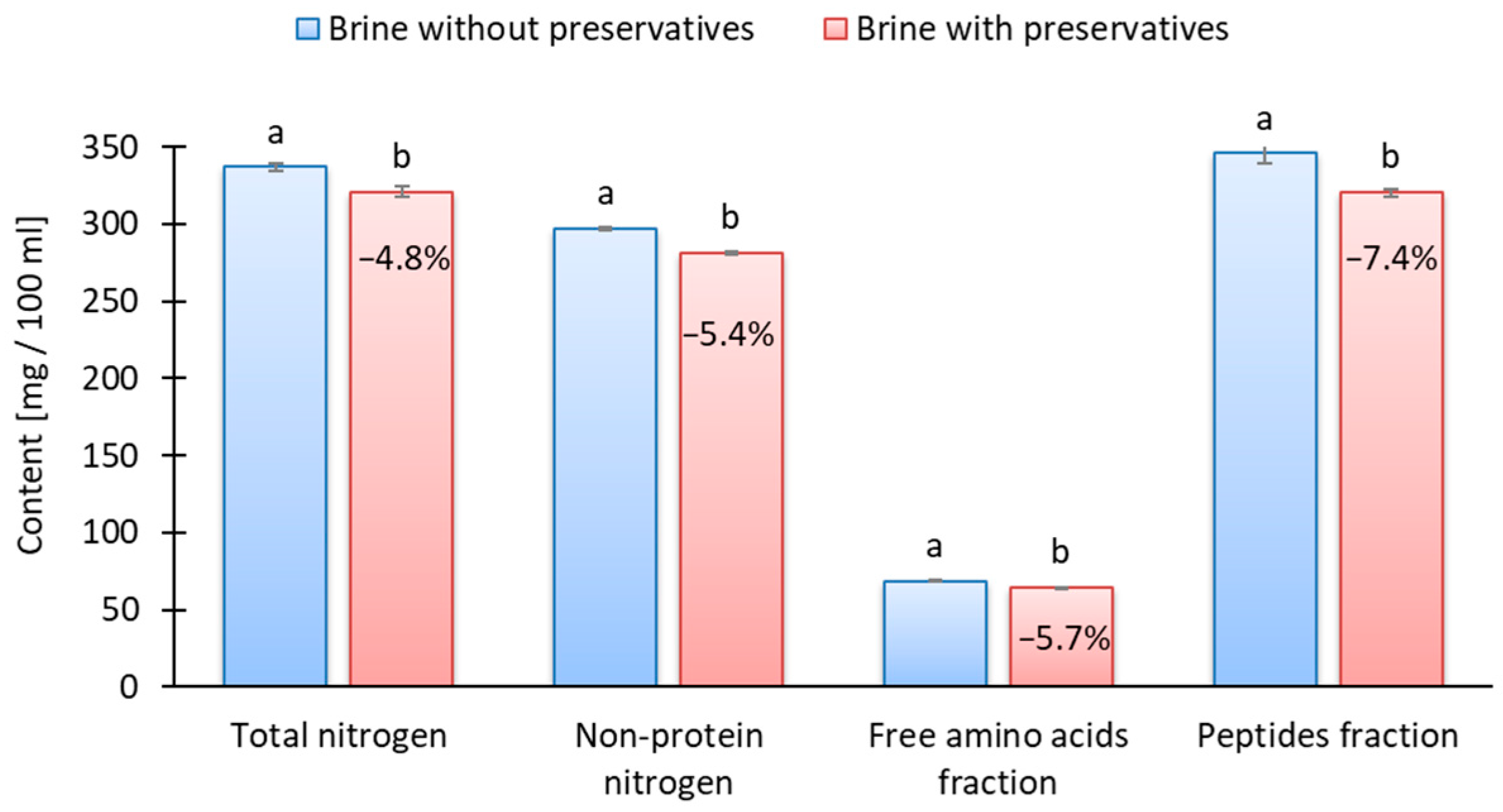
2.3. Nitrogen Fractions and Proteolytic Activity
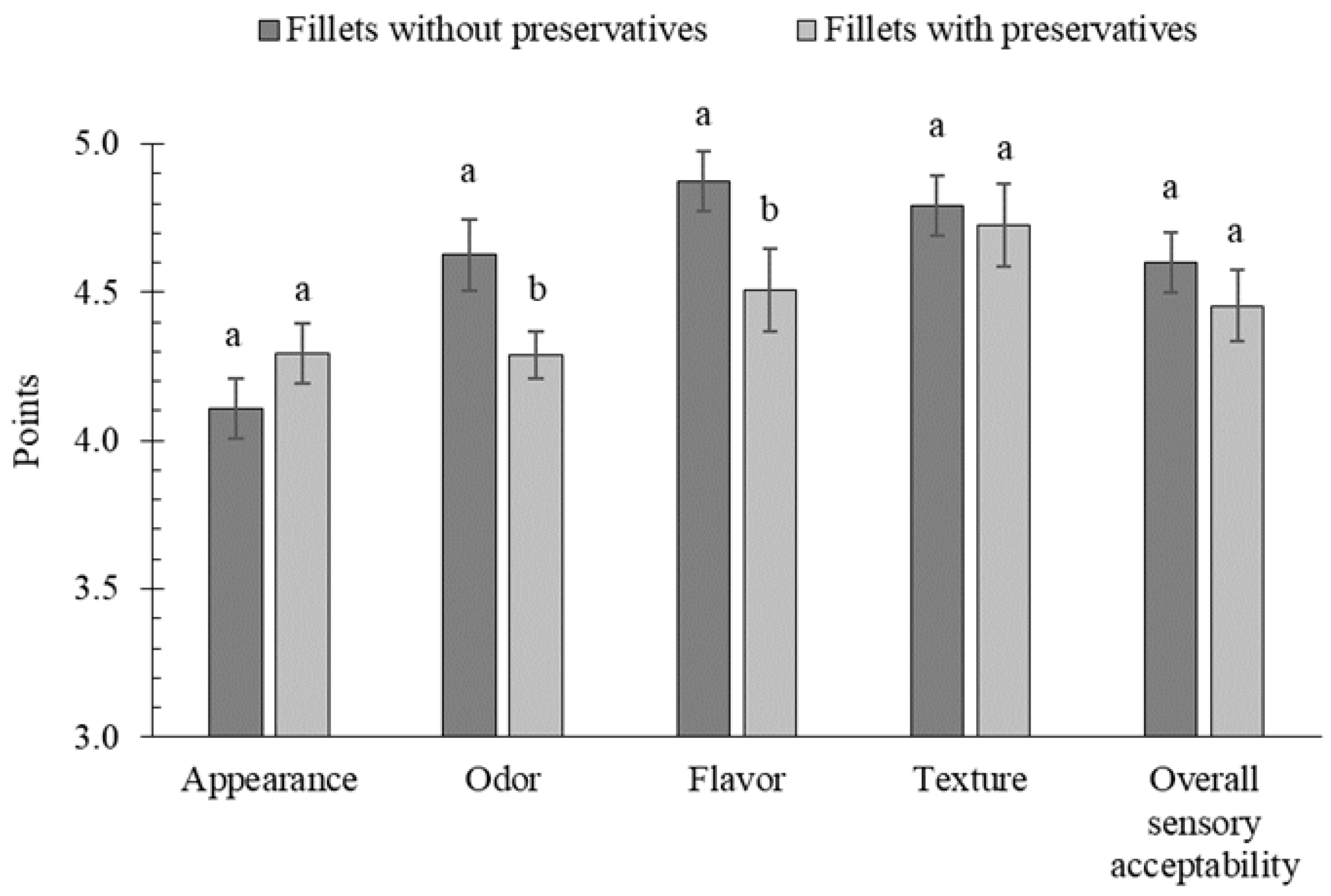
2.4. Colour, Texture Profile, and Sensory Evaluation of Marinated Herring Fillets
2.5. Microbiological Analysis
3. Materials and Methods
3.1. Marinated Herring Fillets
3.2. Total Acidity, pH, Salt Content, and Moisture Content
3.3. Lipid Content and Oxidation Index
3.4. Total Protein and Non-Protein Nitrogen Content
3.5. Cathepsins and Aminopeptidase Activity
3.6. Texture Profile Analyses (TPA) and Colour of Fillets
3.7. Sensory Evaluation
3.8. Microbiology Analyses
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The European Commission. Commission Regulation (EU) 2024/2895 of 20 November 2024 Amending Regulation (EC) No 2073/2005 as Regards Listeria Monocytogenes; The European Commission: Brussels, Belgium, 2024.
- Sampels, S. The effects of processing technologies and preparation on the final quality of fish products. Trends Food Sci. Technol. 2015, 44, 131–146. [Google Scholar] [CrossRef]
- Logrén, N.; Hiidenhovi, J.; Kakko, T.; Välimaa, A.L.; Mäkinen, S.; Rintala, N.; Mattila, P.; Yang, B.; Hopia, A. Effects of weak acids on the microbiological, nutritional and sensory quality of Baltic herring (Clupea harengus membras). Foods 2022, 11, 1717. [Google Scholar] [CrossRef]
- Bykowski, P.; Kalkowska, D.; Pawlikowski, D.; Kowalewski, W. Próby wytwarzania półproduktu do marynat zimnych o przedłużonej trwałości. In Prace Nad Technologią Marynat Rybnych. Studia i Materiały; Seria, D., Ed.; Wydawnictwo Morskiego Instytutu Rybackiego: Gdynia, Poland, 1981; Volume 14, pp. 51–89. (In Polish) [Google Scholar]
- Chan, P.N.A. Chemical Properties and Applications of Food Additives: Preservatives, Dietary Ingredients, and Processing Aids. In Handbook of Food Chemistry; Cheung, P., Mehta, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 75–100. [Google Scholar] [CrossRef]
- El-Shenawy, M.A.; Marth, E.H. Inhibition and inactivation of Listeria monocytogenes by sorbic acid. J. Food Prot. 1988, 51, 842–847. [Google Scholar] [CrossRef]
- El-Shenawy, M.A.; Marth, E.H. Sodium benzoate inhibits growth of or inactivates Listeria monocytogenes. J. Food Prot. 1988, 51, 525–530. [Google Scholar] [CrossRef]
- Thakur, B.R.; Patel, T.R. Sorbates in fish and fish products—A review. Food Rev. Int. 1994, 10, 93–107. [Google Scholar] [CrossRef]
- Meyer, V. Marinades. In Fish as Food; Borgstrom, G., Ed.; Academic Press: New York, NY, USA, 1965; Volume 3, Part 1. [Google Scholar]
- Murray, J. Absorption of benzoic and sorbic acid by herring and prawns in marinades. Int. J. Food Sci. Technol. 1988, 23, 171–175. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the re-evaluation of sorbic acid (E 200), potassium sorbate (E 202) and calcium sorbate (E 203) as food additives. EFSA J. 2015, 13, 4144. [Google Scholar] [CrossRef]
- Laub-Ekgreen, M.H. The Influence of Processing Conditions on the Weight Change of Single Herring (Clupea harengus) Fillets during Marinating. Ph.D. Thesis, Technical University of Denmark (DTU), Lyngby, Denmark, 2018. Available online: https://orbit.dtu.dk/files/162533538/PHD_Maria_Helbo_Laub_Ekgreen.pdf (accessed on 15 September 2025).
- Puolanne, E.; Peltonen, J. The effects of high salt and low pH on the water-holding of meat. Meat Sci. 2013, 93, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Ewald, G.; Bremle, G.; Karlsson, A. Differences between Bligh and Dyer and Soxhlet extractions of PCBs and lipids from fat and lean fish muscle: Implications for data evaluation. Mar. Pollut. Bull. 1998, 36, 222–230. [Google Scholar] [CrossRef]
- Szymczak, M.; Szymczak, B.; Koronkiewicz, A.; Felisiak, K.; Bednarek, M. Effect of cover brine type on the quality of meat from herring marinades. J. Food Sci. 2013, 78, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Domiszewski, Z.; Bienkiewicz, G.; Plust, D.; Kulasa, M. Quality of lipids in marinated herring. Electron. J. Pol. Agric. Univ. 2011, 14, #09. [Google Scholar]
- Bartosz, G.; Kołakowska, A. Lipid Oxidation in Food Systems. In Chemical and Functional Properties of Food Lipids, 2nd ed.; Sikorski, E.Z., Kołakowska, A., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 163–184. [Google Scholar]
- Richards, M.P.; Modra, A.M.; Li, R. Role of deoxyhemoglobin in lipid oxidation of washed cod muscle mediated by trout, poultry and beef hemoglobins. Meat Sci. 2002, 62, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Undeland, I.; Stading, M.; Lingnert, H. Influence of skinning on lipid oxidation in different horizontal layers of herring (Clupea harengus) during frozen storage. J. Sci. Food Agric. 1998, 78, 441–450. [Google Scholar] [CrossRef]
- Undeland, I.; Ekstrand, B.; Lingnert, H. Lipid Oxidation in Minced Herring (Clupea harengus) during Frozen Storage. Effect of Washing and Precooking. J. Agric. Food Chem. 1998, 46, 2319–2328. [Google Scholar] [CrossRef]
- Wu, H.; Forghani, B.; Abdollahi, M.; Undeland, I. Lipid oxidation in sorted herring (Clupea harengus) filleting co-products from two seasons and its relationship to composition. Food Chem. 2022, 373, 131523. [Google Scholar] [CrossRef]
- Grunwald, E.W.; Richards, M.P. Mechanisms of Heme Protein-Mediated Lipid Oxidation Using Hemoglobin and Myoglobin Variants in Raw and Heated Washed Muscle. J. Agric. Food Chem. 2006, 54, 8271–8280. [Google Scholar] [CrossRef]
- Maestre, R.; Pazos, M.; Medina, I. Involvement of methemoglobin (MetHb) formation and hemin loss in the pro-oxidant activity of fish hemoglobins. J. Agric. Food Chem. 2009, 57, 7013–7021. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.S.; Jacobsen, C. Retardation of lipid oxidation in fish oil-enriched fish pâté combination effects. J. Food Biochem. 2013, 37, 88–97. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- Albertos, I.; Gringer, N.; Rico, D.; Baron, C.P. Salted herring brine as a coating or additive for herring (Clupea harengus) products—A source of natural antioxidants? Innov. Food Sci. Emerg. Technol. 2016, 37, 286–292. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, B.; Li, J.; Cheong, K.L.; Li, R.; Chen, J.; Xu, Y. Emulsion-filled surimi gel: A promising approach for enhancing gel properties, water holding capacity, and flavor. Trends Food Sci. Technol. 2024, 152, 104663. [Google Scholar] [CrossRef]
- Domiszewski, Z.; Mierzejewska, S.; Michalska-Pożoga, I.; Rybka, K.; Rydzkowski, T. Effect of graphene and graphene oxide addition to polyethylene film on lipid quality of African catfish (Clarias gariepinus) fillets during refrigerated storage. Coatings 2024, 14, 1506. [Google Scholar] [CrossRef]
- Sohn, J.-H.; Taki, Y.; Ushio, H.; Kohata, T.; Shioya, I.; Ohshima, T. Lipid Oxidations in Ordinary and Dark Muscles of Fish: Influences on Rancid Off-Odor Development and Color Darkening of Yellowtail Flesh During Ice Storage. J. Food Sci. 2006, 70, S490–S496. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schwarz, K. Sensory Odour Profiling and Lipid Oxidation Status of Fish Oil and Microencapsulated Fish Oil. Food Chem. 2010, 123, 968–975. [Google Scholar] [CrossRef]
- Secci, G.; Parisi, G. From Farm to Fork: Lipid Oxidation in Fish Products—A Review. Ital. J. Food Sci. 2016, 28, 124–136. [Google Scholar] [CrossRef]
- Domiszewski, Z.; Mierzejewska, S. Effect of technological process on true retention rate of eicosapentaenoic and docosahexaenoic acids, lipid oxidation and physical properties of canned smoked sprat (Sprattus sprattus). Int. J. Food Sci. 2021, 2021, 5539376. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, M.; Kamiński, P.; Turło, M.; Bucholska, J.; Mogut, D.; Minkiewicz, P.; Mizielińska, M.; Stobińska, M. Effect of marinating temperature of Atlantic herring on meat ripening, peptides fractions proportion and antioxidant activity of meat and brine. Appl. Sci. 2023, 13, 7225. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Asanova, A.A.; Deeva, A.A.; Kratasyuk, V.A. Inhibition effect of food preservatives on endoproteinases. Food Chem. 2017, 235, 294–297. [Google Scholar] [CrossRef]
- Hernández-Herrero, M.M.; Duflos, G.; Malle, P.; Bouquelet, S. Collagenase Activity and Protein Hydrolysis as Related to Spoilage of Iced Cod (Gadus morhua). Food Res. Int. 2003, 36, 141–147. [Google Scholar] [CrossRef]
- Kamiński, P.; Szymczak, M.; Szymczak, B. The effect of previous storage condition on the diffusion and contribution of digestive proteases in the ripening of marinated Baltic herring (Clupea harengus). LWT–Food Sci. Technol. 2022, 165, 113723. [Google Scholar] [CrossRef]
- ASTM D2244-23; Standard Practice for Calculation of Color Tolerances and Color Differences from Instrumentally Measured Color Coordinates. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Nielsen, D.; Hyldig, G.; Nielsen, J.; Nielsen, H.H. Sensory properties of marinated herring (Clupea harengus) processed from raw material from commercial landings. J. Sci. Food Agric. 2005, 85, 127–134. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Dias, M.A. Processed Fishery Products. In Practical Notions on Fish Health and Production, 1st ed.; Monteiro de Oliveira, M.M.C., Robalo, J.I.E.S., Bernardo, F.M.D., Eds.; Bentham Science Publishers Ltd.: Sharjah, United Arab Emirates, 2016; Chapter 8; pp. 249–317. [Google Scholar] [CrossRef][Green Version]
- Özogul, Y.; Özogul, F.; Olgunoglu, I.A.; Kuley, E. Bacteriological and biochemical assessment of marinating cephalopods, crustaceans and gastropoda during 24 weeks of storage. Int. J. Food Sci. Nutr. 2008, 59, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Françoise, L. Occurrence and role of lactic acid bacteria in seafood products. Food Microbiol. 2010, 27, 698–709. [Google Scholar] [CrossRef]
- ISO 6885:2008; Animal and Vegetable Oils and Fats—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2008.
- Pietrzyk, C. Spectrophotometric determination of lipid peroxides by thiocyanate technique. Rocz. Państw. Zakł. Hig. 1958, 9, 75–84. [Google Scholar]
- Kołakowski, E. Analysis of proteins, peptides, and amino acids in foods. In Methods of Analysis of Food Components and Additives, 2nd ed.; Ötles, S., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 59–96. [Google Scholar]
- ISO 8586:2014-03; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors (ISO 8586:2012, Corrected Version 2014-06-15). ISO: Geneva, Switzerland, 2014.
- ISO 11035:1999; Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. ISO: Geneva, Switzerland, 1999.
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 17410:2019; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. ISO: Geneva, Switzerland, 2019.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds. Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0.95. ISO: Geneva, Switzerland, 2008.
- ISO 21871:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Determination of Low Numbers of Presumptive Bacillus cereus—Most probable Number Technique and Detection Method. ISO: Geneva, Switzerland, 2006.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017.
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection method. ISO: Geneva, Switzerland, 2017.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- ISO 6888-1:2021/Amd 1:2023; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. ISO: Geneva, Switzerland, 2023.
| Analysis | Raw Fillet | Marinades—No Preservatives | Marinades—With Preservatives | Difference [%] |
|---|---|---|---|---|
| Physical properties: | ||||
| Mass of fillets [g] | 60.3 ± 3.5 a,* | 54.3 ± 2.9 b | 51.5 ± 4.5 b | −5.2 |
| Length of fillets [cm] | 14.6 ± 1.1 a | 15.6 ± 1.4 a | 14.9 ± 0.9 a | −4.5 |
| Fillet thickness [mm] | 14.21 ± 0.54 a | 12.26 ± 0.97 b | 11.81 ± 0.74 b | −3.7 |
| pH value | 6.65 ± 0.01 a | 3.98 ± 0.03 b | 3.97 ± 0.03 b | −0.2 |
| Proximate composition: | ||||
| Total acidity [g/100 g] | 0.22 ± 0.05 c | 1.78 ± 0.02 b | 1.84 ± 0.01 a | 3.4 |
| Salt [g/100 g] | 0.20 ± 0.02 b | 2.90 ± 0.03 a | 2.96 ± 0.02 a | 2.1 |
| Moisture [g/100 g] | 67.2 ± 0.1 a | 60.6 ± 0.06 c | 60.8 ± 0.06 b | 0.3 |
| Lipids by Soxhlet [g/100 g] | 12.8 ± 0.06 c | 17.0 ± 0.02 a | 16.5 ± 0.1 b | −2.9 |
| Lipids by Bligh–Dyer [g/100 g] | 13.9 ± 0.26 b | 18.4 ± 0.42 a | 18.0 ± 0.23 a | −2.3 |
| Crude Protein [g/100 g] | 12.95 ± 0.1 a | 13.05 ± 0.22 a | 13.33 ± 0.12 a | 2.1 |
| Analysis | Raw Fillet | Marinades—No Preservatives | Marinades—With Preservatives | Difference [%] |
|---|---|---|---|---|
| NPN [mg/100 g] | 235 ± 8.2 a,* | 254.2 ± 13.0 a | 246.9 ± 8.3 a | −2.9 |
| FAA [mg/100 g] | 22.3 ± 1.1 c | 78.0 ± 1.9 a | 73.3 ± 0.9 b | −6.0 |
| Peptides [mg/100 g] | 56 ± 3.2 c | 604.3 ± 6.2 a | 557.2 ± 7.3 b | −8.8 |
| Cathepsin D [U] | 2887 ± 32 c | 7228 ± 20 a | 7099 ± 24 b | −1.8 |
| Cathepsin B [U] | 1504 ± 17 a | 805.0 ± 11 b | 786.0 ± 6.0 c | −2.4 |
| Cathepsin L [U] | 487 ± 11 c | 1420 ± 21 a | 1370 ± 22 b | −3.5 |
| LAP [U] | 153 ± 6.1 a | 4.22 ± 0.3 b | 2.33 ± 0.3 c | −44.8 |
| AAP [U] | 114 ± 3.4 a | 3.0 ± 0.2 b | 2.34 ± 0.3 c | −22 |
| Analysis | Raw Fillet | Marinades—No Preservatives | Marinades—With Preservatives | Difference [%] |
|---|---|---|---|---|
| Colour of fillets’ surface: | ||||
| L* (brightness) | 61.25 ± 0.87 b,* | 67.66 ± 1.08 a | 65.57 ± 1.15 a | −3.1 |
| a* (redness) | 4.30 ± 0.32 a | 3.21 ± 0.40 b | 3.21 ± 0.39 b | 0.0 |
| b* (yellowness) | 3.54 ± 0.18 a | 2.24 ± 0.26 b | 3.03 ± 0.37 a | 26.1 |
| Colour difference (ΔE) between marinades: | 2.23 unit | |||
| Texture profile analysis: | ||||
| Hardness [N] | 9.12 ± 1.29 a | 8.13 ± 1.09 a | 7.39 ± 1.01 a | −9.1 |
| Adhesiveness [mJ] | 4.56 ± 0.90 a | 4.01 ± 0.99 a | 3.66 ± 0.88 a | 8.7 |
| Springiness [m] | 2.12 ± 0.11 a | 0.82 ± 0.06 b | 0.81 ± 0.06 b | −1.2 |
| Cohesiveness [-] | 0.80 ± 0.04 a | 0.51 ± 0.03 b | 0.48 ± 0.04 b | −5.9 |
| Gumminess [N] | 6.21 ± 0.42 a | 4.25 ± 0.61 b | 3.38 ± 0.45 b | −20.5 |
| Chewiness [J] | 4.11 ± 0.60 a | 3.6 ± 0.68 ab | 2.71 ± 0.48 b | −24.7 |
| Resilience [N] | 0.235 ± 0.007 a | 0.153 ± 0.011 b | 0.135 ± 0.009 b | −11.8 |
| Analyses | Herring Fillet Raw Material | Fillets Without Preservatives | Fillets with Preservatives |
|---|---|---|---|
| Psychrophiles | 5 × 101 | 12 × 102 | <10 |
| Mesophiles | <10 | <10 | <10 |
| Yeasts | <10 | 9 × 102 | <10 |
| Moulds | <10 | <10 | <10 |
| L. monocytogenes | absent | absent | absent |
| S. aureus | <10 | <10 | <10 |
| Salmonella | absent | absent | absent |
| B. cereus | <10 | <10 | <10 |
| Enterobacteriaceae | <10 | <10 | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, M.; Kamiński, P.; Szymczak, B.; Undeland, I.; Dmytrów, I. Sodium Benzoate and Potassium Sorbate Inhibit Proteolysis and Promote Lipid Oxidation in Atlantic Herring Marinades Produced on an Industrial Scale. Molecules 2025, 30, 4103. https://doi.org/10.3390/molecules30204103
Szymczak M, Kamiński P, Szymczak B, Undeland I, Dmytrów I. Sodium Benzoate and Potassium Sorbate Inhibit Proteolysis and Promote Lipid Oxidation in Atlantic Herring Marinades Produced on an Industrial Scale. Molecules. 2025; 30(20):4103. https://doi.org/10.3390/molecules30204103
Chicago/Turabian StyleSzymczak, Mariusz, Patryk Kamiński, Barbara Szymczak, Ingrid Undeland, and Izabela Dmytrów. 2025. "Sodium Benzoate and Potassium Sorbate Inhibit Proteolysis and Promote Lipid Oxidation in Atlantic Herring Marinades Produced on an Industrial Scale" Molecules 30, no. 20: 4103. https://doi.org/10.3390/molecules30204103
APA StyleSzymczak, M., Kamiński, P., Szymczak, B., Undeland, I., & Dmytrów, I. (2025). Sodium Benzoate and Potassium Sorbate Inhibit Proteolysis and Promote Lipid Oxidation in Atlantic Herring Marinades Produced on an Industrial Scale. Molecules, 30(20), 4103. https://doi.org/10.3390/molecules30204103







