Abstract
The overuse of antibiotics in human and veterinary medicine has contributed significantly to their persistent presence in the environment, creating conditions that promote the spread of antimicrobial resistance (AMR). In this context, innovative approaches that reduce antibiotic doses without compromising therapeutic efficacy are urgently needed. This review explores the emerging role of plant-derived secondary metabolites, particularly saponins, as bioactive excipients capable of enhancing antibiotic activity through synergistic mechanisms. By improving membrane permeability, inhibiting resistance pathways, and modulating host responses, these natural adjuvants may allow for lower antibiotic concentrations in clinical treatments, ultimately reducing pharmaceutical residues entering the environment. We discuss the potential of such combined therapies not only to mitigate the evolution and dissemination of AMR in natural microbial communities but also to provide more sustainable, biodegradable, and ecologically safer alternatives to synthetic formulation agents. Plant-derived compounds, inherently shaped by co-evolution with microbes, offer a dynamic and adaptive molecular diversity that may be less prone to long-term microbial resistance. In addition to reviewing current knowledge, this article highlights the environmental and public health implications of integrating phytochemical excipients into antibiotic regimens, and calls for further interdisciplinary efforts to evaluate their safety, efficacy, and role in shaping future antimicrobial stewardship.
1. Introduction
The advent of antibiotics in the last century significantly reduced mortality and morbidity associated with infectious diseases. However, the misuse and overuse of these drugs have precipitated the emergence of resistant microbial strains. Pathogenic bacteria have developed intrinsic resistance to antibiotics through mechanisms such as the alteration of target sites, active drug efflux, and enzymatic degradation [1]. This growing issue has sparked increased interest in medicinal plants, as 25–50% of current pharmaceuticals are derived from plant sources [2]. Crude extracts from these plants represent a promising alternative for resistance-modifying agents due to their diverse array of secondary metabolites. Compounds such as saponins in these extracts possess potential antimicrobial properties and can act as resistance modifiers [3]. Consequently, medicinal plants may also serve as effective modulators of host-related cellular processes, including immune response, mitosis, apoptosis, and signal transduction. Thus, their activity extends beyond merely eliminating microorganisms, as they may interfere with critical stages of the pathogenic process. This multifaceted approach may reduce the ability of bacteria to develop resistance to botanical agents.
This narrative, mechanism-driven review sets out to articulate a membrane-centric rationale for combining licensed antibiotics with herbal bioenhancers, with saponins presented as a translational exemplar. The novelty of this review lies in integrating membrane biophysics with the pharmacology of botanical adjuvants and mapping these mechanisms onto clinically used antibiotic classes and delivery strategies.
2. Why We Need New Antimicrobials
The bulk of antibiotic classes in use today were identified during the “golden era” of antibiotic discovery, which occurred between the 1940s and 1980s [1]. During this period, screening efforts focused on compounds capable of inhibiting or killing rapidly dividing bacteria. Consequently, most antibiotics from this era are designed to interfere with crucial bacterial growth processes, such as the synthesis of proteins, peptidoglycan, folic acid, DNA, and RNA [4]. As bacterial resistance to these antibiotics emerged, chemical modifications were applied to existing drug classes to develop analogues with improved potency. These analogues, much like their predecessors, primarily target metabolically active, fast-replicating bacteria. However, in certain infections, bacteria may encounter hostile environments that push them into a quiescent state characterized by minimal or no growth. These metabolically dormant bacteria are capable of surviving high antibiotic concentrations, necessitating prolonged treatment for efficacy [5,6,7,8,9]. Once antibiotic levels drop below the threshold required to kill or inhibit these cells, the dormant bacteria can reactivate, leading to a recurrence of symptoms. Provided that these reactivated bacteria have not developed genetic resistance, subsequent treatment cycles can eliminate them. The shift from active division to dormancy in bacterial cells is complex and often triggered by environmental stresses, such as nutrient and oxygen shortages, and acidic pH, which collectively slow down or halt bacterial growth (as shown in Figure 1).
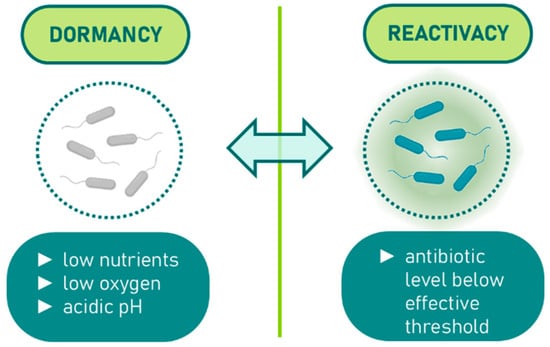
Figure 1.
Dormancy induction in bacteria under environmental stress.
Infections associated with biofilms and tuberculosis (TB) present significant treatment challenges due to the presence of slow-growing or dormant bacteria. Since the mid-1990s, awareness of biofilm-associated diseases has significantly increased, with the US CDC (United States Centers for Disease Control) estimating that biofilms contribute to up to 65% of all infections in developed countries [10]. These infections range from those linked to medical device implants, such as bloodline catheters and heart valves, to conditions related to cystic fibrosis, wounds, and superficial skin infections. The critical need for improved TB therapies also became apparent in the mid-1990s, particularly with the rise in drug-resistant strains and the deadly interaction between TB and HIV [11,12]. TB remains one of the leading causes of death from bacterial infections, resulting in over 1 million deaths annually [13]. It is estimated that approximately one-third of the global population is infected with dormant, asymptomatic Mycobacterium tuberculosis, from which around 8 million new active TB cases arise each year [14].
Over the past decade, extensive research has highlighted the potential of antimicrobials that disrupt bacterial membrane integrity as a novel approach to treating persistent infections. Additionally, bacteria capable of dormancy, such as M. tuberculosis and biofilm-forming species like Staphylococcus aureus and Pseudomonas aeruginosa, may utilize anaerobic metabolism (e.g., substrate-level phosphorylation and anaerobic respiration) to survive without active growth [15,16,17,18,19,20]. Consequently, enzymes involved in these anaerobic processes are being explored as promising targets for new drug development [20,21].
Antibiotic resistance is a significant contributor to treatment failures [22]. Beyond genetic resistance, pathogenic bacteria often undergo physiological changes that slow or halt their growth, making them difficult to eradicate with bactericidal antibiotics, despite not having developed genetic resistance [23]. These bacteria can regain antibiotic sensitivity once growth resumes. This recalcitrance to treatment arises from two key phenomena: ‘antibiotic persistence’ and ‘antibiotic indifference’ [24,25,26]. Together, these phenomena, are referred to as ‘antibiotic survival,’ and have been illustrated in Figure 2. Antibiotic survival (as used here) denotes non-inherited phenotypes that allow bacteria to avoid killing by bactericidal antibiotics during exposure and later regain susceptibility upon resumption of growth. We highlight two major states: persistence, a specialized non-growing subpopulation that survives lethal exposure [27,28], and indifference, a stress-imposed slow-growth/dormant state (e.g., host responses, low pH, nutrient or oxygen limitation) that reduces antibiotic-induced killing without genetic resistance [29,30]. As framed by Hurdle et al. and O’Neill [31,32], these non-genetic states are key contributors to treatment recalcitrance.
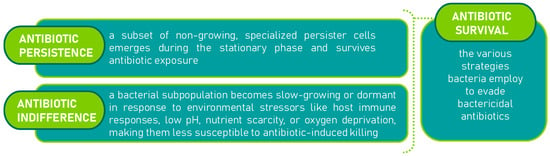
Figure 2.
Mechanisms of antibiotic survival [31,32]: persistence [27,28] and indifference [29,30].
Emerging consensus from various studies suggests that persisting bacteria evade antibiotic action by significantly downregulating biosynthetic processes targeted by most antibiotics, without compromising their survival in a metabolically inactive state. For instance, β-lactam antibiotics rely on active peptidoglycan synthesis during cell division to activate autolysins [33].
3. Targeting the Cell Membrane
Membrane-active agents offer promising antimicrobial properties, making them valuable candidates for drug discovery and therapeutic application. The bacterial membrane is an essential target due to its critical role in maintaining selective permeability, cellular homeostasis, and energy transduction, regardless of the cell’s metabolic state [34,35]. The membrane is composed of approximately one-third of the cell’s proteins and is the site of vital processes, including nutrient and waste transport, bacterial respiration, proton motive force establishment in conjunction with respiratory enzymes, ATP production, and cell–cell communication within biofilms [36]. The antibacterial potential of the membrane is further supported by the action of host-derived antimicrobial peptides and other bioactive molecules that target this structure [37]. Despite the membrane’s importance as an antibacterial target, traditional antibiotic discovery efforts have largely overlooked it. Several synthetic (e.g., ceragenins [38]) and natural compounds (e.g., polymyxins [39]) that damage bacterial membranes, potentially effective against dormant bacteria, have been underexplored, possibly due to concerns about their potential to disrupt mammalian cell membranes and the limited knowledge on optimizing these compounds for pathogen selectivity [40,41].
Membrane-damaging agents often exhibit a complex mode of action, targeting multiple cellular components. These agents can disrupt membrane architecture and functional integrity through the interaction of their lipophilic moieties with the bacterial membrane, sterically inhibit membrane-embedded proteins, or alter the proton motive force, potentially leading to cytosolic content leakage and cell death [42,43,44]. These disruptions can have lethal, pleiotropic effects on quiescent bacteria, as shown in Figure 3.
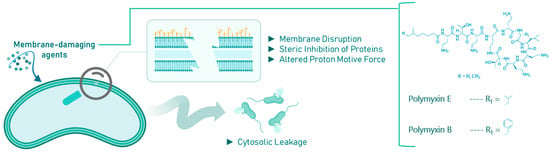
Figure 3.
Effects of membrane-damaging agents (with example compounds) on quiescent bacteria.
However, it is important to note that while dissipation of the proton motive force alone is not universally bactericidal, it can limit energy supply in already metabolically inactive cells. Notably, Mycobacterium tuberculosis differs from other pathogens in its requirement for a fully energized membrane for survival under both aerobic and hypoxic conditions. As a result, ionophores like nigericin or valinomycin, which dissipate the proton motive force, are highly bactericidal to both active and dormant M. tuberculosis. Furthermore, increasing the proton permeability of the membrane enhances the sensitivity of mycobacteria to reactive free radicals, such as nitric oxide and superoxide, within macrophages, a mechanism that is particularly effective at acidic pH levels [45].
4. Outer Membrane Permeability and Antibiotic Resistance
Currently, most antibiotics are designed to target intracellular processes, necessitating their ability to penetrate the bacterial cell envelope. The outer membrane of Gram-negative bacteria presents a significant challenge as a robust barrier that antibiotics must overcome [46,47]. Antibiotics penetrate this outer membrane via two primary routes: a lipid-mediated pathway for hydrophobic drugs and diffusion through porins for hydrophilic drugs [48]. The composition of lipids and proteins within the outer membrane significantly influences bacterial susceptibility to antibiotics, and modifications to these components are a common mechanism of drug resistance [49].
The outer membrane (OM) of Gram-negative bacteria serves the crucial function of adding an extra protective layer while still allowing the exchange of materials necessary for life. This dual function highlights the OM as a complex macromolecular structure whose intricacies have only recently been understood. The OM combines a highly hydrophobic lipid bilayer with pore-forming proteins that have specific size-exclusion properties, making it a selective barrier. This selective permeability has a profound effect on a bacterium’s vulnerability to antibiotics, which are generally aimed at intracellular targets [50]. Small hydrophilic drugs, such as β-lactams, use these porins to access the cell interior, while hydrophobic drugs, like macrolides, pass through the lipid bilayer. The emergence of drug-resistant strains across numerous bacterial species, due to alterations in the OM’s lipid or protein composition, underscores the OM’s critical role in antibiotic sensitivity. This review will detail the properties of the OM’s lipid barrier and porin-mediated permeability and discuss how modifications to these structures contribute to antibiotic resistance.
In most Gram-negative bacteria, the OM is an asymmetric bilayer composed of phospholipids and lipopolysaccharides (LPS), the latter being exclusively located in the outer leaflet. A typical LPS molecule consists of three components as shown in Figure 4 [51,52]. The inner leaflet of the OM closely resembles the cytoplasmic membrane, containing about 80% phosphatidylethanolamine, 15% phosphatidylglycerol, and 5% cardiolipin [53]. In mutants with altered LPS structures, phospholipids may also be found in the outer leaflet, likely due to a reduction in OM protein levels. The OM is populated by various proteins, some of which are extremely abundant. For instance, murein lipoprotein (Lpp) and outer membrane protein A (OmpA) are present at around 100,000 copies per cell [54]. Lpp is anchored in the OM by a fatty acid moiety, and a portion of Lpp is also covalently attached to the peptidoglycan layer, suggesting a role in maintaining OM integrity [55]. Mutants lacking Lpp produce OM vesicles and lose periplasmic enzymes. OmpA, another abundant protein, is believed to contribute to cell shape, and its absence, along with Lpp, compromises cell structure. OmpA also has pore-forming properties, though with low permeation efficiency, and recent evidence suggests it may exist in two conformations: a monomeric β-barrel and an oligomeric form with large open β-barrels, similar to general diffusion porins like OmpF [55]. Beyond general diffusion porins, the OM includes specialized channels and receptors for the uptake of specific substrates, proteins involved in OM biogenesis, and enzymes such as the OmpT protease in E. coli [56]. These components are crucial for various cellular functions, including LPS assembly and the formation of surface appendages like pili and flagella.
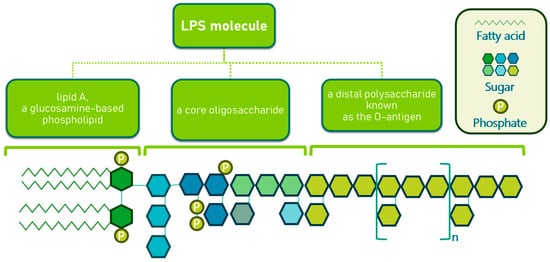
Figure 4.
Structure of lipopolysaccharides in Gram-negative bacteria.
Hydrophobic antibiotics, including aminoglycosides, macrolides, and cationic peptides, can permeate the OM lipid bilayer, with the core region of LPS playing a crucial role in providing a barrier [49]. The anionic groups in the LPS structure bind divalent cations, stabilizing the membrane by compensating for electrostatic repulsion between LPS molecules. The lipid-mediated pathway is particularly significant for the resistance of Gram-negative bacteria to hydrophobic antibiotics [49]. Strains expressing full-length LPS exhibit intrinsic resistance to these antibiotics. However, the application of membrane permeabilizers, such as Tris/EDTA or polymyxin B, can dramatically increase the sensitivity of bacteria like E. coli and Salmonella typhimurium to hydrophobic antibiotics, bringing their sensitivity levels closer to those of deep rough mutants, which have truncated LPS and are more susceptible to lipophilic compounds [46].
Polymyxin B and its nonapeptide derivative, PMBN, disrupt the LPS layer by competing with divalent cations for binding sites, leading to increased lateral diffusion of LPS and destabilization of the OM [57]. This process facilitates the penetration of polymyxin B into the periplasm, where it further permeabilizes the inner membrane, exerting its antibacterial effect. PMBN, though less bactericidal, still enhances OM permeability, demonstrating its potential as an adjuvant in antibiotic therapy.
Targeting the membrane represents a promising strategy for addressing dormant infections, but the discovery and evaluation of membrane-active agents come with several significant challenges and opportunities that need careful consideration. Below, Table 1 has gathered a summary of these key points.

Table 1.
Challenges and opportunities for membrane-active agents.
5. Antimicrobial Effects of Plant Secondary Metabolites (PSMs)
Historically, plants have been used as medicinal remedies for various ailments. The rising challenge of antibiotic-resistant microorganisms has steered researchers towards plants in search of new antimicrobial agents. Numerous plants produce secondary metabolites as a defense mechanism against microbial and pest attacks, with examples gathered in Table 2.

Table 2.
Examples of plant secondary metabolites and their antimicrobial action.
The structural and chemical complexity of natural products significantly distinguishes them from synthetic drugs. Natural compounds generally contain less nitrogen, sulfur, phosphorus, and halogens, but they exhibit greater molecular diversity, including varied ring systems, carbohydrate content, and stereochemical configurations. These unique properties allow plant products to modulate protein–protein interactions effectively, making them potent agents in immune response regulation, mitosis, apoptosis, and signal transduction [72]. Moreover, the complexity and multiplicity of chemical components in plant extracts make it difficult for bacteria to develop resistance compared to single-compound synthetic drugs [73,74].
6. Combating Resistance Through Synergism Among Phytoconstituents
Traditionally, research on medicinal plants has focused on isolating single active compounds responsible for therapeutic effects. However, this approach can lead to a reduction in the efficacy of the extract, as isolation may lose the synergy between different constituents. The enhanced activity of plant extracts is often due to the complex interplay of secondary metabolites, which may function in defense and cell signaling, thereby increasing the overall biological activity of the plant [75]. The combined action of various compounds within a single extract targets multiple sites within pathogens, including receptors, enzymes, ion channels, and transport proteins. This multitargeted approach slows the development of bacterial resistance compared to treatments involving a single active compound.
Combining plant compounds with conventional antibiotics can enhance the latter’s effectiveness and reduce side effects. Understanding the synergistic interactions between various phytochemicals can further promote the use of medicinal plants, whether used alone, in combination with each other, or alongside antibiotics. For instance, Shibata et al. (2005) found that ethyl gallate increased the susceptibility of Staphylococcus aureus strains to β-lactam antibiotics [76]. This synergy was specific to β-lactams and did not affect other antibiotic classes tested.
Another study highlighted the synergistic effect of 5-methoxyhydnocarpin, a compound from chaulmoogra oil, which enhances the antibacterial activity of berberine against Staphylococcus aureus [77]. Although 5-methoxyhydnocarpin itself lacked antimicrobial activity, it significantly increased berberine accumulation within bacterial cells by inhibiting the bacteria’s multidrug resistance pumps, illustrating how weak antimicrobial agents can be combined with other compounds to potentiate their activity.
7. Combining Traditional and Modern Medicine—Bioenhancers
In the face of growing antibiotic resistance, the development of new, effective antibiotics has become increasingly challenging. Current global drug development efforts may not yield new antibiotics within the next decade, despite advances in pharmacology and chemistry aimed at modifying existing antibiotics or identifying new enzyme targets [78]. Given the rising resistance to conventional antibiotics, it is logical to explore combination therapies that pair standard antibiotics with plant extracts known for their bioenhancing properties, aiming to achieve bactericidal synergy. Such combination therapies could offer novel treatment options for infectious diseases by expanding the antimicrobial spectrum, preventing the emergence of resistant strains, and reducing toxicity [79] (Table 3).

Table 3.
Mechanisms of bioenhancer-mediated synergy with antibiotics.
Bioenhancers can function through various mechanisms: enhancing drug absorption, modulating drug biotransformation in the liver or intestines, influencing active transport, decreasing drug elimination, or exerting immunomodulatory effects (Figure 5) [58].
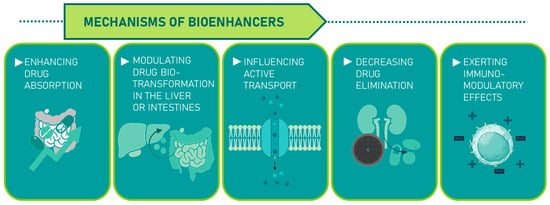
Figure 5.
Plant-derived bioenhancers and their mechanisms.
Traditional Chinese Medicine (TCM) is one of the oldest and most popular practices to this day, which is based on the use of bioactive compounds of plant origin, including in particular extracts containing numerous bio-compounds classified as plant secondary metabolites bioenhancers. Nowadays, the increasing popularity of TCM raises significant concerns regarding its safety, regulation, efficacy, and mode of action. In some instances, the use of TCM has been associated with serious adverse effects, including nephropathy and hepatitis [84,85]. Variations in the chemical composition of different brands of the same herb, often due to inadequate processing or adulteration with cheaper substitutes, have been linked to cases of poisoning. Additionally, contamination with heavy metals further exacerbates safety concerns.
TCM is deeply rooted in medical theory, and discarding this traditional knowledge while using the drugs can lead to severe consequences. It is important for individuals using dietary supplements, including Chinese herbal medicines, to have a deeper understanding of the underlying medical theories and the rationale for their use. A notable example is the misuse of the ancient formula xiao-chai-hu-tang in Japan, originally intended for treating febrile diseases in the Shaoyang meridian (imbalances in the body’s energy pathways) but later widely prescribed for long-term hepatitis treatment. This misuse led to severe adverse effects, including fatalities [84,85].
The historical success of TCM suggests its potential to broaden healthcare options, whether through single-chemical entities or complex botanical drugs. As the global population ages and chronic and degenerative diseases become more prevalent, TCM and complementary and alternative medicine (CAM) are likely to gain further acceptance. With advances in systems biology, pharmacogenomics, synergistic medicine, and personalized medicine, there is hope for the eventual integration of TCM and Western medicine, combining the strengths of both traditions to benefit patients.
8. Saponins
Saponins are a class of secondary metabolites produced by certain plants, some insects and marine organisms. The term “saponin” originates from the Latin word “sapo,” meaning soap, which reflects their ability to form a stable, soap-like foam when an aqueous solution of saponins is vigorously shaken [86]. Saponins can be extracted from various parts of a plant, including roots, leaves, fruits, pericarps, flowers, and seeds. The composition and concentration of saponins can vary not only between different plants but also among different parts of the same plant [87,88]. These variations in saponin content are influenced by environmental factors during the plant’s development, as well as the methods used for extraction. Saponins are naturally occurring amphiphilic glycosides, characterized by their structure, which includes polar glycone components (sugar moieties) and nonpolar aglycone components (sapogenins) [87,88]. These compounds are categorized based on the type of aglycone they contain into two main groups: steroidal saponins and triterpenoid saponins as shown in Figure 6. Saponins are also categorized based on the number of sugar units attached to the aglycone (Figure 7). The sugar chains attached to the aglycone can be either branched or linear, typically comprising D-glucose, D-galactose, L-arabinose, L-rhamnose, D-xylose, D-fucose, and glucuronic acid [89].
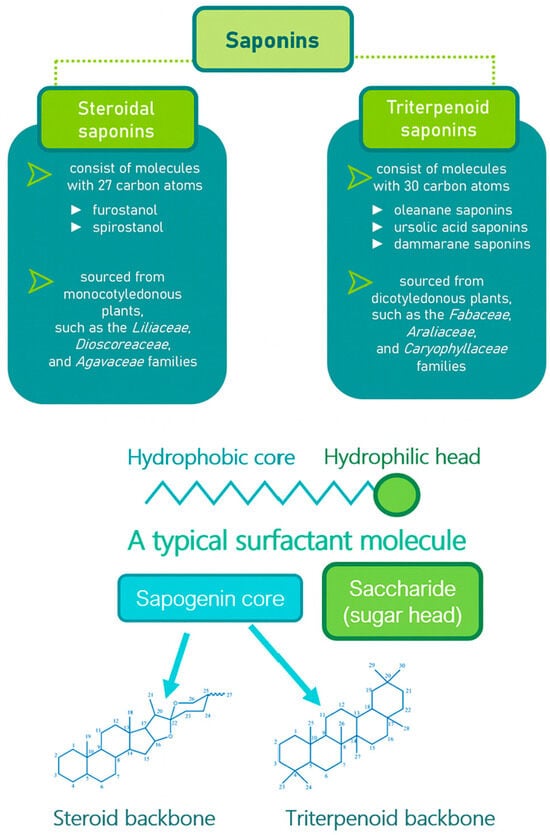
Figure 6.
Classification of saponins by aglycone structure.
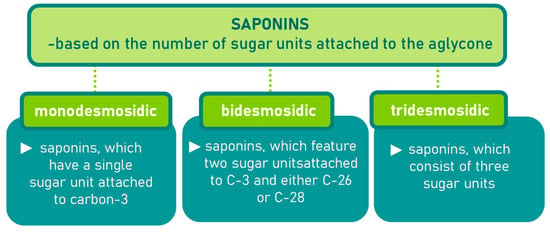
Figure 7.
Classification of saponins by sugar unit attachment.
Due to the combination of lipophilic aglycones and hydrophilic sugar chains, saponins function as natural surfactants, making them potential substitutes for chemical surfactants. Beyond this, saponins have a wide range of applications, including in the pharmaceutical, food, and cosmetic industries [87] (Figure 8).
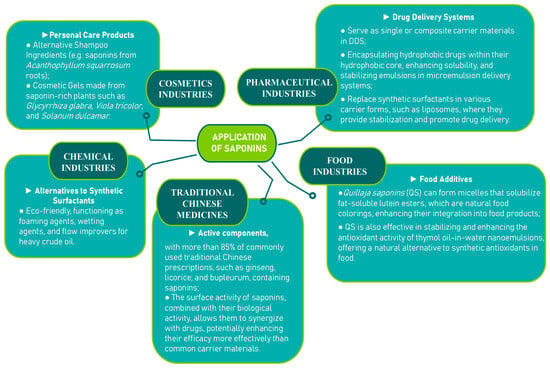
Figure 8.
Application of saponins [90,91,92,93,94,95,96].
They exhibit numerous bioactivities, such as expectorant, anti-inflammatory, vasoprotective, hypocholesterolemic, immunomodulatory, hypoglycemic, antifungal, and antiparasitic effects [97,98,99,100,101,102] (Table 4).

Table 4.
The bioactivity of saponins.
Plants rich in saponins, like Panax ginseng and Glycyrrhiza glabra, have been used medicinally for centuries, particularly in Asian cultures [102]. In modern times, saponins are also employed as adjuvants in vaccine production [114,115,116]. In addition, steroidal sapogenins derived from saponins are important raw materials for the pharmaceutical industry, especially in the synthesis of steroidal hormones (Suresh et al., 2021 [117]). Recent research has highlighted the role of saponins as chemopreventive and antitumor agents across various models. Notable examples include the chemopreventive effects of ginsenosides and diosgenins, as well as the anticancer properties of saikosaponins and glycyrrhizins [118,119,120,121]. Saponins inhibit tumor growth through various mechanisms, such as inducing apoptosis and autophagy, causing mitotic arrest, reducing nitric oxide (NO) production, suppressing MMP-2 and MMP-9, and activating caspase 2 [122,123,124]. Among saponins, cycloartanes, dammaranes, oleananes, spirostanes, and furostanes have been identified as particularly potent anticancer agents [125]. Saponins serve as well with their multiple functions within Drug Delivery Systems (DDS), including traditional roles like enhancing bioavailability, minimizing side effects, enabling controlled release, and improving targeting [126,127,128]. Moreover, due to their inherent biological activity, saponins can act synergistically with drugs [99,127,129,130] (Table 5).

Table 5.
Functions of saponins in Drug Delivery Systems (DDS).
In summary, saponins are promising natural surfactants in DDS, offering benefits such as improved bioavailability, reduced side effects, enhanced targeting, and synergistic effects with drugs, particularly in cancer therapy. Their ability to replace traditional carrier materials like cholesterol further highlights their versatility and potential in pharmaceutical applications.
9. Challenges in Bioavailability of Saponins
One of the primary obstacles in the development of saponins as therapeutic agents is their low bioavailability. Despite the identification of over 20,000 naturally occurring saponins and more than 4000 sapogenins, their clinical potential remains limited [145]. A recent analysis highlights a trend of more than 500 new saponins being discovered annually [146,147,148]. The first report of saponins exhibiting antitumor activity dates back to 1960, involving an extract from sea cucumber [149]. Since then, around 500 studies have explored the chemopreventive or anticancer properties of saponins. However, the majority of these investigations were conducted in vitro, with only about 10% involving in vivo models, typically in mice. The poor bioavailability of saponins has often precluded their progression to human clinical trials, particularly as oral agents [150,151,152,153]. For instance, ginsenoside Rh2, a promising chemopreventive compound, has shown bioavailability rates ranging from 1% to 24.8% in animal models such as mice and dogs [153,154]. Nonetheless, in China, ginsenosides have been successfully utilized as adjuvant therapies, with some even gaining approval as pharmaceutical drugs. The low oral bioavailability of saponins presents significant challenges in drug development, particularly for human clinical trials (Figure 9) [155]. Therefore, enhancing the bioavailability of saponins is crucial, as pharmaceutical companies typically refrain from developing drugs with oral bioavailability below 30%. Given this, our review delves into the mechanisms underlying the absorption and distribution of saponins both in vitro and in vivo.
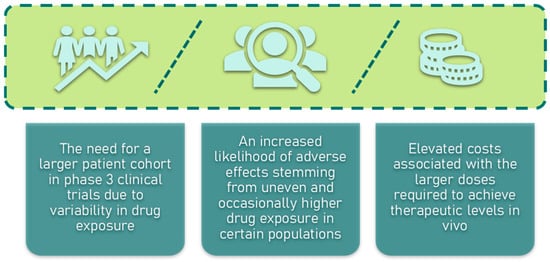
Figure 9.
Challenges in drug development of low oral bioavailability of saponins.
It is noteworthy that various sapogenins, which are saponin precursors, have demonstrated better bioavailability and bioactivity than their saponin counterparts [156]. This improvement is due to more favorable chemical properties, such as the absence of sugar chains. However, the relatively poor solubility of sapogenins compared to saponins is a limiting factor that also impedes a significant increase in bioavailability. Despite these challenges, there remains a scarcity of comprehensive data on the digestion, bioaccessibility, and bioavailability of saponins and sapogenins. Given the chemical diversity and complexity of saponins, it is difficult to generalize their bioavailability profiles. This underscores the need for further research to establish safe and effective oral dosing regimens.
In addition to the role of gut microbiota in modulating the bioactivity of dietary saponins, it is important to consider the impact of saponins on the microbiota itself and the subsequent health outcomes. Although current data on the relationship between saponins, microbiota, and health are limited, emerging research suggests that saponins may act as “prebiotic-like” compounds, similar to polyphenols [156]. This hypothesis is supported by recent findings, and a patent “Use of herbal saponins to regulate gut microflora” has even been filed for the use of herbal saponins to regulate gut microflora [157]. The invention proposes that plant-based saponins can promote anticancer and anti-inflammatory effects by balancing gut microbiota and maintaining a healthy epithelial environment in the gut.
The bioavailability of saponins is primarily influenced by two factors: the physicochemical properties of the saponin itself (such as molecular weight, hydrogen bond donors and acceptors, solubility, and chemical stability) and the biological barriers that hinder its absorption into the systemic circulation. These barriers include drug transport (efflux and uptake), metabolism by gut microflora, and first-pass metabolism in the intestine and liver; strategies to enhance the bioavailability of saponins are presented in Table 6.

Table 6.
Strategies to enhance bioavailability.
10. Summary
The integration of saponins as bio-enhancers in antibiotic therapies represents a significant advance in the field of drug delivery, particularly within the context of the ongoing debate over the use of herbal excipients from complementary and alternative medicine (CAM). This scientific review has explored the multifaceted roles of saponins, particularly their ability to enhance the efficacy of antibiotics, and has addressed the challenges and controversies surrounding their use.
Saponins, as natural surfactants, have demonstrated considerable potential in enhancing the bioavailability and potency of antibiotics. Their amphiphilic nature allows them to interact with cellular membranes, potentially improving drug penetration and reducing the necessary dosage of antibiotics. This interaction is crucial in the context of antibiotic resistance, where saponins could provide a complementary mechanism to restore or enhance the activity of existing drugs. Moreover, their ability to disrupt bacterial membranes offers a promising approach to combat persistent and dormant infections, which are often resistant to conventional treatments. By enabling lower antibiotic doses, such combinatory therapies may also reduce pharmaceutical residues in the environment, contributing to the mitigation of selective pressure that drives the evolution of antimicrobial resistance (AMR) in natural ecosystems.
However, the application of saponins is not without its challenges. One of the primary concerns is their hemolytic toxicity, which raises significant safety issues when considering their use as drug carriers. The hemolytic activity of saponins, particularly at higher concentrations, necessitates careful dose optimization and possibly the development of less toxic alternatives.
Another critical challenge is the variability in saponin solubilization properties, which can either enhance or inhibit the solubility of different drugs. This variability underscores the need for a tailored approach to saponin selection and formulation, ensuring that the chosen saponin not only enhances the target drug’s bioavailability but also maintains consistent and predictable pharmacokinetic properties.
Furthermore, the practical application of saponins in drug delivery systems (DDS) remains largely at the experimental stage. The high cost of extraction and purification, along with the sustainability concerns due to the plant-based origin of most saponins, limits their widespread adoption. The example of the Quillaja tree, where only a small fraction of the plant is utilized, highlights the inefficiency and environmental impact of current extraction methods. Developing environmentally sustainable sourcing and processing strategies, including cultivation of medicinal plants and green extraction technologies, will be essential to ensure the ecological viability of these excipients in future pharmaceutical systems.
Despite these obstacles, the potential benefits of combining saponins with antibiotics are significant. The ability of saponins to act as bio-enhancers aligns with the broader trend of integrating CAM with modern pharmacotherapy, offering a complementary strategy to traditional antibiotic treatments. This approach not only has the potential to improve drug efficacy but also to reduce the development of antibiotic resistance by lowering the required dosage and enhancing the drug’s mechanism of action. Importantly, such strategies may also align with global efforts to reduce the environmental burden of pharmaceuticals and limit the spread of resistance-conferring genes across microbial communities.
11. Conclusions
The promise of saponins as antibiotic bioenhancers is clear, yet realizing this potential in clinical practice will require close attention to safety, manufacturing variability, and application pathways. The ongoing research into less-toxic saponin variants, improved extraction methods, and optimized drug formulations will be crucial in overcoming these challenges. Given their dual potential to enhance human therapeutic outcomes and reduce environmental risk factors associated with antibiotic misuse, saponins represent a promising and sustainable innovation in future antimicrobial strategies. As the field progresses, the combination of saponins with antibiotics could become a powerful tool in the fight against resistant infections, bridging the gap between CAM and conventional medicine and paving the way for more effective and sustainable therapeutic strategies.
Author Contributions
Conceptualization, A.M.; software, A.M.; validation, A.M., W.S. and E.K.; formal analysis, A.M.; investigation, A.M.; resources, E.K.; data curation, A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.M., W.S. and E.K.; visualization, A.M.; supervision, W.S. and E.K.; project administration, A.M., W.S. and E.K.; funding acquisition, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center, Poland, grant number 2020/39/B/NZ9/03196.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study, data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TB | Tuberculosis |
| HIV | Human Immunodeficiency Virus |
| ATP | Adenosine Triphosphate |
| US CDC | United States Centers for Disease Control |
| OM | Outer Membrane |
| OmpA | Outer membrane protein A |
| OmpF | Outer membrane protein F |
| OmpT | Outer membrane protein T |
| LPS | Lipopolysacharides |
| Lpp | Lipoprotein |
| PMBN | Polymyxin B Nonapeptide |
| MMP-2 | Metalloproteinase 2 |
| MMP-9 | Metalloproteinase 9 |
| TCM | Traditional Chinese Medicine |
| CAM | Complementary and Alternative Medicine |
| DDS | Drug Delivery System |
| GA | Glycyrhhizic Acid |
| PSMs | Plant Secondary Metabolites |
| MIC | Minimum Inhibitory Concentration |
| LD50 | Median lethal dose/lethal dose, 50% |
| QS | Quillaja Saponins |
References
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic Discovery: History, Methods and Perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Ghosh, S.; Mukherjee, G.; Acharya Chowdhury, A. Secondary Metabolites: Treasure Trove for Future Medicine. In Plant Specialized Metabolites; Springer: Cham, Switzerland, 2023; pp. 1–45. [Google Scholar]
- Upmanyu, N.; Malviya, V.N. Antibiotics: Mechanisms of Action and Modern Challenges. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 367–382. [Google Scholar]
- Coates, A.R.M.; Hu, Y. Targeting Non-Multiplying Organisms as a Way to Develop Novel Antimicrobials. Trends Pharmacol. Sci. 2008, 29, 143–150. [Google Scholar] [CrossRef]
- Hu, Y.; Coates, A.R.; Mitchison, D.A. Comparison of the Sterilising Activities of the Nitroimidazopyran PA-824 and Moxifloxacin against Persisting Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2008, 12, 69–73. [Google Scholar]
- Lenaerts, A.J.; Gruppo, V.; Marietta, K.S.; Johnson, C.M.; Driscoll, D.K.; Tompkins, N.M.; Rose, J.D.; Reynolds, R.C.; Orme, I.M. Preclinical Testing of the Nitroimidazopyran PA-824 for Activity against Mycobacterium tuberculosis in a Series of In Vitro and In Vivo Models. Antimicrob. Agents Chemother. 2005, 49, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Poppens, P.T. Impact of Biofilm on the in Vitro Activity of Vancomycin Alone and in Combination with Tigecycline and Rifampicin against Staphylococcus aureus. J. Antimicrob. Chemoth 2009, 63, 485–488. [Google Scholar] [CrossRef]
- Roveta, S.; Schito, A.M.; Marchese, A.; Schito, G.C. Activity of Moxifloxacin on Biofilms Produced in Vitro by Bacterial Pathogens Involved in Acute Exacerbations of Chronic Bronchitis. Int. J. Antimicrob. Agents 2007, 30, 415–421. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Yusuf Aliyu, A.; Adeleke, O.A. Latest Progress on Tuberculosis and HIV Co-Infection: A Closer Look at People of Different Ages. Adv. Ther. 2025, 8, 2400033. [Google Scholar] [CrossRef]
- Lelisho, M.E.; Wotale, T.W.; Tareke, S.A.; Alemu, B.D.; Hassen, S.S.; Yemane, D.M.; Korsa, B.B.; Bedaso, N.G. Survival Rate and Predictors of Mortality among TB/HIV Co-Infected Adult Patients: Retrospective Cohort Study. Sci. Rep. 2022, 12, 18360. [Google Scholar] [CrossRef]
- Ledesma, J.R.; Ma, J.; Zhang, M.; Basting, A.V.L.; Chu, H.T.; Vongpradith, A.; Novotney, A.; LeGrand, K.E.; Xu, Y.Y.; Dai, X.; et al. Global, Regional, and National Age-Specific Progress towards the 2020 Milestones of the WHO End TB Strategy: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 698–725. [Google Scholar] [CrossRef]
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The Spectrum of Latent Tuberculosis: Rethinking the Biology and Intervention Strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef]
- Schobert, M.; Tielen, P. Contribution of Oxygen-Limiting Conditions to Persistent Infection of Pseudomonas aeruginosa. Future Microbiol. 2010, 5, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Cuppoletti, J.; Trapnell, B.; Lymar, S.V.; Rowe, J.J.; Sun Yoon, S.; Hilliard, G.M.; Parvatiyar, K.; Kamani, M.C.; Wozniak, D.J.; et al. Anaerobic Metabolism and Quorum Sensing by Pseudomonas aeruginosa Biofilms in Chronically Infected Cystic Fibrosis Airways: Rethinking Antibiotic Treatment Strategies and Drug Targets. Adv. Drug Deliv. Rev. 2002, 54, 1425–1443. [Google Scholar] [CrossRef] [PubMed]
- Bavesh, D.K.; Edith; Machowski, E.; Schechter, N.; Teh, J.-S.; Rubin, H.; Mizrahi, V. Mycobacterium: Genomics and Molecular Biology; Parish, T., Brown, A., Eds.; Caister Academic Press: Norfolk, UK, 2010; ISBN 9781912530144. [Google Scholar]
- Somerville, G.A.; Proctor, R.A. At the Crossroads of Bacterial Metabolism and Virulence Factor Synthesis in Staphylococci. Microbiol. Mol. Biol. Rev. 2009, 73, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Pané-Farré, J.; Kohler, C.; Hecker, M.; Engelmann, S. Anaerobic Gene Expression in Staphylococcus aureus. J. Bacteriol. 2007, 189, 4275–4289. [Google Scholar] [CrossRef]
- Bald, D.; Koul, A. Respiratory ATP Synthesis: The New Generation of Mycobacterial Drug Targets? FEMS Microbiol. Lett. 2010, 308, 1–7. [Google Scholar] [CrossRef]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial Persisters: Molecular Mechanisms and Therapeutic Development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial Resistance: A Concise Update. Lancet Microbe 2025, 6, 100947. [Google Scholar] [CrossRef]
- Ferrara, F.; Castagna, T.; Pantolini, B.; Campanardi, M.C.; Roperti, M.; Grotto, A.; Fattori, M.; Dal Maso, L.; Carrara, F.; Zambarbieri, G.; et al. The Challenge of Antimicrobial Resistance (AMR): Current Status and Future Prospects. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 9603–9615. [Google Scholar] [CrossRef]
- Bakkeren, E.; Diard, M.; Hardt, W.-D. Evolutionary Causes and Consequences of Bacterial Antibiotic Persistence. Nat. Rev. Microbiol. 2020, 18, 479–490. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and Guidelines for Research on Antibiotic Persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Bigger, J.W. Treatment of Staphylococcal Infections with Penicillin by Intermittent Sterilisation. Lancet 1944, 244, 497–500. [Google Scholar] [CrossRef]
- Lewis, K. Persister Cells, Dormancy and Infectious Disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Tuomanen, E.; Durack, D.T.; Tomasz, A. Antibiotic Tolerance among Clinical Isolates of Bacteria. Antimicrob. Agents Chemother. 1986, 30, 521–527. [Google Scholar] [CrossRef]
- Levin, B.R.; Rozen, D.E. Non-Inherited Antibiotic Resistance. Nat. Rev. Microbiol. 2006, 4, 556–562. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting Bacterial Membrane Function: An Underexploited Mechanism for Treating Persistent Infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.J. Bacterial Phenotypes Refractory to Antibiotic-Mediated Killing: Mechanisms and Mitigation. In Emerging Trends in Antibacterial Discovery: Answering the Call to Arms; Miller, A.A., Miller, P.F., Eds.; Caister Academic Press: Poole, UK, 2011; pp. 195–210. [Google Scholar]
- Shaku, M.; Ealand, C.; Matlhabe, O.; Lala, R.; Kana, B.D. Peptidoglycan Biosynthesis and Remodeling Revisited. Adv. Appl. Microbiol. 2020, 112, 67–103. [Google Scholar] [PubMed]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical Properties of the Bacterial Outer Membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef]
- Saha, S.; Lach, S.R.; Konovalova, A. Homeostasis of the Gram-Negative Cell Envelope. Curr. Opin. Microbiol. 2021, 61, 99–106. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Transcriptional Regulation in Bacterial Membrane Lipid Synthesis. J. Lipid Res. 2009, 50, S115–S119. [Google Scholar] [CrossRef]
- Chen, E.H.-L.; Wang, C.-H.; Liao, Y.-T.; Chan, F.-Y.; Kanaoka, Y.; Uchihashi, T.; Kato, K.; Lai, L.; Chang, Y.-W.; Ho, M.-C.; et al. Visualizing the Membrane Disruption Action of Antimicrobial Peptides by Cryo-Electron Tomography. Nat. Commun. 2023, 14, 5464. [Google Scholar] [CrossRef]
- Karasiński, M.; Wnorowska, U.; Durnaś, B.; Król, G.; Daniluk, T.; Skłodowski, K.; Głuszek, K.; Piktel, E.; Okła, S.; Bucki, R. Ceragenins and Ceragenin-Based Core-Shell Nanosystems as New Antibacterial Agents against Gram-Negative Rods Causing Nosocomial Infections. Pathogens 2023, 12, 1346. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C. Polymyxins and Bacterial Membranes: A Review of Antibacterial Activity and Mechanisms of Resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Verkleij, A.J.; Zwaal, R.F.A.; Roelofsen, B.; Comfurius, P.; Kastelijn, D.; van Deenen, L.L.M. The Asymmetric Distribution of Phospholipids in the Human Red Cell Membrane. A Combined Study Using Phospholipases and Freeze-Etch Electron Microscopy. Biochim. Biophys. Acta (BBA)-Biomembr. 1973, 323, 178–193. [Google Scholar] [CrossRef]
- Regen, S.L. Membrane-Disrupting Molecules as Therapeutic Agents: A Cautionary Note. JACS Au 2021, 1, 3–7. [Google Scholar] [CrossRef]
- Zhang, Y. Mode of Action of Pyrazinamide: Disruption of Mycobacterium Tuberculosis Membrane Transport and Energetics by Pyrazinoic Acid. J. Antimicrob. Chemoth 2003, 52, 790–795. [Google Scholar] [CrossRef]
- Koul, A.; Vranckx, L.; Dendouga, N.; Balemans, W.; Van den Wyngaert, I.; Vergauwen, K.; Göhlmann, H.W.H.; Willebrords, R.; Poncelet, A.; Guillemont, J.; et al. Diarylquinolines Are Bactericidal for Dormant Mycobacteria as a Result of Disturbed ATP Homeostasis. J. Biol. Chem. 2008, 283, 25273–25280. [Google Scholar] [CrossRef]
- Rao, S.P.S.; Alonso, S.; Rand, L.; Dick, T.; Pethe, K. The Protonmotive Force Is Required for Maintaining ATP Homeostasis and Viability of Hypoxic, Nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2008, 105, 11945–11950. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer Membrane Permeability and Antibiotic Resistance. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- Muñoz, K.A.; Hergenrother, P.J. Facilitating Compound Entry as a Means to Discover Antibiotics for Gram-Negative Bacteria. Acc. Chem. Res. 2021, 54, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Ghai, I.; Ghai, S. Understanding Antibiotic Resistance via Outer Membrane Permeability. Infect. Drug Resist. 2018, 11, 523–530. [Google Scholar] [CrossRef]
- Saxena, D.; Maitra, R.; Bormon, R.; Czekanska, M.; Meiers, J.; Titz, A.; Verma, S.; Chopra, S. Tackling the Outer Membrane: Facilitating Compound Entry into Gram-Negative Bacterial Pathogens. npj Antimicrob. Resist. 2023, 1, 17. [Google Scholar] [CrossRef]
- Matamoros-Recio, A.; Franco-Gonzalez, J.F.; Forgione, R.E.; Torres-Mozas, A.; Silipo, A.; Martín-Santamaría, S. Understanding the Antibacterial Resistance: Computational Explorations in Bacterial Membranes. ACS Omega 2021, 6, 6041–6054. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vello, P.; Di Lorenzo, F.; Zucchetta, D.; Zamyatina, A.; De Castro, C.; Molinaro, A. Lipopolysaccharide Lipid A: A Promising Molecule for New Immunity-Based Therapies and Antibiotics. Pharmacol. Ther. 2022, 230, 107970. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Reeves, P.R. The Variation of O Antigens in Gram-Negative Bacteria. In Endotoxins: Structure, Function and Recognition; Springer: Dordrecht, The Netherlands, 2010; pp. 123–152. [Google Scholar]
- Xiao, X.; Sankaranarayanan, K.; Khosla, C. Biosynthesis and Structure–Activity Relationships of the Lipid a Family of Glycolipids. Curr. Opin. Chem. Biol. 2017, 40, 127–137. [Google Scholar] [CrossRef]
- Ortiz-Suarez, M.L.; Samsudin, F.; Piggot, T.J.; Bond, P.J.; Khalid, S. Full-Length OmpA: Structure, Function, and Membrane Interactions Predicted by Molecular Dynamics Simulations. Biophys. J. 2016, 111, 1692–1702. [Google Scholar] [CrossRef]
- Mathelié-Guinlet, M.; Asmar, A.T.; Collet, J.-F.; Dufrêne, Y.F. Lipoprotein Lpp Regulates the Mechanical Properties of the E. Coli Cell Envelope. Nat. Commun. 2020, 11, 1789. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Guo, Y.; He, Q.-S.; Peng, L.; Wu, S.-C.; Cao, H.; Huang, S.-H. Escherichia Coli Outer Membrane Protease OmpT Confers Resistance to Urinary Cationic Peptides. Microbiol. Immunol. 2010, 54, 452–459. [Google Scholar] [CrossRef]
- Slingerland, C.J.; Martin, N.I. Recent Advances in the Development of Polymyxin Antibiotics: 2010–2023. ACS Infect. Dis. 2024, 10, 1056–1079. [Google Scholar] [CrossRef]
- Thorat, S.S.; Gujar, K.N.; Karale, C.K. Bioenhancers from Mother Nature: An Overview. Futur. J. Pharm. Sci. 2023, 9, 20. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between Hemolytic Activity, Cytotoxicity and Systemic in Vivo Toxicity of Synthetic Antimicrobial Peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Membrane-Active Peptides and Their Potential Biomedical Application. Pharmaceutics 2023, 15, 2091. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.; Zdybicka-Barabas, A.; Pawlikowska-Pawlega, B.; Cytrynska, M.; Wlodarczyk, M.; Grudzinski, W.; Luchowski, R.; Gruszecki, W.I. Modes of the Antibiotic Activity of Amphotericin B against Candida albicans. Sci. Rep. 2019, 9, 17029. [Google Scholar] [CrossRef]
- Melcrová, A.; Klein, C.; Roos, W.H. Membrane-Active Antibiotics Affect Domains in Bacterial Membranes as the First Step of Their Activity. Nano Lett. 2024, 24, 11800–11807. [Google Scholar] [CrossRef]
- Bothra, A.; Arumugam, P.; Panchal, V.; Menon, D.; Srivastava, S.; Shankaran, D.; Nandy, A.; Jaisinghani, N.; Singh, A.; Gokhale, R.S.; et al. Phospholipid Homeostasis, Membrane Tenacity and Survival of Mtb in Lipid Rich Conditions Is Determined by MmpL11 Function. Sci. Rep. 2018, 8, 8317. [Google Scholar] [CrossRef]
- Hu, Y.; Shamaei-Tousi, A.; Liu, Y.; Coates, A. A New Approach for the Discovery of Antibiotics by Targeting Non-Multiplying Bacteria: A Novel Topical Antibiotic for Staphylococcal Infections. PLoS ONE 2010, 5, e11818. [Google Scholar] [CrossRef]
- Ooi, N.; Miller, K.; Randall, C.; Rhys-Williams, W.; Love, W.; Chopra, I. XF-70 and XF-73, Novel Antibacterial Agents Active against Slow-Growing and Non-Dividing Cultures of Staphylococcus aureus Including Biofilms. J. Antimicrob. Chemoth 2010, 65, 72–78. [Google Scholar] [CrossRef]
- Brauner, A.; Shoresh, N.; Fridman, O.; Balaban, N.Q. An Experimental Framework for Quantifying Bacterial Tolerance. Biophys. J. 2017, 112, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.; Agyei, D.; Ali, A. Smart Chitosan Films as Intelligent Food Packaging: An Approach to Monitoring Food Freshness and Biomarkers. Food Packag. Shelf Life 2024, 46, 101370. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Abhary, M.; Al-Hazmi, A.-A. Antibacterial Activity of Miswak (Salvadora persica L.) Extracts on Oral Hygiene. J. Taibah Univ. Sci. 2016, 10, 513–520. [Google Scholar] [CrossRef]
- Shehadi, M.; Awada, F.; Oleik, R.; Chokr, A.; Hamze, K.; Hamdan, H.A.; Harb, A.; Kobaissi, A. Comparative Analysis of the Anti-Bacterial Activity of Four Plant Extracts. Int. J. Curr. Res. Aca Rev. 2014, 8, 83–94. [Google Scholar]
- Rajashekara, S.; Reena, D.; Mainavi, M.V.; Sandhya, L.S.; Baro, U. Biological Isolation and Characterization of Catharanthus roseus (L.) G. Don Methanolic Leaves Extracts and Their Assessment for Antimicrobial, Cytotoxic, and Apoptotic Activities. BMC Complement. Med. Ther. 2022, 22, 328. [Google Scholar] [CrossRef]
- Hooda, P.; Malik, R.; Bhatia, S.; Al-Harrasi, A.; Najmi, A.; Zoghebi, K.; Halawi, M.A.; Makeen, H.A.; Mohan, S. Phytoimmunomodulators: A Review of Natural Modulators for Complex Immune System. Heliyon 2024, 10, e23790. [Google Scholar] [CrossRef]
- Chowdhury, S.K.; Misra, D.; Mandal, V. Medicinal Plant-Derived Antimicrobials’ Fight Against Multidrug-Resistant Pathogens. In Medicinal and Aromatic Plants; Springer International Publishing: Cham, Switzerland, 2021; pp. 391–427. [Google Scholar]
- Keita, K.; Darkoh, C.; Okafor, F. Secondary Plant Metabolites as Potent Drug Candidates against Antimicrobial-Resistant Pathogens. SN Appl. Sci. 2022, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kondo, K.; Katsuyama, R.; Kawazoe, K.; Sato, Y.; Murakami, K.; Takaishi, Y.; Arakaki, N.; Higuti, T. Alkyl Gallates, Intensifiers of β-Lactam Susceptibility in Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 549–555. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a Medicinal Plant: Antimicrobial Action of Berberine Potentiated by 5′-Methoxyhydnocarpin, a Multidrug Pump Inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef]
- Piddock, L.J.V.; Alimi, Y.; Anderson, J.; de Felice, D.; Moore, C.E.; Røttingen, J.-A.; Skinner, H.; Beyer, P. Advancing Global Antibiotic Research, Development and Access. Nat. Med. 2024, 30, 2432–2443. [Google Scholar] [CrossRef]
- Cheesman, M.; Ilanko, A.; Blonk, B.; Cock, I. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [CrossRef]
- Shiota, S.; Shimizu, M.; Sugiyama, J.; Morita, Y.; Mizushima, T.; Tsuchiya, T. Mechanisms of Action of Corilagin and Tellimagrandin I That Remarkably Potentiate the Activity of Β-Lactams against Methicillin-Resistant Staphylococcus aureus. Microbiol. Immunol. 2004, 48, 67–73. [Google Scholar] [CrossRef]
- De Oliveira, S.M.S.; Falcão-Silva, V.S.; Siqueira-Junior, J.P.; Costa, M.J.d.C.; Diniz, M.d.F.F.d.M. Modulation of Drug Resistance in Staphylococcus aureus by Extract of Mango (Mangifera indica L., Anacardiaceae) Peel. Rev. Bras. De. Farmacogn. 2011, 21, 190–193. [Google Scholar] [CrossRef]
- Purushotham, K.G.; Arun, P.; Johnsy Jayarani, J.; Vasnthakumari, R.; Sankar, L.; Reddy, B. Synergistic in Vitro Antibacterial Activity of Tectona Grandis Leaves with Tetracycline. Int. J. Pharmtech Res. 2010, 2, 519–523. [Google Scholar]
- Li, Z.; Zhu, B.; Chen, W.; Hu, J.; Xue, Y.; Yin, H.; Hu, X.; Liu, W. Pseudolaric Acid A: A Promising Antifungal Agent Against Prevalent Non-Albicans Candida Species. Infect. Drug Resist. 2023, 16, 5953–5964. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M. Perspective: Herbal Dangers. Nature 2011, 480, S97. [Google Scholar] [CrossRef]
- Itoh, S.; Marutani, K.; Nishijima, T.; Matsuo, S.; Itabashi, M. Liver Injuries Induced by Herbal Medicine, Syo-Saiko-to (Xiao-Chai-Hu-Tang). Dig. Dis. Sci. 1995, 40, 1845–1848. [Google Scholar] [CrossRef]
- Jolly, A.; Hour, Y.; Lee, Y.-C. An Outlook on the Versatility of Plant Saponins: A Review. Fitoterapia 2024, 174, 105858. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Mishra, V.; Gupta, S.; Sharma, S.; Vaishnav, A.; Singh, S.V. Industrial and Environmental Applications of Plant-Derived Saponins: An Overview and Future Prospective. J. Plant Growth Regul. 2024, 43, 3012–3026. [Google Scholar] [CrossRef]
- Oleszek, M.; Oleszek, W. Saponins in Food. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2020; pp. 1–40. [Google Scholar]
- Akbari, S.; Abdurahman, N.H.; Kudrashou, V. Surface Activity and Emulsification Properties of Saponins as Biosurfactants. In Advancements in Biosurfactants Research; Springer International Publishing: Cham, Switzerland, 2023; pp. 137–153. [Google Scholar]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yang, J.; Lv, L.; Dai, X.; Shi, X. Saponin Surfactants Used in Drug Delivery Systems: A New Application for Natural Medicine Components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Ortiz, Y.; Arturo, D.; Navarro, Y.; Chejne, F. Saponins: Natural Surfactants and Their Alternative Sustainability in the Formulation of Bio-Based Detergents to Mitigate Environmental Pollution. Waste Biomass Valorization 2024, 15, 5965–5982. [Google Scholar] [CrossRef]
- Tippel, J.; Lehmann, M.; von Klitzing, R.; Drusch, S. Interfacial Properties of Quillaja Saponins and Its Use for Micellisation of Lutein Esters. Food Chem. 2016, 212, 35–42. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Van Camp, J.; Dewettinck, K.; Van der Meeren, P. Production of Thymol Nanoemulsions Stabilized Using Quillaja Saponin as a Biosurfactant: Antioxidant Activity Enhancement. Food Chem. 2019, 293, 134–143. [Google Scholar] [CrossRef]
- Aghel, N.; Moghimipour, E.; Dana, A.R. Formulation of a Herbal Shampoo Using Total Saponins of Acanthophyllum squarrosum. Iran. J. Pharm. Res. 2007, 6, 167–172. [Google Scholar]
- Moghimipour, E.; Jasemnezhad, M.; Mohammad Soleymani, S.; Salimi, A. Preparation and Evaluation of a Free Surfactant Herbal Shampoo with Acanthophyllum squarrosum Saponins. J. Cosmet. Dermatol. 2021, 20, 181–187. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Bujak, T. Saponins as Natural Raw Materials for Increasing the Safety of Bodywash Cosmetic Use. J. Surfactants Deterg. 2018, 21, 767–776. [Google Scholar] [CrossRef]
- Sharma, K.; Kaur, R.; Kumar, S.; Saini, R.K.; Sharma, S.; Pawde, S.V.; Kumar, V. Saponins: A Concise Review on Food Related Aspects, Applications and Health Implications. Food Chem. Adv. 2023, 2, 100191. [Google Scholar] [CrossRef]
- Aqib, A.I.; Atta, K.; Muneer, A.; Arslan, M.; Shafeeq, M.; Rahim, K. Saponin and Its Derivatives (Glycyrrhizin) and SARS-CoV-2. In Application of Natural Products in SARS-CoV-2; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–46. [Google Scholar]
- Wijesekara, T.; Luo, J.; Xu, B. Critical Review on Anti-inflammation Effects of Saponins and Their Molecular Mechanisms. Phytother. Res. 2024, 38, 2007–2022. [Google Scholar] [CrossRef]
- Zhang, W.; Li, F.; Cheng, J.; Wang, Y.; Zheng, Y.; Li, H.; Lin, M.; Ruan, J.; Zhang, Y.; Wang, T. Saponins from Dolichos Lablab Seeds with Anti-Inflammatory Activity. Bioorg Chem. 2024, 151, 107692. [Google Scholar] [CrossRef]
- Panda, S.P.; Soren, D.; Roy, P.; Mohanty, D.; Meena, D.K.; Sahoo, A.K.; Das, B.K. Immunomodulatory Effect of Saponin Treatment and Microbial Infections Provoke the Expression of Mx Gene in Catla (Labeo catla). Aquac. Int. 2024, 32, 2203–2221. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J.; Nikabadi, S.; Varma, A. Importance of Medicinal and Aromatic Plants in Human Life. In Medicinal Plants and Environmental Challenges; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar]
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial Activity and Mechanism of Action Saponins from Chenopodium quinoa Willd. Husks against Foodborne Pathogenic Bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Alviano, D.S.; Barreto, D.W.; Coelho, M.A.Z. Functional Properties of Saponins from Sisal (Agave sisalana) and Juá (Ziziphus joazeiro): Critical Micellar Concentration, Antioxidant and Antimicrobial Activities. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 736–743. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Li, J.; Liu, J.; Zhao, Y.; Zhang, P.; Zhang, M.-X.; Zhang, L. Saponins Isolated from the Root of Panax notoginseng Showed Significant Anti-Diabetic Effects in KK-Ay Mice. Am. J. Chin. Med. 2008, 36, 939–951. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Zhao, Y.; Shen, L.; Jiang, X.; Xie, Z.; Liang, N.; Zhang, L.; Chen, Z. Anti-Diabetic Effects of Panax Notoginseng Saponins and Its Major Anti-Hyperglycemic Components. J. Ethnopharmacol. 2010, 130, 231–236. [Google Scholar] [CrossRef]
- Zheng, T.; Shu, G.; Yang, Z.; Mo, S.; Zhao, Y.; Mei, Z. Antidiabetic Effect of Total Saponins from Entada phaseoloides (L.) Merr. in Type 2 Diabetic Rats. J. Ethnopharmacol. 2012, 139, 814–821. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Feng, T.; Chen, Y.; Yang, G. Hypoglycemic and Hypolipidemic Effects of Total Saponins from Stauntonia chinensis in Diabetic Db/Db Mice. J. Cell Mol. Med. 2018, 22, 6026–6038. [Google Scholar] [CrossRef]
- SUN, H. Haemolytic Activities and Adjuvant Effect of Bupleurum Chinese Saponins on the Immune Responses to Ovalbumin in Mice. Vaccine 2006, 24, 1324–1331. [Google Scholar] [CrossRef]
- Fleck, J.D.; Kauffmann, C.; Spilki, F.; Lencina, C.L.; Roehe, P.M.; Gosmann, G. Adjuvant Activity of Quillaja brasiliensis Saponins on the Immune Responses to Bovine Herpesvirus Type 1 in Mice. Vaccine 2006, 24, 7129–7134. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Liu, Q.; Meng, H.; Zhao, D. Haemolytic Activity and Adjuvant Effect of Soyasaponins and Some of Their Derivatives on the Immune Responses to Ovalbumin in Mice. Int. Immunopharmacol. 2014, 18, 333–339. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, H.; Li, M.; Li, Y.; Guan, S.; Liu, J. Immunological Adjuvant Effect of Japanese Ginseng Saponins (JGS) on Specific Antibody and Cellular Response to Ovalbumin and Its Haemolytic Activities. Vaccine 2008, 26, 5911–5917. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.; Mal, G.; Varshney, R. A Comparative Analysis of Saponin-Enriched Fraction from Silene vulgaris (Moench) Garcke, Sapindus mukorossi (Gaertn) and Chlorophytum borivilianum (Santapau and Fernandes): An in Vitro Hemolytic and Cytotoxicity Evaluation. Anim. Biotechnol. 2022, 33, 193–199. [Google Scholar] [CrossRef]
- Reed, J.; Orme, A.; El-Demerdash, A.; Owen, C.; Martin, L.B.B.; Misra, R.C.; Kikuchi, S.; Rejzek, M.; Martin, A.C.; Harkess, A.; et al. Elucidation of the Pathway for Biosynthesis of Saponin Adjuvants from the Soapbark Tree. Science 2023, 379, 1252–1264. [Google Scholar] [CrossRef]
- Ou, B.S.; Baillet, J.; Filsinger Interrante, M.V.; Adamska, J.Z.; Zhou, X.; Saouaf, O.M.; Yan, J.; Klich, J.H.; Jons, C.K.; Meany, E.L.; et al. Saponin Nanoparticle Adjuvants Incorporating Toll-like Receptor Agonists Drive Distinct Immune Signatures and Potent Vaccine Responses. Sci. Adv. 2024, 10, eadn7187. [Google Scholar] [CrossRef]
- Stertman, L.; Palm, A.-K.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-MTM Adjuvant: A Critical Component of Vaccines for the 21st Century. Hum. Vaccin. Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef]
- Suresh, P.S.; Bhatt, V.; Singh, P.P.; Sharma, U. Steroidal Sapogenins from Genus Trillium: Chemistry, Synthesis, and Opportunities in Neuro-Active Steroids Designing. Stud. Nat. Prod. Chem. 2021, 68, 67–95. [Google Scholar]
- Han, Y.; Sheng, W.; Liu, X.; Liu, H.; Jia, X.; Li, H.; Wang, C.; Wang, B.; Hu, T.; Ma, Y. Glycyrrhizin Ameliorates Colorectal Cancer Progression by Regulating NHEJ Pathway through Inhibiting HMGB1-Induced DNA Damage Response. Sci. Rep. 2024, 14, 24948. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Gao, C. Saikosaponins Targeting Programmed Cell Death as Anticancer Agents: Mechanisms and Future Perspectives. Drug Des. Devel Ther. 2024, 18, 3697–3714. [Google Scholar] [PubMed]
- Yang, Y.; Nan, Y.; Du, Y.; Liu, W.; Ning, N.; Chen, G.; Gu, Q.; Yuan, L. Ginsenosides in Cancer: Proliferation, Metastasis, and Drug Resistance. Biomed. Pharmacother. 2024, 177, 117049. [Google Scholar] [CrossRef]
- Raju, J.; Mehta, R. Cancer Chemopreventive and Therapeutic Effects of Diosgenin, a Food Saponin. Nutr. Cancer 2009, 61, 27–35. [Google Scholar] [CrossRef]
- Zhou, Y.; Farooqi, A.A.; Xu, B. Comprehensive Review on Signaling Pathways of Dietary Saponins in Cancer Cells Suppression. Crit. Rev. Food Sci. Nutr. 2023, 63, 4325–4350. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Li, X.; Dai, Y.; Zhao, C.; Man, S.; Wang, Y.; Gao, W. Dioscin-6′-O-Acetate Impairs Migration of Lung Cancer Cells through Attenuations of MMP-2 and MMP-9 via NF-ΚB Suppression. Med. Chem. Res. 2019, 28, 1–12. [Google Scholar] [CrossRef]
- Ma, B.; Zhu, J.; Zhao, A.; Zhang, J.; Wang, Y.; Zhang, H.; Zhang, L.; Zhang, Q. Raddeanin A, a Natural Triterpenoid Saponin Compound, Exerts Anticancer Effect on Human Osteosarcoma via the ROS/JNK and NF-ΚB Signal Pathway. Toxicol. Appl. Pharmacol. 2018, 353, 87–101. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical Study and Medical Application of Saponins as Anti-Cancer Agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Luo, M.; Jiang, Q.; Liu, S.; Jia, Q.; Bai, Z.; Wu, F.; Xie, J. Advancements and Challenges in Pharmacokinetic and Pharmacodynamic Research on the Traditional Chinese Medicine Saponins: A Comprehensive Review. Front. Pharmacol. 2024, 15, 1393409. [Google Scholar] [CrossRef]
- Parama, D.; Boruah, M.; Yachna, K.; Rana, V.; Banik, K.; Harsha, C.; Thakur, K.K.; Dutta, U.; Arya, A.; Mao, X.; et al. Diosgenin, a Steroidal Saponin, and Its Analogs: Effective Therapies against Different Chronic Diseases. Life Sci. 2020, 260, 118182. [Google Scholar] [CrossRef] [PubMed]
- Nazemoroaya, Z.; Sarafbidabad, M.; Mahdieh, A.; Zeini, D.; Nyström, B. Use of Saponinosomes from Ziziphus Spina-Christi as Anticancer Drug Carriers. ACS Omega 2022, 7, 28421–28433. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Dai, X.; Yang, C.; Zhang, Y.; Tian, Y.; Qu, Q.; Sheng, M.; Li, Z.; Peng, X.; Cen, S.; et al. Unification of Medicines and Excipients: The Roles of Natural Excipients for Promoting Drug Delivery. Expert. Opin. Drug Deliv. 2023, 20, 597–620. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, C. Herb–Drug Interactions between Panax Notoginseng or Its Biologically Active Compounds and Therapeutic Drugs: A Comprehensive Pharmacodynamic and Pharmacokinetic Review. J. Ethnopharmacol. 2023, 307, 116156. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dungan, S.R. Cholesterol Solubilization in Aqueous Micellar Solutions of Quillaja saponin, Bile Salts, or Nonionic Surfactants. J. Agric. Food Chem. 2001, 49, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Jaspers, T.C.C.; van Hasselt, P.M.; Hennink, W.E.; van Nostrum, C.F. A Mixed Micelle Formulation for Oral Delivery of Vitamin K. Pharm. Res. 2016, 33, 2168–2179. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Improving Curcumin Solubility and Bioavailability by Encapsulation in Saponin-Coated Curcumin Nanoparticles Prepared Using a Simple PH-Driven Loading Method. Food Funct. 2018, 9, 1829–1839. [Google Scholar] [CrossRef]
- Kong, R.; Zhu, X.; Meteleva, E.S.; Polyakov, N.E.; Khvostov, M.V.; Baev, D.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Atorvastatin Calcium Inclusion Complexation with Polysaccharide Arabinogalactan and Saponin Disodium Glycyrrhizate for Increasing of Solubility and Bioavailability. Drug Deliv. Transl. Res. 2018, 8, 1200–1213. [Google Scholar] [CrossRef]
- Meteleva, E.S.; Chistyachenko, Y.S.; Suntsova, L.P.; Khvostov, M.V.; Polyakov, N.E.; Selyutina, O.Y.; Tolstikova, T.G.; Frolova, T.S.; Mordvinov, V.A.; Dushkin, A.V.; et al. Disodium Salt of Glycyrrhizic Acid—A Novel Supramolecular Delivery System for Anthelmintic Drug Praziquantel. J. Drug Deliv. Sci. Technol. 2019, 50, 66–77. [Google Scholar] [CrossRef]
- Lorent, J.; Le Duff, C.S.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. Induction of Highly Curved Structures in Relation to Membrane Permeabilization and Budding by the Triterpenoid Saponins, α- and δ-Hederin. J. Biol. Chem. 2013, 288, 14000–14017. [Google Scholar] [CrossRef]
- Sharif Makhmal Zadeh, B.; Esfahani, G.; Salimi, A. Permeability of Ciprofloxacin-Loaded Polymeric Micelles Including Ginsenoside as P-Glycoprotein Inhibitor through a Caco-2 Cells Monolayer as an Intestinal Absorption Model. Molecules 2018, 23, 1904. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A.K. Transdermal Delivery of Carvedilol Containing Glycyrrhizin and Chitosan as Permeation Enhancers: Biochemical, Biophysical, Microscopic and Pharmacodynamic Evaluation. Drug Deliv. 2008, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lan, J.; Li, X.; Xin, M.; Wang, H.; Zhang, F.; Lu, X.; Zhuang, Z.; Wu, X. Novel Ultra-Small Micelles Based on Ginsenoside Rb1: A Potential Nanoplatform for Ocular Drug Delivery. Drug Deliv. 2019, 26, 481–489. [Google Scholar] [CrossRef]
- Tolstikova, T.; Khvostov, M.; Bryzgalov, A. The Complexes of Drugs with Carbohydrate-Containing Plant Metabolites as Pharmacologically Promising Agents. Mini Rev. Med. Chem. 2009, 9, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Influence of Glycyrrhizin on Permeability and Elasticity of Cell Membrane: Perspectives for Drugs Delivery. Drug Deliv. 2016, 23, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, J.; Cai, Y.; Zhan, J.; Gao, J.; Song, M.; Shi, Y.; Yang, Z. A Glycyrrhetinic Acid-Modified Curcumin Supramolecular Hydrogel for Liver Tumor Targeting Therapy. Sci. Rep. 2017, 7, 44210. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel Ginsenoside-Based Multifunctional Liposomal Delivery System for Combination Therapy of Gastric Cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef]
- Lu, L.; Ding, Y.; Zhang, Y.; Ho, R.J.; Zhao, Y.; Zhang, T.; Guo, C. Antibody-Modified Liposomes for Tumor-Targeting Delivery of Timosaponin AIII. Int. J. Nanomed. 2018, 13, 1927–1944. [Google Scholar] [CrossRef] [PubMed]
- Rafat, M.; Fong, K.W.; Goldsipe, A.; Stephenson, B.C.; Coradetti, S.T.; Sambandan, T.G.; Sinskey, A.J.; Rha, C. Association (Micellization) and Partitioning of Aglycon Triterpenoids. J. Colloid. Interface Sci. 2008, 325, 324–330. [Google Scholar] [CrossRef]
- Sun, I.C.; Kashiwada, Y.; Morris-Natschke, S.L.; Lee, K.H. Plant-Derived Terpenoids and Analogues as Anti-HIV Agents. Curr. Top. Med. Chem. 2003, 3, 155–169. [Google Scholar] [CrossRef]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2005, 22, 487–503. [Google Scholar] [CrossRef]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962–998. [Google Scholar] [CrossRef]
- Fries, S.L.; Standaert, F.G.; Whitcomb, E.R.; Nigrelli, R.F.; Chanley, J.D.; Sobotka, H. Some Pharmacologic Properties Of Holothurin A, A Glycosidic Mixture from the Sea Cucurmber. Ann. N. Y. Acad. Sci. 1960, 90, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Büchele, B.; Simmet, T. Analysis of 12 Different Pentacyclic Triterpenic Acids from Frankincense in Human Plasma by High-Performance Liquid Chromatography and Photodiode Array Detection. J. Chromatogr. B 2003, 795, 355–362. [Google Scholar] [CrossRef]
- Kaunzinger, A.; Baumeister, A.; Cuda, K.; Häring, N.; Schug, B.; Blume, H.H.; Raddatz, K.; Fischer, G.; Schubert-Zsilavecz, M. Determination of 11-Keto-Boswellic Acid in Human Plasma. J. Pharm. Biomed. Anal. 2002, 28, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Thawani, V.; Hingorani, L.; Shrivastava, M.; Bhate, V.R.; Khiyani, R. Pharmacokinetic Study of 11-Keto β-Boswellic Acid. Phytomedicine 2004, 11, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, S.; Wang, J.; Yin, T.; Teng, Y.; Wu, B.; You, M.; Jiang, Z.; Hu, M. Enhancement of Oral Bioavailability of 20(S)-Ginsenoside Rh2 through Improved Understanding of Its Absorption and Efflux Mechanisms. Drug Metab. Dispos. 2011, 39, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, G.-J.; Sun, J.-G.; Jia, Y.-W.; Wang, W.; Xu, M.-J.; Lv, T.; Zheng, Y.-T.; Sai, Y. Pharmacokinetic Characterization of Ginsenoside Rh2, an Anticancer Nutrient from Ginseng, in Rats and Dogs. Food Chem. Toxicol. 2009, 47, 2257–2268. [Google Scholar] [CrossRef]
- Thomas, V.H.; Bhattachar, S.; Hitchingham, L.; Zocharski, P.; Naath, M.; Surendran, N.; Stoner, C.L.; El-Kattan, A. The Road Map to Oral Bioavailability: An Industrial Perspective. Expert. Opin. Drug Metab. Toxicol. 2006, 2, 591–608. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Herrera, T.; Fornari, T.; Reglero, G.; Martin, D. The Gastrointestinal Behavior of Saponins and Its Significance for Their Bioavailability and Bioactivities. J. Funct. Foods 2018, 40, 484–497. [Google Scholar] [CrossRef]
- Wendy Hsiao, W.L.; Chen, L. Use of Herbal Saponins to Regulate Gut Microflora. U.S. Patent US10456436B2, 29 October 2019. [Google Scholar]
- Dantzig, A.; Law, K.; Cao, J.; Starling, J. Reversal of Multidrug Resistance by the P-Glycoprotein Modulator, LY335979, from the Bench to the Clinic. Curr. Med. Chem. 2001, 8, 39–50. [Google Scholar] [CrossRef]
- He, J.-X.; Akao, T.; Tani, T. Repetitive Administration of Shaoyao-Gancao-Tang to Rats Restores the Bioavailability of Glycyrrhizin Reduced by Antibiotic Treatment. J. Pharm. Pharmacol. 2003, 55, 1569–1575. [Google Scholar] [CrossRef]
- Krief, S.; Thoison, O.; Sévenet, T.; Wrangham, R.W.; Lavaud, C. Triterpenoid Saponin Anthranilates from Albizia g randibracteata Leaves Ingested by Primates in Uganda. J. Nat. Prod. 2005, 68, 897–903. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).