Keggin Heteropolyacid Immobilized on Nanosilica as a Heterogeneous Catalyst for Sugar Dehydration in an Aqueous Medium
Abstract
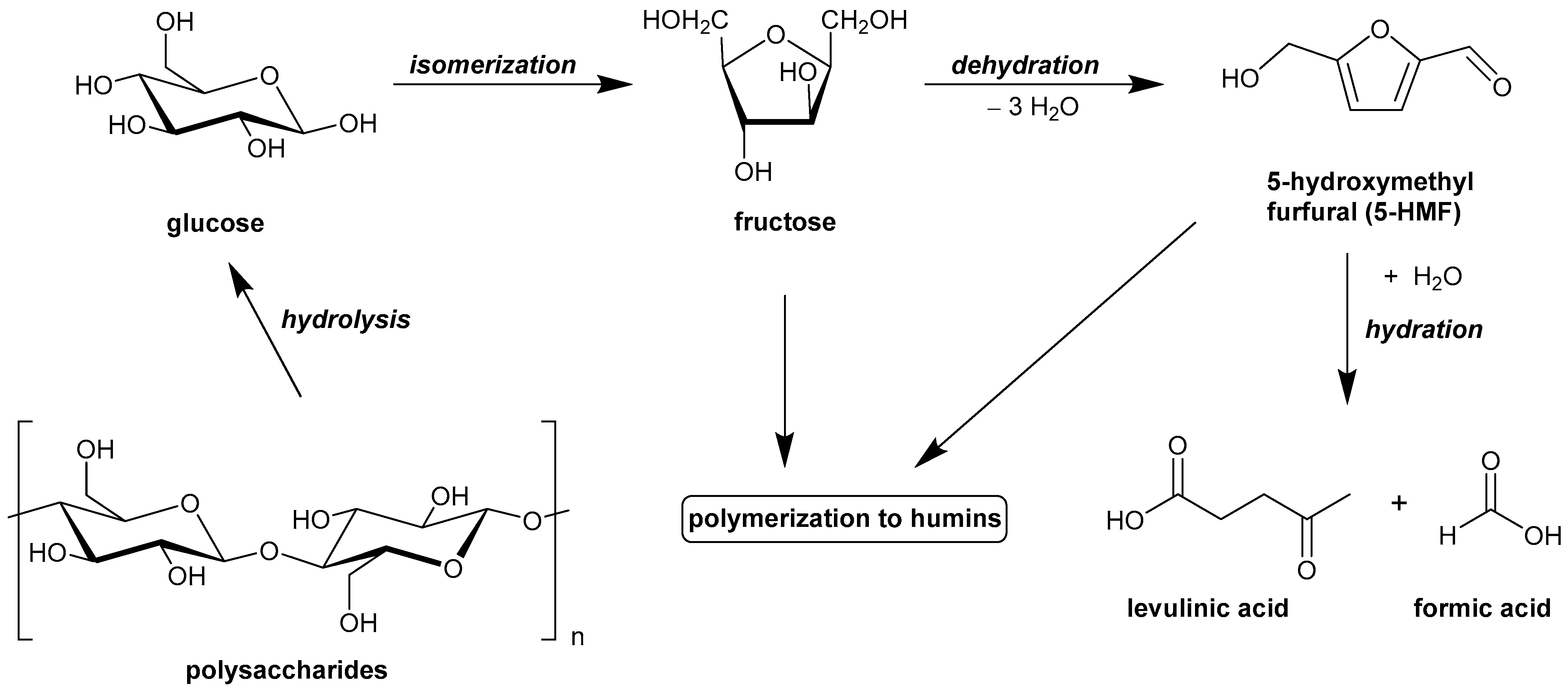
1. Introduction
2. Results and Discussion
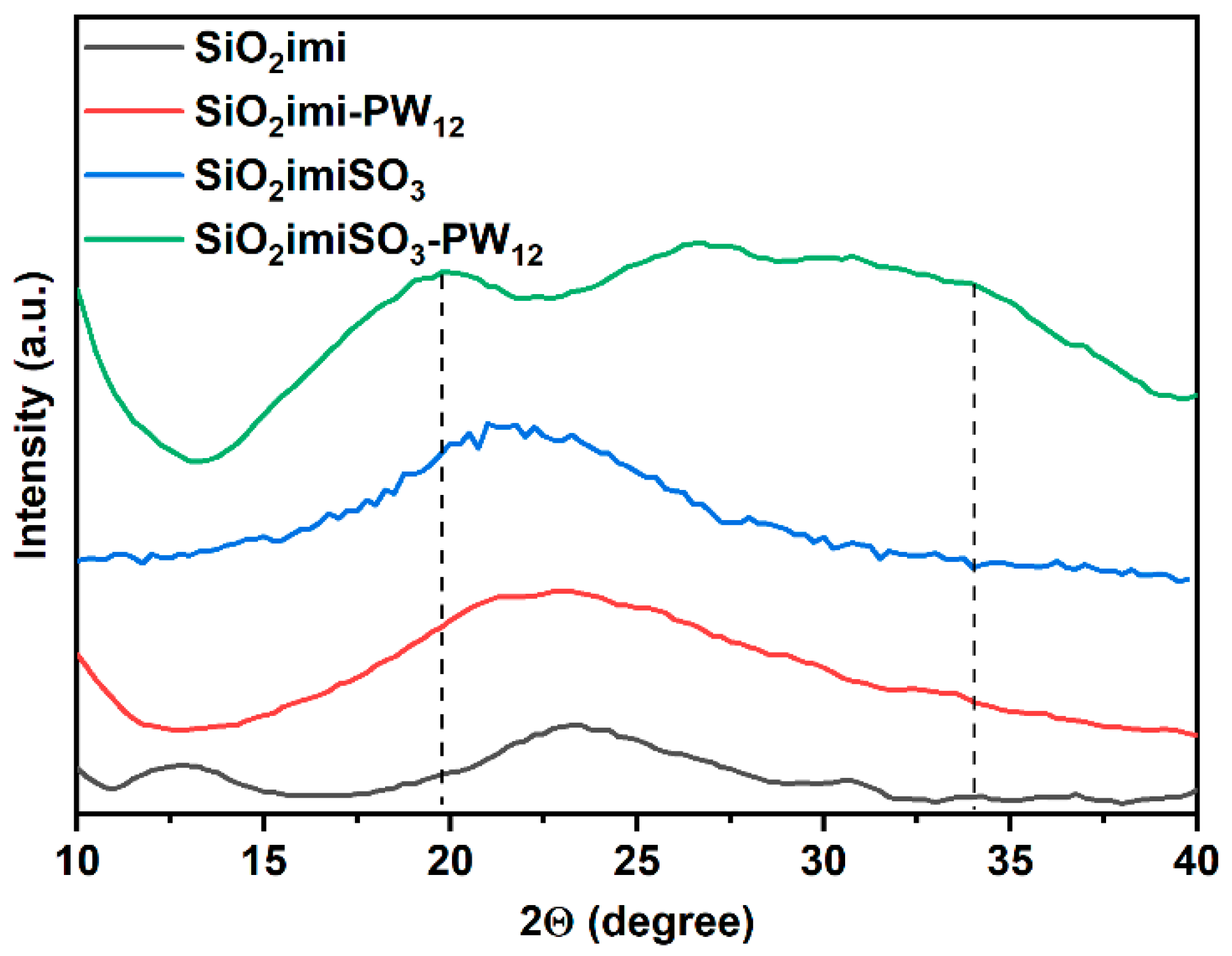
2.1. Characterization of the Catalysts
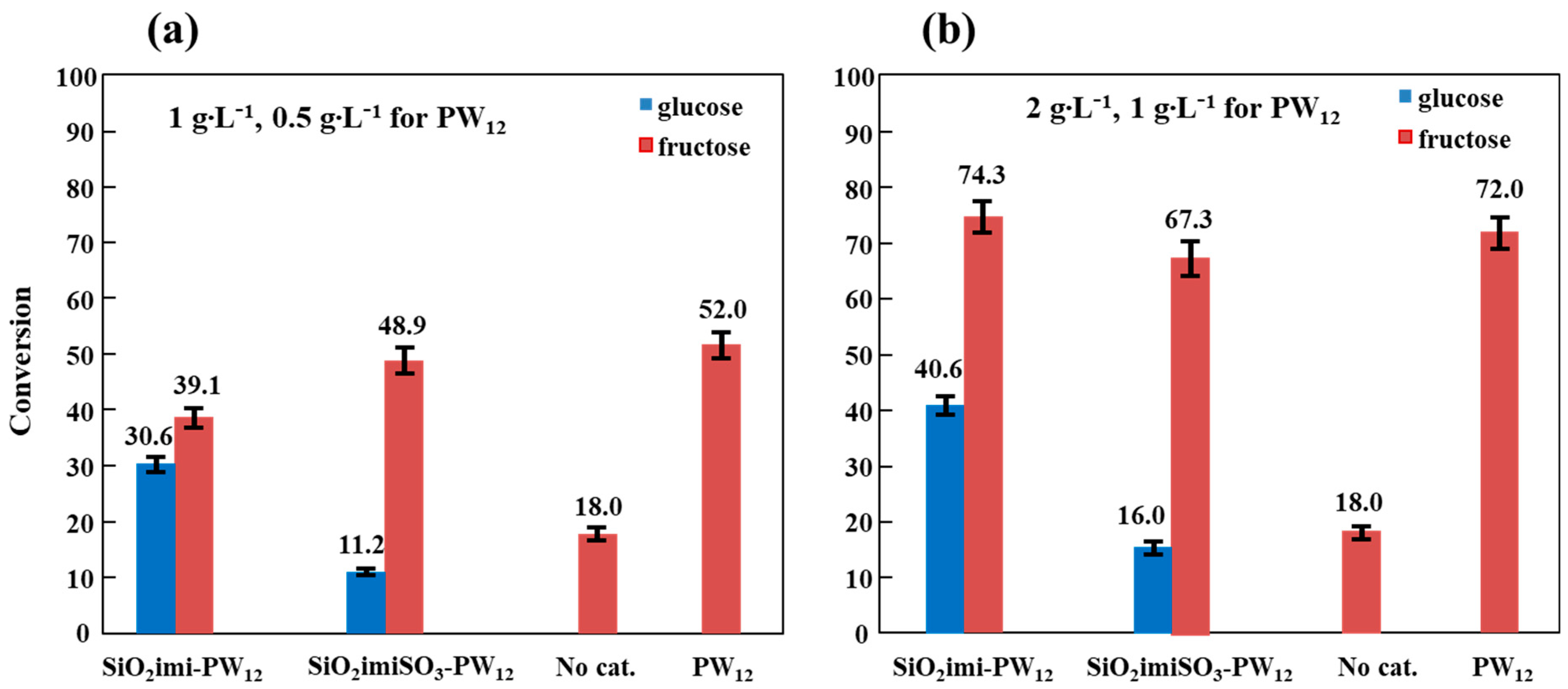
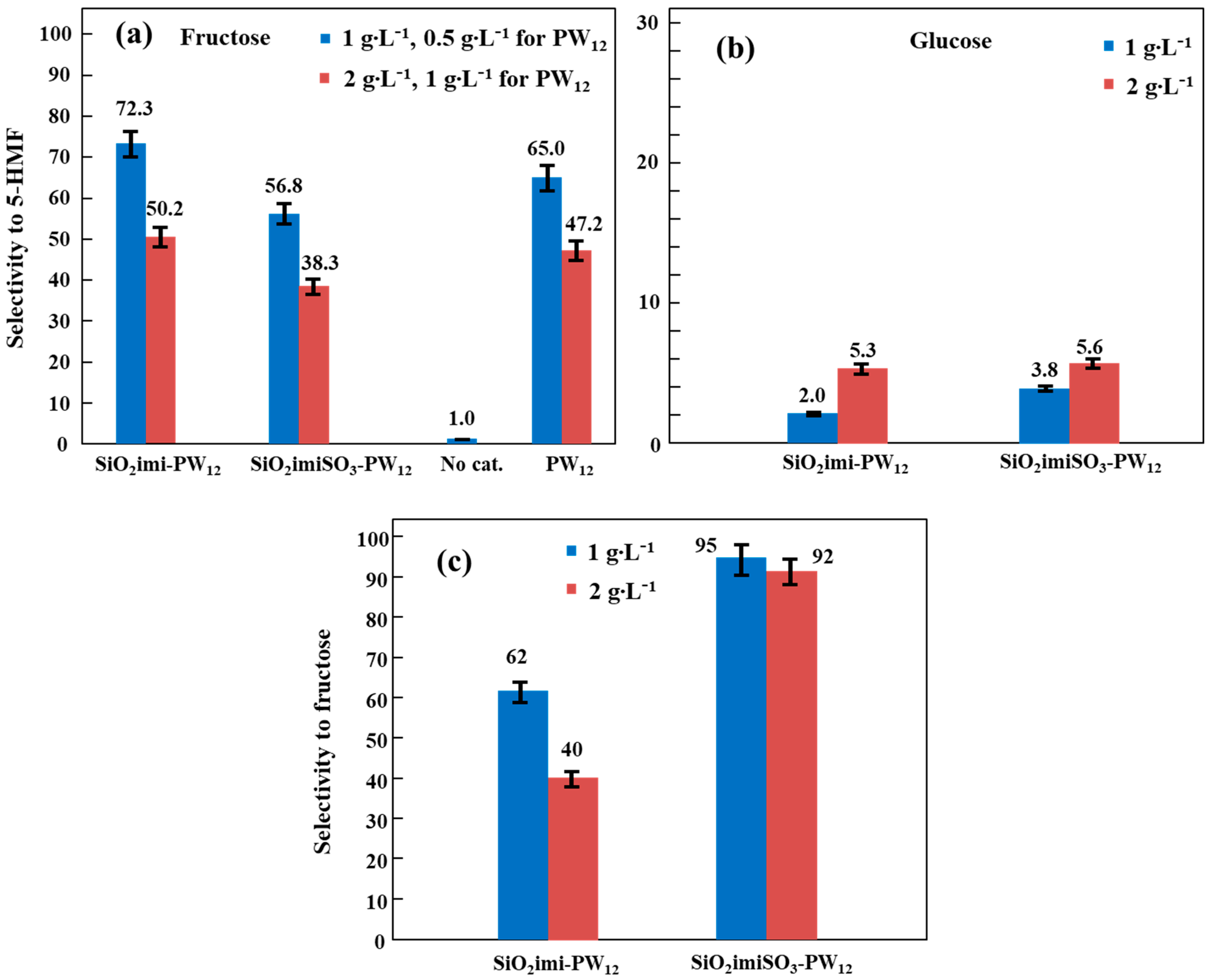
2.2. Catalytic Hexoses (Fructose/Glucose) Dehydration Activity
2.3. Catalytic Treatment of Wastewater Deriving from the Brewing Industry
3. Experimental
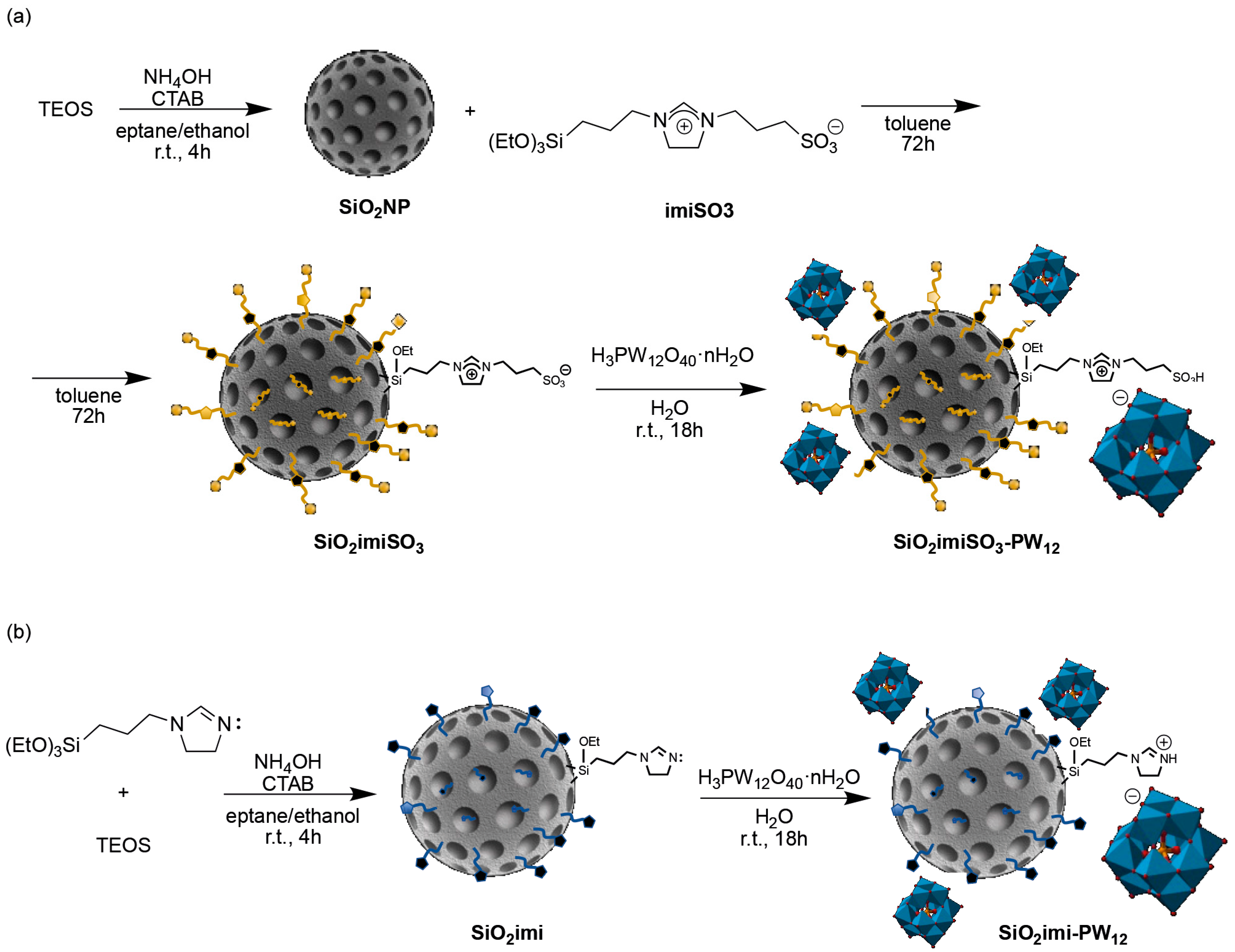
3.1. Preparation of the Supported Heteropolyacid Catalysts
3.1.1. Preparation of SiO2NP
3.1.2. Preparation of SiO2imiSO3
3.1.3. Preparation of SiO2imiSO3-PW12
3.1.4. Preparation of SiO2imi
3.1.5. Preparation of SiO2imi-PW12
3.2. Characterization of the Catalysts
3.3. Catalytic Hexoses Dehydration Experiments
3.4. Catalytic Valorization of Sugars Contained in Wastewater Deriving from the Brewing Industry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galkin, K.I.; Ananikov, V.P. The Increasing Value of Biomass: Moving From C6 Carbohydrates to Multifunctionalized Building Blocks via 5-(hydroxymethyl)furfural. ChemistryOpen 2020, 9, 1135–1148. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Y.; Hu, D.; Guo, R.; Yan, K.; Luque, R. Chemical transformations of 5-hydroxymethylfurfural into highly added value products: Present and future. Green Chem. 2023, 25, 871–892. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Selective Conversion of D-Fructose to 5-Hydroxymethylfurfural by Ion-Exchange Resin in Acetone/Dimethyl sulfoxide Solvent Mixtures. Ind. Eng. Chem. Res. 2008, 47, 9234–9239. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the interplay of lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl)furfural and levulinic acid in aqueous media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Lima, S.; García-López, E.I.; Krivtsov, I.; Ilkaeva, M.; Bornes, C.; Mafra, L.; Liotta, L.F.; Villar-Rodil, S.; Paredes, J.I.; Marcì, G.; et al. Valorisation of microalga Chlorella sp. into furans in the presence of Nb2O5 catalysts. J. Catal. 2024, 433, 115457. [Google Scholar] [CrossRef]
- Lima, S.; García-López, E.I.; Adawi, A.; Marci, G.; Scargiali, F. Valorisation of Chlorella sp. biomass in 5-HMF through a two-step conversion in the presence of Nb2O5 and NbOPO4 and optimisation through reactive extraction. Chem. Eng. J. 2023, 471, 144583. [Google Scholar] [CrossRef]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub- and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Zhao, H.B.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Schwegler, M.A.; Vinke, P.; van der Eijk, M.; van Bekkum, H. Activated carbon as a support for heteropolyanion catalysts. Appl. Catal. A 1992, 80, 41–57. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, P.; Han, B.; Wu, T.; Wu, Y. The Synthesis of 5-Hydroxymethylfurfural from Glucose in Biphasic System by Phosphotungstic Acidified Titanium–Zirconium Dioxide. Waste Biomass Valorization 2018, 9, 657–668. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural-A promising biomass-derived building block. Chem. Rev. 2010, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, W.; Yang, J.; Yang, T.; Liu, Z.; Zhang, M. The Effects of Support Specific Surface Area and Active Metal on the Performance of Biphenyl Selective Hydrogenation to Cyclohexylbenzene. Catalysts 2024, 14, 727. [Google Scholar] [CrossRef]
- Meenakshi, R.; Shakeela, K.; Kutti Rani, S.; Ranga Rao, G. Oxidation of Aniline to Nitrobenzene Catalysed by 1-Butyl-3-methyl imidazolium phosphotungstate Hybrid Material Using m-chloroperbenzoic Acid as an Oxidant. Catal Lett. 2018, 148, 246–257. [Google Scholar] [CrossRef]
- Gervasini, A.; Auroux, A. Acidity and basicity of metal oxide surfaces II. Determination by catalytic decomposition of isopropanol. J. Catal. 1991, 131, 190–198. [Google Scholar] [CrossRef]
- Essayem, N.; Holmqvist, A.; Gayraud, P.Y.; Vedrine, J.C.; Taarit, Y.B. In situ FTIR studies of the protonic sites of H3PW12O40 and its acidic cesium salts MxH3−xPW12O40. J. Catal. 2001, 197, 273–280. [Google Scholar] [CrossRef]
- Bielański, A.; Lubańska, A. FTIR investigation on Wells-Dawson and Keggin type heteropolyacids: Dehydration and ethanol sorption. J. Mol. Catal. A 2004, 224, 179–187. [Google Scholar] [CrossRef]
- Jalil, P.A.; Faiz, M.; Tabet, N.; Hamdan, N.M.; Hussain, Z. A study of the stability of tungstophosphoric acid, H3PW12O40, using synchrotron XPS, XANES, hexane cracking, XRD, and IR spectroscopy. J. Catal. 2003, 217, 292–297. [Google Scholar] [CrossRef]
- Mosa, J.; Larramona, G.; Aparicio, M. Synthesis and characterization of P2O5-ZrO2-SiO2 membranes doped with tungstophosphoric acid (PWA) for applications in PEMFC. J. Memb. Sci. 2008, 307, 21–27. [Google Scholar] [CrossRef]
- Lavrencic Stangar, U.; Groselj, N.; Orel, B.; Schmitz, A.; Colomban, P. Proton-conducting sol-gel hybrids containing heteropoly acids. Solid State Ion. 2001, 145, 109–118. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Lee, D.; Choi, Y.; Jung, N.; Choi, J. Poly(styrene sulfonic acid)-Grafted Carbon Black Synthesized by Surface-Initiated Atom Transfer Radical Polymerization. Molecules 2023, 28, 4168. [Google Scholar] [CrossRef] [PubMed]
- Ilbeygi, H.; Kim, I.Y.; Kim, M.G.; Cha, W.; Sahaya, P.; Kumar, M.; Park, D.H.; Vinu, A. Highly Crystalline Mesoporous Phosphotungstic Acid: A High-Performance Electrode Material for Energy-Storage Applications. Angew. Chem. Int. Ed. 2019, 58, 10849–10854. [Google Scholar] [CrossRef] [PubMed]
- Mastikhin, V.M.; Kulikov, S.M.; Nosov, A.V.; Kozhevnikov, I.V.; Mudrakovsky, I.L.; Timofeeva, M.N. 1H and 31P MAS NMR studies of solid heteropolyacids and H3PW12O40 supported on SiO2. J. Mol. Catal. 1990, 60, 65–70. [Google Scholar] [CrossRef]
- Essayem, N.; Tong, Y.Y.; Jobic, H.; Vedrine, J.C. Characterization of protonic sites in H3PW12O40 and Cs1.9H1.1PW12O40: A solid-state 1H, 2H, 31P MAS-NMR and inelastic neutron scattering study on samples prepared under standard reaction conditions. Appl. Catal. A Gen. 2000, 194–195, 109–122. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- García-López, E.I.; Pomilla, F.R.; Megna, B.; Testa, M.L.; Liotta, L.F.; Marcì, G. Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural in Aqueous Medium over Nb2O5-Based Catalysts. Nanomaterials 2021, 11, 1821. [Google Scholar] [CrossRef]
- Turek, W.; Krowiak, A. Evaluation of oxide catalysts’ properties based on isopropyl alcohol conversion. Appl. Catal. A 2012, 417–418, 102–110. [Google Scholar] [CrossRef]
- García-López, E.I.; Marcì, G.; Pomilla, F.R.; Kirpsza, A.; Micek-Ilnicka, A.; Palmisano, L. Supported H3PW12O40 for 2-propanol (photo-assisted) catalytic dehydration in gas-solid regime: The role of the support and of the pseudo-liquid phase in the (photo)activity. Appl. Catal. B 2016, 189, 252–265. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heterogeneous Catalysis. Chem. Rev. 1998, 98, 199–218. [Google Scholar] [CrossRef]
- Wiesfeld, J.J.; Gaquere, R.; Hensen, E.J.M. Mesoporous Doped Tungsten Oxide for Glucose Dehydration to 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 7552–7562. [Google Scholar] [CrossRef]
- Pimenta Lorenti, J.; Scolari, E.; Cabral, N.M.; Bisio, C.; Gallo, J.M.R. Isomerization and Epimerization of Glucose Catalyzed by Sn-Containing Mesoporous Silica. Ind. Eng. Chem. Res. 2021, 60, 12821–12833. [Google Scholar] [CrossRef]
- Presentato, A.; Armetta, F.; Spinella, A.; Chillura Martino, D.; Alduina, R.; Saladino, M.L. Formulation of mesoporous silica nanoparticles for controlled release of antimicrobials for stone preventive conservation. Front. Chem. 2020, 8, 699. [Google Scholar] [CrossRef] [PubMed]
- Vafaeezadeh, M.; Wilhelm, C.; Breuninger, P.; Ernst, S.; Antonyuk, S.; Thiel, W.R. A Janus-type Heterogeneous Surfactant for Adipic Acid Synthesis. ChemCatChem 2020, 12, 2695. [Google Scholar] [CrossRef]
- Vitale, F.; Saladino, M.L.; Armetta, F.; Presentato, A.; Alduina, R.; Mercadante, A.; La Parola, V.; Giacalone, F. New biocides based on imidazolinium functionalised hybrid mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2022, 343, 112142. [Google Scholar] [CrossRef]
- Gottuso, A.; Armetta, F.; Cataldo, A.; Mollica Nardo, V.; Parrino, F.; Saladino, M.L. Functionalization of mesoporous silica nanoparticles through one-pot co-condensation in w/o emulsion. Microporous Mesoporous Mater. 2022, 335, 111833. [Google Scholar] [CrossRef]
- Lim, M.H.; Blandford, C.F.; Stein, A. Synthesis of Ordered Microporous Silicates with Organosulfur Surface Groups and Their Applications as Solid Acid Catalysts. Chem. Mater. 1998, 10, 467–470. [Google Scholar] [CrossRef]
- Putz, A.M.; Almásy, L.; Len, A.; Ianăşi, C. Functionalized silica materials synthesized via co-condensation and post-grafting methods. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 323–332. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
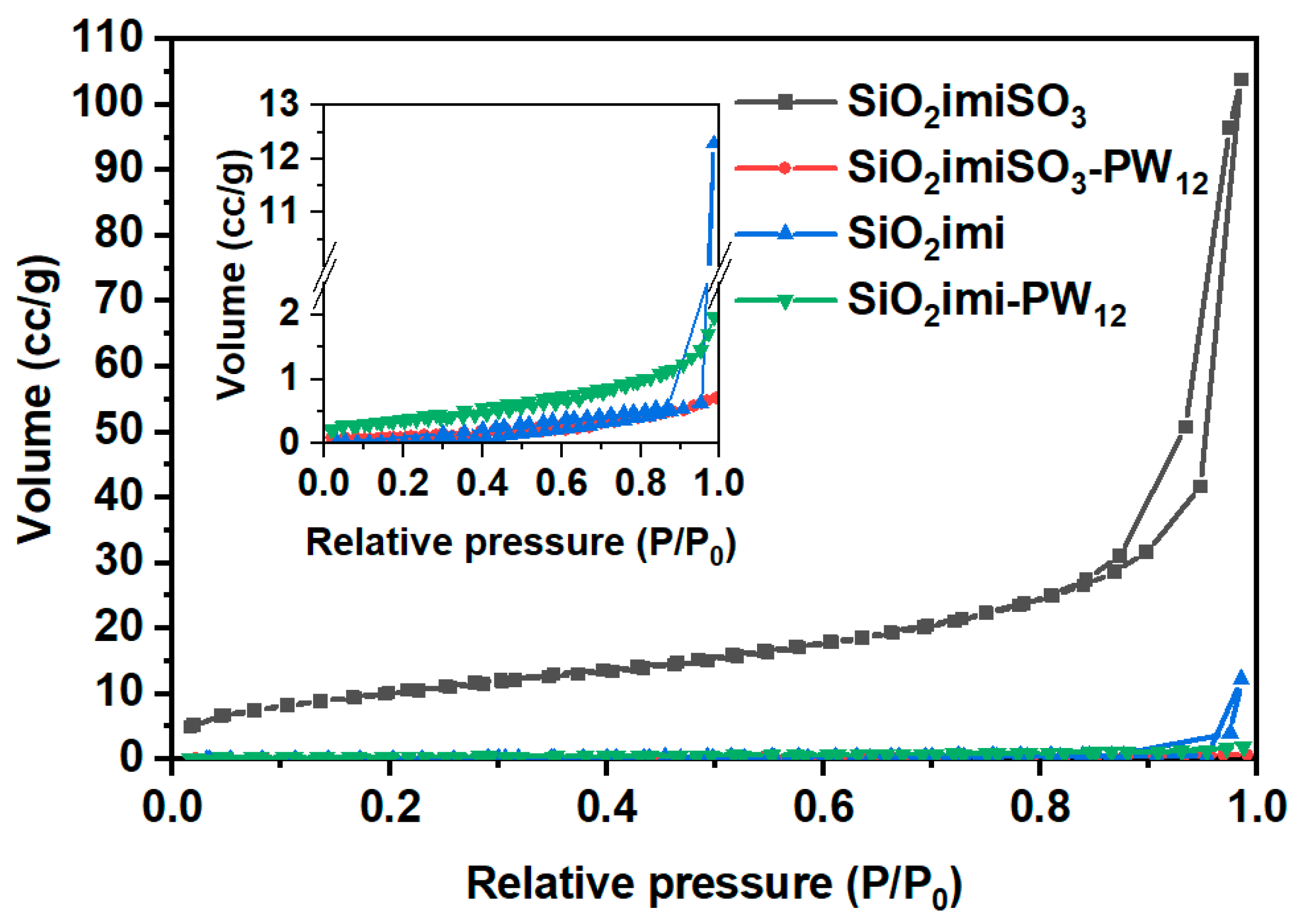
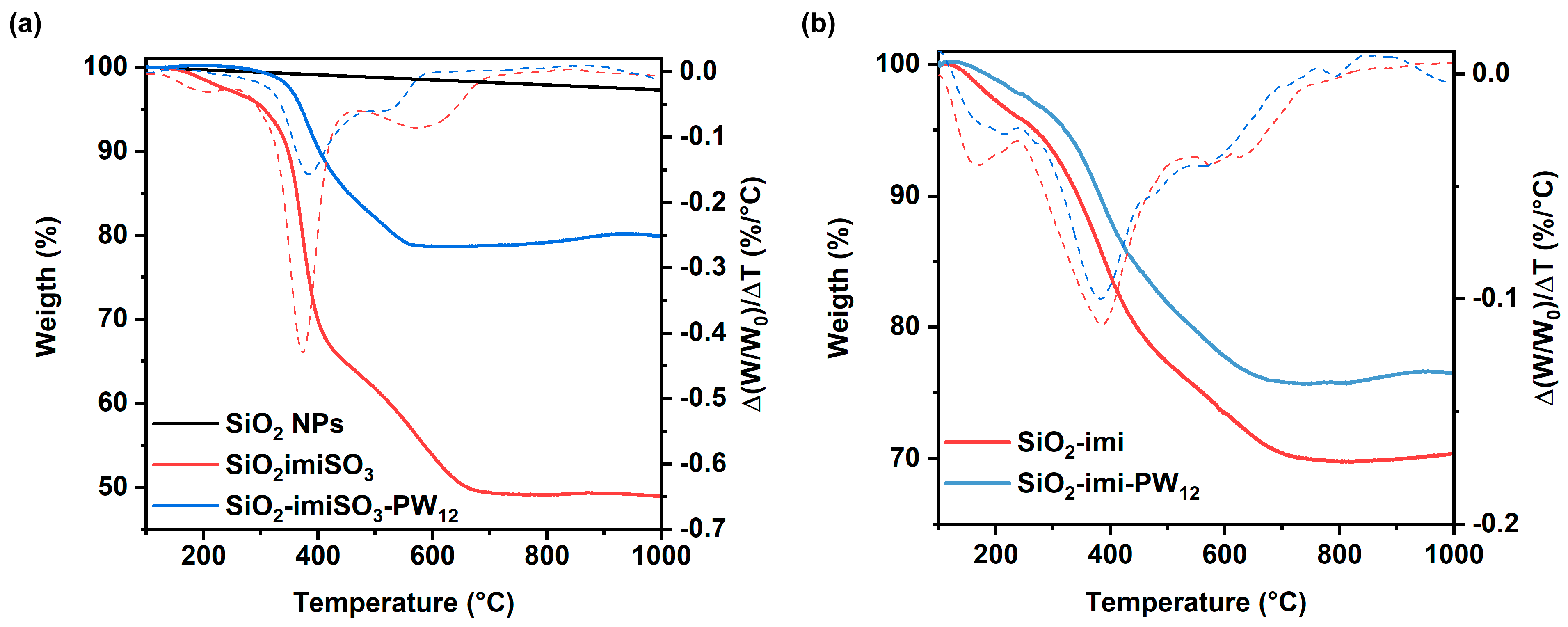
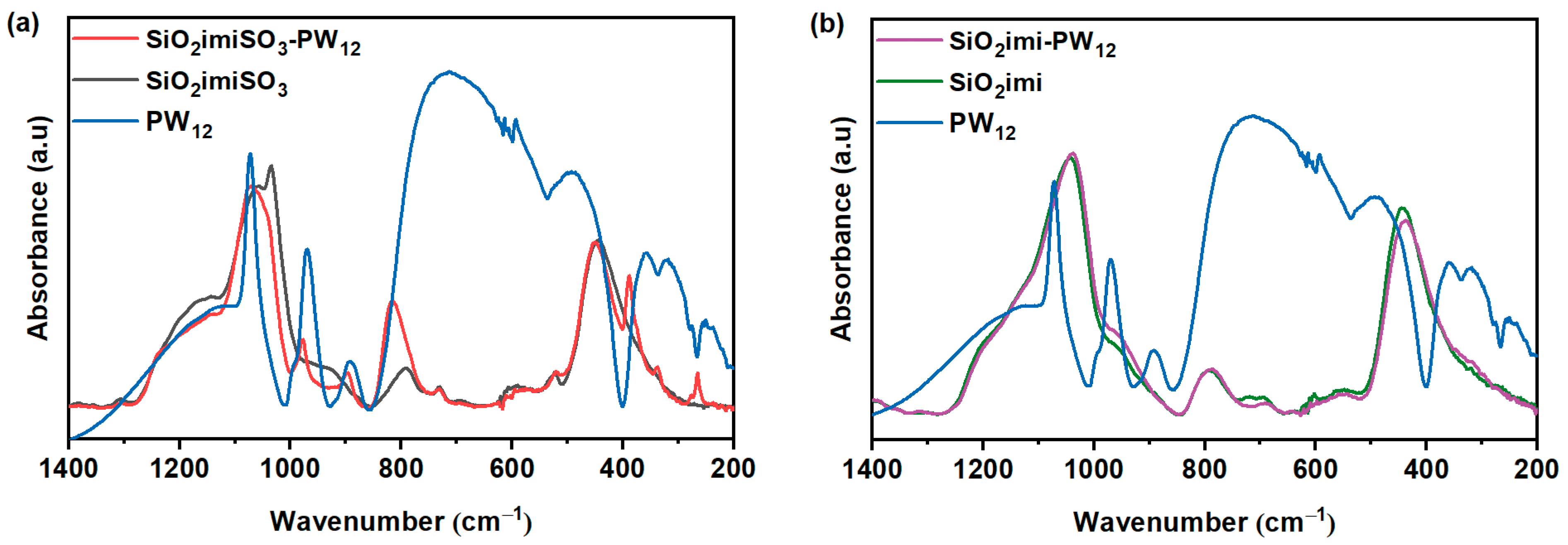
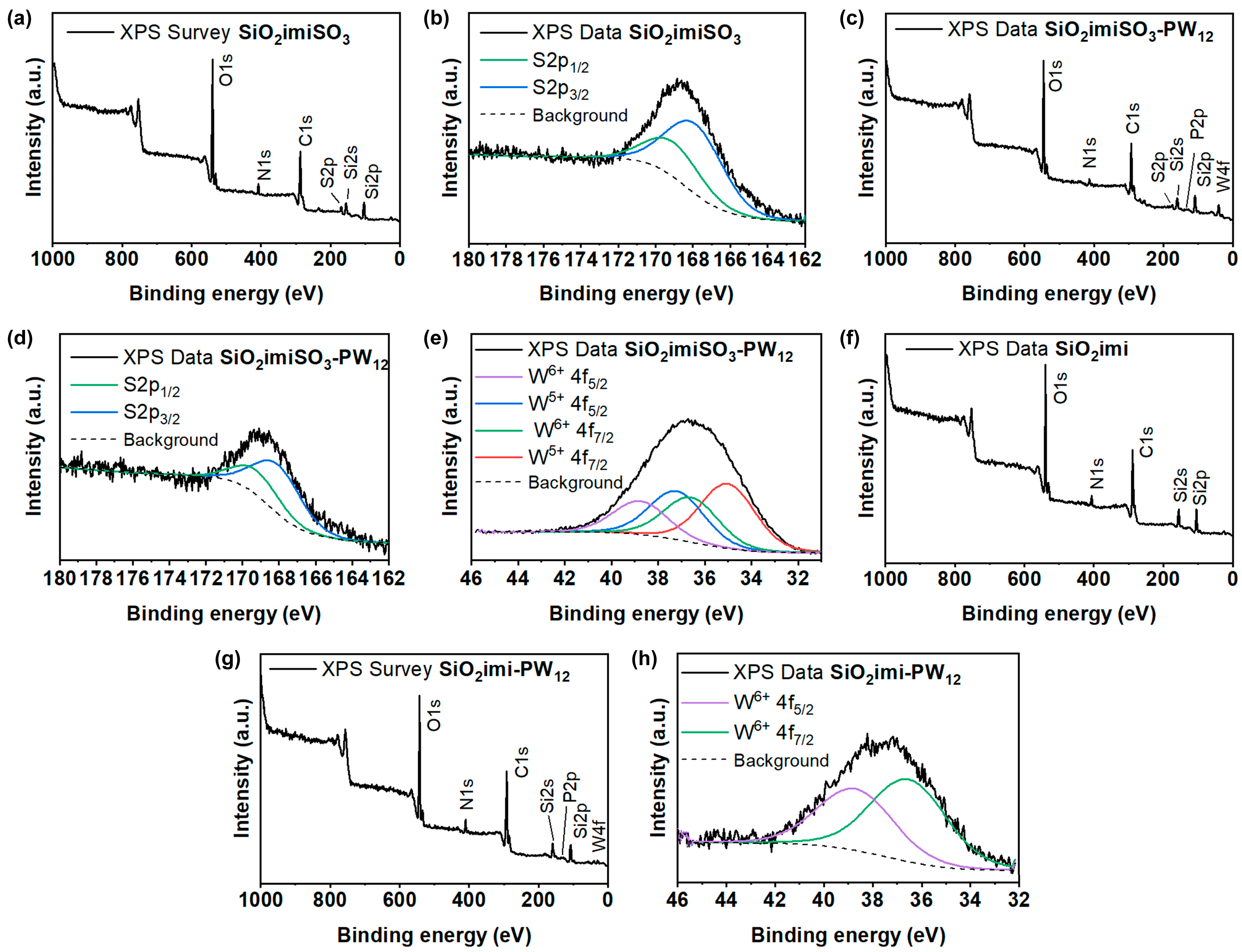
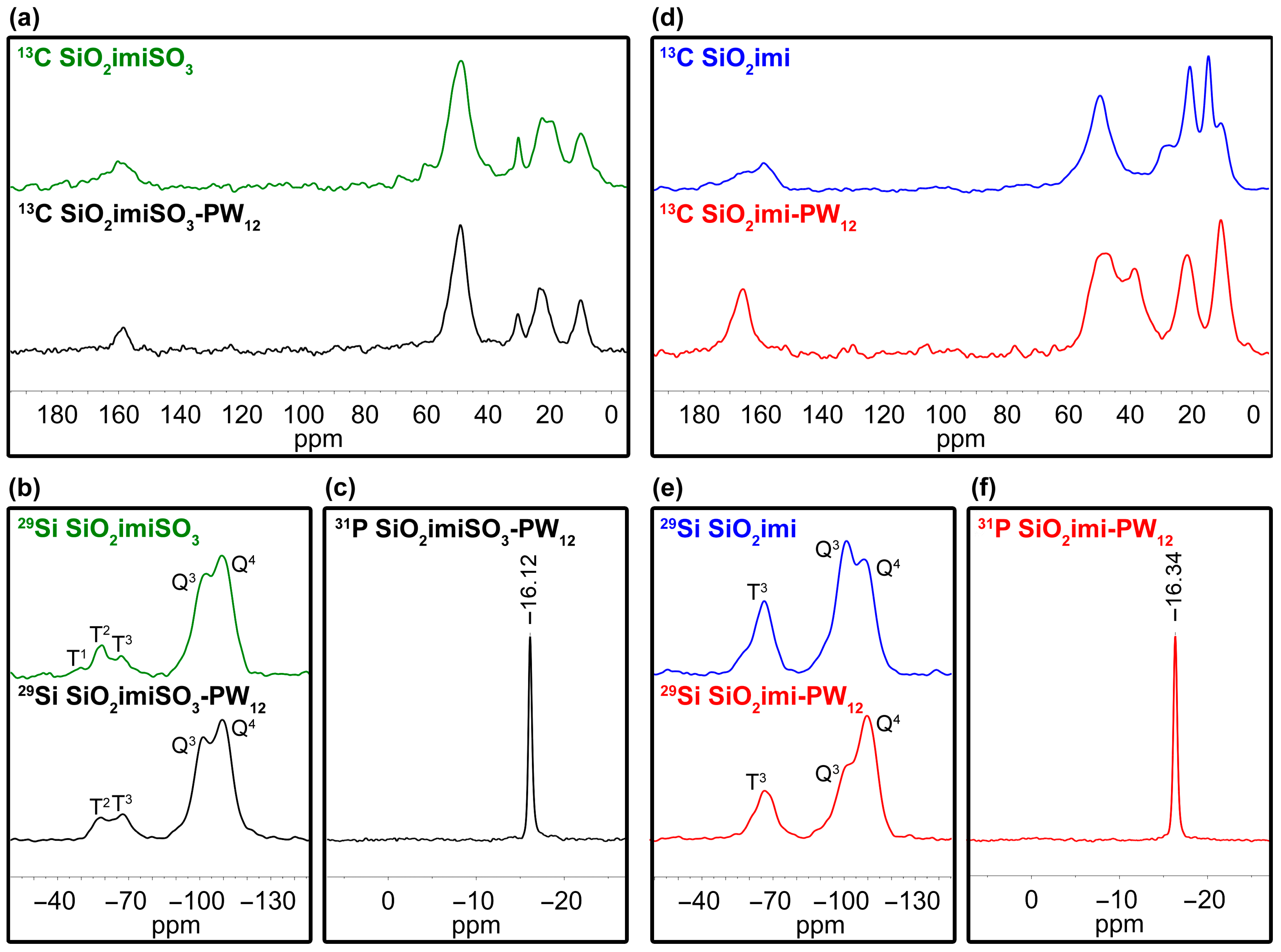
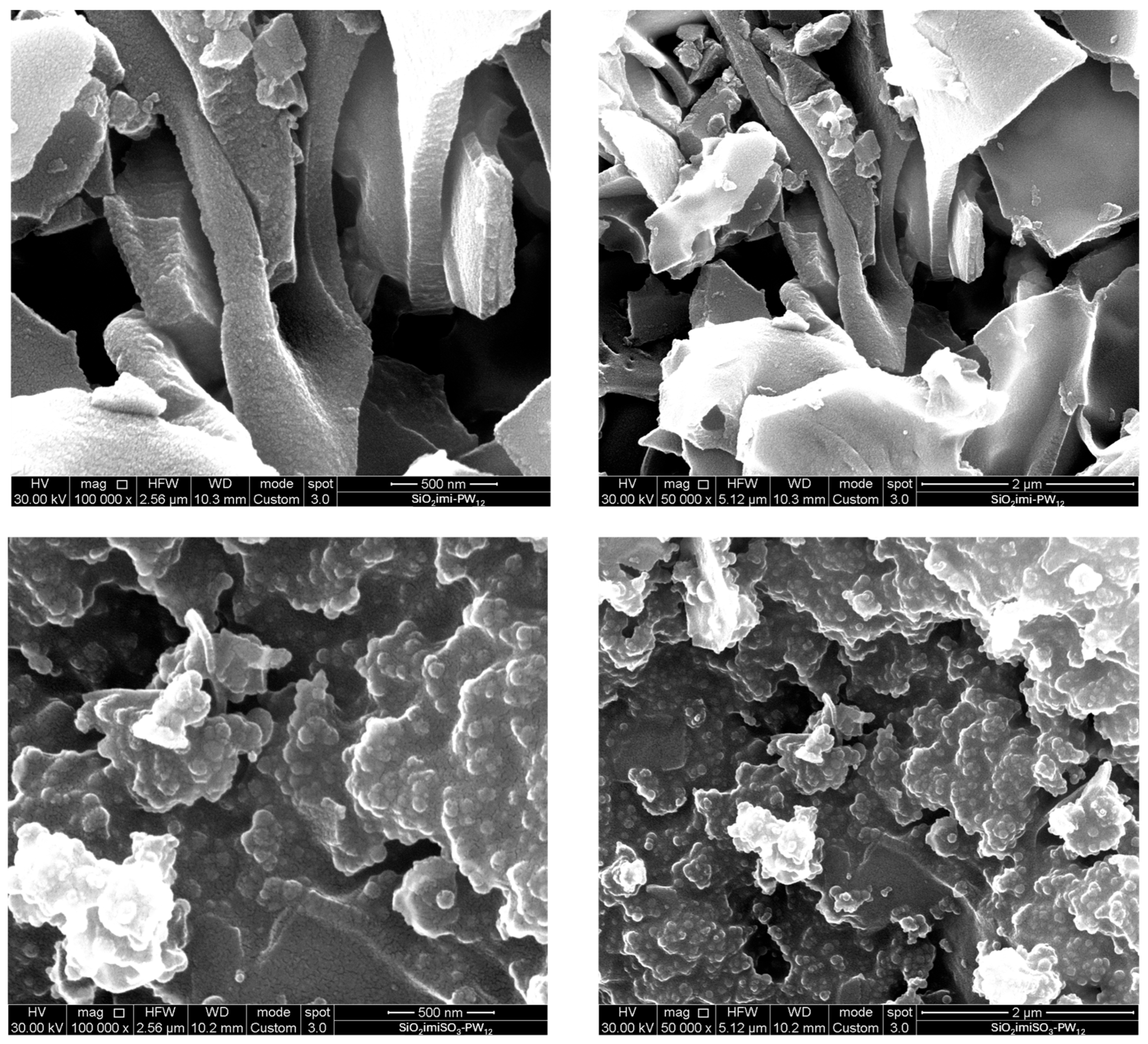
| Sample | SBET (m2∙g−1) | Vt (cm3∙g−1) | wBJH (nm) |
|---|---|---|---|
| SiO2imiSO3 | 37(1) | 0.15(8) | 1.9(1) |
| SiO2imiSO3-PW12 | 3.2(2) | 0.012(1) | 2.0(1) |
| SiO2imi | 3.9(2) | 0.045(2) | 1.8(1) |
| SiO2imi-PW12 | 15(8) | 0.032(1) | 1.7(1) |
| Catalyst | Amount (g∙L−1) | Xglucose | S5-HMF | Sfructose | Y5-HMF | Yfructose |
|---|---|---|---|---|---|---|
| SiO2imi-PW12 | 1 | 30.6 | 2.0 | 62 | 0.6 | 19 |
| 2 | 40.6 | 5.3 | 40 | 2.1 | 16 | |
| SiO2imiSO3-PW12 | 1 | 11.2 | 3.8 | 95 | 0.4 | 11 |
| 2 | 16.0 | 5.6 | 92 | 0.9 | 15 |
| Concentration [mM] | ||
|---|---|---|
| Carbohydrate | Before Enzymatic Treatment | After Enzymatic Treatment |
| Maltose/sucrose | 273.4 | - |
| Glucose | 88.6 | 307.3 |
| Fructose | 28.3 | 98.3 |
| Catalyst | X | S | Y |
|---|---|---|---|
| SiO2imi-PW12 | 58.9 | 2.4 | 1.4 |
| SiO2imiSO3-PW12 | 48.9 | 4.0 | 2.0 |
| PW12 | 44.4 | 3.3 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campisciano, V.; Lima, S.; Marcì, G.; Vitale, F.; Saladino, M.L.; Giacalone, F.; García-López, E.I. Keggin Heteropolyacid Immobilized on Nanosilica as a Heterogeneous Catalyst for Sugar Dehydration in an Aqueous Medium. Molecules 2025, 30, 4097. https://doi.org/10.3390/molecules30204097
Campisciano V, Lima S, Marcì G, Vitale F, Saladino ML, Giacalone F, García-López EI. Keggin Heteropolyacid Immobilized on Nanosilica as a Heterogeneous Catalyst for Sugar Dehydration in an Aqueous Medium. Molecules. 2025; 30(20):4097. https://doi.org/10.3390/molecules30204097
Chicago/Turabian StyleCampisciano, Vincenzo, Serena Lima, Giuseppe Marcì, Filippo Vitale, Maria Luisa Saladino, Francesco Giacalone, and Elisa I. García-López. 2025. "Keggin Heteropolyacid Immobilized on Nanosilica as a Heterogeneous Catalyst for Sugar Dehydration in an Aqueous Medium" Molecules 30, no. 20: 4097. https://doi.org/10.3390/molecules30204097
APA StyleCampisciano, V., Lima, S., Marcì, G., Vitale, F., Saladino, M. L., Giacalone, F., & García-López, E. I. (2025). Keggin Heteropolyacid Immobilized on Nanosilica as a Heterogeneous Catalyst for Sugar Dehydration in an Aqueous Medium. Molecules, 30(20), 4097. https://doi.org/10.3390/molecules30204097











