Centipeda minima: A Review of Phytochemistry, Pharmacology, and Predictive Analysis on Quality Markers
Abstract
1. Introduction
2. Chemical Composition
2.1. Volatile Oils
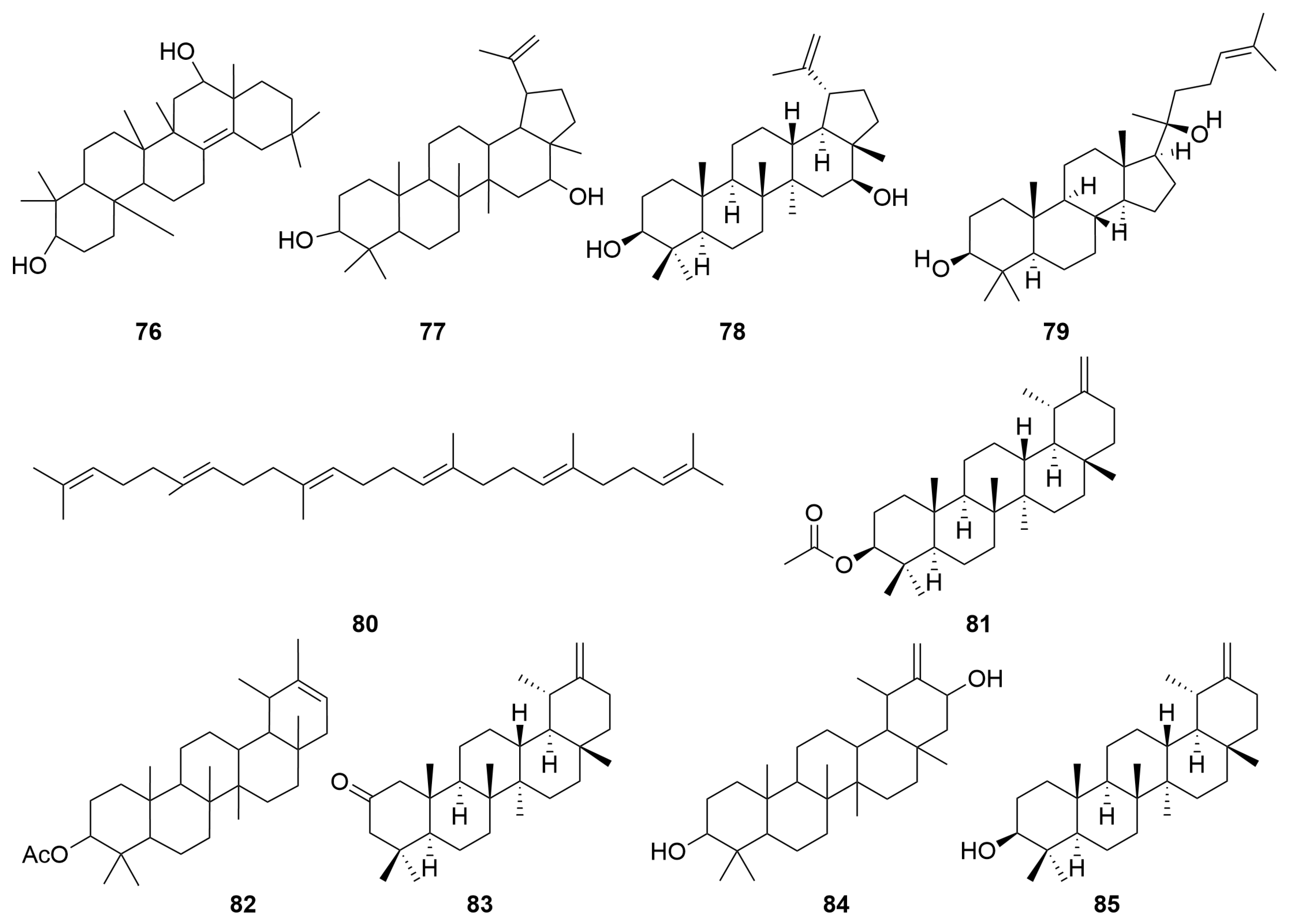
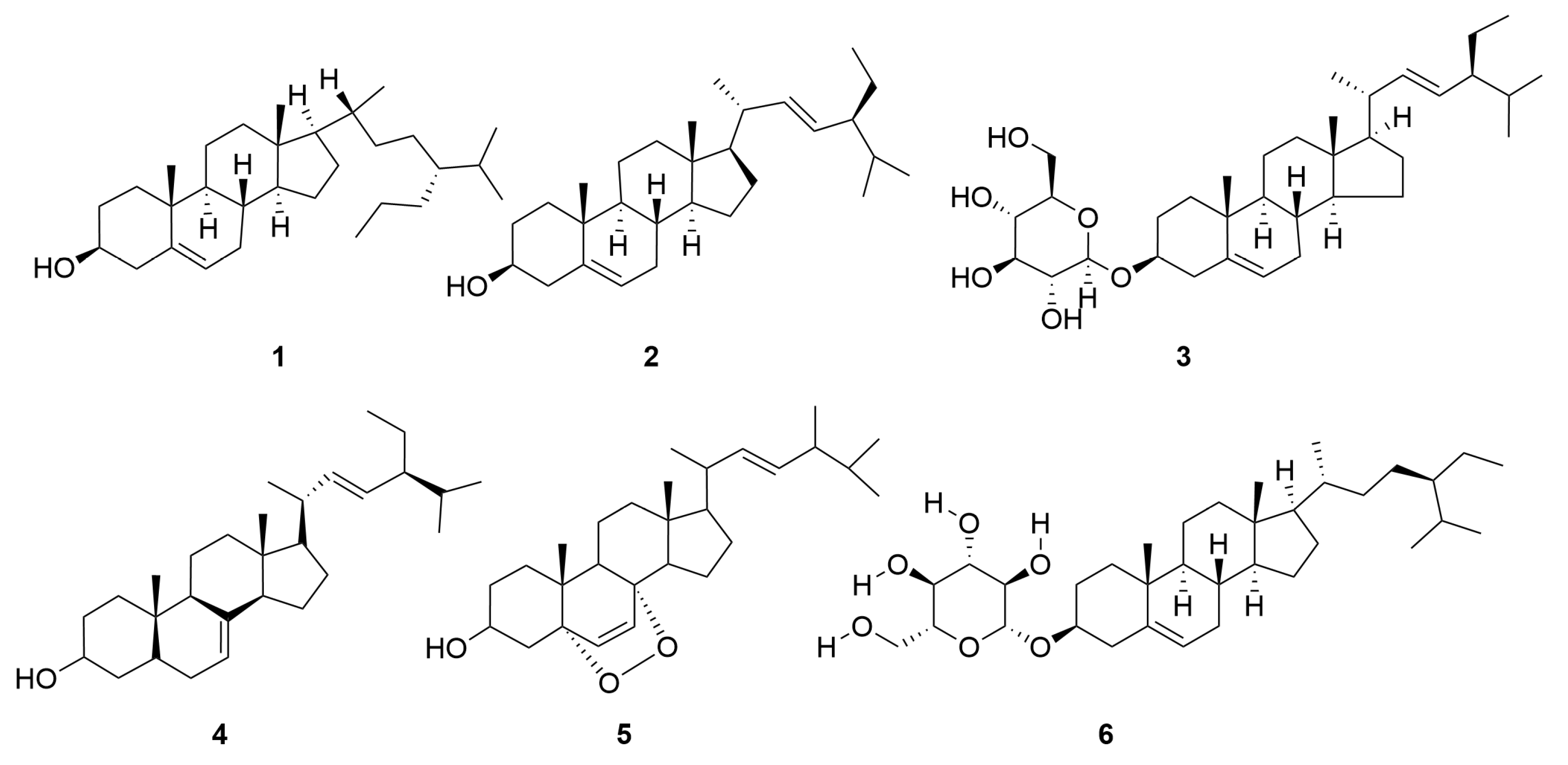
2.2. Terpenoids and Their Glycosides
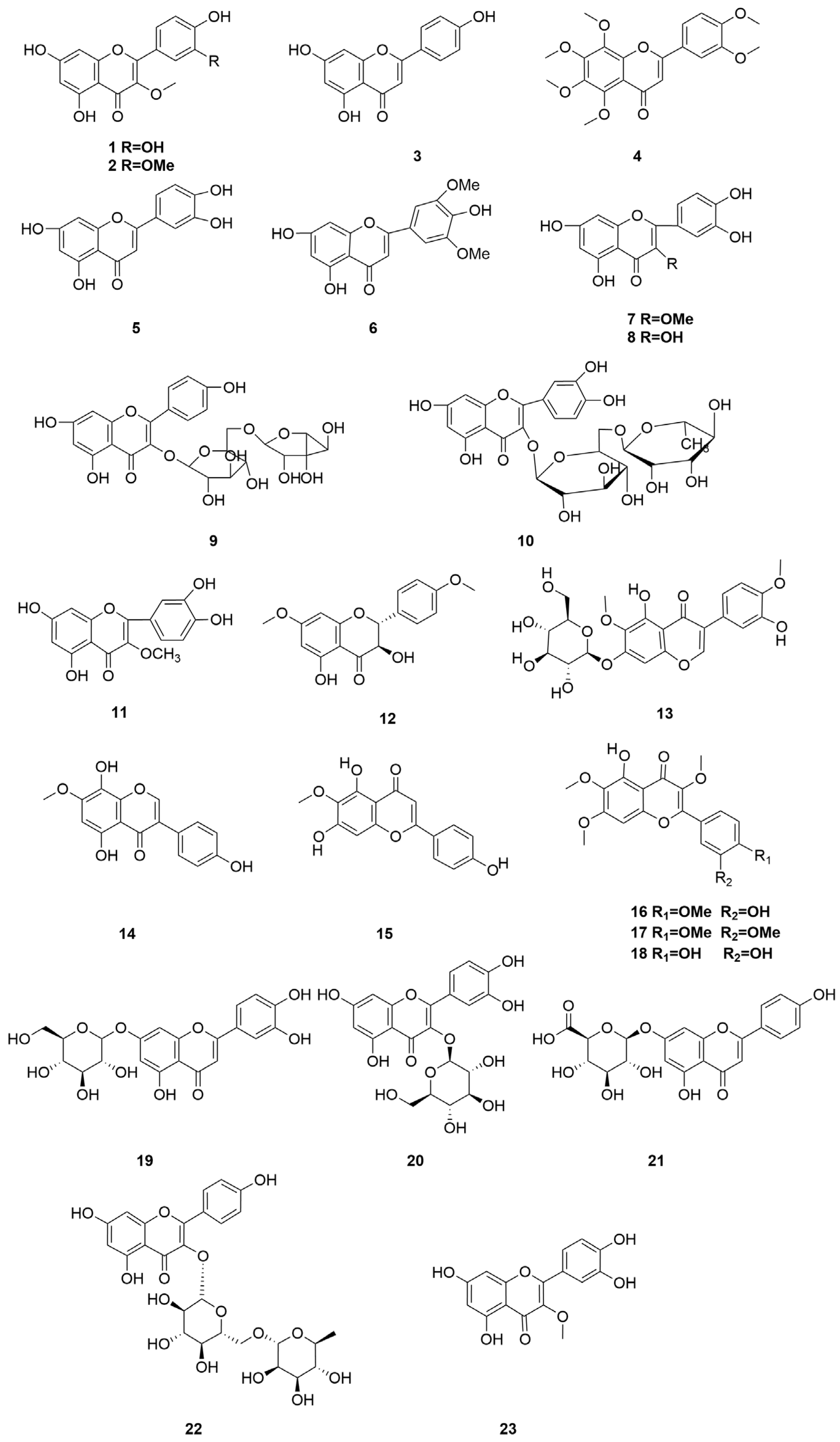
2.3. Flavonoids and Their Glycosides
2.4. Sterols
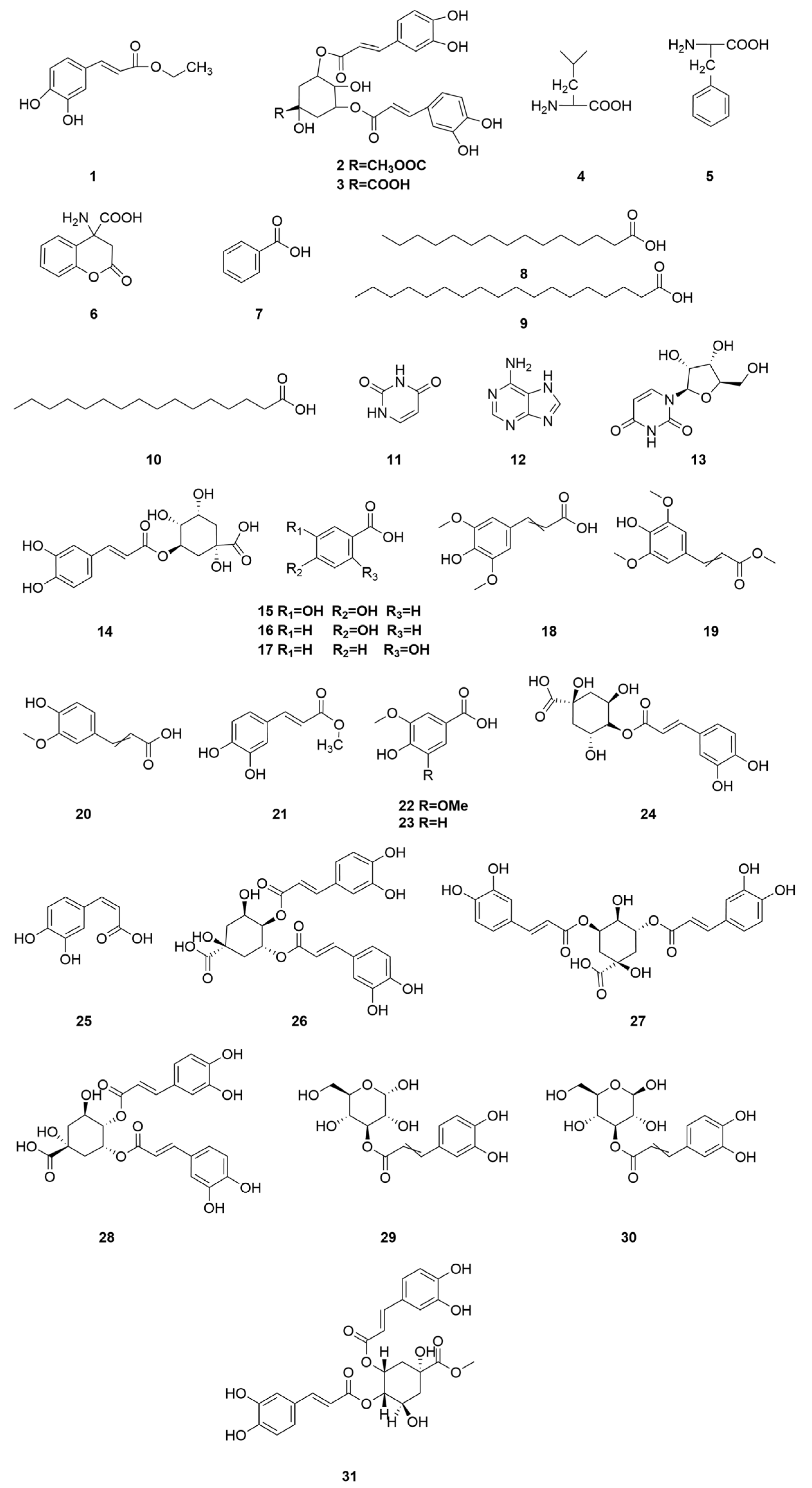
2.5. Organic Acids
2.6. Other Chemical Compositions
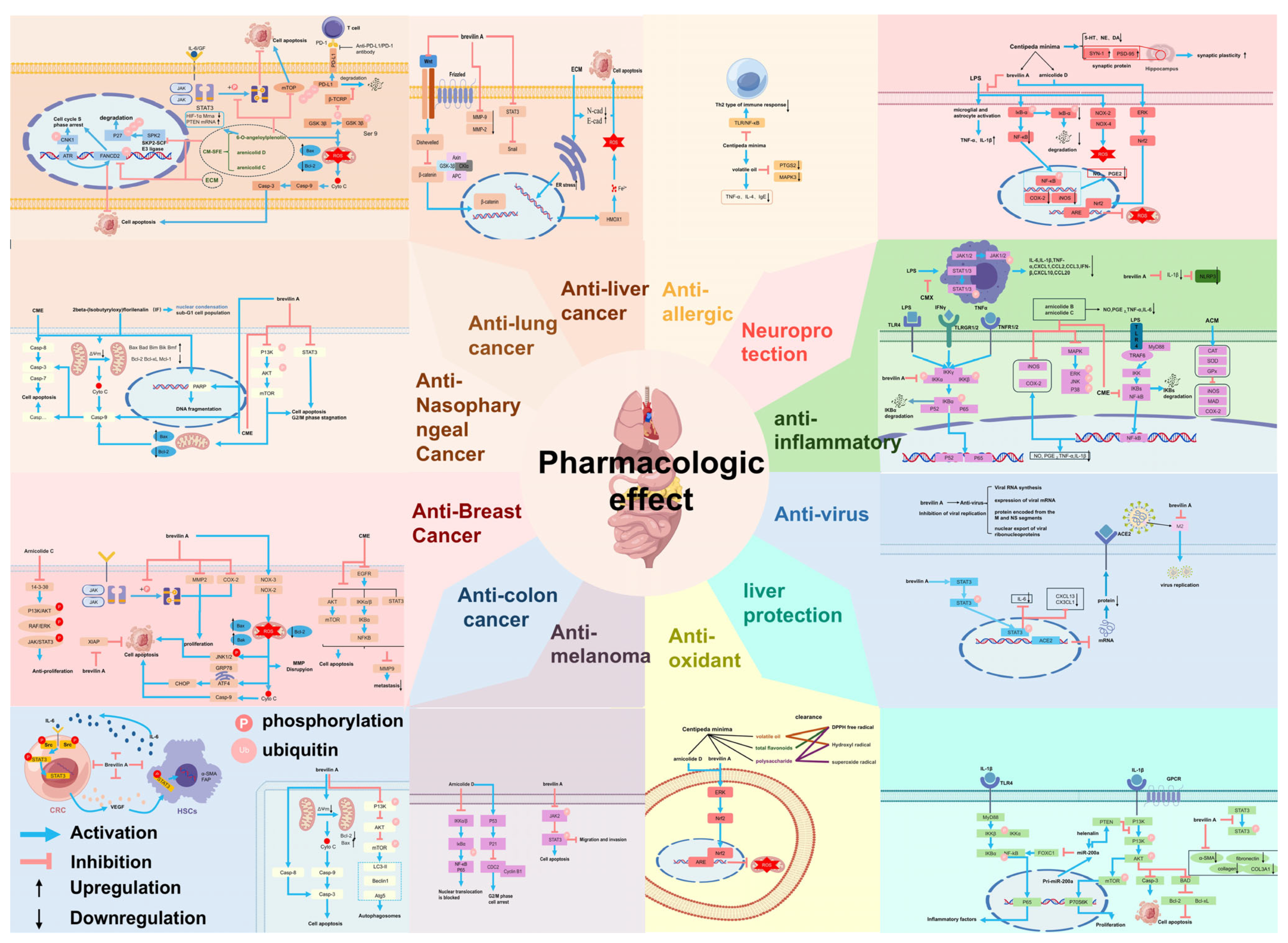
3. Pharmacological Effects
3.1. Anti-Allergy
3.2. Anti-Tumour
3.2.1. Anti-Lung Cancer
3.2.2. Anti-Nasopharyngeal Cancer
3.2.3. Anti-Liver Cancer
3.2.4. Anti-Breast Cancer
3.2.5. Anti-Colon Cancer
3.2.6. Anti-Melanoma
3.2.7. Others
3.3. Anti-Depressant (Neuroprotective)
3.4. Antioxidant
3.5. Antibacterial and Anti-Inflammatory
3.6. Hepatoprotective Effect
3.7. Antiviral
3.8. Others
4. Security Evaluation
5. Clinical Applications
6. Quality Marker Prediction
6.1. Predictive Analysis of Quality Markers Based on Plant Affinities and Chemical Specificity
6.2. Predictive Analysis of Quality Markers Based on Correlation Between Composition and Efficacy
6.3. Predictive Analysis of Quality Markers Based on Chemical Composition Measurability
6.4. Predictive Analysis of Quality Markers Based on Incoming Blood Components
6.5. Predictive Analysis of Quality Markers Based on Correlation of Ingredients with Modern Clinical Use
6.6. Summary
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China 2020; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 1902. [Google Scholar]
- Tan, J.; Qiao, Z.; Meng, M.; Zhang, F.; Kwan, H.Y.; Zhong, K.; Yang, C.; Wang, Y.; Zhang, M.; Liu, Z.; et al. Centipeda minima: An update on its phytochemistry, pharmacology and safety. J. Ethnopharmacol. 2022, 292, 115027. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, G.; Zhan, X.; Wang, Z.; Wang, Y.; Liu, H.; Hou, X.; Shi, W.; Ma, J.; Bai, Z.; et al. Brevilin A inhibits NLRP3 inflammasome activation in vivo and in vitro by acting on the upstream of NLRP3-induced ASC oligomerization. Mol. Immunol. 2021, 135, 116–126. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, J.; Wang, Y.; Zhang, X.; Shi, Y.; Liang, Y.; Guo, D.; Yang, M. Mechanism of allergic rhinitis treated by Centipeda minima from different geographic areas. Pharm. Biol. 2021, 59, 606–618. [Google Scholar] [CrossRef]
- Liu, C.-X.; Cheng, Y.-Y.; Guo, D.-A.; Zhang, T.-J.; Li, Y.-Z.; Hou, W.-B.; Huang, L.-Q.; Xu, H.-Y. A New Concept on Quality Marker for Quality Assessment and Process Control of Chinese Medicines. Chin. Herb. Med. 2017, 9, 3–13. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y. Research progress on essential oils of Cenpiteda minima (L.), A. Br. Aschers. Shandong Chem. Ind. 2018, 47, 66–68. [Google Scholar]
- Su, F.; Yang, G.; Hu, D.; Ruan, C.; Wang, J.; Zhang, Y.; Zhu, Q. Chemical Composition, Antibacterial and Antioxidant Activities of Essential Oil from Centipeda minima. Molecules 2023, 28, 824. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, H.; Tu, W. Essential Oil GC-MS study of Centipeda minima. Chin. Herb. Med. 2002, 33, 20–21. [Google Scholar]
- Hsu, C.Y.; Rajabi, S.; Hamzeloo-Moghadam, M.; Kumar, A.; Maresca, M.; Ghildiyal, P. Sesquiterpene lactones as emerging biomolecules to cease cancer by targeting apoptosis. Front. Pharmacol. 2024, 15, 1371002. [Google Scholar] [CrossRef]
- Jialing, S.; Ze, Z.; Zedan, Z.; Cuiyun, G.; Ruyu, W.; Huafeng, P.; Xiangzhen, F. Research progress of chemical constituents and pharmacological actions of Ebushicao (Centipeda minima). J. Hunan Univ. Chin. Med. 2024, 44, 516–530. [Google Scholar]
- Pu, S. Studies on the Chemical Constituents and their Anti-Timor Activity in Centipeda minima Lour. & Hydrocotyle sibthorpioides Lam. Ph.D. Thesis, Tianjin University, Tianjin, China, 2009. [Google Scholar]
- Pu, S.; Guo, Y.; Gao, W. Chemical constituents from Centipeda minima. Chin. Tradit. Herb. Drugs 2009, 40, 363–365. [Google Scholar]
- Pu, S.; Guo, Y.; Gao, W. Chemical constituents from Centipeda minima. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Medica 2009, 34, 1520–1522. [Google Scholar]
- Wu, H.; Liu, Y.; Yang, Y.; Xiao, M. Study on Chemical Constituents from Ethyl Acetate Fraction of Centipeda minima. Shizhen Guoyi Guoyao 2010, 21, 1096–1098. [Google Scholar]
- Wang, Y. Study on the chemical constituents of Centipeda minima. Hai Xia Yao Xue 2019, 31, 84–86. [Google Scholar]
- Yang, L.; Gao, Q.; Zhou, L. Study on chemical constituents of Centipeda minima and anti-inflammatory activity. J. Chin. Med. Mater. 2023, 46, 1924–1930. [Google Scholar]
- Xie, J. Studies on the Chemical Composition of Centipeda minimca and Helleborus thibetanus. Master’s Thesis, University of Jinan, Jinan, China, 2021. [Google Scholar]
- Liang, H.; Bao, F.; Dong, X.; Tan, R.; Zhang, C.; Lu, Q.; Cheng, Y. Antibacterial thymol derivatives isolated from Centipeda minima. Molecules 2007, 12, 1606–1613. [Google Scholar] [CrossRef]
- Chan, C.O.; Xie, X.J.; Wan, S.W.; Zhou, G.L.; Yuen, A.C.; Mok, D.K.; Chen, S.B. Qualitative and quantitative analysis of sesquiterpene lactones in Centipeda minima by UPLC-Orbitrap-MS & UPLC-QQQ-MS. J. Pharm. Biomed. Anal. 2019, 174, 360–366. [Google Scholar]
- Chan, C.O.; Jin, D.P.; Dong, N.P.; Chen, S.B.; Mok, D.K. Qualitative and quantitative analysis of chemical constituents of Centipeda minima by HPLC-QTOF-MS & HPLC-DAD. J. Pharm. Biomed. Anal. 2016, 125, 400–407. [Google Scholar]
- Lin, Y.; Lv, Q.; Chen, S. Isolation structural elucidation of sesquiterpene lactones from Centipeda minima (L.), A. Br. et Aschers. and bioassay on their anticancer activities. Cent. South Pharm. 2019, 17, 356–359. [Google Scholar]
- Xie, X. Study on the Anti-Tumor Active Components and Their Fingerprints of Centipeda minima. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2007. [Google Scholar]
- Wu, P.; Su, M.X.; Wang, Y.; Wang, G.C.; Ye, W.C.; Chung, H.Y.; Li, J.; Jiang, R.W.; Li, Y.L. Supercritical fluid extraction assisted isolation of sesquiterpene lactones with antiproliferative effects from Centipeda minima. Phytochemistry 2012, 76, 133–140. [Google Scholar] [CrossRef]
- Wu, P.; Li, X.G.; Liang, N.; Wang, G.C.; Ye, W.C.; Zhou, G.X.; Li, Y.L. Two new sesquiterpene lactones from the supercritical fluid extract of Centipeda minima. J. Asian Nat. Prod. Res. 2012, 14, 515–520. [Google Scholar] [CrossRef]
- Wu, J.B.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: Sesquiterpenes of potential anti-allergy activity. Chem. Pharm. Bull. 1991, 39, 3272–3275. [Google Scholar] [CrossRef]
- Su, M.; Li, Y.; Chung, H.Y.; Ye, W. 2beta-(Isobutyryloxy)florilenalin, a sesquiterpene lactone isolated from the medicinal plant Centipeda minima, induces apoptosis in human nasopharyngeal carcinoma CNE cells. Molecules 2009, 14, 2135–2146. [Google Scholar] [CrossRef]
- Ding, L.F.; Liu, Y.; Liang, H.X.; Liu, D.P.; Zhou, G.B.; Cheng, Y.X. Two new terpene glucosides and antitumor agents from Centipeda minima. J. Asian Nat. Prod. Res. 2009, 11, 732–736. [Google Scholar] [CrossRef]
- Liang, H.X.; Bao, F.K.; Dong, X.P.; Zhu, H.J.; Lu, X.J.; Shi, M.; Lu, Q.; Cheng, Y.X. Two new antibacterial sesquiterpenoids from Centipeda minima. Chem. Biodivers. 2007, 4, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.-H. Chemical constituents of triterpenes from Centipeda minima and its anti-inflammatory activities. Zhong Cao Yao 2020, 51, 4907–4915. [Google Scholar]
- Heng-Xing, L.; Fu-Kai, B.; Xiao-Ping, D.; Qing, L.; Yong-Xian, C. Antibacterial triterpenes from Centipeda minima (Compositae). Plant Divers. 2007, 29, 479. [Google Scholar]
- Wang, J.; Xue, P.; Guang, C.; Wang, Y.; Ma, T.; Qiu, F. Study on chemical constituents from n-BuOHextract of Centipeda minima (L.), A. Br. et Aschers. J. Tianjin Univ. Trad. Chin. Med. 2021, 40, 253–259. [Google Scholar]
- Chao, Y. Screening of Effective Sites for Goose-paste Allergy-resistant Rhinitis and the Preparation Process of Its Nasal Spray. Master’s Thesis, Hubei University of Traditional Chinese Medicine, Wuhan, China, 2019. [Google Scholar]
- Gao, C.; Pan, H.; Ma, F.; Zhang, Z.; Zhao, Z.; Song, J.; Li, W.; Fan, X. Centipeda minima active components and mechanisms in lung cancer. BMC Complement. Med. Ther. 2023, 23, 89. [Google Scholar] [CrossRef]
- Cao, J.; Li, G. Chemical constituents of Centipeda minima. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Medica 2012, 37, 2301–2303. [Google Scholar]
- Jiaojiao, Z.; Zhiming, B.; Yan, H.; Lijuan, L. Chemical Constituents of Centipeda minima. Pharm. Clin. Res. 2013, 21, 133–134. [Google Scholar]
- Wu, L.; Liu, Y.; Chen, M.; Bi, Z.; Wang, H.; Liu, E. Chemical constituents of Centipeda minima. Cent. South Pharm. 2016, 14, 351–354. [Google Scholar]
- Zhu, Y. Study on the Chemical Constituents and the Antitumor Activity of Centipeda minima. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2012. [Google Scholar]
- Cordeiro, M.; Martins, V.; Silva, A.P.D.; Rocha, H.A.O.; Rachetti, V.P.S.; Scortecci, K.C. Phenolic Acids as Antidepressant Agents. Nutrients 2022, 14, 4309. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Yan, B.; Zhang, Y.; Wu, H. Study on the chemical constituents of n-butanol extract fraction from Centipeda minima. Shizhen Guoyi Guoyao 2013, 24, 2358–2359. [Google Scholar]
- Bingwu, Z. Study on the Chemical Constituents of N-butanol Extract Fraction and Quality Analysis of Centipeda minima. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2013. [Google Scholar]
- Wu, J.B.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: Isolation of a new plenolin ester and the antiallergy activity of sesquiterpene lactones. Chem. Pharm. Bull. 1985, 33, 4091–4094. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Yu, H.-M.; Wen, S.-l.; Liu, Y.-L. Histopathological study on allergic rhinitis treated with Centipeda minima. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Medica 2005, 30, 292–294. [Google Scholar]
- Jun, L.; Chengyi, S.; Jianle, X.; Zhuoping, L. Basic Study on the Regulation of Th2 Immune Response in Rats with Allergic Rhinitis by Centipeda minima Extract Product via Mediating Toll-like Receptor-Nuclear Factor Kappa B Signaling Pathway. Chin. Arch. Tradit. Chin. 2024, 1–11. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Z.; Chen, Y.F.; Zhang, S.B.; He, D.H.; Wei, S.F.; Wang, Q.; Pan, H.F.; Liu, Y.Q. Centipeda minima extract sensitizes lung cancer cells to DNA-crosslinking agents via targeting Fanconi anemia pathway. Phytomedicine 2021, 91, 153689. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xue, P.-H.; Yu, H.; Wang, M.; Zhao, X.-C.; Qiu, F.; Zhou, G.-B. Screening of anti-lung cancer compounds and in vitro mechanism of helenalin-isovalerate for Centipeda minima. Chin. Trad. Pat. Med. 2020, 42, 3151–3157. [Google Scholar]
- Wang, H.C.; Wu, P.E.; He, W.D.; Chen, C.Y.; Zheng, R.Q.; Pang, Y.C.; Wu, L.C.; Cheng, Y.X.; Liu, Y.Q. Centipeda minima extracts and the active sesquiterpene lactones have therapeutic efficacy in non-small cell lung cancer by suppressing Skp2/p27 signaling pathway. J. Ethnopharmacol. 2025, 340, 119277. [Google Scholar] [CrossRef]
- Wang, M.; Guo, H.; Sun, B.B.; Jie, X.L.; Shi, X.Y.; Liu, Y.Q.; Shi, X.L.; Ding, L.Q.; Xue, P.H.; Qiu, F.; et al. Centipeda minima and 6-O-angeloylplenolin enhance the efficacy of immune checkpoint inhibitors in non-small cell lung cancer. Phytomedicine 2024, 132, 155825. [Google Scholar] [CrossRef]
- Wang, R.; Gao, C.; Yu, M.; Song, J.; Feng, Z.; Wang, R.; Pan, H.; Liu, H.; Li, W.; Fan, X. Mechanistic prediction and validation of Brevilin A Therapeutic effects in Lung Cancer. BMC Complement. Med. Ther. 2024, 24, 214. [Google Scholar] [CrossRef]
- Khan, M.; Maryam, A.; Saleem, M.Z.; Shakir, H.A.; Qazi, J.I.; Li, Y.; Ma, T. Brevilin A induces ROS-dependent apoptosis and suppresses STAT3 activation by direct binding in human lung cancer cells. J. Cancer 2020, 11, 3725–3735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, R.Y.; Zhang, J.; Zhang, W.X.; Huang, Z.H.; Hu, H.F.; Li, Y.L.; Li, B.; He, Q.Y. Inhibition of Nrf2 enhances the anticancer effect of 6-O-angeloylenolin in lung adenocarcinoma. Biochem. Pharmacol. 2017, 129, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Q.; Sun, H.Y.; Chan, C.O.; Liu, B.B.; Wu, J.H.; Chan, S.W.; Mok, D.K.; Tse, A.K.; Yu, Z.L.; Chen, S.B. Centipeda minima (Ebushicao) extract inhibits PI3K-Akt-mTOR signaling in nasopharyngeal carcinoma CNE-1 cells. Chin. Med. 2015, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qu, Z.; Lin, Y.; Lee, C.S.; Tai, W.C.; Chen, S. Brevilin A Induces Cell Cycle Arrest and Apoptosis in Nasopharyngeal Carcinoma. Front. Pharmacol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Liu, R.; Dow Chan, B.; Mok, D.K.; Lee, C.S.; Tai, W.C.; Chen, S. Arnicolide D, from the herb Centipeda minima, Is a Therapeutic Candidate against Nasopharyngeal Carcinoma. Molecules 2019, 24, 1908. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Chapter One–Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. In Advances in Cancer Research; Sarkar, D., Fisher, P.B., Eds.; Academic Press: New York, NY, USA, 2021; Volume 149, pp. 1–61. [Google Scholar]
- Wei, J.; Zhao, X.; Wu, B.; Tan, Z.; Xie, Y.; Wei, M.; Wu, L. Centipeda minima extract exhibits anti-liver cancer effects via the ER stress/HMOX1/Fe2+/ROS pathway. Phytomedicine 2025, 139, 156487. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, H. In vitro evaluation of anti-hepatoma activity of brevilin A: Involvement of Stat3/Snail and Wnt/β-catenin pathways. RSC Adv. 2019, 9, 4390–4396. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, X.; Chen, J.; Zhang, B.; Xie, S.; Yuan, Y.; Sun, L.; Yuan, S.; Yu, H.; Ding, J.; et al. Arnicolide C Suppresses Tumor Progression by Targeting 14-3-3θ in Breast Cancer. Pharmaceuticals 2024, 17, 224. [Google Scholar] [CrossRef]
- Wen, W.; Jin, K.; Che, Y.; Du, L.Y.; Wang, L.N. Arnicolide D Inhibits Oxidative Stress-induced Breast Cancer Cell Growth and Invasion through Apoptosis, Ferroptosis, and Parthanatos. Anticancer. Agents Med. Chem. 2024, 24, 836–844. [Google Scholar] [CrossRef]
- Lee, M.M.; Chan, B.D.; Wong, W.Y.; Qu, Z.; Chan, M.S.; Leung, T.W.; Lin, Y.; Mok, D.K.; Chen, S.; Tai, W.C. Anti-cancer Activity of Centipeda minima Extract in Triple Negative Breast Cancer via Inhibition of AKT, NF-κB, and STAT3 Signaling Pathways. Front. Oncol. 2020, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.Z.; Nisar, M.A.; Alshwmi, M.; Din, S.R.U.; Gamallat, Y.; Khan, M.; Ma, T. Brevilin A Inhibits STAT3 Signaling and Induces ROS-Dependent Apoptosis, Mitochondrial Stress and Endoplasmic Reticulum Stress in MCF-7 Breast Cancer Cells. Onco Targets Ther. 2020, 13, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Ngeow, J.; Eng, C. Oh GxE! The Complexity of Body Mass Index and Colon Cancer Risk. J. Natl. Cancer Inst. 2021, 113, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Meng, M.; Li, B.; Chen, H.; Tan, J.; Xu, K.; Xiao, S.; Kwan, H.Y.; Liu, Z.; Su, T. Brevilin A is a potent anti-metastatic CRC agent that targets the VEGF-IL6-STAT3 axis in the HSCs-CRC interplay. J. Transl. Med. 2023, 21, 260. [Google Scholar] [CrossRef]
- You, P.; Wu, H.; Deng, M.; Peng, J.; Li, F.; Yang, Y. Brevilin A induces apoptosis and autophagy of colon adenocarcinoma cell CT26 via mitochondrial pathway and PI3K/AKT/mTOR inactivation. Biomed. Pharmacother. 2018, 98, 619–625. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Su, T.; Wang, Y.P.; Wang, X.N.; Li, C.Y.; Zhu, P.L.; Huang, Y.M.; Yang, Z.Y.; Chen, S.B.; Yu, Z.L. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of brevilin A. Life Sci. 2020, 241, 117169. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, Z.; Fu, X.; Li, J.; Yin, C.; Chou, J.; Wang, Y.; Liu, Y.; Chen, Y.; Bai, J.; et al. Arnicolide D exerts anti-melanoma effects and inhibits the NF-κB pathway. Phytomedicine 2019, 64, 153065. [Google Scholar] [CrossRef]
- You, P.; Tang, L.; Zhu, Y.; Tian, Y. Brevilin A shows an anti-tumor role in prostate cancer via the lncRNA H19/miR-194/E2F3 signaling pathway. Aging 2023, 15, 4411–4428. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, R.; Hu, Y.; Yang, Y.; Tian, Y. Arnicolide D Inhibits Proliferation and Induces Apoptosis of Osteosarcoma Cells through PI3K/Akt/mTOR Pathway. Anticancer. Agents Med. Chem. 2024, 24, 1288–1294. [Google Scholar] [CrossRef]
- Lee, D.; Kwak, H.J.; Kim, B.H.; Kim, D.W.; Kim, H.Y.; Kim, S.H.; Kang, K.S. Brevilin A Isolated from Centipeda minima Induces Apoptosis in Human Gastric Cancer Cells via an Extrinsic Apoptotic Signaling Pathway. Plants 2022, 11, 1658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Cui, X.; Lv, D.; Jin, L.; Khan, M.; Ma, T. Brevilin A promotes oxidative stress and induces mitochondrial apoptosis in U87 glioblastoma cells. Onco Targets Ther. 2018, 11, 7031–7040. [Google Scholar] [CrossRef] [PubMed]
- Changlong, L.; Hezhen, W.; Yongping, H.; Yanfang, Y.; Yanwen, L.; Jianwen, L. 6-O-Angeloylenolin induces apoptosis through a mitochondrial/caspase and NF-kappaB pathway in human leukemia HL60 cells. Biomed. Pharmacother. 2008, 62, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shiying, D. Centipeda minima Improves Reserpine-Induced Depression-Like Behavior in Rat. Master’s Thesis, University of South China, Hengyang, China, 2023. [Google Scholar]
- Zhou, Y.L.; Yan, Y.M.; Li, S.Y.; He, D.H.; Xiong, S.; Wei, S.F.; Liu, W.; Hu, L.; Wang, Q.; Pan, H.F.; et al. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol. Sin. 2020, 41, 10–21. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, X.Y.; Hao, X.Y.; Yan, Y.M.; Hong, M.; Wei, S.F.; Zhou, Y.L.; Wang, Q.; Cheng, Y.X.; Liu, Y.Q. Ethanol Extract of Centipeda minima Exerts Antioxidant and Neuroprotective Effects via Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9421037. [Google Scholar] [CrossRef]
- Tan, Y.; He, F.; Chen, Z.-W.; Zhang, Y.-L.; Zhang, C.; Gu, R.-X.; Zhang, D.-Y.; Wang, X.; Hua, Q. Scalp mechanical stimulation promotes moderate vasodilation and the permeability of blood-brain barrier in anesthetized mice. Chin. J. Pharmacol. Toxicol. 2019, 6, 41. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ya, J.; Guanghui, Z.; Meihuan, W. Extraction process optimization and antioxidant activity of total flavonoids from Centipeda minima. J. Int. Pharm. Res. 2020, 47, 666–670. [Google Scholar]
- Yang, G.; Su, F.; Hu, D.; Ruan, C.; Che, P.; Zhang, Y.; Wang, J. Optimization of the Extraction Process and Antioxidant Activity of Polysaccharide Extracted from Centipeda minima. Chem. Biodivers. 2023, 20, e202200626. [Google Scholar] [CrossRef]
- Taylor, R.S.; Towers, G.H. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry 1998, 47, 631–634. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.; Leung, T.W.; Shum, T.Y.; Cho, W.C.; Chen, S.; Tai, W.C. Centipeda minima Extract Attenuates Dextran Sodium Sulfate-Induced Acute Colitis in Mice by Inhibiting Macrophage Activation and Monocyte Chemotaxis. Front. Pharmacol. 2021, 12, 738139. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Chiu, C.S.; Lin, T.H.; Lee, M.M.; Lee, C.Y.; Chang, S.J.; Hou, W.C.; Huang, G.J.; Deng, J.S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhou, Y.L.; He, D.H.; Liu, W.; Fan, X.Z.; Wang, Q.; Pan, H.F.; Cheng, Y.X.; Liu, Y.Q. Centipeda minima extract exerts antineuroinflammatory effects via the inhibition of NF-κB signaling pathway. Phytomedicine 2020, 67, 153164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kim, B.H.; Yun, S.K.; Roh, Y.S. Centipeda minima Extract Inhibits Inflammation and Cell Proliferation by Regulating JAK/STAT Signaling in Macrophages and Keratinocytes. Molecules 2023, 28, 1723. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Zhang, Q.; Mou, X.; Zhao, Y.; Fan, H.; Xu, H.; Chen, D.; Qiu, F.; Zhao, F. Two sesquiterpene lactones, arnicolide B and arnicolide C, isolated from Centipeda minima, exert anti-inflammatory effects in LPS stimulated RAW 264.7 macrophages via inactivation of the MAPK pathway. Nat. Prod. Res. 2023, 37, 2969–2972. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Jiang, Y.; Yuan, Y.; Yang, L.; Hu, Q.; Tang, J.; Meng, X.; Xie, C.; Shen, X. Brevilin A Ameliorates Acute Lung Injury and Inflammation Through Inhibition of NF-κB Signaling via Targeting IKKα/β. Front. Pharmacol. 2022, 13, 911157. [Google Scholar]
- Xue, P.H.; Zhang, N.; Liu, D.; Zhang, Q.R.; Duan, J.S.; Yu, Y.Q.; Li, J.Y.; Cao, S.J.; Zhao, F.; Kang, N.; et al. Cytotoxic and Anti-Inflammatory Sesquiterpenes from the Whole Plants of Centipeda minima. J. Nat. Prod. 2021, 84, 247–258. [Google Scholar] [CrossRef]
- Fang, B.; Wen, S.; Li, Y.; Bai, F.; Wei, Y.; Xiong, Y.; Huang, Q.; Lin, X. Prediction and verification of target of helenalin against hepatic stellate cell activation based on miR-200a-mediated PI3K/Akt and NF-κB pathways. Int. Immunopharmacol. 2021, 92, 107208. [Google Scholar] [CrossRef]
- Park, Y.J.; Jeon, M.S.; Lee, S.; Kim, J.K.; Jang, T.S.; Chung, K.H.; Kim, K.H. Anti-fibrotic effects of brevilin A in hepatic fibrosis via inhibiting the STAT3 signaling pathway. Bioorg. Med. Chem. Lett. 2021, 41, 127989. [Google Scholar] [CrossRef]
- Liang, L.J.; Wang, D.; Yu, H.; Wang, J.; Zhang, H.; Sun, B.B.; Yang, F.Y.; Wang, Z.; Xie, D.W.; Feng, R.E.; et al. Transcriptional regulation and small compound targeting of ACE2 in lung epithelial cells. Acta Pharmacol. Sin. 2022, 43, 2895–2904. [Google Scholar] [CrossRef]
- Zhang, X.L.; He, J.; Huang, W.H.; Huang, H.B.; Zhang, Z.M.; Wang, J.J.; Yang, L.; Wang, G.C.; Wang, Y.F.; Li, Y.L. Antiviral Activity of the Sesquiterpene Lactones from Centipeda minima against Influenza A Virus in vitro. Nat. Prod. Commun. 2018, 13, 115–119. [Google Scholar] [CrossRef]
- Zhang, X.L.; Xia, Y.P.; Yang, L.; He, J.; Li, Y.L.; Xia, C. Brevilin A, a Sesquiterpene Lactone, Inhibits the Replication of Influenza A Virus In Vitro and In Vivo. Viruses 2019, 11, 835. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, C.; Zhu, B.; Peng, H.; Ni, F. Study on the antiasthmatic effects of essential oil from Centipeda minima. Chin. J. Mod. Appl. Pharm. 2010, 27, 473–476. [Google Scholar]
- Baek, J.Y.; Kim, B.H.; Kim, D.W.; Lee, W.Y.; Kim, C.E.; Kim, H.Y.; Pyo, J.; Park, E.S.; Kang, K.S. Hair Growth Effect of DN106212 in C57BL/6 Mouse and Its Network Pharmacological Mechanism of Action. Curr. Issues Mol. Biol. 2023, 45, 5071–5083. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, M.J.; Lee, W.Y.; Pyo, J.; Shin, M.S.; Hwang, G.S.; Shin, D.; Kim, C.E.; Park, E.S.; Kang, K.S. Hair Growth Stimulation Effect of Centipeda minima Extract: Identification of Active Compounds and Anagen-Activating Signaling Pathways. Biomolecules 2021, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, F.; Wu, Q.; Li, S.; Ruan, G.; Yang, J.; Yu, C.; Jiang, N.; Xiao, Y.; Liu, Y. Brevilin A enhances innate immunity and the resistance of oxidative stress in Caenorhabditis elegans via p38 MAPK pathway. Int. Immunopharmacol. 2022, 113 Pt A, 109385. [Google Scholar] [CrossRef]
- Huang, W.; Yu, X.; Liang, N.; Ge, W.; Kwok, H.F.; Lau, C.B.; Li, Y.; Chung, H.Y. Anti-angiogenic Activity and Mechanism of Sesquiterpene Lactones from Centipeda minima. Nat. Prod. Commun. 2016, 11, 435–438. [Google Scholar] [CrossRef]
- Chabert, P.; Fougerousse, A.; Brouillard, R. Anti-mitotic properties of resveratrol analog (Z)-3,5,4′-trimethoxystilbene. Biofactors 2006, 27, 37–46. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, L.; Wang, C. Two cases of serious adverse reactions caused by internal administration of Centipeda minima its discussion. Zhejiang J. Tradit. Chin. Med. 2020, 55, 509. [Google Scholar]
- Ruan, Q.; Wang, C.; Zhang, Y.; Sun, J. Brevilin A attenuates cartilage destruction in osteoarthritis mouse model by inhibiting inflammation and ferroptosis via SIRT1/Nrf2/GPX4 signaling pathway. Int. Immunopharmacol. 2023, 124 Pt B, 110924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y. Progress of clinical application and pharmacological research of Centipeda minima. Agric. Jilin 2015, 364, 76–77. [Google Scholar]
- Wang, X.; Wang, Z.; Cao, L.; He, H.; Wang, Z.; Li, X.; Miao, M. Medication rule analysis and pharmacological analysis of traditional Chinese medicine treating allergic rhinitis based on data mining, network pharmacology and experimental validation. Nat. Prod. Res. Dev. 2024, 1, 137–154. [Google Scholar]
- Xiong, Y.A.; Li, W.; Lv, J.; Chen, L.; Ou, S.; Wang, S. Study on Material Basis of Miao Medicine Bi-Ning Spray in Allergic Rhinitis Treatment. World Sci. Technol.-Mod. Tradit. Chin. Med. 2017, 28, 880–884. [Google Scholar]
- Zherui, G.; Baijian, G. Clinical application of Cough Relief and Anti-inflammatory Powder. J. Tradit. Chin. Med. 1981, 43. [Google Scholar]
- Lu, L.; Bin, J.; Suyi, L.; Xiaobin, C.; Hui, Z.; Pei, T. Study on UPLC Fingerprints and QAMS of Centipeda minima Dispensing Granules. Chin. J. Ethnomed. Ethnopharmacy 2024, 33, 27–34. [Google Scholar]
- Shi, G.L.; Lu, X.; Zheng, Y.H.; Yang, T.; Zhu, E.Y.; Song, Y.H.; Huang, P.T. Insights into the potential dual-antibacterial mechanism of Kelisha capsule on Escherichia coli. BMC Complement. Med. Ther. 2024, 24, 207. [Google Scholar] [CrossRef]
- Sijun, Z. Emergency Medicine for Children with High Fever–Zekepine Injection. Chin. Pract. J. Rural Dr. 2007, 64. [Google Scholar]
- Yip, K.M.; Lee, K.M.; Ng, T.B.; Xu, S.J.; Yung, K.K.L.; Qu, S.G.; Cheung, A.K.L.; Sze, S.C.W. An anti-inflammatory and anti-fibrotic proprietary Chinese medicine nasal spray designated as Allergic Rhinitis Nose Drops (ARND) with potential to prevent SARS-CoV-2 coronavirus infection by targeting RBD (Delta)-angiotensin converting enzyme 2 (ACE2) binding. Chin. Med. 2022, 17, 88. [Google Scholar]
- Xiao-hui, T.; Jing, H.; Chao-xiang, R.; Jie, S.; Jin, P.; Qing-hua, W.; Jiang, C. Analysis of genetic diversity relationship in medicinal plants of Compositae by, I.S.S.R. Nat. Prod. Res. Dev. 2018, 30, 1764. [Google Scholar]
- Borgo, J.; Wagner, M.S.; Laurella, L.C.; Elso, O.G.; Selener, M.G.; Clavin, M.; Bach, H.; Catalán, C.A.N.; Bivona, A.E.; Sepúlveda, C.S.; et al. Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties. Molecules 2024, 29, 814. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus Essential Oil: An Update of Its Phytochemistry, Biological Activities, and Safety Profile. Oxid. Med. Cell. Longev. 2022, 2022, 8442734. [Google Scholar] [CrossRef] [PubMed]
- Wanqing, A.; Tong, S.; Yadan, L.; Jiayi, Z.; Xiaowen, O.; Qiaoqiao, Z.; Yebing, Z.; Lei, Z.; Hongquan, Z. Research Progress on Chemical Constituents and Pharmacological Effects of Chrysanthemum Plants. Mod. Chin. Med. 2025, 27, 562–574. [Google Scholar]
- Sun, Y.-P. Expression of pungent-taste herbs and their applications in clinical compatibility. Zhongcaoyao 2015, 46, 785–790. [Google Scholar]
- Ruiqi, L.; Mingsan, M. Modern research and relationship of warm Chinese medicine properties. Acta Chin. Med. 2012, 27, 1456–1459. [Google Scholar]
- Wang, X.; Lu, S.; Zheng, S. Empirical analysis of the channel tropism traditional Chinese medicine in chemical constituent, pharmacological effects and clinical application. Zhong Hua Zhong Yi Yao Za Zhi 2018, 33, 5193–5197. [Google Scholar]
- Liu, J.; Zheng, W.; He, Y.; Zhang, W.; Luo, Z.; Liu, X.; Jiang, X.; Meng, F.; Wu, L. A Review of the Research Applications of Centipeda minima. Molecules 2023, 29, 108. [Google Scholar] [CrossRef]
- Zhang, T.-J.; Bai, G. The concept, core theory and research methods of Chinesemedicine quality markers. Yao Xue Xue Bao 2019, 54, 187–196. [Google Scholar]
- Zan, K.; Xie, Y.; Guo, L.; Zheng, J.; Ma, S. Study on characteristic chromatogram and quantitation method of seven components for Centipedae Herba. Chin. J. Pharm. Anal. 2018, 38, 151–157. [Google Scholar]
- Ren, H.; Lv, Q.; Peng, Y. Simultaneous determination of five sesquiterpene lactones in Centipeda minima by HPLC/UV Assay. Mod. Chin. Med. 2019, 21, 616–621. [Google Scholar]
- Ying, G.; Dan, C.; Yili, L.; Huaping, Z.; Shubin, L. Thin-layer chromatographic identification and gas chromatographic determination of Centipeda minima. Fujian J. Tradit. Chin. Med. 2016, 47, 14–17. [Google Scholar]
- Chen, W.; Yang, H.; Chen, Y.; Wang, H.; Chen, X. Quality evaluation of Centipedae Herba from different origins based on multi-component quantification combined with multivariate statistical analysis. Nat. Prod. Res. Dev. 2024, 36, 1573–1583. [Google Scholar]
- Rongfa, Z. Metabolism of Centipeda minima in Rats. Master’s Thesis, Kunming Medical University, Kunming, China, 2022. [Google Scholar]
- Liu, Y.; Chen, X.Q.; Liang, H.X.; Zhang, F.X.; Zhang, B.; Jin, J.; Chen, Y.L.; Cheng, Y.X.; Zhou, G.B. Small compound 6-O-angeloylplenolin induces mitotic arrest and exhibits therapeutic potentials in multiple myeloma. PLoS ONE 2011, 6, e21930. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Xiong, Y.A.; Ou, S.; Wang, Y. Study on HPLC Fingerprint of Bining Nasal Spray. China Pharm. 2017, 4278–4282. [Google Scholar]
- Liu, J.; Wang, S.-Y.; Dou, H.-Q. GC Determination of Chrysanthenyl Acetate in Bisaitong Spray. Chin. J. Pharm. Anal. 2005, 25, 840–842. [Google Scholar]
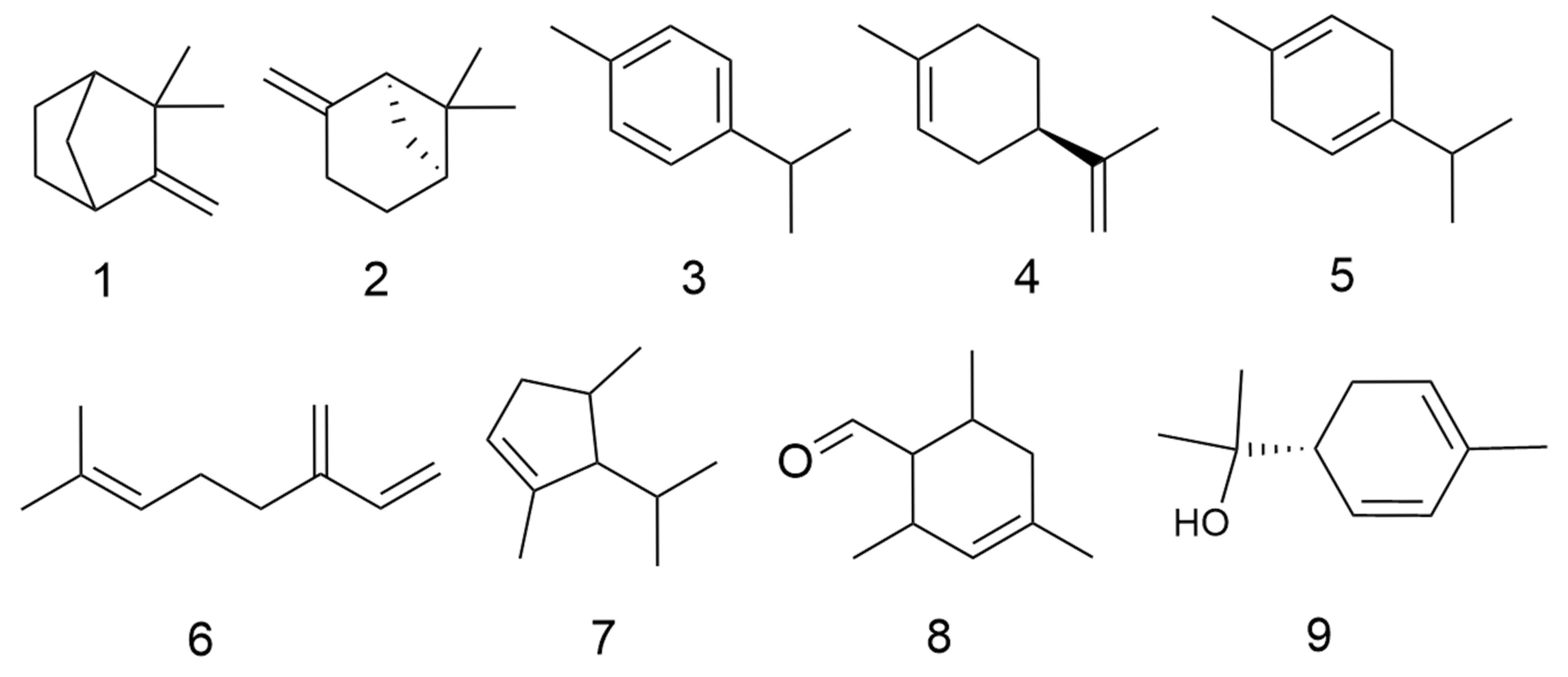
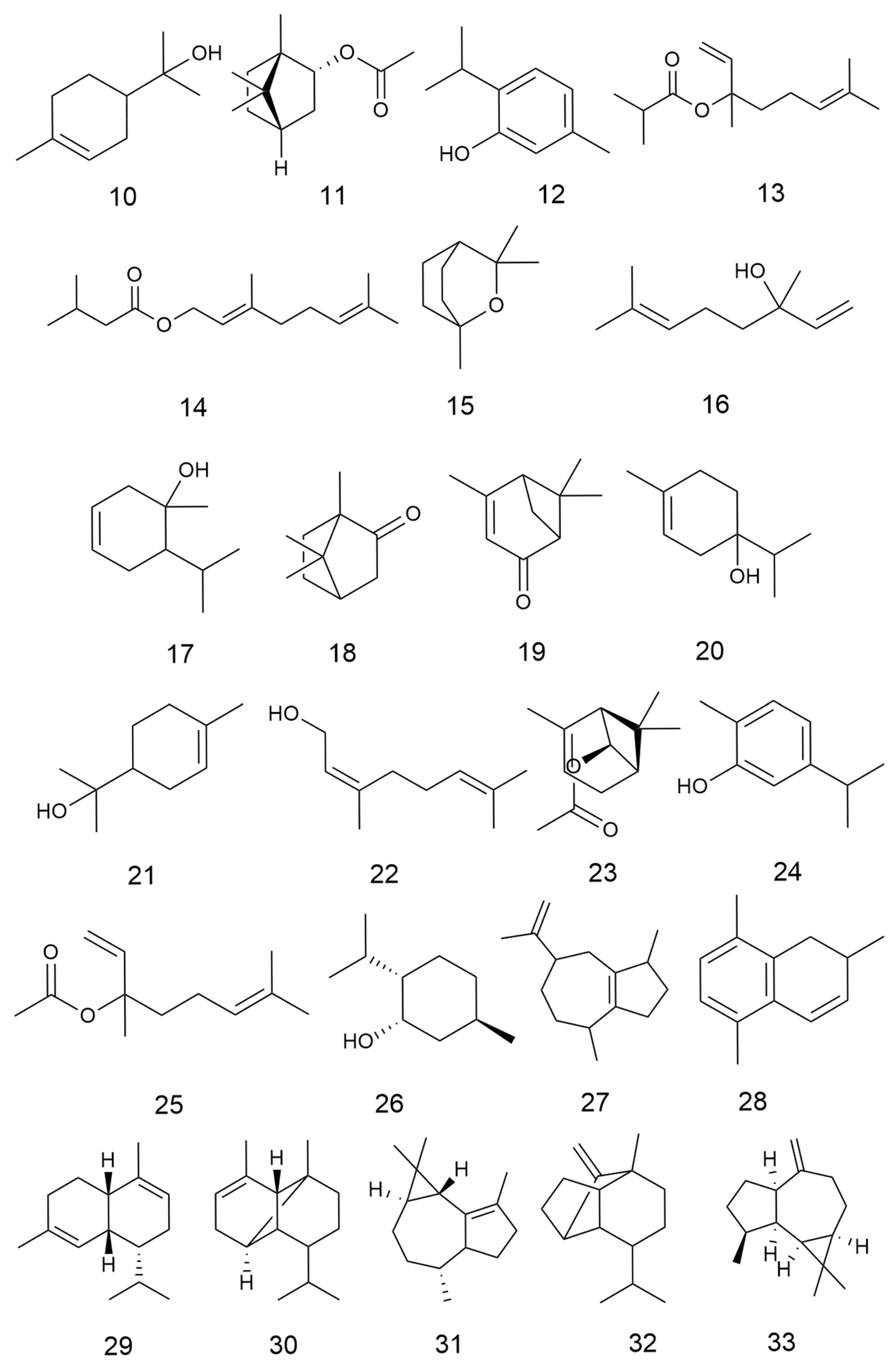
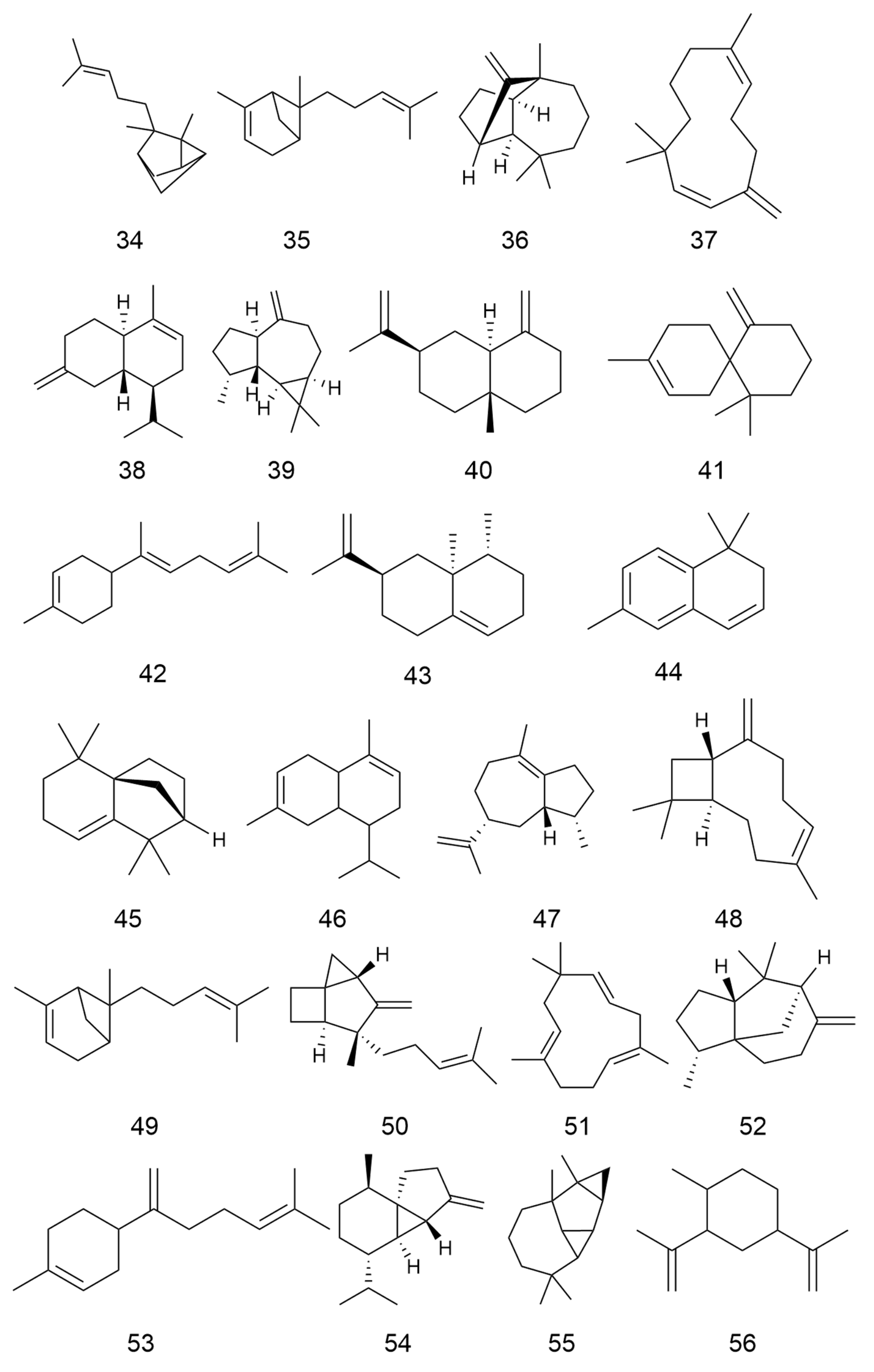
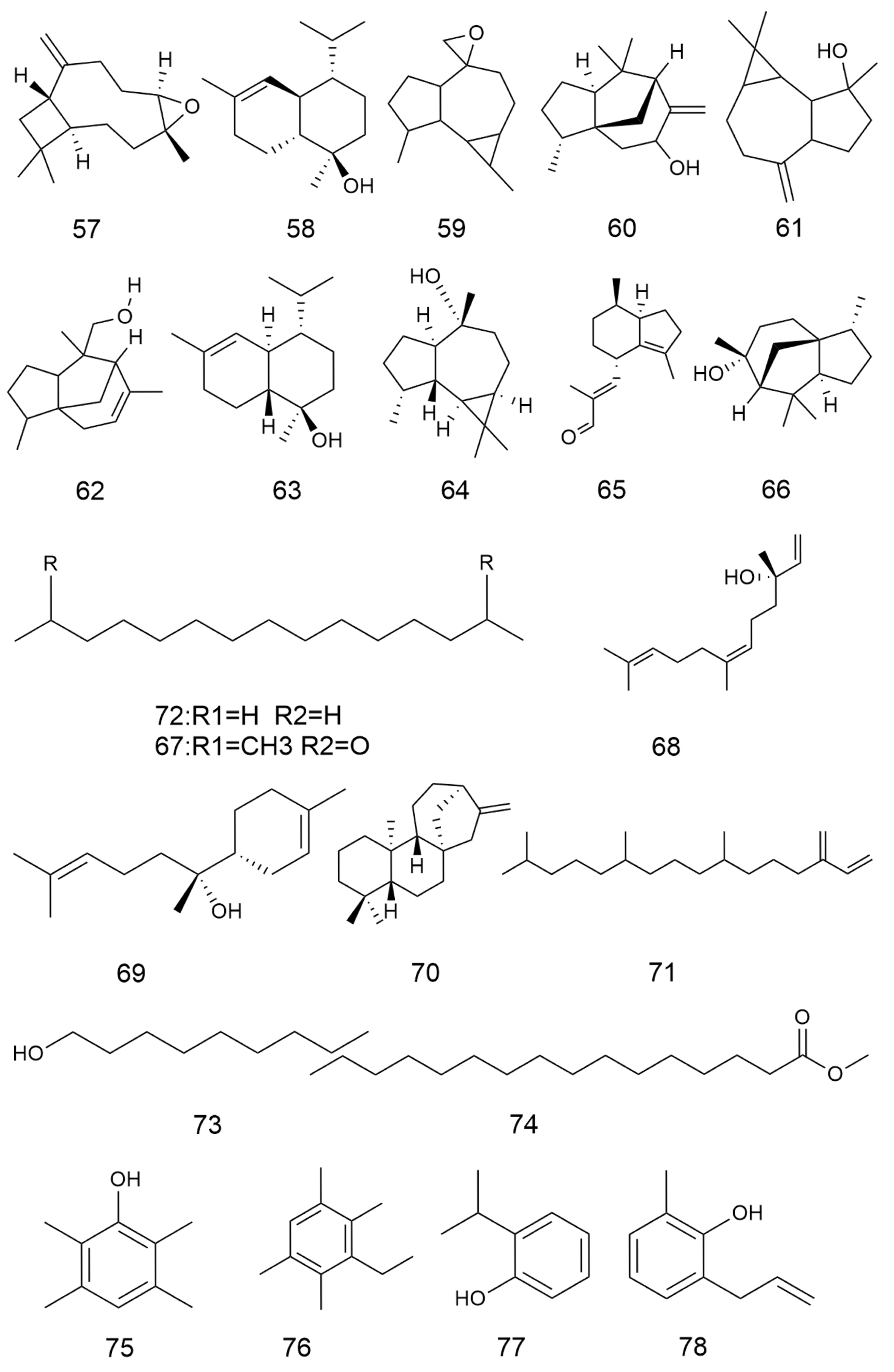
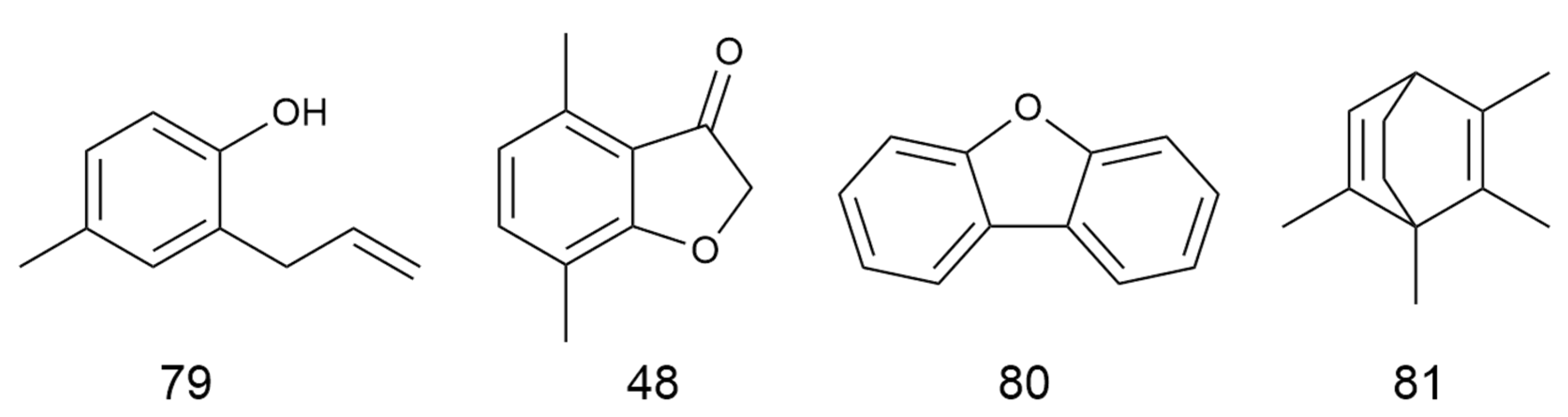
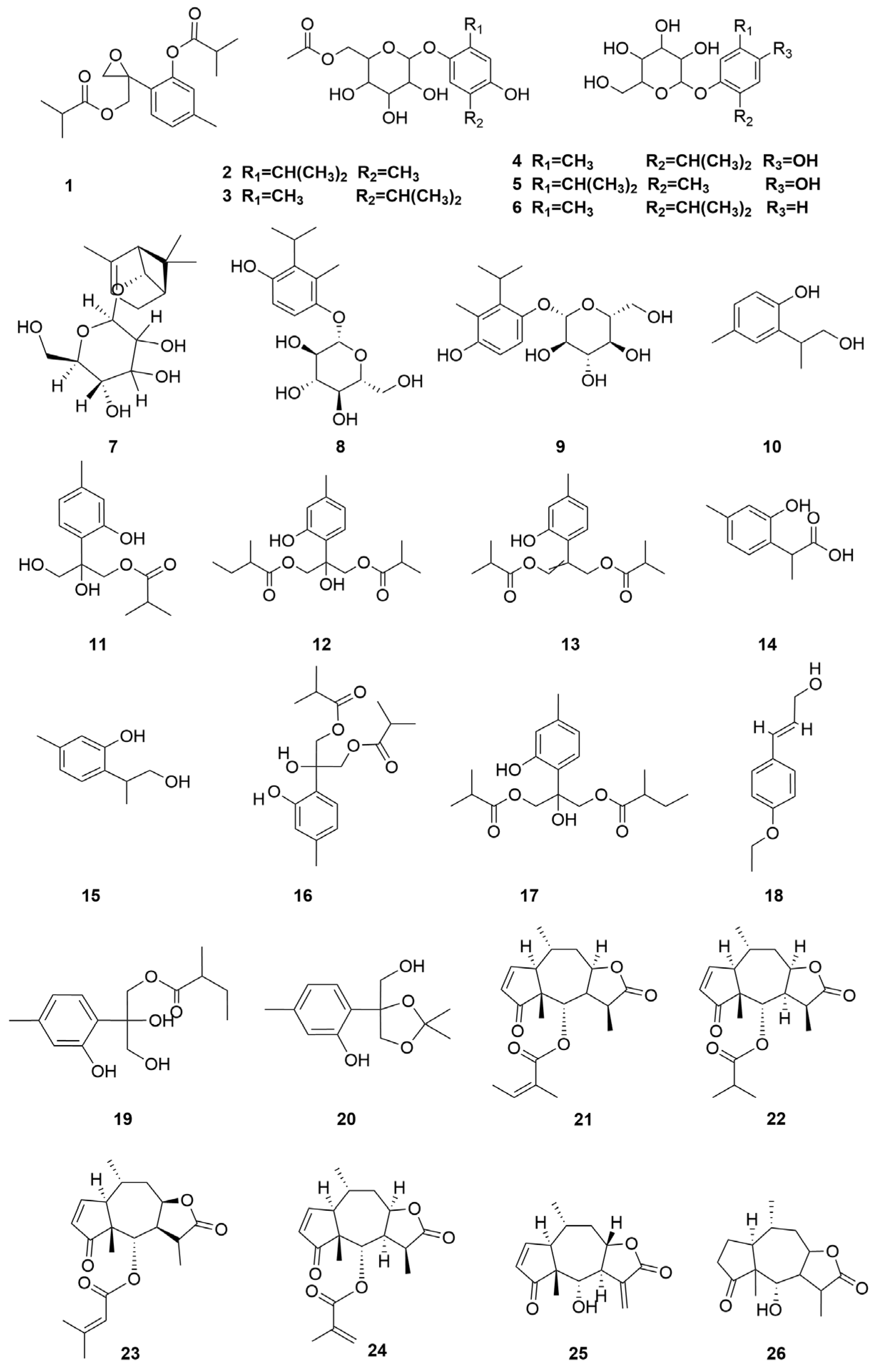
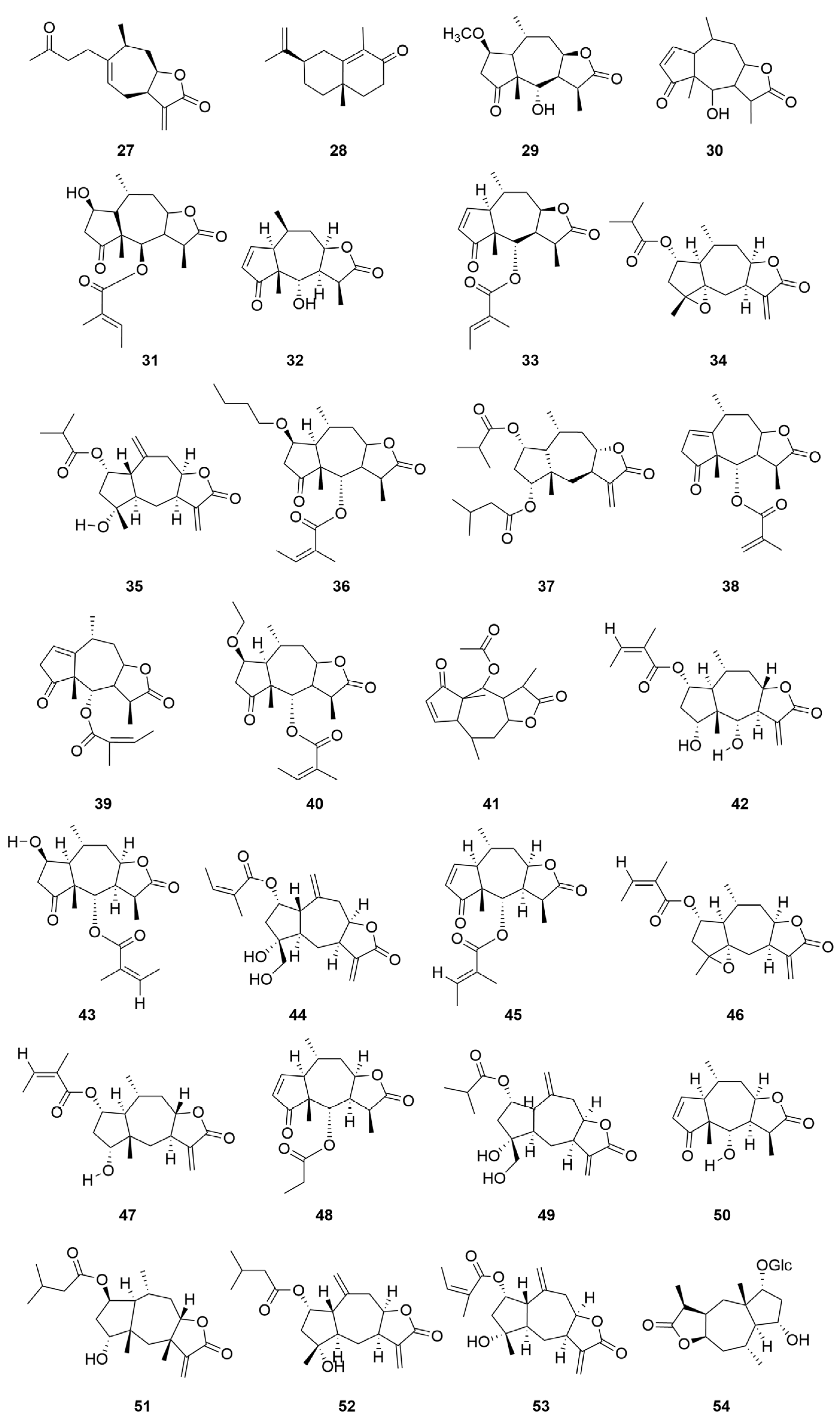
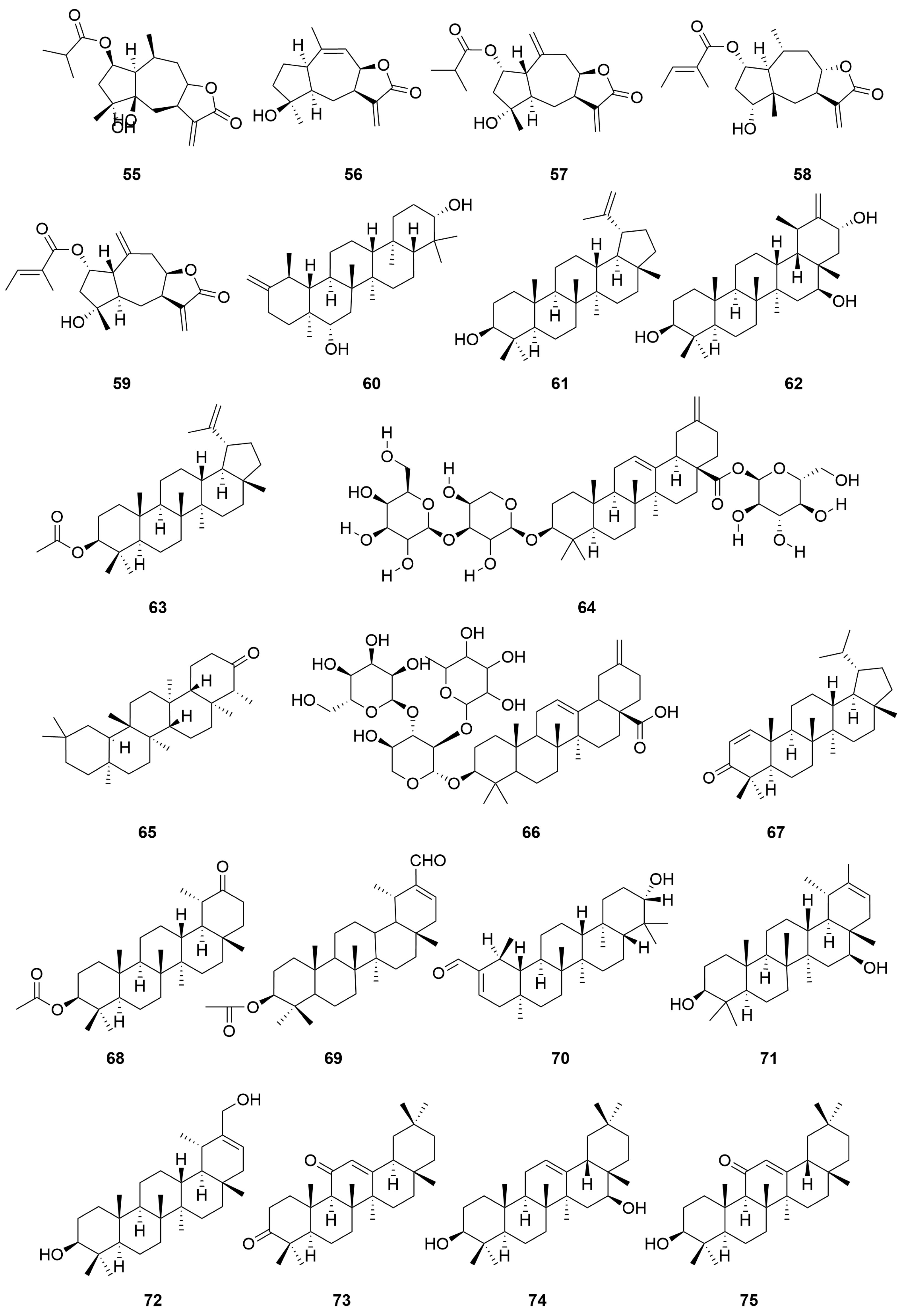
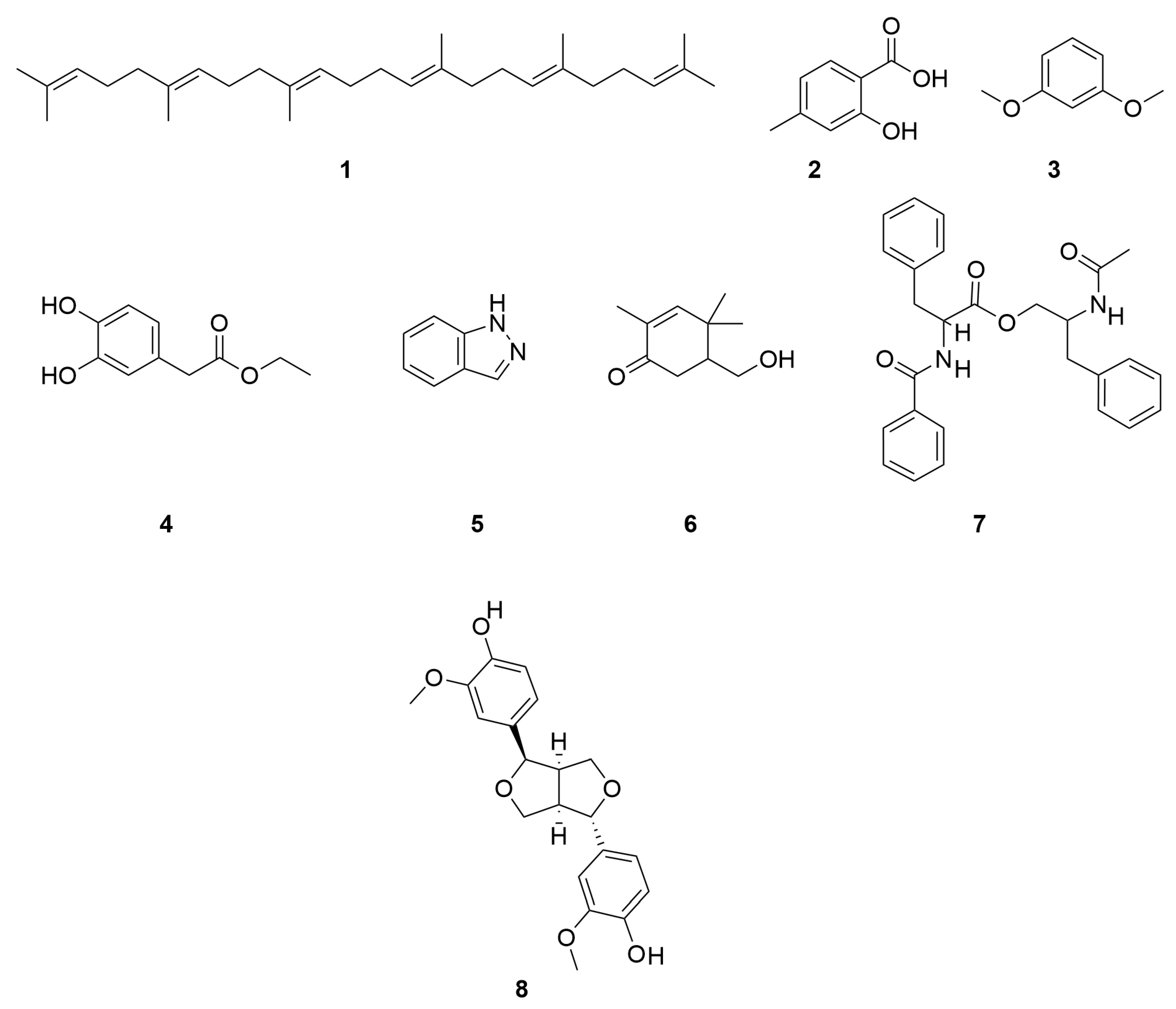
| No. | Categories | Chemical Compound | Chemical Formula | Relative Content (%) | Reference |
|---|---|---|---|---|---|
| 1 | Monoterpene | Camphene | C10H16 | 0.08 | [8] |
| 2 | Beta-pinene | C10H16 | 0.11 | [8] | |
| 3 | P-cymene | C10H14 | 0.24 | [8] | |
| 4 | (+)-Limonene | C10H16 | 0.27 | [8] | |
| 5 | γ-terpinene | C10H16 | 0.13 | [8] | |
| 6 | Myrcene | C10H16 | 0.32 | [8] | |
| 7 | 1,4-Dimethyl-5-isopropylcyclopentene | C10H18 | 0.22 | [8] | |
| 8 | Oxygenated monoterpene | 2,4,6-Trimethyl-3-cyclohexene-1-Carboxaldehyde | C10H16O | 0.84 | [7] |
| 9 | α-Phellandrene-8-ol | C10H16O | 0.14 | [7] | |
| 10 | α-Terpineol | C10H18O | 0.22 | [7] | |
| 11 | Bornyl acetate | C12H20O2 | 0.29 | [7] | |
| 12 | Thymol | C10H14O | 5.69 | [7] | |
| 13 | Linalyl isobutyrate | C14H24O2 | 1.57 | [7] | |
| 14 | Geranyl isovalerate | C15H26O2 | 1.66 | [7] | |
| 15 | Cineole | C10H18O | 1.53 | [8] | |
| 16 | Linalool | C10H18O | 0.51 | [7,8] | |
| 17 | (E)-para-2-menthen-1-ol | C10H18O | 0.11 | [8] | |
| 18 | Camphor | C10H16O | 0.35 | [7,8] | |
| 19 | (−)-Verbenone | C10H14O | 2.76 | [8] | |
| 20 | 4-Methyl-1-isopropyl-3-cyclohexenol | C10H18O | 0.78 | [8] | |
| 21 | Terpineol | C10H18O | 0.22 | [8] | |
| 22 | Nerol | C10H18O | 0.15 | [7,8] | |
| 23 | Trans-Chrysanthenyl acetate | C12H18O2 | 20.23 | [7,8] | |
| 24 | Carvacrol | C10H14O | 0.52 | [7,8] | |
| 25 | Linalyl acetate | C12H20O2 | 2.20 | [8] | |
| 26 | Neo-Menthol | C10H20O | 1.51 | [7] | |
| 27 | Sesquiterpene | α-Guaiene | C15H24 | 1.00 | [7] |
| 28 | 1,4,6-trimethyl-5,6-dihydronaphthalene | C13H16 | 0.14 | [7] | |
| 29 | α-Amorphene | C15H24 | 0.15 | [7] | |
| 30 | α-Copaene | C15H24 | 1.27 | [7] | |
| 31 | α-Gurjunene | C15H24 | 0.91 | [7] | |
| 32 | Sativen | C15H24 | 0.17 | [7] | |
| 33 | Allo-Aromadendrene | C15H24 | 1.20 | [7] | |
| 34 | α-Santalene | C15H24 | 0.87 | [7] | |
| 35 | α-Bergamotene | C15H24 | 0.23 | [7] | |
| 36 | Longifolene | C15H24 | 0.65 | [7] | |
| 37 | Humule[7,8]ne | C15H24 | 1.83 | [7] | |
| 38 | γ-Cadinene | C15H24 | 0.31 | [7] | |
| 39 | Aromadendrene | C15H24 | 11.47 | [7] | |
| 40 | β-Selinene | C15H24 | 0.18 | [7] | |
| 41 | β-Chamigrene | C15H24 | 0.89 | [7] | |
| 42 | α-Bisabolene | C15H24 | 0.21 | [7] | |
| 43 | Valencene | C15H24 | 0.57 | [7] | |
| 44 | 1,1,6-trimethyl-1,2-dihydronaphthalene | C13H16 | 0.29 | [7] | |
| 45 | Isolongifolene | C15H24 | 1.09 | [7,8] | |
| 46 | 1-Isopropyl-4,7-dimethyl-1,2,4a,5,8,8a-hexahydronaphthalene | C15H24 | 0.43 | [8] | |
| 47 | α-bulnesene | C15H24 | 0.71 | [8] | |
| 48 | Isocaryophyllene | C15H24 | 0.73 | [8] | |
| 49 | α-bergamotene | C15H24 | 1.87 | [8] | |
| 50 | β-Santalene | C15H24 | 2.29 | [7,8] | |
| 51 | β-caryophyllene | C15H24 | 5.26 | [7,8] | |
| 52 | β-Cedrene | C15H24 | 0.21 | [8] | |
| 53 | β-bisabolene | C15H24 | 0.55 | [7,8] | |
| 54 | β-cubebene | C15H24 | 0.11 | [8] | |
| 55 | Longicyclene | C15H24 | 0.59 | [7] | |
| 56 | 1-Methyl-2,4-diisopropenylcyclohexane | C13H22 | 0.22 | [8] | |
| 57 | Oxygenated sesquiterpene | β-Caryophyllene oxide | C15H24O | 1.03 | [7] |
| 58 | α-Cadinol | C15H26O | 0.23 | [7] | |
| 59 | Aromadendrene oxide | C15H24O | 0.48 | [7] | |
| 60 | Cedrenol | C15H24O | 0.47 | [7] | |
| 61 | Spathulenol | C15H24O | 0.38 | [7] | |
| 62 | Cedr-8-en-13-ol | C15H24O | 0.35 | [7] | |
| 63 | Muurolol | C15H26O | 0.33 | [7] | |
| 64 | Globulol | C15H26O | 0.53 | [7] | |
| 65 | Valerenal | C15H22O | 0.56 | [7] | |
| 66 | Cedrol | C15H26O | 0.25 | [7] | |
| 67 | Hexahydrofarnesyl acetone | C18H36O | 1.28 | [7] | |
| 68 | D-Nerolidol | C15H26O | 0.54 | [7] | |
| 69 | α-Bisabolol | C15H26O | 0.22 | [8] | |
| 70 | Diterpenes | Kaur-16-ene | C20H32 | 0.53 | [7] |
| 71 | Neophytadiene | C20H38 | 0.74 | [7] | |
| 72 | Aliphatic hydrocarbons | Pentadecane | C15H32 | 0.25 | [7] |
| 73 | Oxygenated aliphatic | 1-Nonanol | C9H20O | 0.11 | [8] |
| 74 | Methyl hexadecanoate | C17H34O2 | 0.59 | [7] | |
| 75 | Aromatic compounds | 2,3,5,6-tetramethyl-Phenol | C10H14O | 2.49 | [7] |
| 76 | 3-Ethyl-1,2,4,5-tetramethylbenzene | C12H18 | 1.05 | [7] | |
| 77 | 2-isopropylphenol | C9H12O | 0.44 | [8] | |
| 78 | Phenylbutazone | 2-Allyl-6-methylphenol | C10H120 | 0.20 | [7] |
| 79 | 4-methyl-2-prop-2-enyl-phenol | C10H12O | 0.38 | [8] | |
| 80 | 4,7-dimethyl-3(2H)-Benzofuranone | C10H10O2 | 0.49 | [7] | |
| 81 | Others | Dibenzofuran | C12H8O | 4.24 | [7] |
| 82 | 1,2,3,6-Tetramethylbicyclo [2.2.2]octa-2,5-diene | C12H18 | 2.79 | [7,8] |
| No. | Categories | Chemical Compound | Chemical Formula | References |
|---|---|---|---|---|
| 1 | Monoterpene | 10-Isobutyryloxy-8,9-epoxythymol isobutyrate | C18H24O5 | [10] |
| 2 | Thymohydroquinone-3-O-β-6′-acetyl-Glc | C18H26O8 | [11] | |
| 3 | Thymohydroquinone-6-O-β-6′-acetyl-Glc | C18H26O8 | [11] | |
| 4 | Zataroside-A | C16H24O7 | [11] | |
| 5 | Zataroside-B | C16H24O7 | [11] | |
| 6 | Thymol-3-O-β-glucoside | C16H24O6 | [11,12] | |
| 7 | (−)-Cis-chrysanthenol-O-β-D-glucopyranoside | C16H26O6 | [11,13] | |
| 8 | Thymoquinol 2-O-β-glucopyranoside | C16H24O7 | [12] | |
| 9 | Thymoquinol 5-O-β-glucopyranoside | C16H24O7 | [12] | |
| 10 | [2-(1-Hydroxypropan-2-yl)-4-methylphenol] | C10H14O2 | [14] | |
| 11 | 10-Dihydroxy-9-isobutyryloxythymol | C14H20O5 | [15,16] | |
| 12 | 8-Hydroxy-10-isobutyryloxy-9-(2-methylbutanoyl) | C19H28O6 | [15] | |
| 13 | Isobutyric acid 2-(2-hydroxy-4-methyl-phenyl)-3-isobutyryloxy-allyl ester | C18H24O5 | [15] | |
| 14 | 9-Carboxylthymol | C10H12O3 | [15] | |
| 15 | 9-Hydroxythymol | C10H14O2 | [15,16] | |
| 16 | 8-Hydroxy-9,10-diisobutyryloxythymol | C18H26O6 | [15,17,18] | |
| 17 | 8-Hydroxy-9-isobutyryloxy-10(2)-methylbutyryloxythymol | C19H28O6 | [16] | |
| 18 | E-p-coumaryl alcohol ethyl ether | C11H14O2 | [16] | |
| 19 | 8,10-Dihydroxy-9(2)-methylbutyryloxythymol | C15H22O5 | [18] | |
| 20 | 10-Hydroxy-8,9-dioxyisopropylidenethymol | C13H18O4 | [18] | |
| 21 | Sesquiterpene | Brevilin A | C20H26O5 | [11,13] [15,17,19,20,21] |
| 22 | Arnicolide C | C19H26O5 | [11,13,15,19,20,21] | |
| 23 | Senecioylplenolin | C20H26O5 | [22] | |
| 24 | Arnicolide D | C19H24O5 | [11,13,15,17,19,20,21,23] | |
| 25 | Helenalin | C15H18O4 | [11,14,24] | |
| 26 | Tetrahydrohelenalin | C15H22O4 | [22] | |
| 27 | Tomentosin | C15H20O3 | [22] | |
| 28 | α-Cyperone | C15H22O | [25] | |
| 29 | 2-Methoxytetrahydrohelenalin | C16H24O5 | [11] | |
| 30 | Dihydrohelenalin | C15H20O4 | [11,12] | |
| 31 | 2β-Hydroxyl-2,3-dihydrogen-6-O-angeloplenolin | C20H28O6 | [14] | |
| 32 | 11,13-Dihydrohelenalin | C15H20O4 | [14] | |
| 33 | Microhenalin C | C20H26O5 | [15,17,21] | |
| 34 | Minimolide F | C19H26O5 | [15,19,21,23] | |
| 35 | 2-β-(Isobutyryloxy)florilenalin | C19H26O5 | [15] | |
| 36 | (1S,2R,5R,6S,7R,8R,10R,11S)-4-Oxo-2β-butoxy-6α-angeloyloxypseudoguaia-8β,12-olide | C24H36O6 | [17] | |
| 37 | Centiplide A | C24H36O6 | [17] | |
| 38 | Cenminolide C | C19H24O5 | [17] | |
| 39 | Cenminolide A | C20H26O5 | [17] | |
| 40 | Minimolide B | C22H32O6 | [23] | |
| 41 | Arnicolide A | C17H22O5 | [19] | |
| 42 | Minimolide C | C20H28O6 | [19,23] | |
| 43 | Minimolide A | C20H28O6 | [19,23] | |
| 44 | Minimolide H | C20H26O6 | [19,24] | |
| 45 | Microhelenin C | C20H26O5 | [19] | |
| 46 | Minimolide E | C20H26O5 | [19,23] | |
| 47 | Minimolide D | C20H28O5 | [19,23] | |
| 48 | Arnicolide G | C18H24O5 | [19] | |
| 49 | Minimolide G | C19H26O6 | [24,26] | |
| 50 | Plenolin | C15H20O4 | [23] | |
| 51 | Pulchellin-2α-O-isovalerate | C21H32O5 | [23] | |
| 52 | Florilenalin-2α-O-isovalerate | C20H28O5 | [23] | |
| 53 | Florilenalin-2α-O-angelate | C20H26O5 | [23] | |
| 54 | Minimaoside B | C21H34O9 | [27] | |
| 55 | 4,5b-Dihydroxy-2β-(isobutyryloxy)-10βH-guai-11(13)-en-12,8β-olide | C19H28O6 | [28] | |
| 56 | 4-Hydroxy-1βH-guaia-9,11(13)-dien-12,8α-olide | C15H20O3 | [28] | |
| 57 | 2β-(Isobutyryloxy)florilenalin | C19H26O5 | [28] | |
| 58 | Pulchellin-2α-O-tiglate | C20H28O5 | [28] | |
| 59 | Florilenalin-2α-O-tiglate | C20H26O5 | [28] | |
| 60 | Triterpene | Arnidiol | C30H50O2 | [29] |
| 61 | Lupeol | C30H50O | [29] | |
| 62 | Ursane-20(30)-en-3β,16β,21α-triol | C30H50O3 | [30] | |
| 63 | Lupeol acetate | C32H52O2 | [16] | |
| 64 | Friedelin | C30H50O | [16] | |
| 65 | Guaiacin D | C46H72O16 | [31] | |
| 66 | Nudicaucin A | C46H72O17 | [31] | |
| 67 | Lupenone | C30H48O | [16] | |
| 68 | 20-Oxo-30-nortaraxastan-3β-yl acetate | C31H50O3 | [29] | |
| 69 | 3β-Acetoxytaraxaster-20-en-30-al | C32H50O3 | [29] | |
| 70 | 3β-Hydroxytaraxaster-20-en-30-al | C30H48O2 | [29] | |
| 71 | Faradiol | C30H50O2 | [29] | |
| 72 | Taraxast-20-ene-3β,30-diol | C30H50O2 | [29] | |
| 73 | Taraxast-20-ene-3β,30-diol | C30H46O2 | [29] | |
| 74 | 18α-Oleana-12-en-3,11-dione | C30H50O2 | [29] | |
| 75 | Maniladiol | C30H48O2 | [29] | |
| 76 | 3β-Hydroxy-oleana-12-en-11-one | C30H50O2 | [29] | |
| 77 | Coflodiol | C30H50O2 | [29] | |
| 78 | 3β,16β-Hydroxy-lupine diol | C30H50O2 | [29] | |
| 79 | Lup-20(29)-ene-3b,16b-diol | C30H52O2 | [29] | |
| 80 | Garcinielliptone Q | C30H50 | [16] | |
| 81 | Taraxasterol acetate | C32H52O2 | [16] | |
| 82 | Pesudotaraxasterol acetate | C32H52O2 | [16,29] | |
| 83 | Taraxasterone | C30H48O | [16] | |
| 84 | 3β,21β-Dihydroxy-20(30)-en-taraxastane | C30H50O2 | [29] | |
| 85 | Taraxasterol | C30H50O | [14,16,29] |
| No. | Chemical Compound | Chemical Formula | References |
|---|---|---|---|
| 1 | Quercetin-3-methyl-ether | C16H12O7 | [2] |
| 2 | Quercetin 3,3′-dimethyl ether | C17H14O7 | [2] |
| 3 | Apigenin | C15H10O5 | [11] |
| 4 | Nobiletin | C21H22O8 | [33] |
| 5 | Luteolin | C15H10O6 | [11] |
| 6 | Tricin | C17H14O7 | [11,13] |
| 7 | 2-O-Methylquercetin | C16H12O7 | [11] |
| 8 | Quercetin | C15H10O7 | [11,12,34] |
| 9 | Rutin | C27H30O16 | [11,20] |
| 10 | Kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyrano side | C27H30O15 | [11] |
| 11 | 3-O-Methylquercetin | C16H12O7 | [17,34] |
| 12 | (2R,3R)-(+)-7,4′-Di-O-methyldihydrokaempferol | C17H16O6 | [34] |
| 13 | Iristectorin A | C23H24O12 | [34] |
| 14 | Isocutellarein 7-methyl ether | C16H12O6 | [34] |
| 15 | Hispidulin | C16H12O6 | [34] |
| 16 | Casticin | C19H18O8 | [35] |
| 17 | Artemitin | C20H20O8 | [35] |
| 18 | Chrysosplenol D | C18H16O8 | [36] |
| 19 | Luteolin-7-O-glucoside | C21H20O11 | [31] |
| 20 | Quercetin-3-O-β-D-glucoside | C21H20O12 | [31] |
| 21 | Apigenin-7-O-β-D-glucuronide | C21H18O11 | [31] |
| 22 | Kaempferol-3-O-rutinoside | C27H30O15 | [20] |
| 23 | Isorhamnetin | C16H12O7 | [20] |
| No. | Chemical Compound | Chemical Formula | References |
|---|---|---|---|
| 1 | β-sitosterol | C30H52O | [11,12,16,37] |
| 2 | Stigmasterol | C29H48O | [11,12,17] |
| 3 | Stigmasterol-3-O-β-D-glucopyranoside | C35H58O6 | [14] |
| 4 | Spinasterol | C29H48O | [37] |
| 5 | [5α,8α-epidioxy-(22E,20S,24R)-ergosta-6,22-dien-3β-ol] | C28H44O3 | [37] |
| 6 | β-Sitosterylpalmitate | C35H60O6 | [14] |
| No. | Chemical Compound | Chemical Formula | References |
|---|---|---|---|
| 1 | Ethyl caffeate | C11H12O4 | [11,12] |
| 2 | Methy 3,5-Di-O-caffeoylquinate | C26H26O12 | [11,13] |
| 3 | 3,5-Di-O-caffeoylquinicacid | C25H24O12 | [11,13] |
| 4 | DL-leucine | C6H13NO2 | [11,13] |
| 5 | Dl-Phenylalanine | C9H11NO2 | [11,13] |
| 6 | 4-Amino-2-oxo-3H-chromene-4-carboxylic acid | C10H9NO4 | [11,13] |
| 7 | Benzoic acid | C7H6O2 | [35,36] |
| 8 | Pentadecanoic acid | C15H30O2 | [39,40] |
| 9 | Stearic Acid | C18H36O2 | [39,40] |
| 10 | Uracil | C4H4N2O2 | [39,40] |
| 11 | N-hexadecanoic acid | C16H32O2 | [39] |
| 12 | Adenine | C5H5N5 | [40] |
| 13 | Uridine | C9H12N2O6 | [40] |
| 14 | Chlorogenic acid | C16H18O9 | [20,31] |
| 15 | Protocatechuicacid | C7H6O4 | [15] |
| 16 | Paraben | C7H6O3 | [31] |
| 17 | Salicylic acid | C7H6O3 | [31] |
| 18 | Sinapic acid | C11H12O5 | [31] |
| 19 | Methyl sinapate | C12H14O5 | [31] |
| 20 | Ferulic acid | C10H10O4 | [31] |
| 21 | Methyl caffeate acid | C10H10O4 | [31] |
| 22 | Syringic acid | C9H10O5 | [31] |
| 23 | Vanillic acid | C8H8O4 | [16] |
| 24 | Cryptochlorogenic acid | C16H18O9 | [20] |
| 25 | Caffeic acid | C9H8O4 | [20] |
| 26 | Isochlorogenic acid B | C25H24O12 | [20] |
| 27 | Isochlorogenic acid A | C25H24O12 | [20] |
| 28 | Isochlorogenic acid C | C25H24O12 | [20] |
| 29 | 3-O-caffeoyl-α-glueopyranose | C15H18O9 | [34] |
| 30 | 3-O-caffeoyl-β-glucopyranose | C15H18O9 | [34] |
| 31 | 4,5-Di-O-caffeoylquinic acid methyl ester | C26H26O12 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Z.; Wang, Y.; Zhu, T.; Niu, W.; Lu, T.; Lou, R. Centipeda minima: A Review of Phytochemistry, Pharmacology, and Predictive Analysis on Quality Markers. Molecules 2025, 30, 4072. https://doi.org/10.3390/molecules30204072
Shang Z, Wang Y, Zhu T, Niu W, Lu T, Lou R. Centipeda minima: A Review of Phytochemistry, Pharmacology, and Predictive Analysis on Quality Markers. Molecules. 2025; 30(20):4072. https://doi.org/10.3390/molecules30204072
Chicago/Turabian StyleShang, Zhihong, Yishuo Wang, Tianxin Zhu, Wenjing Niu, Tianai Lu, and Rui Lou. 2025. "Centipeda minima: A Review of Phytochemistry, Pharmacology, and Predictive Analysis on Quality Markers" Molecules 30, no. 20: 4072. https://doi.org/10.3390/molecules30204072
APA StyleShang, Z., Wang, Y., Zhu, T., Niu, W., Lu, T., & Lou, R. (2025). Centipeda minima: A Review of Phytochemistry, Pharmacology, and Predictive Analysis on Quality Markers. Molecules, 30(20), 4072. https://doi.org/10.3390/molecules30204072






