Abstract
As a traditional Chinese medicinal herb, Centipeda minima (L.) A. Braun & Asch is known for its effects in dispersing wind-cold, clearing nasal passages, and relieving coughs. Current research has identified various chemical constituents isolated from C. minima, including volatile oils, flavonoids, organic acids, and terpenoids. These compounds demonstrate multiple pharmacological activities such as anti-inflammatory, antioxidant, anti-allergic, and anti-tumour effects. This review summarizes the chemical constituents, pharmacological effects, and clinical applications of C. minima. Furthermore, based on the concept of Quality Markers (Q-Markers) in Chinese medicine, potential Q-Markers for C. minima are predicted and analyzed from five perspectives: botanical phylogeny, specificity of chemical composition, measurability of constituents, traditional efficacy, and medicinal properties. Compounds including brevilin A, arnicolide C, arnicolide D, and helenalin are proposed as candidate Q-Markers for C. minima, providing a scientific basis for elucidating its pharmacologically active substances and establishing quality evaluation criteria.
1. Introduction
Centipeda minima is the dried whole plant of Centipeda minima (L.) A. Br. et Aschers. (Asteraceae), also commonly known as chicken intestine herb, stone coriander, or ground coriander. It has a pungent taste and warm nature, acting on the lung meridian, and functions to disperse wind-cold, unblock the nasal passages, and relieve cough. It is clinically used for symptoms such as wind-cold headache, cough with phlegm, nasal congestion, and runny nose [1]. Modern studies have shown that it is rich in sesquiterpene lactones, flavonoids, triterpenoids, and volatile oils [2]. Among these, pseudoguaiacolide-type sesquiterpenes (e.g., brevilin A) have demonstrated significant anti-inflammatory and anti-allergic effects [3,4], showing potential applications in anti-allergy, anti-tumour, antioxidant, and neuroprotective activities. However, current research still has notable limitations: Firstly, the quality control method for C. minima specified in the Pharmacopoeia of the People’s Republic of China (2020 edition) includes only qualitative methods such as thin-layer chromatography and microscopic identification, with quantitation limited to a single compound (brevilin A), which is insufficient for comprehensively reflecting the herb’s quality. Secondly, the relationship between chemical constituents and medicinal efficacy has not been systematically studied, and critical quality attributes (CQAs) remain unclear. Thirdly, research on predicting quality markers (Q-Markers) based on a multi-dimensional ‘constituent-activity-mechanism’ network is still in its early stages, hindering the improvement of its quality standards.
The concept of Q-Markers offers a new paradigm for quality control of traditional Chinese medicine. It emphasizes the selection of quality indicators that are specific and traceable based on biosynthetic pathways, efficacy correlation, and component measurability, among other dimensions [5]. There is an urgent need to integrate chemomics, network pharmacology, and metabolomics to systematically analyze the ‘chemical composition–pharmacological activity–clinical application’ network and thereby construct a scientific and rational Q-Marker prediction model for C. minima.
This review retrieved relevant literature from databases including Web of Science (https://webofscience.clarivate.cn), PubMed (https://pubmed.ncbi.nlm.nih.gov), China National Knowledge Infrastructure (https://www.cnki.net), Wanfang Data (https://www.wanfangdata.com.cn), and Google Scholar (https://scholar.google.com), using keywords such as “C. minima”, “chemical composition”, and “pharmacological action”. After removing duplicate and irrelevant records, the collected literature and classical texts on Chinese medicine were summarized. Based on the Q-Marker theoretical framework, potential quality markers were explored from the perspectives of component specificity, bioactivity, deliverability, and measurability, aiming to provide a theoretical basis for improving the quality standards and supporting innovative drug development related to C. minima.
2. Chemical Composition
2.1. Volatile Oils
C. minima is rich in volatile oil components with anti-inflammatory, antibacterial, antitumour, asthma and antiallergic activities [6]. Fan Su et al. [7] found that by extracting the volatile oils of C. minima and detecting them by GC-MS, the volatile oils of C. minima were mainly terpenes and esters, with the highest content of trans-chrysanthemum acetate, followed by aromatophilus, thymol, and β-stigmastanene, among others. A total of 86 volatile oil components have been identified. The specific composition is shown in Table 1. The compound structure is shown in Figure 1.

Table 1.
Chemical composition of volatile oils.
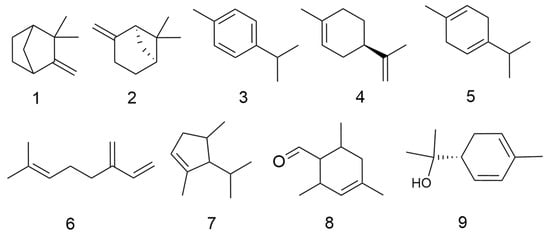
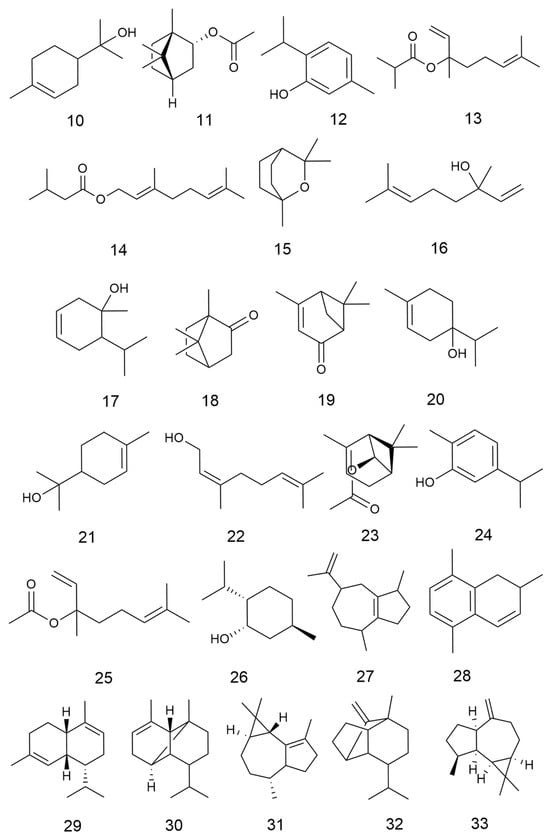
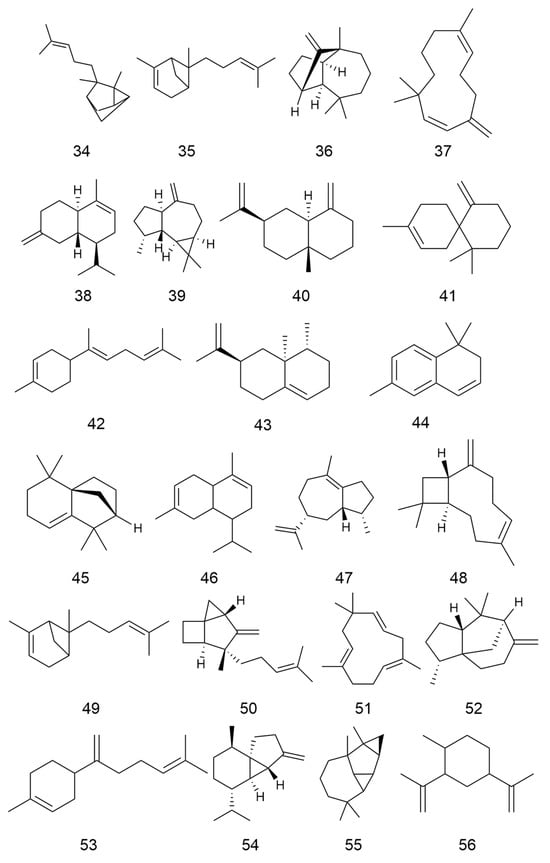
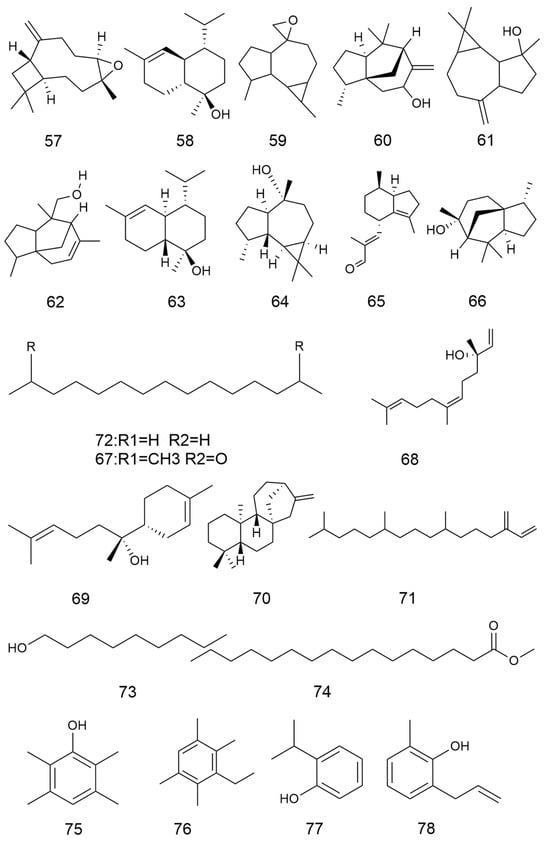
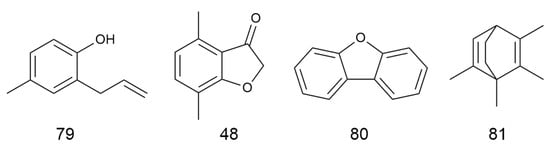
Figure 1.
Volatile oils structure of C. minima. The numbers in the diagram correspond to those in Table 1.
2.2. Terpenoids and Their Glycosides
C. minima contains a large number of terpenoids, of which guaiacolane sesquiterpene lactones are the main active constituents with biological activities such as anti-tumour, anti-inflammatory, anti-viral, anti-microbial, anti-malarial, anticancer, anti-diabetic, and analgesic [9], in addition to monoterpene glycosides, sesquiterpenes, and triterpene constituents, where triterpene compounds are mainly present in the form of pentacyclic triterpene saponins and their glycosides. Among these, the sesquiterpene lactones brevilin A, arnicolide D, and arnicolide C constitute the principal active constituents of C. minima. See Table 2 for details. The specific composition is shown in Table 2. The compound structure is shown in Figure 2.

Table 2.
Chemical composition of terpenoids and their glycosides.
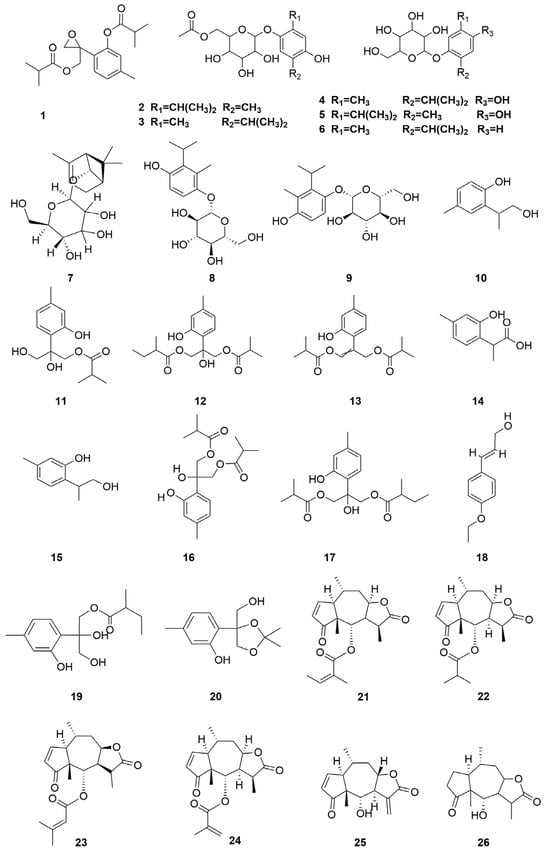
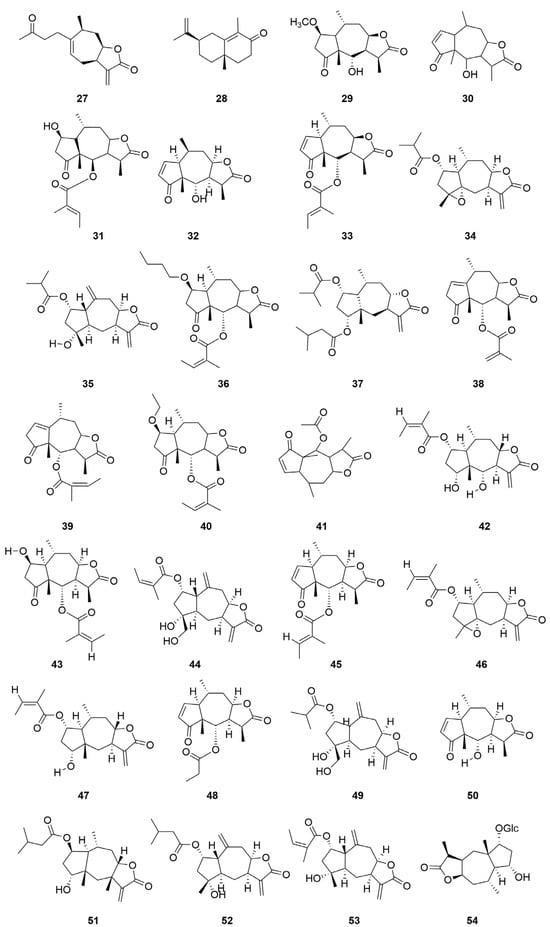
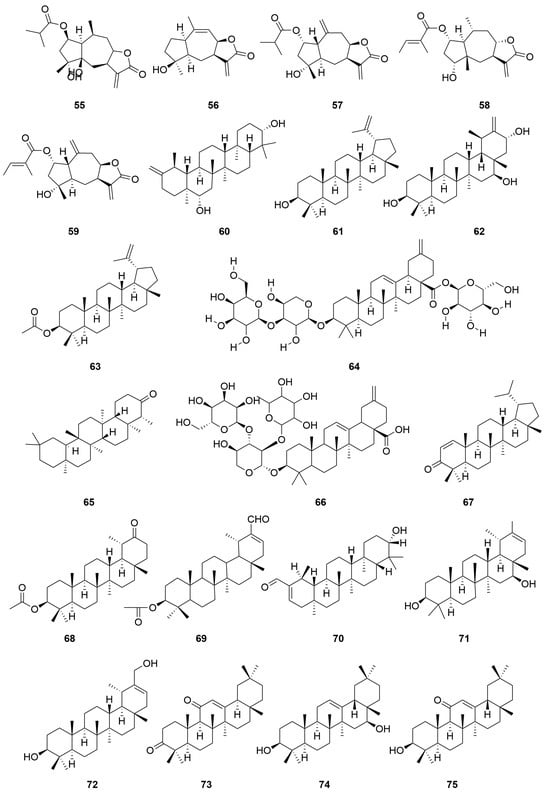
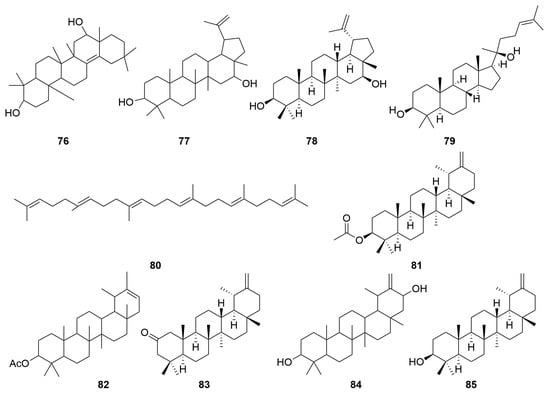
Figure 2.
Terpenoids and their glycosides structure of C. minima. The numbers in the diagram correspond to those in Table 2.
2.3. Flavonoids and Their Glycosides
C. minima is rich in flavonoids, with significant pharmacological activity, commonly used in clinical treatment of allergic rhinitis, and the anti-allergic effect of flavonoids in goose bugloss is slightly stronger than that of volatile oil components [32]. The specific composition is shown in Table 3. The compound structure is shown in Figure 3.

Table 3.
Chemical composition of flavonoids and their glycosides.
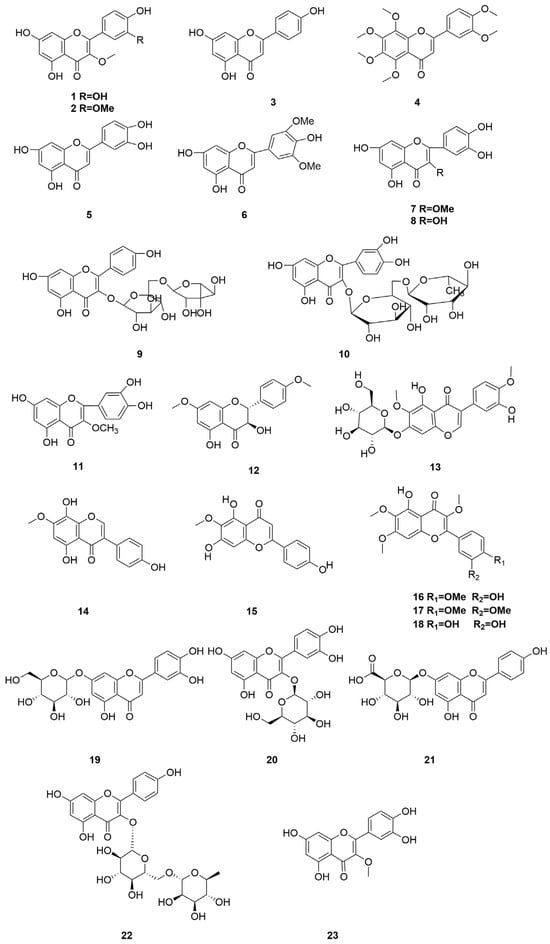
Figure 3.
Terpenoids and their glycosides structure of C. minima. The numbers in the diagram correspond to those in Table 3.
2.4. Sterols
A variety of sterol components have been extracted from C. minima, including β-sitosterol, stigmasterol and stigmasterol glucoside, totalling six. The specific composition is shown in Table 4. The compound structure is shown in Figure 4.

Table 4.
Chemical composition of sterols.
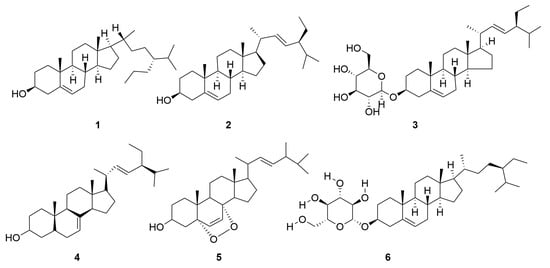
Figure 4.
Sterols of C. minima. The numbers in the diagram correspond to those in Table 4.
2.5. Organic Acids
Organic acid components are important chemical constituents in C. minima, and the phenolic acid components have pharmacological activities such as antioxidant, antitumour, antimicrobial, antiviral, anti-inflammatory, antidepressant, and anxiolytic [38], but there are fewer studies on the activity of organic acids in C. minima. Nowadays, 30 organic acid components have been identified from C. minima. The specific composition is shown in Table 5. The compound structure is shown in Figure 5.

Table 5.
Chemical composition of organic acids.
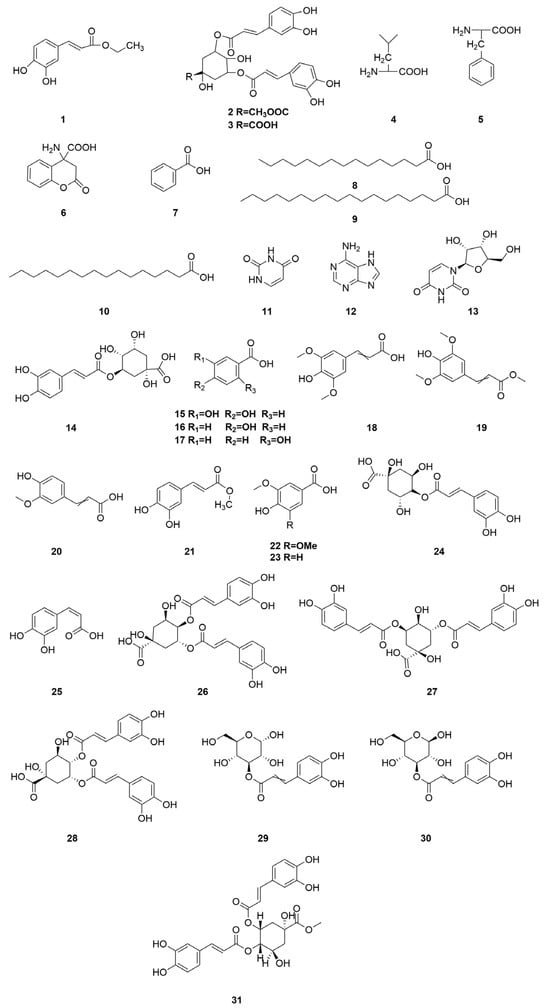
Figure 5.
Organic acids structure of C. minima. The numbers in the diagram correspond to those in Table 5.
2.6. Other Chemical Compositions
Yang Liu et al. also identified Squalene (1), 2-hydroxy-4-methy-lbenzoic acid (2), 1,3-Dimethoxybenzene (3), ethyl-2-(3,4-dihydroxyphenyl)acetate (4), 1H-Indazole (5), 5-(hydroxymethyl)-2,4,4-trimethyl-2-cyclohexen-1-one (6) and patriscabratine (7) [16]. In addition to the above mentioned constituents, C. minima also contains epipinoresinol (8) and other constituents [33]. The compound structure is shown in Figure 6.
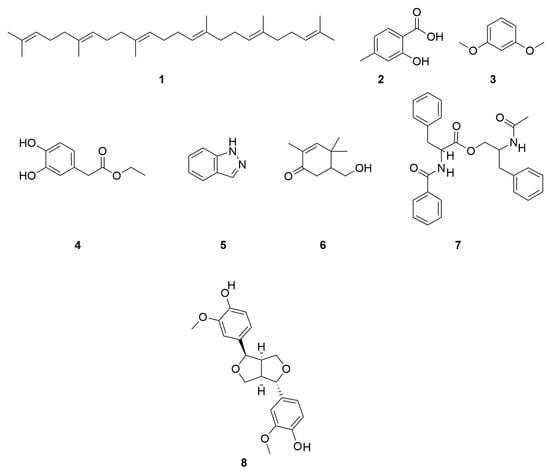
Figure 6.
Other chemical compositions structure of C. minima.
3. Pharmacological Effects
3.1. Anti-Allergy
C. minima has potential components for anti-allergic effects and is mostly used clinically for the treatment of allergic rhinitis [25,41]. In the model group of allergic rhinitis, C. minima significantly improved the nasal epithelium with a large number of lysosomes, vacuoles of organelles, deformed nuclei, misplaced cells in the appropriate layer of connective tissue, damaged organelles, and infiltration of eosinophils and mast cells in the connective tissue [42]. It has been found that C. minima extract can effectively inhibit the TLR/NF-κΒ pathway involved in the cellular immune response and inflammatory response, which in turn attenuates the intensity of Th2-type immune response and treats allergic rhinitis [43]. Jia Yet al. [4] showed that C. minima volatile oil reduced the levels of TNF-α, IL-4, and IgE in the serum and significantly lowered the PTGS2 and MAPK3 protein levels, thereby treating allergic rhinitis. The specific mechanism of action is shown in Figure 7.
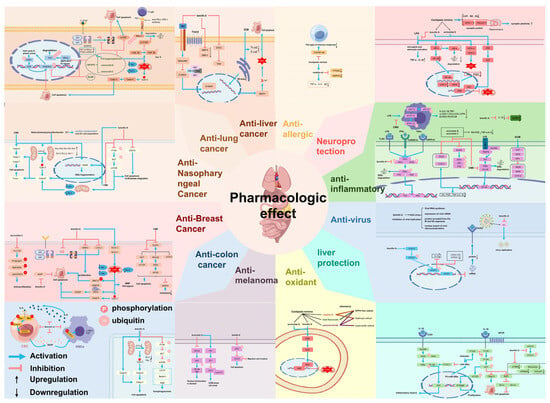
Figure 7.
An overview of the pharmacological mechanism of action of the C. minima.
3.2. Anti-Tumour
3.2.1. Anti-Lung Cancer
With 2.2 million new cases, lung cancer is the second most commonly diagnosed cancer and the leading type of cancer death in 2020 [44]. The most common types of lung cancer by tissue type are non-small cell lung cancer and small cell carcinoma. Fan XZet al. [45] found that the ethanol extract of C. minima could inhibit the Fanconi anemia pathway induced by DNA cross-linking agents by decreasing the expression of FANCD2 and mono-ubiquitylation, thus exerting synergistic anticancer effects with DNA cross-linking agents in the treatment of non-small cell lung cancer (NSCLC). Hu Qian et al. [46] found that alidiol, brevilin A, and helenalin-isovalerate exhibited a stronger, concentration-dependent inhibitory effect on lung cancer cells within the concentration range of 2.5–20 μmol/L, and the apoptosis-inducing effect of lung cancer cells was enhanced with the increase in the concentration of the components, and found that the mechanism of their action might be related to the inhibition of p-ERK, p-AKT, and NF-κB expression of p-ERK, p-AKT and NF-κB. Wang HC et al. [47] found that brevilin A, arnicolide D and arnicolide C in C. minima could exert anti-small cell lung cancer activity by inhibiting the Skp2/p27 signalling pathway, which induced cell cycle arrest and inhibited the migration of non-small cell lung cancer cells. brevilin A could play an anti-small cell lung cancer role by enhancing cancer cell killing activity through the enhancement of CD8 T cytotoxicity and the inhibition of GSK-3β-β-TRCP-mediated ubiquitylation and degradation de-inducing the expression of PD-L1 [48]. Wang R et al. [49] found that brevilin A significantly decreased HIF-1α mRNA expression and increased PTEN mRNA levels using network pharmacology and in vitro experimental validation, and hypothesized that C. minima could involve the HIF pathway in the treatment of lung cancer. Khan M et al. [50] found that brevilin A from C. minima inhibited proliferation and induced morphological changes in A549 and NCI-H1650 non-small cell lung cancer cells in a dose-dependent manner and increased ROS production and bax/bcl-2 ratio, while decreasing intracellular glutathione (GSH) and mitochondrial membrane potential, leading to induction of apoptosis. brevilin A also directly binds to STAT3, thereby inhibiting its activation and enhancing cytotoxicity. It has been found that brevilin A exerts its anticancer effect by generating ROS, and significantly inhibits the cell viability of lung cancer cells H1299 and A549 by inhibiting the Nrf2 antioxidant system, and does not have significant toxicity on non-cancer cells HB [51]. The specific mechanism of action is shown in Figure 7.
3.2.2. Anti-Nasopharyngeal Cancer
C. minima is also effective against nasopharyngeal carcinoma. Lin Yusan et al. [21]. tested the inhibitory effect of compounds on tumour cell proliferation by MTT assay, and found that brevilin A, Arnicolide C, and Arnicolide D had a more obvious inhibitory effect on nasopharyngeal carcinoma cells CNE-1 and CNE-2, and their IC50 values were all less than 10 μmol/L-1, which was close to that of the positive drug, cisplatin. Su M [26] showed significant dose- and time-dependent inhibition of human nasopharyngeal carcinoma epithelial (CNE) cell growth by 2beta-(Isobutyryloxy)florilenalin (IF) isolated from C. minima, inducing apoptosis of CNE cells, resulting in accumulation of sub-G1 cell population, DNA fragmentation and nuclear condensation, Caspase-3 activation and PARP cleavage, which in turn leads to caspase cleavage and cell death. It was found that goosefoot extract inhibited PI3K-Akt-mTOR signalling and cell proliferation in nasopharyngeal carcinoma CNE-1 cells [52]. Brevilin A and Arnicolide D can activate the Caspase signalling pathway by regulating cell cycle proteins, inhibit the PI3K/AKT/mTOR and STAT3 signalling pathways, and thus inhibit the nasopharyngeal carcinoma cell growth [53,54]. The specific mechanism of action is shown in Figure 7.
3.2.3. Anti-Liver Cancer
Liver cancer is the fifth most common cancer and the fourth leading cause of cancer-related deaths worldwide. There are two main types of primary liver cancer—hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC)—and approximately 70% of colorectal cancer patients develop secondary liver cancer [55]. Wei J et al. found that ethanolic extract of goosefoot significantly inhibited the G2/M cell cycle, up-regulated HMOX1 expression through ER stress, leading to Fe2+, ROS accumulation, and apoptosis, and inhibited invasion and cell migration of hepatocellular carcinoma through down-regulation of N-cad and up-regulation of E-cad [56]. Qin Y et al. found that brevilin A-treated HepG2 and SMMC-7221 cells showed a significant increase in apoptosis and a decrease in the expression levels of MMP-2 and MMP-9 [57]. In addition, brevilin A inhibited the Stat3/Snail and Wnt/β-catenin signalling pathways in HCC cells, which led to the inhibition of hepatocellular carcinoma. The specific mechanism of action is shown in Figure 7.
3.2.4. Anti-Breast Cancer
Since 2020, breast cancer has surpassed lung cancer as the most prevalent malignancy in women, and it is one of the leading causes of cancer-related deaths globally [58]. Liu Z et al. [59] found that Arnicolide C in C. minima induced cell cycle arrest and increased apoptosis, and hypothesized that it exerts anti-breast cancer effects by affecting the level of 14-3-3θ protein expression Wen W et al. [60] found that arnicolide D inhibited oxidative stress-induced breast cancer cell growth and invasion through apoptosis and iron death. Lee MM et al. [61] found that the total extract of C. minima significantly reduced cell viability and proliferation and induced a significant reduction in cell viability and proliferation in a dose- and time-dependent manner in MDA-MB-231 xenograft mice. They found that in MDA-MB-231 xenograft mice, C. minima extract could significantly reduce cell viability and proliferation in a dose-dependent and time-dependent manner, induce apoptosis, and inhibit cancer cell migration and invasion, and exert its anti-triple-negative breast cancer effects through multi-targets and multi-pathways, such as the EGFR, PI3K/AKT/mTOR, NF-κB, and STAT3 pathways, and cause a decrease in MMP-9 activity and metastasis inhibition in vitro, without any side-effects. It was also found that brevilin A induced mitotic G2/M block, ROS-dependent apoptosis, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and inhibited STAT3 activation in MCF-7 cells, thereby inhibiting MCF-7 breast cancer cell activity [62]. The specific mechanism of action is shown in Figure 7.
3.2.5. Anti-Colon Cancer
Colorectal cancer (CRC) is one of the most common cancers worldwide and its incidence is increasing every year, with high-fat, low-fibre diets and colonic inflammation as possible contributing factors [63]. Brevilin A was found to target the VEGF-IL6-STAT3 axis in colorectal cancer-hepatic stellate cells (CRC-HSCs) interactions, resulting in significant inhibition of colorectal liver metastasis and further cancer progression in vitro and in vivo [64]. You P [65] found that brevilin A could also induce apoptosis and autophagy in CT26 colon adenocarcinoma cells via the mitochondrial pathway and PI3K/AKT/mTOR inactivation by increasing the ROS level, decreasing the mitochondrial membrane potential (MMP), and inducing apoptosis in CT26 cells in a dose-dependent manner. The apoptosis induced by brevilin A was higher than that induced by adriamycin at the same dose, and the up-regulation of cleaved-caspase-8, cleaved-caspase-9, and cleaved-caspase-3, the increase in Bax protein expression, and the decrease in Bcl-2 were observed after brevilin A treatment. Further studies showed that brevilin A inhibited the phosphorylation of PI3K, AKT and mTOR, and promoted the expression of autophagy-related proteins LC3-II, Beclin1 and Atg5, resulting in the formation of autophagosomes, whereas 3-methyladenine (3-MA), a type III PI3K inhibitor, inhibited the brevilin A-induced autophagosome formation. The specific mechanism of action is shown in Figure 7.
3.2.6. Anti-Melanoma
Melanoma is an extremely aggressive cancer prone to metastasis, primarily affecting the skin and mucous membranes, with a steadily increasing global patient population [66]. C. minima exhibits certain therapeutic efficacy for this disease. Research by Su T et al. [67] revealed that brevilin A reduces cell viability, induces apoptosis, inhibits migration and invasion, and lowers the protein levels of phosphorylated JAK2 (Y1007/1008) and phosphorylated STAT3 (Tyr705). Concurrently, it suppresses STAT3 expression, thereby exerting anti-melanoma effects. Zhu P et al. [68] found that Arnicolide D also exerted an antimelanoma effect, mainly by decreasing cell viability, inducing G2/M cell cycle arrest and apoptosis, and increasing the levels of the cell cycle regulatory proteins p53 and p21 in melanoma cells. Arnicolide D also exerts antimelanoma effects by reducing cell viability, inducing G2/M cell cycle arrest and apoptosis, increasing the levels of p53 and p21, and decreasing the levels of the G2/M checkpoint protein Cdc2 and the cell cycle protein B1, through the mechanisms of inhibiting the activity of IKKα/β, the degradation of IκBα, and the phosphorylation and expression of NF-κB p65 in melanoma cells. The specific mechanism of action is shown in Figure 7.
3.2.7. Others
In addition to the above cancers, C. minima can also be used in the treatment of prostate cancer, osteosarcoma, gastric cancer, etc. You P et al. [69] found that Brevilin A in C. minima inhibited prostate cancer cell proliferation, migration and invasion, suppressed the expression of lncRNA H19 and E2F3, and enhanced the level of miR-194. It regulates prostate cancer cells through the lncRNA H19/miR-194/E2F3 axis. Chen Z et al. [70] also found that Arnicolide D in C. minima inhibited the activation of the PI3K/Akt/mTOR pathway in osteosarcoma cells, which has the effect of inhibiting osteosarcoma cell proliferation and inducing apoptosis. Lee D et al. [71] found that Brevilin A induced apoptosis by up-regulating the expression of cleaved caspase-8 and cleaved caspase-3, reducing the ratio of Bax to Bcl-2, and achieving anti-gastric cancer effects. Wang J et al. [72] found that Brevilin A induced oxidative stress and mitochondrial dysfunction, thereby inhibiting the proliferation of U87 glioblastoma cells and inducing apoptotic cells in a dose-dependent manner and inducing severe morphological changes and apoptotic cell death. Brevilin A also induced apoptosis in human leukemia HL60 cells by stimulating reactive oxygen species (ROS) production, lowering the mitochondrial transmembrane potential (DeltaPsim), and activating caspase-3/7; inhibited the activation of NF-kappaB, regulating the Bcl-2 gene family expression and disruption of mitochondrial function-induced apoptosis; and even inhibited solid cancer growth in the Lewis lung cancer xenograft model [73].
3.3. Anti-Depressant (Neuroprotective)
Depression is a very threatening disease to mental health, and C. minima, a traditional Chinese medicine, has some neuroprotective effects. Dou Shi-Ying [74] and others found that C. minima improved serum monoamine neurotransmitter contents such as 5-HT, NE and DA in rifampicin-induced depressed rats, improved hippocampal tissue morphology damage and increased the content of synaptic proteins SYN-1 and PSD-95 in the hippocampus, and speculated that its mechanism of action might be related to the increase in serum monoamine neurotransmitter contents and the improvement of hippocampal. Zhou, Y.L. [75] revealed that Brevilin A, a natural sesquiterpene lactone from C. minima, dose-dependently inhibited LPS-induced microglia activation, decreased the levels of inflammatory factors such as TNF-α and IL-1β, inhibited the nuclear translocation of NF-κB and the expression of COX-2/iNOS, and reduced the levels of NO and PGE2 production, which can effectively alleviate the inflammatory response of brain tissue, inhibit glial cell activation, and also protect neuronal and synaptic structures, and thus exert neuroprotective effects against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. High levels of ROS and aberrant redox changes significantly induced neuronal death and enhanced the pathogenesis of neurodegenerative diseases, whereas C. minima ethanolic extract possessed antioxidant activity and was able to protect neuronal cells from oxidative stress-induced damage through activation of ERK/Nrf2 signalling pathway, and significantly improved learning and memory ability in mice. Brevilin A and arnicolide D are the main components responsible for activating the Nrf2 signalling pathway and inhibiting ROS production, thus exerting neuroprotective effects [76,77]. The specific mechanism of action is shown in Figure 7.
3.4. Antioxidant
Endogenous reactive oxygen species (ROS) play an important role in the human body, and oxidative stress is a phenomenon caused by an imbalance between the production and accumulation of oxygen-responsive substances (ROS) in cells and tissues and the ability of the biological system to detoxify these reaction products, which leads to oxidative damage, disrupts membrane integrity or leads to cell death, and induces a series of diseases in the body [78]. Wang, Y.J. et al. [76] found that the ethanolic extract of C. minima exhibited significant antioxidant activity and revealed its mechanism of action, isolating brevilin A and arnicolide D as the active compounds responsible for the activation of the Nrf2 signalling pathway and the inhibition of ROS production. The total flavonoids of C. minima showed strong scavenging effects on DPPH radicals [79], and the volatile oil was found to have antioxidant effects on C. minima through the scavenging effect experiments on DPPH radicals and hydroxyl radicals: essential oil > carvacrol > thymol [7].Yang, G. et al. [80] found that C. minima polysaccharides could also exhibit good antioxidant activity with potential to scavenge DPPH radicals, hydroxyl radicals and superoxide radicals. The specific mechanism of action is shown in Figure 7.
3.5. Antibacterial and Anti-Inflammatory
C. minima essential oil inhibits the growth of S. aureus and inhibits biofilm formation by altering cell membrane permeability and interfering with metabolic activities in bacteria in vivo [7]. C. minima possesses three antimicrobial sesquiterpene lactone components 6-O-methylacrylylplenolin, 6-O-isobutyroylplenolin, and 6-O-angeloylplenolin with activity against Bacillus subtilis and Staphylococcus aureus, with 6-O-isobutyroylplenolin being the most active [81].
Total extract of C. minima showed inhibitory effects on NF-κB, STAT3 and MAPK signalling in macrophages and CCL8 expression in activated macrophages and ameliorated inflammatory bowel disease [82]. The aqueous extract of C. minima showed anti-inflammatory activity by decreasing the levels of malondialdehyde (MDA), nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) by increasing the activities of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) [83]. C. minima ethanolic extract protected HT22 neuronal cells from inflammatory damage by reducing the production of pro-inflammatory mediators tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), inhibiting the phosphorylation of NF-κB, and decreasing the expression of COX2, iNOS, NOX2, and NOX4 in hippocampal tissues, and found that LPS-induced microglia activation was significantly attenuated in the hippocampus, whereas a high dose of C. minima ethanol extract possessed stronger anti-inflammatory activity [84]. Penghui Xue et al. [29] determined the inhibitory activity of C. minima extract on lipopolysaccharide (LPS)-induced release of the inflammatory mediator NO from mouse macrophages (RAW264.7) using Griess method, and found that 3β-acetoxytaraxaster-20-en-30-al, 3β,16β-hydroxy-lupinidiol, 16β-hydroxy-lupin-20(29)-en-3-one, and garcinielliptone Q had significant anti-inflammatory activity. C. minima extract significantly inhibited JAK1/2 and STAT1/3 phosphorylation in macrophages and inflammatory response in keratin-forming cells [85]. C. minima arnicolide B and arnicolide C in C. minima not only inhibited the production of inflammatory mediators NO, PGE2, TNF-α and IL-6, but also down-regulated the high expression of inflammatory proteins iNOS and COX-2. In addition, they can inhibit the phosphorylation of ERK, JNK, and p38 proteins in the MAPK signalling pathway, thus exerting anti-inflammatory effects [86]. Liu L et al. [87] found that Brevilin A effectively reduced LPS-induced inflammatory lung injury by targeting IKKα/β and inhibiting NF-κB signalling. Xue PH et al. [88] identified three anti-inflammatory components in C. minima, centiplide A, centiplide H, and helenalin-isovalerate, which exhibited significant inhibitory activity on nitric oxide production in a lipopolysaccharide-activated macrophage cell line of RAW 264.7 mice. Qin Qin et al. [3] found that C. minima could effectively reduce LPS-induced inflammatory lung injury by targeting IKKα/β to inhibit NF-κB signalling. found that Brevilin A in C. minima significantly reduced IL-1β secretion to inhibit NLRP3 inflammatory vesicles. The specific mechanism of action is shown in Figure 7.
3.6. Hepatoprotective Effect
Sesquiterpene lactones from C. minima have hepatoprotective activity. Bin Fang found that Helenalin significantly inhibited the proliferation, migration and colony formation of Hepatic stellate cells (HSCs), induced apoptosis of HSCs, alleviated inflammation, regulated the balance of MMPs/TIMPs, and reduced the synthesis of collagen in activated HSCs, and the mechanism of Helenalin may be related to the up-regulation of miR-200a and the inhibition of NF-κB and PI3K/Akt signalling pathways mediated by miR-200a [89]. Brevilin A significantly inhibited STAT3 phosphorylation, which was increased by TGF-β treatment, and reduced the expression levels of fibronectin and connective tissue growth factor, which in turn exerted an antifibrotic effect in liver fibrosis [90]. The specific mechanism of action is shown in Figure 7.
3.7. Antiviral
Brevilin A from C. minima is a potent Angiotensin-converting enzyme 2 (ACE2) inhibitory component that also inhibits the entry of SARS-CoV-2 S-protein pseudoviruses into the target cells, thereby protecting lung epithelial cells from SARS-CoV-2 infection [91]. Zhang, X.L. et al. [92] tested the anti-influenza virus A/Puerto Rico/8/34 HIN1 (PR8) activity of nine sesquiterpene lactones using the Cellular Pathogenic Effect (CPE) Reduction and Cell Counting Kit 8 (CCK8) assay and found that brevilin A showed the strongest anti-PR8 activity with IC50 values of 0.01 and 0.01, respectively, and that brevilin A affected PR8 replication through down-regulation of the expression of viral M2 protein. It was found that brevilin A exhibited the most potent anti-PR8 activity by down-regulating the expression of the viral M2 protein, affecting the intracellular replication of PR8, and the IC50 value was much lower than that of the positive control, ribavirin. and H9N2) [93]. The specific mechanism of action is shown in Figure 7.
3.8. Others
In addition to this, C. minima has pharmacological effects such as asthma-calming, hair growth, anti-angiogenesis, and anti-mitosis effects.
Chen Qiang et al. [94] used chloroacetylcholine and histamine phosphate to create an asthma model; C. minima volatile oil can significantly extend the effect of guinea pig-induced asthma latency after oral administration, and through the contraction of isolated guinea pig tracheal smooth muscle spiral strip contraction experiments, they found that the volatile oil of C. minima can antagonize the effect of histamine phosphate on the H1 receptor to produce bronchial smooth muscle contraction and exert an asthmatic effect.
A study found that C. minima can enhance the expression of vascular endothelial growth factor (Vegfa) and insulin-like growth factor (Igf1), inhibit the expression of transforming growth factor β 1 (Tgfb1), promote the development of hair follicles, and then promote hair growth in C57BL/6 mice [95]. C. minima extract has also been found to promote hair growth and growth factor secretion through the Wnt/β-catenin, ERK and JNK signalling pathways, and its compounds have been shown to have high bioavailability and gastrointestinal absorption [96].
Brevilin A in C. minima activates p38/PMK-1 in the gut for innate immune responses, increases resistance to oxidative stress and prolongs lifespan through the p38 MAPK pathway, and enhances resistance to pathogens by reducing bacteria in the gut [97].
C. minima and its Brevilin A constituent have been shown to possess antiangiogenic activity [98]. The (Z)-3,5,4′-Trimethoxystilbene in C. minima exerts antimitotic activity by inhibiting microtubule protein polymerisation in a dose-dependent manner [99].
4. Security Evaluation
Oral administration of C. minima exhibits gastrointestinal irritancy. Within a certain period after ingestion, symptoms such as pharyngeal, esophageal, and gastric burning sensations, nausea, vomiting, gastralgia, abdominal pain (sometimes severe), and even back pain and generalized myalgia have been reported in some patients [100].
Using the screening criteria of oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18, active constituents of C. minima were retrieved from the TCMSP platform (https://www.tcmsp-e.com/tcmsp.php) After standardization using the Uniprot database, 197 potential target gene standard abbreviations were obtained. Disease target genes were retrieved and screened using the keywords “gastric irritation” and “gastric mucosal injury” from the GeneCards (http://www.genecards.org/) and OMIM (http://omim.org/) databases. After merging and deduplication, 7110 disease targets were identified. Venn diagram analysis of the constituents and gastric irritation targets yielded 179 common targets, providing corroborating evidence for the documented irritancy.
However, clinically, only a low incidence of adverse reactions has been observed. Furthermore, no documented toxicity exists in historical records of Traditional Chinese Medicine (TCM), indicating that C. minima is a relatively mild and safe medicinal herb with significant therapeutic value, and it is widely used in TCM practice. Pharmacological experiments revealed that oral administration in mice showed no signs of weight loss or hepatorenal toxicity, as evidenced by the levels of serum transaminases, alanine aminotransferase, creatinine, and blood urea nitrogen concentrations [45,101], demonstrating a favourable safety profile. Current research on C. minima is limited. To better assess its safety and potential side effects, further systematic toxicological studies are warranted in the future.
5. Clinical Applications
C. minima is widely used in the clinic, mostly as a single medicine or in compound formulas, and is commonly used for the treatment of allergic rhinitis, whooping cough, stones, headache, malaria, and hookworm tail pass infections [102]. C. minima is also commonly used in combination with Magnolia, Xanthium sibiricum Patr. and Asari Radix et Rhizoma and other drugs to treat allergic rhinitis [103]. Various compounded formulations have been developed, such as Nasonex Nasal Drops [104], Resting Cough and Anti-inflammatory Bulk [105], C. minima-formulated granules [106], Kelisha capsule [107], and Thermo Kepin injection [108]. One of the allergic rhinitis nasal drops (ARND) not only inhibits viral infections and disrupts the affinity between ACE2 and the spiking protein (Delta), but also attenuates the inflammatory response after infection, which may lead to a better prognosis and lower risk of pulmonary fibrosis [109]. Kelisha capsule, by inhibiting the activity of the enzyme ATP synthase in Escherichia coli, reduces the ATP levels, as well as disrupting the cell wall and cell membrane of E. coli, leading to cytoplasmic leakage and bacterial death to exert its antimicrobial effect [107].
6. Quality Marker Prediction
6.1. Predictive Analysis of Quality Markers Based on Plant Affinities and Chemical Specificity
C. minima belongs to the genus Centipeda Lour. in the subtribe Chrysantheminae O.Hoffm., tribe Anthemideae Cass., subfamily Carduoideae Kitam of the Asteraceae family (also known as Compositae). The Asteraceae family comprises approximately 1000 genera and 25,000–30,000 species worldwide, with relatively fewer representatives in tropical regions. In China, this family encompasses over 200 genera and more than 2000 species distributed across the country, including approximately 155 genera and 778 species with medicinal value [110]. The genus Centipeda contains six species globally, distributed across Asia, Oceania, and South America, with C. minima being the sole representative species in China. Plants in the Asteraceae family predominantly contain bioactive compounds such as terpenoids, flavonoids, and phenylpropanoids, with sesquiterpene lactones and flavonoids being particularly ubiquitous [111]. The phytochemical profile of Centipeda species aligns with this pattern, containing terpenoids, flavonoids, phenylpropanoids, volatile oils, sterols, and organic acids. Within the subtribe Chrysantheminae, medicinal genera such as Artemisia and Chrysanthemum are noteworthy. Artemisia species are characterized by essential oils, flavonoids, and organic acids [112], while Chrysanthemum species primarily produce terpenoids, flavonoids, polyacetylenes, phenylpropanoids, alkaloids, and steroids [113]. Comparative analysis reveals that C. minima contains distinctive sesquiterpene lactones, including brevilin A, arnicolide C, and arnicolide D, which are absent in other genera within the family. These unique compounds—brevilin A, arnicolide C, and arnicolide D—may therefore serve as potential Quality Markers (Q-Markers) for the authentication and quality control of C. minima.
6.2. Predictive Analysis of Quality Markers Based on Correlation Between Composition and Efficacy
C. minima is characterized as pungent in flavour, warm in nature, and associated with the lung meridian. It is traditionally used to address wind-cold headaches, cough with excessive phlegm, nasal congestion, and sinusitis with rhinorrhea [1]. The pungent flavour in traditional Chinese medicine (TCM) theory is described as “capable of dispersing and moving,” reflecting its ability to promote circulation and resolve stagnation. Pungent herbs typically contain volatile oils, glycosides, terpenoids, and alkaloids [114]. Warm-natured herbs are often associated with volatile oils, esters, organic acids, carbohydrates, inorganic compounds, alkaloids, and flavonoids, which collectively form their bioactive foundation [115]. Notably, lung meridian-targeting herbs frequently contain volatile oils, glycosides, flavonoids, and terpenoids, exhibiting pharmacological activities such as anti-inflammatory, antimicrobial, and antitumor effects [116]. C. minima predominantly contains volatile oils, flavonoids, terpenoids, and organic acids [117], making these classes of compounds potential quality markers (Q-Markers) for this herb.
Brevilin A, arnicolide C, arnicolide D, and 2β-(isobutyryloxy)florilenalin (IF) demonstrate significant anti-tumour activity, while 6-O-angeloylplenolin and arnicolide D exhibit neuroprotective effects. Anti-inflammatory activity is observed in compounds such as 3β-acetoxytaraxaster-20-en-30-al, 3β,16β-dihydroxylup-20(29)-en-3-one, 16β-hydroxylup-20(29)-en-3-one, brevilin A, centiplide A, centiplide H, helenalin-isovalerate, garcinielliptone Q, arnicolide B, and arnicolide C. Antimicrobial activity is attributed to 6-O-methylacrylylplenolin, 6-O-isobutyroylplenolin, and brevilin A. Additionally, helenalin and brevilin A display hepatoprotective properties, while brevilin A exhibits antiviral activity.
In summary, Brevilin A, 2beta-(Isobutyryloxy)florilenalin (IF), 3beta-acetoxytaraxaster-20-en-30-al, 3beta,16beta-hydroxy-lupine diol, 16beta-hydroxy-lupin-20(29)-en-3-one, centiplide A, centiplide H, helenalin-isovalerate, garcinielliptoneQ, arnicolide B and arnicolide C, 6-O-methylacrylylplenolin, 6-O- isobutyroylplenolin, Helenalin can be used as a reference for the selection of C. minima Q-Markers.
6.3. Predictive Analysis of Quality Markers Based on Chemical Composition Measurability
The chemical components in C. minima are abundant, and the substances that are stable and can be quantitatively determined are clearly defined in order to better establish a quality control evaluation system, and the selected chemical components should have a certain content and be able to have a content determination method that meets the exclusivity [118]. Zan Ke et al. [119] employed high-performance liquid chromatography (HPLC) to establish a characteristic fingerprint of the medicinal herb Achillea millefolium, whilst simultaneously determining the content of chlorogenic acid, cryptoclorogenic acid, caffeic acid, rutin, isochlorogenic acid B, isochlorogenic acid A, and isochlorogenic acid C. Ren Haiqin et al. [120] established a method for the simultaneous measurement of arnicolide D, arnicolide C, minimolide F, microhelenin C and brevilin A by HPLC. Chan CO et al. [19] established a method for the simultaneous measurement of arnicolide D, arnicolide C, minimolide F, microhelenin C, and brevilin A by ultra performance liquid chromatography coupled with triple quadrupole mass spectrometry (UPLC-QQQ-MS) and UPLC-High Resolution Orbitrap Mass Spectrometry (UPLC-Orbitrap-MS) initially identified 15 sesquiterpene lactones in the methanolic extract of C. minima, and found that Brevilin A and arnicolide D were the major sesquiterpene lactone components in C. minima. Chan CO simultaneously determined chlorogenic acid, cryptoclorogenic acid, caffeic acid, rutin, isochlorogenic acid B, kaempferol-3-O-rutinoside, isochlorogenic acid A, isochlorogenic acid C, 3-methoxyquercetin, brevilin A, arnicolide D, arnicolide C [20]. The contents of these acids were determined by HPLC-DAD. Thin-layer chromatography was used to identify C. minima oil, and gas chromatography internal standard method was used to determine the content of chrysanthenyl acetate and thymol in C. minima oil [121]. Chen Wei et al. [122] quantified 16 batches of C. minima from 7 provinces by HPLC and found that chlorogenic acid, isochlorogenic acid A, isochlorogenic acid C, quercetin, kaempferol, 3-methoxyquercetin, arnicolide D, arnicolide C, microhelenin C, short brevilin A, lupinol, β-sitosterol, and taraxasterol varied considerably among provenances.
In summary, chlorogenic acid, quercetin, brevilin A, rutin, isochlorogenic acid B, isochlorogenic acid A and isochlorogenic acid C, arnicolide D, arnicolide C, minimolide F, microhelenin C, cryptochlorogenic acid, caffeic acid, nicotiflorin, 3-methoxyquercetin, chrysanthenyl acetate, and thymol may inform the Q-Marker selection of C. minima.
6.4. Predictive Analysis of Quality Markers Based on Incoming Blood Components
An in vivo pharmacokinetic study of rutin and 3-O-methyl quercetin in the alcoholic extract of C. minima revealed that the rutin prototype was detected in plasma, the liver at 1 h, the kidney at 4 h, brain tissue at 15 min–8 h, and urine at 0–24 h in rats that were orally administered with the extract, and stated that rutin could cross the blood–brain barrier (BBB) in rats and 3-O-methylquercetin was detected in bile [123]. Intravenous and intraperitoneal injection of brevilin A and detection of blood by LC-MS revealed that in intraperitoneally injected rats, the bioavailability of brevilin A was 92.5%, indicating that its therapeutic concentration could be achieved by intravenous and intraperitoneal injection of brevilin A [124].
In conclusion, rutin, 3-O-methylquercetin, and brevilin A can be used as blood-entry components with good pharmacokinetic profiles, and can be used as potential choices for the Q-Marker of C. minima.
6.5. Predictive Analysis of Quality Markers Based on Correlation of Ingredients with Modern Clinical Use
Currently C. minima is available in the market mostly in the form of formulated granules, sprays, capsules, dispersions, and injections, which are commonly used for the treatment of symptoms such as rhinitis, fever, and cough, etc. C. minima formulated granules contain caffeic acid, isochlorogenic acid A, isochlorogenic acid C, isochlorogenic acid B, cryptochlorogenic acid, and neochlorogenic acid [106]. Nasonex spray has been found to contain components such as chlorogenic acid, rutin, isochlorogenic acid A, quercetin, and kaempferol [125]. Nasonex Spray has been found to contain components such as chrysanthenyl acetate [126].
In summary, caffeic acid, isochlorogenic acid A, isochlorogenic acid C, isochlorogenic acid B, cryptochlorogenic acid, and neochlorogenic acid, chrysanthemum acetate, chlorogenic acid, rutin, isochlorogenic acid A, quercetin, and kaempferol may be potential choices for Q-Marker of C. minima.
6.6. Summary
Based on the five principles for selecting Q-Markers, brevilin A, arnicolide C, arnicolide D, and Helenalin possess pharmacological effects such as antitumor activity, indicating effectiveness. These components are present in C. minima at high levels and exhibit specificity compared to closely related species. Furthermore, there are currently accurate and reliable methods for their quantitative determination, ensuring measurability. However, other components such as caffeic acid, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, chlorogenic acid, and rutin are widely distributed in other medicinal materials, lacking specificity and therefore suitability as Q-Marker selections. Therefore, brevilin A, arnicolide C, arnicolide D, and Helenalin can serve as potential Q-Marker candidates for C. minima.
7. Conclusions
This review systematically consolidates the current knowledge on the diverse chemical constituents and multifaceted pharmacological activities of C. minima, underscoring its significant potential as a source of therapeutic agents. The plant produces a rich array of bioactive compounds, including volatile oils, flavonoids, and sesquiterpene lactones, which are responsible for its demonstrated anti-inflammatory, antitumor, and other pharmacological effects. Despite this promising foundation, our analysis reveals that the translation of these findings into standardized and safe clinical applications is impeded by several interconnected research gaps.
Substantial challenges remain across the board. Firstly, the chemical research is incomplete, with critical components like polysaccharides being poorly characterized, and the fundamental structure–activity relationships for most constituents are still unknown. Secondly, the understanding of pharmacological mechanisms is fragmented, particularly for volatile oils, and the complex synergistic interactions between compounds are largely unexplored. Finally, and most critically, the safety profile of C. minima is inadequately defined. It lacks comprehensive pharmacokinetic and toxicological studies, especially concerning its known gastrointestinal irritation and the potential toxicity of lipophilic components.
To bridge these gaps, a more systematic and integrated research strategy is imperative. Future efforts must prioritize the in-depth exploration of structure–activity relationships, the application of multi-omics and other advanced technologies to elucidate complex mechanisms, and rigorous safety assessments that include investigating traditional processing methods to mitigate adverse effects. Concurrently, establishing robust quality control standards, potentially using specific sesquiterpene lactones as quality markers, is essential for ensuring the consistency, efficacy, and safety of C. minima-based preparations. Addressing these priorities cohesively will be crucial for validating its traditional uses and unlocking its full potential in modern evidence-based medicine.
Author Contributions
Z.S.: Data curation, Formal analysis, Visualization, Writing—original draft. T.L.: Writing—review and editing. T.Z.: Supervision, Writing—review and editing. W.N.: Writing—review and editing. R.L.: Supervision, Conceptualization. Y.W.: Conceptualization, Data curation, Project administration, Validation, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Development of Taxus chinensis Chinese Materia Medica Standard grant number [2020DB224] and Open Subject Project of Key Laboratory of Quality Control of Chinese Materia Medica and Drinking Tablets of State Drug Administration [KF202205] And The APC was funded by it.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China 2020; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 1902. [Google Scholar]
- Tan, J.; Qiao, Z.; Meng, M.; Zhang, F.; Kwan, H.Y.; Zhong, K.; Yang, C.; Wang, Y.; Zhang, M.; Liu, Z.; et al. Centipeda minima: An update on its phytochemistry, pharmacology and safety. J. Ethnopharmacol. 2022, 292, 115027. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, G.; Zhan, X.; Wang, Z.; Wang, Y.; Liu, H.; Hou, X.; Shi, W.; Ma, J.; Bai, Z.; et al. Brevilin A inhibits NLRP3 inflammasome activation in vivo and in vitro by acting on the upstream of NLRP3-induced ASC oligomerization. Mol. Immunol. 2021, 135, 116–126. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, J.; Wang, Y.; Zhang, X.; Shi, Y.; Liang, Y.; Guo, D.; Yang, M. Mechanism of allergic rhinitis treated by Centipeda minima from different geographic areas. Pharm. Biol. 2021, 59, 606–618. [Google Scholar] [CrossRef]
- Liu, C.-X.; Cheng, Y.-Y.; Guo, D.-A.; Zhang, T.-J.; Li, Y.-Z.; Hou, W.-B.; Huang, L.-Q.; Xu, H.-Y. A New Concept on Quality Marker for Quality Assessment and Process Control of Chinese Medicines. Chin. Herb. Med. 2017, 9, 3–13. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y. Research progress on essential oils of Cenpiteda minima (L.), A. Br. Aschers. Shandong Chem. Ind. 2018, 47, 66–68. [Google Scholar]
- Su, F.; Yang, G.; Hu, D.; Ruan, C.; Wang, J.; Zhang, Y.; Zhu, Q. Chemical Composition, Antibacterial and Antioxidant Activities of Essential Oil from Centipeda minima. Molecules 2023, 28, 824. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, H.; Tu, W. Essential Oil GC-MS study of Centipeda minima. Chin. Herb. Med. 2002, 33, 20–21. [Google Scholar]
- Hsu, C.Y.; Rajabi, S.; Hamzeloo-Moghadam, M.; Kumar, A.; Maresca, M.; Ghildiyal, P. Sesquiterpene lactones as emerging biomolecules to cease cancer by targeting apoptosis. Front. Pharmacol. 2024, 15, 1371002. [Google Scholar] [CrossRef]
- Jialing, S.; Ze, Z.; Zedan, Z.; Cuiyun, G.; Ruyu, W.; Huafeng, P.; Xiangzhen, F. Research progress of chemical constituents and pharmacological actions of Ebushicao (Centipeda minima). J. Hunan Univ. Chin. Med. 2024, 44, 516–530. [Google Scholar]
- Pu, S. Studies on the Chemical Constituents and their Anti-Timor Activity in Centipeda minima Lour. & Hydrocotyle sibthorpioides Lam. Ph.D. Thesis, Tianjin University, Tianjin, China, 2009. [Google Scholar]
- Pu, S.; Guo, Y.; Gao, W. Chemical constituents from Centipeda minima. Chin. Tradit. Herb. Drugs 2009, 40, 363–365. [Google Scholar]
- Pu, S.; Guo, Y.; Gao, W. Chemical constituents from Centipeda minima. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Medica 2009, 34, 1520–1522. [Google Scholar]
- Wu, H.; Liu, Y.; Yang, Y.; Xiao, M. Study on Chemical Constituents from Ethyl Acetate Fraction of Centipeda minima. Shizhen Guoyi Guoyao 2010, 21, 1096–1098. [Google Scholar]
- Wang, Y. Study on the chemical constituents of Centipeda minima. Hai Xia Yao Xue 2019, 31, 84–86. [Google Scholar]
- Yang, L.; Gao, Q.; Zhou, L. Study on chemical constituents of Centipeda minima and anti-inflammatory activity. J. Chin. Med. Mater. 2023, 46, 1924–1930. [Google Scholar]
- Xie, J. Studies on the Chemical Composition of Centipeda minimca and Helleborus thibetanus. Master’s Thesis, University of Jinan, Jinan, China, 2021. [Google Scholar]
- Liang, H.; Bao, F.; Dong, X.; Tan, R.; Zhang, C.; Lu, Q.; Cheng, Y. Antibacterial thymol derivatives isolated from Centipeda minima. Molecules 2007, 12, 1606–1613. [Google Scholar] [CrossRef]
- Chan, C.O.; Xie, X.J.; Wan, S.W.; Zhou, G.L.; Yuen, A.C.; Mok, D.K.; Chen, S.B. Qualitative and quantitative analysis of sesquiterpene lactones in Centipeda minima by UPLC-Orbitrap-MS & UPLC-QQQ-MS. J. Pharm. Biomed. Anal. 2019, 174, 360–366. [Google Scholar]
- Chan, C.O.; Jin, D.P.; Dong, N.P.; Chen, S.B.; Mok, D.K. Qualitative and quantitative analysis of chemical constituents of Centipeda minima by HPLC-QTOF-MS & HPLC-DAD. J. Pharm. Biomed. Anal. 2016, 125, 400–407. [Google Scholar]
- Lin, Y.; Lv, Q.; Chen, S. Isolation structural elucidation of sesquiterpene lactones from Centipeda minima (L.), A. Br. et Aschers. and bioassay on their anticancer activities. Cent. South Pharm. 2019, 17, 356–359. [Google Scholar]
- Xie, X. Study on the Anti-Tumor Active Components and Their Fingerprints of Centipeda minima. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2007. [Google Scholar]
- Wu, P.; Su, M.X.; Wang, Y.; Wang, G.C.; Ye, W.C.; Chung, H.Y.; Li, J.; Jiang, R.W.; Li, Y.L. Supercritical fluid extraction assisted isolation of sesquiterpene lactones with antiproliferative effects from Centipeda minima. Phytochemistry 2012, 76, 133–140. [Google Scholar] [CrossRef]
- Wu, P.; Li, X.G.; Liang, N.; Wang, G.C.; Ye, W.C.; Zhou, G.X.; Li, Y.L. Two new sesquiterpene lactones from the supercritical fluid extract of Centipeda minima. J. Asian Nat. Prod. Res. 2012, 14, 515–520. [Google Scholar] [CrossRef]
- Wu, J.B.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: Sesquiterpenes of potential anti-allergy activity. Chem. Pharm. Bull. 1991, 39, 3272–3275. [Google Scholar] [CrossRef]
- Su, M.; Li, Y.; Chung, H.Y.; Ye, W. 2beta-(Isobutyryloxy)florilenalin, a sesquiterpene lactone isolated from the medicinal plant Centipeda minima, induces apoptosis in human nasopharyngeal carcinoma CNE cells. Molecules 2009, 14, 2135–2146. [Google Scholar] [CrossRef]
- Ding, L.F.; Liu, Y.; Liang, H.X.; Liu, D.P.; Zhou, G.B.; Cheng, Y.X. Two new terpene glucosides and antitumor agents from Centipeda minima. J. Asian Nat. Prod. Res. 2009, 11, 732–736. [Google Scholar] [CrossRef]
- Liang, H.X.; Bao, F.K.; Dong, X.P.; Zhu, H.J.; Lu, X.J.; Shi, M.; Lu, Q.; Cheng, Y.X. Two new antibacterial sesquiterpenoids from Centipeda minima. Chem. Biodivers. 2007, 4, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.-H. Chemical constituents of triterpenes from Centipeda minima and its anti-inflammatory activities. Zhong Cao Yao 2020, 51, 4907–4915. [Google Scholar]
- Heng-Xing, L.; Fu-Kai, B.; Xiao-Ping, D.; Qing, L.; Yong-Xian, C. Antibacterial triterpenes from Centipeda minima (Compositae). Plant Divers. 2007, 29, 479. [Google Scholar]
- Wang, J.; Xue, P.; Guang, C.; Wang, Y.; Ma, T.; Qiu, F. Study on chemical constituents from n-BuOHextract of Centipeda minima (L.), A. Br. et Aschers. J. Tianjin Univ. Trad. Chin. Med. 2021, 40, 253–259. [Google Scholar]
- Chao, Y. Screening of Effective Sites for Goose-paste Allergy-resistant Rhinitis and the Preparation Process of Its Nasal Spray. Master’s Thesis, Hubei University of Traditional Chinese Medicine, Wuhan, China, 2019. [Google Scholar]
- Gao, C.; Pan, H.; Ma, F.; Zhang, Z.; Zhao, Z.; Song, J.; Li, W.; Fan, X. Centipeda minima active components and mechanisms in lung cancer. BMC Complement. Med. Ther. 2023, 23, 89. [Google Scholar] [CrossRef]
- Cao, J.; Li, G. Chemical constituents of Centipeda minima. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Medica 2012, 37, 2301–2303. [Google Scholar]
- Jiaojiao, Z.; Zhiming, B.; Yan, H.; Lijuan, L. Chemical Constituents of Centipeda minima. Pharm. Clin. Res. 2013, 21, 133–134. [Google Scholar]
- Wu, L.; Liu, Y.; Chen, M.; Bi, Z.; Wang, H.; Liu, E. Chemical constituents of Centipeda minima. Cent. South Pharm. 2016, 14, 351–354. [Google Scholar]
- Zhu, Y. Study on the Chemical Constituents and the Antitumor Activity of Centipeda minima. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2012. [Google Scholar]
- Cordeiro, M.; Martins, V.; Silva, A.P.D.; Rocha, H.A.O.; Rachetti, V.P.S.; Scortecci, K.C. Phenolic Acids as Antidepressant Agents. Nutrients 2022, 14, 4309. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Yan, B.; Zhang, Y.; Wu, H. Study on the chemical constituents of n-butanol extract fraction from Centipeda minima. Shizhen Guoyi Guoyao 2013, 24, 2358–2359. [Google Scholar]
- Bingwu, Z. Study on the Chemical Constituents of N-butanol Extract Fraction and Quality Analysis of Centipeda minima. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2013. [Google Scholar]
- Wu, J.B.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: Isolation of a new plenolin ester and the antiallergy activity of sesquiterpene lactones. Chem. Pharm. Bull. 1985, 33, 4091–4094. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Yu, H.-M.; Wen, S.-l.; Liu, Y.-L. Histopathological study on allergic rhinitis treated with Centipeda minima. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Medica 2005, 30, 292–294. [Google Scholar]
- Jun, L.; Chengyi, S.; Jianle, X.; Zhuoping, L. Basic Study on the Regulation of Th2 Immune Response in Rats with Allergic Rhinitis by Centipeda minima Extract Product via Mediating Toll-like Receptor-Nuclear Factor Kappa B Signaling Pathway. Chin. Arch. Tradit. Chin. 2024, 1–11. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Z.; Chen, Y.F.; Zhang, S.B.; He, D.H.; Wei, S.F.; Wang, Q.; Pan, H.F.; Liu, Y.Q. Centipeda minima extract sensitizes lung cancer cells to DNA-crosslinking agents via targeting Fanconi anemia pathway. Phytomedicine 2021, 91, 153689. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xue, P.-H.; Yu, H.; Wang, M.; Zhao, X.-C.; Qiu, F.; Zhou, G.-B. Screening of anti-lung cancer compounds and in vitro mechanism of helenalin-isovalerate for Centipeda minima. Chin. Trad. Pat. Med. 2020, 42, 3151–3157. [Google Scholar]
- Wang, H.C.; Wu, P.E.; He, W.D.; Chen, C.Y.; Zheng, R.Q.; Pang, Y.C.; Wu, L.C.; Cheng, Y.X.; Liu, Y.Q. Centipeda minima extracts and the active sesquiterpene lactones have therapeutic efficacy in non-small cell lung cancer by suppressing Skp2/p27 signaling pathway. J. Ethnopharmacol. 2025, 340, 119277. [Google Scholar] [CrossRef]
- Wang, M.; Guo, H.; Sun, B.B.; Jie, X.L.; Shi, X.Y.; Liu, Y.Q.; Shi, X.L.; Ding, L.Q.; Xue, P.H.; Qiu, F.; et al. Centipeda minima and 6-O-angeloylplenolin enhance the efficacy of immune checkpoint inhibitors in non-small cell lung cancer. Phytomedicine 2024, 132, 155825. [Google Scholar] [CrossRef]
- Wang, R.; Gao, C.; Yu, M.; Song, J.; Feng, Z.; Wang, R.; Pan, H.; Liu, H.; Li, W.; Fan, X. Mechanistic prediction and validation of Brevilin A Therapeutic effects in Lung Cancer. BMC Complement. Med. Ther. 2024, 24, 214. [Google Scholar] [CrossRef]
- Khan, M.; Maryam, A.; Saleem, M.Z.; Shakir, H.A.; Qazi, J.I.; Li, Y.; Ma, T. Brevilin A induces ROS-dependent apoptosis and suppresses STAT3 activation by direct binding in human lung cancer cells. J. Cancer 2020, 11, 3725–3735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, R.Y.; Zhang, J.; Zhang, W.X.; Huang, Z.H.; Hu, H.F.; Li, Y.L.; Li, B.; He, Q.Y. Inhibition of Nrf2 enhances the anticancer effect of 6-O-angeloylenolin in lung adenocarcinoma. Biochem. Pharmacol. 2017, 129, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Q.; Sun, H.Y.; Chan, C.O.; Liu, B.B.; Wu, J.H.; Chan, S.W.; Mok, D.K.; Tse, A.K.; Yu, Z.L.; Chen, S.B. Centipeda minima (Ebushicao) extract inhibits PI3K-Akt-mTOR signaling in nasopharyngeal carcinoma CNE-1 cells. Chin. Med. 2015, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qu, Z.; Lin, Y.; Lee, C.S.; Tai, W.C.; Chen, S. Brevilin A Induces Cell Cycle Arrest and Apoptosis in Nasopharyngeal Carcinoma. Front. Pharmacol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Liu, R.; Dow Chan, B.; Mok, D.K.; Lee, C.S.; Tai, W.C.; Chen, S. Arnicolide D, from the herb Centipeda minima, Is a Therapeutic Candidate against Nasopharyngeal Carcinoma. Molecules 2019, 24, 1908. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Chapter One–Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. In Advances in Cancer Research; Sarkar, D., Fisher, P.B., Eds.; Academic Press: New York, NY, USA, 2021; Volume 149, pp. 1–61. [Google Scholar]
- Wei, J.; Zhao, X.; Wu, B.; Tan, Z.; Xie, Y.; Wei, M.; Wu, L. Centipeda minima extract exhibits anti-liver cancer effects via the ER stress/HMOX1/Fe2+/ROS pathway. Phytomedicine 2025, 139, 156487. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, H. In vitro evaluation of anti-hepatoma activity of brevilin A: Involvement of Stat3/Snail and Wnt/β-catenin pathways. RSC Adv. 2019, 9, 4390–4396. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, X.; Chen, J.; Zhang, B.; Xie, S.; Yuan, Y.; Sun, L.; Yuan, S.; Yu, H.; Ding, J.; et al. Arnicolide C Suppresses Tumor Progression by Targeting 14-3-3θ in Breast Cancer. Pharmaceuticals 2024, 17, 224. [Google Scholar] [CrossRef]
- Wen, W.; Jin, K.; Che, Y.; Du, L.Y.; Wang, L.N. Arnicolide D Inhibits Oxidative Stress-induced Breast Cancer Cell Growth and Invasion through Apoptosis, Ferroptosis, and Parthanatos. Anticancer. Agents Med. Chem. 2024, 24, 836–844. [Google Scholar] [CrossRef]
- Lee, M.M.; Chan, B.D.; Wong, W.Y.; Qu, Z.; Chan, M.S.; Leung, T.W.; Lin, Y.; Mok, D.K.; Chen, S.; Tai, W.C. Anti-cancer Activity of Centipeda minima Extract in Triple Negative Breast Cancer via Inhibition of AKT, NF-κB, and STAT3 Signaling Pathways. Front. Oncol. 2020, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.Z.; Nisar, M.A.; Alshwmi, M.; Din, S.R.U.; Gamallat, Y.; Khan, M.; Ma, T. Brevilin A Inhibits STAT3 Signaling and Induces ROS-Dependent Apoptosis, Mitochondrial Stress and Endoplasmic Reticulum Stress in MCF-7 Breast Cancer Cells. Onco Targets Ther. 2020, 13, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Ngeow, J.; Eng, C. Oh GxE! The Complexity of Body Mass Index and Colon Cancer Risk. J. Natl. Cancer Inst. 2021, 113, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Meng, M.; Li, B.; Chen, H.; Tan, J.; Xu, K.; Xiao, S.; Kwan, H.Y.; Liu, Z.; Su, T. Brevilin A is a potent anti-metastatic CRC agent that targets the VEGF-IL6-STAT3 axis in the HSCs-CRC interplay. J. Transl. Med. 2023, 21, 260. [Google Scholar] [CrossRef]
- You, P.; Wu, H.; Deng, M.; Peng, J.; Li, F.; Yang, Y. Brevilin A induces apoptosis and autophagy of colon adenocarcinoma cell CT26 via mitochondrial pathway and PI3K/AKT/mTOR inactivation. Biomed. Pharmacother. 2018, 98, 619–625. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Su, T.; Wang, Y.P.; Wang, X.N.; Li, C.Y.; Zhu, P.L.; Huang, Y.M.; Yang, Z.Y.; Chen, S.B.; Yu, Z.L. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of brevilin A. Life Sci. 2020, 241, 117169. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, Z.; Fu, X.; Li, J.; Yin, C.; Chou, J.; Wang, Y.; Liu, Y.; Chen, Y.; Bai, J.; et al. Arnicolide D exerts anti-melanoma effects and inhibits the NF-κB pathway. Phytomedicine 2019, 64, 153065. [Google Scholar] [CrossRef]
- You, P.; Tang, L.; Zhu, Y.; Tian, Y. Brevilin A shows an anti-tumor role in prostate cancer via the lncRNA H19/miR-194/E2F3 signaling pathway. Aging 2023, 15, 4411–4428. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, R.; Hu, Y.; Yang, Y.; Tian, Y. Arnicolide D Inhibits Proliferation and Induces Apoptosis of Osteosarcoma Cells through PI3K/Akt/mTOR Pathway. Anticancer. Agents Med. Chem. 2024, 24, 1288–1294. [Google Scholar] [CrossRef]
- Lee, D.; Kwak, H.J.; Kim, B.H.; Kim, D.W.; Kim, H.Y.; Kim, S.H.; Kang, K.S. Brevilin A Isolated from Centipeda minima Induces Apoptosis in Human Gastric Cancer Cells via an Extrinsic Apoptotic Signaling Pathway. Plants 2022, 11, 1658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Cui, X.; Lv, D.; Jin, L.; Khan, M.; Ma, T. Brevilin A promotes oxidative stress and induces mitochondrial apoptosis in U87 glioblastoma cells. Onco Targets Ther. 2018, 11, 7031–7040. [Google Scholar] [CrossRef] [PubMed]
- Changlong, L.; Hezhen, W.; Yongping, H.; Yanfang, Y.; Yanwen, L.; Jianwen, L. 6-O-Angeloylenolin induces apoptosis through a mitochondrial/caspase and NF-kappaB pathway in human leukemia HL60 cells. Biomed. Pharmacother. 2008, 62, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shiying, D. Centipeda minima Improves Reserpine-Induced Depression-Like Behavior in Rat. Master’s Thesis, University of South China, Hengyang, China, 2023. [Google Scholar]
- Zhou, Y.L.; Yan, Y.M.; Li, S.Y.; He, D.H.; Xiong, S.; Wei, S.F.; Liu, W.; Hu, L.; Wang, Q.; Pan, H.F.; et al. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol. Sin. 2020, 41, 10–21. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, X.Y.; Hao, X.Y.; Yan, Y.M.; Hong, M.; Wei, S.F.; Zhou, Y.L.; Wang, Q.; Cheng, Y.X.; Liu, Y.Q. Ethanol Extract of Centipeda minima Exerts Antioxidant and Neuroprotective Effects via Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9421037. [Google Scholar] [CrossRef]
- Tan, Y.; He, F.; Chen, Z.-W.; Zhang, Y.-L.; Zhang, C.; Gu, R.-X.; Zhang, D.-Y.; Wang, X.; Hua, Q. Scalp mechanical stimulation promotes moderate vasodilation and the permeability of blood-brain barrier in anesthetized mice. Chin. J. Pharmacol. Toxicol. 2019, 6, 41. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ya, J.; Guanghui, Z.; Meihuan, W. Extraction process optimization and antioxidant activity of total flavonoids from Centipeda minima. J. Int. Pharm. Res. 2020, 47, 666–670. [Google Scholar]
- Yang, G.; Su, F.; Hu, D.; Ruan, C.; Che, P.; Zhang, Y.; Wang, J. Optimization of the Extraction Process and Antioxidant Activity of Polysaccharide Extracted from Centipeda minima. Chem. Biodivers. 2023, 20, e202200626. [Google Scholar] [CrossRef]
- Taylor, R.S.; Towers, G.H. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry 1998, 47, 631–634. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.; Leung, T.W.; Shum, T.Y.; Cho, W.C.; Chen, S.; Tai, W.C. Centipeda minima Extract Attenuates Dextran Sodium Sulfate-Induced Acute Colitis in Mice by Inhibiting Macrophage Activation and Monocyte Chemotaxis. Front. Pharmacol. 2021, 12, 738139. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Chiu, C.S.; Lin, T.H.; Lee, M.M.; Lee, C.Y.; Chang, S.J.; Hou, W.C.; Huang, G.J.; Deng, J.S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhou, Y.L.; He, D.H.; Liu, W.; Fan, X.Z.; Wang, Q.; Pan, H.F.; Cheng, Y.X.; Liu, Y.Q. Centipeda minima extract exerts antineuroinflammatory effects via the inhibition of NF-κB signaling pathway. Phytomedicine 2020, 67, 153164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kim, B.H.; Yun, S.K.; Roh, Y.S. Centipeda minima Extract Inhibits Inflammation and Cell Proliferation by Regulating JAK/STAT Signaling in Macrophages and Keratinocytes. Molecules 2023, 28, 1723. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Zhang, Q.; Mou, X.; Zhao, Y.; Fan, H.; Xu, H.; Chen, D.; Qiu, F.; Zhao, F. Two sesquiterpene lactones, arnicolide B and arnicolide C, isolated from Centipeda minima, exert anti-inflammatory effects in LPS stimulated RAW 264.7 macrophages via inactivation of the MAPK pathway. Nat. Prod. Res. 2023, 37, 2969–2972. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Jiang, Y.; Yuan, Y.; Yang, L.; Hu, Q.; Tang, J.; Meng, X.; Xie, C.; Shen, X. Brevilin A Ameliorates Acute Lung Injury and Inflammation Through Inhibition of NF-κB Signaling via Targeting IKKα/β. Front. Pharmacol. 2022, 13, 911157. [Google Scholar]
- Xue, P.H.; Zhang, N.; Liu, D.; Zhang, Q.R.; Duan, J.S.; Yu, Y.Q.; Li, J.Y.; Cao, S.J.; Zhao, F.; Kang, N.; et al. Cytotoxic and Anti-Inflammatory Sesquiterpenes from the Whole Plants of Centipeda minima. J. Nat. Prod. 2021, 84, 247–258. [Google Scholar] [CrossRef]
- Fang, B.; Wen, S.; Li, Y.; Bai, F.; Wei, Y.; Xiong, Y.; Huang, Q.; Lin, X. Prediction and verification of target of helenalin against hepatic stellate cell activation based on miR-200a-mediated PI3K/Akt and NF-κB pathways. Int. Immunopharmacol. 2021, 92, 107208. [Google Scholar] [CrossRef]
- Park, Y.J.; Jeon, M.S.; Lee, S.; Kim, J.K.; Jang, T.S.; Chung, K.H.; Kim, K.H. Anti-fibrotic effects of brevilin A in hepatic fibrosis via inhibiting the STAT3 signaling pathway. Bioorg. Med. Chem. Lett. 2021, 41, 127989. [Google Scholar] [CrossRef]
- Liang, L.J.; Wang, D.; Yu, H.; Wang, J.; Zhang, H.; Sun, B.B.; Yang, F.Y.; Wang, Z.; Xie, D.W.; Feng, R.E.; et al. Transcriptional regulation and small compound targeting of ACE2 in lung epithelial cells. Acta Pharmacol. Sin. 2022, 43, 2895–2904. [Google Scholar] [CrossRef]
- Zhang, X.L.; He, J.; Huang, W.H.; Huang, H.B.; Zhang, Z.M.; Wang, J.J.; Yang, L.; Wang, G.C.; Wang, Y.F.; Li, Y.L. Antiviral Activity of the Sesquiterpene Lactones from Centipeda minima against Influenza A Virus in vitro. Nat. Prod. Commun. 2018, 13, 115–119. [Google Scholar] [CrossRef]
- Zhang, X.L.; Xia, Y.P.; Yang, L.; He, J.; Li, Y.L.; Xia, C. Brevilin A, a Sesquiterpene Lactone, Inhibits the Replication of Influenza A Virus In Vitro and In Vivo. Viruses 2019, 11, 835. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, C.; Zhu, B.; Peng, H.; Ni, F. Study on the antiasthmatic effects of essential oil from Centipeda minima. Chin. J. Mod. Appl. Pharm. 2010, 27, 473–476. [Google Scholar]
- Baek, J.Y.; Kim, B.H.; Kim, D.W.; Lee, W.Y.; Kim, C.E.; Kim, H.Y.; Pyo, J.; Park, E.S.; Kang, K.S. Hair Growth Effect of DN106212 in C57BL/6 Mouse and Its Network Pharmacological Mechanism of Action. Curr. Issues Mol. Biol. 2023, 45, 5071–5083. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, M.J.; Lee, W.Y.; Pyo, J.; Shin, M.S.; Hwang, G.S.; Shin, D.; Kim, C.E.; Park, E.S.; Kang, K.S. Hair Growth Stimulation Effect of Centipeda minima Extract: Identification of Active Compounds and Anagen-Activating Signaling Pathways. Biomolecules 2021, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, F.; Wu, Q.; Li, S.; Ruan, G.; Yang, J.; Yu, C.; Jiang, N.; Xiao, Y.; Liu, Y. Brevilin A enhances innate immunity and the resistance of oxidative stress in Caenorhabditis elegans via p38 MAPK pathway. Int. Immunopharmacol. 2022, 113 Pt A, 109385. [Google Scholar] [CrossRef]
- Huang, W.; Yu, X.; Liang, N.; Ge, W.; Kwok, H.F.; Lau, C.B.; Li, Y.; Chung, H.Y. Anti-angiogenic Activity and Mechanism of Sesquiterpene Lactones from Centipeda minima. Nat. Prod. Commun. 2016, 11, 435–438. [Google Scholar] [CrossRef]
- Chabert, P.; Fougerousse, A.; Brouillard, R. Anti-mitotic properties of resveratrol analog (Z)-3,5,4′-trimethoxystilbene. Biofactors 2006, 27, 37–46. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, L.; Wang, C. Two cases of serious adverse reactions caused by internal administration of Centipeda minima its discussion. Zhejiang J. Tradit. Chin. Med. 2020, 55, 509. [Google Scholar]
- Ruan, Q.; Wang, C.; Zhang, Y.; Sun, J. Brevilin A attenuates cartilage destruction in osteoarthritis mouse model by inhibiting inflammation and ferroptosis via SIRT1/Nrf2/GPX4 signaling pathway. Int. Immunopharmacol. 2023, 124 Pt B, 110924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y. Progress of clinical application and pharmacological research of Centipeda minima. Agric. Jilin 2015, 364, 76–77. [Google Scholar]
- Wang, X.; Wang, Z.; Cao, L.; He, H.; Wang, Z.; Li, X.; Miao, M. Medication rule analysis and pharmacological analysis of traditional Chinese medicine treating allergic rhinitis based on data mining, network pharmacology and experimental validation. Nat. Prod. Res. Dev. 2024, 1, 137–154. [Google Scholar]
- Xiong, Y.A.; Li, W.; Lv, J.; Chen, L.; Ou, S.; Wang, S. Study on Material Basis of Miao Medicine Bi-Ning Spray in Allergic Rhinitis Treatment. World Sci. Technol.-Mod. Tradit. Chin. Med. 2017, 28, 880–884. [Google Scholar]
- Zherui, G.; Baijian, G. Clinical application of Cough Relief and Anti-inflammatory Powder. J. Tradit. Chin. Med. 1981, 43. [Google Scholar]
- Lu, L.; Bin, J.; Suyi, L.; Xiaobin, C.; Hui, Z.; Pei, T. Study on UPLC Fingerprints and QAMS of Centipeda minima Dispensing Granules. Chin. J. Ethnomed. Ethnopharmacy 2024, 33, 27–34. [Google Scholar]
- Shi, G.L.; Lu, X.; Zheng, Y.H.; Yang, T.; Zhu, E.Y.; Song, Y.H.; Huang, P.T. Insights into the potential dual-antibacterial mechanism of Kelisha capsule on Escherichia coli. BMC Complement. Med. Ther. 2024, 24, 207. [Google Scholar] [CrossRef]
- Sijun, Z. Emergency Medicine for Children with High Fever–Zekepine Injection. Chin. Pract. J. Rural Dr. 2007, 64. [Google Scholar]
- Yip, K.M.; Lee, K.M.; Ng, T.B.; Xu, S.J.; Yung, K.K.L.; Qu, S.G.; Cheung, A.K.L.; Sze, S.C.W. An anti-inflammatory and anti-fibrotic proprietary Chinese medicine nasal spray designated as Allergic Rhinitis Nose Drops (ARND) with potential to prevent SARS-CoV-2 coronavirus infection by targeting RBD (Delta)-angiotensin converting enzyme 2 (ACE2) binding. Chin. Med. 2022, 17, 88. [Google Scholar]
- Xiao-hui, T.; Jing, H.; Chao-xiang, R.; Jie, S.; Jin, P.; Qing-hua, W.; Jiang, C. Analysis of genetic diversity relationship in medicinal plants of Compositae by, I.S.S.R. Nat. Prod. Res. Dev. 2018, 30, 1764. [Google Scholar]
- Borgo, J.; Wagner, M.S.; Laurella, L.C.; Elso, O.G.; Selener, M.G.; Clavin, M.; Bach, H.; Catalán, C.A.N.; Bivona, A.E.; Sepúlveda, C.S.; et al. Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties. Molecules 2024, 29, 814. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus Essential Oil: An Update of Its Phytochemistry, Biological Activities, and Safety Profile. Oxid. Med. Cell. Longev. 2022, 2022, 8442734. [Google Scholar] [CrossRef] [PubMed]
- Wanqing, A.; Tong, S.; Yadan, L.; Jiayi, Z.; Xiaowen, O.; Qiaoqiao, Z.; Yebing, Z.; Lei, Z.; Hongquan, Z. Research Progress on Chemical Constituents and Pharmacological Effects of Chrysanthemum Plants. Mod. Chin. Med. 2025, 27, 562–574. [Google Scholar]
- Sun, Y.-P. Expression of pungent-taste herbs and their applications in clinical compatibility. Zhongcaoyao 2015, 46, 785–790. [Google Scholar]
- Ruiqi, L.; Mingsan, M. Modern research and relationship of warm Chinese medicine properties. Acta Chin. Med. 2012, 27, 1456–1459. [Google Scholar]
- Wang, X.; Lu, S.; Zheng, S. Empirical analysis of the channel tropism traditional Chinese medicine in chemical constituent, pharmacological effects and clinical application. Zhong Hua Zhong Yi Yao Za Zhi 2018, 33, 5193–5197. [Google Scholar]
- Liu, J.; Zheng, W.; He, Y.; Zhang, W.; Luo, Z.; Liu, X.; Jiang, X.; Meng, F.; Wu, L. A Review of the Research Applications of Centipeda minima. Molecules 2023, 29, 108. [Google Scholar] [CrossRef]
- Zhang, T.-J.; Bai, G. The concept, core theory and research methods of Chinesemedicine quality markers. Yao Xue Xue Bao 2019, 54, 187–196. [Google Scholar]
- Zan, K.; Xie, Y.; Guo, L.; Zheng, J.; Ma, S. Study on characteristic chromatogram and quantitation method of seven components for Centipedae Herba. Chin. J. Pharm. Anal. 2018, 38, 151–157. [Google Scholar]
- Ren, H.; Lv, Q.; Peng, Y. Simultaneous determination of five sesquiterpene lactones in Centipeda minima by HPLC/UV Assay. Mod. Chin. Med. 2019, 21, 616–621. [Google Scholar]
- Ying, G.; Dan, C.; Yili, L.; Huaping, Z.; Shubin, L. Thin-layer chromatographic identification and gas chromatographic determination of Centipeda minima. Fujian J. Tradit. Chin. Med. 2016, 47, 14–17. [Google Scholar]
- Chen, W.; Yang, H.; Chen, Y.; Wang, H.; Chen, X. Quality evaluation of Centipedae Herba from different origins based on multi-component quantification combined with multivariate statistical analysis. Nat. Prod. Res. Dev. 2024, 36, 1573–1583. [Google Scholar]
- Rongfa, Z. Metabolism of Centipeda minima in Rats. Master’s Thesis, Kunming Medical University, Kunming, China, 2022. [Google Scholar]
- Liu, Y.; Chen, X.Q.; Liang, H.X.; Zhang, F.X.; Zhang, B.; Jin, J.; Chen, Y.L.; Cheng, Y.X.; Zhou, G.B. Small compound 6-O-angeloylplenolin induces mitotic arrest and exhibits therapeutic potentials in multiple myeloma. PLoS ONE 2011, 6, e21930. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Xiong, Y.A.; Ou, S.; Wang, Y. Study on HPLC Fingerprint of Bining Nasal Spray. China Pharm. 2017, 4278–4282. [Google Scholar]
- Liu, J.; Wang, S.-Y.; Dou, H.-Q. GC Determination of Chrysanthenyl Acetate in Bisaitong Spray. Chin. J. Pharm. Anal. 2005, 25, 840–842. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).