Synthetic Cadaver Odorants and the Sulfur Gap: Linking Chemistry and Canine Olfaction in Human Remains Detection
Abstract
1. Introduction
2. Chemical Composition of Decomposition Odor
2.1. Introduction to Thanatochemistry
2.2. Key Volatile Organic Compounds (VOCs) in Decomposition
2.3. Sigma Pseudo™ Corpse Scent: Synthetic Replication of Decomposition
3. Canine Detection of Decomposition Odor
3.1. Introduction to the Biological Basis of Canine Olfaction
3.1.1. Genetics of Canine Olfaction
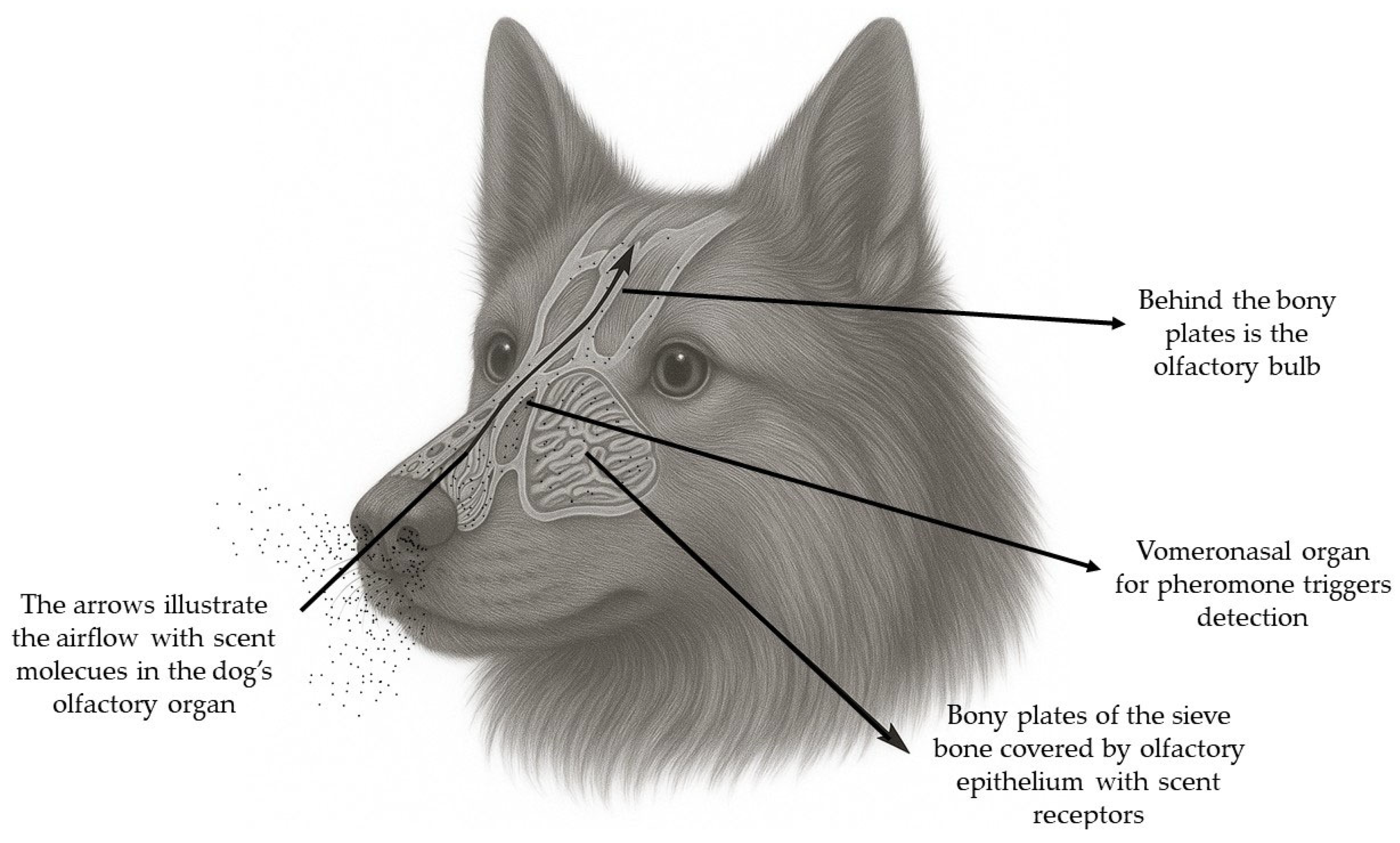
3.1.2. Anatomy of Nasal Cavity and Nasal Turbinates
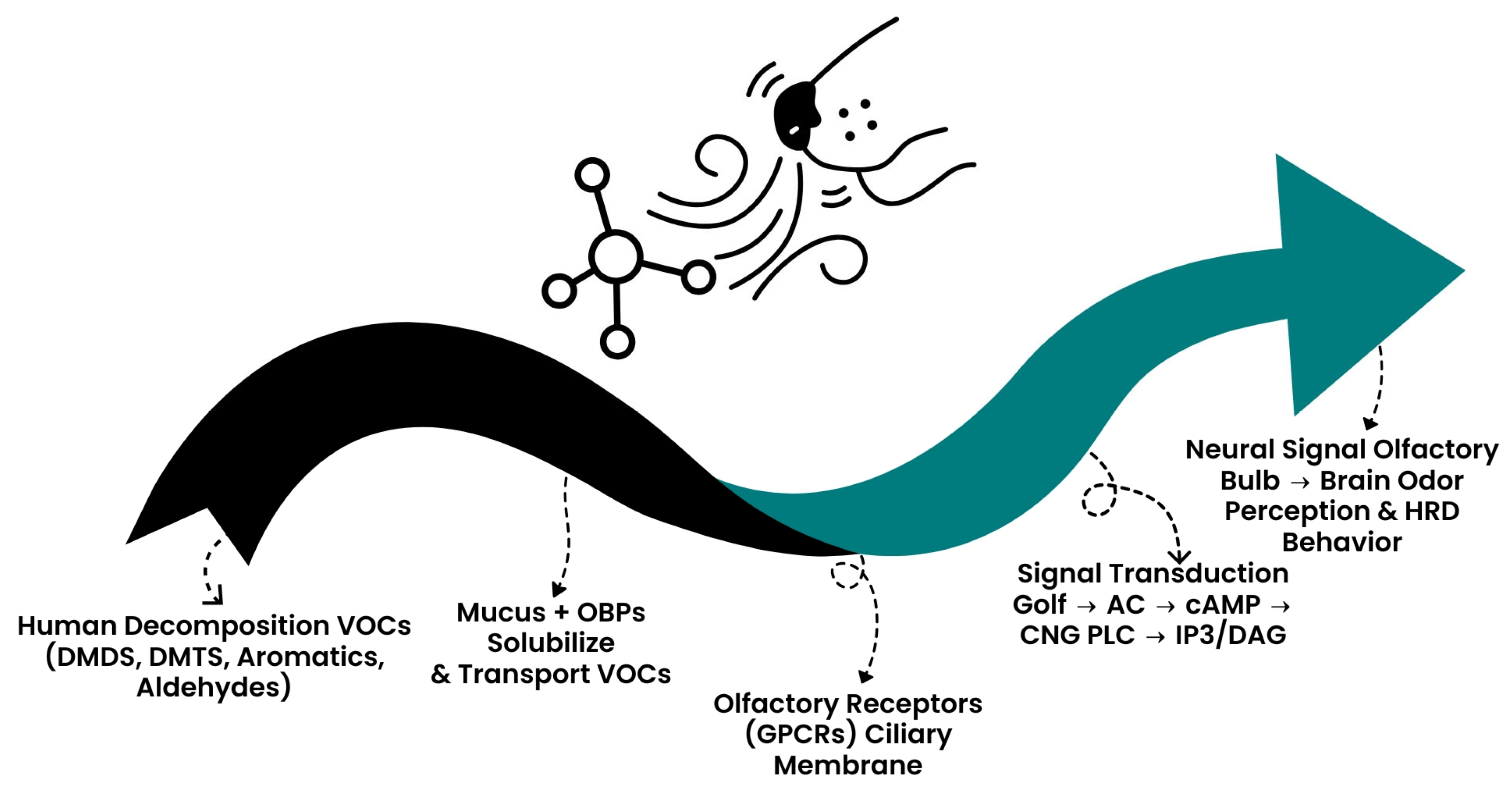
3.1.3. The Molecular Basis of Signal Transduction
3.2. HRD Dog Training and Scent Detection
Examples of Sigma Use in Training Programs
3.3. Performance and Limitations
3.3.1. Sensitivity, Specificity, and False Alerts
3.3.2. Environmental and Operational Challenges
3.3.3. Legal and Ethical Considerations in Canine Evidence
4. Linking Chemistry and Canine Detection: Sigma as a Training Tool
4.1. How Sigma’s Chemical Profile Matches Canine Detection Capabilities
4.2. Current Evidence on Canine Responses to Sigma vs. Real Human Remains
4.3. Molecular Mechanisms of Canine Olfaction as a Framework for Designing Synthetic Training Aids
4.4. Sulfur Gap-Hypothesis
4.5. Importance of Analytical Chemistry in Developing and Validating Synthetic Scents
4.6. Future Directions for Research and Validation Under Operational Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1D | One-dimensional |
| 2D | Two-dimensional |
| ACEM | Automated Chemical Environment Monitor |
| ARF | Anthropology Research Facility |
| ATP | Adenosine triphosphate |
| cAMP | Cyclic adenosine monophosphate |
| CDD | Cadaver-detection dogs |
| CDI | Cadaver Decomposition Island |
| COVID-19 | Coronavirus disease 2019 |
| DHS | Department of Homeland Security |
| DMDS | dimethyl disulfide |
| DMS | dimethyl sulfide |
| DMTS | dimethyl trisulfide |
| DTI | diffusion tensor imaging |
| FBI | Federal Bureau of Investigation |
| FEMA | Federal Emergency Management Agency |
| FET-GC-ITD | Full Evaporation Technique-Gas Chromatography-Ion-Trap Detection |
| fMRI | functional magnetic resonance imaging |
| GABA | 4-Aminobutanoic acid |
| GC | Gas Chromatography |
| GC × GC | Two-dimensional gas chromatography |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| GPCR | G-protein-coupled receptor |
| HRD | human remains detection |
| HRV | heart-rate variability |
| ISO | International Organization for Standardization |
| LFD | life finding dogs |
| MDMPP | 2-methyl-1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl propanoic acid |
| NOAA | National Oceanic and Atmospheric Administration |
| OB | olfactory bulb |
| OR | olfactory receptor |
| ORN | olfactory receptor neuron |
| P/T | Purge and Trap |
| PMI | postmortem interval |
| PSI | Sigma Pseudo™ Corpse Scent Formulation I |
| PSII | Sigma Pseudo™ Corpse Scent Formulation II |
| QBA | quantitative behavior assessment |
| SAR | Search and rescue |
| Sigma | Sigma Pseudo™ Corpse Scent |
| SPME | Solid-Phase Microextraction |
| TAAR | Trace amine-associated receptor |
| TOFMS | Time-of-flight mass spectrometry |
| VNO | vomeronasal organ |
| VOC | volatile organic compound |
References
- Rebmann, A.; David, E.; Sorg, M. Cadaver Dog Handbook: Forensic Training and Tactics for the Recovery of Human Remains; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Martin, C.; Malević, M.; Diederich, C.; Verheggen, F. Copycatting the Smell of Death: Deciphering the Role of Cadaveric Scent Components Used by Detection Dogs to Locate Human Remains. J. Forensic Sci. 2023, 68, 1190–1197. [Google Scholar] [CrossRef]
- DeGreeff, L.E.; Weakley-Jones, B.; Furton, K.G. Creation of Training Aids for Human Remains Detection Canines Utilizing a Non-Contact, Dynamic Airflow Volatile Concentration Technique. Forensic Sci. Int. 2012, 217, 32–38. [Google Scholar] [CrossRef]
- Ouimet, F.; Patel, D.; Tsontakis, M.; Samson, C.; Forbes, S.L. Establishing the Volatile Organic Compound Profile and Detection Capabilities of Human Remain Detection Dogs to Human Bones. Forensic Sci. Int. Synerg. 2025, 10, 100566. [Google Scholar] [CrossRef]
- Jones, K.E.; Dashfield, K.; Downend, A.B.; Otto, C.M. Search-and-Rescue Dogs: An Overview for Veterinarians. J. Am. Vet. Med. Assoc. 2004, 225, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Hare, E.; Buchweitz, J.P.; Kelsey, K.M.; Fitzgerald, S.D. Fifteen-Year Surveillance of Pathological Findings Associated with Death or Euthanasia in Search-and-Rescue Dogs Deployed to the September 11, 2001, Terrorist Attack Sites. J. Am. Vet. Med. Assoc. 2020, 257, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, J. Police and Military Dogs: Criminal Detection, Forensic Evidence, and Judicial Admissibility; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Vass, A.A. Odor Mortis. Forensic Sci. Int. 2012, 222, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Statheropoulos, M.; Spiliopoulou, C.; Agapiou, A. A Study of Volatile Organic Compounds Evolved from the Decaying Human Body. Forensic Sci. Int. 2005, 153, 147–155. [Google Scholar] [CrossRef]
- Barlow, C. Human Subjects Protection and Federal Regulations of Clinical Trials. Semin. Oncol. Nurs. 2020, 36, 151001. [Google Scholar] [CrossRef]
- National Academies’ Institute of Medicine. Medicolegal Death Investigation System: Workshop Summary; National Academies Press: Washington, DC, USA, 2003; ISBN 9780309526425. [Google Scholar]
- Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:102:0048:0058:en:PDF (accessed on 14 July 2025).
- Perrault, K.; Stuart, B.; Forbes, S. A Longitudinal Study of Decomposition Odour in Soil Using Sorbent Tubes and Solid Phase Microextraction. Chromatography 2014, 1, 120–140. [Google Scholar] [CrossRef]
- Cieśla, J.; Skrobisz, J.; Niciński, B.; Kloc, M.; Mazur, K.; Pałasz, A.; Javan, G.T.; Tomsia, M. The Smell of Death. State-of-the-Art and Future Research Directions. Front. Microbiol. 2023, 14, 1260869. [Google Scholar] [CrossRef]
- Okunuga, O.O. Analysis of Volatile Organic Compounds Produced during the Decomposition of Human Analogues. Ph.D. Thesis, University of Leicester, Leicester, UK, 2017. [Google Scholar]
- Caldwell, P.T.; Tipple, C.; Dulgerian, N.; Eckenrode, B.A. Characterization of Pseudo Corpse Scents Used as Canine Training Aids. In Proceedings of the 61st American Chemical Society Southeast Regional Meeting, San Juan, Puerto Rico, 21–22 October 2009. [Google Scholar]
- Martin, C.; Willem, N.; Desablens, S.; Menard, V.; Tajri, S.; Blanchard, S.; Brostaux, Y.; Verheggen, F.; Diederich, C. What a Good Boy! Deciphering the Efficiency of Detection Dogs. Front. Anal. Sci. 2022, 2, 932857. [Google Scholar] [CrossRef]
- Rosier, E.; Loix, S.; Develter, W.; Van de Voorde, W.; Tytgat, J.; Cuypers, E. The Search for a Volatile Human Specific Marker in the Decomposition Process. PLoS ONE 2015, 10, e0137341. [Google Scholar] [CrossRef]
- Jenkins, E.K.; DeChant, M.T.; Perry, E.B. When the Nose Doesn’t Know: Canine Olfactory Function Associated with Health, Management, and Potential Links to Microbiota. Front. Vet. Sci. 2018, 5, 56. [Google Scholar] [CrossRef]
- Azzouzi, N.; Guillory, A.-S.; Chaudieu, G.; Galibert, F. Dog Olfactory Receptor Gene Expression Profiling Using Samples Derived from Nasal Epithelium Brushing. Canine Med. Genet. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Quignon, P.; Giraud, M.; Rimbault, M.; Lavigne, P.; Tacher, S.; Morin, E.; Retout, E.; Valin, A.-S.; Lindblad-Toh, K.; Nicolas, J.; et al. The Dog and Rat Olfactory Receptor Repertoires. Genome Biol. 2005, 6, R83. [Google Scholar] [CrossRef] [PubMed]
- Robin, S.; Tacher, S.; Rimbault, M.; Vaysse, A.; Dréano, S.; André, C.; Hitte, C.; Galibert, F. Genetic Diversity of Canine Olfactory Receptors. BMC Genom. 2009, 10, 21. [Google Scholar] [CrossRef]
- Lesniak, A.; Walczak, M.; Jezierski, T.; Sacharczuk, M.; Gawkowski, M.; Jaszczak, K. Canine Olfactory Receptor Gene Polymorphism and Its Relation to Odor Detection Performance by Sniffer Dogs. J. Hered. 2008, 99, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.M.; Wacker, D.; Roque, M.A.; Baldwin, M.W.; Stevens, R.C.; Liberles, S.D. Agonists for 13 Trace Amine-Associated Receptors Provide Insight into the Molecular Basis of Odor Selectivity. ACS Chem. Biol. 2012, 7, 1184–1189. [Google Scholar] [CrossRef]
- Liberles, S.D. Trace Amine-Associated Receptors Are Olfactory Receptors in Vertebrates. Ann. N. Y. Acad. Sci. 2009, 1170, 168–172. [Google Scholar] [CrossRef]
- Mombaerts, P. Genes and Ligands for Odorant, Vomeronasal and Taste Receptors. Nat. Rev. Neurosci. 2004, 5, 263–278. [Google Scholar] [CrossRef]
- Block, E.; Batista, V.S.; Matsunami, H.; Zhuang, H.; Ahmed, L. The Role of Metals in Mammalian Olfaction of Low Molecular Weight Organosulfur Compounds. Nat. Prod. Rep. 2017, 34, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.M.; O’Sullivan, G.; Stefanuto, P.-H.; Sandau, C.D.; Focant, J.-F. Use of GC×GC for the Characterization of Odours in Forensic Applications. In Characterization of Odorant Patterns by Comprehensive Two-Dimensional Gas Chromatography; Elsevier: Amsterdam, The Netherlands, 2022; pp. 335–365. ISBN 9780323988810. [Google Scholar]
- Srirangarajan, S.; Sindhu, V.; Raju, S.; Rao, R.J.; Prabhu, S.; Rudresh, V. Evaluation of Gingival Tissue Samples for Predicting the Time of Death Using Histological and Biochemical Tests. Forensic Sci. Int. 2021, 324, 110850. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, S.; Schütz, S. Post-Mortem Volatiles of Vertebrate Tissue. Appl. Microbiol. Biotechnol. 2011, 91, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.L.; Perrault, K.A.; Stefanuto, P.-H.; Nizio, K.D.; Focant, J.-F. Comparison of the Decomposition VOC Profile during Winter and Summer in a Moist, Mid-Latitude (Cfb) Climate. PLoS ONE 2014, 9, e113681. [Google Scholar] [CrossRef]
- Schuberth, J. A Full Evaporation Headspace Technique with Capillary GC and ITD: A Means for Quantitating Volatile Organic Compounds in Biological Samples. J. Chromatogr. Sci. 1996, 34, 314–319. [Google Scholar] [CrossRef]
- DeHaan, J.D.; Brien, D.J.; Large, R. Volatile Organic Compounds from the Combustion of Human and Animal Tissue. Sci. Justice 2004, 44, 223–236. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Agapiou, A.; Spiliopoulou, C.; Pallis, G.C.; Sianos, E. Environmental Aspects of VOCs Evolved in the Early Stages of Human Decomposition. Sci. Total Environ. 2007, 385, 221–227. [Google Scholar] [CrossRef]
- Vass, A.A.; Smith, R.R.; Thompson, C.V.; Burnett, M.N.; Dulgerian, N.; Eckenrode, B.A. Odor Analysis of Decomposing Buried Human Remains. J. Forensic Sci. 2008, 53, 384–391. [Google Scholar] [CrossRef]
- Hoffman, E.M.; Curran, A.M.; Dulgerian, N.; Stockham, R.A.; Eckenrode, B.A. Characterization of the Volatile Organic Compounds Present in the Headspace of Decomposing Human Remains. Forensic Sci. Int. 2009, 186, 6–13. [Google Scholar] [CrossRef]
- DeGreeff, L.E.; Furton, K.G. Collection and Identification of Human Remains Volatiles by Non-Contact, Dynamic Airflow Sampling and SPME-GC/MS Using Various Sorbent Materials. Anal. Bioanal. Chem. 2011, 401, 1295–1307. [Google Scholar] [CrossRef]
- Rosier, E.; Cuypers, E.; Dekens, M.; Verplaetse, R.; Develter, W.; Van de Voorde, W.; Maes, D.; Tytgat, J. Development and Validation of a New TD-GC/MS Method and Its Applicability in the Search for Human and Animal Decomposition Products. Anal. Bioanal. Chem. 2014, 406, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Rosier, E.; Loix, S.; Develter, W.; Van de Voorde, W.; Tytgat, J.; Cuypers, E. Time-Dependent VOC-Profile of Decomposed Human and Animal Remains in Laboratory Environment. Forensic Sci. Int. 2016, 266, 164–169. [Google Scholar] [CrossRef]
- Rust, L.; Nizio, K.D.; Forbes, S.L. The Influence of Ageing and Surface Type on the Odour Profile of Blood-Detection Dog Training Aids. Anal. Bioanal. Chem. 2016, 408, 6349–6360. [Google Scholar] [CrossRef]
- Perrault, K.A.; Stefanuto, P.-H.; Dubois, L.M.; Varlet, V.; Grabherr, S.; Focant, J.-F. A Minimally-Invasive Method for Profiling Volatile Organic Compounds within Postmortem Internal Gas Reservoirs. Int. J. Legal Med. 2017, 131, 1271–1281. [Google Scholar] [CrossRef]
- Waters, B.; Hara, K.; Ikematsu, N.; Takayama, M.; Kashiwagi, M.; Matsusue, A.; Kubo, S.-I. Volatile Hydrocarbon Analysis in Blood by Headspace Solid-Phase Microextraction: The Interpretation of VHC Patterns in Fire-Related Incidents. J. Anal. Toxicol. 2017, 41, 300–306. [Google Scholar] [CrossRef]
- DeHaan, J.D.; Taormina, E.I.; Brien, D.J. Detection and Characterization of Volatile Organic Compounds from Burned Human and Animal Remains in Fire Debris. Sci. Justice 2017, 57, 118–127. [Google Scholar] [CrossRef]
- Rosier, E.; Loix, S.; Develter, W.; Van de Voorde, W.; Cuypers, E.; Tytgat, J. Differentiation between Decomposed Remains of Human Origin and Bigger Mammals. J. Forensic Leg. Med. 2017, 50, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.M.; Stefanuto, P.-H.; Heudt, L.; Focant, J.-F.; Perrault, K.A. Characterizing Decomposition Odor from Soil and Adipocere Samples at a Death Scene Using HS-SPME-GC×GC-HRTOFMS. Forensic Chem. 2018, 8, 11–20. [Google Scholar] [CrossRef]
- Chilcote, B.; Rust, L.; Nizio, K.D.; Forbes, S.L. Profiling the Scent of Weathered Training Aids for Blood-Detection Dogs. Sci. Justice 2018, 58, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ikematsu, N.; Kashiwagi, M.; Hara, K.; Waters, B.; Matsusue, A.; Takayama, M.; Kubo, S.-I. Diagnostic Meaning of Blood P-Cresol Concentration in Forensic Autopsy Cases. Leg. Med. 2018, 34, 27–35. [Google Scholar] [CrossRef]
- Rendine, M.; Fiore, C.; Bertozzi, G.; De Carlo, D.; Filetti, V.; Fortarezza, P.; Riezzo, I. Decomposing Human Blood: Canine Detection Odor Signature and Volatile Organic Compounds. J. Forensic Sci. 2019, 64, 587–592. [Google Scholar] [CrossRef]
- Dubois, L.M.; Stefanuto, P.-H.; Perrault, K.A.; Delporte, G.; Delvenne, P.; Focant, J.-F. Comprehensive Approach for Monitoring Human Tissue Degradation. Chromatographia 2019, 82, 857–871. [Google Scholar] [CrossRef]
- Patel, D.; Dargan, R.; Burr, W.S.; Daoust, B.; Forbes, S. Identifying the Early Post-Mortem VOC Profile from Cadavers in a Morgue Environment Using Comprehensive Two-Dimensional Gas Chromatography. Separations 2023, 10, 566. [Google Scholar] [CrossRef]
- Martin, C.; Verheggen, F. All Equal in the Face of Death!—Characterization of the Volatile Cadaveric Compounds of Fresh Stage Human Corpses. Forensic Chem. 2023, 35, 100516. [Google Scholar] [CrossRef]
- Schieweck, A.; Schulz, N.; Amendt, J.; Birngruber, C.; Holz, F. Catch Me If You Can-Emission Patterns of Human Bodies in Relation to Postmortem Changes. Int. J. Legal Med. 2024, 138, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Patel, D.; Burr, W.S.; Samson, C.; Forbes, S.L. Identifying VOCs from Human Remains Detectable in Water Using Comprehensive Two-Dimensional Gas Chromatography. Forensic Chem. 2024, 38, 100561. [Google Scholar] [CrossRef]
- Dargan, R.; Patel, D.; Burr, W.S.; Daoust, B.; Samson, C.; Forbes, S.L. Using Ethically Sourced Training Aids for Human Remains Detection Dog Training. Forensic Chem. 2024, 40, 100589. [Google Scholar] [CrossRef]
- Tsontakis, M.; Patel, D.; Ouimet, F.; Samson, C.; Burr, W.S.; Forbes, S.L. The Comparison of Volatile Organic Compound Profiles between Human and Non-Human Bones and Its Application to Human Remains Detection Dogs. Forensic Chem. 2025, 42, 100642. [Google Scholar] [CrossRef]
- Raymer, J.; Prada-Tiedemann, P.A.; Rojas, J.U. Decomposition Residual Odor Volatiles in Soil from a West Texas Environment. Rev. Crim. 2020, 62, 79–101. [Google Scholar]
- Nizio, K.D.; Ueland, M.; Stuart, B.H.; Forbes, S.L. The Analysis of Textiles Associated with Decomposing Remains as a Natural Training Aid for Cadaver-Detection Dogs. Forensic Chem. 2017, 5, 33–45. [Google Scholar] [CrossRef]
- Gelderman, T.; Stigter, E.; Krap, T.; Amendt, J.; Duijst, W. The Time of Death in Dutch Court; Using the Daubert Criteria to Evaluate Methods to Estimate the PMI Used in Court. Leg. Med. 2021, 53, 101970. [Google Scholar] [CrossRef]
- Patel, D.; Burr, W.S.; Daoust, B.; Forbes, S. Identifying the Transition from Ante-Mortem to Post-Mortem Odor in Cadavers in an Outdoor Environment. Forensic Sci. Int. Synerg. 2025, 11, 100616. [Google Scholar] [CrossRef] [PubMed]
- Thurn, B.; Schotsmans, E.M.J.; Ueland, M. Lime and Odour: A Preliminary Investigation into the Effect of Hydrated Lime on the Volatiles Emitted from Human Remains. Forensic Sci. Int. 2024, 358, 111745. [Google Scholar] [CrossRef] [PubMed]
- Agapiou, A.; Zorba, E.; Mikedi, K.; McGregor, L.; Spiliopoulou, C.; Statheropoulos, M. Analysis of Volatile Organic Compounds Released from the Decay of Surrogate Human Models Simulating Victims of Collapsed Buildings by Thermal Desorption-Comprehensive Two-Dimensional Gas Chromatography-Time of Flight Mass Spectrometry. Anal. Chim. Acta 2015, 883, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Tipple, C.A.; Caldwell, P.T.; Kile, B.M.; Beussman, D.J.; Rushing, B.; Mitchell, N.J.; Whitchurch, C.J.; Grime, M.; Stockham, R.; Eckenrode, B.A. Comprehensive Characterization of Commercially Available Canine Training Aids. Forensic Sci. Int. 2014, 242, 242–254. [Google Scholar] [CrossRef]
- Stadler, S.; Stefanuto, P.-H.; Byer, J.D.; Brokl, M.; Forbes, S.; Focant, J.-F. Analysis of Synthetic Canine Training Aids by Comprehensive Two-Dimensional Gas Chromatography-Time of Flight Mass Spectrometry. J. Chromatogr. A 2012, 1255, 202–206. [Google Scholar] [CrossRef]
- Cadaver—Scentlogix. Available online: https://scentlogix.com/s/cadaver/ (accessed on 13 August 2025).
- Thalmann, O.; Shapiro, B.; Cui, P.; Schuenemann, V.J.; Sawyer, S.K.; Greenfield, D.L.; Germonpré, M.B.; Sablin, M.V.; López-Giráldez, F.; Domingo-Roura, X.; et al. Complete Mitochondrial Genomes of Ancient Canids Suggest a European Origin of Domestic Dogs. Science 2013, 342, 871–874. [Google Scholar] [CrossRef]
- Udell, M.A.R.; Dorey, N.R.; Wynne, C.D.L. What Did Domestication Do to Dogs? A New Account of Dogs’ Sensitivity to Human Actions. Biol. Rev. Camb. Philos. Soc. 2010, 85, 327–345. [Google Scholar] [CrossRef]
- Miklósi, A.; Kubinyi, E.; Topál, J.; Gácsi, M.; Virányi, Z.; Csányi, V. A Simple Reason for a Big Difference: Wolves Do Not Look Back at Humans, but Dogs Do. Curr. Biol. 2003, 13, 763–766. [Google Scholar] [CrossRef]
- Kubinyi, E. Comparative Social Cognition: From Wolf and Dog to Humans. Comp. Cogn. Behav. Rev. 2006, 2, 26–46. [Google Scholar] [CrossRef]
- Aziz, M.; Goyal, H.; Haghbin, H.; Lee-Smith, W.M.; Gajendran, M.; Perisetti, A. The Association of “Loss of Smell” to COVID-19: A Systematic Review and Meta-Analysis. Am. J. Med. Sci. 2021, 361, 216–225. [Google Scholar] [CrossRef]
- Berg, P.; Mappes, T.; Miiamaaria, V.K. Olfaction in the Canine Cognitive and Emotional Processes: From Behavioral and Neural Viewpoints to Measurement Possibilities. Neurosci. Biobehav. Rev. 2024, 157, 105527. [Google Scholar] [CrossRef]
- Mackay-Sim, A.; Royet, J.-P. Structure and Function of the Olfactory System. In Olfaction and the Brain; Brewer, W.J., Castle, D., Pantelis, C., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 3–27. ISBN 9780511543623. [Google Scholar]
- Walker, D.B.; Walker, J.C.; Cavnar, P.J.; Taylor, J.L.; Pickel, D.H.; Hall, S.B.; Suarez, J.C. Naturalistic Quantification of Canine Olfactory Sensitivity. Appl. Anim. Behav. Sci. 2006, 97, 241–254. [Google Scholar] [CrossRef]
- Zhaoping, L. Olfactory Object Recognition, Segmentation, Adaptation, Target Seeking, and Discrimination by the Network of the Olfactory Bulb and Cortex: Computational Model and Experimental Data. Curr. Opin. Behav. Sci. 2016, 11, 30–39. [Google Scholar] [CrossRef]
- Glusman, G.; Yanai, I.; Rubin, I.; Lancet, D. The Complete Human Olfactory Subgenome. Genome Res. 2001, 11, 685–702. [Google Scholar] [CrossRef] [PubMed]
- Ramaihgari, B.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.J.; Wildey, C.; Morrison, E.; Salibi, N.; Katz, J.S.; Denney, T.S.; Vodyanoy, V.J.; et al. Zinc Nanoparticles Enhance Brain Connectivity in the Canine Olfactory Network: Evidence from an fMRI Study in Unrestrained Awake Dogs. Front. Vet. Sci. 2018, 5, 127. [Google Scholar] [CrossRef]
- Available online: https://www.academia.edu/97505494/The_anatomy_and_internal_aerodynamics_of_canine_olfaction?auto=download (accessed on 14 July 2025).
- Sadowski, B. Biologiczne Mechanizmy Zachowania Się Ludzi i Zwierząt; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2001. [Google Scholar]
- Kokocińska-Kusiak, A.; Woszczyło, M.; Zybala, M.; Maciocha, J.; Barłowska, K.; Dzięcioł, M. Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications. Animals 2021, 11, 2463. [Google Scholar] [CrossRef]
- Andrews, E.F.; Pascalau, R.; Horowitz, A.; Lawrence, G.M.; Johnson, P.J. Extensive Connections of the Canine Olfactory Pathway Revealed by Tractography and Dissection. J. Neurosci. 2022, 42, 6392–6407. [Google Scholar] [CrossRef] [PubMed]
- Singletary, M.; Lau, J.W.; Hagerty, S.; Pustovyy, O.; Globa, L.; Vodyanoy, V. Endogenous Zinc Nanoparticles in the Rat Olfactory Epithelium Are Functionally Significant. Sci. Rep. 2020, 10, 18435. [Google Scholar] [CrossRef]
- Available online: https://kryminalistyka.wpia.uw.edu.pl/wp-content/uploads/2012/10/osm3.pdf (accessed on 7 September 2025).
- Tadeusz, J. Zmysł Węchu Psów i Jego Praktyczne Wykorzystanie. Jastrzębiec: Polska Akademia Nauk. Instytut Genetyki i Hodowli Zwierząt; Polska Akademia Nauk: Warsaw, Poland, 2008. [Google Scholar]
- Turin, L. A Spectroscopic Mechanism for Primary Olfactory Reception. Chem. Senses 1996, 21, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.J.; Nash, R.; Elledge, H.S. Electro-Olfactography: A Technique with Potential for Diagnosis of Anosmia in the Dog. Am. J. Vet. Res. 1984, 45, 2296–2298. [Google Scholar] [CrossRef]
- Jia, H.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.J.; Schumacher, J.; Wildey, C.; Barrett, J.; Morrison, E.; Salibi, N.; Denney, T.S.; et al. Functional MRI of the Olfactory System in Conscious Dogs. PLoS ONE 2014, 9, e86362. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.J.; Hanrahan, L.A.; Swango, L.J.; Nusbaum, K.E. Anosmia Associated with Canine Distemper. Am. J. Vet. Res. 1988, 49, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Abrams, K.L.; Ward, D.A.; Sabiniewicz, A.; Hummel, T. Olfaction Evaluation in Dogs with Sudden Acquired Retinal Degeneration Syndrome. Vet. Ophthalmol. 2024, 27, 127–138. [Google Scholar] [CrossRef]
- Grosmaitre, X.; Santarelli, L.C.; Tan, J.; Luo, M.; Ma, M. Dual Functions of Mammalian Olfactory Sensory Neurons as Odor Detectors and Mechanical Sensors. Nat. Neurosci. 2007, 10, 348–354. [Google Scholar] [CrossRef]
- Ghatpande, A.S.; Reisert, J. Olfactory Receptor Neuron Responses Coding for Rapid Odour Sampling: Rapid Odour Sampling by Olfactory Receptor Neurons. J. Physiol. 2011, 589, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Margrie, T.W.; Sakmann, B.; Urban, N.N. Action Potential Propagation in Mitral Cell Lateral Dendrites Is Decremental and Controls Recurrent and Lateral Inhibition in the Mammalian Olfactory Bulb. Proc. Natl. Acad. Sci. USA 2001, 98, 319–324. [Google Scholar] [CrossRef]
- Schoppa, N.E.; Westbrook, G.L. AMPA Autoreceptors Drive Correlated Spiking in Olfactory Bulb Glomeruli. Nat. Neurosci. 2002, 5, 1194–1202. [Google Scholar] [CrossRef]
- Kashiwadani, H.; Sasaki, Y.F.; Uchida, N.; Mori, K. Synchronized Oscillatory Discharges of Mitral/Tufted Cells with Different Molecular Receptive Ranges in the Rabbit Olfactory Bulb. J. Neurophysiol. 1999, 82, 1786–1792. [Google Scholar] [CrossRef]
- Dargan, R.; Samson, C.; Burr, W.S.; Daoust, B.; Forbes, S.L. Validating the Use of Amputated Limbs Used as Cadaver Detection Dog Training Aids. Front. Anal. Sci. 2022, 2, 934639. [Google Scholar] [CrossRef]
- Dargan, R.; Forbes, S.L. Cadaver-detection Dogs: A Review of Their Capabilities and the Volatile Organic Compound Profile of Their Associated Training Aids. WIRES Forensic Sci. 2021, 3, e1409. [Google Scholar] [CrossRef]
- Martin, C.; Diederich, C.; Verheggen, F. Cadaver Dogs and the Deathly Hallows—A Survey and Literature Review on Selection and Training Procedure. Animals 2020, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Sidel, S.B.; Gandenberger, J.; Murphy, K.; Morris, K.N. Recognizing and Mitigating Canine Stress in Human–Canine Interaction Research: Proposed Guidelines. Animals 2025, 15, 1665. [Google Scholar] [CrossRef] [PubMed]
- Shegani, A.; Kealey, S.; Luzi, F.; Basagni, F.; Machado, J.d.M.; Ekici, S.D.; Ferocino, A.; Gee, A.D.; Bongarzone, S. Radiosynthesis, Preclinical, and Clinical Positron Emission Tomography Studies of Carbon-11 Labeled Endogenous and Natural Exogenous Compounds. Chem. Rev. 2023, 123, 105–229. [Google Scholar] [CrossRef] [PubMed]
- Canines’ Role in Urban Search & Rescue. Available online: https://www.fema.gov/emergency-managers/national-preparedness/frameworks/urban-search-rescue/canines?utm (accessed on 14 July 2025).
- Available online: https://www.elitek9.com/PDF/CSdoc.pdf (accessed on 14 July 2025).
- Jantorno, G.M.; Xavier, C.H.; Magalhães, M.E.P.; de Castro, M.B.; McManus, C.; de Melo, C.B. Detection Dogs Fighting Transnational Narcotraffic: Performance and Challenges under Real Customs Scenario in Brazil. Front. Vet. Sci. 2024, 11, 1380415. [Google Scholar] [CrossRef]
- Welcome to SARDA CanTech. Available online: https://www.cantech.org.uk/ (accessed on 7 September 2025).
- Sigma Pseudo Leichengeruch. Available online: https://vermisstefinden.de/oeffentlichkeit/ (accessed on 7 September 2025).
- Available online: https://www.sigmaaldrich.com/PL/pl/product/sial/p4304?srsltid=AfmBOoqRI90_2pDbBoBaM088VwofQGpcYBIhRZEdd2Q3IymOr_gr20z7 (accessed on 7 September 2025).
- Sigma Pseudo Corpse Scent (Drowned Victim), MilliporeSigma Supelco 50 Caps. Available online: https://www.fishersci.com/shop/products/sigma-pseudo-corpse-scent-drowned-victim-milliporesigma-supelco-2/111004441 (accessed on 7 September 2025).
- Alexander, M.B.; Hodges, T.K.; Bytheway, J.; Aitkenhead-Peterson, J.A. Application of Soil in Forensic Science: Residual Odor and HRD Dogs. Forensic Sci. Int. 2015, 249, 304–313. [Google Scholar] [CrossRef]
- Ensminger, J.J.; Jezierski, T.; McCulloch, M. Scent Identification in Criminal Investigations and Prosecutions: New Protocol Designs Improve Forensic Reliability. SSRN Electron. J. 2010. [Google Scholar] [CrossRef][Green Version]



| Parameter | Sigma Pseudo™ Corpse Scent Formulation I | Sigma Pseudo™ Corpse Scent Formulation II |
|---|---|---|
| Direct Liquid GC-MS Analysis | ||
| Primary Components | 2-Pyrrolidinone (28 ± 4%), 4-Aminobutanoic acid (71 ± 5%) | Putrescine (11 ± 1%), Cadaverine (11 ± 1%), 2-Pyrrolidinone (24 ± 5%), 4-Aminobutanoic acid (54 ± 7%) |
| Minor Components | 3-Methyl-2-pyrrolidinone, 4-Methyl-2-pyrrolidinone (≤1%) | 3-Methyl-2-pyrrolidinone, 4-Methyl-2-pyrrolidinone, trace 5-amino-pentanol, ethanol, butyrolactone, acetone, methanol, 1,4-dioxane |
| Solid phase microextraction (SPME) | ||
| Compounds observed | Acetone, 2-Butanone, 1-Butanol, Heptane, 2,4-dimethyl-furan, Methyl isobutyl ketone, Octane, Methoxy-phenyl-oxime, Heptanal, 2-Butoxy-ethanol, Phenol, 2,4,6-Trimethyl-pyridine, Benzothiazole, 1,3-Bis(1,1-dimethylethyl)-benzene, Tetradecane, 2-Methyl-,1(1,1-dimethylethyl)-2-methyl-1,3-propanediyl propanoic acid | Acetone, 1,4-Dioxane, 2,4-dimethyl-furan, 1-Pentanol, 2-Heptanone, Phenol, 2,4,6-Trimethyl-pyridine, Octanal, 2-Ethyl-1-hexanol, 4-Ethyl-1,3-benzenediol, 2-Nonanone, 2-Decanone, Benzothiazole, 2-Methyl-,1(1,1-dimethylethyl)-2-methyl-1,3-propanediyl propanoic acid, Benzophenones, 24-Bis(1,1-dimethylethyl)-phenol |
| Purge and trap (P/T) analysis | ||
| Major compounds detected | Acetone (93 ± 3%), isopropanol (1.7 ± 0.1%), 2-butanone (1.3 ± 0.2%) | Acetone (19 ± 2%), 1-vinyl aziridine (28 ± 6%), 1,4-dioxane (14 ± 3%), 1-pentanol (28 ± 10%) |
| Automated Chemical Environment Monitor (ACEM) headspace analysis | ||
| Key component detected | Acetone | Acetone |
| Other compounds | α-methylstyrene, acetophenone, nonadecane, phthalate, 2-butanone, 2-pentanone, 3-methyl-2-pentanone, heptanal, and 2-butoxyethanol | 2-butanone, 1-butanol, 1,4-dioxane, 1-pentanol, 2,3,4,5-tetrahydropyridazine, α-methylstyrene, acetophenone, diphenyl sulfone, phthalate, 3-methyl-2-pentanone and 2-cyclopenten-1-one |
| Cryogenic preconcentration analysis | ||
| Major compounds detected | Acetone and isopropanol (86%) | Acetone and ethanol (62%) |
| Other compounds | Ethanol (4.5%), 1,1-difluoroethane (2.9%), octane (1.2%), and 78 trace compounds (<1%) | 1,4-dioxane (4.4%), pentyl formate, (3.6%), 1-bromo-2-propanol (2.7%), 2-butanone (2.2%), 3,4-dimethyldihydrofuran-2,5-dione (1.2%), acetaldehyde (1.1%), 2-amino-1-propanol (1.0%), and 68 trace compounds (<1%) |
| Aspect | Findings in Dogs | Relevance for HRD | Ref. |
|---|---|---|---|
| OR gene repertoire | ~1094 functional OR genes, one of the largest among mammals | Explains high olfactory acuity compared to humans (~400 ORs) | [21] |
| Genomic organization | ~40 OR clusters, major loci on chromosomes 18 and 21 | Suggests evolutionary expansion and specialization of olfaction | [22] |
| Genetic diversity | High SNP frequency, many leading to amino acid substitutions | Provides a molecular basis for inter-individual variability | [22] |
| Breed-related selection | Allelic variants associated with detection performance; enriched in scent-oriented breeds | May explain why retrievers/spaniels excel in HRD work | [23] |
| Expression variability | Transcript levels vary > 10,000-fold among ORs; age and environment modulate expression | Suggests dynamic adaptation of olfaction over the lifetime and the environment | [20] |
| Specialized receptors (TAARs) | Detect volatile amines (putrescine, cadaverine, trimethylamine) linked to decay | Supports the role of amines in the cadaveric odor signature | [24,25] |
| Country | Organization/Unit | Sigma Scent Type (SKU/Name) | Usage Context/Notes | Ref. |
|---|---|---|---|---|
| USA | K-9 Specialty Search School (North Franklin, Connecticut)—“K9 Connecticut” | Corpse Scent: Formulation I (P4304), Formulation II (P3929) | Training materials titled “Basic Cadaver Training Using Sigma Pseudo™…”. Direct, practical use for HRD training. | [99] |
| USA | FEMA US&R Canine Program | (HRD pseudo-odors permitted; no brand specified) | FEMA standards describe the use of odor training aids for LF/HRD; no public confirmation that Sigma is used. | [98] |
| UK | SARDA CanTech (Search And Rescue Dog Association—Canine Technical) | Drowned Victim (P7184) | Declared training of dogs using Sigma’s “Drowned Victim” scent; water-recovery HRD applications. | [101] |
| Germany | Vermisstefinden e.V. | Formulation I (P4304), Formulation II (P3929) | The organization states the active use of Merck (Darmstadt, Germany)/Sigma Pseudo Scents for training “Leichenspürhunde” (cadaver dogs). | [102] |
| Poland | Trainer market/distribution (e.g., Thor Working Dogs) | PSI (P4304), PSII (P3929), PSDV (P7184) | Availability and use in the HRD training community; police training programs exist, but do not specify brand. | [103] |
| Canada | Fisher Scientific Canada (distribution) | PSII (P3929), PSDV (P7184) | Official Merck/MilliporeSigma distribution; indirect evidence (availability to institutions), no public brand confirmation by specific services. | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk-Jabłońska, I.; Zieniuk, B.; Pawełkowicz, M. Synthetic Cadaver Odorants and the Sulfur Gap: Linking Chemistry and Canine Olfaction in Human Remains Detection. Molecules 2025, 30, 4066. https://doi.org/10.3390/molecules30204066
Kowalczyk-Jabłońska I, Zieniuk B, Pawełkowicz M. Synthetic Cadaver Odorants and the Sulfur Gap: Linking Chemistry and Canine Olfaction in Human Remains Detection. Molecules. 2025; 30(20):4066. https://doi.org/10.3390/molecules30204066
Chicago/Turabian StyleKowalczyk-Jabłońska, Iwona, Bartłomiej Zieniuk, and Magdalena Pawełkowicz. 2025. "Synthetic Cadaver Odorants and the Sulfur Gap: Linking Chemistry and Canine Olfaction in Human Remains Detection" Molecules 30, no. 20: 4066. https://doi.org/10.3390/molecules30204066
APA StyleKowalczyk-Jabłońska, I., Zieniuk, B., & Pawełkowicz, M. (2025). Synthetic Cadaver Odorants and the Sulfur Gap: Linking Chemistry and Canine Olfaction in Human Remains Detection. Molecules, 30(20), 4066. https://doi.org/10.3390/molecules30204066







