Rational Design of Cu@Pd Core–Shell Nanostructures via Galvanic Replacement for Dual Electrochemical Applications: Hydrogen Evolution and Nitrate Reduction Reactions
Abstract
1. Introduction
2. Results and Discussion
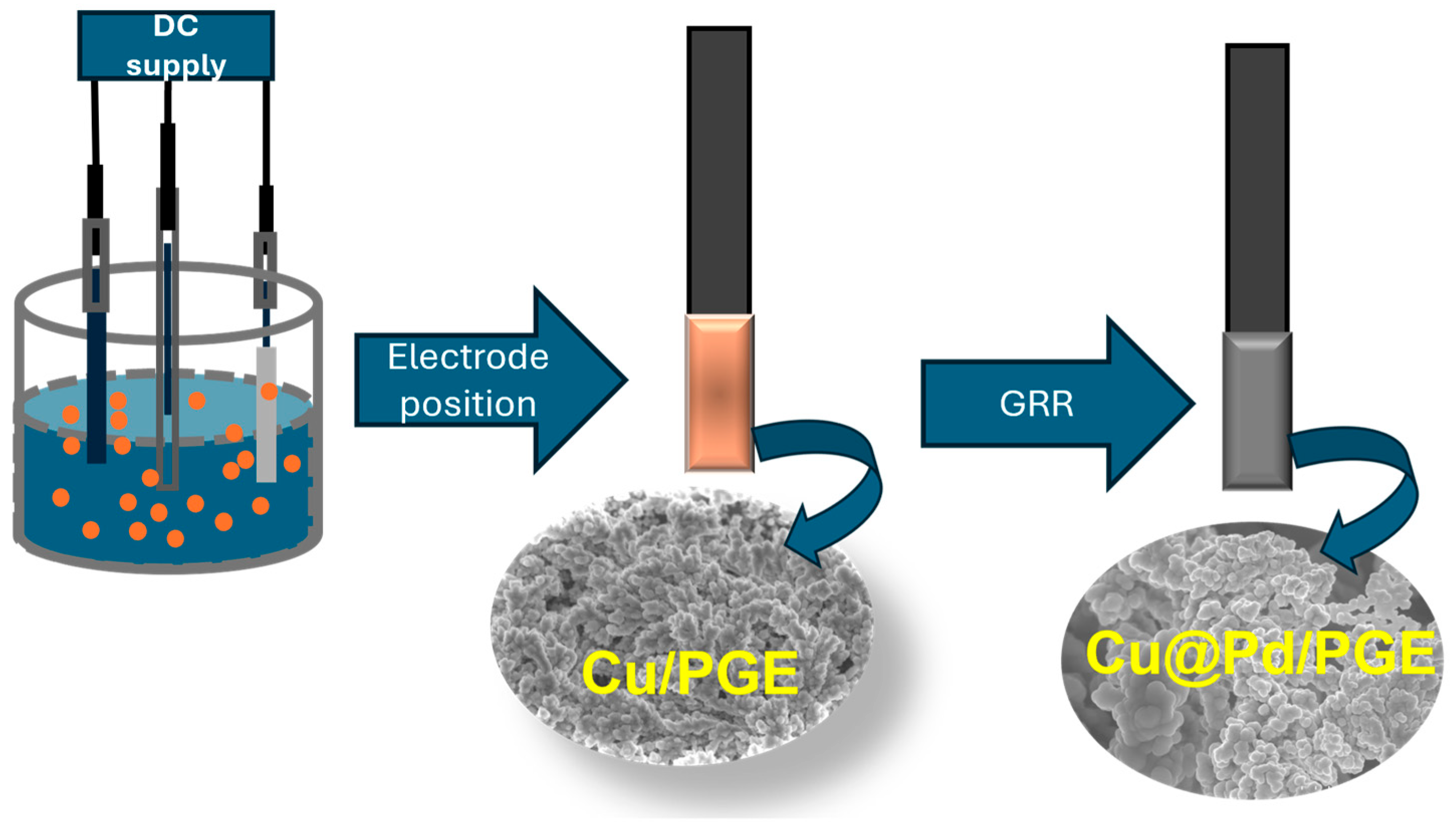
2.1. Material Characterisation
2.2. Hydrogen Evolution Reaction (HER)
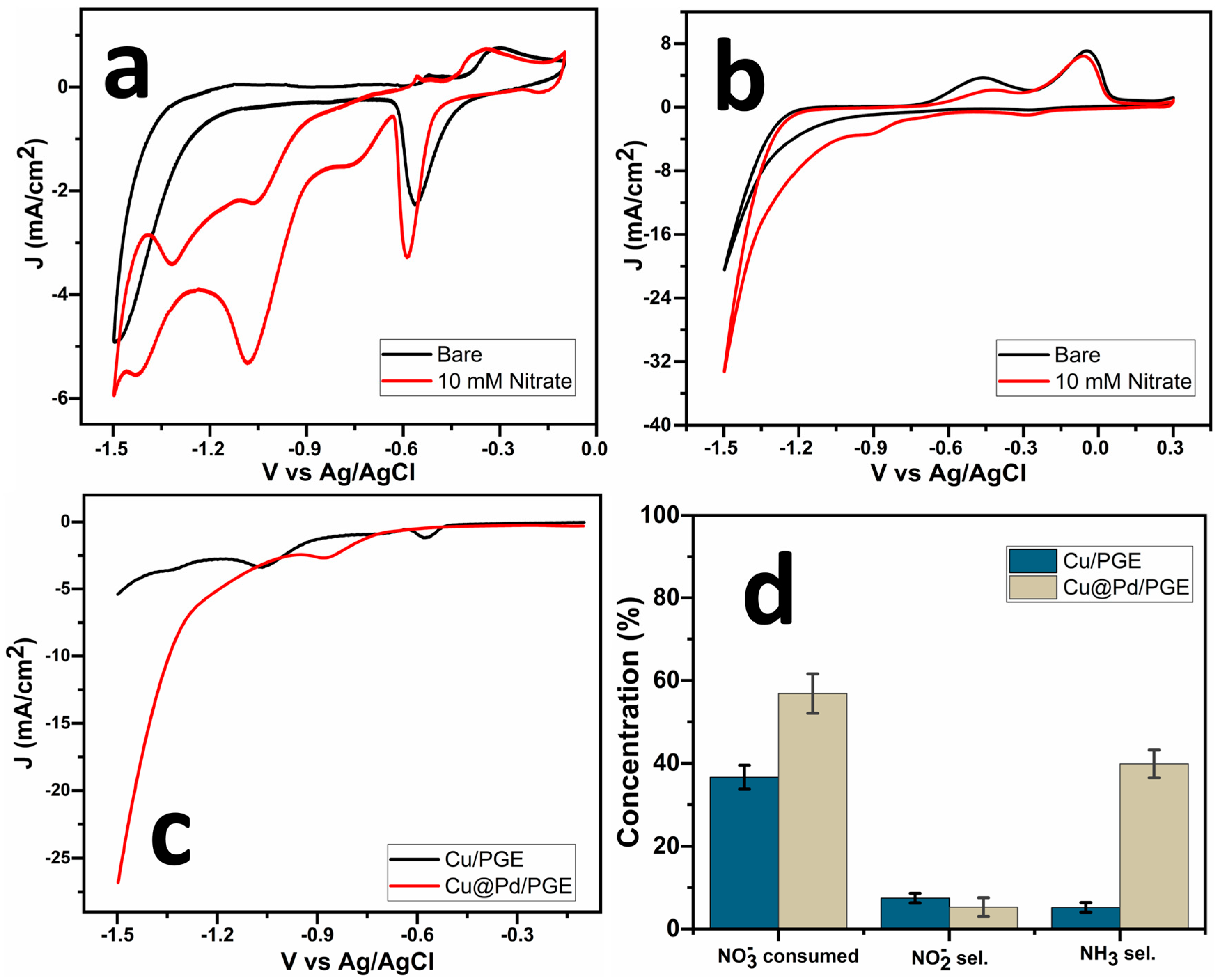
2.3. Nitrate Reduction Reaction (NRR)
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qadeer, M.A.; Zhang, X.; Farid, M.A.; Tanveer, M.; Du, S.; Huang, Z.-F.; Tahir, M.; Zou, J.-J. A Review on Fundamentals for Designing Hydrogen Evolution Electrocatalyst. J. Power Sources 2024, 613, 234856. [Google Scholar] [CrossRef]
- Sorensen Montoya, E. Birth Outcome Effects of Nitrate Contamination in Drinking Water. J. Environ. Econ. Manag. 2025, 133, 103197. [Google Scholar] [CrossRef]
- Dong, H.; Chen, H.; SenGupta, A.K. CO2 Utilization for Water Treatment: Ion Exchange Nitrate Removal Driven by CO2 without Producing Spent Brine Regenerant. ACS EST Water 2021, 1, 2275–2283. [Google Scholar] [CrossRef]
- Shelly, Y.; Kuk, M.; Menashe, O.; Zeira, G.; Azerrad, S.; Kurzbaum, E. Nitrate Removal from a Nitrate-Rich Reverse Osmosis Concentrate: Superior Efficiency Using the Bioaugmentation of an Acinetobacter Biofilm. J. Water Process Eng. 2021, 44, 102425. [Google Scholar] [CrossRef]
- Li, S.; Liao, Y.; Pang, Y.; Dong, X.; Strous, M.; Ji, G. Denitrification and Dissimilatory Nitrate Reduction to Ammonia in Long-Term Lake Sediment Microcosms with Iron(II). Sci. Total Environ. 2022, 807, 150835. [Google Scholar] [CrossRef]
- Bommireddy, N.; Palathedath, S.K. Surfactant Mediated Electrodeposition of Copper Nanostructures for Environmental Electrochemistry: Influence of Morphology on Electrochemical Nitrate Reduction Reaction. J. Solid State Electrochem. 2022, 26, 2733–2742. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Zeng, G.; Liu, X.; Fang, C.; Li, C. A Three-Dimensional Cu Nanobelt Cathode for Highly Efficient Electrocatalytic Nitrate Reduction. Nanoscale 2020, 12, 9385–9391. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Wang, Z.; Dinh, C.-T.; Sinton, D.; Zheng, G.; Sargent, E.H. Enhanced Nitrate-to-Ammonia Activity on Copper–Nickel Alloys via Tuning of Intermediate Adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhan, G.; Yang, J.; Quan, F.; Mao, C.; Liu, Y.; Wang, B.; Du, Y.; Hao, W.; Wong, P.K.; et al. Efficient Ammonia Electrosynthesis from Nitrate on Strained Ruthenium Nanoclusters. J. Am. Chem. Soc. 2020, 142, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
- Braley, S.E.; Xie, J.; Losovyj, Y.; Smith, J.M. Graphite Conjugation of a Macrocyclic Cobalt Complex Enhances Nitrite Electroreduction to Ammonia. J. Am. Chem. Soc. 2021, 143, 7203–7208. [Google Scholar] [CrossRef]
- Liu, M.; Mao, Q.; Shi, K.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Electroreduction of Nitrate to Ammonia on Palladium–Cobalt–Oxygen Nanowire Arrays. ACS Appl. Mater. Interfaces 2022, 14, 13169–13176. [Google Scholar] [CrossRef]
- Exner, K.S. Paradigm Change in Hydrogen Electrocatalysis: The Volcano’s Apex Is Located at Weak Bonding of the Reaction Intermediate. Int. J. Hydrog Energy. 2020, 45, 27221–27229. [Google Scholar] [CrossRef]
- Gao, G.; Zhu, G.; Chen, X.; Sun, Z.; Cabot, A. Optimizing Pt-Based Alloy Electrocatalysts for Improved Hydrogen Evolution Performance in Alkaline Electrolytes: A Comprehensive Review. ACS Nano 2023, 17, 20804–20824. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Feidenhans, A.A.; Regmi, Y.N.; Wei, C.; Xia, D.; Kibsgaard, J.; King, L.A. Precious Metal Free Hydrogen Evolution Catalyst Design and Application. Chem. Rev. 2024, 124, 5617–5667. [Google Scholar] [CrossRef]
- Naveen, B.; Rajendra Kumar Reddy, G.; Suresh Kumar, P. Copper-Palladium Core-Shell Bifunctional Nanoelectrocatalyst for Ethanol Oxidation and Hydrogen Evolution Reactions. J. Electrochem. Soc. 2022, 169, 056501. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An Overview on Pd-Based Electrocatalysts for the Hydrogen Evolution Reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Li, H.; Li, G. Novel Palladium-Based Nanomaterials for Multifunctional ORR/OER/HER Electrocatalysis. J. Mater. Chem. A 2023, 11, 9383–9400. [Google Scholar] [CrossRef]
- Assad, S.; Tariq, T.; Zaeem Idrees, M.; Mannan Butt, A.; Bakhat, K.; Shamraiz, U. Recent Progress in Pd Based Electrocatalysts for Electrochemical Nitrogen Reduction to Ammonia. J. Electroanal. Chem. 2023, 931, 117174. [Google Scholar] [CrossRef]
- Johansson, M.; Skúlason, E.; Nielsen, G.; Murphy, S.; Nielsen, R.M.; Chorkendorff, I. Hydrogen Adsorption on Palladium and Palladium Hydride at 1 Bar. Surf. Sci. 2010, 604, 718–729. [Google Scholar] [CrossRef]
- Naveen, B.; Lee, S.-W. Template-Assisted Electrodeposited Copper Nanostructres for Selective Detection of Hydrogen Peroxide. Molecules 2024, 29, 4571. [Google Scholar] [CrossRef]
- Naveen, B.; Lee, S.-W. Surfactant Mediated Electrodeposition of Cu-Ag Nanostructures on Pencil Graphite Electrodes for Amperometric Detection of Nitrate. Microchem. J. 2025, 214, 114090. [Google Scholar] [CrossRef]
- Naveen, B.; Reddy, B.P.; Palathedath, S.K. Dual Performing Copper–Platinum Core–Shell Nanozyme for Environmental Electrochemistry–Electrocatalytic Oxidation and Electroanalysis of Ammonia. Environ. Sci. Nano 2021, 8, 3603–3612. [Google Scholar] [CrossRef]
- Xu, H.; Wu, J.; Luo, W.; Li, Q.; Zhang, W.; Yang, J. Dendritic Cell-Inspired Designed Architectures toward Highly Efficient Electrocatalysts for Nitrate Reduction Reaction. Small 2020, 16. [Google Scholar] [CrossRef]
- de Sousa, P.V.F.; de Oliveira, A.F.; da Silva, A.A.; Lopes, R.P. Environmental Remediation Processes by Zero Valence Copper: Reaction Mechanisms. Env. Sci Pollut Res 2019, 26, 14883–14903. [Google Scholar] [CrossRef]
- Choi, J.H.; Kang, J.W.; Koo, H.Y.; Kang, Y.C. Lithiophilic and Conductive CuO-Cu2O-Cu Microspheres with Controlled Void Structure via Spray Pyrolysis for Improved Lithium Metal Anode Performance. Int. J. Energy Res. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Aftab, F.; Ali, M.; Ahmed, B.A.; Farooq, W.; Hakeem, A.S.; Nazar, M.F. Synergistic Engineering of Palladium-Cobalt Nanoalloys on Graphite Sheet for Efficient and Sustainable Hydrogen Evolution Reaction. Mater. Today Sustain. 2024, 27, 100836. [Google Scholar] [CrossRef]
- Joya, K.S.; Ehsan, M.A.; Babar, N.-U.-A.; Sohail, M.; Yamani, Z.H. Nanoscale Palladium as a New Benchmark Electrocatalyst for Water Oxidation at Low Overpotential. J. Mater. Chem. A 2019, 7, 9137–9144. [Google Scholar] [CrossRef]
- She, S.; Chen, C.; Fan, K.; Chen, G.; Zhu, Y.; Leung, C.W.; Tsang, Y.H.; Huang, H. Optimizing the Ru Catalyst–Support Interaction via Tunnel Size of MnO2 Support for Enhanced Acidic Water Oxidation. J. Am. Chem. Soc. 2025, 147, 24392–24402. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.J.; Wu, Z.L.; Lin, C.Y.; Huang, Y.H.; Huang, C.P. Manipulating the Crystalline Morphology and Facet Orientation of Copper and Copper-Palladium Nanocatalysts Supported on Stainless Steel Mesh with the Aid of Cationic Surfactant to Improve the Electrochemical Reduction of Nitrate and N2 Selectivity. Appl. Catal. B Environ. 2020, 273, 119053. [Google Scholar] [CrossRef]
- Jeanmairet, G.; Rotenberg, B.; Salanne, M. Microscopic Simulations of Electrochemical Double-Layer Capacitors. Chem. Rev. 2022, 122, 10860–10898. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, C.; Lu, S.; Zou, Z.; Gu, S.; Zhang, Y.; Li, C.M. Rationally Tuning the Atomic Ratio of Electrodeposited NiP for Greatly Enhanced Hydrogen Evolution in Alkaline Media. Chem. Commun. 2018, 54, 12408–12411. [Google Scholar] [CrossRef]
- Smiljanic, M.; Bele, M.; Moriau, L.; Ruiz-Zepeda, F.; Sala, M.; Hodnik, N. Electrochemical Stability and Degradation of Commercial Pd/C Catalyst in Acidic Media. J. Phys. Chem. C 2021, 125, 27534–27542. [Google Scholar] [CrossRef]
- Sahin, N.E.; Pech-Rodriguez, W.J. Scrutinizing the Basis of Pd Electrochemistry: An Accurate Assessment of the Electrochemically Active Surface Area. Electroanalysis 2025, 37. [Google Scholar] [CrossRef]
- Conway, B.E.; Jerkiewicz, G. Relation of Energies and Coverages of Underpotential and Overpotential Deposited H at Pt and Other Metals to the ‘Volcano Curve’ for Cathodic H2 Evolution Kinetics. Electrochim. Acta 2000, 45, 4075–4083. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A Comprehensive Review on the Electrochemical Parameters and Recent Material Development of Electrochemical Water Splitting Electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef]
- Nie, M.; Sun, H.; Lei, D.; Kang, S.; Liao, J.; Guo, P.; Xue, Z.; Xue, F. Novel Pd/MOF Electrocatalyst for Hydrogen Evolution Reaction. Mater. Chem. Phys. 2020, 254, 123481. [Google Scholar] [CrossRef]
- Bhowmik, T.; Kundu, M.K.; Barman, S. Palladium Nanoparticle–Graphitic Carbon Nitride Porous Synergistic Catalyst for Hydrogen Evolution/Oxidation Reactions over a Broad Range of PH and Correlation of Its Catalytic Activity with Measured Hydrogen Binding Energy. ACS Catal. 2016, 6, 1929–1941. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Jebakumar Immanuel Edison, T.N.; Sethuraman, M.G. Electrocatalytic Performance of Carbon Dots/Palladium Nanoparticles Composite towards Hydrogen Evolution Reaction in Acid Medium. Int. J. Hydrog Energy 2020, 45, 28800–28811. [Google Scholar] [CrossRef]
- Pitchai, C.; Vedanarayanan, M.; Mathur Gopalakrishnan, S. Efficient Hydrogen Evolution Electrocatalysis Using Nitrogen Doped Carbon Dot Decorated Palladium Copper Nanocomposites in Acid Medium. New J. Chem. 2023, 47, 14355–14363. [Google Scholar] [CrossRef]
- Sharma, M.D.; Mahala, C.; Basu, M. Nanosheets of MoSe2@M (M = Pd and Rh) Function as Widespread PH Tolerable Hydrogen Evolution Catalyst. J. Colloid Interface Sci. 2019, 534, 131–141. [Google Scholar] [CrossRef]
- Xiao, P.; Buijnsters, J.G.; Zhao, Y.; Yu, H.; Xu, X.; Zhu, Y.; Tang, D.; Zhu, J.; Zhao, Z. Fullerene-like WS2 Supported Pd Catalyst for Hydrogen Evolution Reaction. J. Catal. 2019, 380, 215–223. [Google Scholar] [CrossRef]
- Perez-Gallent, E.; Figueiredo, M.C.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic Reduction of Nitrate on Copper Single Crystals in Acidic and Alkaline Solutions. Electrochim. Acta 2017, 227, 77–84. [Google Scholar] [CrossRef]
- Choi, H.; Jun, M.; Kang, W.; Kim, T.; Jung, Y.; Jin, K.; Lee, K. Synergy in Pd/Cu2O Heteronanostructure Boosts the Electrochemical Conversion of Nitrate to Ammonia. Chem Catal. 2024, 4, 101029. [Google Scholar] [CrossRef]
- Lim, J.; Chen, Y.; Cullen, D.A.; Lee, S.W.; Senftle, T.P.; Hatzell, M.C. PdCu Electrocatalysts for Selective Nitrate and Nitrite Reduction to Nitrogen. ACS Catal. 2023, 13, 87–98. [Google Scholar] [CrossRef]
- Yoon, A.; Bai, L.; Yang, F.; Franco, F.; Monteiro, M.C.d.O.; Chee, S.W.; Roldan Cuenya, B. Revealing Catalyst Restructuring and Composition during Nitrate Electroreduction through Correlated Operando Microscopy and Spectroscopy. Nat. Mater. 2025, 24, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wan, Z.; Hu, X.; Huang, J.; Kang, X. Restructuring of Copper Catalysts by Potential Cycling and Enhanced Two-Carbon Production for Electroreduction of Carbon Dioxide. J. CO2 Util. 2022, 56, 101846. [Google Scholar] [CrossRef]
- Lim, J.; Cullen, D.A.; Stavitski, E.; Lee, S.W.; Hatzell, M.C. Atomically Ordered PdCu Electrocatalysts for Selective and Stable Electrochemical Nitrate Reduction. ACS Energy Lett. 2023, 8, 4746–4752. [Google Scholar] [CrossRef]
- Aouina, N.; Cachet, H.; Debiemme-chouvy, C.; Tran, T.T.M. Insight into the Electroreduction of Nitrate Ions at a Copper Electrode, in Neutral Solution, after Determination of Their Diffusion Coefficient by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2010, 55, 7341–7345. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Fan, X.; Orfao, J.J.M.; Lapkin, A.A.; Pereira, M.F.R. Kinetic Modeling of Nitrate Reduction Catalyzed by Pd–Cu Supported on Carbon Nanotubes. Ind. Eng. Chem. Res. 2012, 51, 4854–4860. [Google Scholar] [CrossRef]
- Su, J.F.; Ruzybayev, I.; Shah, I.; Huang, C.P. The Electrochemical Reduction of Nitrate over Micro-Architectured Metal Electrodes with Stainless Steel Scaffold. Appl. Catal. B Environ. 2016, 180, 199–209. [Google Scholar] [CrossRef]
- Li, L.; Yun, Y.; Zhang, Y.; Huang, Y.; Xu, Z. Electrolytic Reduction of Nitrate on Copper and Its Binary Composite Electrodes. J. Alloys Compd. 2018, 766, 157–160. [Google Scholar] [CrossRef]
- Bai, Z.; Dong, J.; Liu, G.; Nkambule, T.T.I.; Hu, C.; Qu, J. Denitrification Enhancement by Electro-Sorption/Reduction Using a Layered Metal Oxide Electrode Loaded with Pd-Cu Nanoparticles. Electrochem. Commun. 2020, 110, 106607. [Google Scholar] [CrossRef]
- Gao, W.; Gao, L.; Li, D.; Huang, K.; Cui, L.; Meng, J.; Liang, J. Removal of Nitrate from Water by the Electrocatalytic Denitrification on the Cu-Bi Electrode. J. Electroanal. Chem. 2018, 817, 202–209. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Ben Aoun, S.; Rahman, M.M.; Asiri, A.M.; Mohamed, N. Lean Cu-Immobilized Pt and Pd Films/–H+ Conducting Membrane Assemblies: Relative Electrocatalytic Nitrate Reduction Activities. J. Ind. Eng. Chem. 2015, 28, 131–137. [Google Scholar] [CrossRef]
- Koay, P.P.; Alam, M.S.; Alam, M.M.; Etesami, M.; Hasnat, M.A.; Mohamed, N. Electrocatalytic Reduction of Nitrate Ions at a Poly Crystalline SnCu Modified Platinum Surface by Using an H+ Conducting Solid Polymer in a Sandwich Type Membrane Reactor. J. Environ. Chem. Eng. 2016, 4, 4494–4502. [Google Scholar] [CrossRef]
- Shih, Y.J.; Wu, Z.L.; Huang, Y.H.; Huang, C.P. Electrochemical Nitrate Reduction as Affected by the Crystal Morphology and Facet of Copper Nanoparticles Supported on Nickel Foam Electrodes (Cu/Ni). Chem. Eng. J. 2020, 383, 123157. [Google Scholar] [CrossRef]
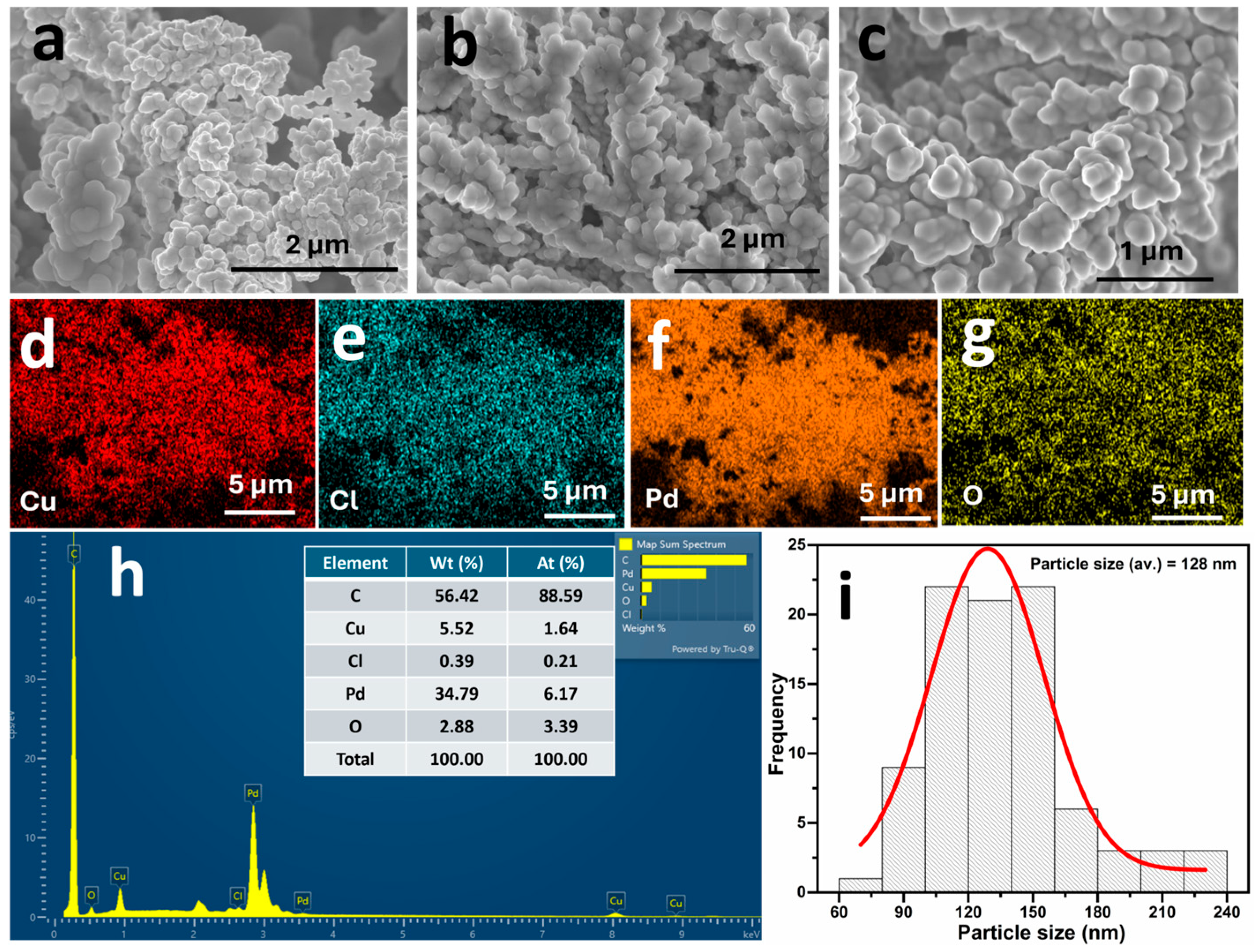
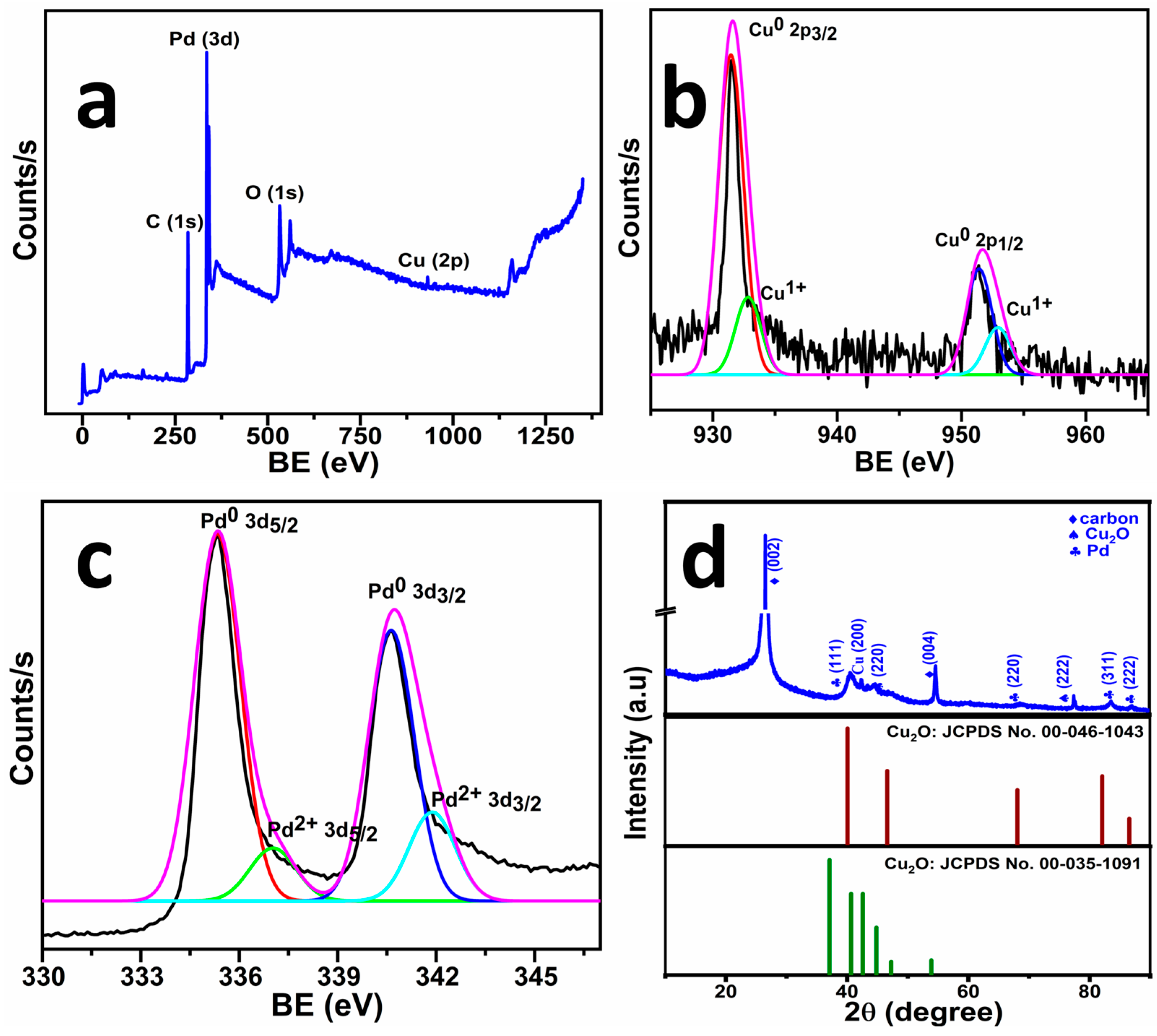
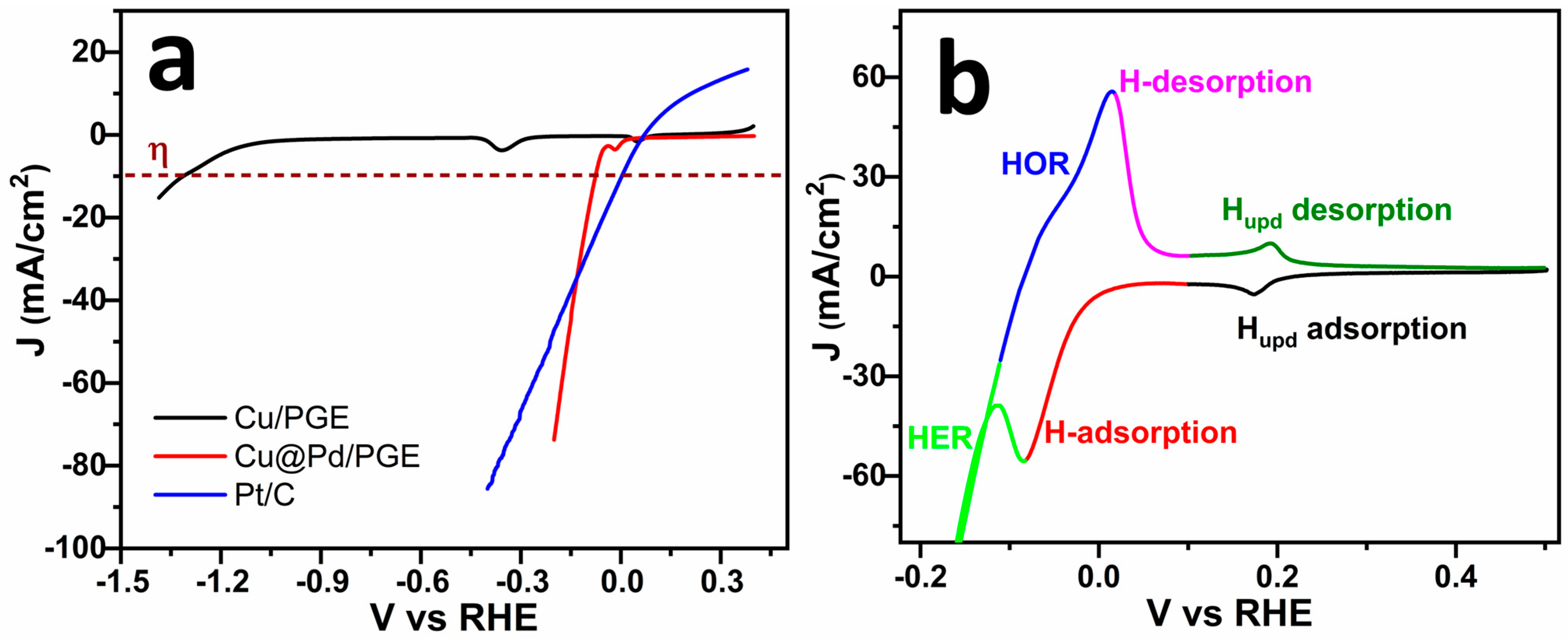

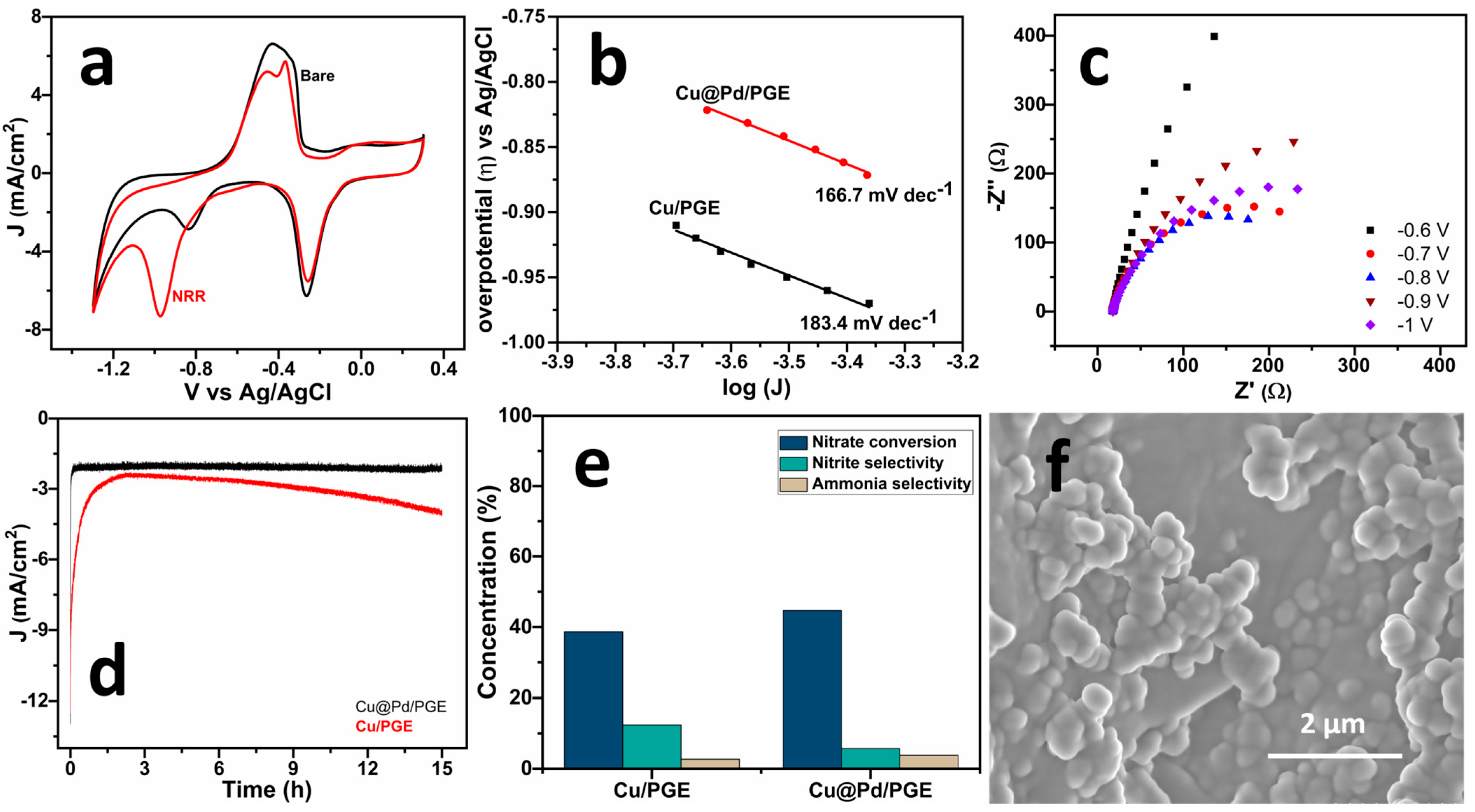
| Catalyst | Electrolyte | Overpotential (η) (mV vs. RHE) | Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|---|
| Pd/MOF | 0.5 M H2SO4 | 105 | 85 | [38] |
| Pd-CNx | 0.5 M H2SO4 | 55 | 35 | [39] |
| n-Pd@NDCDs | 0.5 M H2SO4 | 291 | 135 | [40] |
| n-PdCu@NDCDs | 0.5 M H2SO4 | 115 | - | [41] |
| MoSe2/Pd | 0.5 M H2SO4 | 231 | 69 | [42] |
| Pd/WS2 | 0.5 M H2SO4 | 130 | 82.4 | [43] |
| (NiPd)17Se15 | 0.5 M H2SO4 | 197 | 91.5 | [43] |
| Cu@Pd/PGE | 0.5 M H2SO4 | 150 | 33.8 | Present study |
| Catalyst | Methodology | Electrolyte | Efficiency | Ref |
|---|---|---|---|---|
| Pd40Cu60/SS | Electrodeposition | 50 mg/L NO3-N in 0.05 M NaClO4 | Potential = −0.3 V, N2 selectivity = 40% | [52] |
| Pd20Cu80/Cu | Ball milling | 0.1 M NO3-N in 0.1 M NaOH | NH3 selectivity = 61% N2 selectivity = 39% | [53] |
| Pd80Cu20/NiAl | Coprecipitation | 3 mM NO3-N in 1 M KOH | Sorption of NO3-N achieved 2 mg/g at 1.0 V, removal rate = 68% | [54] |
| Bi10Cu90/Cu | Electrodeposition | 100 mg/L NO3-N in 0.1 M Na2SO4 | Removal rate = 87.5% at 6 mA/cm2 | [55] |
| Pt-Cu/NF, Pd-Cu/NF | Electrodeposition | 0.05 M NO3-N in 0.5 M KCl | NH3 selectivity = 24%, removal rate = 93% at 0.1 A/cm2 | [56] |
| Sn-Cu/Pt-NF | Electrodeposition | 0.05 M NO3-N in 0.05 M KCl | NH3 selectivity = 39% and Sn enhanced selectivity towards N2 | [57] |
| Cu/Ni | Electroless deposition | 50 mg/L NO3-N in 0.1 M Na2SO4 | High nitrate removal (97%) at 3 mA/cm2 | [58] |
| Cu@Pd/PGE | Electrodeposition | 10 mM in 0.1 M KOH | Nitrate removal of 46% at −1.0 V | This work |
| Cu@Pd/PGE | Electrodeposition | 10 mM in 0.05 M Na2SO4 | Nitrate removal of 59% at −1.3 V with high ammonia selectivity | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naveen, B.; Lee, S.-W. Rational Design of Cu@Pd Core–Shell Nanostructures via Galvanic Replacement for Dual Electrochemical Applications: Hydrogen Evolution and Nitrate Reduction Reactions. Molecules 2025, 30, 4062. https://doi.org/10.3390/molecules30204062
Naveen B, Lee S-W. Rational Design of Cu@Pd Core–Shell Nanostructures via Galvanic Replacement for Dual Electrochemical Applications: Hydrogen Evolution and Nitrate Reduction Reactions. Molecules. 2025; 30(20):4062. https://doi.org/10.3390/molecules30204062
Chicago/Turabian StyleNaveen, Bommireddy, and Sang-Wha Lee. 2025. "Rational Design of Cu@Pd Core–Shell Nanostructures via Galvanic Replacement for Dual Electrochemical Applications: Hydrogen Evolution and Nitrate Reduction Reactions" Molecules 30, no. 20: 4062. https://doi.org/10.3390/molecules30204062
APA StyleNaveen, B., & Lee, S.-W. (2025). Rational Design of Cu@Pd Core–Shell Nanostructures via Galvanic Replacement for Dual Electrochemical Applications: Hydrogen Evolution and Nitrate Reduction Reactions. Molecules, 30(20), 4062. https://doi.org/10.3390/molecules30204062









