Potential of Nb2O5 as a Catalyst in Biodiesel Production: A Study with Different Feedstock
Abstract
1. Introduction
2. Results and Discussion
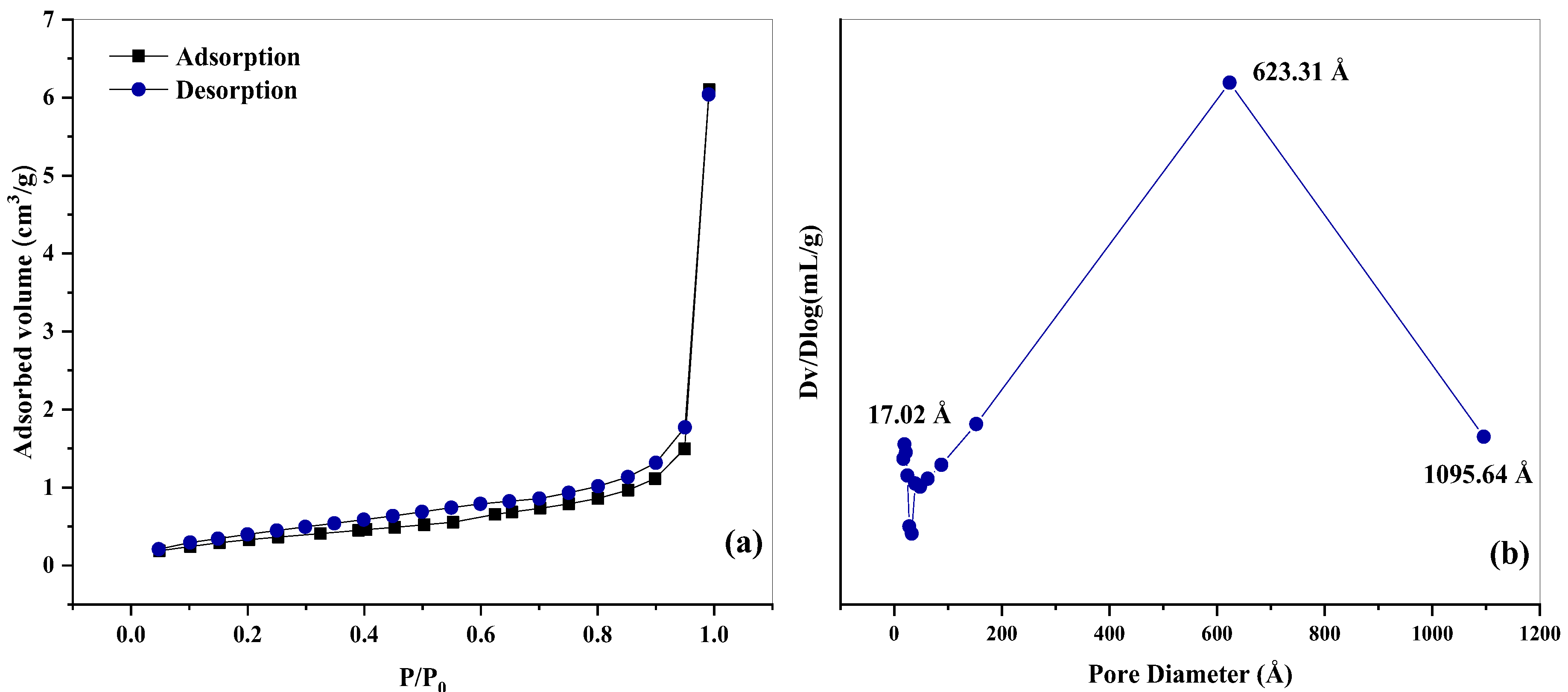
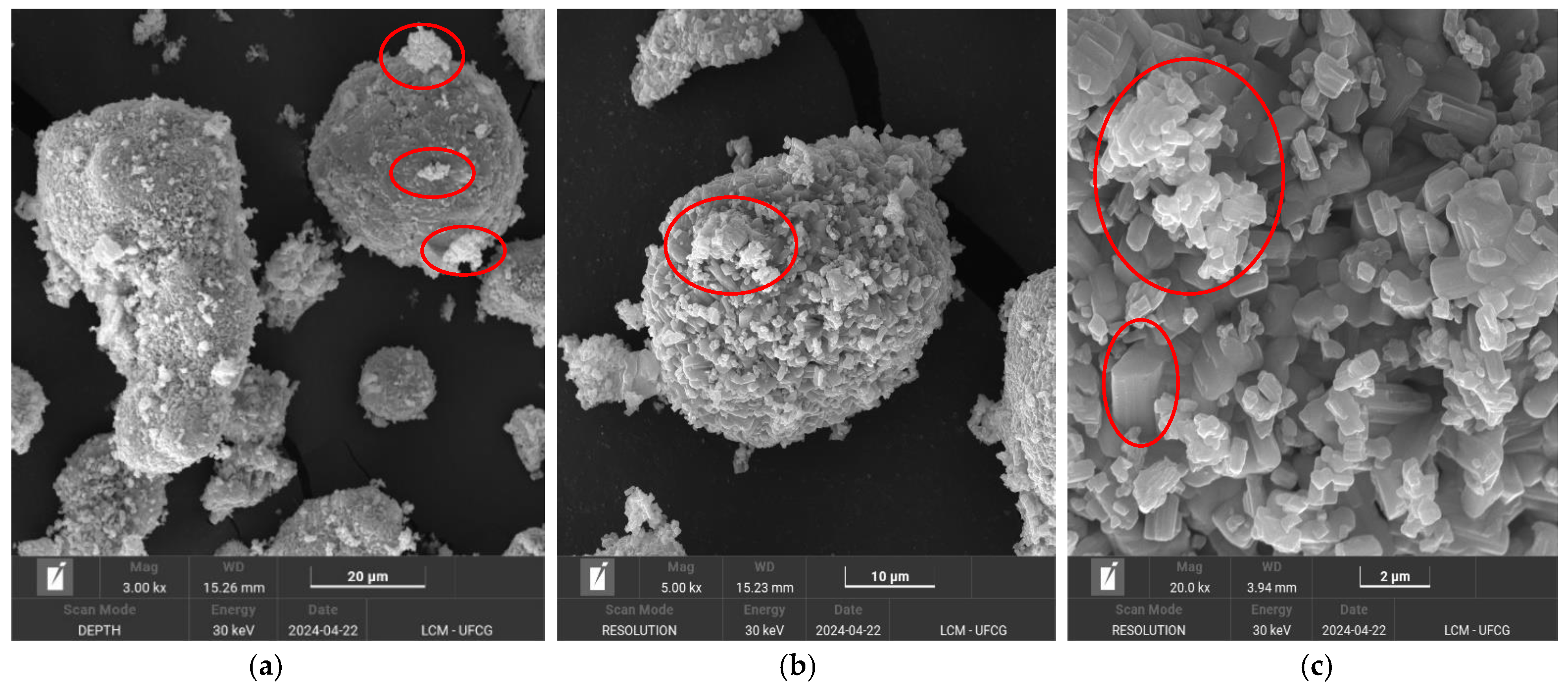
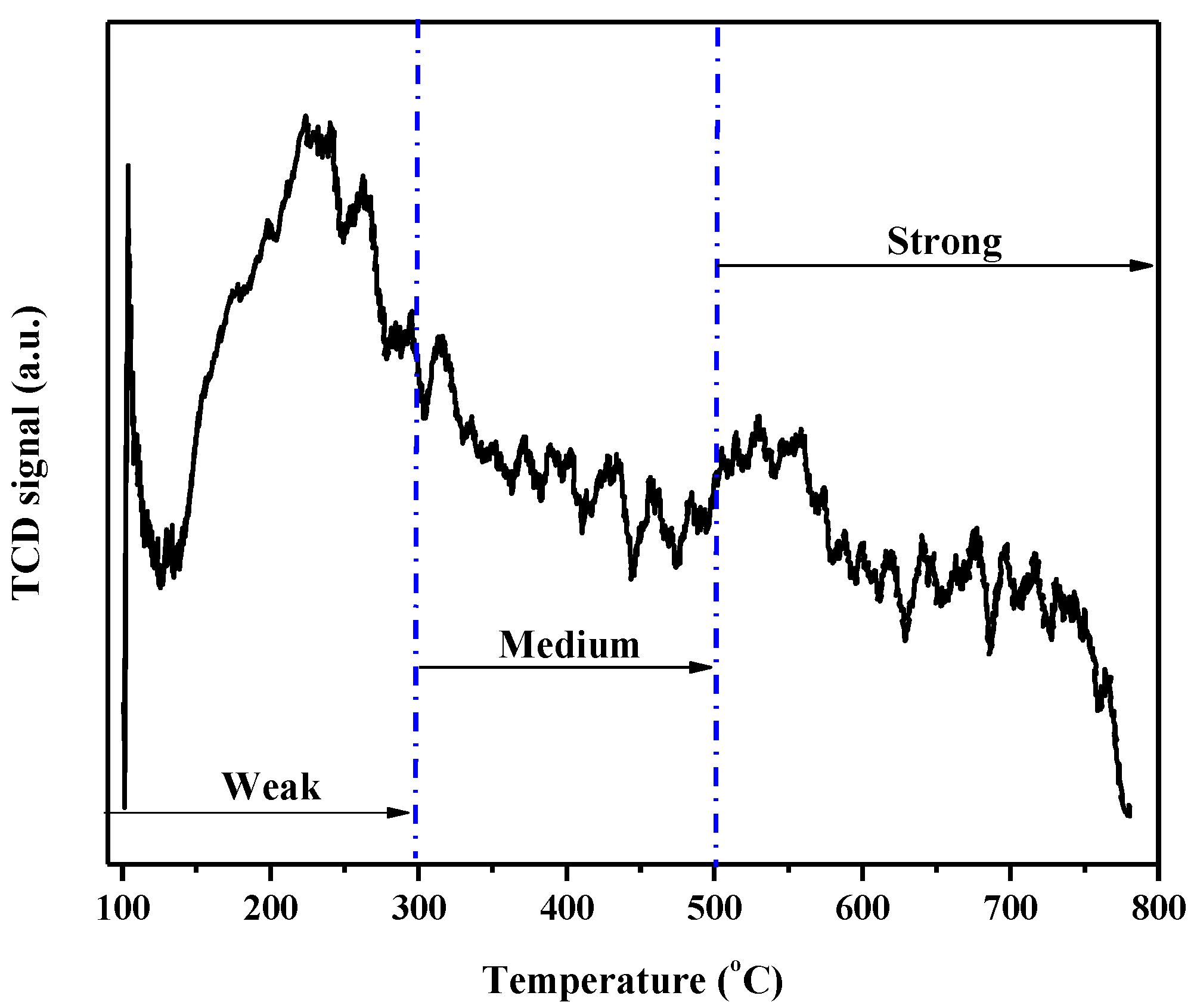
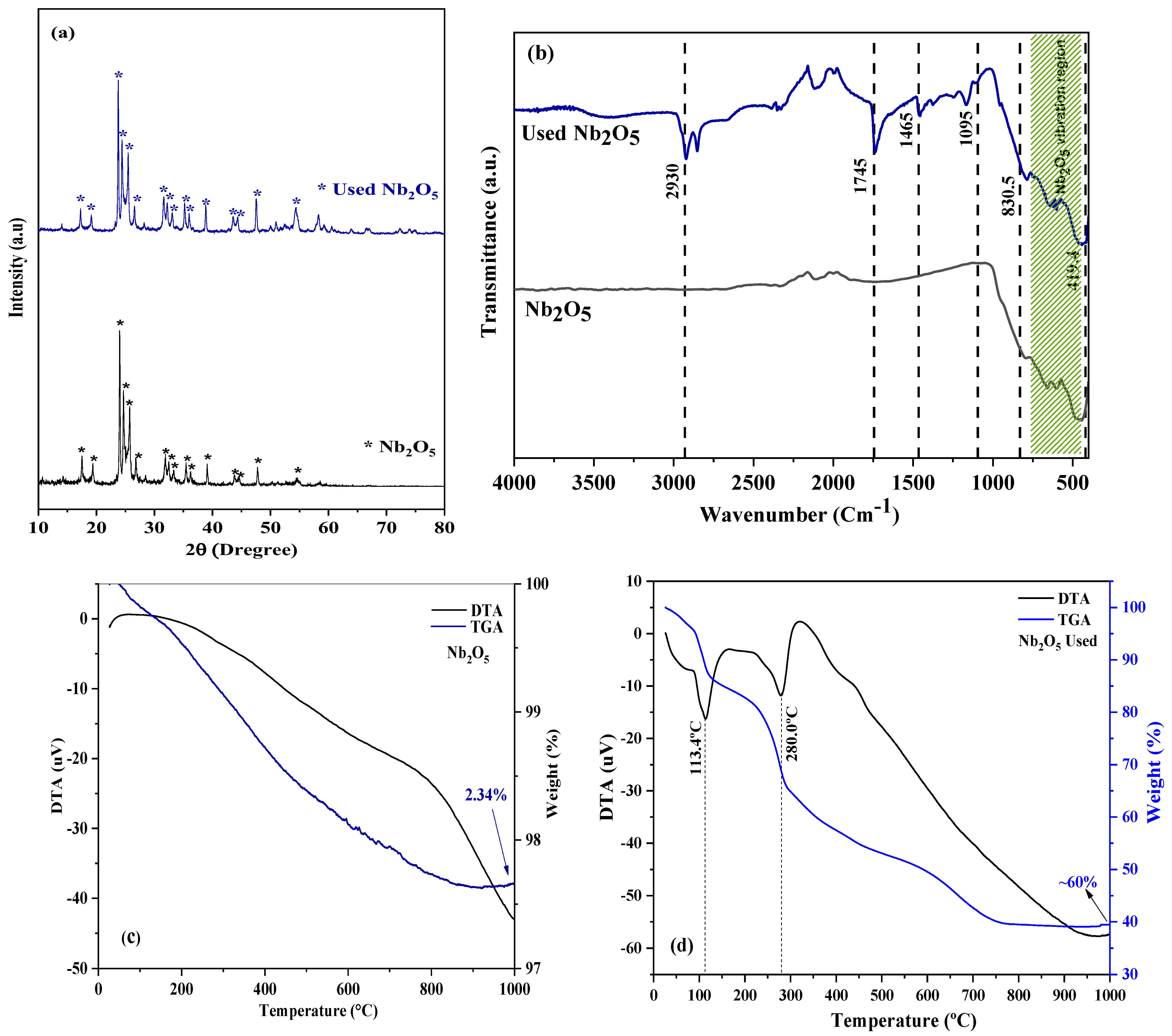
2.1. Catalyst Characterization
2.2. Catalytic Tests
2.2.1. Effect of Feedstock
2.2.2. Effect of the Route Used
2.2.3. Catalyst Reuse
Characterization of the Reused Catalyst
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Catalyst Preparation
3.2.2. Physico-Chemical Characterization
3.3. Catalytic Test
Catalyst Reuse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, G.H.M.; Andrade, R.R.; Mohallem, N.D.S. Investigation of phase transition employing strain mapping in TT- and T-Nb2O5 obtained by HRTEM micrographs. Micron 2021, 148, 103112. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-M.; Jia, Y.-F.; Tang, B.; Yang, F.; Zhong, M.; Liu, Q.-J. Theoretical study on structural, excitonic and electronic properties of Nb2O5 with five crystal structures. Mater. Sci. Semicond. Process. 2021, 130, 105831. [Google Scholar] [CrossRef]
- Carvalho, R.C.; Mendonça, M.E.V.; Tavares, M.S.; Moreira, E.; Azevedo, D.L. Optoelectronic and thermodynamic properties, infrared and Raman spectra of NbO2 and Nb2O5 from DFT formalism. J. Phys. Chem. Solids 2022, 163, 110549. [Google Scholar] [CrossRef]
- Mitterhuber, L.; Veerapandiyan, V.; Deluca, M.; Misture, S.; Schaeperkoetter, J.; Tkadletz, M.; Mitterer, C.; Spitaler, J. Anomalous thermal conductivity in amorphous niobium pentoxide thin films: A correlation study between structure and thermal properties. Materialia 2022, 26, 101601. [Google Scholar] [CrossRef]
- Islam, K.; Sultana, R.; Satpati, B.; Chakraborty, S. Studies on structural and dielectric properties of NbO2-Nb2O5 thin-film-based devices. Vacuum 2022, 195, 110675. [Google Scholar] [CrossRef]
- Díaz-Moreno, C.A.; Khanal, N.; Macías, A.H.; Noveron, J.; López, J.A. Structural and second harmonic generation properties of nanogel of niobium oxide nanoparticles. Mater. Chem. Phys. 2020, 255, 123579. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, S.; Dhiman, P.; Sharma, G.; Stadler, F.J. Current progress in heterojunctions based on Nb2O5 for photocatalytic water treatment and energy applications. J. Mol. Liq. 2024, 399, 124360. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, J.; Sun, D.; Yu, J.; Wu, X.; Zhao, S. Efficient removal of Hg0 by regulating the bonding degree between CuO and carrier Nb2O5. Sep. Purif. Technol. 2024, 332, 125796. [Google Scholar] [CrossRef]
- De Lima, A.A.; Tractz, G.T.; Macedo, A.G.; Thomazi, F.; Pinto Rodrigues, P.R.; Dartora, C.A. Electrochemical investigation of PEDOT:PSS and Nb2O5 composites as counter electrodes for dye-sensitized solar cells. Opt. Mater. 2022, 132, 112763. [Google Scholar] [CrossRef]
- Chen, X.; Cui, P.; Chen, X.; Naveed, A.; Zhou, Y.; Dou, A.; Su, M.; Liu, Y. W6+-doped Nb2O5 as high-rate anode materials for lithium-ion batteries. J. Alloys Compd. 2023, 967, 171846. [Google Scholar] [CrossRef]
- Faria, A.L.A.; Centurion, H.A.; Torres, J.A.; Gonçalves, R.V.; Ribeiro, L.S.; Riberio, C.; da Cruz, J.C.; Nogueira, F.G.E. Enhancing Nb2O5 activity for CO2 photoreduction through Cu nanoparticles cocatalyst deposited by DC-magnetron sputtering. J. CO2 Util. 2021, 53, 101739. [Google Scholar] [CrossRef]
- Sturt, N.R.M.; Vieira, S.S.; Moura, F.C.C. Catalytic activity of sulfated niobium oxide for oleic acid esterification. J. Environ. Chem. Eng. 2019, 7, 102866. [Google Scholar] [CrossRef]
- Ding, H.; Song, Z.; Feng, K.; Zhang, H.; Zhang, H.; Li, X. Controlled synthesis of pure-phase metastable tetragonal Nb2O5 anode material for high-performance lithium batteries. J. Solid State Chem. 2021, 299, 122136. [Google Scholar] [CrossRef]
- García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.M.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Niobium-containing MCM-41 silica catalysts for biodiesel production. Appl. Catal. B Environ. 2011, 108–109, 161–167. [Google Scholar] [CrossRef]
- Mejía, C.H.; Verbart, D.M.A.; de Jong, K.P. Niobium-based solid acids in combination with a methanol synthesis catalyst for the direct production of dimethyl ether from synthesis gas. Catal. Today 2021, 369, 77–87. [Google Scholar] [CrossRef]
- Ammar, S.H.; Salman, M.D.; Shafi, R.F. Catalytic activity of niobium oxide supported bimetallic nanocatalysts (IrxPt1-x/Nb2O5) for oxidation of formaldehyde at moderate temperature. Colloid Interface Sci. Commun. 2020, 38, 100305. [Google Scholar] [CrossRef]
- Genco, A.; García-López, E.I.; Megna, B.; Ania, C.; Marcì, G. Nb2O5 based photocatalysts for efficient generation of H2 by Photoreforming of aqueous solutions of ethanol. Catal. Today 2025, 447, 115147. [Google Scholar] [CrossRef]
- Lima, S.; García-López, E.I.; Adawy, A.; Marcì, G.; Scargiali, F. Valorisation of Chlorella sp. biomass in 5-HMF through a two-step conversion in the presence of Nb2O5 and NbOPO4 and optimisation through reactive extraction. Chem. Eng. J. 2023, 471, 144583. [Google Scholar] [CrossRef]
- Putla, S.B.; Subha, P.; Swapna, B.; Singh, N.; Sudarsanam, P. Valorizing biomass waste glycerol to fuel additive at room temperature using a nanostructured WO3/Nb2O5 catalyst. Catal. Commun. 2024, 186, 106827. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Liu, M.; Zhang, P.; Cui, H.; He, J. Enhanced esterification performance by SO42−/ZrO2 anchored Nb2O5 nanotube. J. Ind. Eng. Chem. 2024, 129, 321–330. [Google Scholar] [CrossRef]
- Bousba, D.; Sobhi, C.; Zouaoui, E.; Rouibah, K.; Boublia, A.; Ferkous, H.; Haddad, A.; Gouasmia, A.; Avramova, I.; Mohammed, Z.; et al. Efficient biodiesel production from recycled cooking oil using a NaOH/CoFe2O4 magnetic nano-catalyst: Synthesis, characterization, and process enhancement for sustainability. Energy Convers. Manag. 2024, 300, 118021. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, A.; Quan, W.; Gong, W. Efficient synthesis of biodiesel from Hyoscyamus niger L. seed oil by base catalysis. Fuel Process. Technol. 2023, 241, 107630. [Google Scholar] [CrossRef]
- Ali, A.H.; Wanderlind, E.H.; Almerindo, G.I. Activated carbon obtained from malt bagasse as a support in heterogeneous catalysis for biodiesel production. Renew. Energy 2024, 220, 119656. [Google Scholar] [CrossRef]
- Scaldaferri, C.A.; Pasa, V.M.D. Production of jet fuel and green diesel range biohydrocarbons by hydroprocessing of soybean oil over niobium phosphate catalyst. Fuel 2019, 245, 458–466. [Google Scholar] [CrossRef]
- Alotaibi, M.A.; Naeem, A.; Wali Khan, I.; Farooq, M.; Ud Din, I.; Saharun, M.S. Optimization and cost analysis evaluation studies of the biodiesel production from waste cooking oil using Na–Si/Ce-500 heterogeneous catalyst. Biomass Bioenergy 2024, 182, 107078. [Google Scholar] [CrossRef]
- Bento, H.B.S.; Reis, C.E.R.; Cunha, P.G.; Carvalho, A.K.F.; De Castro, H.F. One-pot fungal biomass-to-biodiesel process: Influence of the molar ratio and the concentration of acid heterogenous catalyst on reaction yield and costs. Fuel 2021, 300, 120968. [Google Scholar] [CrossRef]
- Farokhi, G.; Saidi, M. Catalytic activity of bimetallic spinel magnetic catalysts (NiZnFe2O4, CoZnFe2O4 and CuZnFe2O4) in biodiesel production process from neem oil: Process evaluation and optimization. Chem. Eng. Process. Process Intensif. 2022, 181, 109170. [Google Scholar] [CrossRef]
- Karimi, S.; Saidi, M. Converting neem seed-derived oil into biodiesel using zeolite ZSM-5 as an efficient catalyst via electrosynthesis procedure: Optimization of operating variables using response surface methodology (RSM). Process Saf. Environ. Prot. 2024, 183, 111–123. [Google Scholar] [CrossRef]
- Okolie, J.A.; Ivan Escobar, J.; Umenweke, G.; Khanday, W.; Okoye, P.U. Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Fuel 2022, 307, 121821. [Google Scholar] [CrossRef]
- Topare, N.S.; Gujarathi, V.S.; Bhattacharya, A.A.; Bhoyar, V.M.; Shastri, T.J.; Manewal, S.P.; Gomkar, C.S.; Khedkar, S.V.; Khan, A.; Asiri, A.M. A review on application of nano-catalysts for production of biodiesel using different feedstocks. Mater. Today Proc. 2023, 72, 324–335. [Google Scholar] [CrossRef]
- Ferreira, R.D.M.; Brackmann, R.; Pereira, E.B.; Costa da Rocha, R.D. Immobilization of lipase from Candida rugosa onto niobium oxide. Biocatal. Agric. Biotechnol. 2020, 30, 101812. [Google Scholar] [CrossRef]
- Fidelis, M.Z.; Favaro, Y.B.; Santos, A.S.G.G.d.; Pereira, M.F.R.; Brackmann, R.; Lenzi, G.G.; Soares, O.S.G.P.; Andreo, O.A.B. Enhancing Ibuprofen and 4-Isobutylacetophenone degradation: Exploiting the potential of Nb2O5 sol-gel catalysts in photocatalysis, catalytic ozonation, and photocatalytic ozonation. J. Environ. Chem. Eng. 2023, 11, 110690. [Google Scholar] [CrossRef]
- Katada, N.; Hatanaka, T.; Ota, M.; Yamada, K.; Okumura, K.; Niwa, M. Biodiesel production using heteropoly acid-derived solid acid catalyst H4PNbW11O40/WO3–Nb2O5. Appl. Catal. A Gen. 2009, 363, 164–168. [Google Scholar] [CrossRef]
- Srilatha, K.; Lingaiah, N.; Devi, B.L.A.P.; Prasad, R.B.N.; Venkateswar, S.; Prasad, P.S.S. Esterification of free fatty acids for biodiesel production over heteropoly tungstate supported on niobia catalysts. Appl. Catal. A Gen. 2009, 365, 28–33. [Google Scholar] [CrossRef]
- Da Conceição, L.R.V.; Carneiro, L.M.; Rivaldi, J.D.; de Castro, H.F. Solid acid as catalyst for biodiesel production via simultaneous esterification and transesterification of macaw palm oil. Ind. Crops Prod. 2016, 89, 416–424. [Google Scholar] [CrossRef]
- Silva, A.; Wilson, K.; Lee, A.F.; dos Santos, V.C.; Bacilla, A.C.C.; Mantovani, K.M.; Nakagaki, S. Nb2O5/SBA-15 catalyzed propanoic acid esterification. Appl. Catal. B Environ. 2017, 205, 498–504. [Google Scholar] [CrossRef]
- De Brito, V.L.; Gonçalves, M.A.; dos Santos, H.C.L.; da Rocha Filho, G.N.; da Conceição, L.R.V. Biodiesel production from waste frying oil using molybdenum over niobia as heterogeneous acid catalyst: Process optimization and kinetics study. Renew. Energy 2023, 215, 118947. [Google Scholar] [CrossRef]
- Ahmad, I.; Al-Qattan, A.; Iqbal, M.Z.; Anas, A.; Khasawneh, M.A.; Obaidullah, A.J.; Mahal, A.; Duan, M.; Al Zoubi, W.; Ghadi, Y.Y.; et al. A systematic review on Nb2O5-based photocatalysts: Crystallography, synthetic methods, design strategies, and photocatalytic mechanisms. Adv. Colloid Interface Sci. 2024, 324, 103093. [Google Scholar] [CrossRef]
- Ding, H.; Song, Z.; Zhang, H.; Zhang, H.; Li, X. Niobium-based oxide anodes toward fast and safe energy storage: A review. Mater. Today Nano 2020, 11, 100082. [Google Scholar] [CrossRef]
- Hu, Z.; He, Q.; Liu, Z.; Liu, X.; Qin, M.; Wen, B.; Shi, W.; Zhao, Y.; Li, Q.; Mai, L. Facile formation of tetragonal-Nb2O5 microspheres for high-rate and stable lithium storage with high areal capacity. Sci. Bull. 2020, 65, 1154–1162. [Google Scholar] [CrossRef]
- Kumari, N.; Gaurav, K.; Samdarshi, S.K.; Bhattacharyya, A.S.; Paul, S.; Rajbongshi, B.; Mohanty, K. Dependence of photoactivity of niobium pentoxide (Nb2O5) on crystalline phase and electrokinetic potential of the hydrocolloid. Sol. Energy Mater. Sol. Cells 2020, 208, 110408. [Google Scholar] [CrossRef]
- Weiss, M.; Marschall, R. Syntheses and characterisation of p-type copper niobium oxides for photocatalytic hydrogen generation. Appl. Catal. A Gen. 2023, 661, 119234. [Google Scholar] [CrossRef]
- Pogue, E.A.; Langevin, S.A.; Hamann, T.; Rao, K.K.; Schroeder, M.A.; Le, N.Q.; McHale, C.; Burchfield, Z.; Ko, J.S. Enhancing low-temperature lithium-ion battery performance under high-rate conditions with niobium oxides. Mater. Today Energy 2024, 45, 101663. [Google Scholar] [CrossRef]
- Ding, H.; Luo, Y.; Song, Z.; Chen, C.; Feng, K.; Yang, X.; Zhang, H.; Li, X. Built defects of homogeneous junction to enhance the lithium storage capacity of niobium pentoxide materials. J. Energy Chem. 2024, 92, 730–737. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Athukoralalage, S.S.; Amiralian, N.; Ling, C.D.; Müllner, M. Nanostructuring niobium oxides using polymer-grafted cellulose nanocrystals and nanofibers as sacrificial scaffolds††Electronic supplementary information (ESI) available: Materials, methods, and supplementary results, including polymer and hybrid characterization, electron microscopy, physisorption and electrochemical performance studies. RSC Appl. Polym. 2024, 3, 146–155. [Google Scholar] [CrossRef]
- Da Cruz, J.A.; Volnistem, E.A.; Ferreira, R.F.; Freitas, D.B.; Sales, A.J.M.; Costa, L.C.; Graça, M.P.F. Structural characterization of Brazilian niobium pentoxide and treatment to obtain the single phase (H-Nb2O5). Therm. Sci. Eng. Prog. 2021, 25, 101015. [Google Scholar] [CrossRef]
- Vinodhini, S.P.; Xavier, J.R. Novel multifunctional nanocomposites containing graphitic carbon nitride/silanized Nb2O5 nanofillers for the protection of steel surfaces in the automobile industry. J. Mol. Struct. 2024, 1306, 137875. [Google Scholar] [CrossRef]
- Aimaiti, G.; Ma, Y.; Shi, Y.; Wang, X.; Wang, S.; Wang, Z.; Li, Y.; Li, J.; Qi, X.; Chen, X. Nb2O5/red phosphorus S-scheme heterojunction photocatalyst for removal of organic contaminant and Cr(VI): Electrochemical performance and mechanism. Mater. Sci. Semicond. Process. 2023, 160, 107421. [Google Scholar] [CrossRef]
- Dai, Q.; Yuan, B.; Guo, M.; Zhang, K.; Chen, X.; Song, Z.; Nguyen, T.T.; Wang, X.; Lin, S.; Fan, J.; et al. A novel nano-fibriform C-modified niobium pentoxide by using cellulose templates with highly visible-light photocatalytic performance. Ceram. Int. 2020, 46, 13210–13218. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Li, S.; Cheng, X.; Zhou, W.; Jiang, J.; Feng, C.; Liu, Y.; Li, X.; Zhang, X.; Wang, Z. Morphology dependence of Nb2O5-supported cobalt oxide in catalytic toluene oxidation††Electronic supplementary information (ESI) available. Catal. Sci. Technol. 2024, 14, 5722–5730. [Google Scholar] [CrossRef]
- Lin, M.; Mochizuki, C.; Ishida, T.; Zhang, Y.; Haruta, M.; Murayama, T. Effect of poly(N-vinylpyrrolidone) ligand on catalytic activities of Au nanoparticles supported on Nb2O5 for CO oxidation and furfural oxidation. Catal. Today 2023, 410, 143–149. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zeng, K.; Wang, S.; Zhang, H.; Li, X.; Wang, Z.; Zhang, C. Revealing the strong interaction effect of MnOx nanoparticles and Nb2O5 supports with variable morphologies on catalytic propane oxidation. Appl. Surf. Sci. 2022, 576, 151797. [Google Scholar] [CrossRef]
- Santos, A.L.d.; Dias, J.A.; Tavares, G.T.d.S.; Mendonça, V.o.d.; Giraldi, T.R. Niobium pentoxide as an adsorbent for methylene blue removal: Synthesis, characterization and thermal stability. Mater. Chem. Phys. 2023, 301, 127659. [Google Scholar] [CrossRef]
- Fridriksson, E.G.; Tryggvason, T.K.; Arnalds, U.B.; Ingason, A.S.; Magnus, F. Growth of NbO, NbO2 and Nb2O5 thin films by reactive magnetron sputtering and post-annealing. Vacuum 2022, 202, 111179. [Google Scholar] [CrossRef]
- Silva, A.L.d.; Sales, H.B.; Meneghetti, S.M.P.; Motta, R.J.B.; Silva, B.J.B.d.; Santos, I.M.G.d.; Costa, A.C.F.d.M. Synthesis of the acidic/magnetic catalyst x-MoO3/Ni0.5Zn0.5Fe2O4 by combustion reaction and evaluation of its catalytic potential/reuse in biodiesel production. Ceram. Int. 2024, 50, 43874–43892. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, X.; Liu, D.; Zhou, L.; Zhou, F.; Wei, C.; Wang, Y.; Wen, G. Structural modulation of Nb2O5 for enhanced Lithium-ion battery Performance: Insights from density functional theory and electrochemical characterization. Chem. Eng. Sci. 2024, 298, 120356. [Google Scholar] [CrossRef]
- Silva, A.L.; Farias, A.F.F.; Meneghetti, S.M.P.; dos Santos Filho, E.A.; de Melo Costa, A.C.F. Optimization of biodiesel production via transesterification of soybean oil using α-MoO3 catalyst obtained by the combustion method. Arab. J. Chem. 2022, 15, 104012. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhao, H.; Ke, X. Unraveling the significance of acid sites in determining NH3-SCR mechanisms over WO3 catalyst: A combined experimental and computational study. J. Alloys Compd. 2024, 1002, 175373. [Google Scholar] [CrossRef]
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2016.
- Mishra, S.; Bukkarapu, K.R.; Krishnasamy, A. A composition based approach to predict density, viscosity and surface tension of biodiesel fuels. Fuel 2021, 285, 119056. [Google Scholar] [CrossRef]
- Esmaeili, H. A critical review on the economic aspects and life cycle assessment of biodiesel production using heterogeneous nanocatalysts. Fuel Process. Technol. 2022, 230, 107224. [Google Scholar] [CrossRef]
- Srikumar, K.; Tan, Y.H.; Kansedo, J.; Tan, I.S.; Mubarak, N.M.; Ibrahim, M.L.; Yek, P.N.Y.; Foo, H.C.Y.; Karri, R.R.; Khalid, M. A review on the environmental life cycle assessment of biodiesel production: Selection of catalyst and oil feedstock. Biomass Bioenergy 2024, 185, 107239. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of biodiesel from castor oil: A review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- 66. Rahman, A.; Oktaufik, M.A.M.; Sasongko, T.W.; Guntoro, I.; Soedjati, D.; Abbas, N.; Rahman, A.; Ulfah, F.; Widiarto, A.; Trihadi, S.E.Y.; et al. Current scenario and potential of waste cooking oil as a feedstock for biodiesel production in Indonesia: Life cycle sustainability assessment (LCSA) review. Case Stud. Chem. Environ. Eng. 2025, 11, 101067. [Google Scholar] [CrossRef]
- Erchamo, Y.S.; Mamo, T.T.; Workneh, G.A.; Mekonnen, Y.S. Improved biodiesel production from waste cooking oil with mixed methanol–ethanol using enhanced eggshell-derived CaO nano-catalyst. Sci. Rep. 2021, 11, 6708. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- AOCS. Official Method Cd 3d-63. Acid Value of Fats and Oils. In Official Methods and Recommended Practices of the AOCS; AOCS: Champaign, IL, USA, 2017. [Google Scholar]
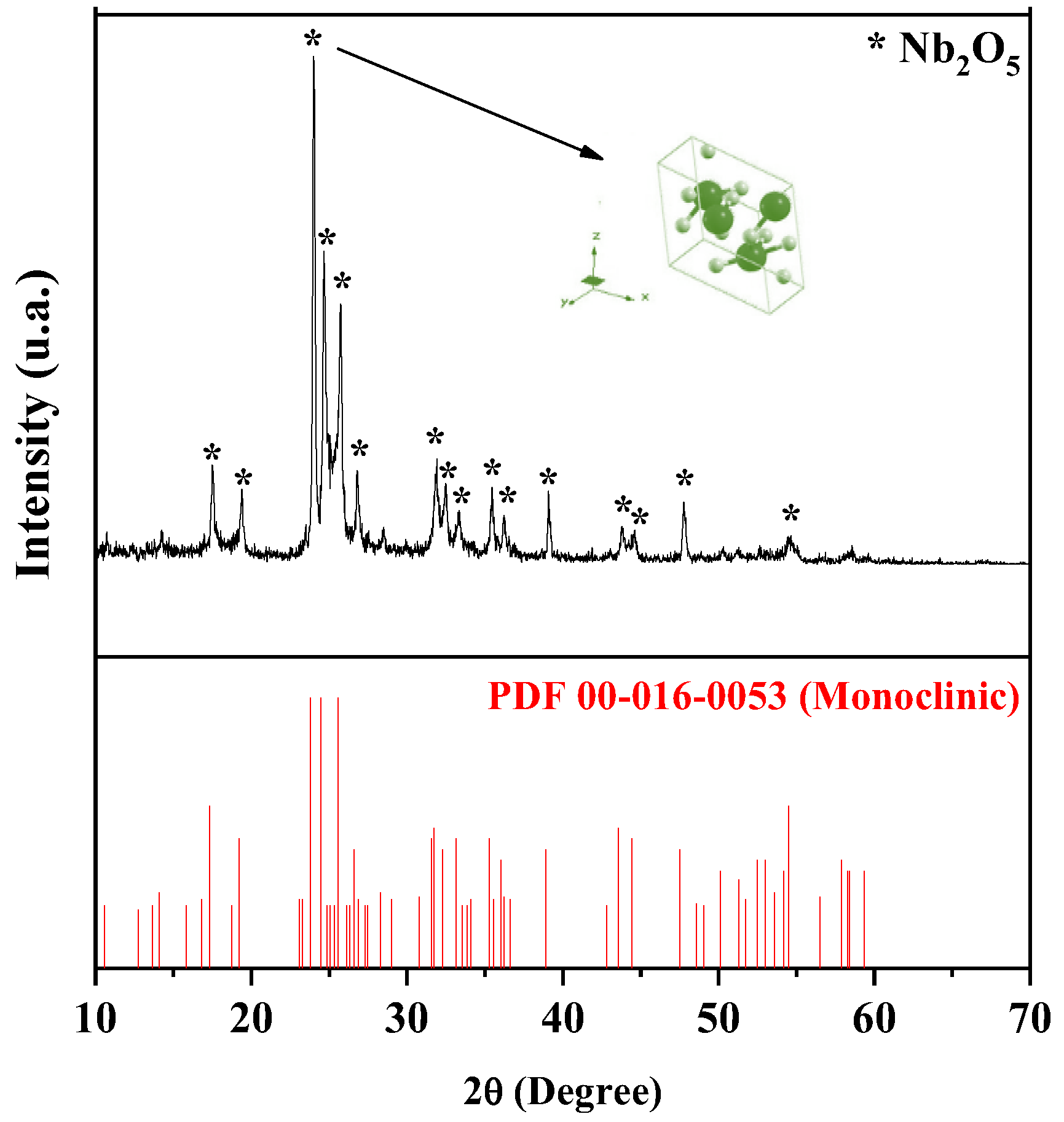
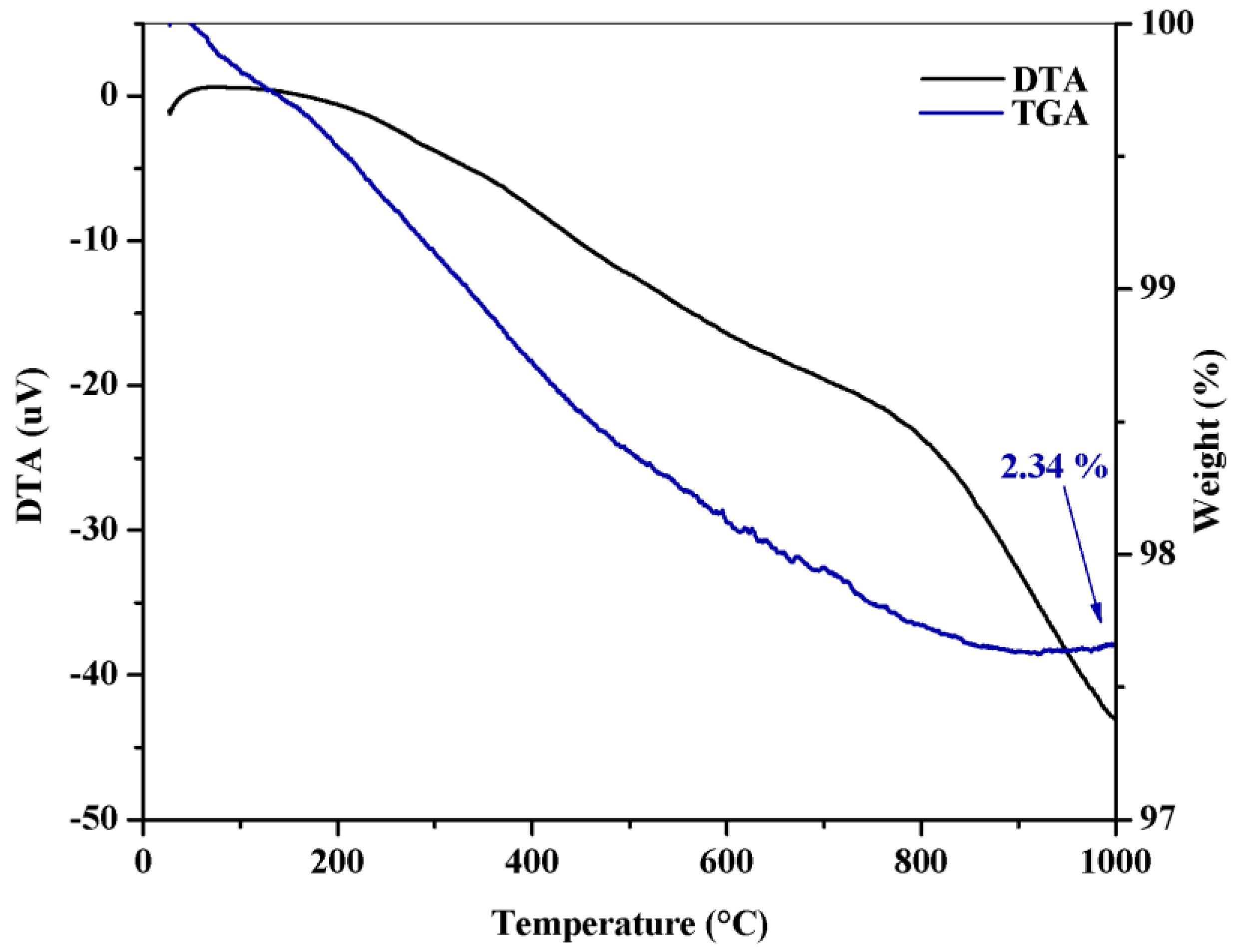
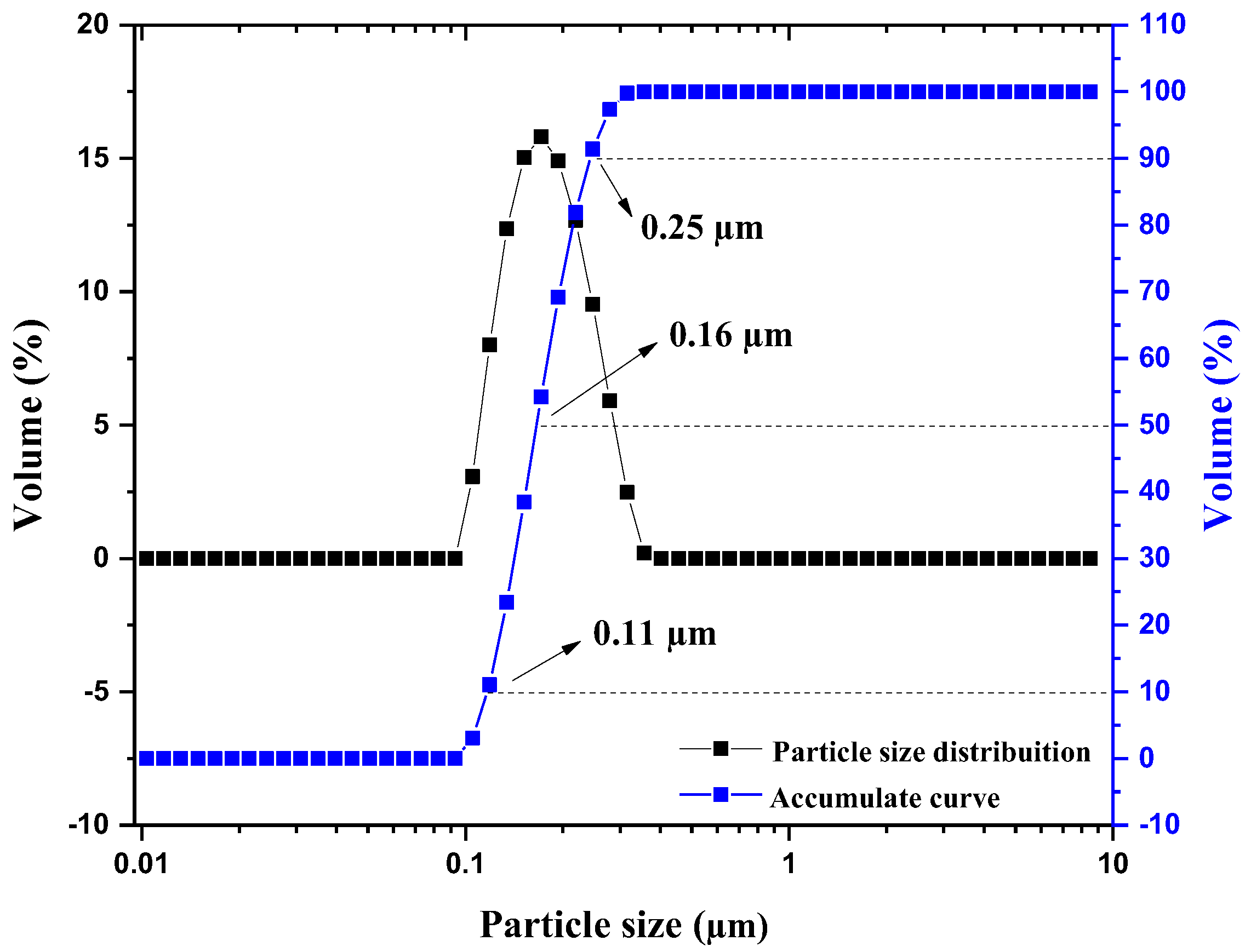
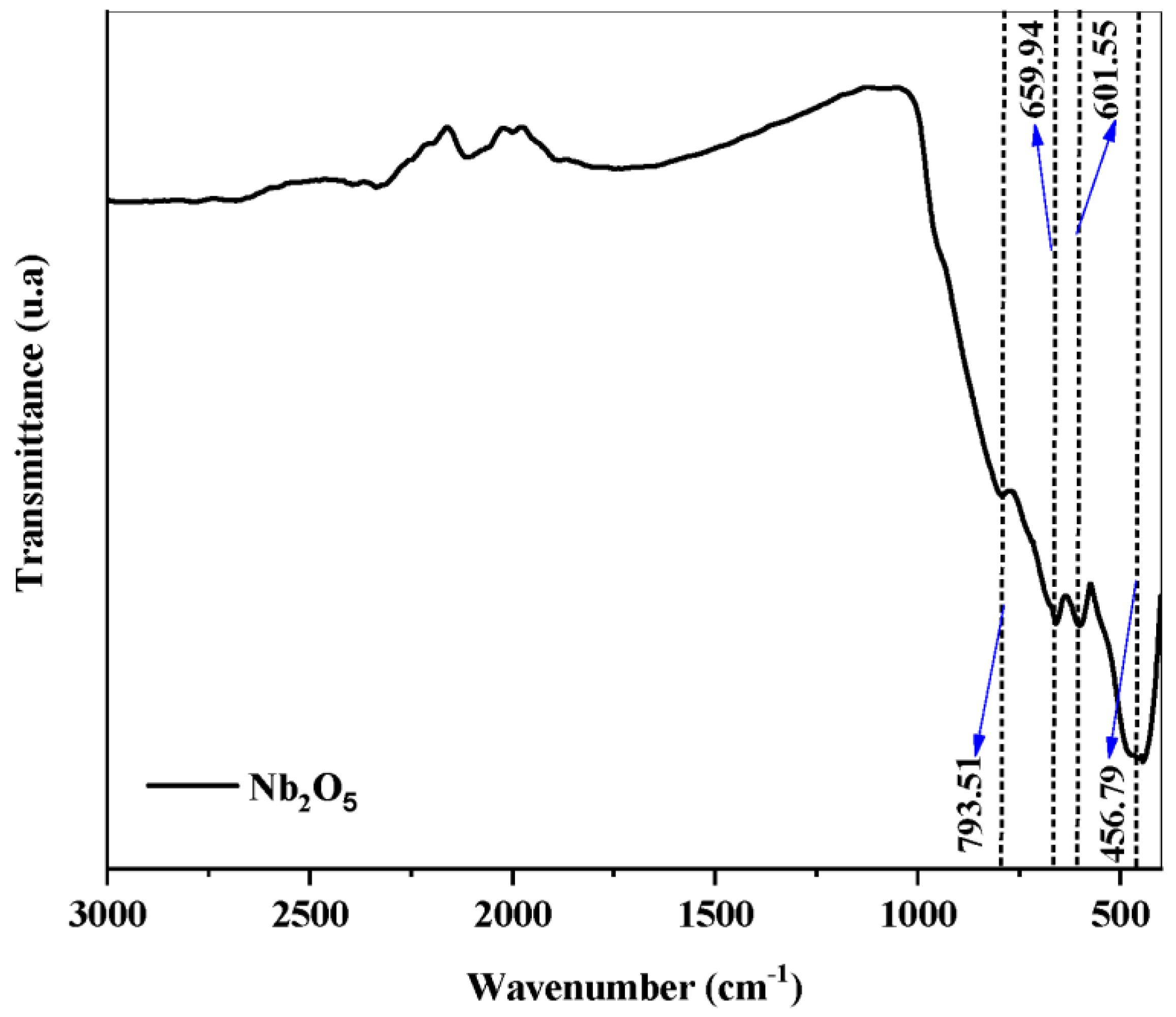
| Catalyst | Crystal Phase | Feedstock | Route | Reaction Conditions | Conversion | Ref. |
|---|---|---|---|---|---|---|
| H4PNbW11O40/WO3–Nb2O5 | - | Oleic acid | Ethanol | AOR * = 15:1, 11.11% wt ** of catalyst, 99.85 °C, 8 h | 70.0% | Katada, Hatanaka [33] |
| 25wt%TPA/Nb2O5 | TT-Nb2O5 | Sunflower oil and Palmitic acid | Methanol | AOR = 13.7:1, 15% wt of catalyst 65 °C, 4 h | 97.3% 99.1% | Srilatha, Lingaiah [34] |
| MCM-41-Nb-8 | - | Sunflower oil | Methanol | AOR = 12:1, 7.5% wt of catalyst 200 °C, 4 h | 95.0% | García et al. [14] |
| Nb2O5/SO4 | TT-Nb2O5 | Palm oil | Ethanol | AOR = 120:1, 30% wt of catalyst, 250 °C, 4 h | 99.2% | da Conceição, Carneiro [35] |
| 2Nb2O5/SBA-15 | Amorphous | Propanoic acid | Methanol | AOR = 15:1, 1% wt of catalyst, 120 °C, 4 h | 92.0% | Silva, Wilson [36] |
| 30MoO3/Nb2O5 | TT-Nb2O5 | Waste oil | Methanol | AOR = 30:1, 5% wt of catalyst, 145 °C, 2.5 h | 94.3% | de Brito, Gonçalves [37] |
| Catalyst | Oxides Presents | |||
|---|---|---|---|---|
| NbO | SiO2 | F2O3 | Others | |
| Nb2O5 | 96.71 | 2.95 | 0.14 | 0.2 |
| Catalyst | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Density (g/cm3) | Total Acidic Sites (μmoles NH3 g−1) | μmol of NH3 m−2 |
|---|---|---|---|---|---|
| Nb2O5 | 1.30 | 0.01 | 4.64 | 301.00 | 231.01 |
| Feedstock | Route | Conversion (%) | Yield (%) | Density (Kg·m−3) | Acidity (mg KOH·g−1) |
|---|---|---|---|---|---|
| Soybean | Methanol | 96.43 ± 0.14 | 65.43 | 908.3 | 0.82 ± 0.07 |
| Ethanol | 21.55 ± 8.40 | 74.63 | 921.1 | 0.82 ± 0.07 | |
| Residual | Methanol | 77.05 ± 6.11 | 74.03 | 951.8 | 5.73 ± 0.07 |
| Ethanol | 69.74 ± 9.52 | 78.76 | 949.6 | 6.29 ± 0.07 | |
| Corn | Methanol | 78.34 ± 13.47 | 79.83 | 910.9 | 1.37 ± 0.07 |
| Ethanol | 13.32 ± 4.05 | 79.82 | 904.6 | 0.55 ± 0.00 | |
| Sunflower | Methanol | 63.04 ± 11.47 | 84.13 | 912.4 | 1.37 ± 0.07 |
| Ethanol | 33.37 ± 9.66 | 75.26 | 918.1 | 1.09 ± 0.00 |
| Cycle | Conversion (%) | Yield (%) |
|---|---|---|
| 1 | 10 ± 1.72 | 79.20 |
| 2 | 19 ± 4.72 | 83.35 |
| 3 | 21 ± 8.85 | 80.18 |
| 4 | 15 ± 5.33 | 81.36 |
| 5 | 18 ± 0.69 | 80.60 |
| Feedstocks | Main Fatty Acids | Acidity (mg KOH·g−1) | ||||
|---|---|---|---|---|---|---|
| Palmitic C16:0 | Stearic C18:0 | Oleic C18:1 | Linoleic C18:2 | Linolenic C18:3> | ||
| Soybean | 1% | 4% | 29% | 63% | 3% | 0.00 |
| Residual | 12% | 4% | 18% | 54% | 12% | 8.19 |
| Corn | 2% | 5% | 31% | 60% | 2% | 0.00 |
| Sunflower | 2% | 1% | 18% | 77% | 2% | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, H.d.L.; Silva, A.L.d.; Luna, C.B.B.; Figueiredo, J.S.B.d.; Meneghetti, S.M.P.; Costa, A.C.F.d.M. Potential of Nb2O5 as a Catalyst in Biodiesel Production: A Study with Different Feedstock. Molecules 2025, 30, 1075. https://doi.org/10.3390/molecules30051075
Pereira HdL, Silva ALd, Luna CBB, Figueiredo JSBd, Meneghetti SMP, Costa ACFdM. Potential of Nb2O5 as a Catalyst in Biodiesel Production: A Study with Different Feedstock. Molecules. 2025; 30(5):1075. https://doi.org/10.3390/molecules30051075
Chicago/Turabian StylePereira, Helder de Lucena, Adriano Lima da Silva, Carlos Bruno Barreto Luna, Joyce Salviano Barros de Figueiredo, Simoni Margareti Plentz Meneghetti, and Ana Cristina Figueiredo de Melo Costa. 2025. "Potential of Nb2O5 as a Catalyst in Biodiesel Production: A Study with Different Feedstock" Molecules 30, no. 5: 1075. https://doi.org/10.3390/molecules30051075
APA StylePereira, H. d. L., Silva, A. L. d., Luna, C. B. B., Figueiredo, J. S. B. d., Meneghetti, S. M. P., & Costa, A. C. F. d. M. (2025). Potential of Nb2O5 as a Catalyst in Biodiesel Production: A Study with Different Feedstock. Molecules, 30(5), 1075. https://doi.org/10.3390/molecules30051075








