Glucose-6-Phosphate Dehydrogenase Modulates Shiraia Hypocrellin A Biosynthesis Through ROS/NO Signaling in Response to Bamboo Polysaccharide Elicitation
Abstract
1. Introduction
2. Results
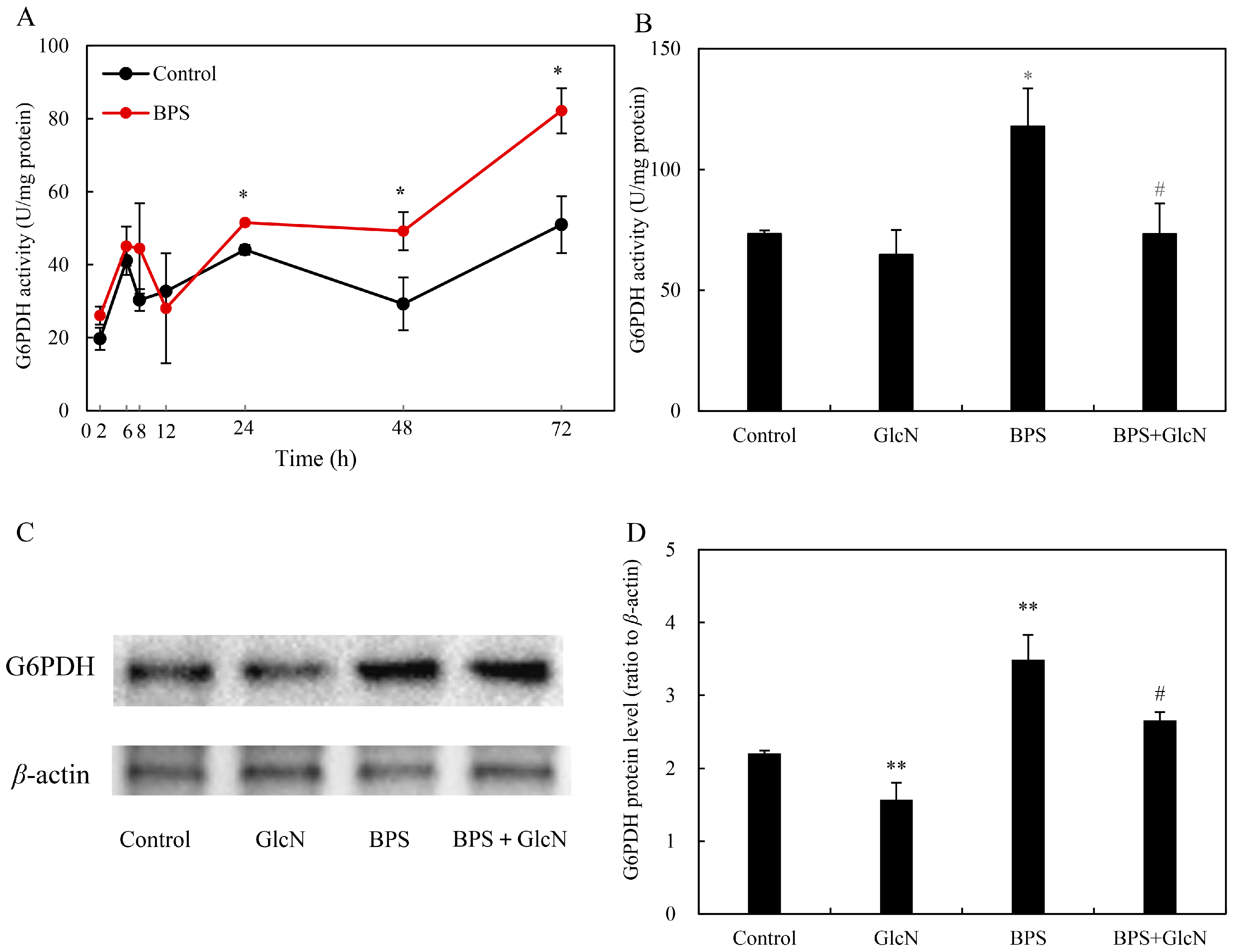
2.1. Eliciting Effects of BPS on Fungal HA Production and G6PDH Activity
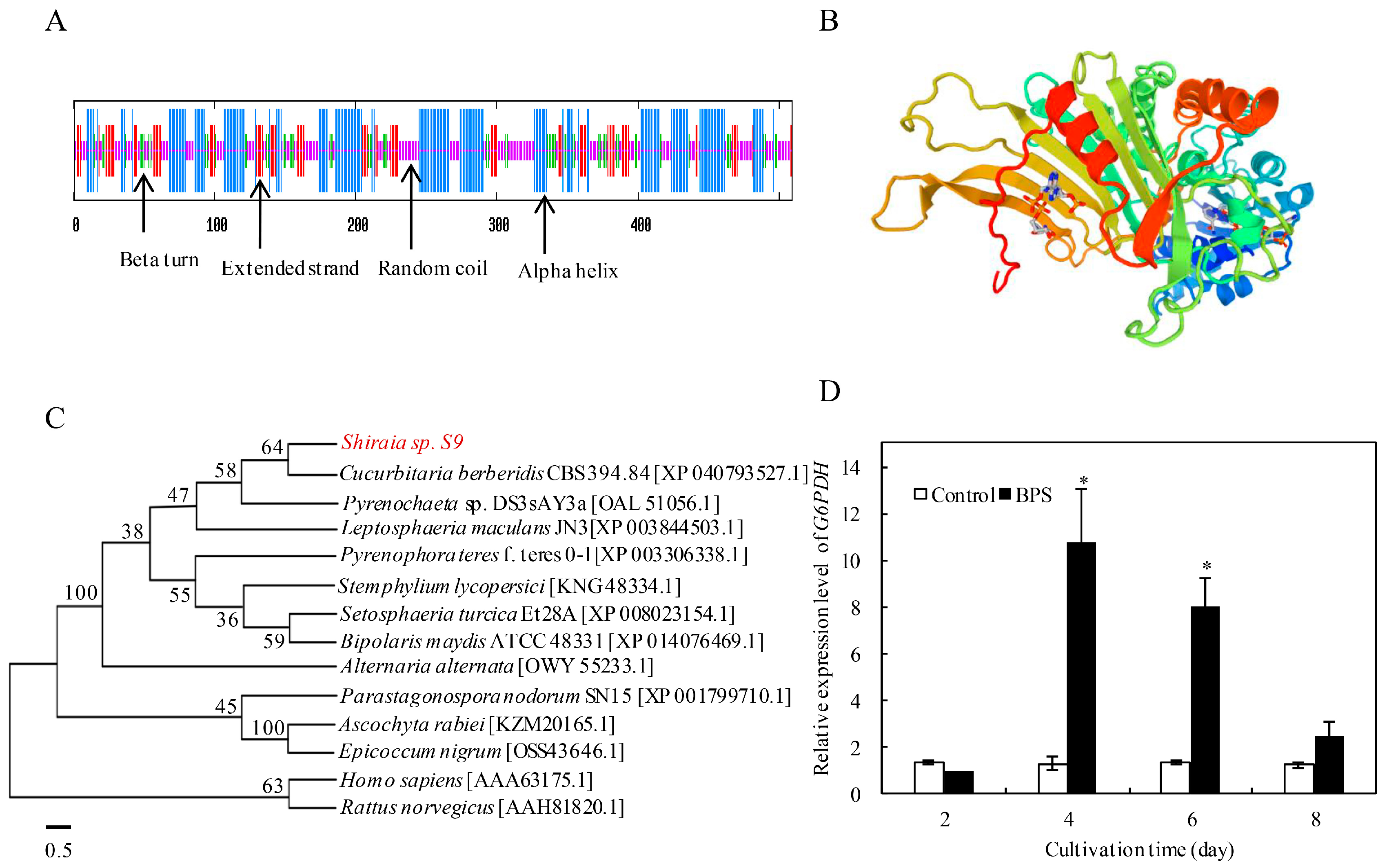
2.2. G6PDH Cloning and Expression Under BPS Treatment
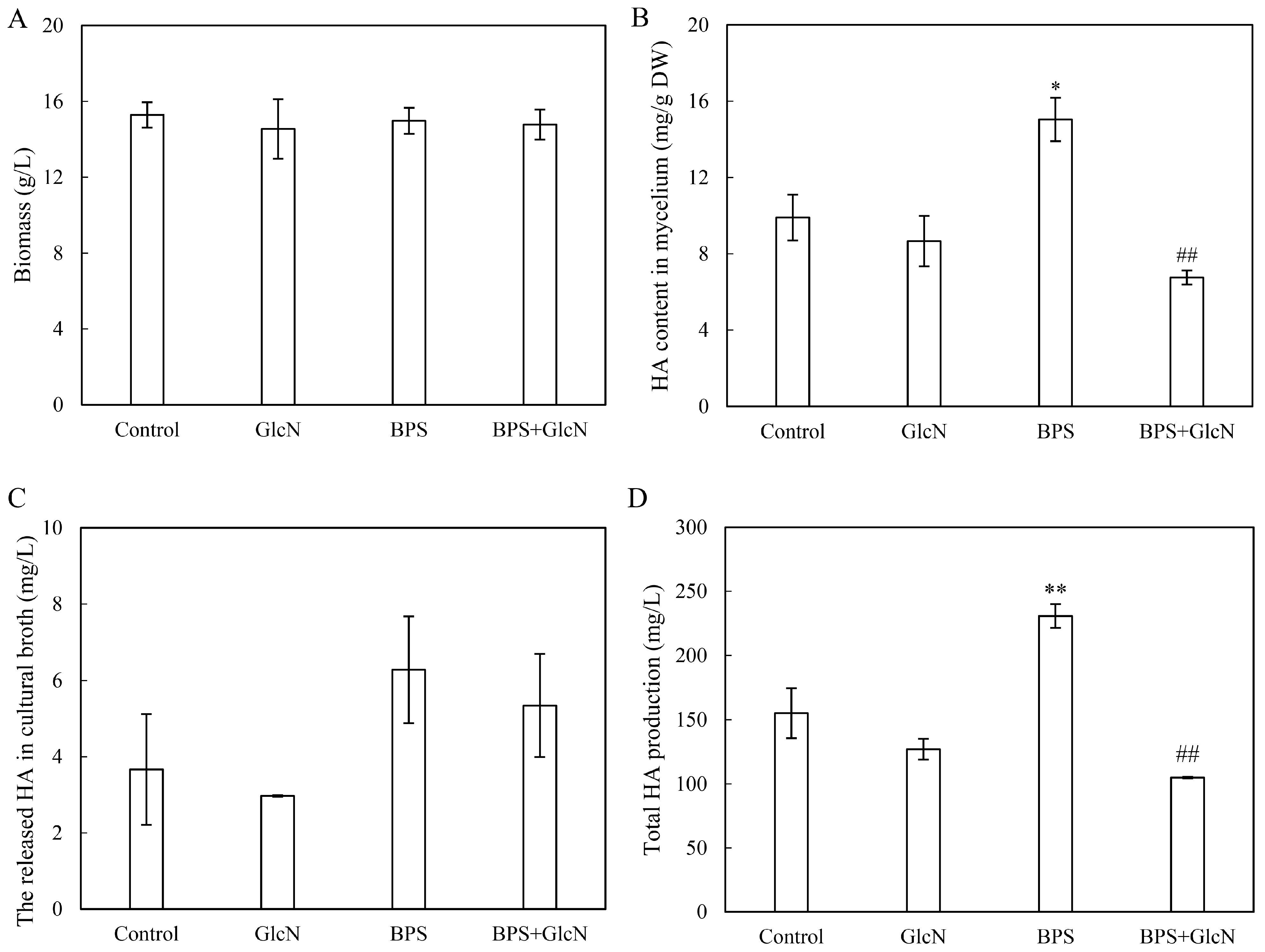
2.3. Effect of G6PDH on BPS-Induced HA Production
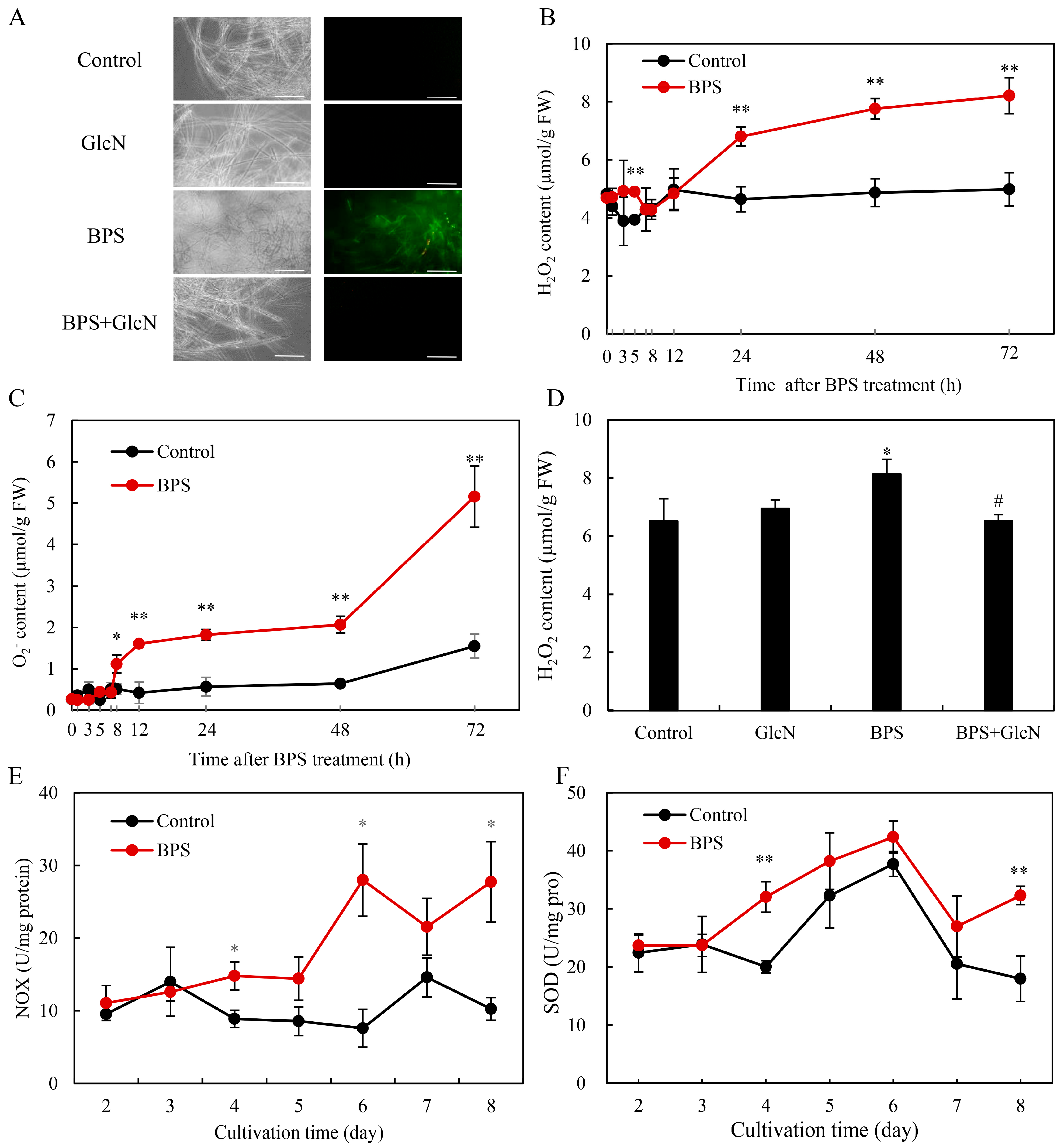
2.4. Effect of G6PDH on BPS-Induced ROS Production
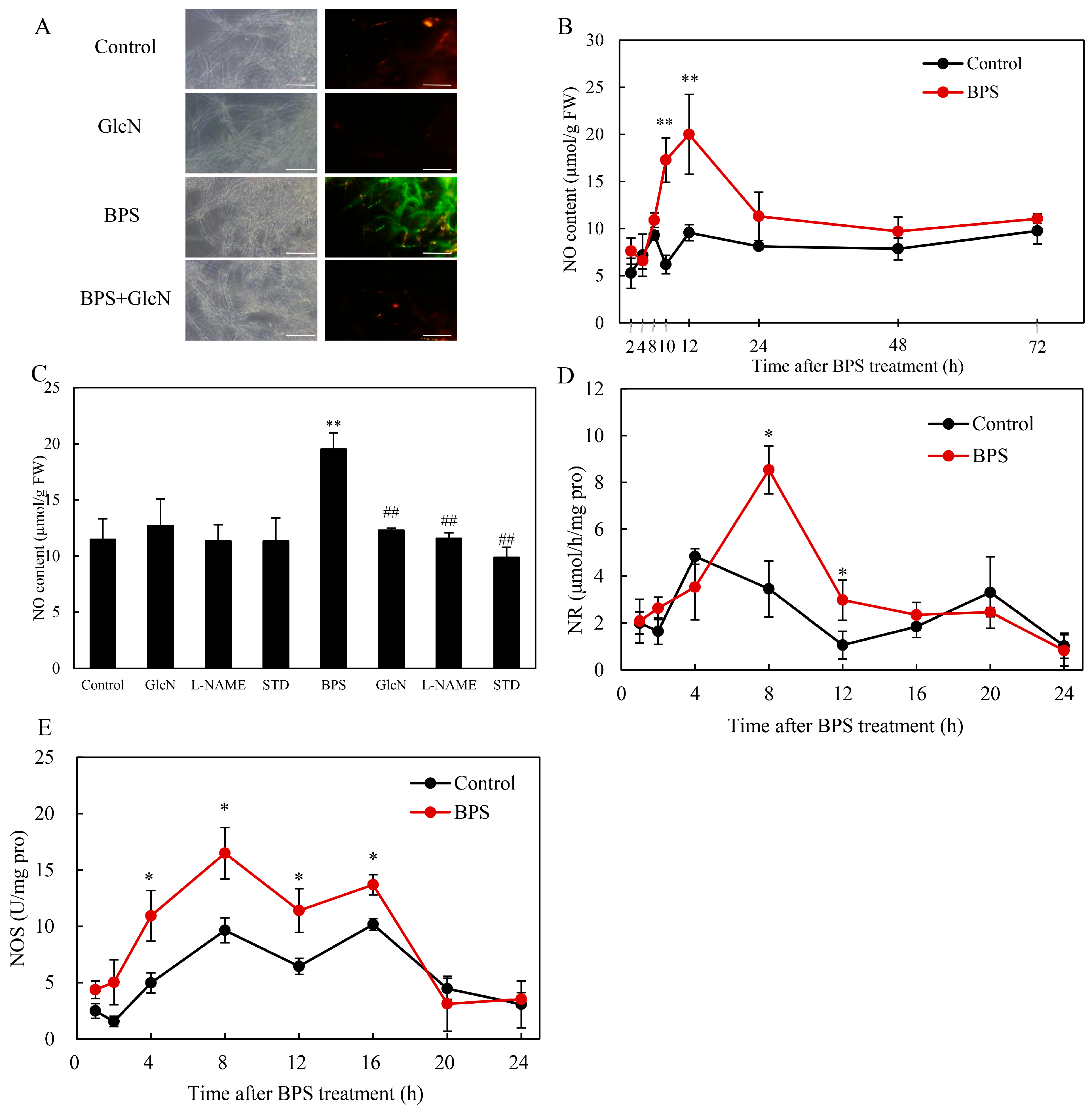
2.5. Effect of G6PDH on BPS-Induced NO Production
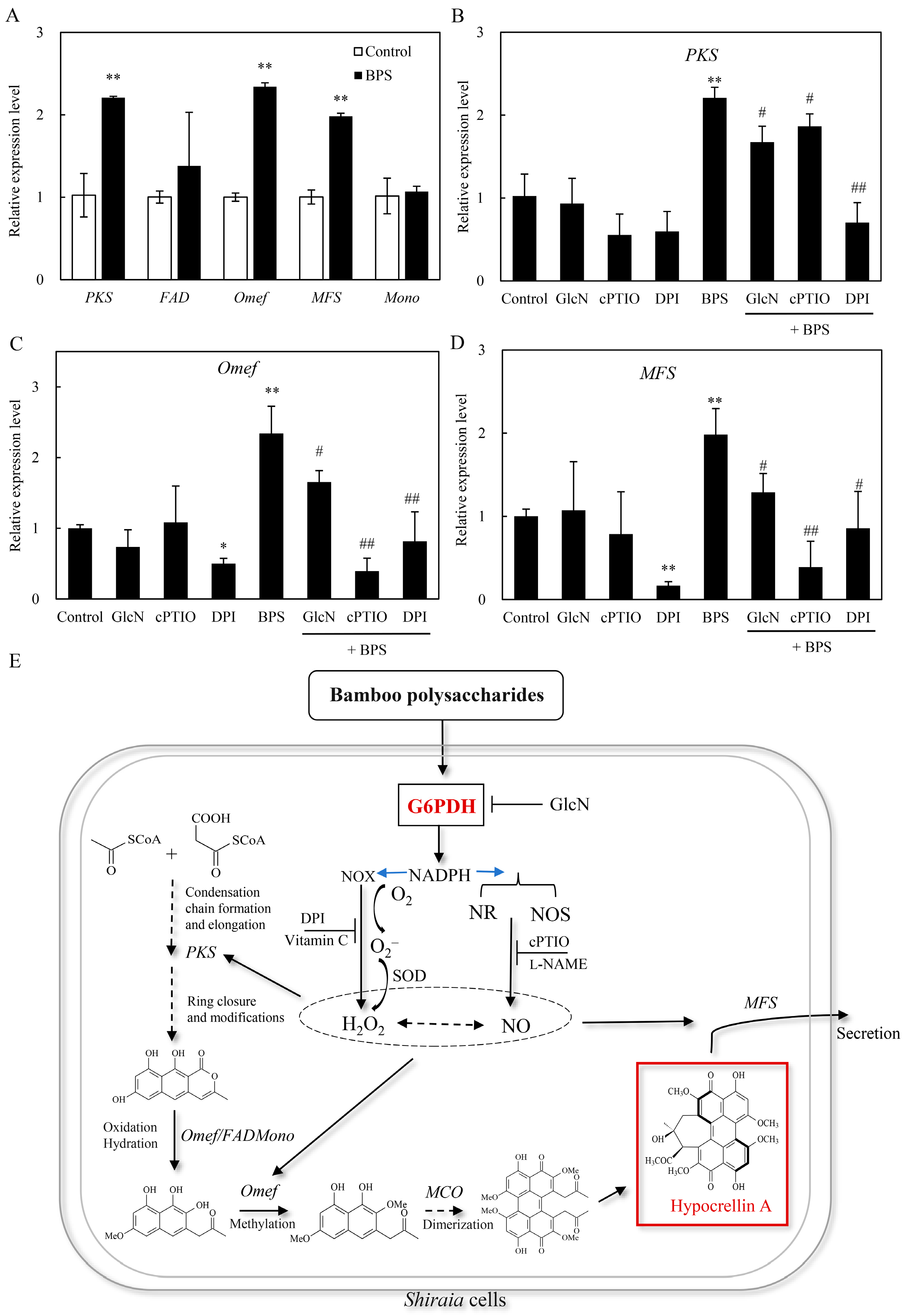
2.6. Effect of G6PDH on BPS-Induced HA Biosynthetic Gene Expression
3. Discussion
4. Materials and Methods
4.1. Strain and Culture Conditions
4.2. Preparation of BPS
4.3. Measurement of G6PDH Activity and Expression
4.4. Cloning of Full-Length G6PDH cDNA
4.5. Measurement of ROS, NO Generation and Enzyme Activities
4.6. Extraction and Quantification of HA
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, Y.G.; Zhang, X.W.; Zhao, W.M.; Zhang, Y.X.; Lang, J.Y.; Shi, Y.H.; Tan, W.F.; Li, M.H.; Zhang, Y.W.; Tong, L.J.; et al. Antiangiogenic effects of shiraiachrome A, a compound isolated from a Chinese folk medicine used to treat rheumatoid arthritis. Euro. J. Pharmacol. 2004, 494, 101–109. [Google Scholar] [CrossRef]
- Diwu, Z.J.; Lown, J.W. Hypocrellins and their use in photosensitization. Photochem. Photobiol. 1990, 52, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Y.; Xie, Y.C.; Xu, C.L.; Zhang, Z.B.; Zhu, D. Biotechnological production and potential applications of hypocrellins. Appl. Microbiol. Biotechnol. 2023, 107, 6421–6438. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Q.L.; Tian, L.W.; Huang, Z.X.; Tang, Y.B.; Wen, Y.D.; Yu, F.Q.; Yan, X.X.; Zhao, Y.C.; Wu, Z.Q.; et al. Advancements and future prospects in hypocrellins production and modification for photodynamic therapy. Fermentation 2024, 10, 559. [Google Scholar] [CrossRef]
- Rajan, S.S.; Chandran, R.; Abrahamse, H. Advancing photodynamic therapy with nano-conjugated hypocrellin: Mechanisms and clinical applications. Int. J. Nanomed. 2024, 19, 11023–11038. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Sun, C.L.; Liang, Z.Q.; Han, Y.F.; Yu, J.P. Antibacterial activity of hypocrellin A against Staphylococcus aureus. World J. Microbiol. Biotechnol. 2012, 28, 3151–3157. [Google Scholar] [CrossRef]
- Yang, Y.J.; Wang, C.L.; Zhuge, Y.Z.; Zhang, J.; Xu, K.; Zhang, Q.L.; Zhang, H.J.; Chen, H.Y.; Chu, M.P.; Jia, C. Photodynamic antifungal activity of hypocrellin A against Candida albicans. Front. Microbiol. 2019, 10, 1810. [Google Scholar] [CrossRef]
- Li, Y.T.; Yang, C.; Wu, Y.; Lv, J.J.; Feng, X.; Tian, X.F.; Zhou, Z.Z.; Pan, X.Y.; Liu, S.W.; Tian, L.W. Axial chiral binaphthoquinone and perylenequinones from the stromata of Hypocrella bambusae are SARS-CoV-2 entry inhibitors. J. Nat. Prod. 2021, 84, 436–443. [Google Scholar] [CrossRef]
- Shi, W.Y.; Lv, P.; Zhang, T.C. Study on fermentation medium for new pigment hypocrellin. Food Res. Devel. 2016, 37, 182–185. (In Chinese) [Google Scholar]
- Su, Y.J.; Si, S.H.; Qiao, L.W.; Cai, Y.J.; Xu, Z.M.; Yang, Y.J. The effect of a hypocrellin A enriched diet on egg yolk quality and hypocrellin A distributions in the meat of laying hens. Eur. Food Res. Technol. 2011, 232, 935–940. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Morgan, B.J.; Mulrooney, C.A.; Carroll, P.J.; Kozlowski, M.C. Perylenequinone natural products: Total synthesis of hypocrellin A. J. Org. Chem. 2010, 75, 57–68. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, Z.Y.; Wongkaew, S. Developing characteristics and relationships of Shiraia bambusicola with bamboo. Songklanakarin J. Sci. Technol. 2012, 34, 17–22. [Google Scholar]
- Li, X.P.; Shen, W.H.; Wang, J.W.; Zheng, L.P. Production of fungal hypocrellin photosensitizers: Exploiting bambusicolous fungi and elicitation strategies in mycelium cultures. Mycology 2025, 16, 593–616. [Google Scholar] [CrossRef]
- Liang, X.H.; Cai, Y.J.; Liao, X.R.; Wu, K.; Wang, L.; Zhang, D.B.; Meng, Q. Isolation and identification of a new hypocrellin A-producing strain Shiraia sp. SUPER-H168. Microbiol. Res. 2009, 164, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Xiao, C.X.; Ma, W.X.; He, G.Q. The production of hypocrellin colorants by submerged cultivation of the medicinal fungus Shiraia bambusicola. Dyes Pigm. 2009, 82, 142–146. [Google Scholar] [CrossRef]
- Cai, Y.J.; Liang, X.H.; Liao, X.R.; Ding, Y.R.; Sun, J.; Li, X.H. High-yield hypocrellin A production in solid-state fermentation by Shiraia sp. SUPER-H168. Appl. Biochem. Biotechnol. 2010, 160, 2275–2286. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, Z.Y.; Yang, Y.L.; Wongkaew, S. Isolation, screening and confirmative identification of high hypocrellin A-producing Shiraia bambusicola isolates. Khon Kaen Agric. J. 2009, 37, 357–364. [Google Scholar]
- Cai, Y.J.; Liao, X.H.; Liang, X.H.; Ding, Y.R.; Sun, J.; Zhang, D.B. Induction of hypocrellin production by Triton X-100 under submerged fermentation with Shiraia sp. SUPER-H168. New Biotechnol. 2011, 28, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.Y.; Zhang, M.Y.; Ma, Y.J.; Wang, J.W. Transcriptomic responses involved in enhanced production of hypocrellin A by addition of Triton X 100 in submerged cultures of Shiraia bambusicola. J. Ind. Microbiol. Biotechnol. 2017, 44, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.X.; Chen, J.J.; Gao, R.J.; Liao, X.R.; Cai, Y.J. Adaptive responses to oxidative stress in the filamentous fungal Shiraia bambusicola. Molecules 2016, 21, 1118. [Google Scholar] [CrossRef]
- Wu, X.Y.; Meng, X.; Xiao, Y.W.; Yang, H.L.; Zhang, Z.B.; Zhu, D. Energy metabolism enhance perylenequinone biosynthesis in Shiraia sp. Slf14 through promoting mitochondrial ROS accumulation. Int. J. Mol. Sci. 2024, 25, 10113. [Google Scholar] [CrossRef]
- Li, X.P.; Ma, Y.J.; Wang, J.W. Adding bamboo charcoal powder to Shiraia bambusicola preculture improves hypocrellin A production. Sustain. Chem. Pharm. 2019, 14, 100191. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, X.P.; Shen, W.H.; Huang, Q.Y.; Cong, R.P.; Zheng, L.P.; Wang, J.W. Nitric oxide mediates red light-induced perylenequinone production in Shiraia mycelium culture. Bioresour. Bioprocess. 2024, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Shen, W.H.; Zhou, L.L.; Huang, Q.Y.; Cong, R.P.; Zheng, L.P.; Wang, J.W. Lipopolysaccharides from a Shiraia fruiting body-associated bacterium elicit host fungal hypocrellin a biosynthesis through nitric oxide generation. Carbohydr. Polym. 2024, 324, 121498. [Google Scholar] [CrossRef]
- Ma, Y.J.; Li, X.P.; Wang, Y.; Wang, J.W. Nitric oxide donor sodium nitroprusside-induced transcriptional changes and hypocrellin biosynthesis of Shiraia sp. S9. Microb. Cell Fact. 2021, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yu, Y.Y.; Yue, Y.X.; Dou, M.Z.; Guo, B.J.; Yan, S.Z.; Chen, S.L. Nitric oxide regulates perylenequinones biosynthesis in Shiraia bambusicola S4201 induced by hydrogen peroxide. Sci. Rep. 2021, 11, 2365. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, Y.; Ma, Y.J.; Wang, J.W.; Zheng, L.P. Nitric oxide and hydrogen peroxide signaling in extractive Shiraia fermentation by triton X-100 for hypocrellin A production. Int. J. Mol. Sci. 2020, 21, 882. [Google Scholar] [CrossRef]
- Jiang, Z.R.; Wang, M.; Nicolas, M.; Ogé, L.; Pérez-Garcia, M.D.; Crespel, L.; Li, G.H.; Ding, Y.F.; Le Gourrierec, J.; Grappin, P.; et al. Glucose-6-phosphate dehydrogenases: The hidden players of plant physiology. Int. J. Mol. Sci. 2022, 23, 16128. [Google Scholar] [CrossRef]
- Rashida, Z.; Laxman, S. The pentose phosphate pathway and organization of metabolic networks enabling growth programs. Curr. Opin. Syst. Biol. 2021, 28, 100390. [Google Scholar] [CrossRef]
- Dal Santo, S.; Stampfl, H.; Krasensky, J.; Kempa, S.; Gibon, Y.; Petutschnig, E.; Rozhon, W.; Heuck, A.; Clausen, T.; Jonak, C. Stress-induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis. Plant Cell 2012, 24, 3380–3392. [Google Scholar] [CrossRef]
- He, Q.; Li, P.; Zhang, W.Y.; Bi, Y.R. Cytoplasmic glucose-6-phosphate dehydrogenase plays an important role in the silicon-enhanced alkaline tolerance in highland barley. Funct. Plant Biol. 2021, 48, 119–130. [Google Scholar] [CrossRef]
- Wang, H.H.; Hou, J.J.; Li, Y.; Zhang, Y.Y.; Huang, J.J.; Liang, W.H. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 2017, 416, 39–52. [Google Scholar] [CrossRef]
- Wang, X.M.; Ruan, M.J.; Wan, Q.; He, W.L.; Yang, L.; Liu, X.Y.; He, L.; Yan, L.L.; Bi, Y.R. Nitric oxide and hydrogen peroxide increase glucose-6-phosphate dehydrogenase activities and expression upon drought stress in soybean roots. Plant Cell Rep. 2020, 39, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Wu, R.R.; Wan, Q.; Xie, G.Q.; Bi, Y.R. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 2007, 48, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Gupte, S.A.; Levine, R.J.; Gupte, R.S.; Young, M.E.; Lionetti, V.; Labinskyy, V.; Floyd, B.C.; Ojaimi, C.; Bellomo, M.; Wolin, M.S.; et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J. Mol. Cell. Cardiol. 2006, 41, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.S.; Floyd, B.C.; Kozicky, M.; George, S.; Ungvari, Z.I.; Neito, V.; Wolin, M.S.; Gupte, S.A. Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic. Biol. Med. 2009, 47, 219–228. [Google Scholar] [CrossRef]
- Park, J.; Choe, S.S.; Choi, A.H.; Kim, K.H.; Yoon, M.J.; Suganami, T.; Ogawa, Y.; Kim, J.B. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes 2006, 55, 2939–2949. [Google Scholar] [CrossRef]
- Minard, K.I.; McAlister-Henn, L. Antioxidant function of cytosolic sources of NADPH in yeast. Free Radic. Biol. Med. 2001, 31, 832–843. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Kitajima, S.; Izawa, S. Importance of glucose-6-phosphate dehydrogenase (G6PDH) for vanillin tolerance in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2014, 118, 263–269. [Google Scholar] [CrossRef]
- Tran, L.T.; Miki, T.; Kamakura, M.; Izawa, S.; Tsujimoto, Y.; Miyabe, S.; Inoue, Y.; Kimura, A. Oxidative stress response in yeast: Induction of glucose-6-phosphate dehydrogenase by lipid hydroperoxide in Hansenula mrakii. J. Ferment. Bioeng. 1995, 80, 606–609. [Google Scholar] [CrossRef]
- Fan, Y.X.; Lu, Y.L.; Zhang, L.W.; Chen, X.L.; Shen, Y.C. Enhancing NADPH regeneration and increasing hydroxylation efficiency with P450 monooxygenase through strengthening expression of glucose-6-phosphate dehydrogenase in industrial filamentous fungi. Biocatal. Agric. Biotechnol. 2017, 11, 307–311. [Google Scholar] [CrossRef]
- Shen, W.H.; Zhou, L.L.; Li, X.P.; Cong, R.P.; Huang, Q.Y.; Zheng, L.P.; Wang, J.W. Bamboo polysaccharides elicit hypocrellin A biosynthesis of a bambusicolous fungus Shiraia sp. S9. World J. Microbiol. Biotechnol. 2023, 39, 341. [Google Scholar] [CrossRef]
- Morakotkarn, D.; Kawasaki, H.; Tanaka, K.; Okane, I.; Seki, T. Taxonomic characterization of Shiraia-like fungi isolated from bamboos in Japan. Mycoscience 2008, 49, 258–265. [Google Scholar] [CrossRef]
- Daub, M.E.; Herrero, S.; Chung, K.R. Photoactivated perylenequinone toxins in fungal pathogenesis of plants. FEMS Microbiol. Lett. 2005, 252, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, B.H.; Woloshuk, C.P. Amylopectin induces fumonisin B1 production by Fusarium verticillioides during colonization of maize kernels. Mol. Plant-Microbe Interact. 2005, 18, 1333–1339. [Google Scholar] [CrossRef]
- Oka, K.; Akamatsu, H.; Kodama, M.; Nakajima, H.; Otani, H. Host-specific AB-toxin production by germinating spores of Alternaria brassicicola is induced by a host-derived oligosaccharide. Physiol. Mol. Plant Pathol. 2005, 66, 12–19. [Google Scholar] [CrossRef]
- Yang, H.L.; Wang, Y.; Zhang, Z.B.; Yan, R.M.; Zhu, D. Whole-genome shotgun assembly and analysis of the genome of Shiraia sp. strain Slf14, a novel endophytic fungus producing huperzine A and hypocrellin A. Genome Announc. 2014, 2, e00011-14. [Google Scholar] [CrossRef]
- Zhao, N.; Lin, X.; Qi, S.S.; Chen, S.L.; Yan, S.Z. De novo transcriptome assembly in Shiraia bambusicola to investigate putative genes involved in the biosynthesis of hypocrellin A. Int. J. Mol. Sci. 2016, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.B.; Wen, Y.D.; Zhang, X.; Gao, Q.; Yu, F.Q.; Wu, Z.Q.; Tian, X.F. Urea-induced enhancement of hypocrellin A synthesis in Shiraia bambusicola GDMCC 60438: Strategies and mechanisms. Fermentation 2024, 10, 381. [Google Scholar] [CrossRef]
- Tsai, J.H.; Schulte, M.; O’Neill, K.; Chi, M.M.; Frolova, A.I.; Moley, K.H. Glucosamine inhibits decidualization of human endometrial stromal cells and decreases litter sizes in mice. Biol. Reprod. 2013, 89, 16. [Google Scholar] [CrossRef]
- Huang, J.J.; Han, R.Z.; Ji, F.; Yu, Y.Y.; Wang, R.Y.; Hai, Z.X.; Liang, W.H.; Wang, H.H. Glucose-6-phosphate dehydrogenase and abscisic acid mediate programmed cell death induced by aluminum toxicity in soybean root tips. J. Hazard. Mater. 2022, 425, 127964. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Yoshioka, M.; Nomura, H.; Tone, C.; Nakajima, K.; Nakane, E.; Doke, N.; Yoshioka, H. A plastidic glucose-6-phosphate dehydrogenase is responsible for hypersensitive response cell death and reactive oxygen species production. J. Gen. Plant Pathol. 2011, 77, 152–162. [Google Scholar] [CrossRef]
- Xu, R.; Li, X.P.; Zhang, X.; Shen, W.H.; Min, C.Y.; Wang, J.W. Contrasting regulation of live Bacillus cereus No. 1 and its volatiles on Shiraia perylenequinone production. Microb. Cell Fact. 2022, 21, 172. [Google Scholar] [CrossRef]
- Wendehenne, D.; Pugin, A.; Klessig, D.F.; Durner, J. Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Leopold, J.A.; Zhang, Y.Y.; Scribner, A.W.; Stanton, R.C.; Loscalzo, J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 411–417. [Google Scholar] [CrossRef]
- Deng, H.X.; Gao, R.J.; Liao, X.R.; Cai, Y.J. Characterization of a major facilitator superfamily transporter in Shiraia bambusicola. Res. Microbiol. 2017, 168, 664–672. [Google Scholar] [CrossRef]
- Li, D.; Zhao, N.; Guo, B.J.; Lin, X.; Chen, S.L.; Yan, S.Z. Gentic overexpression increases production of hypocrellin A in Shiraia bambusicola S4201. J. Microbiol. 2019, 57, 154–162. [Google Scholar] [CrossRef]
- Zhou, L.G.; Lu, S.Q.; Li, Y.; Li, P.Q.; Xu, J.M.; Shan, T.J.; Mou, Y. Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgen in production in cell suspension culture of Dioscorea zingiberensis. Molecules 2011, 16, 9003–9016. [Google Scholar]
- Li, S.F.; Wang, A.J.; Liu, L.N.; Tian, G.R. Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Charact. 2019, 13, 1382–1389. [Google Scholar] [CrossRef]
- Hochberg, M.L.; Sargent, M.L. Rhythms of enzyme activity associated with circadian conidiation in Neurospora crassa. J. Bacteriol. 1974, 120, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Tongul, B.; Tarhan, L. The effect of menadione-induced oxidative stress on the in vivo reactive oxygen species and antioxidant response system of Phanerochaete chrysosporium. Process Biochem. 2014, 49, 195. [Google Scholar] [CrossRef]
| BPS (mg/L) | Biomass (g/L) | HA Content in Mycelium (mg/g DW) | The Released HA in Cultural Broth (mg/L) | Total HA Production (mg/L) |
|---|---|---|---|---|
| 0 | 7.9 ± 0.8 | 12.8 ± 0.2 | 8.3 ± 1.1 | 109.3 ± 11.7 |
| 60 | 8.2 ± 0.5 | 19.9 ± 0.7 ** | 17.9 ± 1.4 ** | 181.8 ± 16.9 ** |
| 100 | 9.8 ± 1.2 | 33.6 ± 0.8 ** | 27.4 ± 2.6 ** | 354.8 ± 30.7 ** |
| 200 | 9.7 ± 1.2 | 30.2 ± 0.8 ** | 17.2 ± 2.0 ** | 310.2 ± 44.3 * |
| Addition Time (day) | Biomass (g/L) | HA Content in Mycelium (mg/g DW) | The Released HA in Cultural Broth (mg/L) | Total HA Production (mg/L) |
|---|---|---|---|---|
| Control | 15.1 ± 0.3 | 17.6 ± 1.9 | 3.4 ± 0.7 | 269.1 ± 26.6 |
| 1 | 14.6 ± 0.5 | 19.5 ± 2.9 | 4.6 ± 1.1 | 287.6 ± 33.2 |
| 2 | 14.8 ± 0.0 | 22.2 ± 1.8 * | 6.8 ± 0.9 * | 334.3 ± 25.3 * |
| 3 | 15.0 ± 0.4 | 28.0 ± 3.1 ** | 9.2 ± 1.0 ** | 428.1 ± 33.5 ** |
| 4 | 14.7 ± 0.5 | 20.8 ± 2.7 | 4.6 ± 0.6 | 309.2 ± 34.4 |
| 5 | 14.8 ± 0.1 | 20.4 ± 2.5 | 3.9 ± 0.5 | 304.9 ± 38.3 |
| 6 | 14.7 ± 0.7 | 18.1 ± 2.8 | 3.3 ± 0.4 | 267.6 ± 29.4 |
| 7 | 15.4 ± 0.6 | 16.2 ± 2.3 | 3.7 ± 1.0 | 251.4 ± 30.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Huang, Q.; Ma, Y.; Zheng, L.; Wang, J. Glucose-6-Phosphate Dehydrogenase Modulates Shiraia Hypocrellin A Biosynthesis Through ROS/NO Signaling in Response to Bamboo Polysaccharide Elicitation. Molecules 2025, 30, 4060. https://doi.org/10.3390/molecules30204060
Li X, Huang Q, Ma Y, Zheng L, Wang J. Glucose-6-Phosphate Dehydrogenase Modulates Shiraia Hypocrellin A Biosynthesis Through ROS/NO Signaling in Response to Bamboo Polysaccharide Elicitation. Molecules. 2025; 30(20):4060. https://doi.org/10.3390/molecules30204060
Chicago/Turabian StyleLi, Xinping, Qunyan Huang, Yanjun Ma, Liping Zheng, and Jianwen Wang. 2025. "Glucose-6-Phosphate Dehydrogenase Modulates Shiraia Hypocrellin A Biosynthesis Through ROS/NO Signaling in Response to Bamboo Polysaccharide Elicitation" Molecules 30, no. 20: 4060. https://doi.org/10.3390/molecules30204060
APA StyleLi, X., Huang, Q., Ma, Y., Zheng, L., & Wang, J. (2025). Glucose-6-Phosphate Dehydrogenase Modulates Shiraia Hypocrellin A Biosynthesis Through ROS/NO Signaling in Response to Bamboo Polysaccharide Elicitation. Molecules, 30(20), 4060. https://doi.org/10.3390/molecules30204060






