Emerging Applications of Thiol-Based Catalysts in Hydrogen Atom Transfer Reactions: A Comprehensive Review
Abstract
1. Introduction
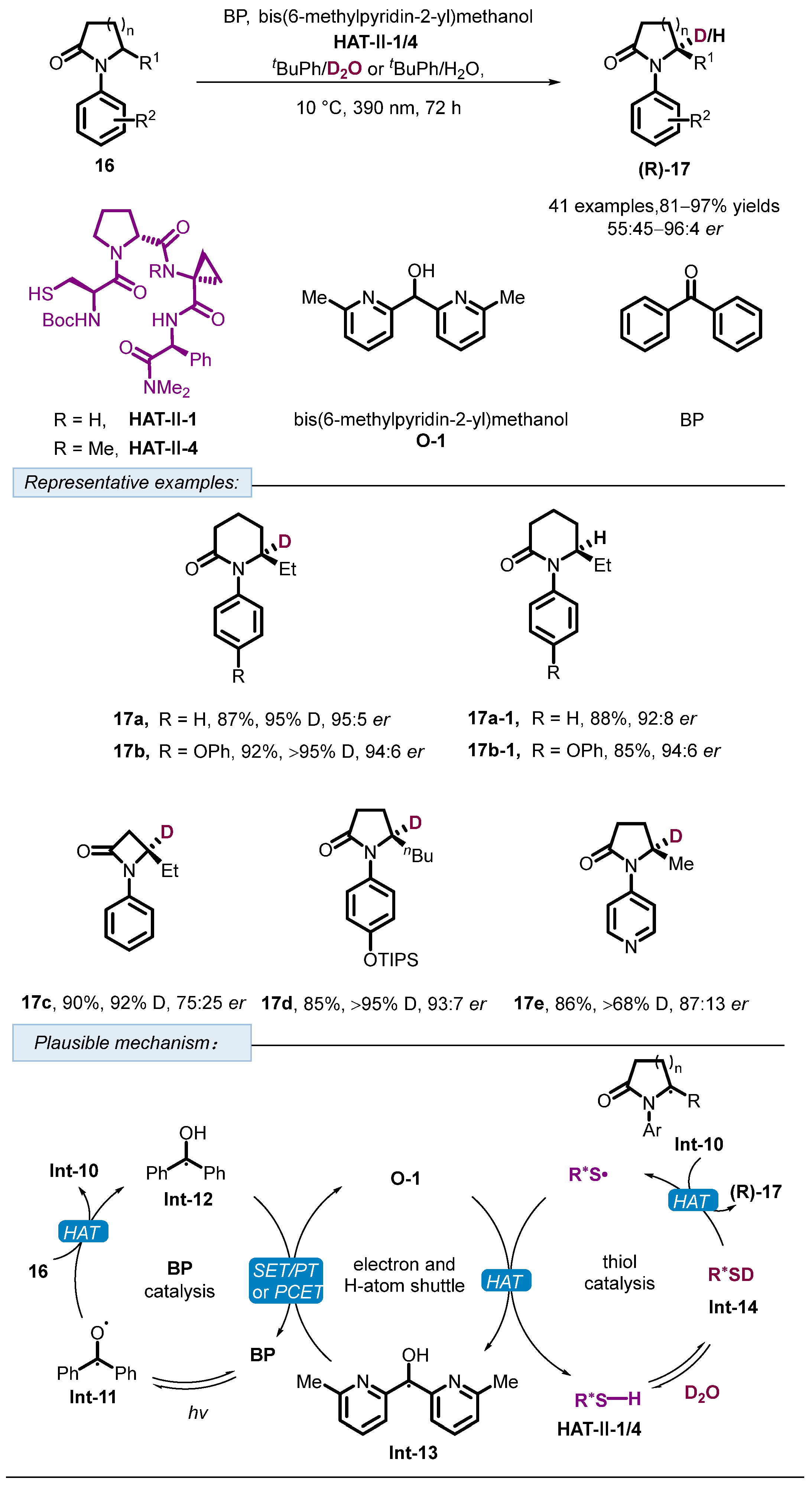
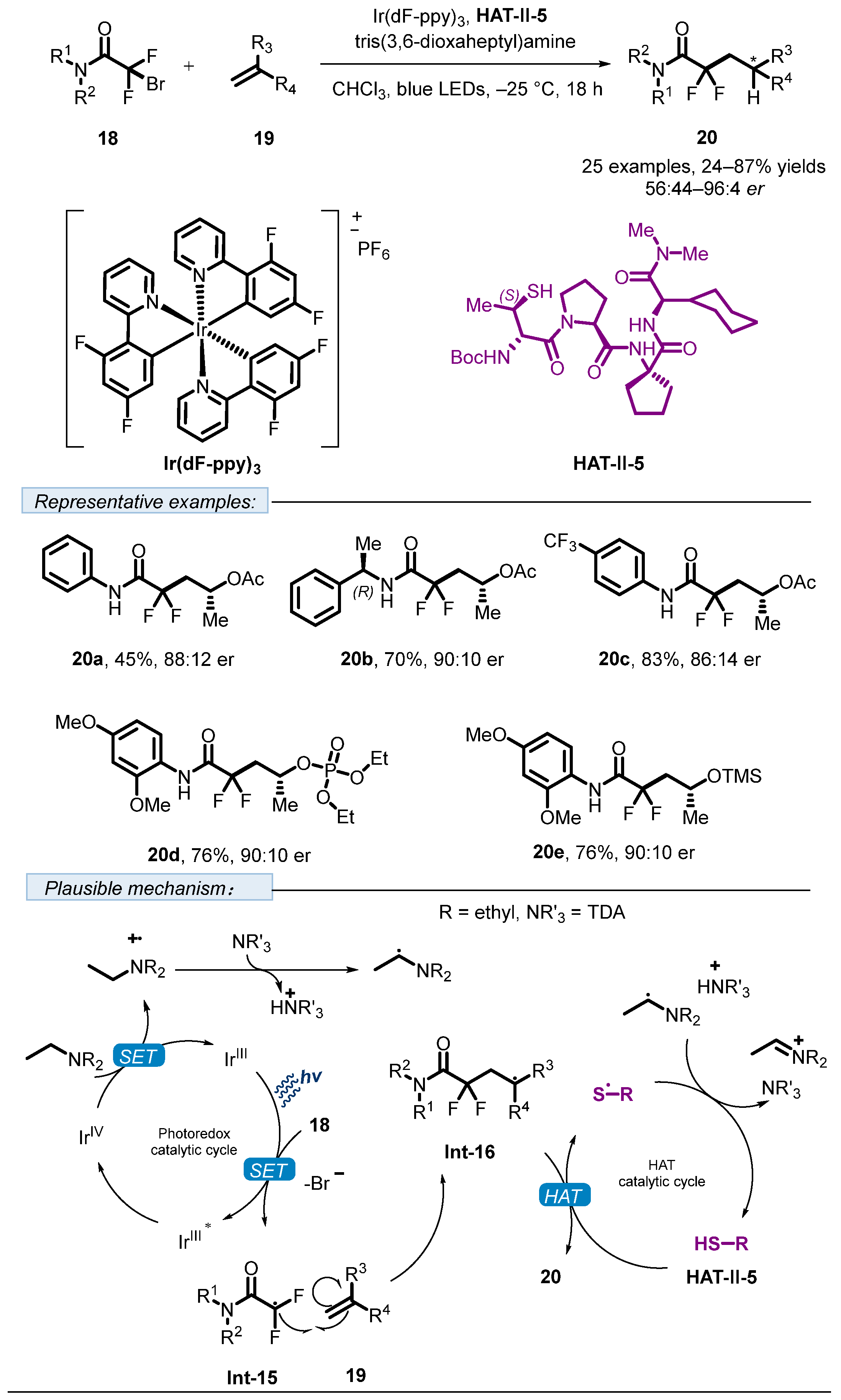
2. Hydrogen Atom Transfer Reagent of Chiral Thiols/Thiophenols
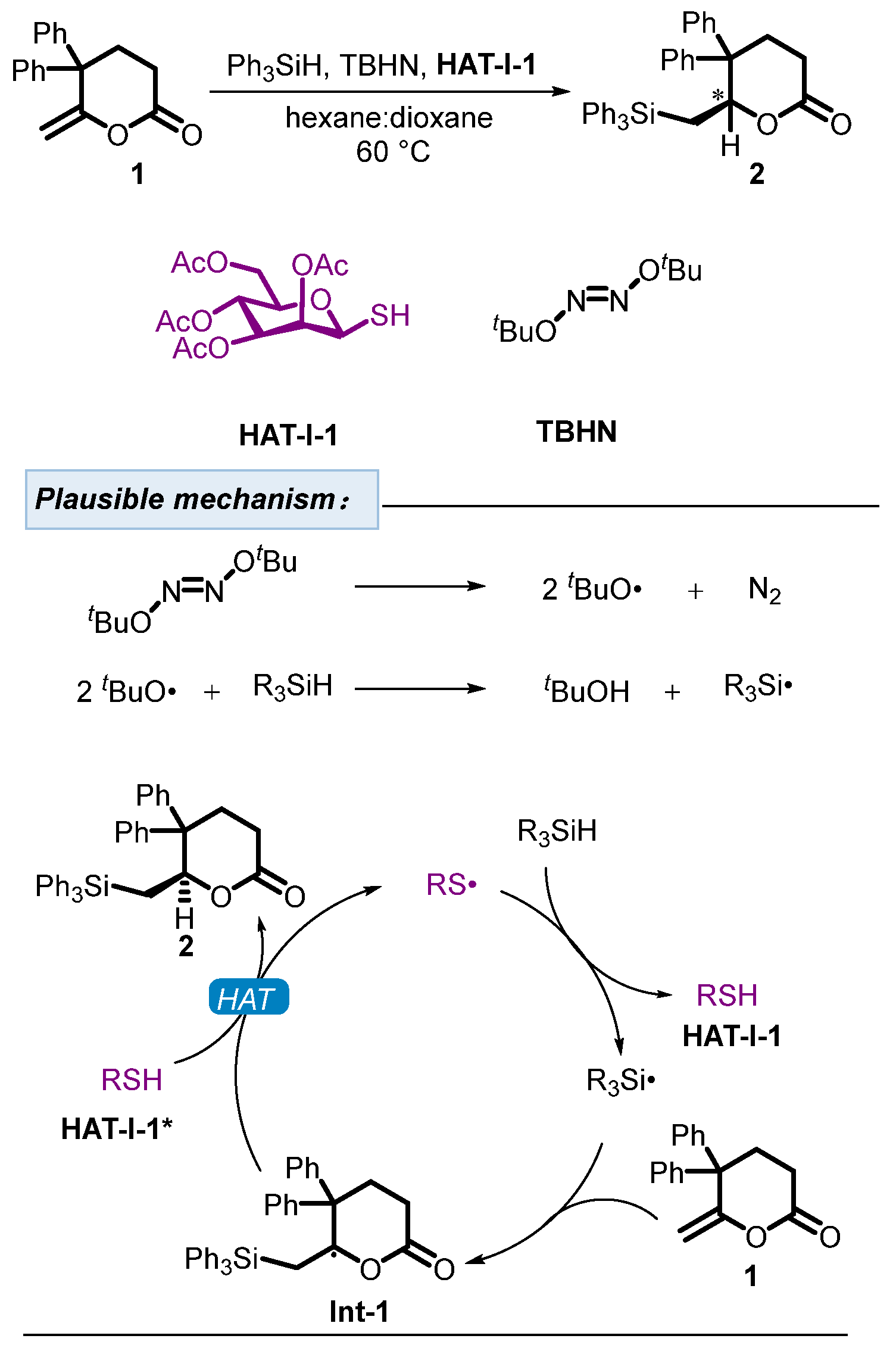
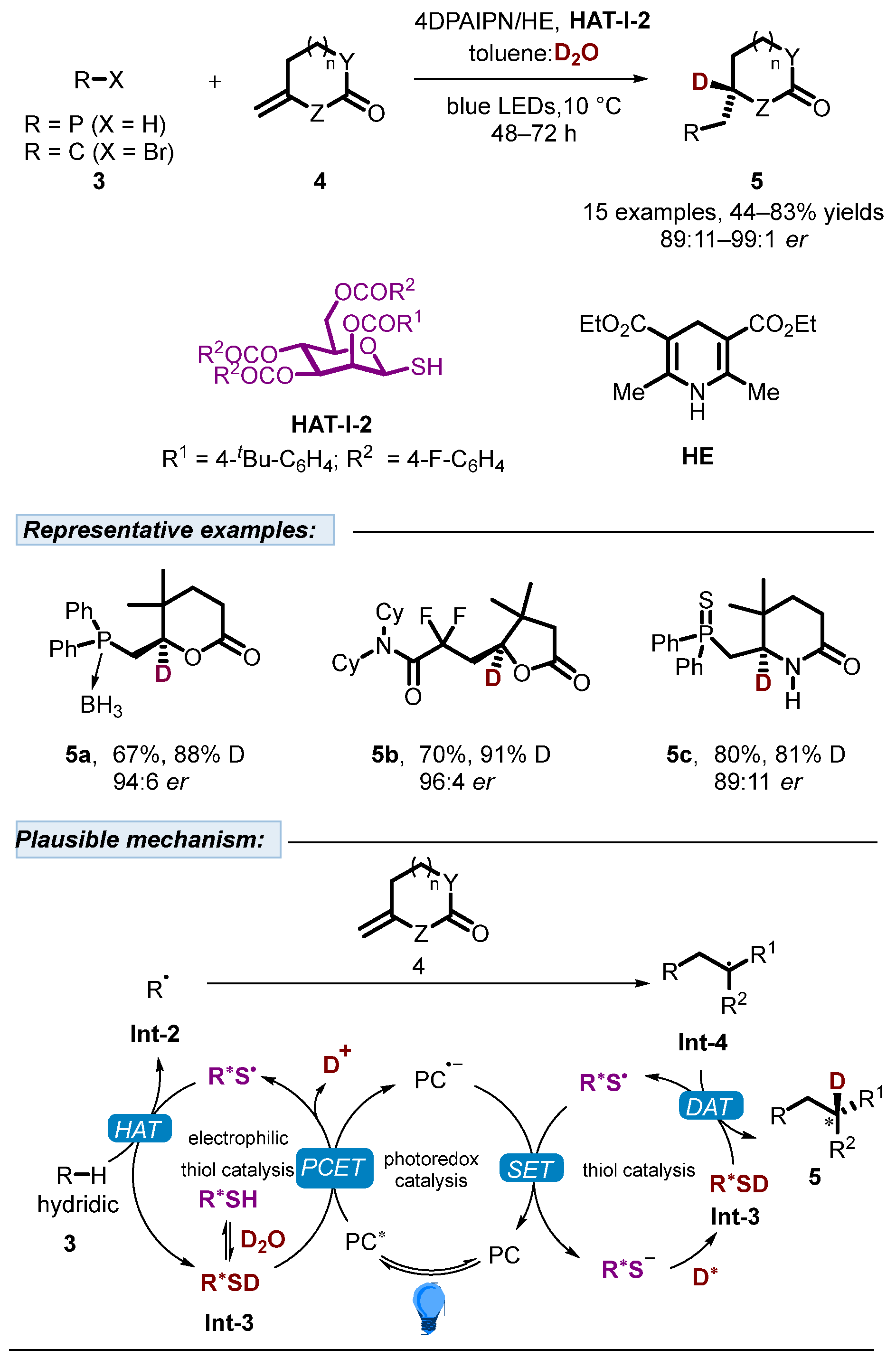
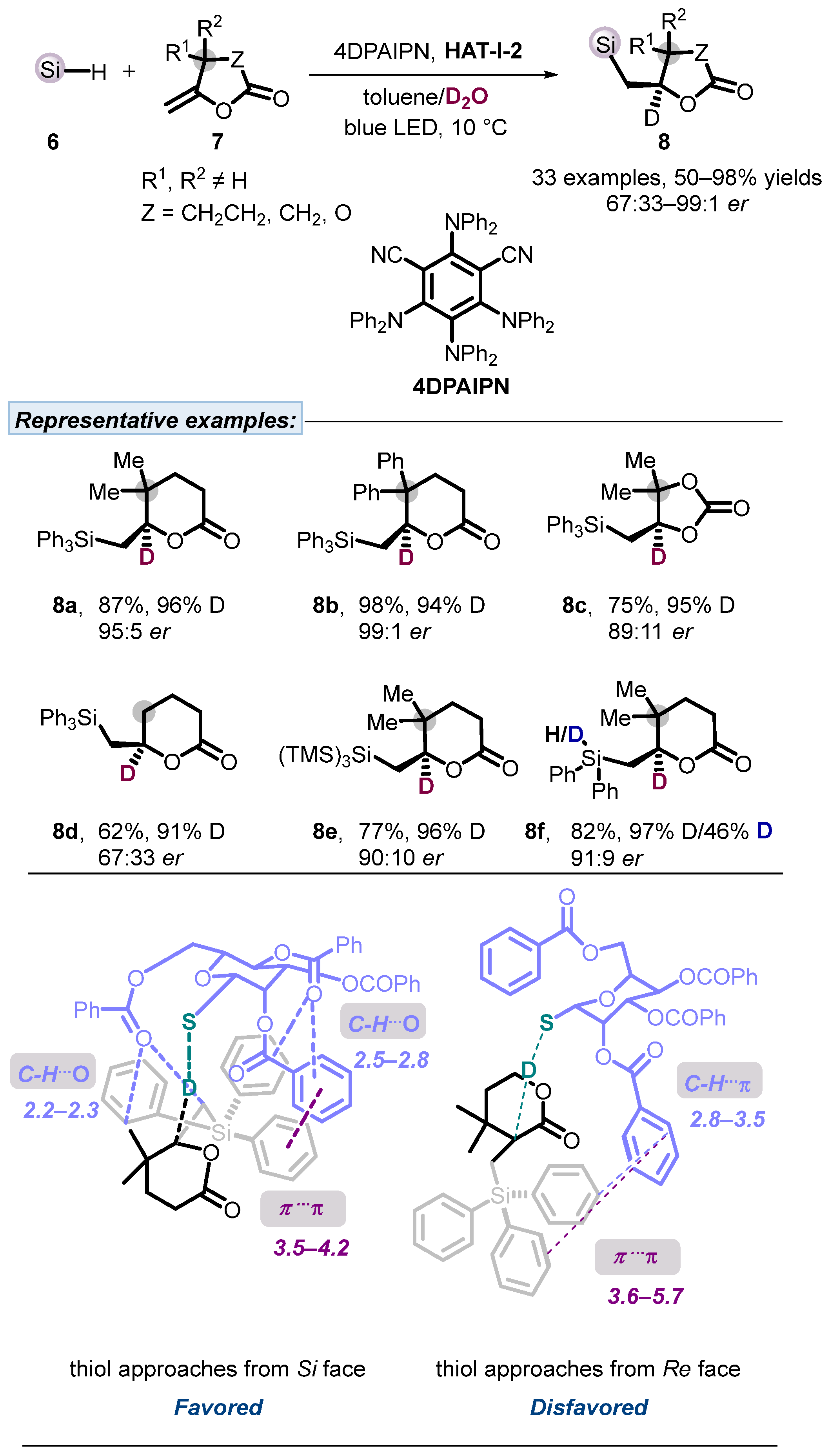
2.1. Hydrogen Atom Transfer Reagent of Sugar-Derived Thiols
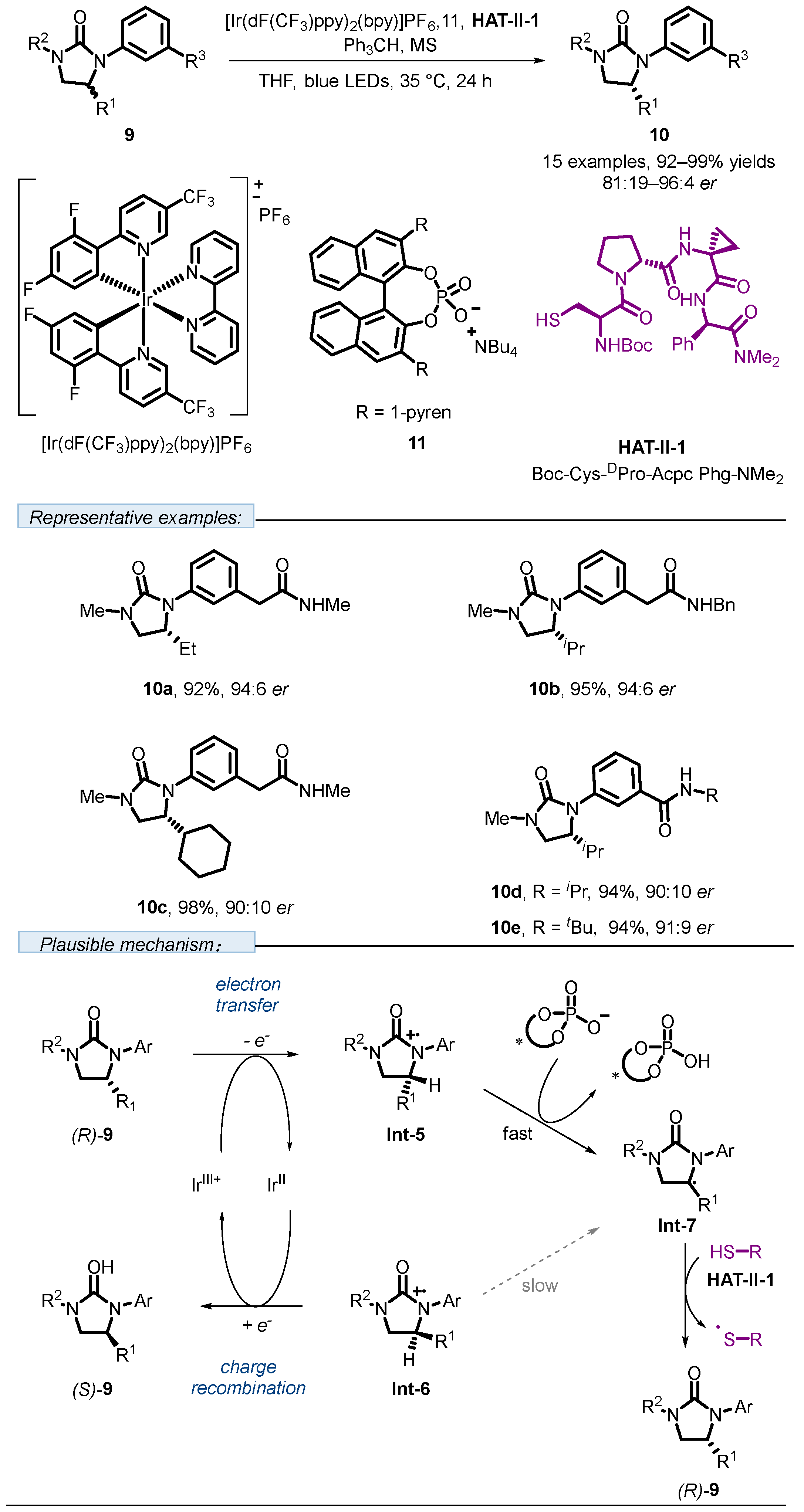
2.2. Hydrogen Atom Transfer Reagent of Peptide-Derived Thiols
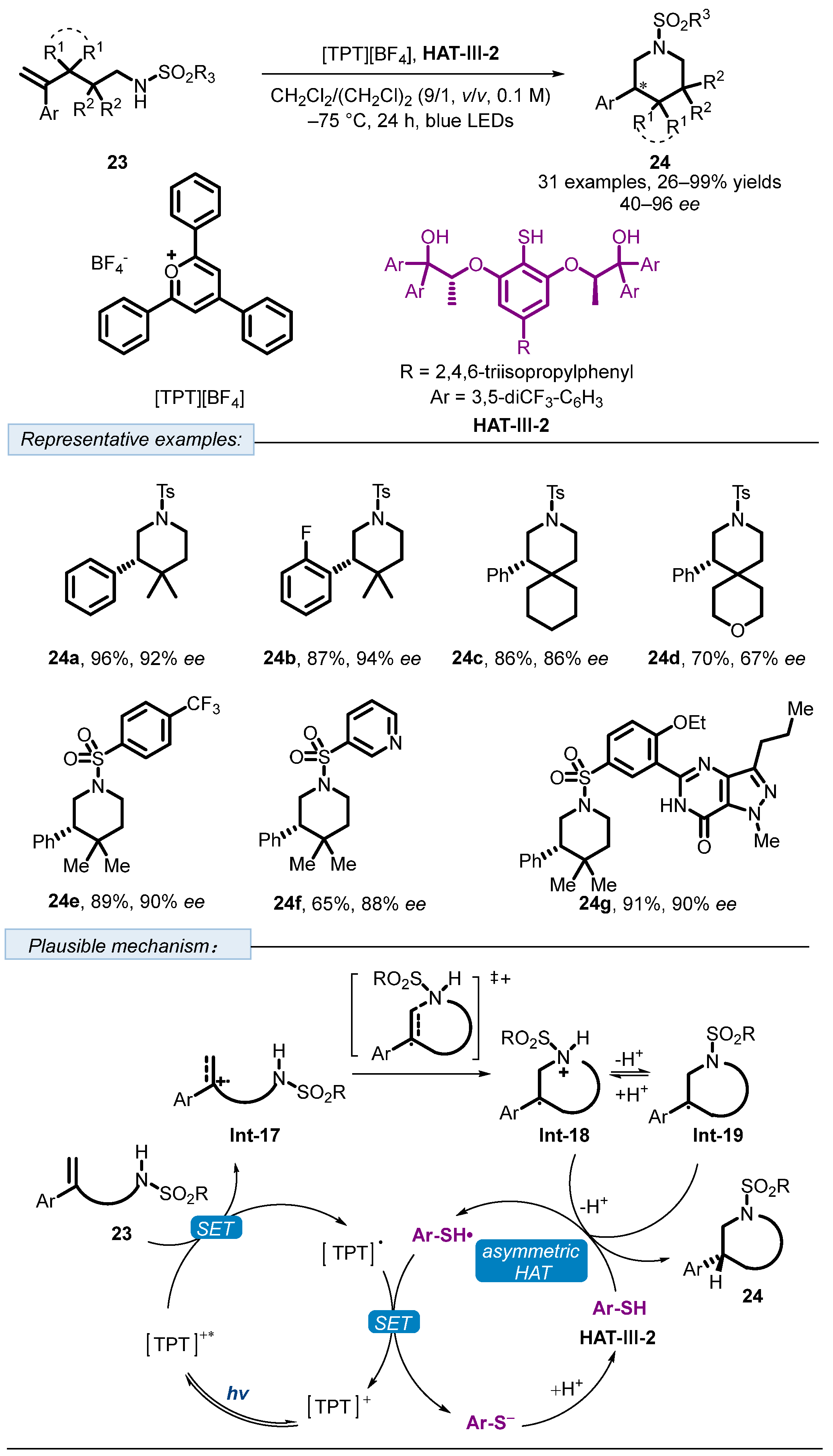
2.3. Hydrogen Atom Transfer Reagent of Chiral Thiophenols
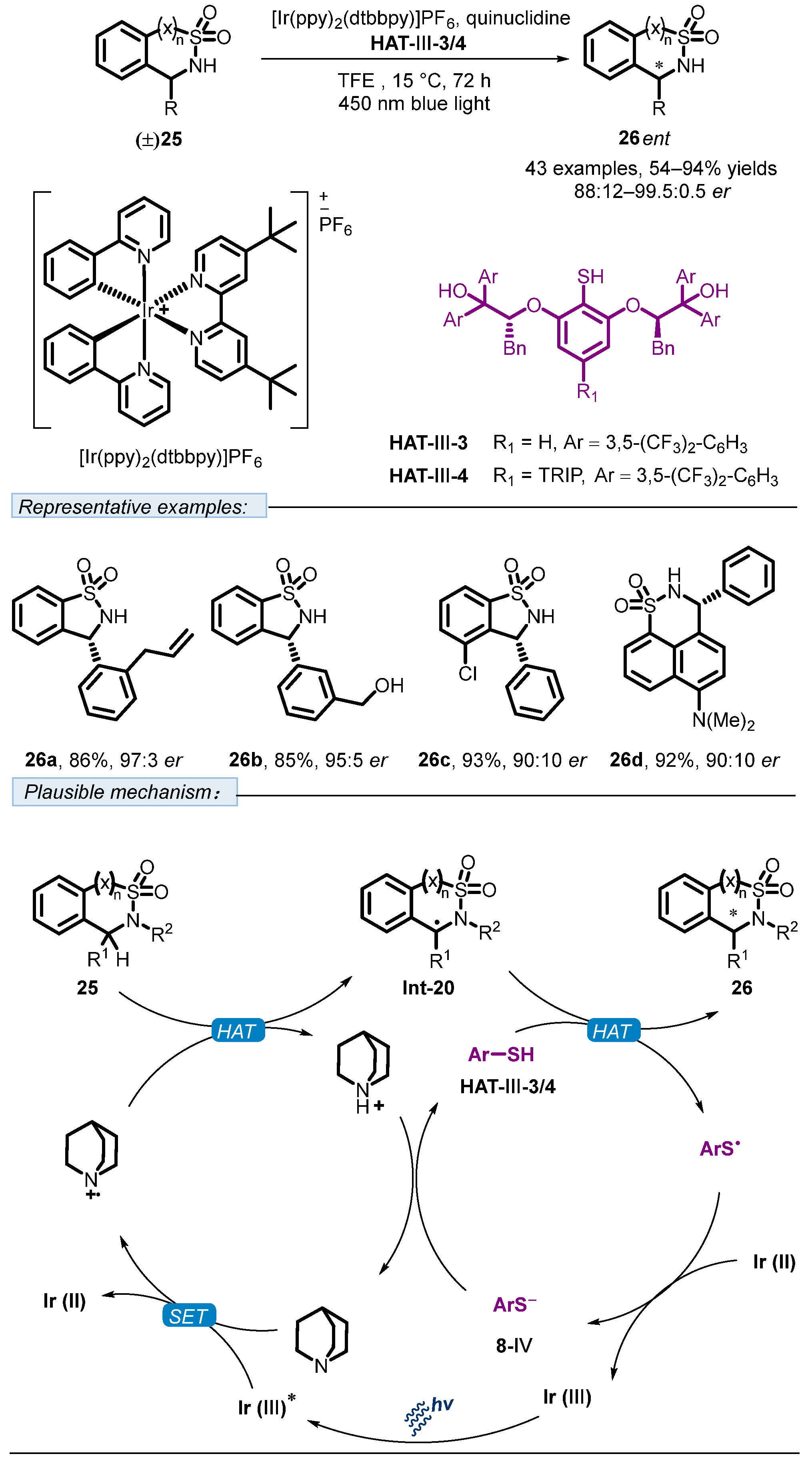
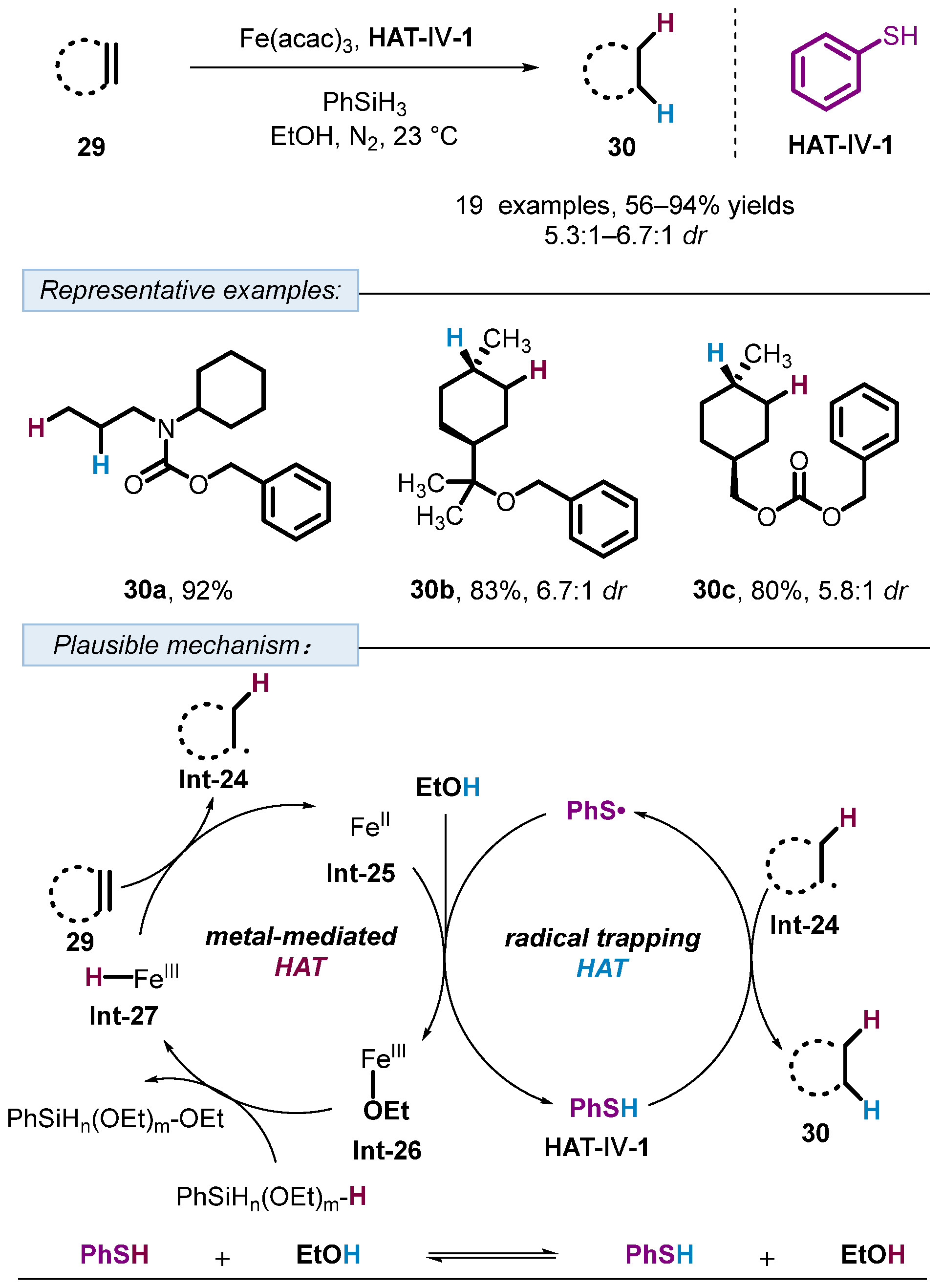
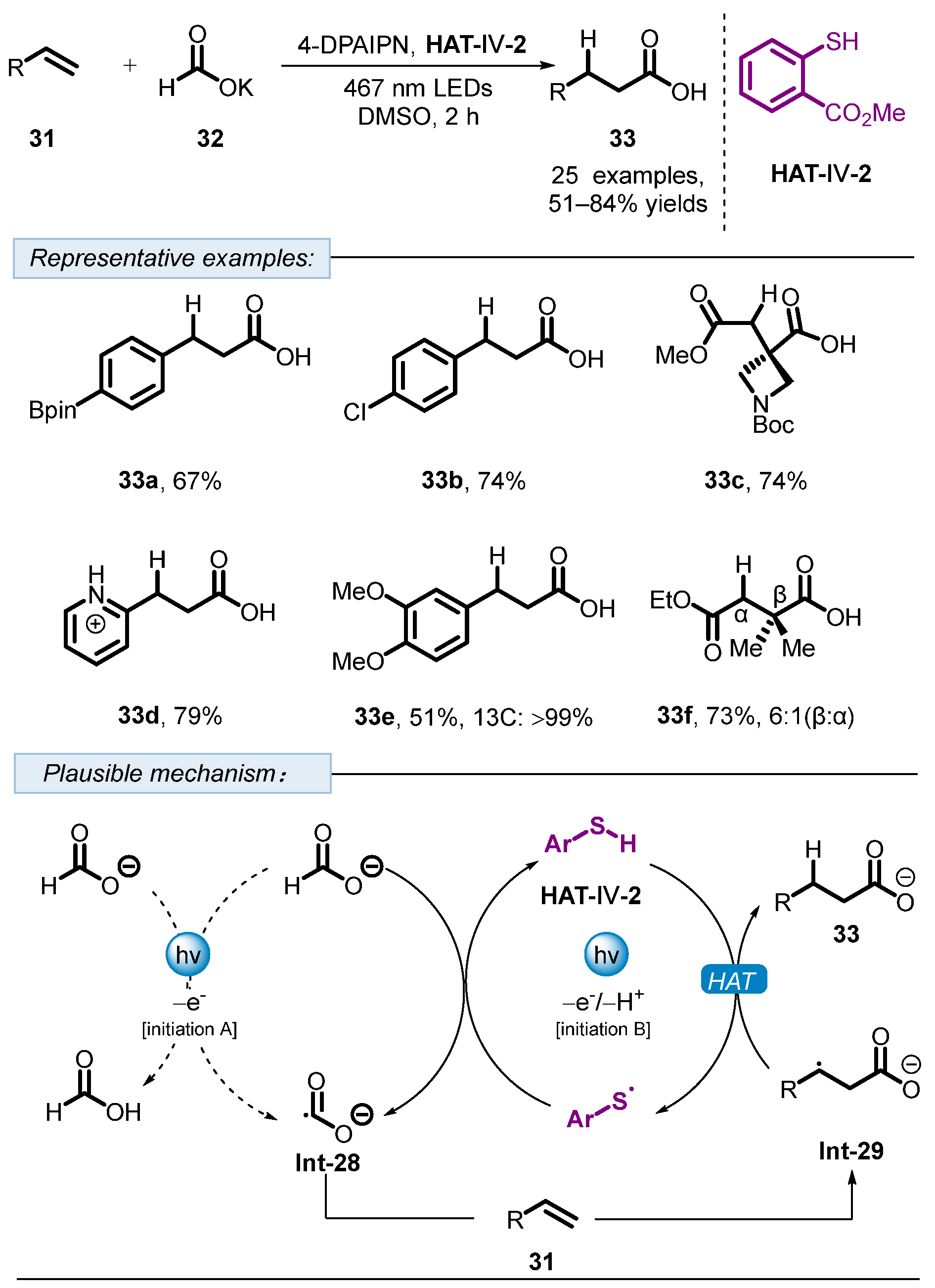
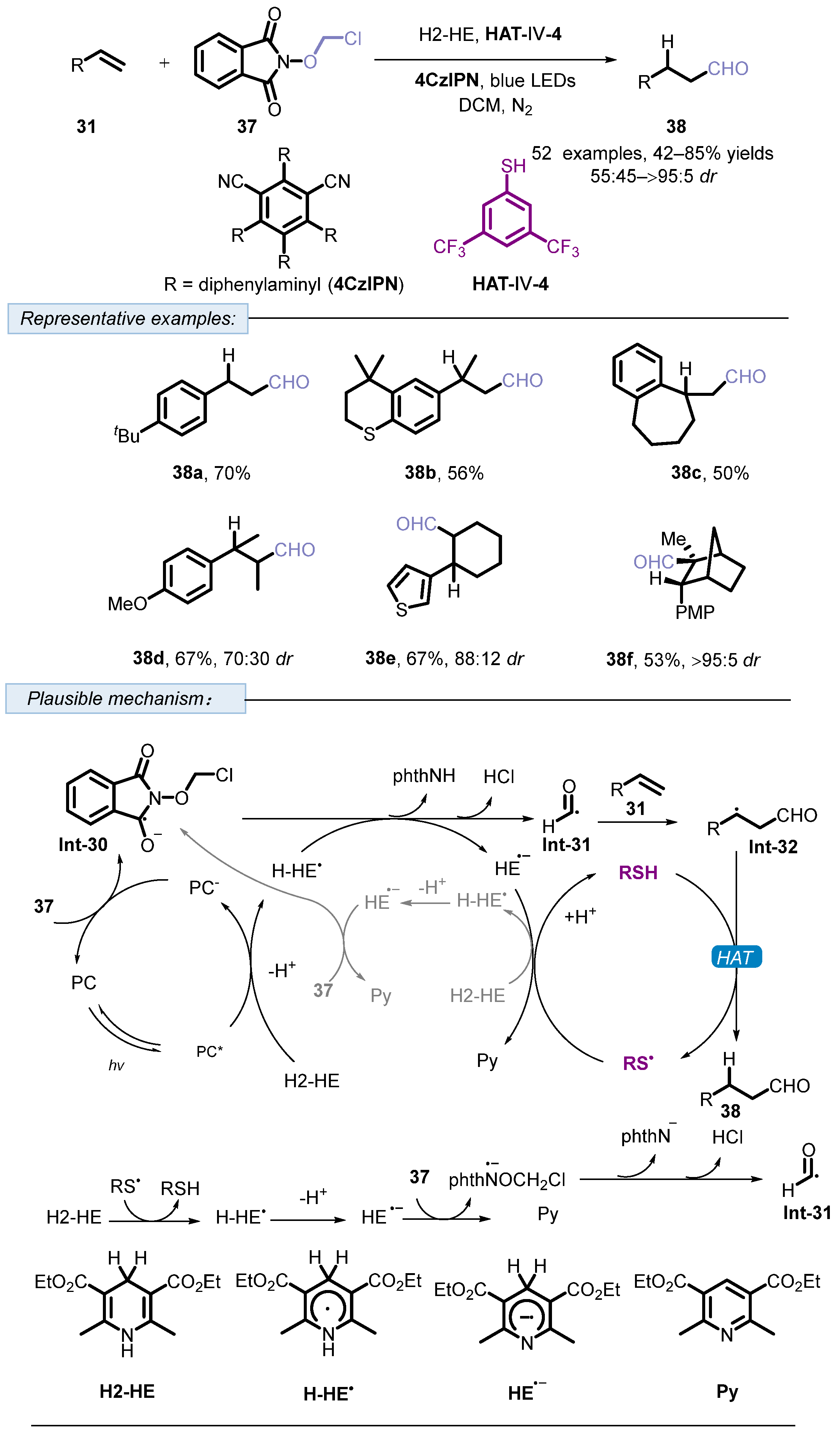
3. Hydrogen Atom Transfer Reagent of Achiral Thiophenols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Photoinduced Intermolecular Hydrogen Atom Transfer Reactions in Organic Synthesis. Chem Catal. 2021, 1, 523–598. [Google Scholar] [CrossRef]
- Li, M.; Harrison, W.; Zhang, Z.; Yuan, Y.; Zhao, H. Remote Stereocontrol with Azaarenes via Enzymatic Hydrogen Atom Transfer. Nat. Chem. 2024, 16, 277–284. [Google Scholar] [CrossRef]
- Subramanian, H.; Sibi, M.P. Stereoselective Hydrogen Atom Transfer in Free Radical Reactions. Asian J. Org. Chem. 2023, 12, e202300175. [Google Scholar] [CrossRef]
- Sandoval, B.A.; Hyster, T.K. Emerging Strategies for Expanding the Toolbox of Enzymes in Biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Darcy, J.W.; Koronkiewicz, B.; Parada, G.A.; Mayer, J.M. A Continuum of Proton-Coupled Electron Transfer Reactivity. Acc. Chem. Res. 2018, 51, 2391–2399. [Google Scholar] [CrossRef]
- Agarwal, R.G.; Coste, S.C.; Groff, B.D.; Heuer, A.M.; Noh, H.; Parada, G.A.; Wise, C.F.; Nichols, E.M.; Warren, J.J.; Mayer, J.M. Free Energies of Proton-Coupled Electron Transfer Reagents and Their Applications. Chem. Rev. 2022, 122, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.M. Understanding Hydrogen Atom Transfer: From Bond Strengths to Marcus Theory. Acc. Chem. Res. 2011, 44, 36–46. [Google Scholar] [CrossRef]
- Warren, J.J.; Tronic, T.A.; Mayer, J.M. Thermochemistry of Proton-Coupled Electron Transfer Reagents and Its Implications. Chem. Rev. 2010, 110, 6961–7001. [Google Scholar] [CrossRef]
- Mao, B.; Yan, J.; Wei, Y.; Shi, M. Hydrogen Atom Transfer Promoted by Carbon-Centered Biradicals via Energy Transfer Catalysis. Acc. Chem. Res. 2025, 58, 2028–2045. [Google Scholar] [CrossRef]
- Chu, J.C.K.; Rovis, T. Complementary Strategies for Directed C(Sp3 )−H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed. 2018, 57, 62–101. [Google Scholar] [CrossRef]
- Galeotti, M.; Salamone, M.; Bietti, M. Electronic Control over Site-Selectivity in Hydrogen Atom Transfer (HAT) Based C(Sp3 )–H Functionalization Promoted by Electrophilic Reagents. Chem. Soc. Rev. 2022, 51, 2171–2223. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, F.-M.; Tu, Y.-Q. Direct Sp3α-C–H Activation and Functionalization of Alcohol and Ether. Chem. Soc. Rev. 2011, 40, 1937. [Google Scholar] [CrossRef]
- Ouyang, J.; Quan, Y. Metal-Organic Framework Catalyzed Hydrogen Atom Transfer for Photocatalytic Organic Synthetic Applications. ChemCatChem 2024, 16, e202400112. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A.K.; Lei, A. Recent Advances in Radical C–H Activation/Radical Cross-Coupling. Chem. Rev. 2017, 117, 9016–9085. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, H.; Shao, T.; Chan, A.S.C.; Meng, S.-S. Mn2(CO)10-Catalyzed Direct Protic Hydrogen Transfer with Unactivated Alkenes. Green Synth. Catal. 2024. [Google Scholar] [CrossRef]
- Zhang, J.; Rueping, M. Metallaphotoredox Catalysis for Sp3 C–H Functionalizations through Hydrogen Atom Transfer (HAT). Chem. Soc. Rev. 2023, 52, 4099–4120. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2017, 2056–2071. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, Y.; Lei, P.; Wang, H.; Yan, Q.; Properzi, R.; Wang, W.; Jing, L.; Chen, F. Visible-Light-Driven Intramolecular Xanthylation of Remote Unactivated C(Sp3)-H Bonds. Green Synth. Catal. 2023, 4, 350–354. [Google Scholar] [CrossRef]
- Liu, L.; Pang, Y.; Ma, C.; Zhou, D.; Zhang, W.; Huang, J.; Sun, J.; Qiu, J.; Liu, Y.; Shen, L.; et al. Direct C(Sp3)-H Functionalization with Thiosulfonates via Photoredox Catalysis. Green Synth. Catal. 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Zhang, T.; Ren, X.; Xu, W.; Wang, B.; Jin, W.; Xia, Y.; Liu, C.; Zhang, Y. Visible-Light-Induced Radical-Cascade Alkylation/Cyclization of N-Methacryloyl-2-Phenylbenzimidazole: Access to Benzoimidazo [2,1-a]Isoquinolin-6(5H)-Ones. Green Synth. Catal. 2024, 5, 319–323. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D.; Fagnoni, M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C–H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Li, W. Photoinduced Hydrogen Atom Transfer Activation of Benzylic C–H Bonds Functionalization. Adv. Synth. Catal. 2025, 367, e202401266. [Google Scholar] [CrossRef]
- Lewis, J.C.; Coelho, P.S.; Arnold, F.H. Enzymatic Functionalization of Carbon–Hydrogen Bonds. Chem. Soc. Rev. 2011, 40, 2003–2021. [Google Scholar] [CrossRef]
- Sandoval, B.A.; Meichan, A.J.; Hyster, T.K. Enantioselective Hydrogen Atom Transfer: Discovery of Catalytic Promiscuity in Flavin-Dependent ‘Ene’-Reductases. J. Am. Chem. Soc. 2017, 139, 11313–11316. [Google Scholar] [CrossRef]
- Huang, X.; Wang, B.; Wang, Y.; Jiang, G.; Feng, J.; Zhao, H. Photoenzymatic Enantioselective Intermolecular Radical Hydroalkylation. Nature 2020, 584, 69–74. [Google Scholar] [CrossRef]
- Bao, X.; Yu, W.; Wang, G. Thiols as Powerful Atom Transfer Catalyst: Opportunities in Photoredox-Mediated Reactions. Adv. Synth. Catal. 2023, 365, 2299–2309. [Google Scholar] [CrossRef]
- Subramanian, H.; Moorthy, R.; Sibi, M.P. Thiyl Radicals: From Simple Radical Additions to Asymmetric Catalysis. Angew. Chem. Int. Ed. 2014, 53, 13660–13662. [Google Scholar] [CrossRef]
- Cai, Y.; Roberts, B.P.; Tocher, D.A. Carbohydrate-Derived Thiols as Protic Polarity-Reversal Catalysts for Enantioselective Radical-Chain Reactions. J. Chem. Soc. Perkin Trans. 2002, 1376–1386. [Google Scholar] [CrossRef]
- Aal E Ali, R.S.; Zhou, Y.; Gong, K.; Jiang, X. Parallel Photoreactor Development with Enhanced Photon Efficiency and Reproducibility Based on Laws of Optics. Green Synth. Catal. 2023, 4, 169–172. [Google Scholar] [CrossRef]
- Davie, E.A.C.; Mennen, S.M.; Xu, Y.; Miller, S.J. Asymmetric Catalysis Mediated by Synthetic Peptides. Chem. Rev. 2007, 107, 5759–5812. [Google Scholar] [CrossRef] [PubMed]
- Metrano, A.J.; Chinn, A.J.; Shugrue, C.R.; Stone, E.A.; Kim, B.; Miller, S.J. Asymmetric Catalysis Mediated by Synthetic Peptides, Version 2.0: Expansion of Scope and Mechanisms. Chem. Rev. 2020, 120, 11479–11615. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, M.; Chang, R.; Ramanathan, D.; Peñin, B.; Funes-Ardoiz, I.; Ye, J. Visible-Light Mediated Catalytic Asymmetric Radical Deuteration at Non-Benzylic Positions. Nat. Commun. 2022, 13, 4453. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, D.; Shi, Q.; Xu, M.; Chang, R.; Peñín, B.; Funes-Ardoiz, I.; Ye, J. Catalytic Asymmetric Deuterosilylation of Exocyclic Olefins with Mannose-Derived Thiols and Deuterium Oxide. Org. Chem. Front. 2023, 10, 1182–1190. [Google Scholar] [CrossRef]
- Kolberg, M. Structure, Function, and Mechanism of Ribonucleotide Reductases. Biochim. Biophys. Acta 2004, 1699, 1. [Google Scholar] [CrossRef]
- Torrents, E.; Aloy, P.; Gibert, I.; Rodríguez-Trelles, F. Ribonucleotide Reductases: Divergent Evolution of an Ancient Enzyme. J. Mol. Evol. 2002, 55, 138–152. [Google Scholar] [CrossRef]
- Shin, N.Y.; Ryss, J.M.; Zhang, X.; Miller, S.J.; Knowles, R.R. Light-Driven Deracemization Enabled by Excited-State Electron Transfer. Science 2019, 366, 364–369. [Google Scholar] [CrossRef]
- Hejna, B.G.; Ganley, J.M.; Shao, H.; Tian, H.; Ellefsen, J.D.; Fastuca, N.J.; Houk, K.N.; Miller, S.J.; Knowles, R.R. Catalytic Asymmetric Hydrogen Atom Transfer: Enantioselective Hydroamination of Alkenes. J. Am. Chem. Soc. 2023, 145, 16118–16129. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Pang, Y.; Zhou, Y.; Chang, R.; Ye, J. Photochemical Deracemization of Lactams with Deuteration Enabled by Dual Hydrogen Atom Transfer. J. Am. Chem. Soc. 2025, 147, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Pinto Pereira Junior, M.V.; Geunes, E.P.; Shao, H.; Zhang, Y.; Cheng, J.; Magpantay, S.V.; Mercado, B.Q.; Mayer, J.M.; Houk, K.N.; Knowles, R.R.; et al. Enantioselective Hydrodifluoroalkylation of Alkenes with Conformationally Tuned Peptidyl Hydrogen Atom Transfer Catalysts. J. Am. Chem. Soc. 2025, 147, 11412–11424. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kawamata, Y.; Maruoka, K. An Organic Thiyl Radical Catalyst for Enantioselective Cyclization. Nat. Chem. 2014, 6, 702–705. [Google Scholar] [CrossRef]
- Tang, L.; Shen, C.; Hao, S.; Dong, K. A Type of Chiral C2 -Symmetric Arylthiol Catalyst for Highly Enantioselective Anti-Markovnikov Hydroamination. J. Am. Chem. Soc. 2024, 146, 16248–16256. [Google Scholar] [CrossRef]
- Dai, L.; Shen, C.; Wang, J.; Li, Y.; Dong, K. Visible Light-Driven Deracemization of Cyclic Sulfonamides by Quinuclidine and Chiral Arylthiol Catalysis. Angew. Chem. Int. Ed. 2025, 137, e202505719. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, C.; Dong, K. Enantioselective Decarboxylative Hydrogen-Atom Transfer Reaction. J. Am. Chem. Soc. 2025, 147, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Kattamuri, P.V.; West, J.G. Hydrogenation of Alkenes via Cooperative Hydrogen Atom Transfer. J. Am. Chem. Soc. 2020, 142, 19316–19326. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chao, E.; Olivier, W.J.; Tilby, M.J.; Leonori, D. Photocatalytic Hydrogenation of Alkenes Using Ammonia-Borane. Chem 2025. [Google Scholar] [CrossRef]
- Bian, K.-J.; Nemoto, D.; Chen, Y.; Lu, Y.-C.; Kao, S.-C.; Chen, X.-W.; Martí, A.A.; West, J.G. Anti-Markovnikov Hydro- and Deuterochlorination of Unsaturated Hydrocarbons Using Iron Photocatalysis. Nat. Synth. 2025, 4, 314–326. [Google Scholar] [CrossRef]
- Bian, K.-J.; Yu, S.; Chen, Y.; Liu, Q.; Chen, X.; Nemoto, D.; Kao, S.-C.; Martí, A.A.; West, J.G. Photocatalytic Anti-Markovnikov Hydro- and Haloazidation of Alkenes. Nat. Commun. 2025, 16, 7906. [Google Scholar] [CrossRef]
- Campbell, M.W.; Polites, V.C.; Patel, S.; Lipson, J.E.; Majhi, J.; Molander, G.A. Photochemical C–F Activation Enables Defluorinative Alkylation of Trifluoroacetates and -Acetamides. J. Am. Chem. Soc. 2021, 143, 19648–19654. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, N.; Shang, R. Photocatalytic Defluoroalkylation and Hydrodefluorination of Trifluoromethyls Using O-Phosphinophenolate. Nat. Commun. 2022, 13, 354. [Google Scholar] [CrossRef]
- Ye, J.; Bellotti, P.; Heusel, C.; Glorius, F. Photoredox-Catalyzed Defluorinative Functionalizations of Polyfluorinated Aliphatic Amides and Esters. Angew. Chem. Int. Ed. 2022, 61, e202115456. [Google Scholar] [CrossRef]
- Alektiar, S.N.; Wickens, Z.K. Photoinduced Hydrocarboxylation via Thiol-Catalyzed Delivery of Formate Across Activated Alkenes. J. Am. Chem. Soc. 2021, 143, 13022–13028. [Google Scholar] [CrossRef] [PubMed]
- Wayner, D.D.M.; Clark, K.B.; Rauk, A.; Yu, D.; Armstrong, D.A. C−H Bond Dissociation Energies of Alkyl Amines: Radical Structures and Stabilization Energies. J. Am. Chem. Soc. 1997, 119, 8925–8932. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wu, L.; Zhang, W.; Chen, P.; Lin, Z.; Liu, G. Site-Specific Allylic C–H Bond Functionalization with a Copper-Bound N-Centred Radical. Nature 2019, 574, 516–521. [Google Scholar] [CrossRef]
- Lei, H.; Rovis, T. A Site-Selective Amination Catalyst Discriminates between Nearly Identical C–H Bonds of Unsymmetrical Disubstituted Alkenes. Nat. Chem. 2020, 12, 725–731. [Google Scholar] [CrossRef]
- Shen, Y.; Funez-Ardoiz, I.; Schoenebeck, F.; Rovis, T. Site-Selective α-C–H Functionalization of Trialkylamines via Reversible Hydrogen Atom Transfer Catalysis. J. Am. Chem. Soc. 2021, 143, 18952–18959. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, P.; Dong, X.; Lan, Y.; Chen, Y. Thiophenol-Catalyzed Radical Hydroformylation of Unactivated Sterically Hindered Alkenes. J. Am. Chem. Soc. 2025, 147, 31662–31670. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Liao, Y.; Guo, H.; Wang, M. Emerging Applications of Thiol-Based Catalysts in Hydrogen Atom Transfer Reactions: A Comprehensive Review. Molecules 2025, 30, 4058. https://doi.org/10.3390/molecules30204058
Yang H, Liao Y, Guo H, Wang M. Emerging Applications of Thiol-Based Catalysts in Hydrogen Atom Transfer Reactions: A Comprehensive Review. Molecules. 2025; 30(20):4058. https://doi.org/10.3390/molecules30204058
Chicago/Turabian StyleYang, Hao, Yanyan Liao, Hao Guo, and Ming Wang. 2025. "Emerging Applications of Thiol-Based Catalysts in Hydrogen Atom Transfer Reactions: A Comprehensive Review" Molecules 30, no. 20: 4058. https://doi.org/10.3390/molecules30204058
APA StyleYang, H., Liao, Y., Guo, H., & Wang, M. (2025). Emerging Applications of Thiol-Based Catalysts in Hydrogen Atom Transfer Reactions: A Comprehensive Review. Molecules, 30(20), 4058. https://doi.org/10.3390/molecules30204058






