1. Introduction
The world’s largest energy source is fossil fuels (oil, gas, and coal). Hydrocarbons and their derivatives are their primary components, with advantages of high energy content, high density, and reasonable cost. However, their use produces GHG emissions, exacerbating global warming, and climate change [
1]. This emphasizes the need for alternative energy sources to meet global energy demands and reduce environmental impact. Multiple renewable energy resources, like wind, solar, hydropower, biomass, and biofuels, can substitute fossil fuels to mitigate their negative consequences. Among them, biofuels (especially biodiesel) have become prominent due to their sustainable and biodegradable features, compatibility with existing energy frameworks, and ability to reduce carbon emissions. Biodiesel, referring to fatty acid alkyl esters (FAAEs), is more viscous and dense than diesel [
2,
3]. Furthermore, biodiesel fuels are mainly made up of saturated and unsaturated alkyl esters with carbon chains of 12 or more atoms in length compounds derived from plant oils or animal fats via the esterification process [
4,
5,
6]. Consequently, the recent large-scale adoption of biodiesel has motivated extensive research initiatives to acquire a systematic understanding of the chemistry underlying biodiesel combustion. For the purpose of understanding fuel combustion (a complex physical and chemical process that includes turbulence, chemical reactions, thermal radiation, and heat transfer), researchers commonly construct comprehensive combustion mechanisms to aid in grasping the combustion process of biodiesel fuels.
The comprehensive combustion mechanism of fuels is inherently complex, typically encompassing thousands of elementary reactions and involving hundreds of distinct chemical species. As the count of carbon atoms present in the fuel molecules increases, there is a corresponding and significant rise in both the numerical value of species and the intricacy of the reactions involved. When it comes to constructing comprehensive reaction mechanisms for alkanes, a systematic approach is generally employed, utilizing automatic mechanism generation programs as referenced in sources. These automatically generated mechanisms are composed of two primary sections: the first section is a reaction base, which encompasses all the reactions that involve small radicals or molecules. A common example of this is the C0~C4 reaction base, which includes reactions of species with up to four carbon atoms. The second section deals with reactions involving larger species. This part is constructed by classifying reactions into distinct reaction classes that are founded on the specific characteristics of the reaction center. A rate rule is systematically applied to each of these classes, serving to assign appropriate rate coefficients to the reactions within the respective class and ensuring the kinetics of the overall mechanism are accurately represented. This dual-component approach allows for a structured and efficient generation of reaction mechanisms, accommodating the vast complexity inherent in the combustion processes of fuels [
7,
8].
The few models [
9,
10] available for ester oxidation have been founded on the same reaction classes as alkanes. Molecular reactions are the only reaction class specific to esters. Detailed chemical kinetic mechanisms for fatty acid alkyl esters (FAAEs) often lack accurate thermo-kinetic parameters for critical unimolecular decomposition pathways, such as alkene elimination, due to the scarcity of experimental data. High-level theoretical calculations provide a powerful alternative to fill this data gap. For instance, Metcalfe et al. [
9] and Schwartz et al. [
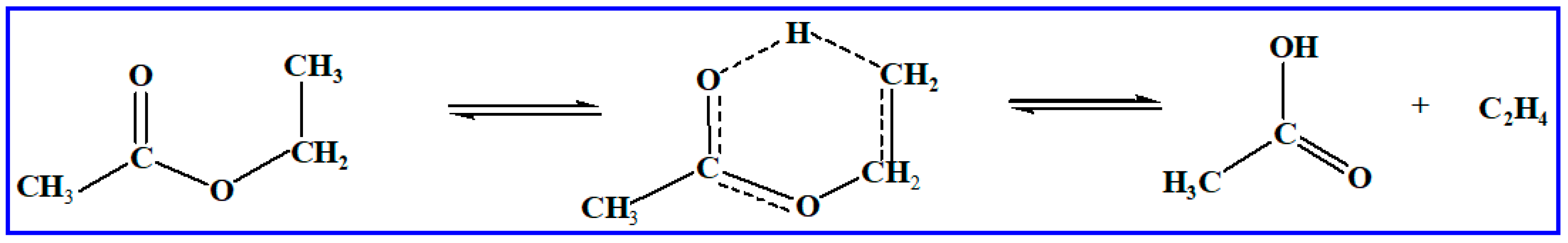
11] proved that the unimolecular alkene elimination reaction (involving a six-membered transition state and producing ethylene and an acid molecule formation) holds importance for ethyl esters. The reaction processes are listed in
Scheme 1. In prior work, several studies have focused on methyl butanoate. As an example, Truhlar et al. [
12] investigated the kinetics of hydrogen abstraction from methyl butanoate by H atoms, while Dibble et al. [
13] explored the isomerization reactions of methyl butanoate. Metcalfe et al. [
9] studied the unimolecular alkene elimination for ethyl propanoate producing propanoic acid and ethylene based on the experiment. However, the rate coefficients for large-size molecules of unimolecular alkene elimination reaction for fatty acid alkyl esters (FAAEs) are scarce. High-level theoretical methods, such as the CCSD(T) and QCISD(T) [
14,
15] methods, are only applied to the small-size systems, and the kinetic parameters corresponding to large-size molecules are usually approximately estimated from similar reaction classes in alkanes. Therefore, a promising method should be proposed to solve this problem.
The unimolecular alkene elimination reaction is a crucial class of decomposition pathways in the reaction mechanism of biodiesel. Within this work, 55 reactions for the FAAEs unimolecular alkene elimination reaction class are chosen, with arrangement based on molecular size in ascending order. The reactions are presented in
Table 1, wherein Reaction R1 is chosen as the reference reaction, and the other reactions within the class are designated as target reactions.
The objectives of this study are as follows: (1) Providing accurate energy barriers, reaction enthalpies, high-pressure-limit rate coefficients, and rate rules for unimolecular alkene elimination reaction of large-size fatty acid alkyl esters (FAAEs), which are usually calculated via analogy to alkanes. (2) Providing the quantitative and systematic influence of alkyl chain architecture—specifically its length and degree of branching—on the energy barriers and rate coefficients. This study aims to establish a comprehensive and theoretically consistent set of high-pressure-limit rate parameters for alkene elimination reaction class of FAAEs. The data presented here are intended to serve as a reliable reference for future kinetic model development and to enhance the predictive understanding of biodiesel combustion, rather than to replicate past experimental measurements. The findings from this study are expected to yield transferable insights valuable for both fundamental chemical knowledge and applied fields such as fuel design and molecular engineering.
3. Methods
All B3LYP level electronic structure calculations were carried out with the BDF software 2025A [
18,
19,
20,
21,
22], whereas high-level CCSD method and G4 method calculation were conducted via the Gaussian 16 software [
23]. The B3LYP hybrid density functional, along with the 6-311G(d, p) basis set, was utilized to optimize the geometries of all species (reactants, transition states, and products) and as the low-level ab initio method within the isodesmic reaction correction scheme. The Gaussian-4 (G4) composite method serves as the high-level ab initio method within the isodesmic reaction correction scheme. It should be note that the G4 energies for transition states were obtained via single-point calculations performed on the fixed geometries optimized at the B3LYP/6-311G(d,p) level. For esters with long alkyl chains, structures were initialized in an extended conformation. Given the localized nature of the reaction, the energetics are expected to be insensitive to the conformation of the carbon chain. The transition states were located using the standard TS (transition state) optimization method. Intrinsic reaction coordinate (IRC) analyses [
16,
24] were conducted for all transition states to confirm that each correctly connects the reactants and products as the appropriate minima. Analytical harmonic frequency calculations were conducted at the B3LYP/6-311G(d,p) level to verify the presence of transition states with precisely one imaginary frequency, and other stationary geometries correspond to true local minimum with no imaginary frequencies. The harmonic frequencies were scaled by a factor of 0.97 for the calculation of thermodynamic properties [
25]. All thermodynamic calculations were performed under the ideal gas approximation. The atomization method was used to calculate the standard enthalpies of formation Δ
fH
θ(298 K), and the experimental Δ
fH
θ(0 K) values for C (169.98 kcal·mol
−1), H (51.63 kcal·mol
−1), and O (58.99 kcal·mol
−1) were utilized [
26].
In the present work, the rate coefficients at the high-pressure-limit were calculated via the reaction class-transition state theory with the ChemRate program 1.5.8 [
27]. The Eckart method [
28] was utilized to account for the quantum mechanical tunneling effect. For traditional transition state theory [
29], the rate coefficients can be expressed as follows:
where
k is the tunneling coefficient; σ is the reaction symmetry number [
30];
kB is Boltzmann constant;
T is temperature;
h is the Planck constant;
Q≠ and
QR are the total partition functions (per unit volume) of the transition state and the reactant, respectively, including translational, rotational, vibrational contributions; Δ
V≠ is the energy barrier, i.e., the difference in the electronic energies between the transition state and the reactant; and
R is the ideal gas constant.
As usual, for small-size molecules (including C0~C4), a combination of high-level ab initio methods and traditional transition state theory is employed to obtain the rate coefficients. The reaction class-transition state theory (RC-TST), developed by Truong et al. [
31,
32,
33], has been introduced into the dynamics calculations for large-size molecules. The reaction class-transition state theory, which builds on traditional transition state theory (TST), posits that all reactions within the same reaction class share the same reaction center, meaning there are certain similarities in the potential energy surface along the reaction coordinate. In RC-TST, a small-sized system within the reaction class is typically selected as the reference reaction, while other reactions in the class serve as target reactions. Then, the rate coefficients for reference reaction can be calculated from first principles using an accurate dynamical theory, with potential energy information computed from a sufficiently high-level of electronic structure theory, and the rate coefficients of target reactions, calculated using a relatively low-level ab initio method, can be corrected to yield results comparable to those from high-level ab initio calculations. Wang [
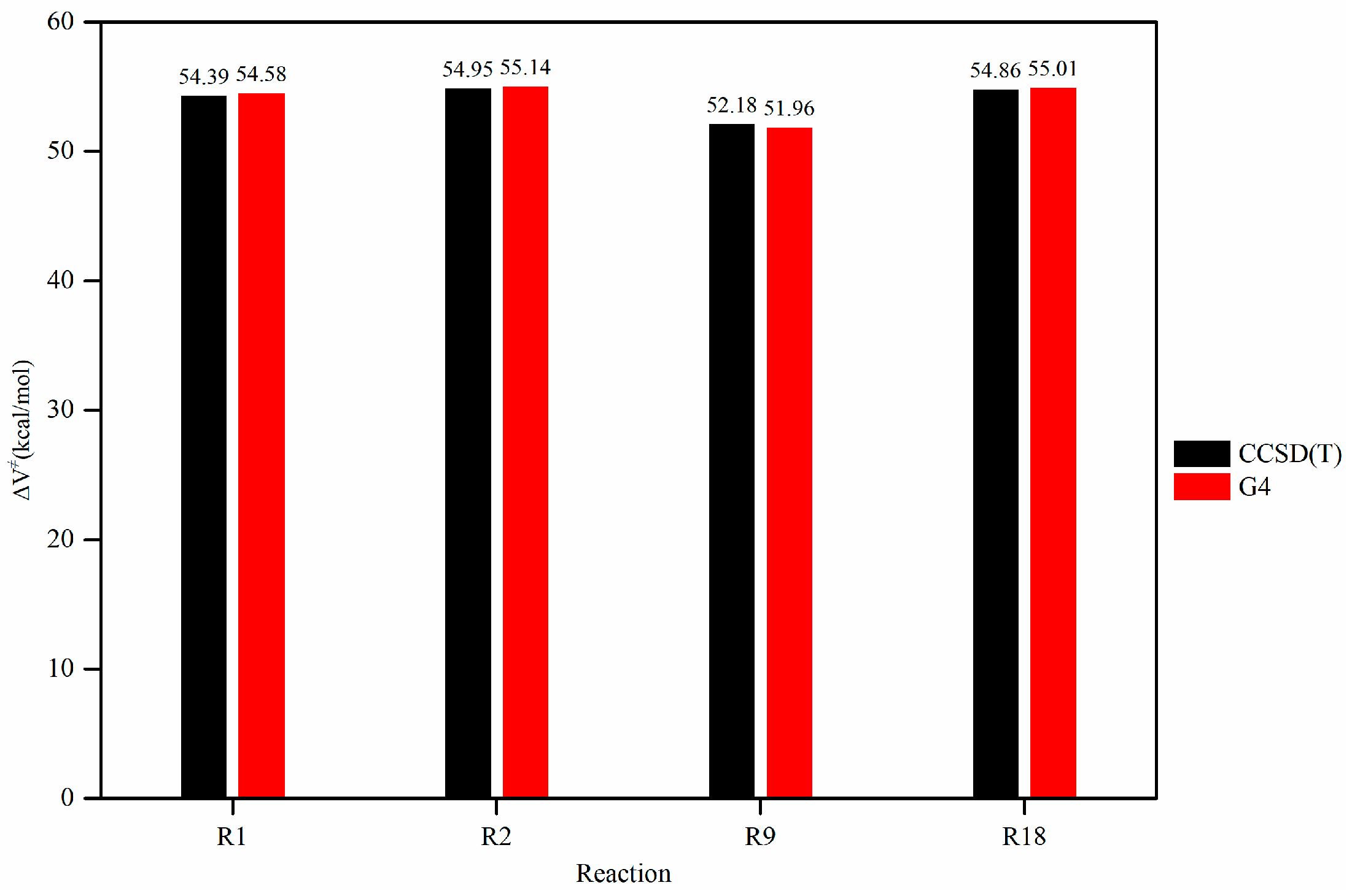
34] and collaborators also provided a clear explanation of this method, which is called the isodesmic reaction method. For the isodesmic reaction method, the selection of an appropriate reference reaction is guided by three key criteria: (1) allowing for energy barrier and rate coefficient calculations at a high-level of theory; (2) ensuring that the reaction center contains the nature of the entire reaction process; and (3) the availability of reliable experimental data for validation. Based on these criteria, the reaction R1 from
Table 1 was chosen as the reference for this study.
In the isodesmic reaction method, the reference reaction and the target reaction, in the process of forming their respective transition states, are defined as follows:
Herein,
and
are the energy barriers for the reference reaction (2) and the target reaction (3), respectively. According to the isodesmic reaction method, the difference between two reactions within a reaction class can also be regarded as an isodesmic reaction, such as reaction (4).
Here,
represents the energy barriers as per the isodesmic reaction method. The values calculated using different ab initio methods exhibit little dependence on the theoretical level, primarily owing to the systematic cancelation of errors stemming from inadequate handling of electron correlation and incompleteness basis sets. The difference in energy barriers for the reference reaction between a low-level (B3LYP) method and a high-level (G4) method can then be expressed as Equation (5) for reference reaction, while Equation (6) (referred to as the isodesmic reaction correction scheme) enables the obtainment of a high-precision energy barrier from low-level ab initio calculations for target reactions. Similarly, accurate reaction enthalpies for target reactions can be derived using Equation (8).
In accordance with the isodesmic reaction correction scheme, the accurate rate coefficients
k′ for target reactions can be obtained by adjusting the approximate rate coefficients
k at a low ab initio level for target reactions using the expression below:
where
k is the rate coefficients for target reaction calculated at the low-level (B3LYP) ab initio method, and
k′ is the corrected rate coefficients for the target reaction within the isodesmic reaction method.
is the correction scheme for energy barriers from the reference reaction in Equation (5).
4. Conclusions
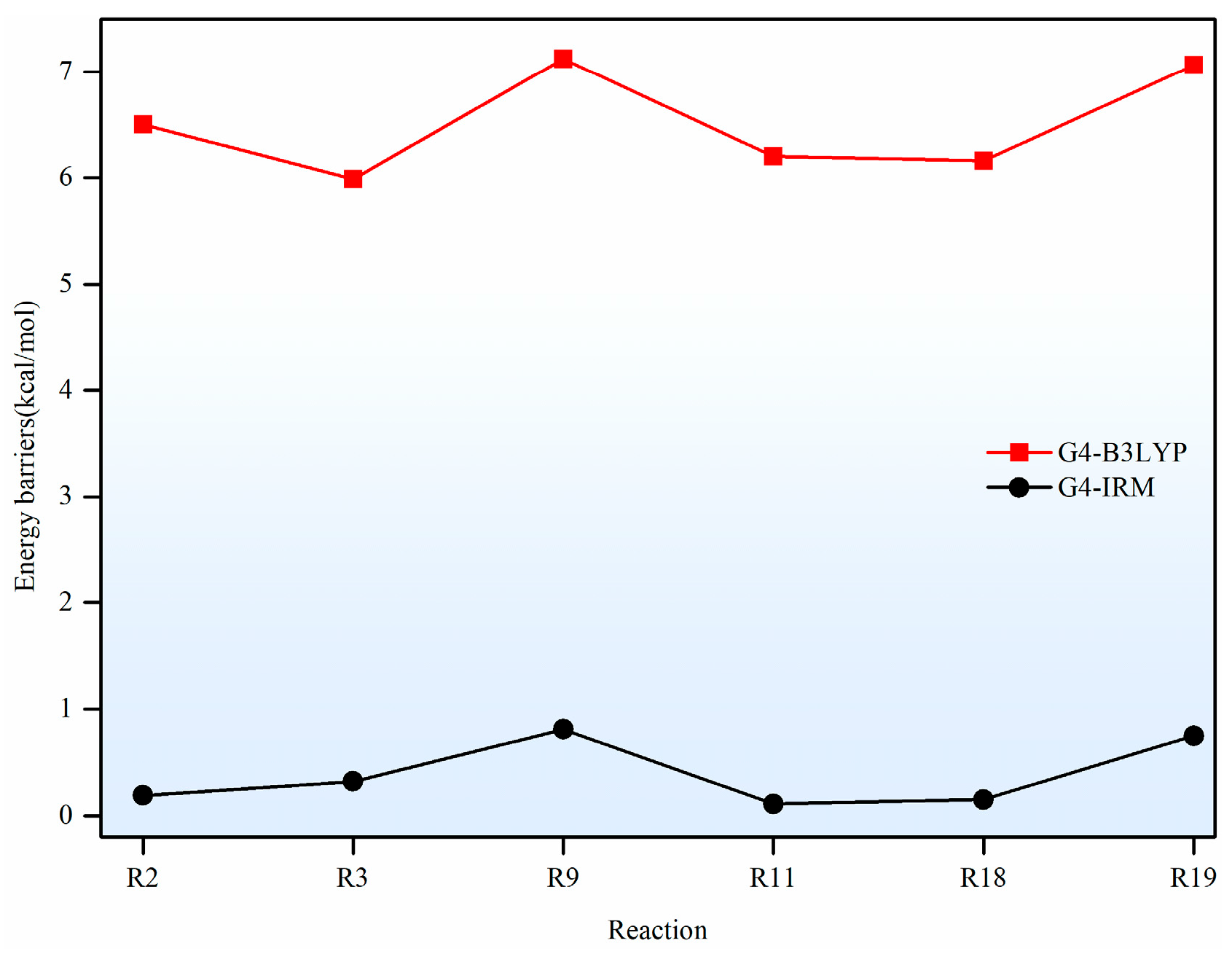
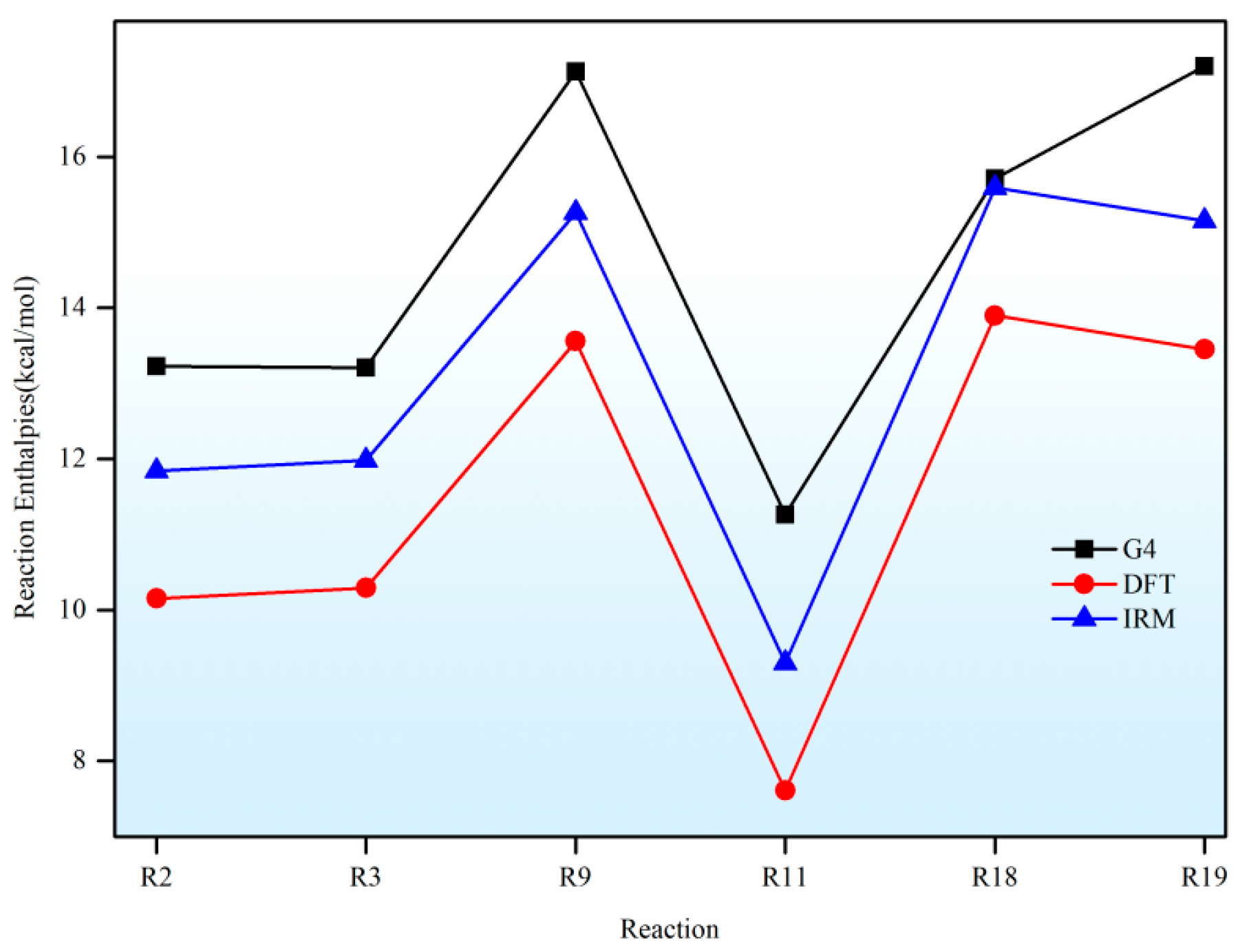
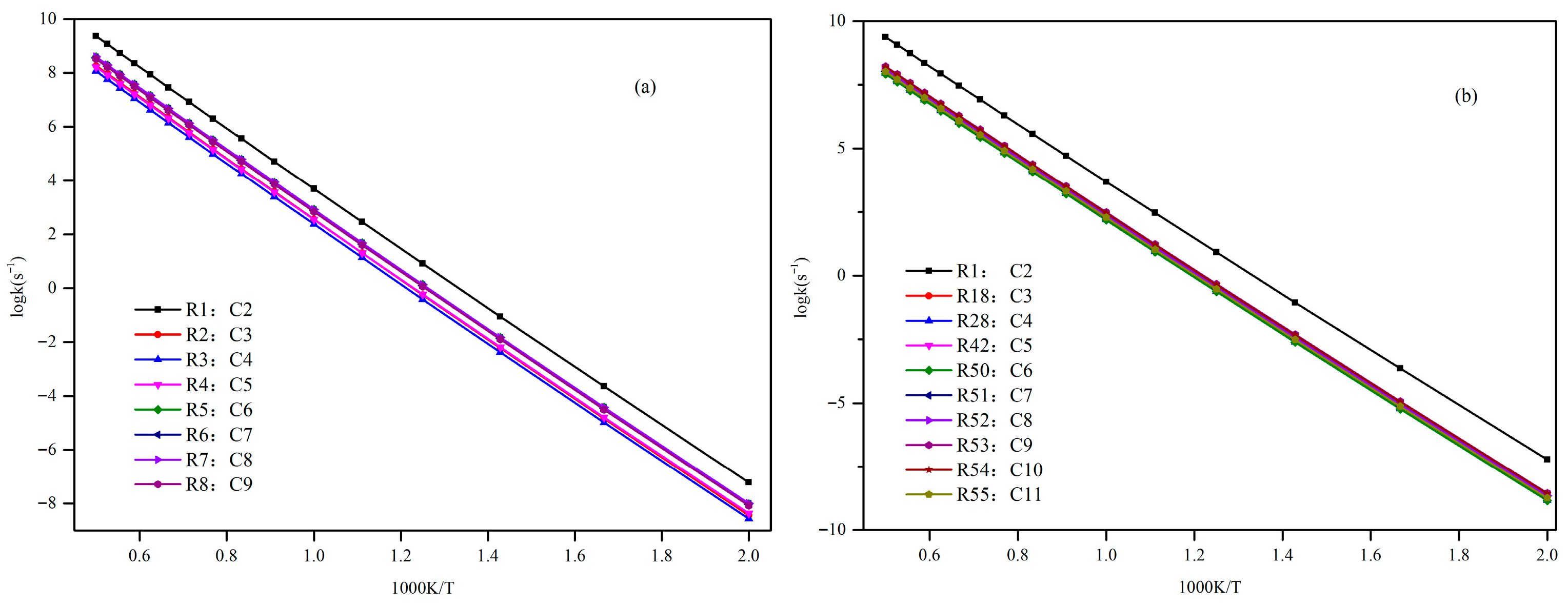
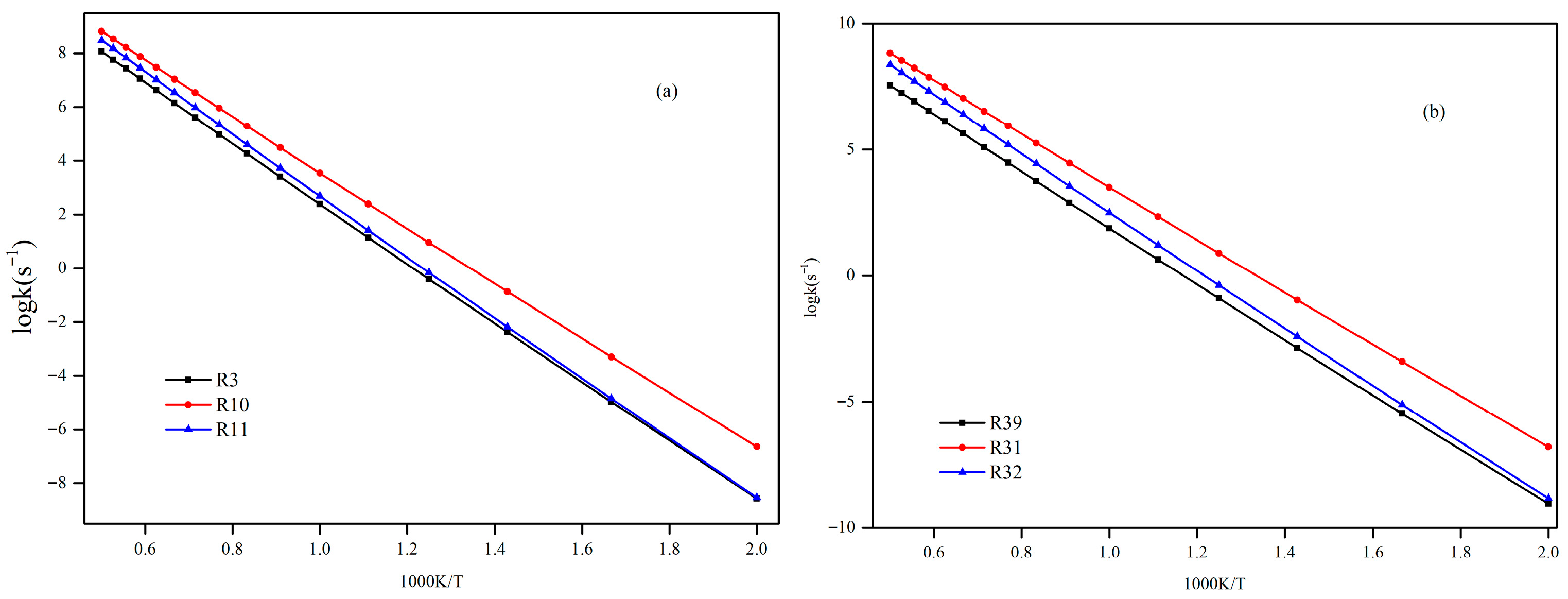
In this paper, a correction scheme within the isodesmic reaction method is utilized to correct the energy barriers, reaction enthalpies, and rate coefficients at the B3LYP level corresponding to the unimolecular alkene elimination reaction class of fatty acid alkyl esters (FAAEs). When comparing the corrected energy barriers and reaction enthalpies with the B3LYP results for a set of target reactions in the class (at the G4 level), it is found that the corrected outcomes are close to the values obtained using the G4 method. Furthermore, the rate coefficients obtained in this paper are comparable to experiment data, demonstrating that the energy barriers, reaction enthalpies, and rate coefficients are acceptable, and that the proposed isodesmic reaction correction scheme represents a viable approach for the accurate calculation of kinetic parameters for large-size molecules. Additionally, the rate rules at the high-pressure-limit are derived by averaging the rate coefficients of a representative set of reactions with different substituent sizes and carbon chain length. The findings show that the uncertainty factor f ranges from 1.21 to 59.5 at 500 K and from 1.07 to 12.9 at 1000 K, indicating that the uncertainty factor f of the rate rules decreases as the temperature increases. Consequently, the rate rules method provides a rapid approach for the mechanism generation program to determine the rate coefficients of large-molecular systems during the creation of the reaction mechanism for large molecules.
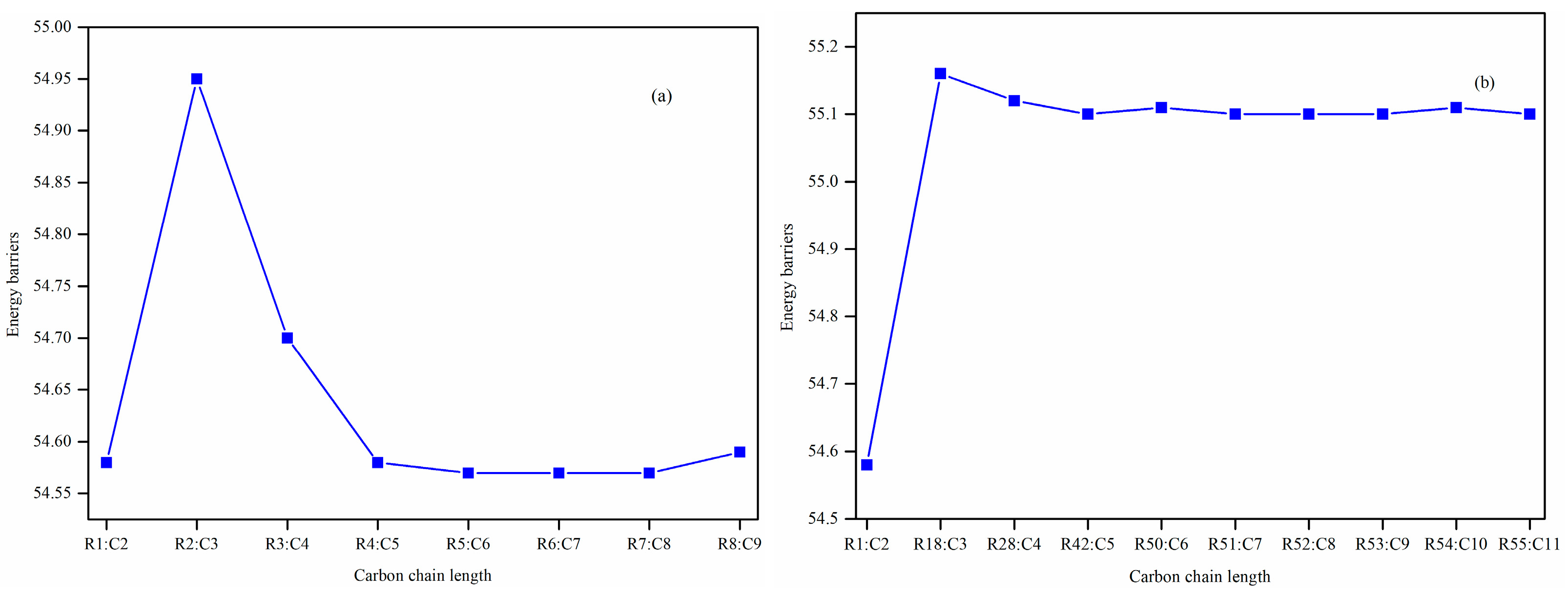
Furthermore, the impacts of the carbon chain length and the branching degree of alkyl groups on the energy barriers and rate coefficients are systematically examined. The results show that as the carbon chain length increases, the change in energy barrier is small. It is attributed to the distal segments of long alkyl chains merely act as non-participating entities, exerting no significant electronic or steric influence that could alter the energy barriers and rate coefficients. Nevertheless, it exhibits a high degree of sensitivity to the degree of the branching on energy barriers, wherein the α-branching can lower the energy barrier, while β-branching leads to an increase in the energy barrier. Meanwhile, the degree of branching in the alkyl chain exerts a profound influence on the rate coefficients. The α-branching consistently provides a significant kinetic advantage by lowering the activation barrier, resulting in a rate acceleration across 500~2000 K. In contrast, the effect of β-branching is markedly weaker. The significant acceleration induced by α-branching can be ascribed to the enhanced electron-donating inductive effect of the branched alkyl group, which effectively stabilizes the cyclic transition state. The results of the β-branching effect confirm that the calculated rate coefficients are comparable or even slightly larger across the investigated temperature range. This observation suggests that the steric hindrance in the β-branched isomer might outweigh the electronic effect, thereby giving rise to a less stable transition state. Additionally, the rate coefficient is also affected by temperature and the pre-exponential factor, which may be due to their combined effects.
In this study, a unified high-level theoretical method has been applied for the first time to a wide range of large-size fatty acid alkyl ester (FAAEs) molecules to obtain accurate kinetic parameters for this reaction class. This allows combustion modelers to incorporate these previously unaccounted-for reaction pathways into reaction mechanisms with a quantifiable level of certainty, directly addressing a well-acknowledged deficiency in biodiesel combustion models. The data presented here will promote more accurate simulations of biodiesel decomposition, thus contributing to a more profound fundamental understanding of the process and its results. The comprehensive kinetic dataset provided in this work establishes a fundamental basis for understanding structural effects in complex reactions in the reaction mechanism of biodiesel fuels, with extensive implications for predicting molecular reactivity and guiding the synthesis of target molecules with tailored kinetic characteristics.