Different Temperature Storage Conditions and Packaging Types Affects Colour Parameters, Amino Acid Composition, Microbial Contamination, and Key Bioactive Molecules of Moringa oleifera Lam. Powder
Abstract
1. Introduction
2. Results and Discussion
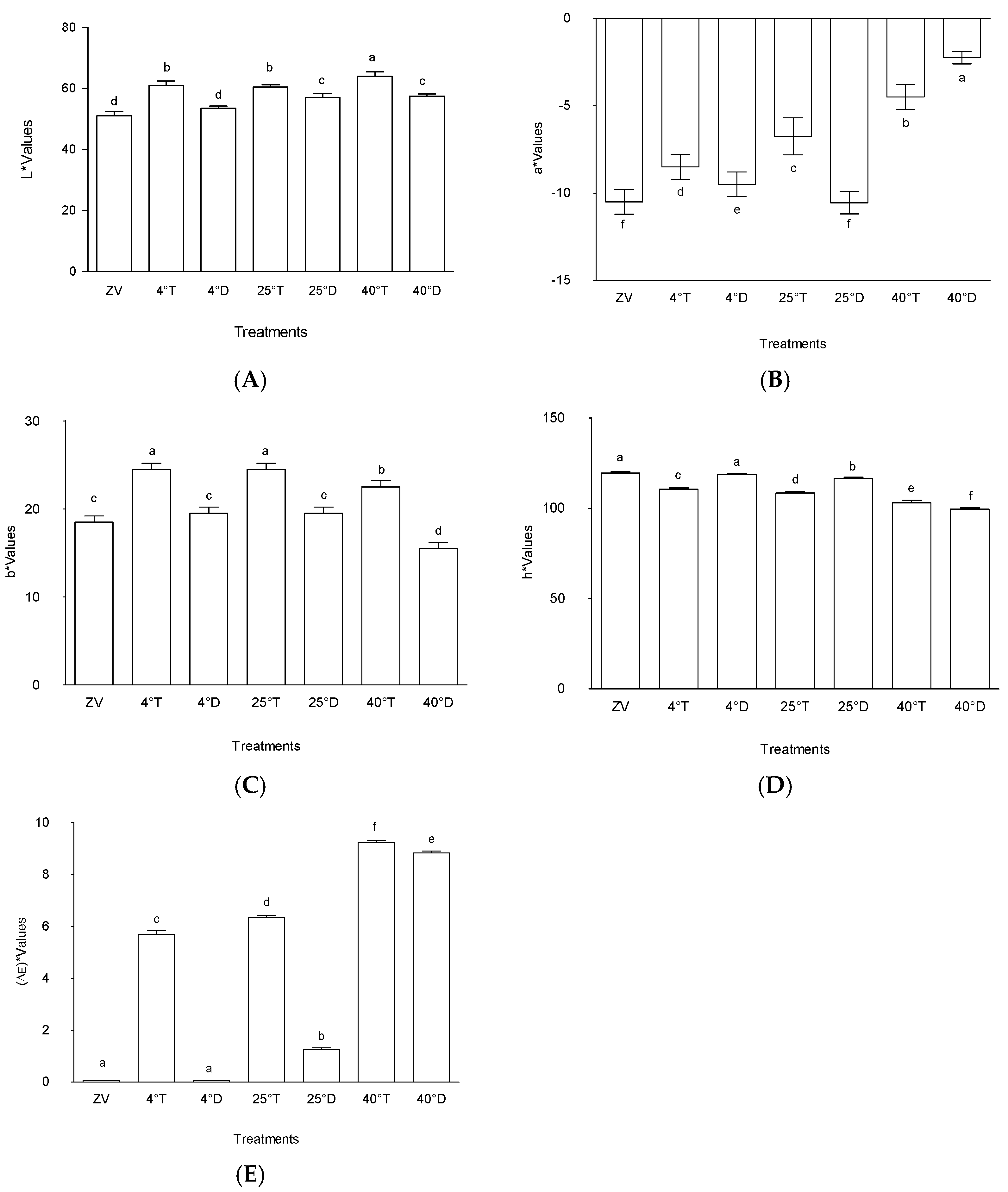
2.1. Colour
2.1.1. Lightness (L*)
2.1.2. Colour Redness (a*)
2.1.3. Colour Yellowness (b*)
2.1.4. Colour (h*)
2.1.5. Colour Difference “(ΔE)”
2.2. Amino Acids
2.3. Microbiological Analysis
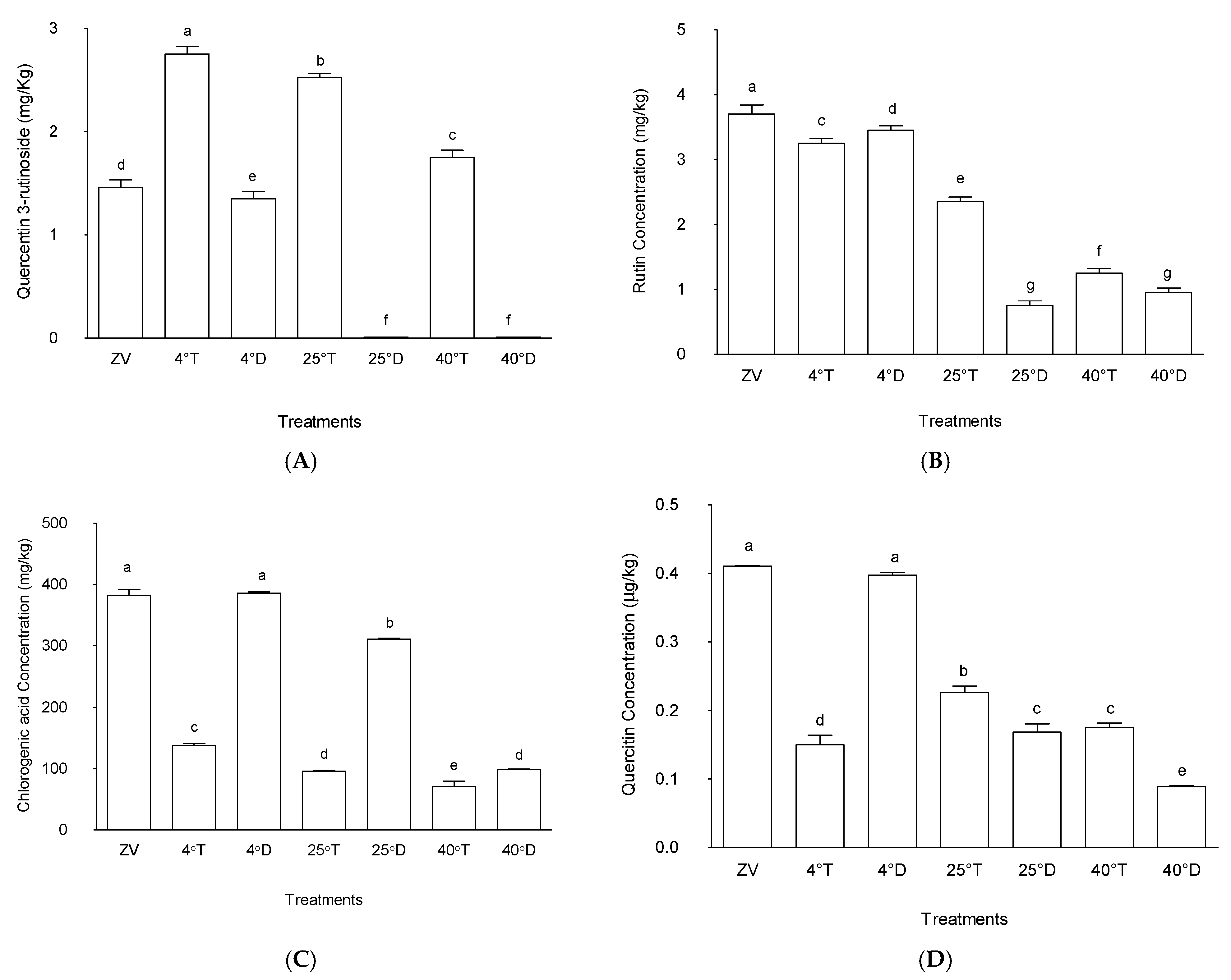
2.4. Phytochemicals
2.4.1. Quercetin-3-Rutinoside
2.4.2. Rutin
2.4.3. Chlorogenic Acid
2.4.4. Quercitrin
3. Materials and Methods
3.1. Plant Material
3.2. Experimental Design and Treatments
3.3. Colour Measurement
3.4. Amino Acids Determination
Derivatization Procedure
3.5. Determination of Microbial Contamination
3.5.1. Sample Dilution
3.5.2. Plating and Incubation
3.5.3. Colony Counting
3.6. Phenolic Variations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schippmann, U.; Leaman, D.J.; Cunningham, A.B. Impact of cultivation and gathering of medicinal plants on biodiversity: Global trends and issues. In Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries; FAO: Rome, Italy, 2002. [Google Scholar]
- Pandey, A.K. Harvesting and post-harvest processing of medicinal plants: Problems and prospects. Pharma Innov. J. 2017, 6, 229–235. [Google Scholar]
- Onukansi, C.P.; Ihekweazu, C.; Oladimeji, A. Investing in traditional medicine: Leveraging evidence and innovative research to strengthen the fight against malaria in Nigeria. Malar. J. 2025, 24, 359. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 1, pp. 285–292. [Google Scholar]
- Mishra, S.P.; Singh, P.; Singh, S. Processing of Moringa oleifera leaves for human consumption. Bull. Environ. Pharmacol. Life Sci. 2012, 2, 1–5. [Google Scholar]
- Dania, S.O.; Akpansubi, P.; Eghagara, O.O. Comparative effects of different fertilizer sources on the growth and nutrient content of moringa (Moringa oleifera) seedling in a greenhouse trial. Adv. Agric. 2014, 726313. [Google Scholar] [CrossRef]
- Abdelazim, S.M.; Hassan, M.A.; El-Sayed, A.M.; Al-Ghamdi, S.A. Moringa oleifera: Recent insights for its biochemical and medicinal applications. J. Food Biochem. 2024, 48, e1270903. [Google Scholar] [CrossRef]
- Fahey, J.W. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Trees Life J. 2005, 1, 1–15. [Google Scholar]
- Ndhlala, A.R.; Tshabalala, T. Diversity in the Nutritional Values of Some Moringa oleifera Lam. Cultivars. Divers. 2023, 15, 834. [Google Scholar] [CrossRef]
- Grubben, G.J.H.; Denton, O.A. Plant Resources of Tropical Africa 2. Vegetables; PROTA Foundation: Wageningen, The Netherlands; Backhuys Publishers: Leiden, The Netherlands; CTA: Wageningen, The Netherlands, 2004; pp. 1–688. [Google Scholar]
- Doerr, B.; Cameron, L.; Moringa Leaf Powder. ECHO Technical Note #51. 2005. Available online: https://www.echocommunity.org/en/resources/ (accessed on 26 May 2024).
- Mensah, M. Effect of Different Packaging Materials on the Quality and Shelf Life of Moringa oleifera Leaf Powder During Storage. Master’s Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2011. [Google Scholar]
- Kayembe, J.; Sekelwa, C.; Bassey, K. Comparative analysis of phytonutrients of Moringa oleifera leaves from South Africa and Nigeria, and their antimicrobial and antioxidant potentials by UPLC-ESI-QToF-MS and OPLS-DA chemometric analysis. Front. Nutr. 2025, 11, 1490484. [Google Scholar] [CrossRef]
- Rico, D.; Martin-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Peter, K.V. Handbook of Herbs and Spices; CRC Press: New York, NY, USA, 2006; Volume 3. [Google Scholar]
- Mayuoni-Kirshinbaum, L.; Daus, A.; Porat, R. Changes in sensory quality and aroma volatile composition during prolonged storage of ‘Wonderful’ pomegranate fruit. Int. J. Food Sci. Technol. 2013, 48, 1569–1578. [Google Scholar] [CrossRef]
- Haile, D.M.; De Smet, S.; Claeys, E.; Vossen, E. Effect of light, packaging condition and dark storage durations on colour and lipid oxidative stability of cooked ham. J. Food Sci. Technol. 2013, 50, 239–247. [Google Scholar] [CrossRef]
- Wang, A.; Duncan, S.E.; Whalley, N.W.; O’Keefe, S.F. Interaction effect of LED colour temperatures and light-protective additive packaging on photo-oxidation in milk displayed in retail dairy case. Food Chem. 2020, 323, 126699. [Google Scholar] [CrossRef]
- Phahom, T.; Kerr, W.L.; Pegg, R.B.; Phoungchandang, S. Effect of packaging types and storage conditions on quality aspects of dried Thunbergia laurifolia leaves and degradation kinetics of bioactive compounds. J. Food Sci. Technol. 2017, 54, 4405–4415. [Google Scholar] [CrossRef] [PubMed]
- Krah, C.Y.; Krisnadi, D. Effect of Harvest and Postharvest Handling on Quality of Moringa Leaf Powder. IOP Conf. Ser. Earth Environ. Sci. 2022, 1038, 012074. [Google Scholar] [CrossRef]
- Meyer, L.H. Food Chemistry; Affiliated East-West Press Pvt Ltd.: New Delhi, India, 1973. [Google Scholar]
- Djaeni, M.; Hadiyanto, H.; Suherman, S.; Handayani, N.A.; Utari, F.D. Production of chlorophyll-rich powder from Moringa oleifera leaves using dehumidification and intermittent drying: Impact on drying characteristics and chlorophyll retention. Int. J. Agric. Technol. 2025, 21, 1687–1702. [Google Scholar] [CrossRef]
- Hasizah, A.; Salengke, S.; Djalal, M.; Arifin, A.S.; Mochtar, A.A. Color change and chlorophyll degradation during fluidized bed drying of Moringa oleifera leaves. AIP Conf. Proc. 2023, 2871, 030007. [Google Scholar]
- Ahad, T.; Gull, A.; Nissar, J.; Masoodi, L.; Rather, A. Effect of storage temperatures, packaging materials and storage periods on antioxidant activity and non-enzymatic browning of antioxidant treated walnut kernels. J. Food Sci. Technol. 2020, 57, 3556–3563. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, H.-M.; Zhu, K.-X.; Guo, X.-N.; Peng, W. Physicochemical changes in the discoloration of dried green tea noodles caused by polyphenol oxidase from wheat flour. LWT 2020, 130, 109614. [Google Scholar] [CrossRef]
- Šopík, T.; Lazárková, Z.; Salek, R.N.; Talár, J.; Purevdorj, K.; Buňková, L.; Foltin, P.; Jančová, P.; Novotný, M.; Gál, R. Changes in the quality attributes of selected long-life food at four different temperatures over prolonged storage. Foods 2022, 11, 2004. [Google Scholar] [CrossRef]
- Östbring, K.; Sjöholm, I.; Rayner, M.; Erlanson-Albertsson, C. Effects of storage conditions on degradation of chlorophyll and emulsifying capacity of thylakoid powders produced by different drying methods. Foods 2020, 9, 669. [Google Scholar] [CrossRef]
- Schnellbaecher, A.; Lindig, A.; Le Mignon, M.; Hofmann, T.; Pardon, B.; Bellmaine, S.; Zimmer, A. Degradation products of tryptophan in cell culture media: Contribution to color and toxicity. Int. J. Mol. Sci. 2021, 22, 6221. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Impact of reactive species on amino acids—Biological relevance in proteins and induced pathologies. Int. J. Mol. Sci. 2022, 23, 14049. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ndhlala, A.R.; Ncube, B.; Abdelgadir, H.A.; Van Staden, J. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. S. Afr. J. Bot. 2020, 129, 106–112. [Google Scholar] [CrossRef]
- Olorode, O.; Bamgbose, A.; Raji, O. Effect of blanching and packaging material on the colour and microbial load of ben oil (Moringa oleifera) leaf powder. In Proceedings of the Outlook 2015 World Association for Sustainable Development Conference Proceedings, Istanbul, Turkey, 1–3 June 2015; pp. 311–320. [Google Scholar]
- Potisate, Y.; Kerr, W.L.; Phoungchandang, S. Changes during storage of dried Moringa oleifera leaves prepared by heat pump-assisted dehumidified air drying. Int. J. Food Sci. Technol. 2015, 50, 1224–1233. [Google Scholar] [CrossRef]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Han, H.J.; Lee, K.-Y.; Kim, A.-N.; Kim, J.C.; Choi, S.-G.; Heo, H.J. Effect of storage temperature on the antioxidant activity and catechins stability of Matcha (Camellia sinensis). Food Sci. Biotechnol. 2020, 29, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Donkor, A.; Nashirudeen, Y.; Suurbaar, J. Stability evaluation and degradation kinetics of rutin in Ficus pumila leaves formulated with oil extracted from Moringa oleifera seeds. J. Anal. Pharm. Res. 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Coz-Bolaños, X.; Campos-Vega, R.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Loarca-Piña, G.F.; Guzmán-Maldonado, S. Moringa Infusion (Moringa oleifera) Rich in Phenolic Compounds and High Antioxidant Capacity Attenuate Nitric Oxide Pro-Inflammatory Mediator In Vitro. Ind. Crops Prod. 2018, 118, 95–101. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The Photostability of Flavanones, Flavonols and Flavones and Evolution of Their Antioxidant Activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Managa, M.G.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Moist Cooking Blanching on Colour, Phenolic Metabolites and Glucosinolate Content in Chinese Cabbage (Brassica rapa L. subsp. chinensis). Foods 2019, 8, 399. [Google Scholar] [CrossRef] [PubMed]


| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| ZV | 4 °T | 4 °D | 25 °T | 25 °D | 40 °T | 40 °D | |
| Histidine | 1.22 ± 0.001 a | 0.87 ± 0.020 cd | 0.94 ± 0.01 b | 0.89 ± 0.010 bc | 0.84 ± 0.020 cd | 0.8 ± 0.010 d | 0.86 ± 0.020 cd |
| Arginine | 2.44 ± 0.001 b | 2.38 ± 0.010 b | 2.07 ± 0.01 c | 2.52 ± 0.050 ab | 1.96 ± 0.040 c | 2.75 ± 0.050 a | 2.1 ± 0.050 c |
| Serine | 1.98 ± 0.020 a | 1.73 ± 0.050 b | 1.64 ± 0.01 b | 1.66 ± 0.020 b | 1.58 ± 0.020 b | 1.95 ± 0.040 a | 1.58 ± 0.010 b |
| Glycine | 2.65 ± 0.001 a | 1.86 ± 0.010 f | 1.96 ± 0.01 e | 2.03 ± 0.010 d | 1.93 ± 0.010 e | 2.18 ± 0.010 b | 2.11 ± 0.010 c |
| Aspartic acid | 4.78 ± 0.001 a | 3.15 ± 0.010 c | 2.56 ± 0.02 d | 2.45 ± 0.020 ef | 2.52 ± 0.010 de | 3.42 ± 0.020 b | 2.35 ± 0.020 f |
| Glutamic | 6.2 ± 0.001 a | 4.54 ± 0.020 c | 4.33 ± 0.02 d | 3.95 ± 0.010 g | 4.15 ± 0.010 e | 5.16 ± 0.010 b | 4.05 ± 0.020 f |
| Threomine | 2.1 ± 0.001 a | 1.6 ± 0.020 cd | 1.67 ± 0.01 c | 1.56 ± 0.020 d | 1.63 ± 0.020 cd | 1.84 ± 0.001 b | 1.63 ± 0.020 cd |
| Alanine | 3.77 ± 0.001 a | 2.05 ± 0.010 c | 1.77 ± 0.01 e | 1.89 ± 0.010 d | 1.8 ± 0.010 e | 2.26 ± 0.010 b | 1.77 ± 0.010 e |
| Proline | 2.98 ± 0.001 a | 1.4 ± 0.010 c | 1.54 ± 0.05 bc | 1.54 ± 0.020 bc | 1.52 ± 0.010 bc | 1.6 ± 0.010 b | 1.59 ± 0.010 b |
| Lysine | 4.56 ± 0.001 a | 2.72 ± 0.020 f | 2.9 ± 0.01 e | 3.43 ± 0.020 c | 2.87 ± 0.010 e | 3.26 ± 0.010 d | 3.59 ± 0.010 b |
| Tyrosine | 2.44 ± 0.001 a | 1.22 ± 0.010 g | 0.97 ± 0.01 f | 0.54 ± 0.010 c | 1.04 ± 0.010 b | 1.12 ± 0.010 e | 0.63 ± 0.010 d |
| Methionine | 1.22 ± 0.001 a | 0.54 ± 0.001 d | 0.54 ± 0.01 d | 0.65 ± 0.001 c | 0.54 ± 0.001 d | 0.88 ± 0.001 b | 0.57 ± 0.001 d |
| Valine | 4.44 ± 0.001 a | 1.91 ± 0.010 de | 1.85 ± 0.02 e | 2 ± 0.020 c | 1.86 ± 0.020 e | 2.14 ± 0.010 b | 1.94 ± 0.010 cd |
| Isoleucine | 2.33 ± 0.001 a | 1.44 ± 0.010 c | 1.49 ± 0.01 c | 1.57 ± 0.010 b | 1.47 ± 0.010 c | 1.6 ± 0.010 b | 1.61 ± 0.010 b |
| Leucine | 4.33 ± 0.001 a | 2.6 ± 0.010 f | 2.83 ± 0.01 e | 2.89 ± 0.010 d | 2.89 ± 0.010 d | 3 ± 0.001 c | 3.13 ± 0.010 b |
| Phenylalanine | 5.33 ± 0.001 a | 2.92 ± 0.010 g | 3.2 ± 0.01 f | 3.75 ± 0.001 c | 3.28 ± 0.010 b | 3.52 ± 0.010 e | 3.96 ± 0.010 d |
| Microorganisms (CFU/g) | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | E.c | S.Sp. | S.a | B.c | Ac | Tc | Y&m |
| ZV | Absent | Absent | Absent | Absent | <10 | 20 | 0.5 × 102 |
| 4 °T | Absent | Absent | Absent | Absent | <10 | 33 | <10 |
| 4 °D | Absent | Absent | Absent | Absent | 10 | 33 | <10 |
| 25 °T | Absent | Absent | Absent | Absent | 20 | 50 | <10 |
| 25 °D | Absent | Absent | Absent | Absent | 40 | 55 | <10 |
| 40 °T | Absent | Absent | Absent | Absent | 60 | 56 | <10 |
| 40 °D | Absent | Absent | Absent | Absent | 40 | 50 | <10 |
| SANS1683:2015 Requirements | 0 | NS | NS | 0 | NS | ≤1.0 × 102 | ≤5.0 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndhlala, A.R.; Ngobeni, G.T.; Mulaudzi, R.; Lebelo, S.L. Different Temperature Storage Conditions and Packaging Types Affects Colour Parameters, Amino Acid Composition, Microbial Contamination, and Key Bioactive Molecules of Moringa oleifera Lam. Powder. Molecules 2025, 30, 4048. https://doi.org/10.3390/molecules30204048
Ndhlala AR, Ngobeni GT, Mulaudzi R, Lebelo SL. Different Temperature Storage Conditions and Packaging Types Affects Colour Parameters, Amino Acid Composition, Microbial Contamination, and Key Bioactive Molecules of Moringa oleifera Lam. Powder. Molecules. 2025; 30(20):4048. https://doi.org/10.3390/molecules30204048
Chicago/Turabian StyleNdhlala, Ashwell R., Gladness T. Ngobeni, Rofhiwa Mulaudzi, and Sogolo L. Lebelo. 2025. "Different Temperature Storage Conditions and Packaging Types Affects Colour Parameters, Amino Acid Composition, Microbial Contamination, and Key Bioactive Molecules of Moringa oleifera Lam. Powder" Molecules 30, no. 20: 4048. https://doi.org/10.3390/molecules30204048
APA StyleNdhlala, A. R., Ngobeni, G. T., Mulaudzi, R., & Lebelo, S. L. (2025). Different Temperature Storage Conditions and Packaging Types Affects Colour Parameters, Amino Acid Composition, Microbial Contamination, and Key Bioactive Molecules of Moringa oleifera Lam. Powder. Molecules, 30(20), 4048. https://doi.org/10.3390/molecules30204048







